Neural basis of protracted developmental changes in visuo-spatial working memory (original) (raw)
Abstract
Developmental studies have shown that visuo-spatial working memory (VSWM) performance improves throughout childhood and adolescence into young adulthood. The neural basis of this protracted development is poorly understood. In this study, we used functional MRI (fMRI) to examine VSWM function in children, adolescents, and young adults, ages 7–22. Subjects performed a 2-back VSWM experiment that required dynamic storage and manipulation of spatial information. Accuracy and response latency on the VSWM task improved gradually, extending into young adulthood. Age-related increases in brain activation were observed in focal regions of the left and right dorsolateral prefrontal cortex, left ventrolateral prefrontal cortex (including Broca's area), left premotor cortex, and left and right posterior parietal cortex. Multiple regression analysis was used to examine the relative contributions of age, accuracy, and response latency on activation. Our analysis showed that age was the most significant predictor of activation in these brain regions. These findings provide strong evidence for a process of protracted functional maturation of bilateral fronto-parietal neural networks involved in VSWM development. At least two neural systems involved in VSWM mature together: (i) a right hemisphere visuo-spatial attentional system, and (ii) a left hemisphere phonological storage and rehearsal system. These observations suggest that visually and verbally mediated mnemonic processes, and their neural representations, develop concurrently during childhood and adolescence and into young adulthood.
The ability to represent and manipulate visuo-spatial information is a key requirement of everyday cognition (1). Visuo-spatial working memory (VSWM), the ability to briefly maintain and manipulate spatial information on line, plays an important role in this process (2). Developmental studies have shown that working memory (WM) performance improves with age throughout childhood and adolescence, into young adulthood (3–6). Zald et al. (3) have shown that spatial WM abilities improve substantially from age 14 to 20, with significant improvements in accuracy and reaction time (RT). The neural bases of these protracted changes are poorly understood. Here, we present the first study to examine the developmental trajectory of concurrent changes in brain function and behavior during the acquisition of VSWM skills in subjects from early childhood to young adulthood (ages 7–22).
Extensive neuroimaging research in adults has shown that several prefrontal and parietal brain regions, including the dorsolateral prefrontal cortex (DLPFC), ventro-lateral prefrontal cortex (VLPFC), premotor cortex (PMC), and the posterior parietal cortex (PPC), play critical and differential roles in VSWM (7–10). In contrast, there have been very few brain imaging studies of WM in children and adolescents. Thomas et al. (11) examined brain activation in six children (8–10 years) and six adults (19–26 years) performing a VSWM task and found activity in the right superior frontal gyrus (SFG), right DLPFC, right superior parietal lobule (SPL), and bilateral inferior parietal cortex (IPC) in both children and adults. However, they could not determine the precise changes in brain function during development because there were no quantitative statistical comparisons between groups. Nelson et al. (12) examined brain activation in 9 children (8–11 years) and found activation in right DLPFC, bilateral SFG, right IPC, and right SPL. Although these studies show that similar brain regions are activated during WM in children and adults, there is no quantitative information available about the functional changes that might contribute to WM development. Thus, our knowledge of the neural basis of protracted developmental changes in VSWM remains limited.
The confounding effects of age and task performance on activation pose a challenge to brain imaging studies of cognitive development. At issue is whether changes in brain activation reflect changes in task performance with age or functional maturation, independent of behavioral changes. Previous functional imaging studies of WM have taken one of two approaches to this question. One approach is to simplify cognitive tasks so that children can perform them (13), thus eliminating performance as a confounding factor. However, the simplified tasks may not capture critical cognitive operations that are evoked during WM. A second approach is to group subjects based on individual performance levels (11), which also poses difficulties in selection of performance criteria and in interpretation of different tasks. To resolve these problems, we took an alternative approach. We examined brain activation in children, adolescents, and young adults who performed a standardized WM task involving the dynamic storage, manipulation, and selection of information in WM. We addressed two questions related to the development of WM function: (i) are there specific brain regions that show linear age-related changes in activation; and (ii) what are the relative contributions of age and performance measures (e.g., accuracy and RT) to changes in brain activation?
In examining age-related changes in brain activation, it is important to know whether activation increases or decreases with age, because increases in activation are typically associated with greater functional maturation, and decreases are associated with less developed or “immature” function. Findings from developmental brain imaging studies have been variable, particularly for PFC function. For example, in studies of response inhibition, Casey et al. (14) reported decreased activation in widespread regions of the PFC; Rubia et al. (15) reported increased activation in the left middle and inferior frontal cortex; and Tamm et al. (16) reported bilateral increases in specific PFC regions known to be involved in response inhibition in adults, whereas decreases were observed in other PFC regions. In a related study of the Stroop color–word interference task, Adelman et al. (17) observed age-related increases in PFC activation from childhood to adulthood, whereas increases in the PPC reached asymptotic levels by adolescence. Schlaggar et al. (18) found that reading single words resulted in increased PFC activity in adults and decreased activity in the visual association cortex in children. The divergent findings of age-related changes could be due to (i) differences in specific ages of subjects studied, (ii) methods for controlling variability in behavioral performance with age, (iii) dissimilar psychological processes and strategies evoked by specific tasks, (iv) differences in methods used to analyze data: whether between-group analyses versus age-related regression are used, or (v) whether voxel-based or region-of-interest (ROI)-based analyses are used.
In this study, we used a voxel-based approach because it provides detailed information about the precise brain regions that show focal changes in WM-related activation with age. Based on our previous findings (16, 17), we hypothesized that, in conjunction with improved task performance with age, key prefrontal and parietal cortical regions known to be involved in WM would reveal focal increases in activation with age. We further hypothesized that focal increases in the DLPFC would be observed even after removing the confounding effects of performance, thus potentially providing evidence for functional maturation independent of performance changes.
Methods
Subjects.
Subjects were selected from 34 healthy individuals who performed a VSWM task as normal comparison subjects in ongoing clinical studies. Subjects (i) had no history of neurological or psychiatric disorders; (ii) were right-handed as assessed by the Edinburgh Handedness Inventory; (iii) were younger than 25 years old; (iv) had a full-scale IQ between 85 and 130; and (v) maintained a maximum head movement of less than 3 mm and rotation less than 3° during fMRI scanning. Subjects were screened for psychiatric conditions by using the Symptom Checklist–Revised (SCL-90-R) (19) or the Child Behavior Checklist (CBCL) (20). Subjects had SCL-90-R or CBCL t scores within 1 standard deviation of the mean of a normative standardized sample. Cognitive functioning was assessed by using the Wechsler Intelligence Scale for Children, Third Edition (WISC-III, ages 7 through 16) and the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III, ages 16 and up).
Of the original 34 subjects, 23 (9 male, 14 female) children, adolescents, and young adults (ages 7–22; mean 14.6 ± 4.6) were included in the study: 8 children (ages 7–12), 8 adolescents (ages 13–17), and 7 adults (ages 18–22).
Experiment.
During functional MRI (fMRI) scanning, subjects performed a visuo-spatial task (21) consisting of 12 alternating 36-s WM and control epochs. Subjects viewed items on a screen and responded to visual cues on a small keypad. During both tasks, subjects viewed the letter “O” once every 2 s, at one of nine distinct locations on the screen. In the WM task, subjects responded if the current location was the same as the location at which the symbol was presented two stimuli back (2-back WM condition). In the control task, subjects responded if the symbol appeared at the center. One-third of the trials in each epoch required a response. In each epoch, 16 stimuli were presented for 500 ms each, with a 1,500-ms interval. Before each epoch, subjects were presented with a 4-s cue about the task they were to perform.
Image Acquisition.
Images were acquired on a 1.5T GE Signa scanner using protocols which have been described in detail elsewhere (16, 17). Eighteen axial slices (6-mm thick, 1-mm skip; 4.35 mm in-plane resolution) were imaged with a temporal resolution of 2 s by using a T2*-weighted gradient echo spiral pulse sequence (22). To aid in localization of functional data, a high-resolution T1-weighted spoiled grass gradient recalled 3D MRI sequence was obtained.
Data Analysis.
fMRI data were processed by using Statistical Parametric Mapping (SPM99) (www.fil.ion.ucl.ac.uk/spm). Our methods are described in detail elsewhere (16, 17). Briefly, images were corrected for movement and normalized to stereotaxic Talairach coordinates (23). Statistical parametric maps were first generated for the WM, compared with control, tasks for each subject using a general linear model. In the second level of analysis, random effects analysis was performed with age as a covariate to determine which voxels showed age-related activation changes across subjects. Significant clusters of age-related activation were determined by using height (P < 0.05) and extent (P < 0.05) thresholds that corrected for spatial correlations (24).
Multiple regression analysis was used to examine the differential contribution of age and performance on brain activation during WM. Significant clusters of activation detected in the group data were defined as the functional ROIs. Within each ROI, two complementary measures of activation were computed for each subject: (i) height of activation, measured as the t statistic at the peak group activation in the ROI, and (ii) extent of activation, measured as the percentage of voxels activated in the ROI. Significance levels were assessed at an α level of 0.05.
Results
Neuropsychological Assessment.
The mean full-scale IQ (FSIQ) score for the sample was 116 ± 10 (93–130). There was no significant correlation between age and FSIQ (r = 0.39; P > 0.05).
Movement.
Mean translational movements in the X (left to right), Y (back to front), and Z (bottom to top) head directions were 0.10 ± 0.11 mm, 0.16 ± 0.03 mm, and 0.65 ± 0.58 mm, respectively. Mean rotational movements about the three axes were 0.44 ± 0.42°, 0.24 ± 0.26°, and 0.18 ± 0.17°, respectively. Movement was not significantly correlated with age for translation in the X (r = −0.09; P = 0.69), Y (r = 0.21; P = 0.35), or Z (r = −1.02; P = 0.64) directions; nor for rotation in the X (r = 0.15; P = 0.51), Y (r = −0.20; P = 0.35), or Z (r = 0.02; P = 0.94) directions.
Behavioral Performance.
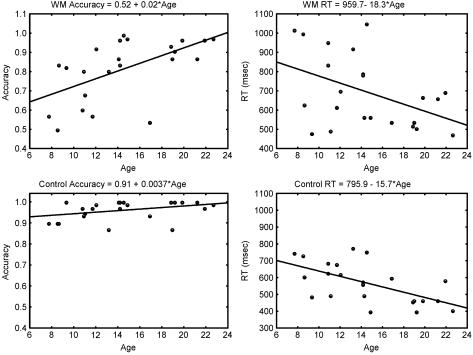
Mean accuracy was 96.01% ± 4.54% on the control task and 81.38% ± 15.89% on the WM task. Mean RT on correct trials was 567 ± 120 ms on the control task and 694 ± 188 ms on the WM task. Accuracy and RT showed significant improvements with age on the WM task with performance improvements extending into young adulthood (Fig. 1 Upper). Accuracy increased with age (_R_2 = 0.33; P = 0.003), and RTs to correct trials (_R_2 = 0.20; P = 0.003) decreased with age during the WM task. Performance reached asymptotic levels by age 8–10 on the control task (Fig. 1 Lower). Accuracy was not significantly correlated with age (_R_2 = 0.14; P = 0.082), but RTs decreased significantly with age (_R_2 = 0.36; P = 0.002). Slopes of RT versus age for the WM and control tasks were not significantly different (F = 0.08; P = 0.78).
Figure 1.
Increase in accuracy and decrease in RT during the WM and control tasks as a function of age. Slopes and intercepts from a regression analysis for each variable are shown above each graph.
Correlation Between Brain Activation and Age.
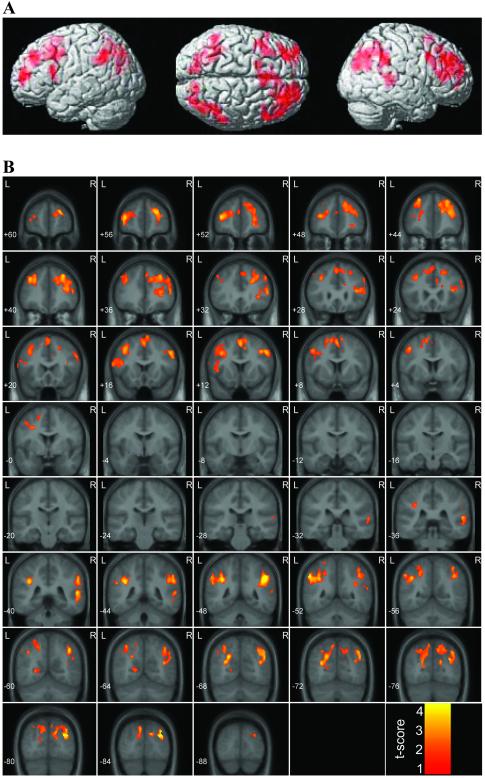
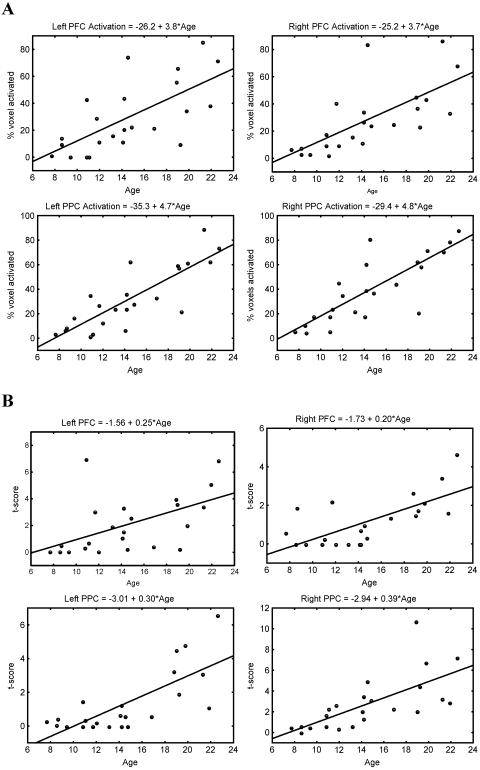
WM-related activation significantly increased with age bilaterally in the PFC and PPC. Within the PFC, activations were observed in the left and right DLPFC, left and right SFG, left VLPFC, and left PMC. Within the PPC, activation was observed primarily in the left and right angular gyrus and adjoining intraparietal sulcus, and to a lesser extent in the left and right SPL (Fig. 2; Table 1). Fig. 3 shows the increase in cluster size and peak t score in each of the PFC and PPC clusters as a function of age. No brain regions showed WM-related decreases in activation with age.
Figure 2.
Surface rendering (A) and coronal views (B) of brain areas that showed significant increases in activation with age during the WM, compared with the control, task. Significant activation was observed in the left and right DLPFC, left VLPFC, left PMC, and left and right PPC.
Table 1.
Brain regions that showed significant increases in activation with age during the WM task
| Regions | Corrected P value | No. of voxels | Maximum Z score | Peak Talairach coordinates, mm |
|---|---|---|---|---|
| Left DLPFC (BA 9/46), VLPFC (BA 44), PMC (BA 6), SFG (BA 8) | <0.001 | 2132 | 3.37 | −38 54 10 |
| Right DLPFC (BA 9/46), SFG (BA 8), SMA | <0.001 | 3780 | 3.38 | 24 58 20 |
| Left IPC (BA 39/40), SPL (BA 7) | <0.001 | 2086 | 3.60 | −54 −52 40 |
| Right IPC (BA 39/40), SPL (BA 7) | <0.001 | 2314 | 4.12 | 48 −48 38 |
Figure 3.
Age-related increase in activation in the left and right PFC and PPC during the WM task. Cluster size (A) and peak t score (B) results are shown for two separate quantitative measures of activation in each cluster.
Multiple Regression Analysis of Brain Activation in Relation to Age and Behavioral Performance.
Multiple linear regression analysis was used to examine the relative contributions of age and task performance (accuracy and RT) to brain activation. Cluster size and magnitude of peak activation were computed separately for each subject, and were used as dependent variables, with age, accuracy, and RT as predictor variables. Results show that age was a significant predictor of brain activation even after factoring out the effect of task performance (Table 2).
Table 2.
Results of brain-behavior analyses using cluster size and peak t score
| Region | _R_2 | Age | Accuracy | RT | ||||
|---|---|---|---|---|---|---|---|---|
| _R_2 | P | β ± SD | P | β ± SD | P | β ± SD | P | |
| Cluster size | ||||||||
| L PFC | 0.27 | 0.101 | 0.60 ± 0.25 | 0.029 | −0.07 ± 0.24 | 0.786 | −0.36 ± 0.22 | 0.118 |
| R PFC | 0.56 | 0.001 | 0.76 ± 0.20 | 0.001 | 0.17 ± 0.19 | 0.540 | −0.30 ± 0.17 | 0.091 |
| L PPC | 0.72 | 0.000 | 0.84 ± 0.16 | 0.000 | 0.11 ± 0.15 | 0.469 | −0.18 ± 0.14 | 0.192 |
| R PPC | 0.69 | 0.000 | 0.77 ± 0.17 | 0.000 | 0.16 ± 0.16 | 0.328 | −0.13 ± 0.14 | 0.379 |
| Peak t score | ||||||||
| L PFC | 0.29 | 0.082 | 0.44 ± 0.25 | 0.091 | 0.11 ± 0.24 | 0.645 | −0.04 ± 0.22 | 0.852 |
| R PFC | 0.53 | 0.002 | 0.75 ± 0.20 | 0.001 | −0.21 ± 0.19 | 0.278 | −0.15 ± 0.17 | 0.374 |
| L PPC | 0.56 | 0.001 | 0.68 ± 0.20 | 0.003 | 0.03 ± 0.19 | 0.885 | −0.10 ± 0.17 | 0.574 |
| R PPC | 0.50 | 0.004 | 0.54 ± 0.21 | 0.019 | 0.18 ± 0.20 | 0.391 | −0.10 ± 0.18 | 0.603 |
Discussion
Our study provides the quantitative analysis of concurrent changes in brain function and behavior during the development of WM in children, adolescents, and young adults from ages 7 to 22. We detected linear changes in brain activation within regions known to play a critical role during VSWM in adults. This linear trajectory is characterized by focal increases in brain activation with age; no brain regions showed WM-related decreases in activation with age. Activation changes were seen in a distributed fronto-parietal network consisting of the left and right DLPFC, left posterior VLPFC, left PMC, and left and right PPC. These regions are known to play a critical role in VSWM in adults (8), and the precise foci of activation are consistent with several previous neuroimaging studies (26_-_31). Our results agree with findings from previous studies that have shown that children activate similar prefrontal and parietal cortex regions as adults (11, 12). We extend these findings by showing that changes in PFC and PPC function underlying WM extend well into young adulthood.
Multiple regression analysis allowed us to examine the relative contributions of age and performance to activation. We used two complementary measures of activation, reflecting the height and extent of activation within PFC and PPC regions; both measures showed similar effects: after accounting for performance, age was a significant predictor of brain activation. Accuracy and RT accounted for a small fraction of the variance in activation. These findings provide the strongest evidence to date for increasing functional specialization of specific brain regions involved in VSWM, independent of performance changes. The pattern of protracted functional developmental of PFC and PPC function during WM is consistent with the prolonged morphological maturation observed in these regions (32, 33). This functional maturation extends beyond the DLPFC to other regions of the PFC as well as the PPC.
With age, accuracy increased and RT decreased during WM performance. Accuracy and RT during WM performance were more variable in younger subjects. Accuracy increased at a slow rate (about 2% per year). To examine whether the relation between age and accuracy depends on the results from children ages 6–8, we reanalyzed the data excluding these children. Accuracy was still significantly correlated with age, indicating that changes in performance are not dependent on the results of the children ages 6–8. Similar findings were observed for RT. Accuracy on the control task reached asymptotic levels by age 10, but RT continued to decrease. Thus, even for simple tasks, which younger subjects perform well, processing speed continues to improve with age. Age-related slopes of RT on the WM and control tasks were not significantly different, suggesting that a common, fundamental mechanism, such as increased signal conduction speed, may underlie improvements in performance speed. These findings are consistent with evidence from morphological studies that have found age-related increases in white matter density from age 8 through young adulthood (33). Our results also suggest that improvements in accuracy are not solely caused by increased processing speed.
Within the left hemisphere, age-related increases were observed in a distributed fronto-parietal network that is known to implement the phonological loop. This loop plays an important role in verbal rehearsal processes involved in WM (34, 35). Almost all brain regions that have been implicated in the phonological loop showed age-related increases in activation, including the left posterior VLPFC, the PMC, the left IPC, and the adjoining intraparietal sulcus. The left posterior VLPFC regions showing activation include the pars opercularis and triangularis regions of the IFG; both regions constitute Broca's area (36). In adults, these regions are thought to subserve distinct functions involved in verbal rehearsal. Broca's area is believed to support the articulatory processes required for phonological recoding of visual stimuli (37), whereas the left dorsal PMC is thought to maintain temporal order, possibly as the location of a timing signal used in the rhythmic organization of rehearsal. Although the precise role of these regions in VSWM is not well understood (38), there is indirect evidence to suggest that even for VSWM, information is recoded and maintained by verbal rehearsal in a phonological short-term store, because it decays if unrehearsed (39). The functional development of this system may underlie the increased ability to recode visually presented information into phonological form. Evidence from behavioral research suggests that verbal recoding of visuo-spatial information is a strategy that develops in children around 7 to 8 years old and continues through adolescence (40), and that the development of VSWM in children is significantly related to using phonological recoding strategies (41). Our study provides direct evidence for protracted development of a brain system involved in this process.
The left and right DLPFC also showed protracted age-related increases. Functional brain imaging studies in adults have consistently demonstrated the involvement of these regions in VSWM (42). The differential contributions of the left and right DLPFC to VSWM are currently unknown. However, most studies suggest that DLPFC activation is more lateralized to the right hemisphere during VSWM, and to the left hemisphere during verbal WM (43). This observation agrees with our finding that age-related increases were greater in the right DLPFC. Experimental studies in humans and animals have consistently shown that the DLPFC plays a critical role in successful WM operations (44–46). The DLPFC is known to be involved in executive control processes subserving WM. Its activation is characteristic of n back WM tasks, like the one we used, which require manipulation and active processing of stored information (10, 47, 48). Furthermore, human electrophysiological studies have shown that DLPFC damage results in decreased neural activity in the PPC (48), suggesting that an excitatory modulation from the DLPFC is necessary to sustain neural activity during WM. These data suggest that the increased bilateral DLPFC activation with age that we found reflects increased functional specialization of modulatory and control processes involved in WM.
Within the PPC, age-related increases were observed in the left and right IPC, primarily in the angular gyrus (Brodmann area 39), as well as the adjoining intraparietal sulcus and to a lesser extent the SPL. Our findings of parallel age-related increases in activation of the DLPFC and the PPC are consistent with electrophysiological studies showing that these regions are co-activated during WM (49). Positron-emission tomography imaging studies in monkeys have also shown simultaneous metabolic activation of PFC and PPC (50). Specifically, electrophysiological studies have shown that neurons in the DLPFC and the PPC are both active during delay periods, when items must be held on-line in WM (51). Neuroimaging studies in humans have shown that although the DLPFC and the PPC are both important for WM (8), the DLPFC is more critically involved in the manipulation and selection of information in WM (8, 52, 53).
In contrast to the left PPC, which is involved in phonological processes subserving WM, the right PPC, including the SPL, the IPC, and the adjoining intraparietal sulcus, is primarily involved in visuo-spatial attention (28). Neuropsychological and functional neuroimaging studies have indicated that there is a substantial overlap in right PPC, as well as right PFC, regions that subserve both visuo-spatial attention and VSWM. Both of these cognitive functions share common processes involved in the dynamic shifting of attentional resources (27), suggesting that visuo-spatial attention and control processes subserved by right hemisphere fronto-parietal networks also mature over an extended time. Behavioral studies have suggested that phonological recoding cannot account for all of the age-related changes in VSWM performance (54), and that the development of visual and verbal processing systems may both be modulated by similar attentional and control mechanisms (54, 55). Our study provides support for this interpretation from the viewpoint of brain systems involved in the development of VSWM.
Thus, with increasing age, at least two neural systems involved in VSWM mature together: a left-hemisphere-based phonological rehearsal system, and a right-hemisphere-based visuo-spatial attentional system, and their associated executive control processes. This finding suggests that alternate verbal- and visual-based strategies and representations and their neural substrates undergo prolonged and concurrent development. Further studies are needed to disentangle the developmental trajectory of different subprocesses involved in WM, such as encoding, rehearsal, manipulation of items in memory, and interference processing.
Acknowledgments
This work was supported by National Institutes of Health Grants HD40761, MH62430, MH01142, HD31715, and MH50047, the Sinclair Fund, and the Packard Foundation.
Abbreviations
DLPFC
dorsolateral prefrontal cortex
fMRI
functional MRI
IPC
inferior parietal cortex
PFC
prefrontal cortex
PMC
premotor cortex
PPC
posterior parietal cortex
RT
reaction time
SFG
superior frontal gyrus
SPL
superior parietal lobule
VLPFC
ventrolateral prefrontal cortex
VSWM
visuo-spatial working memory
WM
working memory
ROI
region of interest
References
- 1.Logie R H. Visuo-Spatial Working Memory. Hillsdale, NJ: L. Erlbaum Associates; 1995. [Google Scholar]
- 2.Baddeley A. Human Memory: Theory and Practice. New York: Oxford Univ. Press; 1997. [Google Scholar]
- 3.Zald D H, Iacono W G. Dev Neuropsychol. 1998;14:563–578. [Google Scholar]
- 4.Kail R, Salthouse T A. Acta Psychol. 1994;86:199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 5.Luciana M, Nelson C A. Neuropsychologia. 1998;36:273–293. doi: 10.1016/s0028-3932(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs E B, Vargha-Khadem F. Br J Dev Psychol. 1989;7:377–380. [Google Scholar]
- 7.Jonides J, Smith E E, Koeppe R A, Awh E, Minoshima S, Mintun M A. Nature (London) 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 8.Smith E E, Jonides J. Proc Natl Acad Sci USA. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan J, Owen A M. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 10.Owen A M. Exp Brain Res. 2000;133:33–43. doi: 10.1007/s002210000398. [DOI] [PubMed] [Google Scholar]
- 11.Thomas K M, King S W, Franzen P L, Welsh T F, Berkowitz A L, Noll D C, Birmaher V, Casey B J. NeuroImage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- 12.Nelson C A, Monk C S, Lin J, Carver L J, Thomas K M, Truwit C L. Dev Psychol. 2000;36:109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- 13.Klingberg T, Forssberg H, Westerberg H. J Cogn Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- 14.Casey B J, Trainor R J, Geidd J, Vauss Y, Vaituzis C K, Hamburger S, Kozuch P, Rappaport J L. J Cogn Neurosci. 1997;9:835–847. [Google Scholar]
- 15.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S C, Simmons A, Andrew C, Bullmore E T. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 17.Adleman N E, Menon V, Blasey C M, White C D, Warsofsky I S, Glover G H, Reiss A L. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- 18.Schlaggar B L, Brown T T, Lugar H M, Visscher K M, Miezin F M, Petersen S E. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- 19.Derogatis L R, Savitz K L. In: The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Maruish M E, editor. Hillsdale, NJ: L. Erlbaum Associates; 1999. pp. 679–724. [Google Scholar]
- 20.Achenbach T M, Edelbrock C S. Monogr Soc Res Child Dev. 1981;46:1–82. [PubMed] [Google Scholar]
- 22.Glover G H, Lai S. Mag Res Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 23.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- 24.Poline J B, Worsley K J, Evans A C, Friston K J. NeuroImage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- 25.Holmes A P, Friston K J. NeuroImage. 1998;7:S754. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 26.Awh E, Anllo-Vento L, Hillyard S A. J Cognit Neurosci. 2000;12:840–847. doi: 10.1162/089892900562444. [DOI] [PubMed] [Google Scholar]
- 27.Awh E, Jonides J. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- 28.Coull J T, Frith C D. NeuroImage. 1998;8:176–187. doi: 10.1006/nimg.1998.0354. [DOI] [PubMed] [Google Scholar]
- 29.Courtney S M, Petit L, Maisog J M, Ungerleider L G, Haxby J V. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 30.D'Esposito M, Aguirre G K, Zarahn E, Ballard D, Shin R K, Lease J. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy G, Puce A, Constable R T, Krystal J H, Gore J C, Goldman-Rakic P. Cereb Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- 32.Sowell E R, Thompson P M, Holmes C J, Batth R, Jernigan T L, Toga A W. NeuroImage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- 33.Giedd J N, Blumenthal J, Jeffries N O, Castellanos F X, Liu H, Zijdenbos A, Paus T, Evans A C, Rapoport J L. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 34.Baddeley A. Curr Opin Neurobiol. 1998;8:234–238. doi: 10.1016/s0959-4388(98)80145-1. [DOI] [PubMed] [Google Scholar]
- 35.Gruber O. Cereb Cortex. 2001;11:1047–1055. doi: 10.1093/cercor/11.11.1047. [DOI] [PubMed] [Google Scholar]
- 36.Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings H B, Zilles K. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Henson R N, Burgess N, Frith C D. Neuropsychologia. 2000;38:426–440. doi: 10.1016/s0028-3932(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 38.Awh E, Jonides J, Reuter-Lorenz P A. J Exp Psychol Hum Percept Perform. 1998;24:780–790. doi: 10.1037//0096-1523.24.3.780. [DOI] [PubMed] [Google Scholar]
- 39.Baddeley A. Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 40.Halliday M S, Hitch G J. In: Growth Points in Cognition. Guy C, editor. London: Routledge; 1988. [Google Scholar]
- 41.Pickering S J, Gathercole S E, Hall M, Lloyd S A. Q J Exp Psychol A. 2001;54:397–420. doi: 10.1080/713755973. [DOI] [PubMed] [Google Scholar]
- 42.D'Esposito M. In: Handbook of Functional Neuroimaging of Cognition. Cabeza R, Kingstone A, editors. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 43.Reuter-Lorenz P A, Jonides J, Smith E E, Hartley A, Miller A, Marshuetz C, Koeppe R A. J Cognit Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 44.Goldman-Rakic P S, Selemon L D. Schizophr Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 45.Levy R, Goldman-Rakic P S. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- 46.Fuster J M. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter P A, Just M A, Reichle E D. Curr Opin Neurobiol. 2000;10:195–199. doi: 10.1016/s0959-4388(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 48.Knight R T, Staines W R, Swick D, Chao L L. Acta Psychol. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 49.Chafee M V, Goldman-Rakic P S. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- 50.Friedman H R, Goldman-Rakic P S. J Neurosci. 1994;14:2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuster J M. Memory in the Cerebral Cortex. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 52.Smith E E, Jonides J. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 53.Fletcher P C, Henson R N. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 54.Pickering S J. Memory. 2001;9:423–432. doi: 10.1080/09658210143000182. [DOI] [PubMed] [Google Scholar]
- 55.Cowan N. In: The Development of Memory in Childhood. Cowan N, editor. Hove, U.K.: Psychology Press; 1997. pp. 163–199. [Google Scholar]