Molecular determinants of emerging excitability in rat embryonic motoneurons (original) (raw)
Abstract
Molecular determinants of excitability were studied in pure cultures of rat embryonic motoneurons. Using RT-PCR, we have shown here that the spike-generating Na+ current is supported by Nav1.2 and/or Nav1.3 α-subunits. Nav1.1 and Nav1.6 transcripts were also identified. We have demonstrated that alternatively spliced isoforms of Nav1.1 and Nav1.6, resulting in truncated proteins, were predominant during the first week in culture. However, Nav1.6 protein could be detected after 12 days in vitro. The Navβ2.1 transcript was not detected, whereas the Nav β1.1 transcript was present. Even in the absence of Navβ2.1, α-subunits were correctly inserted into the initial segment. RT-PCR (at semi-quantitative and single-cell levels) and immunocytochemistry showed that transient K+ currents result from the expression of Kv4.2 and Kv4.3 subunits. This is the first identification of subunits responsible for a transient K+ current in spinal motoneurons. The blockage of Kv4.2/Kv4.3 using a specific toxin modified the shape of the action potential demonstrating the involvement of these conductance channels in regulating spike repolarization and the discharge frequency. Among the other Kv α-subunits (Kv1.3, 1.4, 1.6, 2.1, 3.1 and 3.3), we showed that the Kv1.6 subunit was partly responsible for the sustained K+ current. In conclusion, this study has established the first correlation between the molecular nature of voltage-dependent Na+ and K+ channels expressed in embryonic rat motoneurons in culture and their electrophysiological characteristics in the period when excitability appears.
The development of excitability in mammal motoneurons is a complex progression of events (Xie & Ziskind-Conhaim, 1995; Gao & Ziskind-Conhaim, 1998). In the rat, motoneurons become excitable at embryonic day 14 (E14), immediately after they cluster in the ventral horn (Ziskind-Conhaim, 1988). Afferent fibres coming from the dorsal horn enter the grey matter at E17 and at the same time, motor axons form initial contacts with muscle fibres (Grinnell, 1995). During late embryonic and post-natal development, motoneuron excitability increases (Gao & Ziskind-Conhaim, 1998), and these changes partially interfere with important phenomena such as apoptosis, axon terminal branching and synapse elimination (Mynlieff & Beam, 1992; Grinnell, 1995).
To better understand the molecular mechanisms involved in the maturation of motoneuronal excitability, it is necessary to identify the ion channels, and their constitutive subunits, expressed at the different stages of differentiation. The basis of spike generation in mammal and chick spinal motoneurons is relatively constant during development. Na+-dependent action potentials are predominant from the onset of excitability (MacDermott & Westbrook, 1986; Ziskind-Conhaim, 1988; McCobb et al. 1990) and the activation of transient and sustained K+ currents is observed during spike repolarization (Takahashi, 1990). Calcium currents are also observed (McCobb et al. 1989), but their participation in spike generation is not as crucial as that described in Xenopus motoneurons (O'Dowd et al. 1988).
Some of the Na+ and K+ channels expressed in adult rat spinal motoneurons have already been identified (Black et al. 1994; Veh et al. 1995; Rudy et al. 1999; Schaller & Caldwell, 2000). However, until now, no information has been available concerning the subunits expressed at the onset of excitability.
In the present study, we established a correlation between the molecular nature of voltage-dependent Na+ and K+ channels expressed in rat motoneurons and their electrophysiological characteristics in the period when excitability appears. For this purpose, we used spinal motoneurons isolated from rat embryos at E14. This well characterized model (Henderson et al. 1994) has mainly been used to study the requirement for trophic factors in motoneuron survival (Oppenheim, 1996; Henderson et al. 1998). We have shown that TTX-sensitive Na+ currents are likely to be the consequence of the expression of Nav1.2 and Nav1.3 α-subunits. Nav1.1 and Nav1.6 α-subunits were identified in spliced forms corresponding to truncated proteins, while the auxiliary Nav β-2.1 subunit was surprisingly absent. We identified Kv4.3 and Kv4.2 α-subunits as the molecular determinants of the transient K+ current. The nature of the proteins underlying the sustained K+ currents was not fully characterized despite evidence that Kv1.6 was partially involved.
METHODS
Motoneuron cell culture
Rats were anaesthetized by 4 % halothane inhalation and killed with an excess of CO2. This procedure was in agreement with the French Ministry of Agriculture and the European Community Council Directive no. 86/609/EEC. Rat spinal motoneurons were purified from ventral cords of E14 embryos as described by Henderson et al. (1994). Purified motoneurons were plated in 35 mm dishes (Nalge Nunc, USA) or on glass coverslips (CML, Angers, France) coated with polyornithine-laminin (Sigma, St Louis, MO, USA). The culture medium was a chemically defined medium composed of neurobasal (Gibco-BRL) supplemented with 2 % B27 (Gibco-BRL), 2 % horse serum, 2.5 × 10−5m 2-mercaptoethanol and containing glial cell line-derived neurotrophic factor (100 pg ml−1) and ciliary neurotrophic factor (1 ng ml−1) (Calbiochem, La Jolla, CA, USA).
Total RNA isolation
RNA extraction was performed on motoneurons grown in 35 mm dishes at a density of 4000-6000 cells per dish. Motoneurons were quantified in Petri dishes by direct counting under phase-contrast. Cultured cells were subjected to RQ1 DNase (100 U ml−1) digestion (for 40 min at 37 °C) in order to remove DNA attached to nuclear debris. Total RNA was then extracted with 0.8 ml Tri Reagent (Sigma) containing 60 μg ml−1 yeast tRNA (Roche, Mannheim, Germany), according to the manufacturer's instructions. The RNA pellet was solubilized in 20 μl of diethyl pyrocarbonate-treated water and DNase treatment was performed for 1 h at 37 °C in 30 μl of 1 × reverse transcription buffer (Life Technologies) containing 6 mm dithiothreitol, 1000 U ml−1 ribonuclease inhibitor (Rnasin, Promega, Madison, WI, USA) and 70 U ml−1 RQ1 DNase (Promega). RQ1 DNase was then heat-inactivated for 5 min at 75 °C and DNase-treated RNA was divided into aliquots and stored at −80 °C until use.
Semi-quantitative RT-PCR
Reverse transcription was mainly performed as described by Lambolez et al. (1992). In brief, RNA was denatured at 95 °C for 1 min and reverse transcription initiated as described (Lambolez et al. 1992) with 1 μl of reverse transcriptase (Superscript II, Life Technologies). Amplification of DNA was conventionally performed by PCR using an MJ Research thermal cycler (Wathman, MA, USA). Primers used in this study were obtained from Genosys (Pampisford, UK).They were either designed with appropriate software (Oligo software, Medprobe, Oslo, Norway) or using information taken from the literature (Song et al. 1998). The main characteristics of the primers used in the present study are summarized in Tables 1 and 2. PCRs were routinely prepared as described (Lambolez et al. 1992) using Taq polymerase (Ampli Tag Gold, Perkin Elmer, Foster City, CA, USA). The reaction mixture was usually divided into two 50 μl aliquots, which were amplified for 34 and 39 cycles respectively, allowing control of the linearity of the reaction. The thermal cycling programme included an initial activation step at 95 °C for 15 min followed by amplification cycles (94 °C for 45 s, 58 (or 63 °C) for 80 s and 68 °C for 90 s).
Table 1.
Characteristics of primer pairs used in PCR of different sodium channel transcripts
| mRNA | Primer pair | Primer position | Sequence | Product length | Accession number |
|---|---|---|---|---|---|
| Nav1.1 | Na1-1 | 6179 | gatgtccactgcagcttgt | 371 | X03638 |
| 6549 | tccctacagtctgcatag | ||||
| Nav1.1 | Na-ex 18 | 3983 | gcgaaagacaatcaagaccat | 331 | X03638 |
| 4313 | gcaaaccagaagcacattca | ||||
| Nav1.2 | Na2-1 | 6323 | tgtatctgtgactccctcagg | 315 | X03639 |
| 6637 | atggcaggtgtggcagtta | ||||
| Nav1.3 | Na3-1 | 6064 | agggaaggattgacttgcc | 295 | Y00766 |
| 6358 | tggacctctccttagagtcca | ||||
| Nav1.6 | Na6-1 | 6137 | cagatctcctatcgcttggg | 401 | L39018 |
| 6537 | agtcctcctcagcctgaaac | ||||
| Nav1.6 | Na 6N | tttaatgggagcttctggga | — | L39018 | |
| 4301 | atgtagatgttgccctcgtag | ||||
| Nav1.6 | Na 6A | 3912 | acgatttgaagggatgagggt | 294 | L39018 |
| 4205 | tgaaggttgccacttgaagaa | ||||
| Nav1.1-1.6 | Nacom | ttcaccaatgcctggtg | — | — | |
| Gccgaagatgatgaaga | |||||
| Naβ1.1 | GNB1-1 | 485 | gtgtggtgtggaacggtagt | 338 | M91808 |
| 822 | caggtattccgaggcattct | ||||
| Naβ1.1A | GNB1-1A | 487 | agggacagatggaaagaagg | 211 | AF182949 |
| 698 | ggaatacacccgtcagagg | ||||
| Naβ2.1 | GNB2-1 | 355 | ctctctgaactggacttacc | 402 | U37026 |
| 756 | ttcaggtcatccgtgctcag | ||||
| GAPDH | GAPDH | 591 | accacagtccatgccatcac | 452 | X02231 |
| 1042 | tccaccaccctgttgctgta | ||||
| ChAT | ChAT-P | tggcttactacaggctttaccagagactg | 338 | Promega | |
| Ggacaaaccggttgctcatcagg | (Ref. G5760) |
Table 2.
Characteristics of primer pairs used in potassium channel PCR
| mRNA | Primer pair | Primer position | Sequence | Product length | Accession number |
|---|---|---|---|---|---|
| Kv 1.1 | 11-2 | 254 | gtgatgtcaggggagaatgc | 603 | X12589 |
| 856 | gtgaatggtgcccgtgaagt | ||||
| 11-3 | 1573 | ccgccgcagctcctctactatca | 209 | ||
| 1781 | caagggttttgtttgggggctttt | ||||
| Kv 1.2 | 12-1 | 581 | gacccagtggatgaggctgc | 640 | X16003 |
| 1220 | cggtgaaggaggtggactgc | ||||
| 12-2 | 1816 | ccgggagacagagggagagg | 537 | ||
| 2352 | ttgatatggtgtgggggctatga | ||||
| Kv 1.3 | 13-3 | 2453 | ccatagcaacccgtgtttc | 280 | X16001 |
| 2732 | agacgacagtttcctttctga | ||||
| 13-4 | 782 | gagctgctggtgcgattcttt | 207 | ||
| 988 | gagcttgaagatgcggaagac | ||||
| Kv 1.4 | 14-1 | 2310 | ctgggggacaagtcagagtatcta | 434 | X16002 |
| 2743 | actctcctcgggaccacct | ||||
| Kv 1.5 | 15-1 | 2415 | tttgcaagactgggggttcc | 251 | M27158 |
| 2665 | gtgggcttaaatactcggtggtgt | ||||
| Kv 1.6 | 16-1 | 1836 | agacggagcaggaggaacaa | 370 | X17621 |
| 2205 | gaggagcgagcaacagtcta | ||||
| 16-2 | 1559 | ctcgggctactcatcttcttc | 201 | ||
| 1759 | acacagtgagcccacaatctt | ||||
| Kv 2.1 | 2.1-4 | 594 | gatcatgttcatcgtcctgtc | 250 | X16476 |
| 844 | gctcttgttggattctgtgag | ||||
| 2.1-5 | 229 | gacgactacagccttgaggac | 197 | ||
| 426 | tcgttcatctgctccttcttt | ||||
| Kv 2.2 | 2.2-4 | 550 | aggtgtgcgatgactacaac | 344 | M77482 |
| 878 | cggatgagttcggcttc | ||||
| 2.2-5 | 682 | ttggccaagagcttgattac | 322 | ||
| 987 | ttgcccaaattcatcgtt | ||||
| Kv 3.1 | 31-3 | 2063 | aagctgccaaggacgtgc | 277 | M68880 |
| 2323 | ccaccagaagccgatggg | ||||
| 31-5 | 788 | ccccatcatctctcccctcct | 233 | ||
| 1020 | gccacccaccccaaccaacga | ||||
| Kv 3.2 | 32-2 | 1058 | ccatcctccccttctactta | 274 | M84202 |
| 1331 | acagcccaccagaaaccaat | ||||
| Kv 3.3 | 33-3 | 1316 | tcatgcgggtcaccttctgc | 511 | M84211 |
| 1826 | ggcatggcaatggtcagcac | ||||
| Kv 3.4 | 34-1 | 1292 | tggttcgcattgtgtgctgc | 676 | X62841 |
| 1967 | tcactgtaggtgctgtcccg | ||||
| Kv 4.1 | 41-1 | 1386 | cggacaaatgctgtgcgttag | 467 | M64226 |
| 1852 | taggggaggaaggttgactttcat | ||||
| Kv 4.2 | 42-1 | 2165 | ccgaatcccaaatgccaatgtg | 265 | S64320 |
| 2429 | cctgacgatgtttcctcccgaata | ||||
| 42-3 | 2030 | tgtggatgaacaagtctttga | 524 | ||
| 2553 | ttgcttggctgtcttaatatg | ||||
| Kv 4.3 | 43-1 | 1389(1331) | gcaagcgcaatggactcctcaa | 217(274) | U42975 |
| 1605(1604) | gaagggcttctggtggatgggtag | (AB003887) | |||
| 43-2 | 165 | agcggcaagatgagctaatc | 180 | U42975 | |
| 344 | tgagcacacaacggaacact | ||||
| 43-3 | 1206 | ttggctccatctgctccctaa | 254 | ||
| 1459 | cttgcccatgtgctcctcttc |
Amplification products were analysed by electrophoresis on 2 % agarose gels containing 5 μg ml−1 ethidium bromide. Photographs of the gels were digitized and images processed using Molecular Analyst software (Biorad, Hercules, CA, USA) for quantification of the signal.
In order to achieve a reliable relative estimation of the abundance of the different mRNAs initially present in motoneurons, we set several criteria in the PCR procedure. We first determined the limits of linearity of the amplification reaction by running PCRs in different cycles with different dilutions of cDNA. Linear intensity of the signal as a function of cDNA concentration was observed between 34 and 36 cycles. Thereafter (as indicated above), PCRs were limited to two series of cycles (34 and 39 cycles respectively). After a 34 cycle series, amplification was included in the linearity zone and thus used for semi-quantification. The second series (39 cycles) allowed us to ensure that the plateau was reached. Variation of amplification efficacy is inherent in the use of different sets of primers for different cDNA targets. To circumvent this problem we decided to simultaneously amplify a given amount of rat genomic DNA which contains a constant amount of target copies and use this as a standard. The amplification signal from any couple of primers directed towards any cDNA target could thus be compared to the corresponding amplification signal of genomic DNA. This approach, which was only possible when the two primers were present on the same exon, imposes drastic controls to eliminate the effect of DNA contamination. PCR reactions on DNase-treated motoneuron RNA which had not been previously subjected to RT were thus systematically performed.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA amplification was used to normalize RT-PCR obtained from different origins.
Specificity of the RT-PCR was controlled after analysis of the size of each amplification product before and after restriction analysis. RT-PCR studies of each transcript were usually performed with two different sets of primers.
Analysis of alternative splicing of Nav1.6 exon 18
Analysis of alternative splicing of the different α-subunit genes in regions homologous to mouse SCN8A/exon 18 was conducted by RT-PCR and subsequent cloning of the reaction products. We designed a set of primers (Nacom, Table 1), chosen in highly conserved regions on each side of exon 18 of SCN8A. The sequence of these primers perfectly matched that of all four central nervous system α-subunits. RT-PCR products were subcloned into PGEM-T vector (Promega) and DNA sequencing was performed on 24 clones.
A search for alternative splicing of the Nav1.1 transcript in a homologous region was completed with specific primers (Na1-ex18) located in Nav1.1 regions homologous to exons 17 and 19 SCN8A. Amplification products were purified by gel electrophoresis, submitted to a new run of amplification and then sequenced on both strands using either primer of the Na1-ex18 pair.
Single cell RT-PCR analysis for Kv4.2 and Kv4.3 transcripts
Single cell RT-PCR analysis was conducted as described by (Lambolez et al. 1992). In brief, after membrane rupture, cell content was harvested under visual control in the recording pipette containing 10 μl intracellular solution. Two successive amplifications (25 and 35 cycles respectively) were performed as described, except that Taq polymerase was from Qiagen, Switzerland. The following multiplexed primers were used: for Kv4.2, upstream primer caaatgccaatgtgtcaggaa (nucleotides 2173-2193) and downstream primer ttccatagtcagggctcccat (nucleotides 2489-2469) of Shal1 cDNA (Gene Bank accession number S64320); for Kv4.3, upstream primer ttggctccatcgctccctaa (nucleotides 1206-1226) and downstream primer cttgcccatgtgctcctcttc (nucleotides 1459-1439) of Shal-related K+ channel cDNA (Gene Bank accession number U42975).
Immunofluorescence labelling
Motoneurons were prefixed by adding paraformaldehyde (PAF, 2 % final concentration) in the dish (2 min at room temperature) and subsequently fixed for 30 min with 4 % PAF. After quenching the excess aldehyde groups with 50 mm ammonium chloride, cells were washed and permeabilized with 0.1 % Triton X-100 in 1 % BSA containing PBS. Monoclonal antibodies anti-Kv1.1, Kv1.2, Kv1.4 or Kv1.6 were from Upstate Biotechnology (Lake Placid, NY, USA). Polyclonal antibody anti-Kv4.3 was kindly given by Dr J. Nerbonne (Barry et al. 1995). All primary antibodies were used at 10 μg ml−1 and incubated for 1 h at room temperature. After washing, cells were treated for 1 h with 5 μg ml−1 of either biotinylated goat anti-mouse or anti-rabbit second antibody (Vector, Burligame, CA, USA). Biotinylated antibody was revealed with fluorescein-avidin (Vector). Images were collected under a confocal microscope (Leica, Heidelberg, Germany).
Monoclonal pan anti-sodium channel α-subunit antibody (clone 58/35, Sigma) was used at 10 μg ml−1 and revealed with anti-mouse Alexa 546 antibody (1:800 dilution) (Molecular Probes). Polyclonal anti-Nav1.2 (Upstate Biotechnology) and anti-Nav1.6 (Chemicon, Temecula, CA, USA) antibodies were respectively used at 1:20 and 1:100 dilution and revealed with anti-rabbit Alexa 488 (1:400 dilution) (Molecular Probes).
Whole-cell patch clamp recording
Motoneurons were studied using the conventional whole-cell patch clamp technique. The bath solution contained (mm): 140 NaCl, 2 KCl, 0.8 MgCl2, 1.8 CaCl2, 0.4 Na2HPO4 and 10 Hepes, pH 7.3. Drugs were added to the bath solution or applied under pressure with a broken pipette placed close to the soma of the recorded motoneuron. We are grateful to S. Diochot (CNRS, UPR 411, Sophia-Antipolis, France) for the generous gift of Phrixotoxin 2 (PaTX2).
Experiments were carried out at room temperature (20-24 °C) with a List EPC7-patch-amplifier. Patch clamp electrodes with resistances of 3.5 to 5 MΩ were filled with (mm): 120 KCl, 10 NaCl, 2 MgCl2, 0.56 CaCl2, 1 EGTA and 10 Hepes, pH 7.4. H. Chagneux (CNRS, Marseille, France) drew up the software programs used for stimulation, data acquisition and analysis. Current or voltage traces were low-pass filtered at 2 kHz and digitized on a hard disk at 2-10 kHz. Capacity transient compensation was routinely performed in the cell-attached mode before patch membrane rupture. In the whole-cell voltage clamp configuration, capacitive transients and leakage currents were subtracted using a factorized hyperpolarizing pulse, without additional transient or series resistance compensation. A junction potential of 5 mV was subtracted from applied voltages.
RESULTS
Characterization of Na+ channel subunits expressed in cultured motoneurons
RT-PCR analysis of Na+ channels was restricted to Nav1.1, Nav1.2, Nav1.3 and Nav1.6 and to the two auxiliary subunit Navβ1.1 and Navβ2.1 mRNAs. As shown in Fig. 1_A,B_, Nav1.3, and to a lesser extent Nav1.2, were the most abundant α-subunit mRNAs, with a lower expression of Nav1.1 and Nav1.6 (Fig. 1_A_). No significant changes were observed between day 0 and day 7 of culture for these transcripts (Fig. 1_B_). In contrast, the amount of choline acetyl transferase (ChAT) amplification product increased during this period (Fig. 1_D_).
Figure 1. RT-PCR analysis of sodium channel transcripts in cultured motoneurons.

A, cDNA obtained from four different motoneuron cultures (1-4) at DIV 0 (0) or DIV 7 (7) was amplified by PCR with primer pairs Na1-1, Na2-1, Na3-1 and Na6-1 (specific for Nav1.1, Nav1.2, Nav1.3 and Nav1.6, respectively) and with primer pair GAPDH. Simultaneous amplification of gDNA was carried out. Positive controls on cDNA from adult rat brain (Ad brain) were also performed (equivalent to 1 ng total RNA for α-subunits or 100 pg for GAPDH amplification). PCR products after 34 (no asterisk) and 39 cycles (asterisk) of amplification are shown for each cDNA and for gDNA. B, relative expression of different Na+ channel α-subunits in cultured motoneurons. Semi-quantification of the signal amplified from motoneuron cDNA was achieved after normalization to the corresponding signal amplified from genomic DNA. Experimental conditions were the same as illustrated in A. Measurements of the signal were performed at 34 cycles (linear zone). Results are expressed as mean + s.e.m. of normalized values obtained from three (DIV 0, ▪) or two (DIV 7, □) independent cultures. C, top: cDNA obtained from cultured motoneurons at day 7 (MN 7) of culture (corresponding to 25 cells) was amplified by PCR with primer pairs GNB1-1 (specific for Navβ1) and with primer pair GAPDH. Simultaneous amplification of cDNA from adult rat brain (adult brain) was performed (2 and 0.25 ng of total RNA). Bottom: PCR amplification of Nav β2.1 with GNB2.1 primer set. cDNA from 10 motoneurons on day 0 (MN 0) or day 7 (MN 7) of culture are compared with cDNA from adult brain (Ad, 50 pg). PCR products after 34 (no asterisk) and 39 cycles (asterisk) of amplification are shown. D, cDNA obtained from cultured motoneurons at day 0 (MN 0) and day 7 (MN 7) of culture (corresponding to 25 cells) was amplified by PCR with primer pairs CHAT (specific for ChAT) and with primer pair GAPDH. Simultaneous amplification of genomic DNA (gDNA) was carried out.
Since alternative splicing of exon 18 in the mouse and human SCN8A gene, generating a truncated protein, was reported to occur during development (Plummer et al. 1997), we investigated whether this phenomenon took place in cultured rat motoneurons. RT-PCR was performed with primers (Nacom) located on either side of exon 18 (Table 1). The perfect sequence match between primers and all four α-subunits enabled us to investigate possible alternative splicing in other α-subunits in addition to that described for Nav1.6 transcripts. After subcloning the RT-PCR products, sequence analysis of 22 independent clones confirmed the predominance of Nav1.3 and Nav1.2 transcripts, without any alternative splicing. This set of primers did not allow us to identify any clones related to the Nav1.1 isoform. However, we identified two Nav1.6 clones corresponding to the neonatal forms of exon 18 in the mouse orthologue gene (Plummer et al. 1997). Sequence analysis of these clones revealed a 100 % sequence identity between the mouse and the rat. By this approach we found only one clone matching the expected sequence of the adult form of Nav1.6 and identified two other clones corresponding to a truncation of exon 18. We further analysed the alternative splicing of exon 18 by RT-PCR, using two different sets of primers (Na6N and Na6A) (Table 1), specifically designed to amplify either the neonatal or the adult isoforms of Nav1.6 respectively. In each of these primer sets, the location of the upstream primer within the exon 18 boundaries impedes the visualization of the truncated transcript (Δ 18). As shown in Fig. 2_A_, we confirmed the large predominance of the neonatal form of exon 18 in motoneurons on day 0. Expression of this neonatal form decreased after 7 days in culture; meanwhile the adult transcript began to be detected. On day 7 of culture, immunodetection of Nav1.6 isoform was negative (not shown). However, Nav1.6 was clearly detected at the initial segment of the axon on day 12 of culture (Fig. 5_F_).
Figure 2. Developmental regulation of alternative splicing of Nav1.6 and Nav1.1 transcripts in regions homologous to exon 18 of the mouse SCN8A gene.

A, Nav1.6 alternative splicing: PCR was performed in parallel on cDNA obtained from motoneurons (50 cells) at 0 (MN 0) or 7 days (MN 7) of culture and on cDNA from 1 and 0.1 ng (100 pg) total RNA from adult rat brain (adult brain). PCR was conducted with the Na6A primer pair specific for the adult form of exon 18 (exon 18A) or with the Na6N primer pair specific for the neonatal form of exon 18 (exon 18N). PCR were performed as indicated in Fig. 1 legend with 34 and 39 cycles (asterisk). B, Nav1.1 alternative splicing in a region homologous to Nav1.1 exon 18: Comparative PCR (39 cycles) on cDNA from cultured motoneurons at 0 and 7 days of culture (MN 0 and MN 7) and on adult rat brain cDNA (adult brain), using primer pair Na-ex 18, clearly demonstrates a different amplification profile. The major band in adult brain cDNA had the expected size (331 bp) according to the primer pair position on Nav1.1 sequence. Conversely, PCR from motoneuron cDNA generated a preponderant band slightly above 400 bp.
Figure 5. Immunocytochemical characterization of Kv1.6, Kv1.4, Kv4.3 and α-subunit of sodium channels in cultured motoneurons.

A, different optical sections (from left to right and top to bottom) obtained by confocal microscopy illustrate strong labelling of the different neuronal compartments with anti-Kv1.6 antibody. The bottom left image corresponds to the maximal projection of the different sections. B, anti-Kv1.4 faintly stains the intra-cellular compartment. C, immunolabelling of motoneurons with anti-Kv4.3 was granular and present in the different compartments including the axon (maximal projection from confocal microscopy). D, pan anti-sodium channel α-subunit clearly stains the initial segment of the axon (arrow) (DIV 7). E, anti-Nav1.2 labelling of axonal initial segment of a motoneuron at DIV 7 (arrow). F, on DIV 12, motoneuron initial segment is labelled by anti-Nav1.6 antibody.
RT-PCR was performed with specific primers for Nav1.1 (Na1-ex18 primers) to amplify cultured motoneurons and adult brain cDNAs (Fig. 2_B_). Gel electrophoresis revealed two major bands. The lower band was predominant in adult brain. The upper band was found mainly in cultured motoneurons. After gel purification of these two bands and direct sequencing of the resulting PCR products, the lower band corresponded to the expected sequence of rat Nav1.1, while the upper one contained a 61 base pair (bp) insert at position 4254 of the rat Nav1.1 sequence (Gene Bank accession number X03638). This insertion resulted in a shift of the open reading frame and premature ending of translation. The insert does not have the canonical features of an intron.
Levels of Nav β1.1 mRNA in cultured motoneurons were estimated to represent one-fifth of that of rat adult brain (Fig. 1_C_). No major changes in Nav β1.1 transcript levels could be detected between day 0 and day 7 of culture (not shown). We also detected the presence of Navβ1.1A transcript, corresponding to an embryonic isoform (Kazen-Gillespie et al. 2000) derived from Navβ1.1 by alternative splicing (result not shown). In contrast Nav β2.1 mRNA was not detected (Fig. 1_C_).
In a previous report (Alessandri-Haber et al. 1999) we found, using 125I-iodinated α-scorpion toxin, that Na+ channels were exclusively distributed in axons and absent from soma and dendrites. This labelling may thus correspond to the expression of the isoforms, Nav1.2 and Nav1.3. Using a pan anti-sodium antibody we show that the majority of motoneurons in culture (DIV 7) displayed a dense labelling for sodium channels at the initial segment (identified with anti-ankyrin G antibody, not shown) (Fig. 5_D_). A granular intracellular staining was also visible and probably due to newly synthesized channels (Fig. 5_D_). Nav1.2 antibody revealed the same typical labelling of the initial segment (DIV 7) in 20 % of the cell population (Fig. 5_E_). As formerly mentioned, Nav1.6 immuno-reactivity appeared later (DIV 12, Fig. 5_F_).
Characterization of voltage-dependent K+ channel subunits expressed in cultured motoneurons
RT-PCR analysis of the majority of Kv subunits was carried out on cDNAs from cultured motoneurons using the primer sets described in Table 2. Results are summarized in Table 3.
Table 3.
Relative frequency of Kv channel mRNAs in cultured motoneurons
| Kv family | Level of expression | Kv family | Level of expression |
|---|---|---|---|
| Shaker-related | Shaw-related | ||
| Kv1.1 | + | Kv3.1 | ++ |
| Kv1.2 | ± | Kv3.2 | ± |
| Kv1.3 | ++ | Kv3.3 | ++ |
| Kv1.4 | + | Kv3.4 | ± |
| Kv1.5 | + | ||
| Kv1.6 | +++ | ||
| Shab-related | Shal-related | ||
| Kv 2.1 | ++ | Kv4.1 | + |
| Kv2.2 | ± | Kv4.2 | +++ |
| Kv4.3 | +++ |
Shaker-related subfamily
Kv1.1 and Kv1.2 transcripts were not detected by RT-PCR (Fig. 3_A_) with primer sets 11-2 and 12-1 (Table 2), respectively. We designed two other sets of primers in order to control these results (primer sets 11-3 and 12-2 respectively specific for Kv1.1 and Kv1.2, Table 2). These second sets of primers were apparently more efficient, since they allowed detection of the corresponding transcripts in cultured motoneurons. However, subsequent semi-quantification indicated that both transcripts were barely expressed. Similarly, Kv1.4 and Kv1.5 transcript levels were low. Kv1.3 levels were moderate while Kv1.6 transcripts were abundant (Fig. 3_A_ and Table 3). The high level of Kv1.6 transcript expression was in good agreement with the immunodetection of the corresponding protein in cultured motoneurons (Fig. 5_A_). The labelling of Kv1.6 was intense and present in all motoneuronal compartments, especially the plasma membrane and axon, while that of Kv1.1, Kv1.2, Kv1.5 (not shown) remained at background level as illustrated for Kv1.4 (Fig. 5_B_).
Figure 3. RT-PCR analysis of potassium channel transcripts in cultured motoneurons.

A, K+ channel α-subunits: RT-PCR was performed on cDNA from cultured motoneurons (corresponding to 8 cells) on day 0 (0) or 7 (7), on an equivalent amount of RNA (negative control) from the same cells and on 140 pg of rat gDNA (used as a standard allowing relative comparison of different transcripts in the same experiment). Amplification was performed as described in Fig. 1_A_ legend (same amount of initial target and same number of cycles; asterisk indicates the 39th cycle). The transcripts corresponding to Kv1.1 (primer pair 11-2), Kv1.2 (primer pair 12 1) were not detected in motoneurons. A comparison of the intensity of cDNA and gDNA products indicates that Kv1.6, Kv4.2 and Kv4.3 are the most abundant transcripts. The weak signal with Kv3.4 on day 7 was not reproduced in other experiments in different cultures. B, K+ channel β-subunits: RT-PCR with specific primers for GAPDH, Kv β1 and Kv β2 was performed on cDNA prepared from 25 motoneurons from three different cultures on day 7 (MN 7) (1 to 3) and on rat adult brain cDNA (adult brain, 2 ng and 500 pg).
Shab-related subfamily
Kv2.1 transcripts were moderately expressed while Kv2.2 transcripts were poorly expressed (Table 3). Interestingly we could detect the expression of Kv2.3r transcripts (Castellano et al. 1997) homologous to the regulatory α-subunit Kv8.1 (Salinas et al. 1997), but their levels were low (not shown).
Shaw-related subfamily
Kv3.1 (not shown) and Kv3.3 (Fig. 3_A_) mRNAs were moderately expressed. Kv3.2 and Kv3.4 mRNAs were barely detected or not at all (Table 3).
Shal-related subfamily
Levels of Kv4.2 and particularly of Kv4.3 transcripts (Fig. 3_A_ and Table 3) were high suggesting that the corresponding proteins may play an important role in the establishment of electrical activity in cultured motoneurons. These results were confirmed with different sets of primers (not shown). In particular, a set of primers for Kv4.3 (Song et al. 1998) allowed us to visualize a differential splicing of Kv4.3. The long form of Kv4.3 was predominant in motoneurons. Sequence analysis of the two forms was in agreement with previously published sequences (Ohya et al. 1997). Kv4.1 expression was low (Fig. 3_A_).
The possibility that both Kv4.2 and Kv4.3 transcripts could exist in the same motoneuron was assessed by single-cell RT-PCR (Fig. 4). Of five cells analysed by this technique, four were positive for Kv4.2 and 5 for Kv4.3.
Figure 4. Detection of Kv4.2 and Kv4.3 transcripts in single cultured motoneurons.

Single cell RT-PCR was performed as described in Methods. The same cells were analysed for Kv4.2 and Kv4.3 mRNA expression. The first round of PCR was conducted with multiplexed primers and the second round with specific primers for Kv4.3 (A) or Kv4.2 (B). Out of 5 cells analysed (numbered 1 to 5), 4 were positive for Kv4.2 (signal for cell 1 is faint) and 5 were positive for Kv4.3.
Immunolabelling with an antibody against Kv4.3 confirmed that the protein was expressed. Granular immunolabelling was visible in axons, soma and dendrites of motoneurons (Fig. 5_C_). This pattern was very close to that reported by Wu et al. (1998) in hippocampal cultured neurons, using the same anti-Kv4.3 antibody.
Auxiliary subunits
Kvβ1 and Kvβ2 mRNAs were both detected (Fig. 3_B_) but not Kvα3 mRNA (not shown). Comparisons of Kvβ1 and Kvβ2 amplifications (normalized with GAPDH) were indicative of significant levels of Kvβ1 and, to a lesser degree, of Kvβ2 transcripts in cultured motoneurons. No significant difference was observed between day 0 and day 7 of culture for Kv subunits.
Transient K+ currents are generated by Kv4.2 and Kv4.3 channel subunits
In a previous report, we identified a transient (A-type current) and a sustained K+ current in cultured motoneurons (Alessandri-Haber et al. 1999). The A-type current was isolated by subtracting the outward currents obtained at a holding potential of −100 mV from currents obtained at a holding potential of −60 mV (Fig. 6_A_). The voltage-dependent activation had a half value at −20 ± 1 mV with a Boltzmann factor of 23 ± 1 mV, and a threshold at −70 mV. The steady-state voltage dependence of inactivation was studied by applying a conditioning step (1.4 s, −140 mV to −10 mV) before a test pulse to 20 mV. Normalized peak current amplitude was fitted to a Boltzmann function having a half-value at −101 ± 0.5 mV and a slope factor of −12 ± 1 mV (Fig. 6_A_). The current was fully inactivated at −30 mV. Inactivation was fitted to a single exponential function. The time constant (τ) decreased slightly as a function of the voltage with a slope of 0.27 ms mV−1 and a value of 16 ± 3 ms at 0 mV (Fig. 6_B_). The time course of recovery from inactivation was best fitted to two exponentials, a fast one with a time constant (τ1) of 28 ms and a slow one with a time constant (τ2) of 353 ms (Fig. 6_C_).
Figure 6. Activation and inactivation of the transient K+ current in cultured motoneurons.

A, left traces: transient K+ currents obtained by subtracting the currents elicited by depolarizing pulses (-50 to 60 mV, in 10 mV step) from a holding potential of −60 mV from the currents elicited from −80 to 60 mV (10 mV step) from a holding potential of −100 mV. Right traces: transient K+ currents evoked by a depolarizing step to 20 mV following successive pre-pulses (1.4 s) from −120 to −10 mV. The test pulse recorded after a conditioning pulse of −10 mV was subtracted from each current trace. Plots: plot of the relative peak conductance (▪, right) as a function of the step voltage, and relative peak current (•, left) as a function of the conditioning potential. The continuous lines are Boltzmann functions. Each point is the mean of n = 5-8 experiments and error bars indicate s.e.m. The bath solution contained 3 μM TTX. B, plot of mean ± s.e.m. inactivation time constant (τ) as a function of step voltage from −50 to 60 mV (n = 12). The transient K+ currents were obtained using the procedure described in Fig. 6_A_. C, inset: the recovery from inactivation was determined by increasing the length of the interpulse (to −100 mV) applied between two depolarizing pulses. The conditioning and test pulses were elicited at 50 mV. The test pulse current recorded with an inter-pulse of 1 ms was subtracted from each recording. Plot: plot of the peak of the test pulse as a function of the inter-pulse duration. Data were fitted to the sum of two exponentials with time constants of 28 and 353 ms.
On the basis of these characteristics and of RT-PCR analysis, the transient K+ current may either result from the expression of Kv1.4 or Kv4 α-subunits, or from the coexpression of the Kvβ1 subunit with Kv1.3 or Kv1.5 α-subunits (Heinemann et al. 1996). This latter hypothesis can be eliminated since (i) heterologous coexpression of Kv1.3 and Kvβ1 subunits generates a K+ current that inactivates much more slowly than the A-type current present in motoneurons; (ii) coexpression of Kv1.5 and Kvβ1 subunits generates an A-type current that recovers from inactivation much more slowly (time constant of 2.4 s) (Heinemann et al. 1996) than the motoneuron current.
In contrast, the biophysical properties of Kv4 or Kv1.4 channels (Tseng-Crank et al. 1990; Serodio et al. 1994) are very similar to those of the transient current in motoneurons. However, these two channels can be distinguished on the basis of three criteria: (i) Kv1.4 and Kv4 channels differ in their rates of recovery from inactivation, (ii) Kv4 channels but not Kv1.4 are blocked by phrixotoxins 1 and 2 (PaTX1/PaTX2, (Diochot et al. 1999) and by an arachidonic acid derivative, 5,8,11,14-eicosatetraynoic acid (ETYA) (Villarroel, 1993) and (iii) the voltage dependence of the steady-state inactivation curve of Kv4.2 and Kv4.3 but not that of Kv1.4 is shifted by cadmium (Wickenden et al. 1999).
Figure 7 illustrates the pharmacological properties of the transient K+ current in motoneurons. This current was (i) insensitive to 20 mm tetraethylammonium (TEA) which reduced the sustained K+ current (Fig. 7_A_); (ii) strongly reduced (80 %) by 1 mm 4-aminopyridine (4AP) (Fig. 7_A_) which partly (18 %) inhibited the sustained K+ current (not illustrated); (iii) completely blocked by 10 μM ETYA (Fig. 7_B_) and strongly reduced (80 %) by 100 nm PaTX2 (Fig. 7_D_). Cadmium chloride (500 μM) induced a 31 mV rightward shift in the steady state inactivation curve of the A-type current (Fig. 7_C_). All these biophysical and pharmacological properties strongly favour a dominant role of Kv4.2/Kv4.3 channels in supporting the transient K+ current in cultured motoneurons. Finally, to detect the function of the A-type K+ current, we recorded single spikes elicited from a resting membrane potential of −74 mV in control conditions and in the presence of PaTX2 (100 nm). PaTX2 broadened and increased the overshoot of a spike (Fig. 7_E_).
Figure 7. Pharmacological characterization of the transient K+ current.
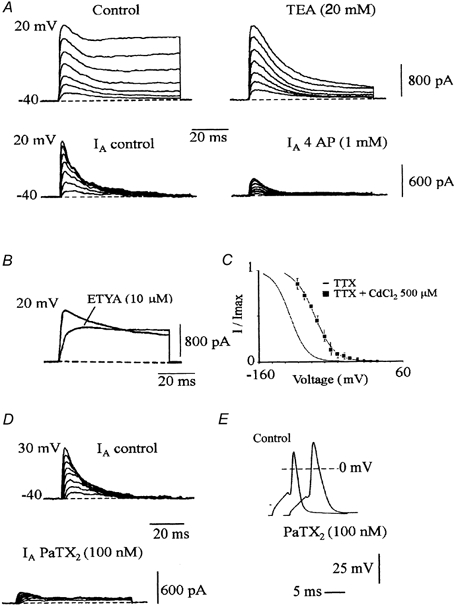
A, upper traces: outward currents evoked by a series of depolarizing steps from −40 to 20 mV from a holding potential of −100 mV. Currents were recorded in control conditions and after application of TEA (20 mm). Lower traces: transient K+ currents (_I_A) resulting from the subtraction of currents evoked by various steps from −40 to 20 mV from two holding potentials −100 and −60 mV. Currents were recorded in control conditions and after application of 4 AP (1 mm). B, effects of ETYA on a global K+ current evoked by a step to 20 mV from a holding potential of −100 mV. C, plot of the normalized peak current as a function of the conditioning voltage, in the presence of CdCl2 (500 μM) in the bath saline. Each point was the mean ± s.e.m. of n = 4 experiments. The continuous curve was determined as in Fig. 6_A_ and the inactivation curve of Fig. 6_A_ was also drawn. D, A-type current obtained from the subtraction of K+ currents evoked from two holding potentials of −100 and −60 mV, in control conditions and after application of PaTX2 (100 nm). E, single spike elicited by current stimulation from −74 mV. Application of PaTX2 (100 nm) increased overshoot and spike duration.
Concerning the sustained K+ current, in addition to the data given in the previous report (Alessandri-Haber et al. 1999), current trace analysis revealed a half-activation of the conductance at 0 ± 3 mV and a Boltzmann coefficient of 8 ± 1 mV. The sustained Kv current did not inactivate with a 1 s depolarizing test pulse and was blocked by TEA (IC50 = 7 mm, not illustrated).
DISCUSSION
Molecular determinants of fast-inactivating Na+ current
In pure cultured motoneurons from rat embryos (E14), fast-inactivating Na+ currents were detected after 18 h in culture and Na+-dependent action potentials were generated as soon as the second day in vitro (Alessandri-Haber et al. 1999). However, the corresponding molecular determinants remained unknown. We show here that the Na+ current mainly resulted from Nav1.2 and/or Nav1.3 expression during the first week in culture. First, mRNAs coding for these subunits displayed the highest levels of expression among the different α-subunits capable of supporting Na+ currents. In addition, Nav1.6 and Nav1.1 mRNAs underwent alternative splicing leading to truncated proteins as previously described in other species or tissues (Plummer et al. 1997; Oh & Waxman, 1998). Indeed, in a previous investigation, we demonstrated that the initial segment of axons could be labelled with 125I-iodinated α-scorpion toxin (Alessandri-Haber et al. 1999). This toxin interacts with the conserved S3-S4 loop in domain IV of Nav1.2 and Nav1.3 (Rogers et al. 1996). In this previous study, we demonstrated that most of the Na+ currents were restricted to the axon and that action potentials were generated from the axon while K+ currents remained somato-dendritic (Alessandri-Haber et al. 1999). Our results are in agreement with previous in situ hybridization studies (Black et al. 1994; Felts et al. 1997).
Sodium channel mRNA detection was performed at the time of motoneuron purification (DIV 0) and 1 week after culture maturation (DIV 7). We thus consider that subunits detected on DIV 0 may reflect the in vivo mRNA expression at E14. Adult motoneurons express high levels of Nav1.6 mRNA (Schaller et al. 1995) and protein (Krzemien et al. 2000). In addition, Nav1.6 is the main Na+ channel detected in the nodes of Ranvier of sciatic nerves (Caldwell et al. 2000). Nav1.6 protein expression is strongly enhanced during the first post-natal week (Schaller & Caldwell, 2000) and is the major contributor to Na+ current increase in motoneurons during the postnatal period (Garcia et al. 1998). Nav1.1 protein (Westenbroek et al. 1989) and mRNA (Black et al. 1994) are also expressed in adult motoneurons.
Two alternatively spliced exons, 18N and 18A of the Nav1.6 channel (encoded by the SCN8A gene) have been identified in mice and humans (Plummer et al. 1997). This alternative splicing is developmentally regulated. Exon 18N is expressed in fetal brain (as well as in some non-neuronal tissues) and leads to a truncated channel because of a conserved in-frame stop codon. This phenomenon also exists in cultured motoneurons with a perfect conservation of the sequence of exon 18N between the rat and mouse. Nav1.6 transcripts were devoid of exon 18 (Δ 18), which also results in premature ending of the translated protein (Plummer et al. 1997). Despite significant levels of Nav1.6 transcripts during the first week in culture, alternative splicing of exon 18 may lead to the absence of function or aberrant addressing and/or accelerated degradation of the corresponding protein. However, we showed by immunocytochemistry that the Nav1.6 protein could be detected and correctly located to the initial segment of the axon as motoneurons mature in vitro (12 days of culture). This indicates that a pure culture of motoneurons may reflect the in vivo situation, in which Nav1.2 is progressively replaced by Nav1.6 (Boiko et al. 2001; Kaplan et al. 2001). However, the important role of glia in this phenomenon could not be studied in our system.
We did not find alternatively spliced forms of Nav1.2 and Nav1.3 in regions homologous to Nav1.6 exon 18, but we identified Nav1.1 transcripts with an insertion of 61 bases (located at base 4254 of SCN1A, access number X0363) which interrupts the open reading frame. Alternative splicing of Nav1.1 in domain III has been described in astrocytes and neuroblastoma cells (Oh & Waxman, 1998).
Particular attention must be paid to the Navβ2.1 auxiliary subunit which is totally absent from our culture model although it is present in adult motoneurons (Levy-Mozziconacci et al. 1998). Thus in embryonic motoneurons, Nav β2.1 is not necessary to correctly target functional Na+ channels to the initial segment of axons. Indirect evidence from northern blotting are indicative of the low levels of Navβ2.1 transcripts in the spinal cord especially during embryonic life (Isom et al. 1995), and it has been shown that Nav1.2 channels are not associated with Navβ2.1 subunits during the first two postnatal weeks in the rat brain (Gong et al. 1999).
We found that both Navβ1.1 and Navβ1.1A isoforms were expressed in cultured motoneurons, but we could not achieve semi-quantitative estimation of their expression because of the localization of both primer pairs on different exons. This prevents comparison with the amplification of genomic DNA. However, the level of expression of Navβ1.1 in cultured motoneurons is not negligible compared to that of adult brain. In vivo, mRNA encoding the auxiliary subunit Navβ1.1 is detected on postnatal day 2 and increases strongly thereafter in motoneurons (Sashihara et al. 1995). The Navβ1.1A isoform results from alternative splicing of the SCN1B gene and is expressed early in development, in particular in spinal motoneurons (Kazen-Gillespie et al. 2000). The simultaneous presence of both Navβ1.1 isoforms may affect α-subunit activity in cultured motoneurons.
The sustained K+ current is partly supported by Kv1.6
Again, we did not observe any difference in the K+ mRNAs detected at DIV 0 and DIV 7 and we conclude that the subunits amplified might correspond to K+ channel subunits expressed in rat embryos at E14. Previous studies have shown that the heterologous expression of Kv1.6 induced a slowly inactivating current, sensitive to TEA (IC50 = 7 mm), with a threshold for activation of −50 mV, a half-voltage for conductance activation of around −3 mV and a Boltzmann slope factor of 6 mV (Grupe et al. 1990; Swanson et al. 1990). These values are in good agreement with the characteristics of the sustained K+ current measured in our cultures. In addition, the detection of the messenger for Kv1.6 and intense immunolabelling of the entire motoneuron using a specific antibody, demonstrate that Kv1.6 is a component of the sustained K+ current in embryonic motoneurons. However, pharmacological experiments performed in a previous study (Alessandri-Haber et al. 1999) showed that only part (around 30 %) of this current was blocked with high concentrations of α-DTX (200 nm), a blocker of Kv1.1, Kv1.2 and Kv1.6 channels (Harvey, 1997).
Results from RT-PCR, suggest a participation of Kv1.3, Kv2.1, Kv3.1 and Kv3.3 in the sustained K+ current. However, Kv1.3 and Kv3.3 currents inactivate within 1 s (Vega-Saenz de Miera et al. 1992; Grissmer et al. 1994); Kv1.3 current is activated at negative potentials (voltage for half conductance activation = −26 mV) and is very sensitive to kaliotoxin (Mourre et al. 1999). These data do not correlate well with the absence of inactivation and the low sensitivity to kaliotoxin (Alessandri-Haber et al. 1999). The mRNA for Kv3.1, present in our culture at a moderate level, has also been detected in adult rat motoneurons (Perney et al. 1992; Weiser et al. 1994; Rudy et al. 1999). The role of the Kv2.1 subunit remains to be determined. Finally, the most important difference we observed in our preparations compared with adult motoneurons is the absence of Kv1.1 and Kv1.2. In the ventral horn of adult spinal cord, Kv1.1 and Kv1.2 mRNAs have been detected and the corresponding proteins are localized in the somata of the largest neurons (Veh et al. 1995) and in paranodal regions of all the myelinated fibres of the sciatic nerve (Rasband & Shrager, 2000).
Kv4 subunits are major determinants of the transient K+ current
Single-cell RT-PCR, Kv4.3 immunocytochemistry and blockade of most of the transient K+ current by ETYA and PaTX2 demonstrate that the transient K+ current results from Kv4.3 and Kv4.2 expression in cultured motoneurons. This observation is novel because: (i) this is the first description of a molecular determinant of the transient K+ current in mammalian motoneurons, (ii) Kv4.2 and Kv4.3 are frequently expressed in the same motoneuron (in contrast to the complementary expression pattern noted in some brain areas; Serodio et al. 1996), (iii) the somato-dendritic compartment contains Kv4.2/4.3 channels as observed in other cell types (Sheng et al. 1992; Hoffman & Johnston, 1998), but in motoneurons the axon is also labelled and (iv) we demonstrate that channel activation modulates spike duration and the time course of repolarization. The transient K+ current in motoneurons is blocked by ETYA, which suggests a possible modulation of dendritic signals by metabolites of the arachidonic acid pathway, as proposed for some central (Colbert & Pan, 1999) and peripheral (Villarroel, 1993) neurons.
The transient current supported by Kv4.2/4.3 may participate in the long-term potentiation observed in motoneurons (Arvanov et al. 2000) during the early post-natal period, as has been proposed to occur in the dendites of CA1 hippocampal neurons (Adams et al. 2000).
In conclusion, our results emphasize the fact that the expression of different Kv channel subunits is regulated during development of rat spinal motoneurons. They also suggest that differential splicing of the Nav1.1 and Nav1.6 genes occurs between E14 and the adult stage and that truncated proteins in the embryo are replaced by functional proteins in adults.
Acknowledgments
We are indebted to Dr J. Nerbonne (Washington University, St Louis, MO, USA) for generous gift of antibodies. We are grateful to Dr M. Seagar (INSERM U464, Marseille, France), to C. Henderson and V. Arce (INSERM U382, Marseille, France) M. Gola and G. Jacquet (CNRS UPR 9024, Marseille, France) for generous advice. We thank the group of Dr J. Rossier (ESPCI, Paris, France) for their support in RT-PCR. This work was supported by a grant of the ‘Association Française contre les Myopathies’ (AFM). C. Deleuze and C. Manrique are recipient of grants from the AFM.
REFERENCES
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel kv4. 2 is a substrate for the mitogen-activated protein kinase ERK. Journal of Neurochemistry. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Paillart C, Arsac C, Gola M, Couraud F, Crest M. Specific distribution of sodium channels in axons of rat embryo spinal motoneurones. Journal of Physiology. 1999;518:203–214. doi: 10.1111/j.1469-7793.1999.0203r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanov VL, Seebach BS, Mendell LM. NT-3 evokes an LTP-like facilitation of AMPA/kainate receptor-mediated synaptic transmission in the neonatal rat spinal cord. Journal of Neurophysiology. 2000;84:752–758. doi: 10.1152/jn.2000.84.2.752. [DOI] [PubMed] [Google Scholar]
- Barry DM, Trimmer JS, Merlie JP, Nerbonne JM. Differential expression of voltage-gated K+ channel subunits in adult rat heart. Relation to functional K+ channels? Circulation Research. 1995;77:361–369. doi: 10.1161/01.res.77.2.361. [DOI] [PubMed] [Google Scholar]
- Black JA, Yokoyama S, Higashida H, Ransom BR, Waxman SG. Sodium channel mRNAs I, II and III in the CNS: cell-specific expression. Brain Research. Molecular Brain Research. 1994;22:275–289. doi: 10.1016/0169-328x(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1. 6 is localized at nodes of ranvier, dendrites, and synapses. Proceedings of the National Academy of Sciences of the USA. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano A, Chiara MD, Mellstrom B, Molina A, Monje F, Naranjo JR, Lopez-Barneo J. Identification and functional characterization of a K+ channel alpha-subunit with regulatory properties specific to brain. Journal of Neuroscience. 1997;17:4652–4661. doi: 10.1523/JNEUROSCI.17-12-04652.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K(+) channels in hippocampal CA1 pyramidal neurons. Journal of Neuroscience. 1999;19:8163–8171. doi: 10.1523/JNEUROSCI.19-19-08163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Drici MD, Moinier D, Fink M, Lazdunski M. Effects of phrixotoxins on the Kv4 family of potassium channels and implications for the role of Ito1 in cardiac electrogenesis. British Journal of Pharmacology. 1999;126:251–263. doi: 10.1038/sj.bjp.0702283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG. Sodium channel alpha-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): different expression patterns in developing rat nervous system. Brain Research. Molecular Brain Research. 1997;45:71–82. doi: 10.1016/s0169-328x(96)00241-0. [DOI] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. Journal of Neurophysiology. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- Garcia KD, Sprunger LK, Meisler MH, Beam KG. The sodium channel Scn8a is the major contributor to the postnatal developmental increase of sodium current density in spinal motoneurons. Journal of Neuroscience. 1998;18:5234–5239. doi: 10.1523/JNEUROSCI.18-14-05234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Rhodes KJ, Bekele-Arcuri Z, Trimmer JS. Type I and type II Na(+) channel alpha-subunit polypeptides exhibit distinct spatial and temporal patterning, and association with auxiliary subunits in rat brain. Journal of Comparative Neurology. 1999;412:342–352. [PubMed] [Google Scholar]
- Grinnell AD. Dynamics of nerve-muscle interaction in developing and mature neuromuscular junctions. Physiological Reviews. 1995;75:789–834. doi: 10.1152/physrev.1995.75.4.789. [DOI] [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1. 1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Molecular Pharmacology. 1994;45:1227–1234. [PubMed] [Google Scholar]
- Grupe A, Schroter KH, Ruppersberg JP, Stocker M, Drewes T, Beckh S, Pongs O. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. EMBO Journal. 1990;9:1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AL. Recent studies on dendrotoxins and potassium ion channels. General Pharmacology. 1997;28:7–12. doi: 10.1016/s0306-3623(96)00173-5. [DOI] [PubMed] [Google Scholar]
- Heinemann SH, Rettig J, Graack HR, Pongs O. Functional characterization of Kv channel beta-subunits from rat brain. Journal of Physiology. 1996;493:625–633. doi: 10.1113/jphysiol.1996.sp021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Koliastos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle [published erratum appears in Science (1995) Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. 10, 267(5199)777] [DOI] [PubMed] [Google Scholar]
- Henderson CE, Yamamoto Y, Livet J, Arce V, Garces A, Delapeyriere O. Role of neurotrophic factors in motoneuron development. Journal of Physiology. 1998;92:279–281. doi: 10.1016/s0928-4257(98)80033-8. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. Journal of Neuroscience. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, Barres BA. Differential control of clustering of the sodium channels Na(v)1. 2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron. 2001;30:105–119. doi: 10.1016/s0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Kazen-Gillespie KA, Ragsdale DS, D'Andrea MR, Mattei LN, Rogers KE, Isom LL. Cloning, localization, and functional expression of sodium channel beta1A subunits. Journal of Biological Chemistry. 2000;275:1079–1088. doi: 10.1074/jbc.275.2.1079. [DOI] [PubMed] [Google Scholar]
- Krzemien DM, Schaller KL, Levinson SR, Caldwell JH. Immunolocalization of sodium channel isoform NaCh6 in the nervous system. Journal of Comparative Neurology. 2000;420:70–83. [PubMed] [Google Scholar]
- Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- Levy-Mozziconacci A, Alcaraz G, Giraud P, Boudier JA, Caillol G, Couraud F, Autillo-Touati A. Expression of the mRNA for the beta 2 subunit of the voltage-dependent sodium channel in rat CNS. European Journal of Neuroscience. 1998;10:2757–2767. doi: 10.1046/j.1460-9568.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- McCobb DP, Best PM, Beam KG. Development alters the expression of calcium currents in chick limb motoneurons. Neuron. 1989;2:1633–1643. doi: 10.1016/0896-6273(89)90052-4. [DOI] [PubMed] [Google Scholar]
- McCobb DP, Best PM, Beam KG. The differentiation of excitability in embryonic chick limb motoneurons. Journal of Neuroscience. 1990;10:2974–2984. doi: 10.1523/JNEUROSCI.10-09-02974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott AB, Westbrook GL. Early development of voltage-dependent sodium currents in cultured mouse spinal cord neurons. Developmental Biology. 1986;113:317–326. doi: 10.1016/0012-1606(86)90167-3. [DOI] [PubMed] [Google Scholar]
- Mourre C, Chernova MN, Martin-Eauclaire MF, Bessone R, Jacquet G, Gola M, Alper SL, Crest M. Distribution in rat brain of binding sites of kaliotoxin, a blocker of Kv1. 1 and Kv1.3 alpha-subunits. Journal of Pharmacology and Experimental Therapeutics. 1999;291:943–952. [PubMed] [Google Scholar]
- Mynlieff M, Beam KG. Developmental expression of voltage-dependent calcium currents in identified mouse motoneurons. Developmental Biology. 1992;152:407–410. doi: 10.1016/0012-1606(92)90148-a. [DOI] [PubMed] [Google Scholar]
- O'Dowd DK, Ribera AB, Spitzer NC. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. Journal of Neuroscience. 1988;8:792–805. doi: 10.1523/JNEUROSCI.08-03-00792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Waxman SG. Novel splice variants of the voltage-sensitive sodium channel alpha subunit. NeuroReport. 1998;9:1267–1272. doi: 10.1097/00001756-199805110-00002. [DOI] [PubMed] [Google Scholar]
- Ohya S, Tanaka M, Oku T, Asai Y, Watanabe M, Giles WR, Imaizumi Y. Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel alpha-subunit, Kv4. 3 in the rat. FEBS Letters. 1997;420:47–53. doi: 10.1016/s0014-5793(97)01483-x. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- Perney TM, Marshall J, Martin KA, Hockfield S, Kaczmarek LK. Expression of the mRNAs for the Kv3. 1 potassium channel gene in the adult and developing rat brain. Journal of Neurophysiology. 1992;68:756–766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- Plummer NW, McBurney MW, Meisler MH. Alternative splicing of the sodium channel SCN8A predicts a truncated two-domain protein in fetal brain and non-neuronal cells. Journal of Biological Chemistry. 1997;272:24008–24015. doi: 10.1074/jbc.272.38.24008. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Shrager P. Ion channel sequestration in central nervous system axons. Journal of Physiology. 2000;525:63–73. doi: 10.1111/j.1469-7793.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JC, Qu Y, Tanada TN, Scheuer T, Catterall WA. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3-S4 extracellular loop in domain IV of the Na+ channel alpha subunit. Journal of Biological Chemistry. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal MS, Hernandez-Pineda R, Hernandez-Cruz A, Erisir A, Leonard C, Vega-Saenz de Miera E. Contributions of Kv3 channels to neuronal excitability. Annals of the New York Academy of Sciences. 1999;868:304–343. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- Salinas M, De Weille J, Guillemare E, Lazdunski M, Hugnot JP. Modes of regulation of shab K+ channel activity by the Kv8. 1 subunit. Journal of Biological Chemistry. 1997;272:8774–8780. doi: 10.1074/jbc.272.13.8774. [DOI] [PubMed] [Google Scholar]
- Sashihara S, Oh Y, Black JA, Waxman SG. Na+ channel beta 1 subunit mRNA expression in developing rat central nervous system. Brain Research. Molecular Brain Research. 1995;34:239–250. doi: 10.1016/0169-328x(95)00168-r. [DOI] [PubMed] [Google Scholar]
- Schaller KL, Caldwell JH. Developmental and regional expression of sodium channel isoform NaCh6 in the rat central nervous system. Journal of Comparative Neurology. 2000;420:84–97. [PubMed] [Google Scholar]
- Schaller KL, Krzemien DM, Yarowsky PJ, Krueger BK, Caldwell JH. A novel, abundant sodium channel expressed in neurons and glia. Journal of Neuroscience. 1995;15:3231–3242. doi: 10.1523/JNEUROSCI.15-05-03231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serodio P, Kentros C, Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. Journal of Neurophysiology. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Serodio P, Vega-Saenz de Miera E, Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. Journal of Neurophysiology. 1996;75:2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur MI, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4. 2 and Kv4.1 subunits. Journal of Neuroscience. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R, Marshall J, Smith JS, Williams JB, Boyle MB, Folander K, Luneau CJ, Antanavage J, Oliva C, Buhrow SA. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990;4:929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. Journal of Physiology. 1990;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng-Crank JC, Tseng GN, Schwartz A, Tanouye MA. Molecular cloning and functional expression of a potassium channel cDNA isolated from a rat cardiac library. FEBS Letters. 1990;268:63–68. doi: 10.1016/0014-5793(90)80973-m. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz de Miera E, Moreno H, Fruhling D, Kentros C, Rudy B. Cloning of ShIII (Shaw-like) cDNAs encoding a novel high-voltage-activating, TEA-sensitive, type-A K+ channel. Proceedings of the Royal Society B. 1992;248:9–18. doi: 10.1098/rspb.1992.0036. [DOI] [PubMed] [Google Scholar]
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. European Journal of Neuroscience. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Villarroel A. Suppression of neuronal potassium A-current by arachidonic acid. FEBS Letters. 1993;335:184–188. doi: 10.1016/0014-5793(93)80726-b. [DOI] [PubMed] [Google Scholar]
- Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. Journal of Neuroscience. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Wickenden AD, Tsushima RG, Losito VA, Kaprielian R, Backx PH. Effect of Cd2+ on Kv4. 2 and Kv1.4 expressed in Xenopus oocytes and on the transient outward currents in rat and rabbit ventricular myocytes. Cellular Physiology and Biochemistry. 1999;9:11–28. doi: 10.1159/000016299. [DOI] [PubMed] [Google Scholar]
- Wu RL, Butler DM, Barish ME. Potassium current development and its linkage to membrane expansion during growth of cultured embryonic mouse hippocampal neurons: sensitivity to inhibitors of phosphatidylinositol 3-kinase and other protein kinases. Journal of Neuroscience. 1998;18:6261–6278. doi: 10.1523/JNEUROSCI.18-16-06261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Ziskind-Conhaim L. Blocking Ca2+-dependent synaptic release delays motoneuron differentiation in the rat spinal cord. Journal of Neuroscience. 1995;15:5900–5911. doi: 10.1523/JNEUROSCI.15-09-05900.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. Electrical properties of motoneurons in the spinal cord of rat embryos. Developmental Biology. 1988;128:21–29. doi: 10.1016/0012-1606(88)90262-x. [DOI] [PubMed] [Google Scholar]