Cathepsin L Regulates CD4+ T Cell Selection Independently of Its Effect on Invariant Chain: A Role in the Generation of Positively Selecting Peptide Ligands (original) (raw)
Abstract
CD4+ T cells are positively selected in the thymus on peptides presented in the context of major histocompatibility complex class II molecules expressed on cortical thymic epithelial cells. Molecules regulating this peptide presentation play a role in determining the outcome of positive selection. Cathepsin L mediates invariant chain processing in cortical thymic epithelial cells, and animals of the I-Ab haplotype deficient in this enzyme exhibit impaired CD4+ T cell selection. To determine whether the selection defect is due solely to the block in invariant chain cleavage we analyzed cathepsin L–deficient mice expressing the I-Aq haplotype which has little dependence upon invariant chain processing for peptide presentation. Our data indicate the cathepsin L defect in CD4+ T cell selection is haplotype independent, and thus imply it is independent of invariant chain degradation. This was confirmed by analysis of I-Ab mice deficient in both cathepsin L and invariant chain. We show that the defect in positive selection in the cathepsin L−/− thymus is specific for CD4+ T cells that can be selected in a wild-type and provide evidence that the repertoire of T cells selected differs from that in wild-type mice, suggesting cortical thymic epithelial cells in cathepsin L knockout mice express an altered peptide repertoire. Thus, we propose a novel role for cathepsin L in regulating positive selection by generating the major histocompatibility complex class II bound peptide ligands presented by cortical thymic epithelial cells.
Keywords: cathepsin, positive selection, CD4 T cells, invariant chain, epitope generation
Introduction
The diversity of the peripheral T cell repertoire is determined in the thymus by positive and negative selection, two events affected primarily by distinct APCs (1). Cortical thymic epithelial cells (cTECs)* mediate positive selection of thymocytes expressing a TCR with low avidity for peptide–MHC class II complexes. Negative selection of potentially autoreactive T cells with high avidity for peptide–MHC class II complexes is induced by bone marrow–derived APCs. The interaction of the TCR with the MHC class II–peptide complex is central to both positive and negative selection, and therefore, the make-up of the thymic MHC class II peptide repertoire plays a key role in determining whether or not a T cell is selected (2, 3). Loading of peptide into the MHC class II peptide binding groove is tightly regulated, and requires that degradation of Ag and maturation of MHC class II occurs in the same or interconnected intracellular compartments (4, 5). Thus, the cellular proteins involved in these processes impact the thymic MHC class II peptide repertoire and hence CD4+ T cell selection.
MHC class II heterodimers are assembled in the endoplasmic reticulum with the assistance of the chaperone molecule invariant chain (Ii) (6, 7). The class II–associated Ii peptide (CLIP) region of Ii interacts with the MHC class II peptide binding groove to prevent inappropriate peptide loading (8, 9) and signals in the cytoplasmic tail of Ii target the Ii–MHC class II complex to the endosomal pathway (10, 11). Entry of the Ii–MHC class II complex into the endocytic pathway exposes Ii to endosomal proteases known as cathepsins (12, 13). Cathepsins degrade Ii in a step-wise manner, leaving CLIP associated with the MHC class II heterodimer. Cathepsins have also been implicated in generation of the antigenic peptide fragments presented in the context of MHC class II molecules (14–17), although the extent to which individual enzymes play a role in this process in different APCs remains to be defined. Exchange of CLIP for this diverse array of peptides is catalyzed by the MHC class II–like protein HLA-DM (H-2M in mice; 18–20) and precedes transport of the peptide–MHC class II complex to the cell surface.
Recent studies in knockout animals have shown that the lysosomal cysteine proteases cathepsin S (catS) and cathepsin L (catL) are differentially expressed and play an important role in MHC class II presentation by discrete populations of APCs (21–24). CatL activity is detected in cTECs, catS activity is observed in B cells and dendritic cells while both enzymes are expressed by macrophages. Analysis of distinct subsets of APCs from catS−/− and catL−/− animals indicated these enzymes mediate the late stages of Ii degradation and are critical for presentation of peptide in the context of MHC class II (21, 23, 24). In catL−/− mice the MHC class II presentation defect results in severely impaired selection of CD4+ T cells (21) and has thus far been interpreted to be due to the critical role played by catL in Ii cleavage. However, it is difficult to determine whether catL is also directly involved in other processes impacting MHC class II presentation, e.g., epitope generation or accessory molecule degradation, as presentation of peptides in the context of I-Ab molecules is severely impaired by Ii defects (25, 26). To circumvent this problem, in the studies described here, we used mice deficient in both catL and Ii to determine whether catL impacts MHC class II presentation independently of its effect on Ii processing.
Studies comparing mice deficient in both Ii and H-2M with Ii single knockout animals have shown that there is a substantial decrease in the number of CD4+ T cells selected if the diversity and level of expression of endogenous peptide–MHC class II complexes is significantly reduced (3, 27–29). By measuring CD4+ T cell numbers in catL-deficient mice, we have analyzed the role played by catL in generating the positively selecting cTEC MHC class II peptide epitopes. We show that catL is required to mediate efficient positive selection of MHC class II restricted TCR transgenic thymocytes and that CD4+ T cell selection is impaired equally in catL deficient mice of both the I-Ab (Ii degradation dependent) and I-Aq haplotype (Ii degradation independent). Most significantly, we demonstrate that in animals deficient in both catL and Ii the low level of CD4+ T cell selection observed in catL and Ii single knockout animals is further reduced, almost to the background level detected in MHC class II–deficient animals. These results indicate a role for catL in MHC class II presentation that is independent of its effect on Ii cleavage. We further provide evidence to suggest that positive selection of an altered T cell repertoire occurs in the absence of catL, and thus we hypothesize this enzyme is involved in generating the positively selecting peptide ligands in cTECs.
Materials and Methods
Mice.
C57BL/6 (BL6), DBA/1, recombination-activating gene (RAG)−/−, and MHC class II–deficient mice (Aβ−/−) were purchased from The Jackson Laboratory and maintained under specific pathogen-free conditions at the University of Washington. CatL−/− (H-2b; reference 21), Ii−/− on a BL6 background (purchased from The Jackson Laboratory), and the TCli (30) and OT-1 (provided by M. Bevan, University of Washington) TCR transgenics, were all bred and maintained under these same conditions. The TCR transgenic mice used in these studies were all maintained on a homozygous RAG−/− background. RAG−/− Ii−/− mice were generated by breeding RAG−/− and Ii−/− animals. CatL−/−Ii−/− double knockout mice were generated by crossing catL−/− and Ii−/− animals. CatL−/− (H-2q) animals were generated by breeding catL−/− (H-2b) mice onto a DBA/1 background. All procedures and care of the animals was in accordance with University of Washington guidelines.
Antibodies.
The following antibodies directed toward mouse cell surface antigens were purchased from BD PharMingen: FITC-conjugated anti-CD4, FITC-conjugated anti-Vβ5, FITC-conjugated anti-CD11c, PE-conjugated anti-CD8α, PE-conjugated anti-Vβ6, PE-conjugated BP-1, PerCP-conjugated anti-CD4. Horseradish peroxidase (HRP)-conjugated donkey anti–rabbit IgG was purchased from Amersham Pharmacia Biotech and HRP-conjugated mouse anti–rat IgG was purchased from Accurate Chemical and Scientific Corp. The polyclonal rabbit antiserum to mouse catL was a gift of A. Erickson (University of North Carolina at Chapel Hill, Chapel Hill, NC). IN-1 (anti-Ii), M5/114 (anti-I-Ab,d,q, anti-Ed,k), and biotin conjugated Y3P (anti-I-Ab) have been described previously (21, 23, 31–33).
Bone Marrow Chimeras.
Bone marrow cells were obtained from the femoral and tibial bone donors, pooled and washed with supplemented RPMI. T cells were depleted by incubating the cells in a 1:1 mixture of anti-CD4 (GK1.5) and anti-CD8 (3.168.8) Ab supernatants, followed by treatment with low-toxin rabbit complement (Cedarlane). The cells were washed in supplemented RPMI, resuspended in PBS at the required cell density, and 0.2 ml cell suspension was injected intravenously into the recipient. Host animals were administered a lethal dose of irradiation (950 rads) 24 h before bone marrow transplantation. Mice were maintained on water containing neomycin (25 μg/ml) and polymyxin B (13 μg/ml) from irradiation until the experiment was terminated. Mice were killed for analysis 6–8 wk after bone marrow transplantation.
Enrichment for cTECs and Flow Cytometry.
Enrichment for cTECs was performed as described previously (21). Briefly, thymi from 6–8-wk-old mice were mechanically disrupted and dissociated with an enzyme mixture containing collagenase (1 mg/ml), dispase (1 mg/ml), and DNase1 (50 μg/ml; all Roche Molecular Biochemicals). The cell suspension was further enriched for cTECs by centrifugation over a discontinuous Percoll gradient. Low density fractions were harvested and cTECs identified by flow cytometry as BP-1hiCD11c−CD4−.
The cell surface phenotype of splenocytes, thymocytes, and cTECs from chimeric and knockout animals was determined by four color flow cytometry. Single cell suspensions were depleted of erythrocytes and ∼1 × 106 cells were incubated in the presence of conjugated antibodies. Binding of biotin conjugated antibodies was detected by allophycocyanin-conjugated streptavidin (BD PharMingen). Data was collected using a FACSCalibur™ flow cytometer (Becton Dickinson) and analyzed using CELL Quest™ software (Becton Dickinson). Typically 50,000 events were recorded for splenocyte analysis and 100,000 events for thymocyte and cTEC analysis.
Immunoblotting and Immunoprecipitation.
Thymic stroma was washed in PBS and lysed in cell lysis buffer (0.5% NP-40, 0.15 M NaCl, 5 mM EDTA, 50 mM Tris-HCl, pH 7.2) supplemented with a cocktail of protease inhibitors (Roche Molecular Biochemicals). Debris was removed by centrifugation at 8,000 rpm for 10 min and the lysates analyzed for protein content using Coomassie® Plus Protein Assay Reagent (Pierce Chemical Co.). Lysate samples containing the indicated amount of total protein were boiled for 5 min in SDS-reducing buffer and separated by 12% SDS-PAGE. The proteins were electrophoretically transferred onto nitrocellulose membrane and this was probed using the indicated primary Ab. Binding was detected using the appropriate HRP-conjugated secondary Ab diluted 1:1,500 and visualized by chemiluminescence (ECL; Amersham Pharmacia Biotech).
For immunoprecipitation, thymic stroma was lysed in 1% NP-40, 0.01 M Tris pH 7.3, 0.15 M NaCl supplemented with a cocktail of protease inhibitors and then assayed for protein content, as described above. Lysate aliquots containing 100 μg of total protein were precleared with protein G-sepharose (Amersham Pharmacia Biotech) and with 18 μg normal rat IgG (Caltag Laboratories Inc.) before precipitation of MHC class II molecules using the Ab M5/114. Precipitated proteins were boiled in SDS-reducing buffer, separated by 12% SDS-PAGE, transferred electrophoretically onto nitrocellulose, and immunoblotted as described above.
Cysteine Protease Active Site Labeling.
Thymic stromal lysate aliquots, prepared as described above, containing 100 μg total protein were incubated for 2 h at 37°C in the presence of 0.25 μM cysteine protease inhibitor Bio-Tyr-Ala-FMK. This biotinylated inhibitor is an analogue of the previously described Cbz-125I-Tyr-Ala-CN2 (34) and it binds irreversibly to the active site cysteine of the enzyme via a thioester bond. Lysates were boiled in SDS-reducing buffer, separated by 12% SDS-PAGE, transferred onto nitrocellose, and immunoblotted as described above, using streptavidin-conjugated HRP.
Results
Selection of CD4+ But Not CD8+ T Cell Receptor Transgenic T Cells Is Impaired in Cathepsin L–deficient Mice.
We have shown previously that cTEC expression of catL is required for efficient selection of CD4+ T cells (21). Furthermore, we suggested that the defect in CD4+ T cell selection in catL−/− mice was due to expression of an altered MHC class II peptide repertoire by cTECs in these animals. However, it remained possible that this defect in selection was less specific and was a result of a global suppression of the positively selecting thymic environment. To address this possibility we sought to determine whether the effect of catL deficiency on selection was restricted to the development of CD4+ T cells, or whether CD8+ T cell maturation was also impaired.
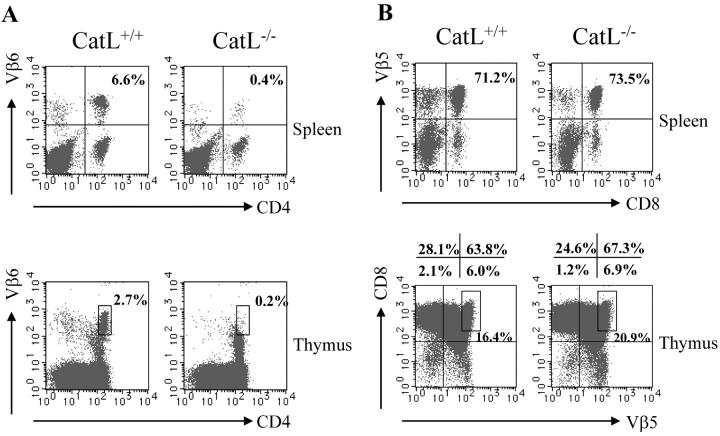
We transferred bone marrow from either a MHC class II–restricted TCR transgenic mouse (TCli) or a MHC class I–restricted TCR transgenic mouse (OT-1) into lethally irradiated catL−/− or wild-type mice. The TCR transgenic donors were on a RAG−/− background to prevent endogenous α-chain rearrangement during selection. Chimerism was >92% in all animals analyzed (data not shown). Analysis of splenocytes and thymocytes from TCli bone marrow recipients indicated that selection of the transgenic T cells (CD4+ Vβ6+) was almost completely abrogated in catL deficient recipients (Fig. 1 A). Selection of OT-1 TCR transgenic T cells (CD8+ Vβ5+), however, occurred normally in catL−/− hosts (Fig. 1 B). This observation that transgenic CD4+ T cells cannot be positively selected by the catL-deficient thymic environment while selection of CD8+ T cells is not impaired, indicates that catL is not essential for maintenance of a viable positive selection environment but that its effect is specific for CD4+ T cells. Thus, this result suggests the MHC class II peptide repertoire expressed at the surface of catL−/− cTECs is altered such that it cannot support efficient selection of CD4+ T cells that develop in wild-type mice.
Figure 1.
Impaired selection of CD4+ but not CD8+ T cell receptor transgenic T cells in cathepsin L–deficient mice. Bone marrow isolated from either CD4+ TCR transgenic (TCli) or CD8+ TCR transgenic (OT-1) mice was transplanted into lethally irradiated catL−/− and BL6 mice. The spleen and thymus of chimeric mice were analyzed by flow cytometry 8 wk after bone marrow transplantation. The percentage of donor T cells was determined by CD4+ Vβ6+ staining for TCli recipients (A) and CD8+ Vβ5+ staining for OT-1 recipients (B). The data shown are representative of three independent experiments with two to three mice per group.
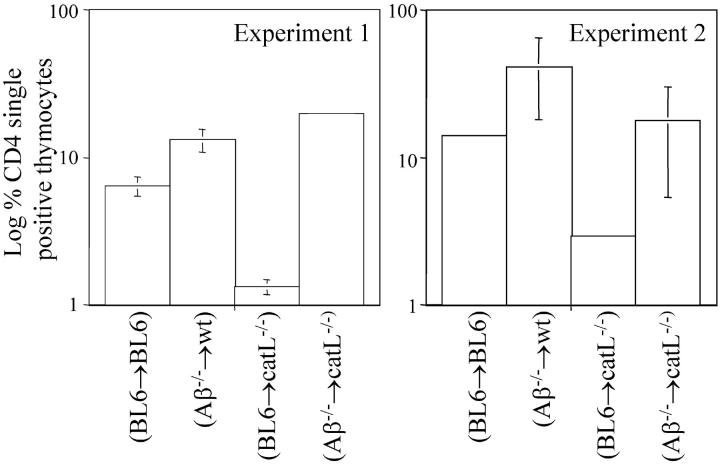
If the MHC class II peptide repertoire of cTECs is altered by the absence of catL, one may predict that an altered T cell repertoire would be positively selected, and then subjected to extensive negative selection on the normal MHC class II peptide repertoire expressed on the surface of bone marrow–derived APCs, thereby resulting in decreased CD4+ T cell selection. We sought to investigate this possibility by transplanting lethally irradiated catL−/− mice and BL6 controls with either BL6 or Aβ−/− bone marrow. Analysis of chimeric animals indicated the percentage of CD4 single-positive thymocytes in (Aβ−/− → BL6) mice was 2–3-fold greater than in (BL6 → BL6) chimeras, while (Aβ−/− → catL−/−) mice exhibited a 5–15-fold increase over (BL6 → catL−/−) chimeras (Fig. 2) . The relative increase in the percentage of CD4 single-positive thymocytes in Aβ−/− bone marrow recipients, when compared with BL6 recipients, correlates directly with the proportion of MHC class II–restricted thymocytes undergoing negative selection (35). Therefore, our observation that CD4 single-positive thymocyte accumulation is enhanced in (Aβ−/− → catL−/−) mice compared with (Aβ−/− → BL6) controls implies that the degree of negative selection in catL-deficient animals is substantially greater than in wild-type mice. This result suggests that the MHC class II peptide repertoire of catL−/− mice elicits positive selection of an altered T cell repertoire and thus, further supports our hypothesis that the MHC class II peptide repertoire is altered in the absence of catL.
Figure 2.
In the absence of negative selection the proportion of CD4 single-positive thymocytes is enhanced in cathepsin L–deficient mice. Bone marrow from either BL6 or Aβ−/− mice was transplanted into lethally irradiated BL6 and catL−/− mice. Mice were killed 6 wk after bone marrow transplantation and the thymi analyzed by flow cytometry to determine the percentage of CD4 single-positive thymocytes. The average percentage of CD4 single positive thymocytes in each group of chimeric animals is shown on a log scale. A total of three mice per group were analyzed in two independent experiments and each experiment is plotted separately.
CD4+ T Cell Selection Is Impaired in H-2q Cathepsin L–deficient Mice.
The observation that selection of CD4+ TCR transgenic thymocytes is severely impaired in catL−/− hosts provides strong evidence that the MHC class II peptide repertoire of cTECs is significantly altered in these animals. This altered peptide repertoire of catL deficient cTECs may be a result of the large number of I-Ab molecules associated with p12 Ii fragments that accumulate in the absence of catL (21). Alternatively, catL may play a direct role in generating the peptide epitopes involved in positive selection of CD4+ T cells. The accumulation of p12 Ii fragments associated with MHC class II in bone marrow–derived APCs from catS-deficient mice is haplotype dependent, as these complexes are readily detected in I-Ab but not I-Aq catS−/− animals (23). This correlates with the observation that presentation of several protein Ags is impaired in I-Ab but not I-Aq catS deficient mice (23) and indicates that the role of catS in regulating presentation of these peptides is mediated via its effect on Ii processing. Thus, the dependence of MHC class II peptide presentation on Ii degradation exhibits haplotype variance in bone marrow–derived APCs. We therefore sought to determine whether a similar difference in the extent of p12 Ii fragment association with MHC class II of the I-Ab and I-Aq haplotypes existed in cTECs isolated from catL−/− mice.
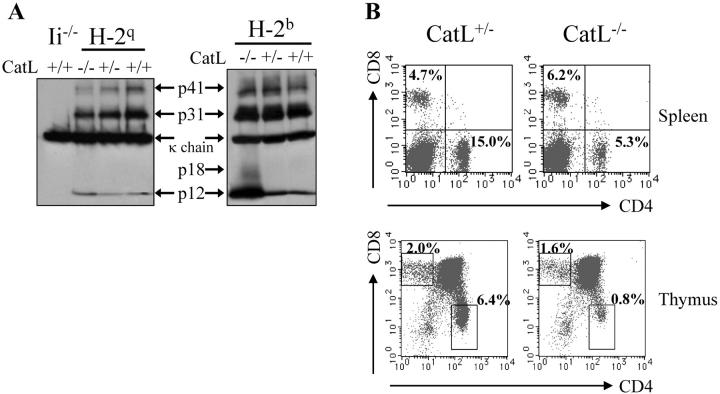
Thymic stromal cell lysates from I-Ab and I-Aq catL−/−, catL+/−, and catL+/+ animals were analyzed by immunoprecipitation with the anti-I-Ab,d,q Ab M5/114 and subsequent immunoblotting with the Ii-specific Ab IN-1 (Fig. 3 A). Thymic stroma from Ii-deficient animals was used as a negative control. The level of MHC class II association with p12 Ii fragments was substantially greater for the I-Ab haplotype than the I-Aq haplotype, both in wild-type and catL deficient animals (Fig. 3 A). Reprobing the immunoblot with rabbit anti-sera specific for the cytoplasmic tails of the MHC class II α and β chains indicated equivalent amounts of I-Ab and I-Aq had been precipitated (data not shown). These observations indicate cTECs exhibit the same difference in haplotype dependence upon Ii degradation as has previously been reported for bone marrow derived APCs (23).
Figure 3.
Selection of CD4+ T cells is impaired in I-Aq catL-deficient mice. (A) Thymic stromal cells were isolated from I-Ab and I-Aq catL−/−, catL+/−, and catL+/+ animals and lysed in the presence of protease inhibitors. 100 μg protein lysate was immunoprecipitated with the I-Ab,d,q specific Ab M5/114, precipitated proteins were separated by 12% SDS-PAGE and transferred to nitrocellulose before immuno-blotting with the Ii specific Ab IN-1. Binding was detected using HRP-conjugated mouse anti–rat IgG. Ii-deficient thymic stromal cells were treated as the other cell samples and used as a control for IN-1 specificity. The data is shown as different exposures of the same blot, a longer exposure was required to clearly visualize Ii fragments associated with I-Aq. The results shown are representative of two independent experiments. (B) Single cell suspensions of splenocytes and thymocytes from I-Aq catL+/− and catL−/− littermates were analyzed by flow cytometry. The CD4/CD8 profiles shown are representative of seven animals analyzed in three independent experiments.
Analysis of splenocytes and thymocytes from I-Aq catL+/− and catL−/− mice indicated that positive selection of CD4+ T cells was severely impaired in the absence of catL (Fig. 3 B). The percentage of CD4+ T cells detected in the spleen was decreased by 65–70% in the absence of catL, as has been observed for the I-Ab haplotype (21; Fig. 4 A). The effect of catL on positive selection of CD4+ T cells is not dose dependent as heterozygous and wild-type littermate control mice exhibited the same CD4+ T cell profiles (data not shown). These observations suggest that the decreased positive selection of CD4+ T cells in catL-deficient animals is not solely due to the block in Ii degradation but that catL has an Ii independent effect on MHC class II presentation by cTECs, e.g., generation of peptide epitopes.
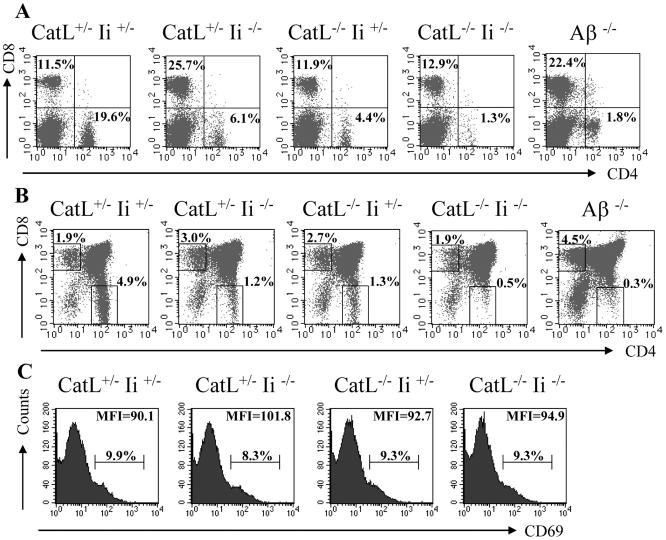
Figure 4.
Selection of CD4+ T cells is reduced to background levels in cathepsin L x invariant chain double-deficient mice. Splenocytes (A) and thymocytes (B) were isolated from catL+/− Ii+/−, catL+/−Ii−/−, catL−/−Ii+/−, and catL−/−Ii−/− mice and analyzed by flow cytometry for CD4 and CD8. Aβ−/− mice were used as a negative control for CD4+ T cell selection. The cellularity of each thymus was ∼100 × 106. The proportion of double-positive thymocytes expressing CD69 was determined by flow cytometry (C). The data shown are representative of eight independent experiments, each with a minimum of one mouse of each genotype.
CD4+ T Cell Selection Is Reduced to Background Levels in Mice Deficient in Both Cathepsin L and Invariant Chain.
The finding that CD4+ T cell selection is severely diminished in I-Aq catL−/− mice suggests catL has an effect on cTEC MHC class II peptide presentation that is independent of its role in Ii processing. To investigate this possibility directly we crossed I-Ab catL−/− mice with Ii−/− mice. The percentage of CD4+ T cells in the spleen (Fig. 4 A) and thymus (Fig. 4 B) of catL−/−Ii−/− animals was reduced almost to the level observed in MHC class II–deficient animals which were used as a negative control. In contrast, selection of CD4+ T cells in catL−/−Ii+/− and catL+/−Ii−/− littermate controls was not completely abrogated but reduced by ∼70%, as has previously been reported (21, 25, 26). This reduction in the efficiency of selection in the absence of both catL and Ii, when compared with single knockout littermates, indicates that these two molecules have distinct roles in regulating positive selection.
Further analysis of these mice indicates that the early stages of thymocyte maturation occur normally in the absence of either catL or Ii, or both, as total thymus cellularity, numbers of double positive thymocytes and their level of expression of CD69 was comparable in these mice and wild type animals (Fig. 4, A and C). Furthermore, the proportion of HSA low cells amongst the CD4 single-positive thymocytes was the same for all the mice (data not shown). In addition, the percentage of CD8+ T cells was the same in wild-type, catL−/−Ii+/−, and catL−/−Ii−/− spleen and thymus, while in MHC class II–deficient animals and catL+/−Ii−/− mice the percentage of these cells was increased (Fig. 4, A and B).
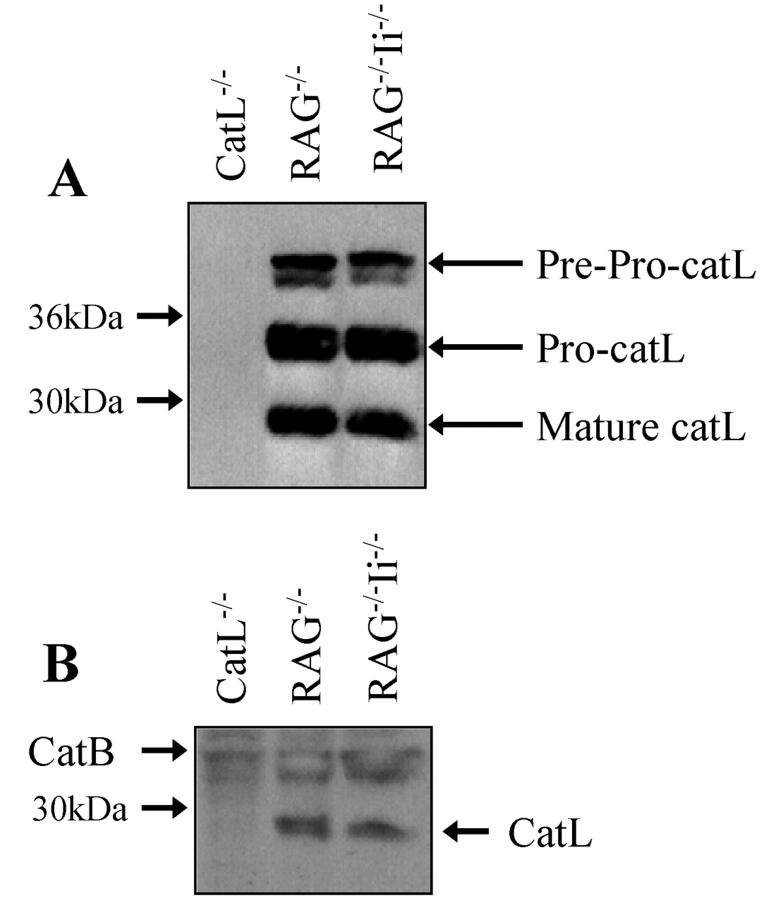
To ensure that our results could be interpreted as indicating that catL can effect cTEC MHC class II presentation independently of its role in Ii processing, we wished to determine whether expression of catL was altered by the presence or absence of Ii. Low levels of catL activity can be detected in thymocytes (unpublished data) and thus we analyzed catL expression in thymic stromal cells isolated from RAG−/− and RAG−/−Ii−/− mice. CatL−/− thymic stroma was used as a negative control. Mature catL protein could be detected in the absence of Ii at a level comparable to that observed in Ii-sufficient cells (Fig. 5 A). Equal protein loading was confirmed by probing the same membranes for actin (data not shown). To determine whether the catL protein detected by immunoblotting was active, thymic stromal cell lysate was incubated in the presence of the irreversible cysteine protease inhibitor Bio-Tyr-Ala-FMK (Fig. 5 B). There was no significant difference in the level of catL activity detected in the presence or absence of Ii, indicating that in thymic epithelial cells catL activity is not substantially altered by a defect in Ii. This result differs from a recently published report in which it was shown that in bone marrow derived macrophages the p41 isoform of Ii is required for catL activity (36).
Figure 5.
Cathepsin L expression is not significantly altered in the absence of invariant chain. Thymic stromal cells were isolated from RAG−/−, RAG−/−Ii−/−, and catL−/− animals and lysed in the presence of protease inhibitors. (A) Cell lysate containing 20 μg protein was reduced, separated by SDS-PAGE in a 12% gel and the proteins electrophoretically transferred to nitrocellulose. The membrane was probed with catL specific rabbit antiserum and binding was detected using HRP-conjugated donkey anti-rabbit IgG. (B) Cell lysate containing 100 μg protein was incubated in the presence of the irreversible cysteine protease inhibitor Bio-Tyr-Ala-FMK, reduced, separated by SDS-PAGE in a 12% gel and the proteins electrophoretically transferred to nitrocellulose. Labeled proteins were detected by probing the membrane with streptavidin-conjugated HRP. These results are representative of two independent experiments.
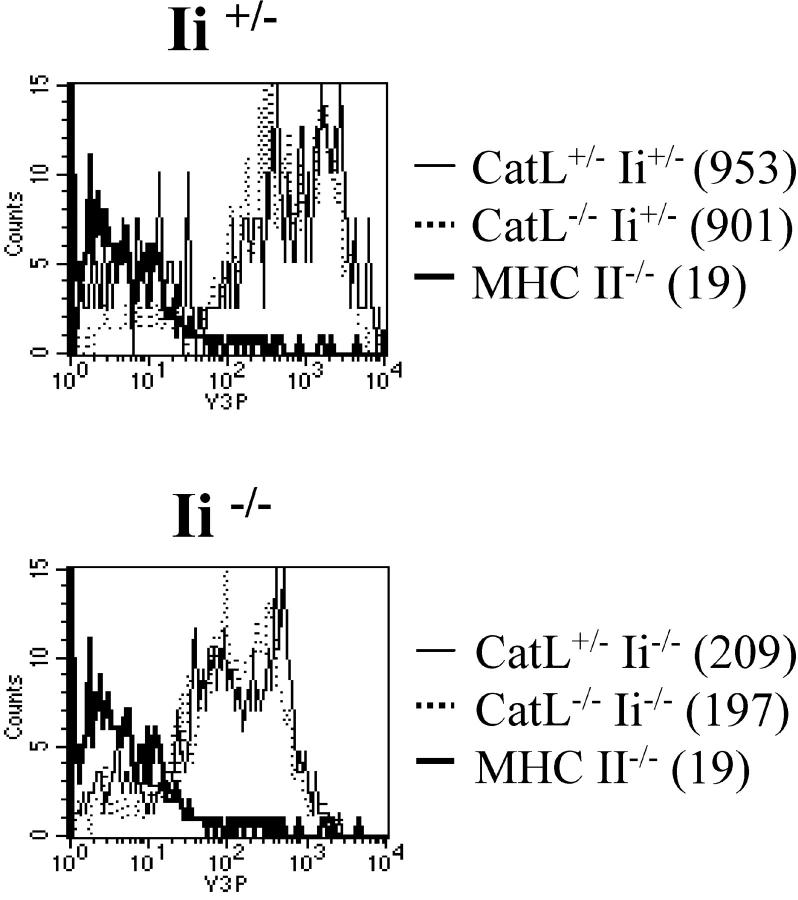
To exclude the possibility that the Ii-independent effect of catL on CD4+ T cell selection was a result of altered MHC class II levels, we analyzed the level of expression of MHC class II molecules on catL deficient cTECs (Fig. 6) . The expression of MHC class II was decreased in the absence of Ii, as has previously been observed (25, 26), however, there was no significant difference in the level of MHC class II expressed on the surface of wild-type and catL−/− cTECs. This observation is consistent with a previously published report in which similar levels of MHC class II were detected on wild-type and catL−/− cTECs (21) and strongly suggests catL plays a role in determining the MHC class II peptide repertoire responsible for CD4+ T cell positive selection.
Figure 6.
Cathepsin L plays no significant role in determining the level of expression of MHC class II on cTECs. Thymi from catL+/−Ii+/−, catL+/−Ii−/−, catL−/−Ii+/−, and catL−/−Ii−/− 6–8 week old mice were enriched for cTECs as described in the Materials and Methods. The cells were analyzed by flow cytometry and the level of expression of MHC class II on the surface of cTECs was determined by gating on CD11c−CD4−BP-1hi cells. The mean fluorescence intensity is shown in parenthesis. The data shown are representative of three independent experiments.
Discussion
The MHC class II peptide repertoire encountered by a CD4+ T cell in the thymus determines whether or not the cell will be selected (2, 3). As a result, the mechanisms that regulate MHC class II peptide generation in thymic APCs directly impact the process of CD4+ T cell selection. This is exemplified by the observation that CD4+ T cell selection is severely impaired in Ii-deficient mice which display a diminished repertoire of MHC class II bound peptides (25, 26). Inefficient selection of CD4+ T cells is also observed in mice lacking catL (21). CatL is highly expressed in the cells mediating positive selection, cTECs, and has been shown to elicit the late stages of Ii degradation (21). Cleavage of Ii is a critical event regulating peptide presentation in the context of MHC class II (37, 38), and the defect in CD4+ T cell selection in catL−/− mice is thought largely to be mediated by altered cTEC MHC class II presentation resulting from the key role played by this protease in Ii degradation. However, as this function of catL is so critical for peptide presentation by I-Ab molecules it masks other effects the enzyme may have, including any potential role in epitope generation. We have previously attempted to assess the repertoire of I-Ab peptides expressed by cTECs deficient in both catL and Ii, or in Ii alone, using T cell hybridomas specific for a number of self peptide–I-Ab complexes (unpublished data). However, we were unable to detect cTEC peptide presentation in this way. Therefore, we use here the previously employed readout of CD4+ T cell selection as an indicator of thymic MHC class II–bound peptide diversity (3, 27–29). We analyzed CD4+ T cell numbers in I-Aq catL−/− mice and I-Ab mice deficient in both catL and Ii to investigate whether the defect in CD4+ T cell selection in the absence of catL was due solely to its role in Ii degradation. We show that in cTECs there is much less association of the p12 fragment of Ii with I-Aq than with I-Ab, a result consistent with our previous observations in bone marrow–derived APCs (23), indicating that the defect in maturation of MHC class II molecules in catL−/− cTECs is haplotype dependent. We used this difference to determine that the defect in CD4+ T cell selection in catL−/− mice is not haplotype dependent, and thus, by implication cannot be explained solely by impaired Ii degradation. Analysis of I-Ab mice deficient in both catL and Ii indicated that CD4+ T cell selection was less efficient in the absence of both molecules than in single knockout animals and thus, we were able to confirm that catL plays a role in CD4+ T cell selection independently of its effect on Ii processing.
Selection of thymocytes expressing a MHC class II–restricted transgenic TCR was shown to be completely abrogated in catL−/− mice. In contrast, selection of both CD8+ TCR transgenic T cells and polyclonal CD8+ T cells occurred normally. These results indicate that the catL defect in T cell selection is specific for CD4+ T cells and that the general ability of cTECs to mediate positive selection is not impaired. The observation that thymic architecture is normal in catL deficient mice further suggests that thymic function is not globally affected by the absence of catL (21, 39). Selection of CD4+ TCR transgenic thymocytes is completely blocked in catL−/− mice while selection of polyclonal CD4+ T cells is not completely abrogated. Therefore, we believe that it is highly unlikely that catL regulates expression of an unknown molecule(s) required for CD4+ T cell selection, as has been suggested by others (4), implying that the effect of catL must be mediated via cTEC MHC class II presentation. Furthermore, we have detected no significant difference in the levels of MHC class II expressed at the surface of wild type and catL−/− cTECs (21; Fig. 5), indicating that the defect in CD4+ T cell selection in catL deficient mice is not a result of quantitative differences in the cTEC MHC class II expression levels. The above data provide indirect evidence of a qualitative difference in MHC class II presentation by catL-deficient cTECs. Further support for such an assertion comes from our results implying that an altered T cell repertoire is selected in catL−/− mice relative to BL6 controls. Thus, we suggest that our data provide support for the hypothesis that the defect in CD4+ T cell selection observed in catL−/− mice is due to expression of an altered cTEC MHC class II peptide repertoire.
Our data provide the first evidence that catL plays a role in CD4+ T cell selection that is distinct from its function as the key regulator of Ii degradation in cTECs. It is therefore likely that catL regulates the MHC class II presentation pathway, and hence CD4+ T cell selection, in several ways, only one of which is through Ii cleavage. Taken together with our results suggesting that the selection defect in catL−/− mice is a result of an altered MHC class II peptide repertoire, this has lead us to propose that catL plays a direct role in generating the MHC class II bound peptides involved in positive selection. Although, we have not formally ruled out the possibility that catL may act upon an accessory molecule, other than Ii, involved in CD4+ T cell development, in view of the available data we consider this unlikely. We believe therefore, that our observations imply catL plays a direct role in generating cTEC MHC class II peptide epitopes.
A role for catL in generating MHC class II peptide epitopes allows the defect in CD4+ T cell selection observed in catL−/− mice to be explained in two nonmutually exclusive ways. In the absence of catL the diversity of the cTEC MHC class II peptide repertoire may be diminished, resulting in inefficient positive selection of CD4+ thymocytes, as has been observed in H-2M−/− mice (20). In addition, catL−/− cTECs may express a novel set of peptide epitopes which support positive selection of an altered T cell repertoire. Such positively selected cells would then be subject to negative selection on bone marrow–derived APCs which express catS and not catL, and therefore, display a normal MHC class II peptide repertoire. Our observation that the increase in the proportion of CD4 single positive thymocytes in the absence of negatively selecting MHC class II+ bone marrow–derived APCs was substantially greater in catL−/− mice (5–15-fold increase) than BL6 controls (2–3-fold increase), provides some evidence to suggest that at least part of the defect in CD4+ T cell selection in catL−/− mice occurs as a result of positive selection of an altered T cell repertoire. In addition, we observed that the decrease in CD4+ T cells in catL−/− mice was accompanied by no change in the percentage of CD8+ T cells when compared with wild-type animals. In mice deficient in either MHC class II or Ii the decrease in CD4+ T cells was, however, concomitant with an increase in CD8+ T cells. The level of MHC class II and the diversity of the peptides expressed by these molecules is dramatically decreased on both positively selecting cTECs and negatively selecting bone marrow–derived APCs in mice deficient in Ii or MHC class II, while in catL−/− mice the MHC class II peptide repertoire is altered only on cTECs. We suggest therefore, that in the absence of catL, any positively selected thymocytes expressing MHC class II–restricted TCRs are efficiently negatively selected by the bone marrow–derived APCs. In contrast, in Ii and MHC class II deficient mice neither positive or negative selection can be mediated efficiently allowing a proportion of the cells expressing MHC class II–restricted TCRs to engage MHC class I molecules with sufficient avidity to be selected as CD8+ T cells. Similarly, both (Aβ−/− → wt) and (Aβ−/− → catL−/−) chimeras, mice which lack the ability to mediate negative selection of MHC class II–restricted thymocytes, exhibited an increase in the proportion of CD8 single-positive thymocytes compared with animals eliciting normal levels of negative selection (data not shown). We suggest these data indicate that while the efficiency of positive selection may be decreased in the absence of catL, a significant proportion of the defect in CD4+ T cell selection in catL-deficient mice is a result of positive selection of an altered T cell repertoire on an altered MHC class II peptide repertoire.
Expression of catL in thymic APCs is limited to cTECs, as the bone marrow–derived APCs that mediate negative selection express only catS (21). Therefore, as we propose that catL plays a role in generating the peptide epitopes bound to cTEC MHC class II molecules, it is possible that the MHC class II peptide repertoire of the cells eliciting positive and negative selection is not completely overlapping, as has previously been suggested by others (40). The extent to which the positively and negatively selecting peptide repertoires differ is however, likely to be limited, as our recent in vitro studies have shown that the majority of peptides bound to MHC class II molecules isolated from a fibroblast cell line engineered to express either catS or catL are identical (41). A small subset of peptides regulated specifically by either catS or catL was identified, although whether these or other disparately generated peptides are expressed in vivo at physiologically significant levels has not been determined. Therefore, elucidating the mechanisms regulating MHC class II peptide generation in different APCs is pivotal to furthering our understanding of thymocyte selection.
The precise mechanisms by which MHC class II bound peptides are generated, in distinct APCs, are ill-defined and our data provide the first description of an enzyme regulating this process in vivo. In vitro assays using purified proteases and substrates have previously indicated a role for two other cathepsins, cathepsin D (catD) (15, 16) and cathepsin E (17), in Ag degradation before MHC class II presentation. Analysis of catD−/− APCs, however, demonstrated that this enzyme is dispensable for such presentation in vivo (42, 43). Asparagine endopeptidase (AEP) has been shown to be a key enzyme in generation of MHC class II-bound peptides derived from the microbial Ag tetanus toxin C (44, 45). However, the in vivo importance of this enzyme remains to be determined.
Recently published data have shown that catL activity in bone marrow–derived macrophages requires the presence of the p41 isoform of Ii (35). We show here, however, that in thymic stromal cells the level of mature catL protein is not significantly decreased in the absence of Ii. Furthermore, catL activity detected in these cells in the absence of Ii, was comparable to that in wild-type cells. Our observation that cTEC expression of catL is not significantly altered by the absence of Ii is consistent with previously published reports that mice lacking the p41 isoform of Ii exhibit no defect in positive selection (46, 47). Similarly, we have observed no change in the level of catL expressed in thioglycollate elicited peritoneal macrophages isolated from Ii sufficient and deficient mice (unpublished data). One potential explanation for the discrepancy between these data is that catL dependence upon p41 was observed in cells differentiated to become APCs in vitro, while no such requirement has been detected in ex vivo–derived cells.
In conclusion, we have shown that the defect in T cell selection in catL knockout mice is not a global effect on the positively selecting thymic environment but that it is specific for CD4+ T cells. This effect on CD4+ T cell selection was not mediated by quantitative differences in the level of MHC class II expressed by cTECs and appears to mediate selection of an altered T cell repertoire susceptible to extensive negative selection on bone marrow–derived APCs; thus implicating catL as pivotal in determining qualitative differences in MHC class II presentation, i.e., the diversity of the positively selecting MHC class II peptide repertoire. The mechanism by which catL determines the repertoire of peptides bound to MHC class II may in part be via its role in late stage Ii degradation. However, we show here that catL also regulates CD4+ T cell selection in an Ii independent manner, and suggest this protease may play a direct role in generating the cTEC MHC class II peptide epitopes eliciting positive selection.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (K. Honey and A. Rudensky) and grants from the National Institutes of Health (A. Rudensky). The authors would like to thank Courtney Beers and Drs. Chyi Hsieh and Peter Gough for helpful discussions and critical review of the manuscript.
Footnotes
*
Abbreviations used in this paper: Aβ−/−, MHC class II–deficient mice; BL6, C57BL/6; catL, cathepsin L; catS, cathepsin S; CLIP, class II–associated Ii peptide; cTEC, cortical thymic epithelial cell; HRP, horseradish peroxidase; Ii, invariant chain; RAG, recombination-activating gene.
References
- 1.Jameson, S.C., and M.J. Bevan. 1998. T-cell selection. Curr. Opin. Immunol. 10:214–219. [DOI] [PubMed] [Google Scholar]
- 2.Sant'Angelo, D.B., P.G. Waterbury, B.E. Cohen, W.D. Martin, L. Van Kaer, A.C. Hayday, and C.A. Janeway, Jr. 1997. The imprint of intrathymic self-peptides on the mature T cell receptor repertoire. Immunity. 7:517–524. [DOI] [PubMed] [Google Scholar]
- 3.Barton, G.M., and A.Y. Rudensky. 1999. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 283:67–70. [DOI] [PubMed] [Google Scholar]
- 4.Villadangos, J.A., and H.L. Ploegh. 2000. Proteolysis in MHC class II antigen presentation: who's in charge? Immunity. 12:233–239. [DOI] [PubMed] [Google Scholar]
- 5.Riese, R.J., and H.A. Chapman. 2000. Cathepsins and compartmentalization in antigen presentation. Curr. Opin. Immunol. 12:107–113. [DOI] [PubMed] [Google Scholar]
- 6.Sant, A.J., and J. Miller. 1994. MHC class II antigen processing: biology of invariant chain. Curr. Opin. Immunol. 6:57–63. [DOI] [PubMed] [Google Scholar]
- 7.Cresswell, P. 1996. Invariant chain structure and MHC class II function. Cell. 84:505–507. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh, P., M. Amaya, E. Mellins, and D.C. Wiley. 1995. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 378:457–462. [DOI] [PubMed] [Google Scholar]
- 9.Morkowski, S., A.W. Goldrath, S. Eastman, L. Ramachandra, D.C. Freed, P. Whiteley, and A. Rudensky. 1995. T cell recognition of major histocompatibility complex class II complexes with invariant chain processing intermediates. J. Exp. Med. 182:1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakke, O., and B. Dobberstein. 1990. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 63:707–716. [DOI] [PubMed] [Google Scholar]
- 11.Lotteau, V., L. Teyton, A. Peleraux, T. Nilsson, L. Karlsson, S.L. Schmid, V. Quaranta, and P.A. Peterson. 1990. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 348:600–605. [DOI] [PubMed] [Google Scholar]
- 12.Maric, M.A., M.D. Taylor, and J.S. Blum. 1994. Endosomal aspartic proteinases are required for invariant-chain processing. Proc. Natl. Acad. Sci. USA. 91:2171–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riese, R.J., P.R. Wolf, D. Bromme, L.R. Natkin, J.A. Villadangos, H.L. Ploegh, and H.A. Chapman. 1996. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 4:357–366. [DOI] [PubMed] [Google Scholar]
- 14.Watts, C. 1997. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 15:821–850. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez, G.M., and S. Diment. 1992. Role of cathepsin D in antigen presentation of ovalbumin. J. Immunol. 149:2894–2898. [PubMed] [Google Scholar]
- 16.van Noort, J.M., and M.J. Jacobs. 1994. Cathepsin D, but not cathepsin B, releases T cell stimulatory fragments from lysozyme that are functional in the context of multiple murine class II MHC molecules. Eur. J. Immunol. 24:2175–2180. [DOI] [PubMed] [Google Scholar]
- 17.Bennett, K., T. Levine, J.S. Ellis, R.J. Peanasky, I.M. Samloff, J. Kay, and B.M. Chain. 1992. Antigen processing for presentation by class II major histocompatibility complex requires cleavage by cathepsin E. Eur. J. Immunol. 22:1519–1524. [DOI] [PubMed] [Google Scholar]
- 18.Denzin, L.K., and P. Cresswell. 1995. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 82:155–165. [DOI] [PubMed] [Google Scholar]
- 19.Sherman, M.A., D.A. Weber, and P.E. Jensen. 1995. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 3:197–205. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki, T., P. Wolf, S. Tourne, C. Waltzinger, A. Dierich, N. Barois, H. Ploegh, C. Benoist, and D. Mathis. 1996. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 84:531–541. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa, T., W. Roth, P. Wong, A. Nelson, A. Farr, J. Deussing, J.A. Villadangos, H. Ploegh, C. Peters, and A.Y. Rudensky. 1998. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 280:450–453. [DOI] [PubMed] [Google Scholar]
- 22.Riese, R.J., R.N. Mitchell, J.A. Villadangos, G.P. Shi, J.T. Palmer, E.R. Karp, G.T. De Sanctis, H.L. Ploegh, and H.A. Chapman. 1998. Cathepsin S activity regulates antigen presentation and immunity. J. Clin. Invest. 101:2351–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa, T.Y., W.H. Brissette, P.D. Lira, R.J. Griffiths, N. Petrushova, J. Stock, J.D. McNeish, S.E. Eastman, E.D. Howard, S.R. Clarke, et al. 1999. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 10:207–217. [DOI] [PubMed] [Google Scholar]
- 24.Shi, G.P., J.A. Villadangos, G. Dranoff, C. Small, L. Gu, K.J. Haley, R. Riese, H.L. Ploegh, and H.A. Chapman. 1999. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 10:197–206. [DOI] [PubMed] [Google Scholar]
- 25.Bikoff, E.K., L.Y. Huang, V. Episkopou, J. van Meerwijk, R.N. Germain, and E.J. Robertson. 1993. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J. Exp. Med. 177:1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viville, S., J. Neefjes, V. Lotteau, A. Dierich, M. Lemeur, H. Ploegh, C. Benoist, and D. Mathis. 1993. Mice lacking the MHC class II-associated invariant chain. Cell. 72:635–648. [DOI] [PubMed] [Google Scholar]
- 27.Tourne, S., T. Miyazaki, P. Wolf, H. Ploegh, C. Benoist, and D. Mathis. 1997. Functionality of major histocompatibility complex class II molecules in mice doubly deficient for invariant chain and H-2M complexes. Proc. Natl. Acad. Sci. USA. 94:9255–9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bikoff, E.K., G. Kenty, and L. Van Kaer. 1998. Distinct peptide loading pathways for MHC class II molecules associated with alternative Ii chain isoforms. J. Immunol. 160:3101–3110. [PubMed] [Google Scholar]
- 29.Kovats, S., C.E. Grubin, S. Eastman, P. deRoos, A. Dongre, L. Van Kaer, and A.Y. Rudensky. 1998. Invariant chain–independent function of H-2M in the formation of endogenous peptide-major histocompatibility complex class II complexes in vivo. J. Exp. Med. 187:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong, P., A.W. Goldrath, and A.Y. Rudensky. 2000. Competition for specific intrathymic ligands limits positive selection in a TCR transgenic model of CD4+ T cell development. J. Immunol. 164:6252–6259. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya, A., M.E. Dorf, and T.A. Springer. 1981. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J. Immunol. 127:2488–2495. [PubMed] [Google Scholar]
- 32.Koch, N., S. Koch, and G.J. Hammerling. 1982. Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature. 299:644–645. [DOI] [PubMed] [Google Scholar]
- 33.Janeway, C.A., Jr., P.J. Conrad, E.A. Lerner, J. Babich, P. Wettstein, and D.B. Murphy. 1984. Monoclonal antibodies specific for Ia glycoproteins raised by immunization with activated T cells: possible role of T cellbound Ia antigens as targets of immunoregulatory T cells. J. Immunol. 132:662–667. [PubMed] [Google Scholar]
- 34.Mason, R.W., L.T. Bartholomew, and B.S. Hardwick. 1989. The use of benzyloxycarbonyl[125I]iodotyrosylalanyldiazomethane as a probe for active cysteine proteinases in human tissues. Biochem. J. 263:945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Meerwijk, J.P., S. Marguerat, R.K. Lees, R.N. Germain, B.J. Fowlkes, and H.R. MacDonald. 1997. Quantitative impact of thymic clonal deletion on the T cell repertoire. J. Exp. Med. 185:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennon-Dumenil, A.M., R.A. Roberts, K. Valentijn, C. Driessen, H.S. Overkleeft, A. Erickson, P.J. Peters, E. Bikoff, H.L. Ploegh, and P. Wolf Bryant. 2001. The p41 isoform of invariant chain is a chaperone for cathepsin L. EMBO J. 20:4055–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa, T.Y., and A.Y. Rudensky. 1999. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunol. Rev. 172:121–129. [DOI] [PubMed] [Google Scholar]
- 38.Villadangos, J.A., R.A. Bryant, J. Deussing, C. Driessen, A.M. Lennon-Dumenil, R.J. Riese, W. Roth, P. Saftig, G.P. Shi, H.A. Chapman, et al. 1999. Proteases involved in MHC class II antigen presentation. Immunol. Rev. 172:109–120. [DOI] [PubMed] [Google Scholar]
- 39.Benavides, F., A. Venables, H. Poetschke Klug, E. Glasscock, A. Rudensky, M. Gomez, N. Martin Palenzuela, J.L. Guenet, E.R. Richie, and C.J. Conti. 2001. The CD4 T cell-deficient mouse mutation nackt (nkt) involves a deletion in the cathepsin L (CtsI) gene. Immunogenetics. 53:233–242. [DOI] [PubMed] [Google Scholar]
- 40.Marrack, P., and J. Kappler. 1987. The T cell receptor. Science. 238:1073–1079. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh, C.S., P. deRoos, K. Honey, C. Beers, and A.Y. Rudensky. 2002. A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J. Immunol. 168:2618–2625. [DOI] [PubMed] [Google Scholar]
- 42.Villadangos, J.A., R.J. Riese, C. Peters, H.A. Chapman, and H.L. Ploegh. 1997. Degradation of mouse invariant chain: roles of cathepsins S and D and the influence of major histocompatibility complex polymorphism. J. Exp. Med. 186:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deussing, J., W. Roth, P. Saftig, C. Peters, H.L. Ploegh, and J.A. Villadangos. 1998. Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc. Natl. Acad. Sci. USA. 95:4516–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manoury, B., E.W. Hewitt, N. Morrice, P.M. Dando, A.J. Barrett, and C. Watts. 1998. An asparaginyl endopeptidase processes a microbial antigen for class II MHC presentation. Nature. 396:695–699. [DOI] [PubMed] [Google Scholar]
- 45.Antoniou, A.N., S.L. Blackwood, D. Mazzeo, and C. Watts. 2000. Control of antigen presentation by a single protease cleavage site. Immunity. 12:391–398. [DOI] [PubMed] [Google Scholar]
- 46.Shachar, I., E.A. Elliott, B. Chasnoff, I.S. Grewal, and R.A. Flavell. 1995. Reconstitution of invariant chain function in transgenic mice in vivo by individual p31 and p41 isoforms. Immunity. 3:373–383. [DOI] [PubMed] [Google Scholar]
- 47.Takaesu, N.T., J.A. Lower, E.J. Robertson, and E.K. Bikoff. 1995. Major histocompatibility class II peptide occupancy, antigen presentation, and CD4+ T cell function in mice lacking the p41 isoform of invariant chain. Immunity. 3:385–396. [DOI] [PubMed] [Google Scholar]