Adaptation of Alphaviruses to Heparan Sulfate: Interaction of Sindbis and Semliki Forest Viruses with Liposomes Containing Lipid-Conjugated Heparin (original) (raw)
Abstract
Passage of Sindbis virus (SIN) in BHK-21 cells has been shown to select for virus mutants with high affinity for the glycosaminoglycan heparan sulfate (HS). Three loci in the viral spike protein E2 (E2:1, E2:70, and E2:114) have been identified that mutate during adaptation and independently confer on the virus the ability to bind to cell surface HS (W. B. Klimstra, K. D. Ryman, and R. E. Johnston, J. Virol. 72:7357-7366, 1998). In this study, we used HS-adapted SIN mutants to evaluate a new model system involving target liposomes containing lipid-conjugated heparin (HepPE) as an HS receptor analog for the virus. HS-adapted SIN, but not nonadapted wild-type SIN TR339, interacted efficiently with HepPE-containing liposomes at neutral pH. Binding was competitively inhibited by soluble heparin. Despite the efficient binding of HS-adapted SIN to HepPE-containing liposomes at neutral pH, there was no fusion under these conditions. Fusion did occur, however, at low pH, consistent with cellular entry of the virus via acidic endosomes. At low pH, wild-type or HS-adapted SIN underwent fusion with liposomes with or without HepPE with similar kinetics, suggesting that interaction with the HS receptor analog at neutral pH has little influence on subsequent fusion of SIN at low pH. Finally, Semliki Forest virus (SFV), passaged frequently on BHK-21 cells, also interacted efficiently with HepPE-containing liposomes, indicating that SFV, like other alphaviruses, readily adapts to cell surface HS. In conclusion, the liposomal model system presented in this paper may serve as a novel tool for the study of receptor interactions and membrane fusion properties of HS-interacting enveloped viruses.
Alphaviruses, such as Ross River virus (RR), Semliki Forest virus (SFV), Sindbis virus (SIN), and Venezuelan equine encephalitis virus (VEE), are enveloped positive-strand RNA viruses belonging to the family Togaviridae. The viral genome consists of a single-stranded RNA molecule, which is complexed with 240 copies of the capsid protein (50). The nucleocapsid is surrounded by a lipid bilayer in which the spike proteins are inserted. A single virion contains 80 hetero-oligomeric spikes, each spike consisting of a trimer of E2/E1 heterodimers. The E1 and E2 glycoproteins mediate the infectious entry of alphaviruses into cells. The E2 glycoprotein is primarily involved in the interaction of the virus particle with an attachment receptor on the cell surface (7, 28, 49), whereas E1 is required for the subsequent fusion process (19, 53).
The spike proteins of RNA viruses are capable of rapid adaptation to their growth environment. Recently, it has been shown that viruses from different families interact with glycosaminoglycans (GAGs), in most cases heparan sulfate (HS), as a cell culture adaptation. Virus families or genera that exhibit such GAG adaptation include alphaviruses (2, 21, 28), flaviviruses (33), pestiviruses (25), picornaviruses (16, 43), and retroviruses (38, 41). GAGs are highly sulfated polymers of disaccharide repeats and hence are negatively charged. They are ubiquitously expressed on cell surfaces but vary with respect to their composition and quantity in different tissues and cell types (3, 52).
Positively charged amino acid substitutions that are responsible for interaction with HS have been identified in the viral spike protein E2 of SIN, RR, and VEE (2, 21, 28). For SIN, three loci in E2 (E2:1, E2:70, and E2:114) appeared to mutate during adaptation of the virus to baby hamster kidney (BHK-21) cells, each mutation independently conferring on the virus the ability to bind to cell surface HS (28). The sequence XBXBBBX or XBBXBX (where X is any residue and B is a basic residue) is a linear binding motif that allows proteins to attach to HS (9). The positive-charge mutation at E2:1 results in the formation (although in the opposite orientation) of a linear HS interaction sequence. The HS-binding motifs are not present in the E2:70 and E2:114 regions, which suggests that these viruses interact with HS in a conformation-dependent manner. This phenomenon is known to occur in foot-and-mouth disease virus type O, structural studies of which have revealed that heparin makes contact with all three major capsid proteins, VP1, VP2, and VP3 (18). Despite the efficient interaction of the selected mutants of SIN, VEE, and RR with HS, the viruses were found to have attenuated virulence in animals compared to wild-type viruses. It has been proposed that HS-adapted mutants can bind to nonproductive cellular structures, such as extracellular membranes and basal laminae, and therefore may be cleared from the blood more rapidly than wild-type viruses (2, 8, 21).
The membrane fusion activities of SIN, SFV, and tick-borne encephalitis (TBE) virus have been investigated by using liposomes lacking a protein or carbohydrate receptor in the target membrane (5, 13, 46, 47). This suggests that receptor interaction is not a prerequisite for fusion of these viruses. However, the characteristics of virus-liposome fusion in the presence of an attachment receptor have not been studied. In this study, we used HS-adapted SIN mutants to evaluate a new model system involving target liposomes supplemented with phosphatidylethanolamine-conjugated heparin (HepPE) as an attachment receptor analog for the virus. With HepPE in the target membrane, we were able to directly investigate the role of HS receptor interaction and its potential function in triggering or influencing the fusion of HS-adapted SIN with target membranes. It is demonstrated that HS-adapted SIN interacts efficiently with HepPE-containing liposomes at neutral pH. Despite the efficient interaction, there was no fusion under these conditions. Fusion was observed only at low pH, consistent with cell entry of SIN via acidic endosomes. Finally, it is shown that SFV, either passaged frequently in BHK-21 cells or derived from the BHK-adapted infectious clone pSFV4, interacts efficiently with HepPE-containing liposomes, indicating that SFV, like SIN, readily adapts to cell surface HS.
MATERIALS AND METHODS
Viruses.
Viruses were generated from cDNA clones. Construction of the consensus SIN AR339 clone pTR339 and the HS-adapted SIN clones p3970 (called p39K70 in previous articles) and pTRSB has been described previously (28, 29, 36). Construction of the SFV clone pSFV4 has been described previously (31). This clone was generated from a laboratory strain of SFV, adapted to growth on BHK-21 cells. A plaque-purified laboratory strain of SFV, also highly adapted to growth on BHK-21 cells, was a generous gift of Margaret Kielian (Albert Einstein College of Medicine, New York, N.Y.).
Viruses were produced by high-efficiency electroporation of BHK-21 cells with in vitro RNA transcripts of linearized cDNA clones as described previously (31). Viruses released from the cells at 20 h posttransfection were harvested, and these stocks were subsequently used for production of pyrene- or [35S]methionine-labeled SIN or SFV particles, as previously described (5, 46). The viruses were characterized by plaque assay on BHK-21 cells (28), phosphate analysis (4), and protein determination (42). The purity of the viruses was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Liposomes.
Liposomes (large unilamellar vesicles) consisted of phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SPM), and cholesterol (Chol) or 6-photocholesterol (photoChol) in a molar ratio of 1:1:1:1.5, supplemented with HepPE as indicated. The phospholipids were obtained from Avanti Polar Lipids (Alabaster, Ala.), and cholesterol was from Sigma (St. Louis, Mo.). The photoactivatable analog of cholesterol, photoChol, was synthesized as described previously (37). The HepPE conjugate, consisting of heparin (from porcine intestinal mucosa; average molecular weight, 10,000; Scientific Protein Laboratories, Wannakee, Wis.) coupled to dipalmitoyl-PE, was synthesized and purified as described previously (51). The liposomes were prepared by _n_-octyl-β-d-glucopyranoside (OGP) dialysis, followed by our freeze-thaw-extrusion protocol described previously (5, 40, 46). OGP was obtained from Calbiochem (Darmstadt, Germany). The OGP dialysis step was included to ensure incorporation of HepPE into the liposomes. Since HepPE is a highly polar lipid and therefore incompletely soluble in chloroform-methanol, uniform HepPE-containing lipid mixtures could not be generated by drying the lipids from chloroform-methanol. Briefly, then, PC-PE-SPM-Chol lipid mixtures were dried from chloroform-methanol and hydrated in 100 mM OGP in 5 mM HEPES-150 mM NaCl-0.1 mM EDTA (pH 7.4) (HNE). Subsequently, HepPE dissolved in 100 mM OGP in HNE was added to the lipid-detergent mixed micelles, and the mixture was dialyzed against HNE to generate liposomes. The liposomes were then subjected to five cycles of freeze-thawing and subsequent extrusion through 0.2-μm-pore-size polycarbonate filters (Nuclepore Inc., Pleasanton, Calif.) in a LipoFast mini-extruder (Avestin, Ottawa, Canada). PhotoChol-containing liposomes were prepared in subdued light. The mean diameter of the liposomes prepared in this fashion was found to be 155 nm, as determined by quasielastic light-scattering analysis in a submicron particle sizer, model 380 ZLS (Nicomp Particle Sizing Systems, Santa Barbara, Calif.). Trypsin-containing liposomes were prepared in a manner similar to that outlined above, except that the lipids were dispersed in 100 mM OGP in HNE containing 10 mg of trypsin (Boehringer, Mannheim, Germany)/ml. The trypsin-containing liposomes were separated from free trypsin by gel filtration on a Sephadex G-100 column in HNE. The phospholipid concentration of the liposomes was determined by phosphate analysis (4).
Binding assays.
Virus binding to BHK-21 cells and heparin- or albumin-agarose beads (both from Sigma) was performed essentially as described previously (28, 48). In the binding assay, 105 to 106 cpm of [35S]methionine-labeled SIN or SFV (approximately 108 to 109 virus particles) was allowed to attach to monolayers of BHK-21 cells or beads for 1 h at 4°C. Subsequently, the cells or beads were washed with HNE plus 1% fetal bovine serum buffer. Virus binding was quantified by liquid scintillation counting.
Binding of the virus to liposomes was assessed by a coflotation assay, as described previously (5, 39, 46). Briefly, [35S]methionine-labeled SIN or SFV (ranging from 105 to 106 cpm) was mixed with liposomes (100 μM phospholipid) and incubated for 1 h at 4°C, unless indicated otherwise. Then 0.1 ml of the mixture was added to 1.4 ml of 50% (wt/vol) sucrose in HNE. On top of this, 1.2-ml volumes of 35, 20, and 5% (wt/vol) sucrose in HNE were layered. After centrifugation at 4°C for 2 h at 150,000 × g in a Beckman SW50 rotor, the gradient was fractionated into 10 samples, starting from the top. The radioactivity found in the top four fractions, relative to the total amount of radioactivity, was taken as a measure of virus-liposome binding. In the heparin competition experiments, soluble heparin (average molecular weight, 6,000; Sigma) was incubated with the virus for 1 h at 4°C before the virus was mixed with the liposomes.
Fusion assays.
Fusion of pyrene-labeled SIN or SFV with liposomes was measured online at 37°C in an AB2 fluorometer (SLM/Aminco, Urbana, Ill.) at excitation and emission wavelengths of 345 and 480 nm, respectively (5, 46, 48). Briefly, pyrene-labeled SIN or SFV (1 μM phospholipid) and liposomes (100 μM phospholipid) were mixed in 0.665 ml of HNE buffer and stirred magnetically in a quartz cuvette. At t = 0 s, fusion was triggered by injection of 35 μl of 0.1 M morpholinoethanesulfonic acid (MES) and 0.2 M acetic acid buffer, pretitrated with NaOH to achieve the final desired pH. Fusion was calibrated such that 0% fusion corresponded to the initial pyrene excimer fluorescence intensity and 100% fusion corresponded to the excimer fluorescence intensity at an infinite dilution of the fluorophore, as induced by addition of 35 μl of 0.2 M octa(ethylene glycol)-_n_-dodecylmonoether (Fluka, Buchs, Switzerland). The initial rate of fusion was determined from the tangent to the initial phase of the curve. The extent of fusion was determined 60 s after acidification.
Mixing of the internal contents of the virus and the liposomes was determined on the basis of the degradation of the viral capsid protein by trypsin, initially encapsulated in the liposomal lumen (54, 46, 47). Briefly, [35S]methionine-labeled SIN (ranging from 105 to 106 cpm) was mixed with trypsin-containing liposomes (100 μM phospholipid) in the presence of 0.125 mg of soybean trypsin inhibitor (Boehringer)/ml in HNE, at 37°C. The mixture was acidified to the desired pH, as described above. After 60 s the reaction mixture was neutralized by addition of a pretitrated volume of NaOH and further incubated for 1 h at 37°C. Control incubations were carried out with empty liposomes, or in the presence of Triton X-100 and absence of trypsin inhibitor. All samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and protein bands were visualized and quantified by phosphorimaging analysis using ImageQuant 3.3 software (Molecular Dynamics, Sunnyvale, Calif.). Capsid degradation was determined by relating the intensity of the capsid protein to the intensity of E1 and E2 in a control experiment in which empty liposomes were used. This ratio was used to calculate the expected intensity of the capsid protein from the reaction in which trypsin-containing liposomes were used. The difference between the expected and the found intensity was taken as a measure of capsid degradation.
RESULTS
Characterization of HS-adapted SIN mutants.
In this study we investigated the HS interaction and membrane fusion activities of the HS-adapted SIN mutants 3970 and TRSB and the nonadapted SIN strain TR339 (carrying the consensus sequence of SIN) (28, 29, 36). SIN mutant 3970 differs from the TR339 clone at position E2:70 (Table 1). The other HS-adapted SIN strain, TRSB, differs from TR339 by a positive-charge amino acid substitution at position E2:1 and a valine-for-alanine substitution at position E1:72 (Table 1).
TABLE 1.
Amino acid differences of HS-adapted SIN mutants
| Virus | Amino acid at position: | |||
|---|---|---|---|---|
| nsP3:528 | E2:1 | E2:70 | E1:72 | |
| TR339 (control) | Arg | Ser | Glu | Ala |
| 3970 | Arg | Ser | Lys | Ala |
| TRSB | Gln | Arg | Glu | Val |
First, the specific infectivity of each of the viruses was determined by a plaque assay on BHK-21 cells (Table 2). In agreement with earlier observations (28, 29), the specific infectivity of TR339 was much lower than the infectivities of the HS-adapted mutants. Next, we determined the extent of binding of the viruses to BHK-21 cells. The results are shown in Table 2. The HS-adapted mutants 3970 and TRSB bound efficiently to monolayers of BHK-21 cells, whereas the TR339 virus bound very poorly under these conditions. Accordingly, TRSB and 3970 bound efficiently to heparin-agarose beads, which served as cell surrogates in a suspension binding assay (Table 2). In a control experiment, none of the viruses bound to albumin-agarose beads (data not shown).
TABLE 2.
Characterization of HS-adapted SIN mutants
| Virus | BHK specific infectivity (PFU/cpm) | Binding (% cpm bound) to: | |
|---|---|---|---|
| BHK cells | HS-agarose beads | ||
| TR339 | 5 | 7 | 10 |
| 3970 | 256 | 42 | 50 |
| TRSB | 186 | 51 | 56 |
Binding of HS-adapted SIN to HepPE-containing liposomes.
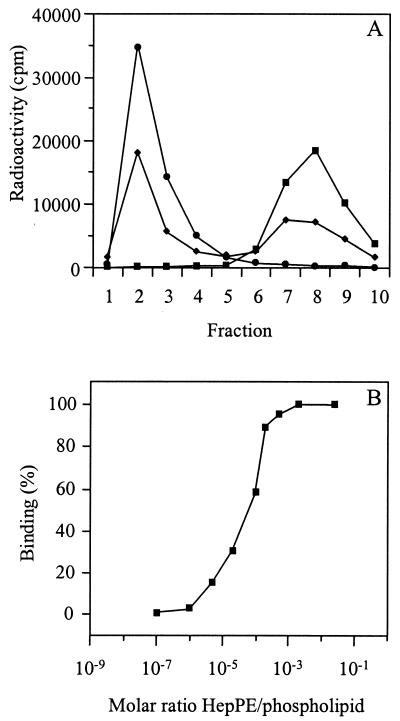
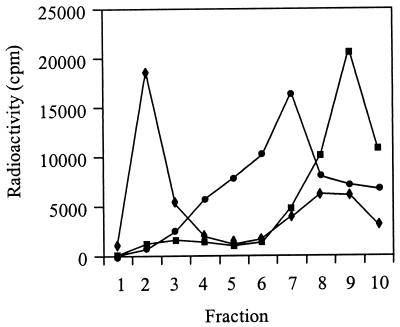
To study the receptor interaction of HS-adapted SIN mutants, we used target liposomes in which HepPE was incorporated in the membrane. Liposomes consisted of PC, PE, SPM, and Chol (molar ratio, 1:1:1:1.5) with various concentrations of HepPE. In the binding experiment, [35S]methionine-labeled SIN was incubated with the liposomes at neutral pH for 1 h at 4°C. Subsequently, the liposome-bound virus was separated from unbound virus by flotation on a sucrose density gradient. In the presence of liposomes supplemented with 0.02 mol% HepPE, essentially all SIN 3970 particles floated to the top of the gradient, demonstrating that the virus bound quantitatively to the liposomes (Fig. 1A). Half-maximal binding was observed with liposomes supplemented with 0.01 mol% HepPE. The virus did not bind to liposomes without HepPE in the membrane.
FIG. 1.
Binding of HS-adapted SIN 3970 to HepPE-containing liposomes. [35S]methionine-labeled SIN (approximately 108 to 109 virus particles) was incubated with HepPE-supplemented PC-PE-SPM-Chol liposomes (100 μM liposomal phospholipid) at pH 7.4 for 1 h at4°C. Binding was determined by flotation analysis on sucrose density gradients as described in Materials and Methods. (A) Gradient profiles obtained after incubation of virus either with control liposomes lacking HepPE (squares) or with liposomes supplemented with 0.01 mol% HepPE (diamonds) or 0.02 mol% HepPE (circles). (B) Extents of binding of SIN 3970 to HepPE-containing liposomes as a function of the molar ratio of HepPE to total phospholipid in the liposomes. Results are averages of triplicate binding measurements.
Figure 1B shows the final extent of binding of SIN 3970 to HepPE-containing liposomes, plotted as a function of the molar ratio of HepPE to total phospholipid in the liposomal membrane. Clearly, binding increased steeply at a ratio of 1 HepPE molecule per 10,000 phospholipid molecules, while binding was maximal at a ratio of 1:5,000. A liposome with a diameter of 155 nm, consisting of phospholipid and Chol in a molar ratio of 2:1, has approximately 100,000 phospholipid molecules in its outer membrane leaflet (55). Thus, maximal binding of SIN 3970 to HepPE-containing liposomes was obtained with, on average, only 20 HepPE molecules exposed on the outer surface of a target liposome, while half-maximal binding occurred with approximately 10 surface-exposed HepPE molecules per liposome.
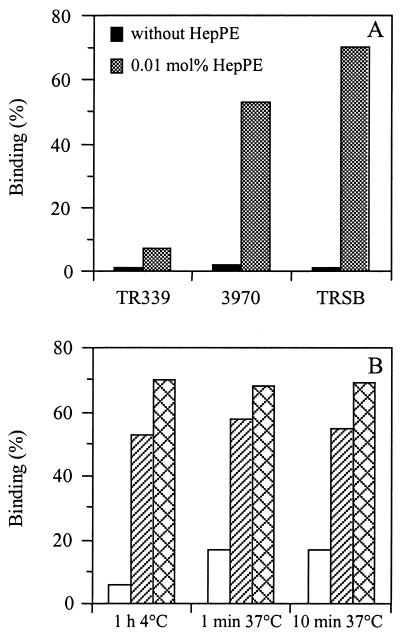
Next, a comparison was made between the liposome binding capacities of HS-adapted SIN 3970 and TRSB and nonadapted SIN TR339. Figure 2A shows the results. For SIN 3970 and TRSB, efficient binding to HepPE-containing liposomes was observed, whereas SIN TR339 bound very poorly to these liposomes. None of the viruses bound to liposomes lacking HepPE in the membrane.
FIG. 2.
Binding of HS-adapted SIN 3970 or TRSB and nonadapted SIN TR339 to liposomes supplemented with 0.01 mol% HepPE. The extent of binding was determined as described in the legend to Fig. 1, unless indicated otherwise. (A) Binding for 1 h at 4°C. Solid bars, PC-PE-SPM-Chol liposomes without HepPE; shaded bars, PC-PE-SPM-Chol liposomes supplemented with 0.01 mol% HepPE. (B) Binding under the conditions indicated. Open bars, TR339; hatched bars, 3970; crosshatched bars, TRSB. Bars represent averages of triplicate binding measurements.
Figure 2B presents the binding of SIN to liposomes supplemented with 0.01 mol% HepPE after incubation for various periods either in the cold or at 37°C. Binding of HS-adapted SIN was fast and efficient at either temperature. Extensive binding of the viruses to HepPE-containing liposomes was observed even after 1 min of incubation at 37°C. Again, nonadapted SIN TR339 bound poorly to the liposomes, although both at 4°C and at 37°C there was a detectable degree of binding, presumably due to a low affinity of the TR339 virus for HS (see also Table 2).
Competition of SIN binding to HepPE-containing liposomes by soluble heparin.
To determine whether the HS-adapted SIN strains interact specifically with the heparin moiety on the liposomal membrane, binding competition experiments were carried out with soluble heparin. SIN was incubated with soluble heparin for 1 h at 4°C. Subsequently, liposomes containing 0.01 mol% HepPE were added and the incubation was continued for 1 h at 4°C in the presence of the soluble heparin. Liposome-bound virus was separated from unbound virus by flotation on a sucrose density gradient. Figure 3 shows the results. In the presence of 5 mg of soluble heparin/ml, SIN 3970 failed to float with the liposomes to the top of the gradient, while in the absence of soluble heparin, efficient binding of the virus to the liposomes was observed. Clearly, soluble heparin blocks binding of the virus to HepPE-containing liposomes, indicating that HS-adapted SIN specifically interacts with the heparin moiety on the liposomal membrane. Maximum competition of SIN 3970 binding to HepPE-containing liposomes required relatively high concentrations of soluble heparin (5 mg/ml). At lower soluble-heparin concentrations (1 mg/ml), we observed migration of the virus to an intermediate position in the gradient, indicating formation of virus-liposome complexes with higher densities than the complexes formed in the absence of soluble heparin. The higher-density complexes presumably arise from interaction between HepPE-containing liposomes and virus aggregated in the presence of soluble heparin. Only at high soluble-heparin concentrations was virus binding to the liposomes blocked completely. This indicates that the interaction of SIN with HepPE-containing liposomes is very tight, suggesting that multiple interactions between a single virion and several HepPE molecules on the liposomal membrane are involved.
FIG. 3.
Effect of soluble heparin on binding of HS-adapted SIN 3970 to PC-PE-SPM-Chol liposomes supplemented with 0.01 mol% HepPE; incubation took place at pH 7.4 for 1 h at 4°C. Binding was determined as described in the legend to Fig. 1. Squares, 5 mg of soluble heparin/ml; circles, 1 mg of soluble heparin/ml; diamonds, control without soluble heparin.
Fusion activity of pyrene-labeled SIN with liposomes with or without HepPE in the target membrane.
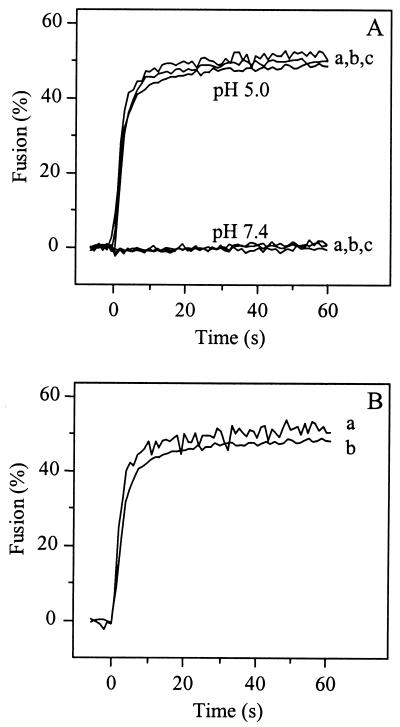
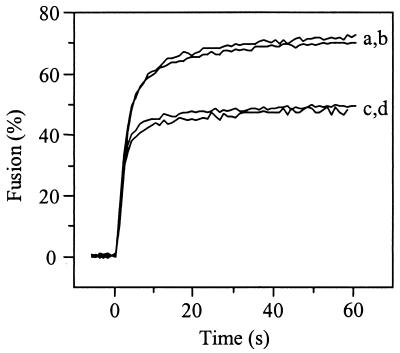
It has been suggested that receptor interaction, rather than low pH, may trigger conformational changes in the viral spike protein of SIN which would subsequently lead to fusion of the viral envelope with the plasma membrane of the cell (1, 17, 24). To study the potential membrane fusion activity of SIN at neutral pH upon interaction with the HepPE attachment receptor, fusion was measured directly by using pyrene-labeled virus, as described previously (46, 47). Pyrene-labeled SIN 3970 or TRSB and liposomes supplemented with 0.01 mol% HepPE were mixed, with continuous stirring, and incubated for 1 min at 37°C and pH 7.4 to achieve binding of the virus to the liposomes (see Fig. 1 and 2). While under these conditions 50 to 60% of the viruses bound to the HepPE receptor on the liposomal target membrane, there was no detectable fusion (Fig. 4A). A control experiment using nonadapted SIN TR339 showed no fusion at neutral pH either. However, all three viruses fused rapidly and efficiently with HepPE-containing liposomes at pH 5.0. Under these conditions, a decrease of 50% in pyrene excimer fluorescence intensity was observed (Fig. 4A). This indicates that SIN, bound to HepPE-containing liposomes at neutral pH, becomes fusion active only upon exposure to an acidic pH.
FIG. 4.
Low-pH-dependent fusion of HS-adapted SIN mutants with HepPE-containing liposomes. Online fusion experiments were performed at 37°C, as described in Materials and Methods. The final virus and liposome concentrations were 1.0 and 100 _μ_M (membrane phospholipid), respectively, unless indicated otherwise. (A) Fusion curves of SIN with PC-PE-SPM-Chol liposomes, supplemented with 0.01 mol% HepPE, at pH 5.0 or pH 7.4. Curves a, TR339; curves b, TRSB; curves c, 3970. (B) Fusion at pH 5.0 of SIN 3970 with liposomes containing 0.01 mol% HepPE after isolation of virus-liposome complexes by flotation on a sucrose density gradient, essentially as in Fig. 1, except that pyrene-labeled virus was used. Peak fractions were collected from the top of the gradient and, after appropriate dilution in HNE, acidified to pH 5.0 at 37°C. Curve a, fusion of isolated virus-liposomes complexes; curve b, fusion of the initial mixture of virus and HepPE-containing liposomes at 1.0 and 100 _μ_M (membrane phospholipid), respectively, before flotation on the sucrose density gradient.
To investigate whether SIN, prebound to HepPE in target liposomes, has an advantage over unbound virus in terms of the kinetics and extent of fusion, we measured fusion on isolated virus-liposome complexes. Pyrene-labeled SIN 3970 was incubated with liposomes containing 0.01 mol% HepPE for 1 h at 4°C. Then liposome-bound and unbound viruses were separated by sucrose density gradient centrifugation, and the membrane fusion activity of virus-liposome complexes collected from the top of the gradient was measured at pH 5.0. The results are shown in Fig. 4B. Clearly, virus prebound to the liposomes fused rapidly and efficiently (curve a), with kinetics very similar to the kinetics of fusion before separation of the unbound virus from the same virus-liposome mixture (curve b). The extent of fusion in the isolated virus-liposome complexes (curve a) was slightly higher than that observed in the initial virus-liposome mixture (curve b).
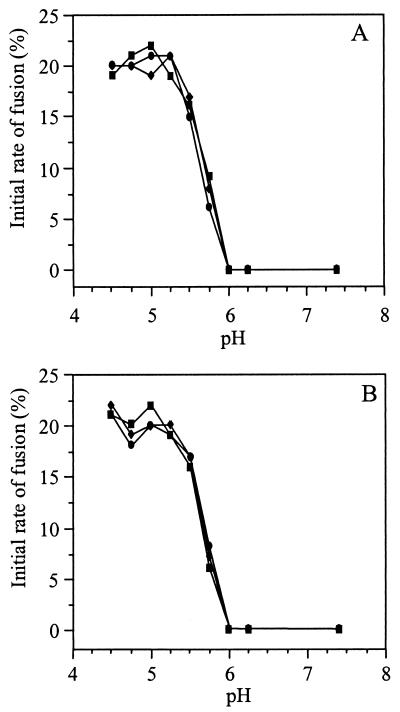
Further support for the idea that prebinding of the virus to the liposomes does not influence the kinetics of the subsequent fusion reaction is presented in Fig. 5. Figure 5A shows the initial rates of fusion of SIN TR339, 3970, and TRSB with liposomes supplemented with 0.01 mol% HepPE as a function of the pH of the medium. Similar fusion kinetics were observed for the HS-adapted SIN 3970 and TRSB versus the nonadapted TR339 virus. Furthermore, using target liposomes without HepPE, we also observed indistinguishable fusion kinetics for HS-adapted SIN and nonadapted SIN TR339 (Fig. 5B). Clearly, all of the SIN strains fused with liposomes in a strictly low-pH-dependent manner, exhibiting similar fusion kinetics irrespective of the presence of HepPE in the target membrane.
FIG. 5.
Kinetics of low-pH-dependent fusion of HS-adapted SIN with HepPE-containing or control liposomes. Online fusion experiments were performed at 37°C, as described in the legend to Fig. 4, at final virus and liposome concentrations of 1.0 and 100 _μ_M (membrane phospholipid), respectively. The initial rate of fusion as a function of pH was determined from the tangents to the first parts of the fusion curves. Squares, 3970; circles, TRSB; diamonds, TR339. (A) Liposomes supplemented with 0.01 mol% HepPE; (B) control PC-PE-SPM-Chol liposomes without HepPE. All fusion measurements were repeated at least three times.
The decrease in pyrene excimer fluorescence intensity by approximately 50%, seen in the above measurements, corresponds to 50% fusion under the assumption that when a virus particle fuses with a liposome, the pyrene probe is diluted infinitely. However, upon fusion of a virus particle with a comparatively small liposome, a residual excimer fluorescence intensity will remain, implying that in this case the actual extent of fusion may be underestimated in the pyrene assay. In this respect it should be noted that the liposomes produced by the OGP dialysis method, as used in our present experiments, tend to be smaller (155 nm in diameter, as indicated above) than the freeze-thaw/extrusion liposomes we use routinely (5, 46, 48). Moreover, inclusion of increasing concentrations of HepPE (>0.02 mol%) in the membrane leads to a further reduction in the size of the liposomes. As a consequence, it is likely that, by using the pyrene excimer fusion assay under these conditions, one in fact underestimates the extent of fusion due to incomplete dilution of the fluorophore.
Recently, it has been shown that a photoactivatable analog of Chol (photoChol) has the capacity to reversibly quench pyrene excimer and monomer fluorescence intensity (37). With this compound we were able to investigate directly whether the pyrene assay does indeed underestimate the extent of fusion. Fusion of pyrene-labeled SIN with photoChol-containing liposomes is monitored not only on the basis of dilution but also on the basis of quenching of the pyrene probe. Figure 6 shows the results. Clearly, at pH 5.0, fusion of SIN 3970 with liposomes consisting of PC, PE, SPM, and photoChol with or without 0.01 mol% HepPE (Fig. 6, curves a and b) appeared more rapid and more efficient than fusion with liposomes containing regular Chol (curves c and d). With photoChol-containing liposomes, the initial rate of fusion was extremely high: an apparent 35 to 40% of the virus particles underwent fusion within the first second after acidification. Furthermore, the apparent extent of fusion was more than 70%. These results indicate that the extent of fusion of SIN with comparatively small liposomes, as assessed by the regular pyrene assay, represents an underestimation of the actual extent of fusion.
FIG. 6.
Fusion of pyrene-labeled SIN 3970 with liposomes containing photoChol at pH 5.0. Fusion was measured online at 37°C as described in the legend to Fig. 4. Curve a, PC-PE-SPM-photoChol liposomes supplemented with 0.01 mol% HepPE; curve b, PC-PE-SPM-photoChol liposomes without HepPE; curve c, PC-PE-SPM-Chol liposomes supplemented with 0.01 mol% HepPE; curve d, PC-PE-SPM-Chol liposomes without HepPE. All fusion measurements were repeated at least three times.
Mixing of contents during fusion of SIN with trypsin-containing liposomes.
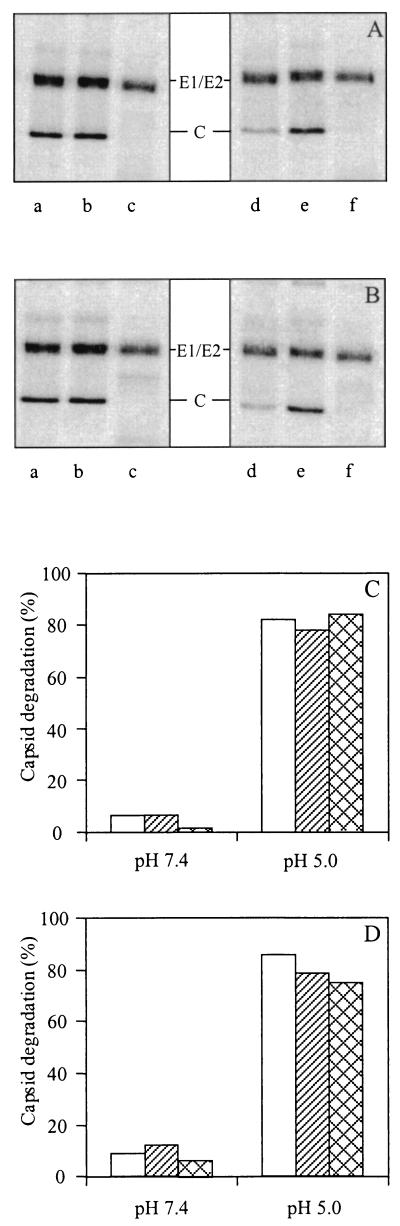
To further quantify the extent of SIN-liposome fusion, we used an entirely different fusion assay based on mixing of the interior of the virus with the liposomal lumen. Mixing of contents was measured as the degradation of the viral capsid protein by trypsin, encapsulated in target liposomes, in the presence of a trypsin inhibitor in the medium (46, 47, 54). [35S]methionine-labeled SIN, either HS-adapted 3970 or nonadapted TR339, and trypsin-containing liposomes supplemented with 0.01 mol% HepPE (100 μM phospholipid) were incubated for 1 min at 37°C and pH 7.4 to allow the virus to bind to the liposomes. Figure 7A and B show that there was very little capsid degradation under these conditions (lanes a). This, again, demonstrates that virus-receptor interaction at neutral pH does not induce fusion of the virus with target liposomes. On the other hand, when SIN 3970 or SIN TR339 was incubated with the liposomes at pH 5.0, almost all of the capsid protein was degraded (Fig. 7A and B, lanes d). In control experiments, in which SIN was incubated with empty HepPE-supplemented liposomes at pH 7.4 or pH 5.0, no capsid degradation was observed (Fig. 7A and B, lanes b and e). The ratio of the radioactivity of the capsid band to the total amount of radioactivity was close to 0.4, as expected on the basis of the number of methionine residues in the structural proteins of SIN. Complete capsid degradation was observed when Triton X-100 was added to the reaction mixture, in the absence of trypsin inhibitor in the medium (Fig. 7A and B, lanes c and f).
FIG. 7.
Fusion of SIN with liposomes, assayed as the degradation of viral capsid protein by liposome-encapsulated trypsin. [35S]methionine-labeled SIN (approximately 108 to 109 virus particles) was incubated with trypsin-containing PC-PE-SPM-Chol liposomes supple-mented with 0.01 mol% HepPE (100 _μ_M liposomal phospholipid) at 37°C, and viral capsid protein degradation was determined as described in Materials and Methods. (A and B) Results for HS-adapted SIN 3970 (A) and nonadapted SIN TR339 (B) with either trypsin-containing liposomes (lanes a and d), empty liposomes (lanes b and e), or trypsin-containing liposomes in the presence of Triton X-100 and absence of a trypsin inhibitor in the medium (lanes c and f) at either pH 7.4 (lanes a to c) or pH 5.0 (lanes d to f). (C and D) Quantification of the extent of capsid protein degradation with liposomes supplemented with 0.01 mol% HepPE (C) or with control liposomes without HepPE (D). Open bars, TR339; hatched bars, 3970; crosshatched bars, TRSB. All capsid degradation experiments were repeated at least twice.
Figure 7C presents the extent of capsid degradation after virus fusion with HepPE-containing liposomes as a function of pH, for HS-adapted SIN 3970 and TRSB or nonadapted SIN TR339. For SIN TR339, little capsid degradation was observed at pH 7.4, whereas at pH 5.0, 82% of the capsid protein was degraded. For HS-adapted SIN 3970 and TRSB, similar results were obtained. Furthermore, in all cases the extent of capsid degradation after fusion of the viruses with liposomes without HepPE was similar to that observed upon fusion with HepPE-containing liposomes (Fig. 7D). The extents of fusion measured with the trypsin assay correspond closely to those observed with the pyrene assay in the presence of photoChol in the target membrane (see Fig. 6). This underlines the above conclusion that the regular pyrene assay underestimates the actual extent of fusion due to incomplete dilution of the probe in the comparatively small dialysis liposomes. Clearly, upon exposure to low pH, under the conditions of our experiments, the large majority of the viruses fuse with the liposomes whether or not these liposomes contain the HepPE attachment receptor.
Interaction of SFV with HepPE-containing liposomes.
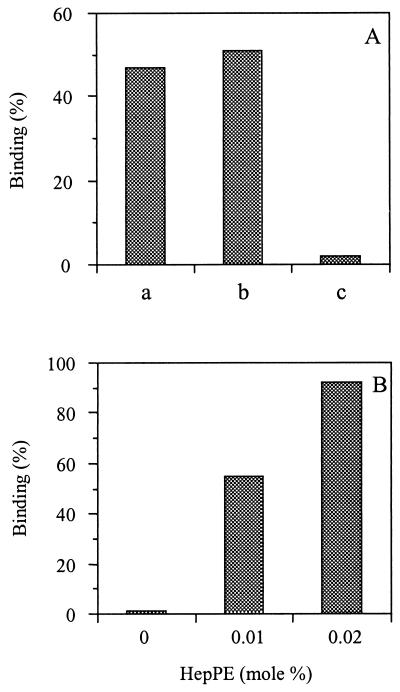
Next, we addressed the question of whether SFV, another member of the Alphavirus genus, has the capacity to adapt to HS during passage in cell culture. To this end, we used SFV derived from the infectious clone pSFV4 as well as a strain of virus passaged many times on BHK-21 cells. The pSFV4 clone was generated from a laboratory strain of SFV, which had also been passaged frequently on BHK-21 cells (31). HS adaptation of SFV derived from the infectious clone pSFV4 was evaluated in binding assays. Figure 8A shows that SFV from pSFV4 bound efficiently to monolayers of BHK-21 cells (bar a). We also used heparin- versus albumin-agarose beads in suspension binding assays (28). The results show that SFV bound efficiently to heparin-agarose beads (Fig. 8A, bar b), whereas the virus did not bind to albumin-agarose beads (bar c).
FIG. 8.
Interaction of SFV with BHK-21 cells, heparin-agarose beads, and HepPE-containing liposomes. (A) [35S]methionine-labeled SFV particles (approximately 108 to 109 virus particles) were added to BHK-21 cell monolayers or heparin- or albumin-agarose beads, and binding was measured after incubation for 1 h at 4°C, as described in Materials and Methods. Bar a, binding to BHK-21 cells; bar b, binding to heparin-agarose beads; bar c, binding to albumin-agarose beads. (B) Binding of [35S]methionine-labeled SFV (approximately 108 to 109 virus particles) to PC-PE-SPM-Chol liposomes supplemented with various concentrations of HepPE (100 _μ_M liposomal phospholipid) during incubation at pH 7.4 for 1 h at 4°C. Binding of SFV to liposomes was assessed as described in the legend to Fig. 1. Each bar represents the average of triplicate binding measurements.
Figure 8B shows that pSFV4-derived virus bound efficiently to HepPE-containing liposomes at a neutral pH. The extent of binding was similar to that obtained with HS-adapted SIN (see Fig. 1 and 2). When liposomes were supplemented with 0.02 mol% HepPE, more than 90% of the virus bound to the liposomes, while with 0.01 mol% HepPE, half-maximal binding was observed. There was no binding to liposomes lacking HepPE in the membrane. Very similar results were obtained with a plaque-purified laboratory-adapted strain of SFV (data not shown). Taken together, these results demonstrate that SFV passaged frequently on BHK-21 cells is strongly adapted to interaction with HS.
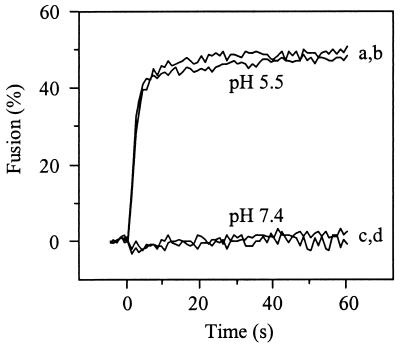
Finally, we investigated the membrane fusion activity of SFV upon interaction with HepPE-containing liposomes. Figure 9 shows the results. Interaction of pyrene-labeled SFV with HepPE in the liposomal membrane did not result in fusion at neutral pH (Fig. 9, curve c). However, the virus fused rapidly and efficiently with HepPE-containing liposomes at pH 5.5 (Fig. 9, curve a). Moreover, similar fusion kinetics were observed with or without HepPE in the target liposomes (Fig. 9, curve a versus curve b). There was no fusion of SFV with liposomes lacking the HepPE receptor analog at neutral pH (Fig. 9, curve d).
FIG. 9.
Low-pH-dependent fusion of pyrene-labeled SFV with liposomes. Fusion of pyrene-labeled SFV with PC-PE-SPM-Chol liposomes with or without 0.01 mol% HepPE (100 _μ_M liposomal phospholipid) at 37°C was determined at pH 5.5 or pH 7.4, essentially as described in the legend to Fig. 4. Curves a and c, PC-PE-SPM-Chol liposomes supplemented with 0.01 mol% HepPE; curves b and d, PC-PE-SPM-Chol liposomes without HepPE. All fusion measurements were repeated at least three times.
DISCUSSION
This paper presents a new liposomal model system in which lipid-conjugated heparin (HepPE) is incorporated in the target membrane as an attachment receptor for HS-adapted alphaviruses. The sulfated polysaccharide heparin is commonly used as an analog for HS in receptor-ligand assays, since interactions of ligands with heparin or HS generally exhibit little qualitative difference (27). It is demonstrated here that HS-adapted SIN 3970 and TRSB interact efficiently, at neutral pH, with liposomes supplemented with remarkably low levels of HepPE in the membrane (Fig. 1 and 2). Without HepPE in the target membrane, the HS-adapted SIN mutants were unable to bind to the liposomes, indicating that the viruses bind specifically to the heparin molecule. Furthermore, SIN strain TR339, which is not adapted to HS, was unable to bind to HepPE-containing liposomes under the same conditions. Moreover, binding competition experiments showed that soluble heparin blocked binding of HS-adapted SIN to HepPE liposomes, further underlining the notion that these viruses specifically interact with the lipid-conjugated heparin moiety on the liposomal membrane. Despite the efficient interaction of SIN with HepPE-containing liposomes at neutral pH, there was no fusion under these conditions (Fig. 4 to 7), as discussed in more detail below.
The interaction of HS-adapted SIN with HepPE-containing liposomes appeared to be extremely efficient. Half-maximal binding was observed with liposomes containing as little as 0.01 mol% HepPE in the membrane, demonstrating that about 10 HepPE molecules on the outer surface of a liposome suffice for efficient binding of the virus to the liposomal membrane. Almost no binding was observed when, on average, 1 to 2 HepPE molecules were incorporated in a single liposome. Therefore, we hypothesize that a SIN particle, after initial binding to a single HepPE molecule in the liposomal membrane, subsequently recruits more HepPE molecules to the site of interaction, resulting in multiple interactions between the virion and several HepPE molecules. This hypothesis is substantiated by the observation that a high concentration of soluble heparin is required for competition. This concentration was at least an order of a magnitude greater than that required for complete competition of binding of HS-adapted SIN to BHK-21 cells (28). An explanation for this difference could be that binding of HS-adapted SIN to cells involves fewer interactions with HS per virus particle than binding to HepPE-supplemented liposomes. This may be related to a limited mobility of HS-carrying core proteins on the cell surface, possibly restricting recruitment of multiple HS moieties to the site of interaction (27).
Similar fusion kinetics were observed for both the HS-adapted SIN 3970 and TRSB and the nonadapted TR339 virus (Fig. 4 and 5). This is a remarkable and, in a sense, counterintuitive observation. One would expect faster kinetics and perhaps higher extents of fusion when the virus is prebound to the target liposomes at the point when the fusion process is triggered by acidification of the medium. Yet all of the SIN strains, whether HS adapted or nonadapted, exhibited almost indistinguishable fusion kinetics irrespective of the presence of HepPE in the target membrane. The explanation for this behavior probably lies in the fact that, in the overall process of virus-liposome binding and fusion, the fusion step is rate-limiting. In previous studies it has been shown that, upon low-pH-triggered SFV- or SIN-liposome binding, fusion occurs only after a distinct lag phase, the length of which increases with increasing pH and decreasing temperature (5, 46). This indicates that, in the absence of prebinding, low-pH-induced virus-liposome binding is a relatively fast process, which is followed by fusion with some delay. Therefore, prebinding of the virus at a neutral pH to HepPE-containing liposomes is in fact unlikely to influence the kinetics of the overall fusion process. This is precisely what is observed. We also studied SIN fusion with liposomes at reduced temperatures and again found that there was no kinetic advantage for virus prebound to HepPE-containing liposomes (data not shown), consistent with an increasing lag phase between virus-liposome binding and the onset of fusion under these conditions (5, 46). With respect to the extent of fusion, our present experiments involving liposomes containing photoChol (Fig. 6) or trypsin (Fig. 7) clearly demonstrate that most of the virus particles (70 to 85%) fuse with the liposomes irrespective of prebinding to HepPE. Therefore, prebinding is unlikely to result in a significant increase in the extent of fusion, since the extent of fusion in the absence of prebinding is already very high.
There is convincing evidence to indicate that SIN, like SFV (22, 23, 34, 35), infects its host cell by receptor-mediated endocytosis and subsequent fusion from within acidic endosomes (15, 20, 46, 47). In this regard, it is intriguing that Hernandez and coworkers recently published a paper (24) in support of earlier observations suggesting that exposure to an acidic compartment within cells may not be an obligatory step in alphavirus infection, but rather that virus-receptor interaction triggers conformational changes in the spike proteins, inducing fusion of the viral membrane with the plasma membrane of the cell (1, 6, 17). The results presented in this study demonstrate that, despite the efficient interaction of SIN with the HepPE in target liposomes at neutral pH, there is no fusion under these conditions. This indicates that HepPE, as an analog of the HS attachment receptor used by cell culture-adapted strains of SIN, has little functional role in triggering membrane fusion activity of the virus. It appears that the principal requirement for SIN-liposome fusion, even after interaction of the virus with the HS receptor analog, remains exposure to a mildly acidic pH. This is in agreement with earlier data demonstrating that not only SIN but also SFV and TBE virus fuse efficiently at low pH with liposomes lacking a protein or carbohydrate receptor (5, 13, 46, 47). Taken together, our present results support the notion that SIN infects its host cells via receptor-mediated endocytosis and low-pH-dependent fusion from within acidic endosomes. The possibility that the initial receptor interaction influences the detailed characteristics of the subsequent pH-dependent membrane fusion process of SIN cannot be excluded, although, as discussed above, in the liposomal model system we observed remarkably similar fusion kinetics for HS-adapted SIN versus nonadapted SIN with or without HepPE in the target membrane. It should be noted that our present study does not address HS-independent virus-receptor interactions. It is likely that some, if not all, alphaviruses use other cellular receptors in addition to or instead of HS (7, 8, 28). It is possible that interaction with these receptors does exert an effect on the low-pH-dependent fusion of alphaviruses.
Newly isolated or unpassaged strains of SIN, RR, and VEE do not bind to heparin and attach poorly to cells in culture relative to laboratory-adapted strains (2, 21, 28). Passage of non-HS-adapted SIN TR339 on BHK-21 cells resulted in virus mutants which bind with high affinity to BHK-21 cells and interact with HS (28). In vivo, these HS-adapted viruses typically exhibit an attenuated phenotype (8, 28). In the present paper, we demonstrate that SFV derived from the infectious clone pSFV4 (31) interacts with HepPE-containing liposomes. In addition, it was found that the virus binds efficiently to BHK-21 cells and that this binding presumably involves cell surface HS. These results suggest that SFV utilizes HS for infection of BHK-21 cells. Thus, adaptation to HS attachment receptors appears to represent a common cell culture-adaptive mechanism among members of the Alphavirus genus.
There is extensive evidence that viruses from different families and genera have the capacity to interact with GAGs, generally HS (2, 7, 10, 11, 12, 14, 26, 28, 30, 32, 33, 44, 56). As for SIN and SFV, binding of flavi-, picorna-, and retroviruses to HS has been found to represent a cell culture adaptation. In other instances, however, this does not appear to be the case. For example, herpes simplex virus type 1 interacts with HS carrying a specific sulfation pattern, serving as an authentic receptor or coreceptor for the virus (45). Since numerous viruses interact with HS, the liposomal model system presented here may serve as a novel tool for the study of basic receptor interactions and membrane fusion properties of these viruses.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (grants HL16660 and AI22186-14), by The Netherlands Organization for Scientific Research (NWO) under the auspices of the Foundation for Chemical Research (CW), and by the Royal Netherlands Academy of Arts and Sciences (KNAW) (travel grant to J.M.S.).
REFERENCES
- 1.Abell, B. A., and D. T. Brown. 1993. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J. Virol. 67**:**5496-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276**:**93-103. [DOI] [PubMed] [Google Scholar]
- 3.Bernfield, M., M. Götte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68**:**729-777. [DOI] [PubMed] [Google Scholar]
- 4.Böttcher, C. J. F., C. M. van Gent, and C. Fries. 1961. A rapid and sensitive sub-micro phosphorus determination. Anal. Chim. Acta 24**:**203-204. [Google Scholar]
- 5.Bron, R., J. M. Wahlberg, H. Garoff, and J. Wilschut. 1993. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 12**:**693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, D. T., and J. Edwards. 1992. Structural changes in alphaviruses accompanying the process of membrane penetration. Semin. Virol. 3**:**519-527. [Google Scholar]
- 7.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72**:**7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrnes, A. P., and D. E. Griffin. 2000. Large plaque-mutants of Sindbis virus show reduced binding to heparan sulfate, heightened viremia and slower clearance from circulation. J. Virol. 74**:**644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardin, A. D., and H. J. R. Weintraub. 1989. Molecular modeling of protein glycoaminoglycan interactions. Arteriosclerosis 9**:**21-32. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3**:**866-871. [DOI] [PubMed] [Google Scholar]
- 11.Chung, C.-S., J.-C. Hsiao, Y.-S. Chang, and W. Chang. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72**:**1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193**:**382-390. [DOI] [PubMed] [Google Scholar]
- 13.Corver, J. A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269**:**37-46. [DOI] [PubMed] [Google Scholar]
- 14.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycoaminoglycans are involved in adenovirus type 5 and type 2 host-cell interactions. Virology 268**:**382-390. [DOI] [PubMed] [Google Scholar]
- 15.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17**:**4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escarmis, C., E. C. Carrillo, M. Ferrer, J. F. Arriaza, N. Lopez, C. Tami, N. Verdaguer, E. Domingo, and M. T. Franze-Fernandez. 1998. Rapid selection in modified BHK-21 cells of a foot-and-mouth disease virus variant showing alterations in cell tropism. J. Virol. 72**:**10172-10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn, D. C., W. J. Meyer, J. M. Mackenzie, and R. E. Johnston. 1990. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interaction. J. Virol. 64**:**3643-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry, E. E., S. M. Lea, T. Jackson, J. W. I. Newman, F. M. Ellard, W. E. Blakemore, R. Abu-Ghazaleh, A. Samuel, A. M. Q. King, and D. I. Stuart. 1999. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 18**:**543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garoff, H., A. M. Frischauf, K. Simons, H. Lehrach, and H. Delius. 1980. Nucleotide sequence of cDNA coding for Semliki Forest virus membrane glycoproteins. Nature 288**:**236-241. [DOI] [PubMed] [Google Scholar]
- 20.Glomb-Reinmund, S., and M. Kielian. 1998. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology 248**:**372-381. [DOI] [PubMed] [Google Scholar]
- 21.Heil, M. L., A. Albee, J. H. Strauss, and R. J. Kuhn. 2001. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J. Virol. 75**:**6303-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helenius, A., J. Kartenbeck, K. Simons, and E. Fries. 1980. On the entry of Semliki Forest virus into BHK-21 cells. J. Cell Biol. 84**:**404-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helenius, A. 1984. Semliki Forest virus penetration from endosomes: a morphological study. Biol. Cell 51**:**181-187. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez, R., T. Luo, and D. T. Brown. 2001. Exposure to low pH is not required for penetration of mosquito cells by Sindbis virus. J. Virol. 75**:**2010-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulst, M. M., H. G. P. van Gennip, and R. J. M. Moormann. 2000. Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein Erns. J. Virol. 74**:**9553-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson, T., F. M. Ellard, R. Abu Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. I. Newman, and A. M. Q. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70**:**5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kjellen, L., and U. Lindahl. 1991. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60**:**443-475. [DOI] [PubMed] [Google Scholar]
- 28.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK-21 cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72**:**7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1999. The furin protease cleavage recognition sequence of Sindbis virus PE2 can mediate virion attachment to cell surface heparan sulfate. J. Virol. 73**:**6299-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krusat, T., and H. J. Streckert. 1997. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch. Virol. 142**:**1247-1254. [DOI] [PubMed] [Google Scholar]
- 31.Liljeström, P., S. Lusa, D. Huylebroek, and H. Garoff. 1991. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J. Virol. 65**:**4107-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, C.-L., C.-S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74**:**3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandl, C. W., H. Kroschewski, S. L. Allison, R. Kofler, H. Holzmann, T. Meixner, and F. X. Heinz. 2001. Adaptation of tick-borne encephalitis virus to BHK-21 cells results in the formation of multiple heparan sulfate binding sites in the envelope protein and attenuation in vivo. J. Virol. 75**:**5627-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsh, M., J. Wellsteed, H. Kern, E. Harms, and A. Helenius. 1982. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc. Natl. Acad. Sci. USA 79**:**5297-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh, M., E. Bolzau, and A. Helenius. 1983. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell 32**:**931-940. [DOI] [PubMed] [Google Scholar]
- 36.McKnight, K. L., D. A. Simpson, S. C. Lin, T. A. Knott, J. M. Polo, D. F. Pence, D. B. Johannesen, H. W. Heidner, N. L. Davis, and R. E. Johnston. 1996. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strain will affect cell culture and in vivo phenotypes. J. Virol. 70**:**1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mintzer, E. A., B.-L. Waarts, J. Wilschut, and R. Bittman. 2002. Behavior of photoactivatable analog of cholesterol, 6-photocholesterol, in model membranes. FEBS Lett. 510**:**181-184. [DOI] [PubMed] [Google Scholar]
- 38.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74**:**1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieva, J. L., R. Bron, J. Corver, and J. Wilschut. 1994. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 13**:**2797-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ollivon, M., S. Lesieur, C. Grabielle-Madelmont, and M. Paternostre. 2000. Vesicle reconstitution from lipid-detergent mixed micelles. Biochim. Biophys. Acta 1508**:**34-50. [DOI] [PubMed] [Google Scholar]
- 41.Patel, M., M. Yanagishita, G. Roderiquez, D. C. Bou-Habib, T. Oravecz, V. C. Vascall, and M. A. Norcross. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retrovir. 9**:**167-174. [DOI] [PubMed] [Google Scholar]
- 42.Peterson, G. L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83**:**346-356. [DOI] [PubMed] [Google Scholar]
- 43.Sa-Carvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 71**:**5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Secchiero, P., D. Sund, A. L. De Vico, R. W. Crowly, M. S. Reitz, Jr., G. Zauli, P. Lusso, and R. C. Gallo. 1997. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J. Virol. 71**:**4571-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99**:**13-22. [DOI] [PubMed] [Google Scholar]
- 46.Smit, J. M., R. Bittman, and J. Wilschut. 1999. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J. Virol. 73**:**8476-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smit, J. M., R. Bittman, and J. Wilschut. 2001. Deacylation of the transmembrane domains of Sindbis virus envelope glycoproteins E1 and E2 does not affect low-pH-induced viral membrane fusion activity. FEBS Lett. 498**:**57-61. [DOI] [PubMed] [Google Scholar]
- 48.Smit, J. M., W. B. Klimstra, K. D. Ryman, R. Bittman, R. E. Johnston, and J. Wilschut. 2001. PE2 cleavage mutants of Sindbis virus: correlation between viral infectivity and pH-dependent membrane fusion activation of the spike heterodimer. J. Virol. 75**:**11196-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, T. J., R. H. Cheng, N. H. Olson, P. Peterson, E. Chase, R. J. Kuhn, and T. S. Baker. 1995. Putative receptor binding sites on Alphaviruses as visualized by cryoelectron microscopy. Proc. Natl. Acad. Sci. USA 92**:**10648-10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strauss, J. H., and E. G. Strauss. 1994. The Alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58**:**491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugiura, N., K. Sakurai, Y. Hori, K. Karasawa, S. Suzuki, and K. Kimata. 1993. Preparation of lipid-derivatized glycoaminoglycans to probe a regulatory function of the carbohydrate moieties of proteoglycans in cell-matrix interaction. J. Biol. Chem. 21**:**15779-15787. [PubMed] [Google Scholar]
- 52.Tumova, S., A. Woods, and J. R. Couchman. 2000. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int. J. Biochem. Cell Biol. 32**:**269-288. [DOI] [PubMed] [Google Scholar]
- 53.Wahlberg, J. M., R. Bron, J. Wilschut, and H. Garoff. 1992. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 66**:**7309-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White, J., and A. Helenius. 1980. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc. Natl. Acad. Sci. USA 77**:**3273-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilschut, J. 1982. Preparation and properties of phospholipid vesicles, p. 9-24. In L. D. Leserman and J. Barbet (ed.), Methodologie des Liposomes, vol. 107. Editions INSERM, Paris, France.
- 56.WuDunn, D., and D. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63**:**52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]