Interleukin-1 (IL-1) Receptor-Associated Kinase-Dependent IL-1-Induced Signaling Complexes Phosphorylate TAK1 and TAB2 at the Plasma Membrane and Activate TAK1 in the Cytosol (original) (raw)
Abstract
Interleukin-1 (IL-1) receptor-associated kinase (IRAK) plays an important role in the sequential formation and activation of IL-1-induced signaling complexes. Previous studies showed that IRAK is recruited to the IL-1-receptor complex, where it is hyperphosphorylated. We now find that the phosphorylated IRAK in turn recruits TRAF6 to the receptor complex (complex I), which differs from the previous concept that IRAK interacts with TRAF6 after it leaves the receptor. IRAK then brings TRAF6 to TAK1, TAB1, and TAB2, which are preassociated on the membrane before stimulation to form the membrane-associated complex II. The formation of complex II leads to the phosphorylation of TAK1 and TAB2 on the membrane by an unknown kinase, followed by the dissociation of TRAF6-TAK1-TAB1-TAB2 (complex III) from IRAK and consequent translocation of complex III to the cytosol. The formation of complex III and its interaction with additional cytosolic factors lead to the activation of TAK1, resulting in NF-κB and JNK activation. Phosphorylated IRAK remains on the membrane and eventually is ubiquitinated and degraded. Taken together, the new data reveal that IRAK plays a critical role in mediating the association and dissociation of IL-1-induced signaling complexes, functioning as an organizer and transporter in IL-1-dependent signaling.
Interleukin-1 (IL-1), a major inflammatory cytokine, exerts its biological effects by activating the transcription of various responsive genes (7). The transcription factors activated by IL-1 include NF-κB, AP1, and ATF (2, 19, 20). The IL-1 receptor complex is composed of the type 1 receptor (IL-1R) and the receptor accessory protein (IL-1RAcp) (8-10). Upon IL-1 stimulation, the cytosolic proteins MyD88 (1, 15, 28) and Tollip (3) are recruited to this receptor complex, where they function as adaptors, recruiting IL-1 receptor-associated kinase (IRAK) in turn. IRAK, a serine-threonine kinase, is phosphorylated at the receptor complex and then interacts with TRAF6 (4, 5, 12-14). Phosphorylated IRAK is eventually ubiquitinated and degraded (31). IRAK4 has recently been shown to be an essential component for the IL-1 signaling pathway and proposed to function as an IRAK kinase (11, 24). IRAK and TRAF6 interact with TAK1, a member of the MAP kinase kinase kinase (MAPKKK) family, and two proteins that bind to it, TAB1 and TAB2 (18, 25). The kinase activity of TAK1 is thus activated upon IL-1 stimulation. While genetic studies show that IRAK is required for the activation of TAK1 (26), in vitro biochemical analyses reveal that TRAF6-mediated ubiquitination may also play an important role in TAK1 activation (27). The activation of TAK1 eventually leads to the activation of IκB kinase (IKK) by an unknown mechanism. Activated IKK phosphorylates the inhibitory IκB proteins, which are then degraded, releasing NF-κB to activate transcription in the nucleus (17, 22, 29, 35). Activated TAK1 has also been implicated in the IL-1-induced activation of MKK6 and JNK (18), leading to the phosphorylation and activation of ATF and AP1, thereby also activating transcription.
We have previously taken a genetic approach to study IL-1-dependent signaling pathways; through random mutagenesis, we generated IL-1-unresponsive cell lines lacking specific components of the pathways. Mutant cell line I1A, which lacks both IRAK protein and mRNA (12, 13), has been used effectively to study structure-function relationships of IRAK in IL-1-dependent signaling (12, 13). Neither NF-κB nor JNK is activated in IL-1-treated I1A cells, but these responses are restored in I1A-IRAK cells, indicating that IRAK is required for both. However, the kinase activity of IRAK is not required for IL-1-dependent signaling (12, 13), since kinase-dead IRAK mutants were still able to restore IL-1 responsiveness in mutant I1A cells. On the other hand, IL-1-induced phosphorylation of IRAK probably plays a critical role in its interaction with TRAF6, TAK1, TAB1, and TAB2 and in the activation of TAK1 and IKK. The IRAK phosphorylated in response to IL-1 is membrane-bound, whereas TRAF6 and TAB2 are localized on the membrane ever before stimulation. Previously, Qian et al. (21) reported that IRAK is required for the IL-1-induced translocation of TRAF6 and TAB2 from the membrane to the cytosol, probably through their signal-dependent interaction on the membrane, and proposed that the translocation of both TAB2 and TRAF6 are required to form a TRAF6-TAK1-TAB1-TAB2 complex in the cytosol, leading to the activation of NF-κB and JNK.
Although much progress has been made in understanding IL-1-mediated signaling, many questions still remain. For example, we do not know exactly how TAK1 is activated, nor do we understand the precise role of IRAK in TAK1 activation. It is also unknown how activated TAK1 leads to the activation of IKK and JNK. We now attempt to elucidate the details of the molecular mechanism of IL-1-dependent signal transduction by investigating the sequential formation and activation of signaling complexes upon IL-1 stimulation. We find that TRAF6 is recruited to the IL-1 receptor complex (complex I) through IRAK upon IL-1 stimulation. IRAK-TRAF6 then leaves the receptor to form complex II with TAK1, TAB1, and TAB2, which are preassociated on the membrane before stimulation. Importantly, formation of this complex II leads to the phosphorylation of TAK1 and TAB2 but not the activation of TAK1 on the membrane. TRAF6, TAB2, TAK1, and TAB1 (complex III) subsequently dissociate from IRAK and translocate from the membrane to the cytosol, where the TAK1 is activated.
MATERIALS AND METHODS
Biological reagents and cell culture.
Recombinant human IL-1β was provided by the National Cancer Institute. Anti-IL-1R and anti-IRAK polyclonal antibodies were a kind gift of Zhaodan Cao (Tularik, South San Francisco, Calif.). Anti-TRAF6 and anti-actin polyclonal antibodies were from Santa Cruz (Santa Cruz, Calif.). Rabbit anti-TAK1, anti-TAB1, and anti-TAB2 polyclonal antibodies were described previously (18, 25). 293-TK/Zeo cells (12), I1A cells, and I1A cells transfected with IRAK deletion mutants (13) were maintained in Dulbecco's modified Eagle's medium, supplemented with 10% fetal calf serum, penicillin G (100 μg/ml), and streptomycin (100 μg/ml).
Recombinant plasmids and stable transfection.
IRAK deletion constructs were described previously (13). pE-selectin-luc, an NF-κB-dependent E-selectin-luciferase reporter plasmid, was described by Schindler and Baichwal (23). For stable transfections, 2 × 105 cells were seeded onto a 10-cm-diameter plate and cotransfected the following day by the calcium phosphate method with 10 μg of each expression vector and 1 μg of pBabePuro. After 48 h, the cells were selected with 1 μg of puromycin per ml until clones appeared.
Coimmunoprecipitation and immunoblotting.
Cells that were not treated or treated with IL-1 (100 U/ml) were lysed in a Triton-containing lysis buffer (0.5% Triton X-100, 20 mM HEPES [pH 7.4], 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 10 mM NaF, 2 mM dithiothreitol, 1 mM sodium orthovanadate, 2 mM EGTA, 20 μM aprotinin, 1 mM phenylmethylsulfonyl fluoride). Cell extracts were incubated with 1 μg of antibody or preimmune serum (negative control) for 2 h, followed by a 2-h incubation with 20 μl of protein A-Sepharose beads (prewashed and resuspended in phosphate-buffered saline at a 1:1 ratio). After incubation, the beads were washed four times with lysis buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon-P membranes (Millipore), and analyzed by immunoblotting.
In vitro phosphorylation assay.
TAK1 and TAB2 immunoprecipitates were incubated with 1 μg of bacterially expressed MKK6 in 20 μl of kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM dithiothreitol, 5 mM MgCl2, and 5 μCi of γ-32P-labeled ATP (3,000 Ci/mmol) at 25°C for 2 min. Samples were subjected to SDS-PAGE again and transferred to Immobilon-P membranes, and the proteins were visualized by autoradiography. The membranes were also analyzed by immunoblotting.
Subcellular fractionation.
The subcellular fractionation method described previously (21) was used with modifications. Cells were lysed in a hypotonic buffer (10 mM HEPES [pH 7.4], 1.5 mM MgCl2, 10 mM KCl, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol) and homogenized on ice with a Dounce homogenizer. To ensure complete breakdown of the cell membrane sheets to small membrane vesicles, 107 cells were lysed in 1 ml of hypotonic buffer (three times more buffer was used than that in the previous protocol [21]) and homogenized with 45 strokes (30 strokes used in the previous protocol [21]). Unlysed cells, cell debris, and nuclei were removed by centrifugation at 1,000 × g for 5 min. Soluble (supernatant [S-100]) and particulate (pellet [P-100]) fractions were generated by centrifugation at 100,000 × g for 1 h at 4°C. P-100 was solubilized in the Triton-containing lysis buffer and were centrifuged at 10,000 × g for 10 min.
RESULTS
IRAK is required for the recruitment of TRAF6 to IL-1R.
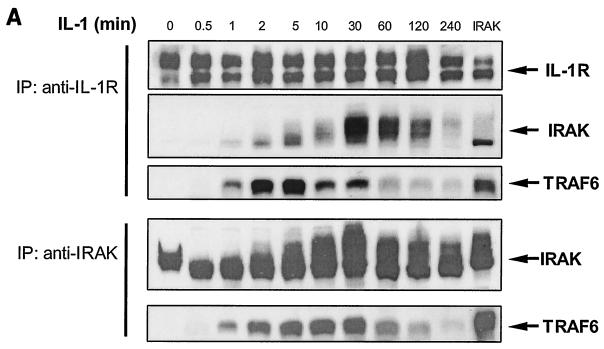
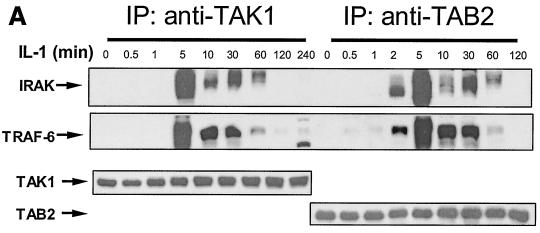
Previously it was shown that IRAK is recruited to the IL-1 receptor and hyperphosphorylated upon IL-1 stimulation (31). It was believed that phosphorylated IRAK then leaves the receptor complex to interact with TRAF6 in the cytoplasm, leading to the activation of NF-κB and JNK. However, TRAF6 is also recruited to the IL-1 receptor upon stimulation (Fig. 1 and 2A). We performed time course experiments to examine the interactions of IRAK and TRAF6 with IL-1R. Extracts of 293 cells treated with IL-1 for different times were immunoprecipitated with anti-IL-1R or anti-IRAK, followed by Western blot analyses with antibodies against IL-1R, IRAK, and TRAF6. IRAK interacts with IL-1R immediately after IL-1 stimulation (Fig. 2A). The recruitment of IRAK peaked at 30 min and was sustained for more than 2 h. Interestingly, TRAF6 was also recruited to the receptor complex, but this binding was more transient. While the recruitment of TRAF6 peaked between 2 and 5 min, most TRAF6 had left IL-1R 10 min after IL-1 stimulation. On the other hand, the interaction of TRAF6 with IRAK peaked at 10 min and was sustained for up to 2 h. Taken together, these results indicate that both IRAK and TRAF6 are recruited to the IL-1R upon IL-1 stimulation, where they form an initial complex, which then dissociates from the receptor to interact with downstream components.
FIG. 1.
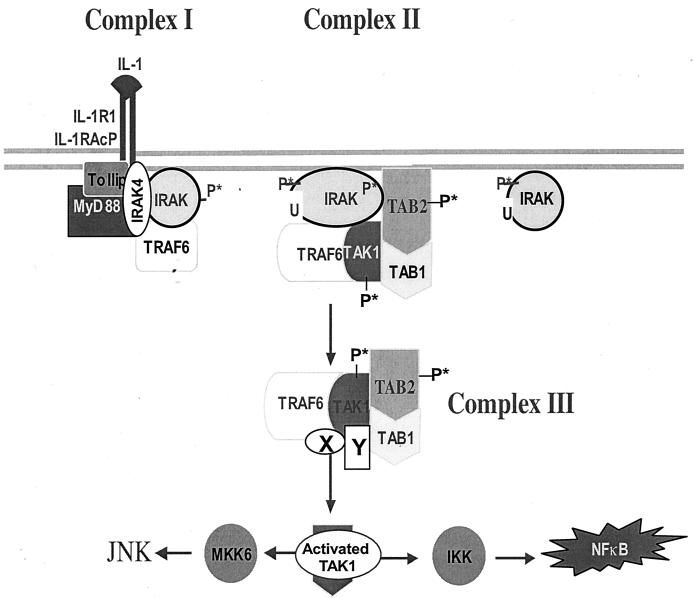
A revised model for IL-1-dependent signaling. Upon IL-1 stimulation, the adaptor molecules MyD88 and Tollip are recruited to the IL-1 receptor complex, which then recruits IRAK. IRAK is hyperphosphorylated, mediating the recruitment of TRAF6 to the receptor complex (complex I). IRAK4 is included in complex I, since it has been suggested that it may function as an IRAK kinase. IRAK-TRAF6 then leaves complex I to interact with preassociated TAK1, TAB1, and TAB2 on the membrane, resulting in the formation of complex II. The formation of complex II leads to the phosphorylation of TAK1 and TAB2, which facilitates the formation and translocation of complex III from the membrane to the cytosol. The formation of complex III and its interaction with additional factors in the cytosol lead to the activation of TAK1. The activated TAK1 causes, directly or indirectly, the activation of IKK and MKK6, resulting in activation of NF-κB and JNK. X and Y are unknown components. P*, phosphorylation; U, ubiquitination.
FIG. 2.
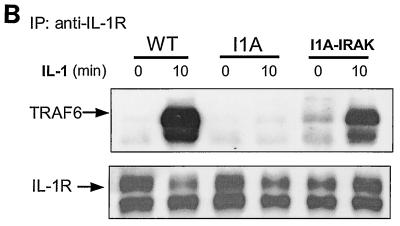
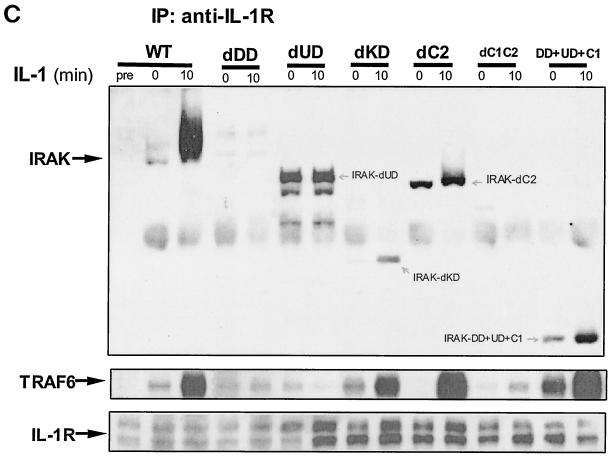
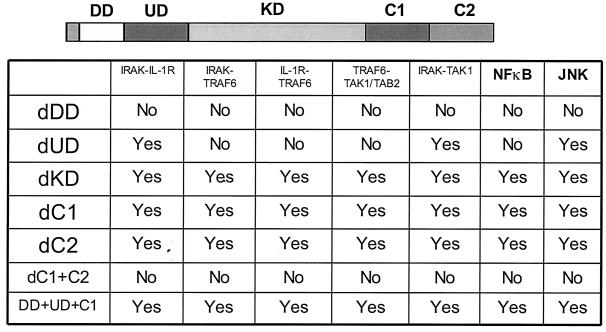
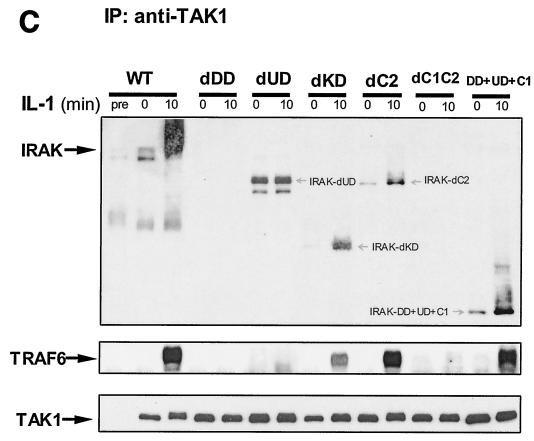
Formation of complex I. (A) Recruitment of TRAF6 to the IL-1 receptor. Extracts of 293 cells either untreated or treated with IL-1 for the indicated times, immunoprecipitated (IP) with anti-IL-1R or anti-IRAK antibody, and subjected to Western blot analysis with anti-IL-1R, anti-IRAK, or anti-TRAF6 antibody. (B) The recruitment of TRAF6 to IL-1 receptor is IRAK dependent. Extracts of 293 cells (wild type [WT]), IRAK-deficient cells (I1A), I1A cells stably transfected with IRAK (I1A-IRAK), either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-IL-1R antibody and subjected to Western blot analysis with anti-TRAF6 and anti-IL-1R antibodies. (C) Domains of IRAK required for the recruitment of IRAK-TRAF6 to IL-1 receptor. Extracts of 293 cells (WT), IRAK-deficient I1A cells transfected with IRAK deletion mutants dDD, dUD, dKD, dC2, dC1C2, and DD+UD+C1 either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-IL-1R antibody and probed with anti-IRAK, anti-TRAF6, or anti-IL-1R antibody.
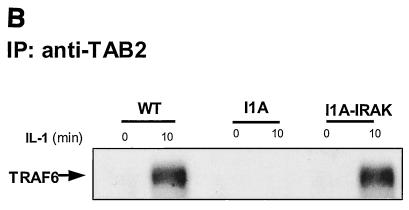
We have previously generated through random mutagenesis IL-1-unresponsive cell lines deficient in specific components of the pathway. Cell line I1A lacks both the IRAK protein and mRNA. To determine whether the recruitment of TRAF6 to the receptor is through IRAK, we studied the TRAF6-IL-1R interaction in I1A cells. Extracts of wild-type 293 and mutant I1A cells, untreated or treated with IL-1, were immunoprecipitated with anti-IL-1R antibody, followed by Western blot analyses with antibodies against TRAF6 and IL-1R. While TRAF6 was recruited to the receptor in wild-type cells, it failed to interact with the receptor in IRAK-deficient I1A cells (Fig. 2B). Transfection of IRAK into the I1A cells restored the recruitment of TRAF6 to the IL-1R. Taken together, the above data indicate that IRAK is required for the recruitment of TRAF6 to the receptor. For convenience, IL-1R-IRAK-TRAF6 is hereafter referred to as complex I (Fig. 1).
IRAK is a multidomain protein containing an N-terminal death domain (DD) (residues 1 to 103), followed by a domain of unknown function (UD) (N-terminal proximal domain) (residues 104 to 198), a kinase domain (KD) (residues 199 to 522), and a two-part C-terminal domain also of unknown function (C1 [residues 523 to 618] and C2 [residues 619 to 712]). IRAK-deficient I1A cells have been used effectively to study the structure-function relationship of IRAK in IL-1-dependent signaling (12). To identify the domains of IRAK required for the formation of complex I, we examined the interactions of IRAK and TRAF6 with IL-1R in IRAK-deficient I1A cells transfected with the various deletion mutants. Coimmunoprecipitation experiments showed that deletion of KD, C1, or C2 of IRAK had no effect on the interactions between IRAK-IL-1R, IRAK-TRAF6, and TRAF6-IL-1R (Fig. 2C and 3), whereas IRAK mutants lacking the N-terminal DD or both C1 and C2 neither interacted with IL-1R or TRAF6 nor supported the recruitment of TRAF6 to the receptor (Fig. 2C and 3), suggesting that DD and the intact C terminus are required for the IL-1-induced recruitment of IRAK-TRAF6 to the IL-1R. On the other hand, the IRAK mutant lacking the N-terminal proximal UD showed only constitutive interaction with IL-1 receptor, but it failed to interact with TRAF6 and was unable to mediate the recruitment of TRAF6 to the receptor (Fig. 2C and 3). Taken together, the above results show that the domains of IRAK required for its interaction with the IL-1 receptor and TRAF6 are also required to recruit TRAF6 to the receptor, further indicating that the interaction of TRAF6 with the IL-1 receptor is through IRAK.
FIG. 3.
IRAK-mediated interactions between signaling components. IL-1-induced interactions between signaling molecules were examined by coimmunoprecipitation in IRAK-deficient I1A cells transfected with different IRAK deletion constructs. DD, death domain; UD, domain of unknown function, KD, kinase domain; dDD, deletion of death domain; dUD, deletion of undetermined domain; dKD, deletion of kinase domain; dC, deletion of C-terminal domain.
Release of IRAK and TRAF6 from the IL-1 receptor precedes their interaction with TAK1 and TAB2.
Previous studies revealed that both IRAK and TRAF6 interact with TAK1, TAB1, and TAB2 upon IL-1 stimulation, probably leading to the activation of TAK1 (18, 21, 22). However, it is not clear how and when these proteins interact with each other. From the data presented above, it is likely that the IRAK-TRAF6 complex forms at the receptor and then leaves to interact with downstream signaling components. To test this point, we first performed time course experiments to examine the interactions of IRAK-TRAF6 with TAK1 and TAB2 upon IL-1 stimulation (Fig. 4A). IRAK and TRAF6 show very similar kinetics of interaction with TAK1 and TAB2, suggesting that IRAK and TRAF6 bind to TAK1 and TAB2 as a complex. Furthermore, the interaction of IL-1R with TAK1 and TAB2 was not detected by coimmunoprecipitation with anti-IL-1R, anti-TAK1, or anti-TAB2 antibody (data not shown), suggesting that the interaction of IRAK-TRAF6 with TAK1 and TAB2 occurs after the release of IRAK-TRAF6 from the receptor complex (complex I [Fig. 1]).
FIG. 4.
Interaction of IRAK-TRAF6 with TAK1 and TAB2. (A) Kinetics. Extracts of 293 cells, either untreated or treated with IL-1, were immunoprecipitated (IP) with anti-TAK1 or anti-TAB2 antibody and subjected to Western blot analyses with anti-IRAK, anti-TRAF6, anti-TAK1, and anti-TAB2 antibodies. (B) The interaction between TRAF6 and TAB2 is IRAK dependent. Extracts of 293 cells (wild type [WT]), IRAK-deficient cells (I1A), and I1A cells stably transfected with IRAK (I1A-IRAK), either untreated or treated with IL-1 for 10 min were immunoprecipitated with anti-TAB2 antibody and probed with anti-TRAF6 antibody. (C) Domains of IRAK required for the interaction of IRAK-TRAF6 with TAK1. Extracts of 293 cells (WT) and IRAK-deficient cells I1A, stably transfected with different IRAK deletion mutants (dDD, dUD, dKD, dC2, dC1C2, and DD+UD+C1), either untreated or treated with IL-1 for 10 min, were immunoprecipitated with anti-TAK1 antibody and probed with anti-IRAK, anti-TRAF6, and anti-TAK1 antibodies.
Using the IRAK-deficient I1A cells, we found that interaction of TRAF6 with TAK1 and TAB2 is IRAK dependent (Fig. 4B and data not shown), further supporting the conclusion that IRAK and TRAF6 bind to TAK1 and TAB2 as a complex. To identify the domains of IRAK required for those interactions, we studied these components in I1A cells transfected with the IRAK deletion mutants (Fig. 3 and 4C). The deletion of KD, C1, or C2 had no effect on the interactions between IRAK-TAK1, IRAK-TAB2, TRAF6-TAK1, and TRAF6-TAB2 (Fig. 3 and 4C). Deletion mutants lacking the whole C terminus or the N-terminal DD neither interacted with TAK1 or TAB2 nor supported the interaction of TRAF6 (Fig. 3 and 4C), indicating that these two domains are required for the interaction of IRAK-TRAF6 with TAK1 and TAB2. Although the deletion mutant lacking UD interacted with TAK1 constitutively, as discussed above, it failed to interact with TRAF6 and was also unable to mediate the interaction of TRAF6 with TAK1 and TAB2 (Fig. 3 and 4C). Taken together, the above results show that the domains of IRAK that are required for its interaction with TRAF6, TAK1, and TAB2 are also required for the interaction of TRAF6 with TAK1 and TAB2, further indicating that the interaction of TRAF6 with TAK1 and TAB2 is through IRAK.
TAK1, TAB1, and TAB2 are preassociated on the membrane.
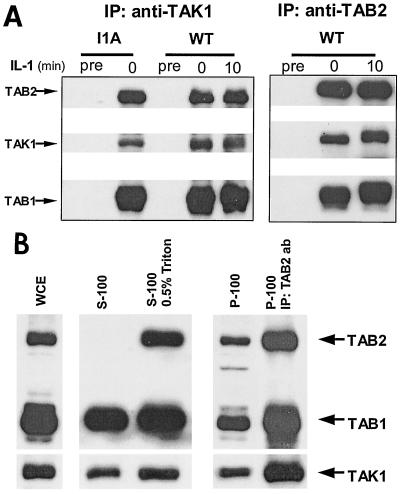
We have previously studied the subcellular localization of IRAK, TRAF6, TAK1, TAB1, and TAB2 (21). While both TRAF6 and TAB2 were localized on the membrane before stimulation, the phosphorylated IRAK induced by IL-1 stimulation was also membrane-bound. Using the IRAK-deficient cells, we reported that IRAK was required for the translocation of TRAF6 and TAB2 from the membrane to the cytosol. On the other hand, TAK1 and TAB1 were mostly localized in the cytosol before or after stimulation, although small amounts of TAK1 and TAB1 were detected in the membrane. Surprisingly, we now found that TAK1, TAB1, and TAB2 are preassociated before stimulation and the association remained after stimulation (Fig. 5A). Further, the preassociation of these three components was independent of IRAK, since their interactions were intact in I1A cells.
FIG. 5.
TAK1, TAB1, and TAB2 are preassociated on the membrane. (A) TAK1, TAB1, and TAB2 are preassociated. Extracts of 293 cells (wild type [WT]) and IRAK-deficient cells (I1A), either untreated or treated with IL-1, were immunoprecipitated with anti-TAK1 and anti-TAB2 antibodies and subjected to Western blot analyses with anti-TAB2, anti-TAK1, and anti-TAB1 antibodies. Preimmune serum (Pre) was used as a control. (B) TAK1, TAB1, and TAB2 form a complex on the membrane. Whole-cell extract (WCE) (prepared with SDS lysis buffer) of 293 cells and membrane and cytosolic fractions prepared from 293 cells with or without Triton (S-100, soluble fraction; S-100/0.5% Triton, soluble fraction prepared with 0.05% Triton; P-100, particulate fraction) were analyzed by the Western blot method with anti-TAB2, anti-TAB1, and anti-TAK1 antibodies. The membrane and cytosolic fractions were prepared in the same volume. Twice as much membrane fraction was loaded compared to the cytosolic fraction. The P-100 fractions were also resuspended in Triton lysis buffer, immunoprecipitated with anti-TAB2 antibody (P-100/IP: TAB2 ab), and analyzed with the same antibodies.
The dilemma is that whereas it is clear that TAK1, TAB1, and TAB2 are preassociated, it was shown previously that TAB2 is membrane-bound and TAK1 and TAB1 are primarily localized in the cytosol (20). Therefore, we reexamined the subcellular localization of TAK1, TAB1, and TAB2. Interestingly, with the modification of the protocol (Materials and Methods), we found that, although the majority of TAK1 and TAB1 was detected in the cytosol (S100), there were comparable amounts of TAK1, TAB1, and TAB2 in the P100 fraction (Fig. 5B and data not shown). Thorough homogenization of the cells in hypotonic buffer ensured complete breakdown of the cell membrane sheets to small vesicles, which helped to enrich the membrane-bound TAK1 and TAB1 proteins in P100 instead of in the cell debris. While TAB2 was exclusively localized in the membrane, Triton in lysis buffer solubilized the membrane-bound TAB2 in the cytosol. To examine whether TAK1, TAB1 and TAB2 form a complex in the membrane, P100 was solubilized in buffer containing 0.5% Triton, immunoprecipitated with anti-TAB2 antibody, and subjected to Western blot analyses with anti-TAB2, anti-TAB1, and anti-TAK1 antibodies (Fig. 5B). TAK1, TAB1, and TAB2 were indeed found in a complex in the membrane fraction. Taken together, the data suggest two pools of TAK1 and TAB1. While the majority resides in the cytosol, some TAK1 and TAB1 are preassociated with TAB2 on the membrane.
Formation of complex II (IRAK-TRAF6-TAK1-TAB1-TAB2) is on the membrane.
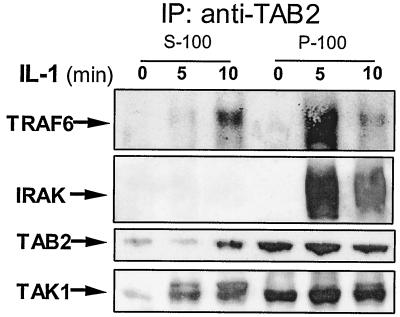
We previously reported that IRAK is required for the IL-1-induced translocation of TRAF6 and TAB2 from the membrane to the cytosol (21). It was proposed that the translocation of these two proteins leads to the formation of a TRAF6-TAK1-TAB1-TAB2 complex in the cytosol, resulting in the activation of TAK1 and IKK. However, the mechanism by which IRAK mediates this translocation event was unclear. As shown in Fig. 4, IRAK-TRAF6 form a second complex with TAK1 and TAB2 after they are released from the receptor complex (complex I), suggesting that the IRAK-mediated translocation is likely to occur through protein-protein interactions. To examine whether the interactions of IRAK-TRAF6 with TAK1 and TAB2 also take place on the membrane, fraction P100 from 293 cells untreated or treated with IL-1, were first solubilized in a Triton-containing buffer, immunoprecipitated with anti-TAB2 antibody, and subjected to Western blot analysis with anti-TRAF6, anti-IRAK, anti-TAB2, and anti-TAK1 antibodies. The same coimmunoprecipitation experiments were also performed with the S100 fractions. The interaction of IRAK-TRAF6 with TAK1 and TAB2 was indeed detected in the membrane fractions 5 min after IL-1 stimulation (Fig. 6). Interestingly, the TRAF6-TAK1-TAB2 complex appeared in the cytosol 10 min after IL-1 treatment (Fig. 6). On the other hand, IRAK was not detected in this TRAF6-TAK1-TAB2 cytosolic complex, which agrees with our previous finding that the modified IRAK stays on the membrane and eventually is degraded. Taken together, these results show that IRAK interacts with TRAF6, TAK1, and TAB2 on the membrane, which probably plays a critical role in mediating the translocation of these proteins from the membrane to the cytosol. We propose that, after IRAK-TRAF6 leaves complex I (the receptor complex), they form a second complex with the preassociated TAK1, TAB1, and TAB2 on the membrane (complex II [Fig. 1]). The formation of complex II probably plays an important role in the formation and translocation of the TRAF6-TAK1-TAB1-TAB2 complex (complex III [Fig. 1]) from the membrane to the cytosol.
FIG. 6.
Formation of complex II on the membrane and translocation of complex III to the cytosol. Membrane (P-100) and cytosolic (S-100) fractions, prepared from 293 cells either untreated or treated with IL-1 for 5 or 10 min, were immunoprecipitated with anti-TAB2 antibody and subjected to Western blot analyses with anti-TRAF6, anti-IRAK, anti-TAB2, and anti-TAK1 antibodies. The P-100 fractions were resuspended in Triton lysis buffer before immunoprecipitation (IP).
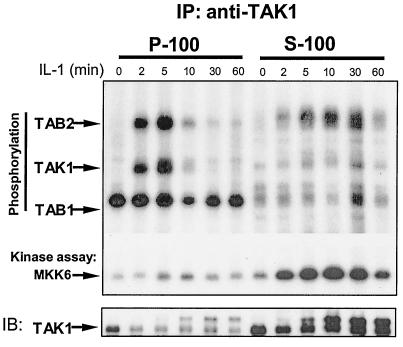
TAK1 and TAB2 are phosphorylated on the membrane, but TAK1 is activated in the cytosol.
Previous studies showed that TAK1 is activated upon IL-1 stimulation, which is required for the activation of IKK, leading to the activation of NF-κB. As discussed above, while complex II was formed on the membrane (Fig. 1 and 6), complex III was detected in the cytosol (Fig. 1 and 6). Is TAK1 activated in complex II on the membrane or in complex III in the cytosol? To address this question, the S-100 and the P-100 fractions from 293 cells treated with IL-1 for different times were immunoprecipitated with anti-TAK1 antibody and then subjected to an in vitro TAK1 kinase assay. Interestingly, while TAK1 and TAB2 were phosphorylated on the membrane upon IL-1 treatment (Fig. 7), the kinase activity of TAK1 was detected only in the cytosol. Taken together, the data suggest that TAK1 and TAB2 are probably phosphorylated in complex II on the membrane, whereas TAK1 is activated in complex III in the cytosol. These results also suggest that phosphorylation of TAK1 might be a prerequisite for TAK1 activation but might not be sufficient to drive the activation.
FIG. 7.
TAK1, TAB1, and TAB2 are phosphorylated on the membrane, and TAK1 is activated in the cytosol. Extracts of 293 cells, either untreated or treated with IL-1 for the indicated times, were immunoprecipitated (IP) with anti-TAK1 antibody. The immunoprecipitates were then incubated with 1 μg of bacterially expressed HA-MKK6 in a kinase buffer containing γ-32P-labeled ATP at 25°C for 2 min. Samples were separated by SDS-PAGE and transferred to membranes, and the proteins were visualized by autoradiography. The same membrane was probed with anti-TAK1 antibody. IB, immunoblotting.
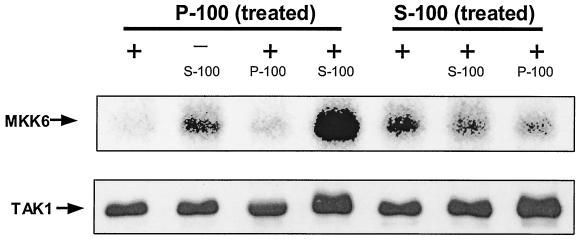
Why is TAK1 activated only in the cytosol? One possibility is that one or more cytosolic factors are required. To test this idea, we set up an in vitro activation assay for TAK1, which was first immunoprecipitated with anti-TAK1 antibody from S-100 and solubilized P-100 and prepared from 293 cells treated with IL-1 for 2 min, during which time TAK1 was probably phosphorylated on the membrane but had not yet translocated to the cytosol. Only the membrane-bound TAK1 immunoprecipitated from IL-1-treated P100 was activated after the incubation with untreated S100 (Fig. 8), suggesting that one or more factors in the cytosol are required for the activation of TAK1 following its phosphorylation and translocation.
FIG. 8.
In vitro activation of TAK1. S-100 and Triton-solubilized P-100 fractions from 293 cells, treated with IL-1 for 2 min, were immunoprecipitated with anti-TAK1 antibody. The immunoprecipitates were incubated (+) or not incubated (−) with 50 μl of S-100 or Triton-solubilized P-100 from untreated 293 cells at 30°C for 10 min, washed with Triton lysis buffer, and then subjected to a TAK1 kinase assay using HA-MKK6 as a substrate. The samples were also studied by use of a Western blot probed with anti-TAK antibody.
DISCUSSION
On the basis of the results presented above, we propose a revised model for IL-1-dependent signaling (Fig. 1). Upon IL-1 stimulation, the adaptor molecules MyD88 and Tollip are recruited to the IL-1 receptor complex, which then recruits IRAK. IRAK is hyperphosphorylated, mediating the recruitment of TRAF6 to the receptor complex (complex I). Since IRAK4 has recently been shown to be an essential component for the IL-1 signaling pathway and proposed to function as an IRAK kinase (11, 24), we have also included IRAK4 in the complex I. IRAK-TRAF6 then leaves complex I to interact with preassociated TAK1, TAB1, and TAB2 on the membrane, resulting in the formation of complex II. The formation of complex II leads to the phosphorylation of TAK1 and TAB2, which facilitates the formation and translocation of complex III from the membrane to the cytosol. The formation of complex III and its interaction with additional factors in the cytosol lead to the activation of TAK1. The activated TAK1 causes, directly or indirectly, the activation of IKK and MKK6, resulting in activation of NF-κB and JNK.
IRAK is necessary for activation of both IL-1-induced NF-κB and JNK, but the kinase activity of IRAK is not required for either, suggesting that IRAK functions through protein-protein interactions. We have now shown that IRAK plays a critical role in the formation of IL-1-induced signaling complexes (complex I). IRAK is not only required for the recruitment of TRAF6 to the receptor to form complex I but also is responsible for bringing TRAF6 to the preassociated TAK1, TAB1, and TAB2 to form complex II on the membrane. We have previously shown that IRAK is required for the translocation of TRAF6 and TAB2 from the membrane to the cytosol. Therefore, IRAK also plays a critical role in mediating the formation and translocation of complex III. The phosphorylated IRAK itself remains on the membrane and eventually is degraded. We propose that IRAK functions as an organizer and transporter in IL-1 signaling pathway, mediating the sequential association and dissociation of different signaling components and leading to the activation of NF-κB and JNK.
In a earlier study (21), we reported that TAK1 and TAB1 were localized primarily in the cytosol with or without stimulation and that only small fractions of TAK1 and TAB1 were detected in the membrane. Therefore, we proposed that IRAK forms a complex with TRAF6 and TAB2 on the membrane upon IL-1 stimulation, followed by the translocation of TRAF6 and TAB2 to the cytosol to interact with the cytosolic TAK1 and TAB1. Our recent observation that TAK1-TAB1-TAB2 are preassociated in a coimmunoprecipitation experiment with whole-cell extract prepared with Triton-containing lysis buffer (Fig. 5A) prompted us to rethink this model. Since TAB2 is localized exclusively on the membrane, we realized that the small amounts of TAK1 and TAB1 detected in the membrane are probably very important. Therefore, we studied the subcellular localization of TAK1, TAB1, and TAB2 more carefully. Through error and trial, we learned that thorough homogenization is very critical to fractionating TAK1 and TAB1 into the P100 membrane fraction. Otherwise we lose large amounts of membrane fraction to the cell debris by the first step, low-speed centrifugation. By modifying our fractionation protocol, it became much easier to detect TAK1 and TAB1 in the P100 membrane fraction (Materials and Methods). It was then very clear that there are two pools of TAK1 and TAB1 in the cells. While the majority is in the cytosol, some TAK1 and TAB1 are preassociated with TAB2 on the membrane. Indeed, we detected the preassociated complex of TAK1-TAB1-TAB2 in the P100 membrane fraction with or without stimulation (Fig. 5B). Furthermore, we have shown that the preassociated TAK1-TAB1-TAB2 forms a complex with IRAK-TRAF6 on the membrane in response to IL-1 stimulation (complex II) (Fig. 6). Moreover, while TAK1 is activated in the cytosol, the IL-1-induced phosphorylation of TAK1 occurs on the membrane (Fig. 7). Taken together, these results yielded the following model. IRAK-TRAF6 complex is first formed on the receptor (complex I) and then is released from the receptor to interact with the preassociated complex TAK1-TAB1-TAB2 on the membrane (complex II), where TAK1 is phosphorylated. TRAF6-TAK1-TAB1-TAB2 then dissociates from IRAK and translocates to the cytosol (complex III), where the kinase activity of TAK1 is activated (Fig. 7 and 8). In addition to its role in IL-1-mediated signaling, TAK1 has also been shown to play a role in Toll-like receptor 2, transforming growth factor β receptor, tumor necrosis factor receptor, and RANK-mediated signaling pathways. Since TAK1 seems to function as an upstream kinase of IKK and MKK6, it is probably involved in many other NF-κB-dependent signaling pathways as well. Therefore, it is plausible that the two pools of TAK1 in the cells may participate in different signaling pathways through the interaction of TAK1 with many other signaling components.
It is important to note that in addition to TAK1, several other MAP3 kinases have also been implicated in activating IKK, including MEKK1, MEKK2, MEKK3, and NIK. However, none of these kinases have been definitively proven to be an IKK kinase in vivo. MEKK1-null mouse embryonic fibroblasts (MEFs) display normal NF-κB activation in response to stimulation (34). Interestingly, although NIK-null cells also display normal NF-κB DNA-binding activity in response to many stimuli (33), they exhibit weak activation of NF-κB-dependent genes specifically in response to lymphotoxin-β receptor (LTβR) signaling. Recently, it has been shown that NIK and IKKα kinase are required specifically for signal-dependent p100 processing, which helps to explain the phenotype of NIK-null cells (16, 30). Curiously, MEKK3, which can phosphorylate IKKβ in vitro, is required for tumor necrosis factor alpha-induced NF-κB activation. MEKK3-null MEFs exhibit a greatly decreased level of IKK activation, IκB degradation, and NF-κB activation in response to tumor necrosis factor alpha (32). Taken together, these findings suggest that there might be more than one IKK kinase.
While TAK1 and TAB2 are phosphorylated on the membrane in response to IL-1 stimulation, the kinase activity of TAK1 is activated only in the cytosol. These results indicate that the phosphorylation of TAK1 on the membrane is probably not due to autophosphorylation. The fact that TAK1 and TAB2 are also phosphorylated in IRAK-deficient cells that express a kinase-dead IRAK mutant indicates that IRAK cannot be the kinase that phosphorylates TAK1. Taken together, these results suggest that another membrane-bound kinase must be responsible for the IL-1-induced phosphorylation of TAK1 and TAB2.
The detailed molecular mechanism for the formation and translocation of complex III is still not clear. Since TAK1 and TAB2 are phosphorylated in complex II on the membrane, it is possible that the phosphorylation of these two proteins results in conformational changes in complex II, which leads to the dissociation of complex III from IRAK, followed by its translocation to the cytosol. The fact that TAK1 is activated only in the cytosol strongly suggests that its activation occurs in complex III, although it is unclear how this is accomplished. Our results from the in vitro TAK1 activation experiments suggest that the activation of TAK1 may involve the interaction of complex III with additional cytosolic factors. This conclusion is supported by previous studies reported by Deng et al. (6) and Wang et al. (27) working in the laboratory of Z. J. Chen. Using an in vitro system to study TRAF6-mediated TAK1 and IKK activation, they showed that TRAF6-mediated TAK1 and IKK activation requires nonclassical ubiquitination catalyzed by the ubiquitination proteins Ubc13 and Uev1A, which play a regulatory role and do not lead to proteasome-mediated degradation. TRAF6, together with Ubc13 and Uev1A, functions as part of a unique E3 complex and is itself the target of ubiquitination. The TAK1-TAB1-TAB complex is activated by association with ubiquitinated TRAF6. Once activated, TAK1 can directly phosphorylate IKK and MKK6, leading to the activation of both the JNK and NF-κB signaling pathways. On the basis of the above information, we propose that complex III may interact with Ubc13 and Uev1A when it is translocated to the cytosol, which triggers the ubiquitination of TRAF6, leading to the activation of TAK1 and IKK. Alternatively, complex III may interact with some unknown components in the cytosol to cause TAK1 activation. These hypotheses will be tested in our in vitro activation system.
Acknowledgments
We thank Huiqin Nie for technical assistance and Zhaodan Cao for providing polyclonal antibodies against IRAK and IL-1R.
This work was supported in part by NIH grant GM 600020 to X.L.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9**:**143-150. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P. J., and M. Karin. 1997. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336**:**1066-1071. [DOI] [PubMed] [Google Scholar]
- 3.Burns, K., J. Clatworthy, L. Martin, F. Martinon, C. Plumpton, B. Maschera, A. Lewis, K. Ray, J. Tschopp, and F. Volpe. 2000. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2**:**346-351. [DOI] [PubMed] [Google Scholar]
- 4.Cao, Z., W. J. Henzel, and X. Gao. 1996. IRAK: a kinase associated with the interleukin-1 receptor. Science 271**:**1128-1131. [DOI] [PubMed] [Google Scholar]
- 5.Cao, Z., J. Xiong, M. Takeuchi, T. Kurama, and D. V. Goeddel. 1996. TRAF6 is a signal transducer for interleukin-1. Nature 383**:**443-446. [DOI] [PubMed] [Google Scholar]
- 6.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z. J. Chen. 2000. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103**:**351-361. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello, C. A. 1996. Biologic basis for interleukin-1 in disease. Blood 87**:**2095-2147. [PubMed] [Google Scholar]
- 8.Greenfeder, S. A., P. Nunes, L. Kwee, M. Labow, R. A. Chizzonite, and G. Ju. 1995. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J. Biol. Chem. 270**:**13757-13765. [DOI] [PubMed] [Google Scholar]
- 9.Huang, J., X. Gao, S. Li, and Z. Cao. 1997. Recruitment of IRAK to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc. Natl. Acad. Sci. USA 94**:**12829-12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korherr, C., R. Hofmeister, H. Wesche, and W. Falk. 1997. A critical role for interleukin-1 receptor accessory protein in interleukin-1 signaling. Eur. J. Immunol. 27**:**262-267. [DOI] [PubMed] [Google Scholar]
- 11.Li, S., A. Strelow, E. J. Fontana, and H. Wesche. 2002. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. USA 99**:**5567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, X., M. Commane, C. Burns, K. Vithalani, Z. Cao, and G. R. Stark. 1999. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell. Biol. 19**:**4643-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, X., M. Commane, Z. Jiang, and G. R. Stark. 2001. IL-1-induced NF-κB and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptor-associated kinase (IRAK). Proc. Natl. Acad. Sci. USA 98**:**4461-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomaga, M. A., W. C. Yeh, I. Sarosi, G. S. Duncan, C. Furlonger, A. Ho, S. Morony, C. Capparelli, G. Van, S. Kaufman, A. van der Heider, A. Itie, A. Wakeham, W. Khoo, T. Sasaki, Z. Cao, J. M. Penninger, C. J. Paige, D. L. Lacey, C. R. Dunstan, W. J. Boyle, D. V. Goeddel, and T. W. Mak. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13**:**1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lord, K. A., B. Hoffman-Liebermann, and D. A. Liebermann. 1990. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene 5**:**1095-1097. [PubMed] [Google Scholar]
- 16.Matsushima, A., T. Kaisho, P. D. Rennert, H. Nakano, K. Kurosawa, D. Uchida, K. Takeda, S. Akira, and M. Matsumoto. 2001. Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase alpha in NF-κB activation through lymphotoxin beta receptor, but not through tumor necrosis factor receptor I. J. Exp. Med. 193**:**631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278**:**860-866. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya-Tsuji, J., K. Kishimoto, A. Hiyama, J. Inoue, Z. Cao, and K. Matsumoto. 1999. The kinase TAK1 can activate the NIK-I κB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398**:**252-256. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill, L. A. 1995. Towards an understanding of the signal transduction pathways for interleukin 1. Biochim. Biophys. Acta 1266**:**31-44. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill, L. A. 1997. Molecular mechanisms underlying the actions of the pro-inflammatory cytokine interleukin 1. R. Irish Acad. Medal Lect. Biochem. Soc. Trans. 25**:**295-302. [DOI] [PubMed] [Google Scholar]
- 21.Qian, Y., M. Commane, J. Ninomiya-Tsuji, K. Matsumoto, and X. Li. 2001. IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NF-κB. J. Biol. Chem. 276**:**41661-41667. [DOI] [PubMed] [Google Scholar]
- 22.Regnier, C. H., H. Y. Song, X. Gao, D. V. Goeddel, Z. Cao, and M. Rothe. 1997. Identification and characterization of an IκB kinase. Cell 90**:**373-383. [DOI] [PubMed] [Google Scholar]
- 23.Schindler, U., and V. R. Baichwal. 1994. Three NF-κB binding sites in the human E-selectin gene required for maximal tumor necrosis factor alpha-induced expression. Mol. Cell. Biol. 14**:**5820-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki, N., S. Suzuki, G. S. Duncan, D. G. Millar, T. Wada, C. Mirtsos, H. Takada, A. Wakeham, A. Itie, S. Li, J. M. Penninger, H. Wesche, P. S. Ohashi, T. W. Mak, and W. C. Yeh. 2002. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416**:**750-756. [DOI] [PubMed] [Google Scholar]
- 25.Takaesu, G., S. Kishida, A. Hiyama, K. Yamaguchi, H. Shibuya, K. Irie, J. Ninomiya-Tsuji, and K. Matsumoto. 2000. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol. Cell 5**:**649-658. [DOI] [PubMed] [Google Scholar]
- 26.Takaesu, G., J. Ninomiya-Tsuji, S. Kishida, X. Li, G. R. Stark, and K. Matsumoto. 2001. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol. Cell. Biol. 21**:**2475-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412**:**346-351. [DOI] [PubMed] [Google Scholar]
- 28.Wesche, H., W. J. Henzel, W. Shillinglaw, S. Li, and Z. Cao. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7**:**837-847. [DOI] [PubMed] [Google Scholar]
- 29.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IκB kinase-beta: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278**:**866-869. [DOI] [PubMed] [Google Scholar]
- 30.Xiao, G., E. W. Harhaj, and S. C. Sun. 2001. NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell 7**:**401-409. [DOI] [PubMed] [Google Scholar]
- 31.Yamin, T. T., and D. K. Miller. 1997. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J. Biol. Chem. 272**:**21540-21547. [DOI] [PubMed] [Google Scholar]
- 32.Yang, J., Y. Lin, Z. Guo, J. Cheng, J. Huang, L. Deng, W. Liao, Z. Chen, Z. Liu, and B. Su. 2001. The essential role of MEKK3 in TNF-induced NF-κB activation. Nat. Immunol. 2**:**620-624. [DOI] [PubMed] [Google Scholar]
- 33.Yin, L., L. Wu, H. Wesche, C. D. Arthur, J. M. White, D. V. Goeddel, and R. D. Schreiber. 2001. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science 291**:**2162-2165. [DOI] [PubMed] [Google Scholar]
- 34.Yujiri, T., M. Ware, C. Widmann, R. Oyer, D. Russell, E. Chan, Y. Zaitsu, P. Clarke, K. Tyler, Y. Oka, G. R. Fanger, P. Henson, and G. L. Johnson. 2000. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-κB activation. Proc. Natl. Acad. Sci. USA 97**:**7272-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91**:**243-252. [DOI] [PubMed] [Google Scholar]