Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus (original) (raw)
Abstract
The 1918 influenza pandemic caused more than 20 million deaths worldwide. Thus, the potential impact of a re-emergent 1918 or 1918-like influenza virus, whether through natural means or as a result of bioterrorism, is of significant concern. The genetic determinants of the virulence of the 1918 virus have not been defined yet, nor have specific clinical prophylaxis and/or treatment interventions that would be effective against a re-emergent 1918 or 1918-like virus been identified. Based on the reported nucleotide sequences, we have reconstructed the hemagglutinin (HA), neuraminidase (NA), and matrix (M) genes of the 1918 virus. Under biosafety level 3 (agricultural) conditions, we have generated recombinant influenza viruses bearing the 1918 HA, NA, or M segments. Strikingly, recombinant viruses possessing both the 1918 HA and 1918 NA were virulent in mice. In contrast, a control virus with the HA and NA from a more recent human isolate was unable to kill mice at any dose tested. The recombinant viruses were also tested for their sensitivity to U.S. Food and Drug Administration-approved antiinfluenza virus drugs in vitro and in vivo. Recombinant viruses possessing the 1918 NA or both the 1918 HA and 1918 NA were inhibited effectively in both tissue culture and mice by the NA inhibitors, zanamivir and oseltamivir. A recombinant virus possessing the 1918 M segment was inhibited effectively both in tissue culture and in vivo by the M2 ion-channel inhibitors amantadine and rimantadine. These data suggest that current antiviral strategies would be effective in curbing the dangers of a re-emergent 1918 or 1918-like virus.
The influenza pandemic of 1918–19 resulted in the deaths of many millions of people worldwide (1) and an estimated 550,000 excess deaths in the United States (2). This exceptionally high mortality rate lowered the average life expectancy in the U.S. by almost 10 years (1). The severity of the 1918 pandemic is unprecedented; by comparison, the influenza pandemics of 1957 and 1968 caused substantially less mortality, ≈70,000 and ≈34,000 deaths in the U.S., respectively. The 1918 pandemic was unusual also in that previously healthy adults suffered a disproportionately high rate of mortality (3, 4). Also of note, the 1918 influenza virus was reported to cause an unusually rapid destruction of respiratory epithelium (5).
The determination of the sequences of the 1918 influenza virus genes has provided new insights into the nature and origin of this pathogen (6–13). Thus far, among the eight viral RNA segments [PA, PB1, PB2, hemagglutinin (HA), neuraminidase (NA), nucleoprotein, matrix (M), and nonstructural (NS)], four complete sequences have been reported: those of HA, NA, NS, and M (6, 9–11). Coupling sequence information with a technique permitting the rescue of influenza A viruses entirely from cDNA (14–16) now permits the generation of recombinant influenza viruses bearing 1918 genes (11). By using this approach, viruses with different combinations of 1918 genes can now be constructed.
The construction of viruses with multiple 1918 influenza virus genes makes a molecular analysis of the virulence of the 1918 pandemic influenza virus possible. In addition, studies on such recombinant viruses will likely provide insight into the pathogenesis of human influenza in general (17). However, reconstruction of all or part of the 1918 virus requires that appropriate precautions be taken to protect laboratory workers and the public. Additionally, the available molecular techniques could be used for the purpose of bioterrorism (17). Moreover, the emergence of a new 1918-like virus from a source in nature cannot be excluded. Thus, it will be important to identify countermeasures targeting 1918 and 1918-like influenza viruses.
Proven measures for influenza prophylaxis include vaccines and antiinfluenza virus drugs (18). A high-containment production facility would likely be required to produce a vaccine derived from an influenza virus expressing the 1918 virus surface glycoproteins. Therefore, development of a 1918 influenza vaccine may be problematic, and alternate approaches such as the identification of avirulent, surrogate vaccine strains or the use of alternate vectors expressing the 1918 HA might be required. An approach more immediately available would be the use, for treatment and/or prophylaxis, of existing antiinfluenza virus drugs, provided they could be shown to be effective against the 1918 influenza virus. The currently available, U.S. Food and Drug Administration-approved antiinfluenza drugs fall into two classes, NA inhibitors and viral M2 ion-channel inhibitors. The viral NA inhibitors target the viral surface glycoprotein NA. The viral NA functions to remove neuraminic (sialic) acid residues from glycoproteins and glycolipids at the cell surface (19–21). This activity removes potential binding sites for the viral HA during the budding process and facilitates virus release. When NA is inhibited, newly formed viral particles aggregate on the surface of infected cells, and viral spread is blocked (20). The M2 inhibitors amantadine and rimantadine block the ion-channel activity of M2, a protein encoded by genome segment 7. The viral M2 ion channel, although not strictly required for viral infectivity in tissue culture (22), performs an important function during viral entry (23). Both classes of drugs, NA inhibitors and M2 inhibitors, can be clinically effective for the treatment or prophylaxis of influenza A virus infections (24).
We have generated recombinant influenza viruses possessing the HA, NA, or M segments of the 1918 pandemic influenza virus. Using these viruses, we demonstrate the efficacy of NA inhibitors against recombinant influenza viruses possessing these reconstructed 1918 virus genes. Because function of the viral NA is tied to HA function (25, 26), analysis of NA-inhibitor activity against the 1918 NA is best performed on a virus bearing both the 1918 HA and 1918 NA. We show that viruses with the 1918 virus NA or both the 1918 HA and 1918 NA are sensitive to NA inhibitors. We also demonstrate the efficacy of the M2 ion-channel inhibitors amantadine and rimantadine against an influenza virus bearing the 1918 virus M segment. These results suggest that the existing antiinfluenza drugs would be effective for prophylaxis against a re-emergent 1918 influenza virus and also might provide benefits when used to treat such infections.
Materials and Methods
Generation of 1918 HA, NA, and M cDNAs and Recombinant Viruses.
The 1918 HA, NA, and M cDNAs were constructed by PCR using overlapping deoxyoligonucleotides corresponding to the published sequence of the influenza A/South Carolina/1/18 (H1N1) virus HA (10) ORF, the influenza A/Brevig Mission/1/18 (H1N1) virus NA (9) ORF, or the influenza A/Brevig Mission/1/18 (H1N1) virus M ORF (6). The noncoding regions of each segment are identical to that of the corresponding segment of influenza A/WSN/33 (H1N1) virus (WSN). Primer sequences and PCR conditions are available on request.
Recombinant viruses were generated by using the reverse genetics system of Fodor et al. (16) following the methods of Basler et al. (11). The generation of viruses possessing 1918 genes was performed under biosafety level 3 (agricultural) containment (27). All subsequent work with live virus also was performed under these containment conditions. The identity of the 1918 influenza virus genes in the recombinant viruses was confirmed by RT-PCR and sequencing.
Mouse Experiments.
Male BALB/c mice, 6–7 weeks old (Simonsen Laboratories, Gilroy, CA), were anesthetized with ketamine/xylazine (1.98 and 0.198 mg per mouse, respectively) and inoculated intranasally with the indicated virus dose. Mice were housed in cages inside stainless steel isolation cabinets, ventilated under negative pressure with HEPA-filtered air. All animal work was performed in specially separated negative-pressured HEPA-filtered rooms within the larger biosafety level 3 (agricultural) building. All personnel wore half-body Rocal hoods with backpack HEPA-filtered air supplies.
Susceptibility of 1918 NA to NA Inhibitors in Vitro.
Zanamivir (Glaxo Wellcome) and oseltamivir carboxylate (GS4071, generously provided by Michael J. M. Hitchcock at Gilead Sciences, Foster City, CA) were tested for their ability to inhibit NA in vitro. NA assays were performed at 37°C by using a phosphate buffer and 4-methyl-umbelliferyl-_N_-acetyl-neuraminic acid as substrate as described (28). Plaque-reduction assays were performed on Madin–Darby canine kidney (MDCK) cells as described (29).
Susceptibility of 1918 NA and 1918 HA/1918 NA Viruses to NA Inhibitors in Vivo.
To measure the ability of oseltamivir (Hoffman–La Roche, distributed by Roche Laboratories, Nutley, NJ) to protect mice from lethal challenge with the 1918 HA/1918 NA virus, mice were administered oseltamivir once daily for 6 days, beginning 24 h before intranasal infection with 10 LD50 units of 1918 HA/1918 NA virus. The drug was administered by oral gavage at a dose of 50 mg/kg of body weight. Mice were examined daily.
Sensitivity of Recombinant Viruses to Amantadine/Rimantadine in Vitro.
Amantadine hydrochloride and rimantadine hydrochloride were obtained from Sigma–Aldrich. Sensitivity of recombinant influenza viruses in tissue culture to amantadine or rimantadine was assessed with a plaque-reduction assay with modifications of methods described previously (29, 30). Virus stocks used for plaque-inhibition assays were grown and titrated on MDCK cell monolayers. Confluent MDCK cell monolayers were washed with serum-free DMEM and inoculated with 100 plaque-forming units (pfu). The 100-μl virus inoculum was allowed to adsorb for 1 h at room temperature, shaking every 15 min to keep the wells covered. The unabsorbed virus then was removed by washing the cells two times with Eagle's minimal essential medium. A 1.6% melted agarose overlay containing equal volumes of 2× L-15 (BioWhittaker) medium and the appropriate drug concentration was added to duplicate monolayers. The drug concentrations applied were 1.5, 0.15, 0.015, and 0.0015 μg/ml. After 48 h at 37°C, plates were stained with 1% crystal violet, and plaques were counted.
Sensitivity of Recombinant Viruses to Rimantadine in Vivo.
Mice were infected via the intranasal route as described above with 10 LD50 (104 pfu) of wild-type WSN virus or 1918 M virus, or mice were infected with 106 pfu of a WSN virus containing the M segment from influenza A/Udorn/72 (H3N2) virus (Udorn M virus) (1 LD67). The mice then were mock-treated with PBS or treated with 40 mg/kg of body weight of rimantadine hydrochloride in PBS beginning 6 h postinfection and once daily for 4 days. One group of mice was left uninfected and treated with rimantadine hydrochloride. Mice were examined daily for weight loss and death.
Results
Construction of Recombinant Viruses with 1918 Influenza Virus Genes.
The genes encoding the 1918 pandemic influenza virus were reconstructed from deoxyoligonucleotides and correspond to the reported 1918 virus coding sequences (6, 8, 9). Genes from other influenza viruses used in the construction of control viruses were generated by RT-PCR from purified viral RNA. Recombinant influenza virus genes were cloned into a plasmid such that each would be expressed in vivo as an influenza A virus viral RNA (31). These segments were rescued into live viruses as described (11, 16). Those viral segments not derived from the 1918 influenza virus were derived from the mouse-adapted WSN virus unless indicated otherwise. The identity of the recombinant viruses was confirmed by analysis of RT-PCR products (data not shown). All work with viruses containing 1918 influenza virus segments was performed under biosafety level 3 (agricultural) containment (27).
Characterization of Viruses Possessing the 1918 HA and/or 1918 NA.
The HA and NA of influenza A viruses have been implicated as virulence factors in birds and mice (32–36). We therefore were interested in the influence of the 1918 HA and 1918 NA on virulence of the recombinant viruses in mice. Introduction into virus of either the 1918 HA or 1918 NA individually led to attenuation in mice, a 56-fold increase in LD50, compared with the isogenic wild-type WSN virus (Table 1). A recombinant virus possessing the HA of influenza A/New Caledonia/20/99 (H1N1) virus (New Cal.) was much more attenuated in mice (Table 1). Interestingly, a virus that possessed both the HA and NA of the 1918 influenza virus (1918 HA/1918 NA virus) was able to kill mice with an efficiency identical to the mouse-adapted WSN virus. This was evidenced by the 50% lethal dose (LD50) titers in 6- to 7-week-old BALB/c mice as well as similar degrees of weight loss and similar lung titers in infected mice (Table 1). In contrast, a recombinant virus with both the HA and NA of the New Cal. virus was highly attenuated relative to the 1918 HA/1918 NA virus or wild-type WSN virus (Table 1).
Table 1.
Properties of recombinant influenza viruses used in this study
| Virus* | Titer†, pfu/ml | Weight loss‡, % | LD50§ | Lung titers¶, EID50/ml ± SE |
|---|---|---|---|---|
| Wild-type WSN virus | 8.6 × 107 | 21.2 | 2.75 | 6.85 ± 0.2 |
| 1918 NA* | 5.0 × 107 | 26.6 | 4.5 | 6.9 ± 0.4 |
| 1918 HA | 2.0 × 107 | 17.1 | 4.5 | 4.3 ± 0.2 |
| 1918 HA/1918 NA | 2.1 × 107 | 20.9 | 2.75 | 7.3 ± 0.1 |
| New Cal. HA | 1.0 × 107 | 8.7 | >6 | 1.7 ± 0.3 |
| New Cal. HA/New Cal. NA | 2.5 × 107 | 0 | >6 | 4.4 ± 0.2 |
| 1918 M | 2.1 × 107 | 21.5 | 3.25 | 6.2 ± 0.1 |
Susceptibility of the 1918 Influenza Virus NA to NA Inhibitors.
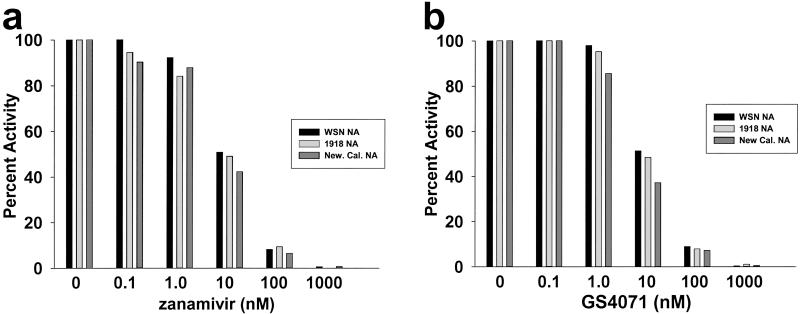
When plasmids that express the influenza A virus polymerase proteins PA, PB1, PB2, and the nucleoprotein are cotransfected into 293T cells, the viral RNA-dependent RNA polymerase is reconstituted. This reconstituted RNA-dependent RNA polymerase is able to transcribe and replicate coexpressed viral RNAs (31). By this method, the 1918 NA and two other N1 NAs, those of the WSN virus and a recent human isolate (New Cal.) were expressed from appropriate plasmids. Lysates were prepared from the transfected cells and assayed for NA activity by using the low molecular weight fluorescent substrate 4-methyl-umbelliferyl-_N_-acetyl-neuraminic acid. The extracts were adjusted to equivalent levels of NA activity and tested for sensitivity to two NA inhibitors, zanamivir and GS4071 (oseltamivir carboxylate), the active form of the pro-drug oseltamivir. The three NAs displayed nearly identical sensitivities to the two drugs (Fig. 1 a and b), and the in vitro sensitivity of the 1918 NA was similar to that reported for other influenza N1 NAs (37–39). No NA activity was detected in the absence of NA plasmid (data not shown).
Figure 1.
Inhibition in vitro of the 1918 influenza virus NA by zanamivir and GS4071 (oseltamivir carboxylate). Extracts expressing WSN NA, New Caledonia NA, or 1918 NA were assayed in the presence of the indicated concentrations of zanamivir (a) or GS4071 (oseltamivir carboxylate) (b). Activities are given as percent activity relative to the no-drug control.
The sensitivity in tissue culture of recombinant viruses that possess the 1918 NA, in combination with the HA of either WSN or the 1918 virus, was measured in a plaque- reduction assay (29). Plaque formation by the 1918 NA or 1918 HA/1918 NA virus was inhibited almost completely by GS4071 at concentrations that also inhibited wild-type WSN virus growth (Fig. 2a). The NA inhibitors also were found to be effective in vivo. Orally administered oseltamivir was able to effectively protect 90% of mice from lethal infection (10 LD50) with the 1918 HA/1918 NA virus (Fig. 2b). These results suggest that NA inhibitors could be used effectively in humans for prophylaxis and possibly treatment of 1918 influenza virus infection.
Figure 2.
Oseltamivir effectively inhibits replication in tissue culture of recombinant influenza viruses possessing the 1918 NA and prevents lethal infection in mice of the recombinant 1918 HA/1918 NA influenza virus. (a) Inhibition of plaque formation by GS4071 (oseltamivir carboxylate). Plaque-reduction assays were performed on MDCK cells by using the indicated concentrations of drug following established methods (29). (b) Oral administration of oseltamivir protects mice from death due to intranasal infection with the 1918 HA/1918 NA virus. Indicated is the percentage of mice surviving intranasal infection at the indicated times postinfection.
An Influenza Virus Possessing the 1918 M Segment Is Sensitive to the M2 Ion-Channel Inhibitors Amantadine and Rimantadine.
A recombinant influenza virus was also constructed that possessed the 1918 M segment (1918 M virus). Its seven remaining genes were derived from the WSN virus. The presence of the 1918 M gene was confirmed by RT-PCR and sequencing (data not shown). This virus replicated similarly to wild-type WSN virus in tissue culture and had an LD50 similar to wild-type WSN virus after intranasal administration to mice (Table 1).
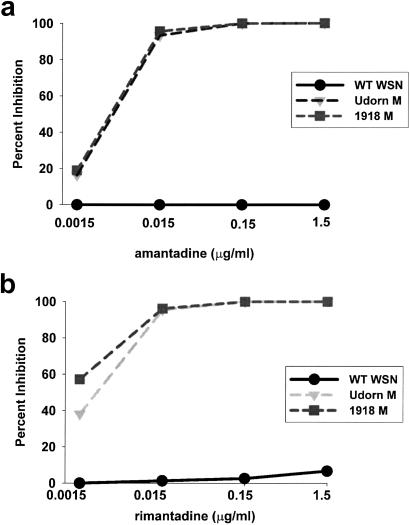
The sensitivity of the 1918 M virus to amantadine or rimantadine was assessed in a plaque-reduction assay (29). As controls, wild-type WSN virus, an amantadine/rimantadine-resistant virus, and a WSN-based virus with the M gene of the amantadine/rimantadine-sensitive Udorn virus (Udorn M virus) (22, 23) also were included. The sensitivities of both the 1918 M and Udorn M virus at various concentrations of amantadine (Fig. 3a) or rimantadine (Fig. 3b) were nearly identical, with >90% inhibition at 15 ng/ml of either drug. As expected, WSN virus was resistant to both drugs (Fig. 3).
Figure 3.
Inhibition of virus plaque formation by amantadine and rimantadine. (a) Percent inhibition of plaque formation in the presence of the indicated concentrations of amantadine hydrochloride. (b) Percent inhibition of plaque formation in the presence of the indicated concentrations of rimantadine hydrochloride. Recombinant, wild-type (WT) WSN virus (circles), Udorn M virus (triangles), and 1918 M virus (squares) were tested for their ability to form plaques in the presence of the indicated concentrations of drug. The data are reported as the percent inhibition of plaque formation as compared with a no-drug control for each virus.
To determine the in vivo susceptibility to rimantadine of viruses encoding the 1918 M2 protein, mice were infected intranasally with lethal doses (10 LD50) of the rimantadine-resistant wild-type WSN virus (23) or the 1918 M virus. Mice also were infected with an ≈67% lethal dose (106 pfu) of Udorn M virus. For the latter virus, this was the highest dose that could be administered intranasally. The mice then were mock-treated or treated via the i.p. route with rimantadine 6 h postinfection and once daily afterward, a method used previously to assess virus sensitivity to rimantadine (40). All mock-treated mice infected with either wild-type WSN or 1918 M virus died (Fig. 4a). In addition, approximately two-thirds of mock-treated mice infected with 106 pfu of the Udorn M virus died (Fig. 4a). [The precise reason for the higher LD50 seen with this virus is not clear, but the generation by reassortment of viruses possessing different M segments has resulted in attenuation in some cases (41, 42).] In all mock-treated mice, illness was apparent as indicated by body-weight loss (Fig. 4b). In contrast to the mock-treated mice, all mice infected with the 1918 M or Udorn M viruses and treated with rimantadine survived (Fig. 4a). These treated mice also suffered relatively mild illness, losing an average of 10% of their body weight versus the ≈30% weight loss seen in lethal infections (Fig. 4b). As expected, mice infected with the rimantadine-resistant wild-type WSN virus succumbed to infection even with rimantadine treatment (Fig. 4a).
Figure 4.
Rimantadine protects mice from lethal infection with an influenza virus possessing the 1918 M segment. (a) Survival after infection with the indicated viruses and either mock treatment with PBS or treatment with rimantadine. An uninfected but treated control group was included also. (b) Average weight of mice from the same groups as in a. Average weight is reported for all mice surviving at the indicated time points. Groups of 8 or 9 mice were infected with 10 LD50 of wild-type (WT) WSN virus (circles) or 1918 M virus (squares) or with 106 pfu of Udorn M virus (1 LD80, triangles). The mice then were mock-treated with PBS (filled symbols) or treated with 40 mg/kg of body weight of rimantadine hydrochloride (open symbols). PBS or drug was administered i.p. beginning 6 h postinfection and once daily afterward. One group of mice was left uninfected and treated with rimantadine (filled diamonds).
Discussion
The availability of prophylaxis and treatment measures for highly lethal human pathogens is a major consideration in planning a response to potential future outbreaks. In the case of the 1918 influenza virus, the availability of such measures could be used to meet the threat of a re-emerging 1918 virus, whether the virus emerged through a natural route or through intentional reconstruction and release. The most successful approach to controlling influenza has been vaccination (18). However, during the period of vaccine development, antiviral drugs could provide an effective method to control virus spread and treat affected individuals, provided that sufficient supplies were available and effective means of distribution were in place (43, 44). The identification of effective strategies to prevent and treat infection by viruses possessing the 1918 NA or M genes should also facilitate the further analysis of these and other viruses possessing 1918 pandemic influenza virus genes; the availability of effective prophylaxis and/or treatment measures for laboratory workers addresses a major safety concern related to research on highly virulent pathogens.
Of the antiinfluenza virus drugs available, the M2 inhibitors have a longer history of use than NA inhibitors. Studies performed during the 1968 H3N2 and 1977 H1N1 pandemics reported chemoprophylaxis efficacies of 59–100 and 31–71%, respectively (43). Based on an analysis of the sequence of the 1918 virus M2 transmembrane domain, sensitivity of the 1918 M2 ion channel to amantadine and rimantadine would be predicted (6), and our data confirm this prediction. Mice were protected completely from death after administration of 10 LD50 of the 1918 M virus by using a postinfection treatment regimen where drug was administered shortly (6 h) after infection. These data predict that amantadine or rimantadine would be effective also if used for prophylaxis against the 1918 M virus, because sensitivity of influenza A viruses to either prophylaxis or treatment depends on the properties of the M2 ion-channel protein. The effectiveness of M2 inhibitors in humans for prophylaxis against a complete 1918 virus therefore is highly likely, and they also might provide benefits when used as a treatment.
Our recombinant viruses possessing the 1918 NA gene are susceptible to the NA inhibitors zanamivir and oseltamivir. These drugs have been shown to be effective for human prophylaxis against both influenza A and B viruses, although there is no experience using them during an influenza pandemic (43, 45–48). NA inhibitors have be used also to treat infection, the primary clinical benefit being an increased (1–1.5 days) rate of recovery, but as with the M2 inhibitors, clinical efficacy requires early administration (24).
Although resistant mutants can be generated with NA inhibitors, these mutants may prove less problematic than amantadine/rimantadine-resistant mutants (49, 50). The frequency of resistant mutant isolation has been relatively low with NA inhibitors (24), and data from animal models suggest that the resistant mutants are attenuated, showing decreased virulence and transmission (51, 52). Previously described NA inhibitor-resistant viruses possess mutations in either the HA or NA gene. Mutations in the NA conferring resistance of the enzyme to inhibitors include substitutions at active site residues 119, 152, 274, and 292 (using N2 NA numbering; residues 119,152, 275, and 293 of the 1918 NA) (53). The 1918 NA possesses the conserved wild-type residues at these positions (9). Mutations in the HA that confer on viruses resistance to NA inhibitors include substitutions that decrease the affinity of HA for sialic acid (53). Because it is possible that mutations other than those already identified will also be able confer resistance to NA inhibitors, it was important to assess the sensitivity of both the 1918 NA enzyme and viruses possessing the 1918 NA and HA for sensitivity to currently approved NA inhibitors. Both zanamivir and oseltamivir carboxylate were found to inhibit in vitro the 1918 NA to the same degree as the NA of the WSN or New Cal. viruses (Fig. 1). Oseltamivir treatment was found also to protect mice from death after administration of drug beginning 24 h before intranasal infection with 10 LD50 units of 1918 HA/1918 NA virus (Fig. 2). Given the effectiveness of NA inhibitors in mice against our recombinant viruses, it seems likely that prophylaxis of humans with NA inhibitors would be effective against a complete 1918 or a 1918-like virus. Our data also suggest the possibility that prompt treatment with these drugs would provide a benefit to individuals suffering from infection with such a virus.
The virulence of the recombinant virus with both the 1918 HA and 1918 NA is intriguing. It was also surprising given that the 1918 HA and 1918 NA genes were derived directly from a human virus without prior mouse adaptation. Typically, strains of influenza A viruses become lethal in mice only after they are adapted to growth in these animals. Exceptions include some of the H5N1 viruses isolated in Hong Kong in 1997, which displayed high virulence in mice without prior mouse adaptation (53–56); these viruses were also highly virulent in humans (57, 58). One of the possible explanations for the virulence of the 1918 HA/1918 NA virus in mice is that its HA and NA are “compatible” with each other and this compatibility, in combination with six mouse-adapted genes, is sufficient for virulence. However, when both the HA and NA of the New Caledonia strain were tested in a WSN background, the virus was highly attenuated (Table 1). These data suggest that the 1918 HA and NA genes might possess intrinsic high-virulence properties.
Acknowledgments
We thank Dr. Michael J. M. Hitchcock from Gilead Sciences for generously providing GS4071. We also thank Svetlana Bourmakina for generating the WSN:Udorn M virus. This work was partially supported by grants from the National Institutes of Health (to P.P., A.G.-S., and C.F.B.) and by a Bristol-Myers Squibb Company Unrestricted Infectious Disease Research Grant (to P.P.). C.F.B. is an Ellison Medical Foundation New Scholar in Global Infectious Diseases. T.M.T. was supported by U.S. Department of Agriculture, Agricultural Research Service Current Research Information System (CRIS) 6612-32000-022-93.
Abbreviations
HA
hemagglutinin
NA
neuraminidase
M
matrix
WSN
influenza A/WSN/33 (H1N1) virus
MDCK
Madin–Darby canine kidney
pfu
plaque-forming unit(s)
Udorn virus
influenza A/Udorn/72 (H3N2) virus
New Cal.
influenza A/New Caledonia/20/99 (H1N1) virus
References
- 1.Reid A H, Taubenberger J K, Fanning T G. Microbes Infect. 2001;3:81–87. doi: 10.1016/s1286-4579(00)01351-4. [DOI] [PubMed] [Google Scholar]
- 2.Kilbourne E D. The Influenza Viruses. New York: Academic; 1975. pp. 483–538. [Google Scholar]
- 3.U.S. Department of Commerce. Historical Statistics in the United States: Colonial Times to 1970. Washington, DC: U.S. Government Printing Office; 1976. p. 58. [Google Scholar]
- 4.Crosby A. America's Forgotten Pandemic. Cambridge, U.K.: Cambridge Univ. Press; 1989. [Google Scholar]
- 5.Winternitz M C, Wason I M, McNamara F P. Pathology of Influenza. New Haven, CT: Yale Univ. Press; 1920. [Google Scholar]
- Reid, A. H., Fanning, T. G., Janczewski, T. A., McCall, S. & Taubenberger, J. K. (2002) J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 7.Taubenberger J K, Reid A H, Fanning T G. Virology. 2000;274:241–245. doi: 10.1006/viro.2000.0495. [DOI] [PubMed] [Google Scholar]
- 8.Taubenberger J K, Reid A H, Krafft A E, Bijwaard K E, Fanning T G. Science. 1997;275:1793–1796. doi: 10.1126/science.275.5307.1793. [DOI] [PubMed] [Google Scholar]
- 9.Reid A H, Fanning T G, Janczewski T A, Taubenberger J K. Proc Natl Acad Sci USA. 2000;97:6785–6790. doi: 10.1073/pnas.100140097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid A H, Fanning T G, Hultin J V, Taubenberger J K. Proc Natl Acad Sci USA. 1999;96:1651–1656. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basler C, Reid A, Dybing J, Janczewski T, Fanning T, Zheng H, Salvatore M, Perdue M, Swayne D, García-Sastre A, et al. Proc Natl Acad Sci USA. 2001;98:2746–2751. doi: 10.1073/pnas.031575198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs M J, Armstrong J S, Gibbs A J. Science. 2001;293:1842–1845. doi: 10.1126/science.1061662. [DOI] [PubMed] [Google Scholar]
- 13.Fanning T G, Slemans R D, Reid A H, Janczewski T A, Dean J, Taubenberger J K. J Virol. 2002;76:7860–7862. doi: 10.1128/JVI.76.15.7860-7862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster R G. Proc Natl Acad Sci USA. 2000;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez D R, Donis R, Hoffmann E, et al. Proc Natl Acad Sci USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fodor E, Devenish L, Engelhardt O G, Palese P, Brownlee G G, García-Sastre A. J Virol. 1999;73:9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederberg J. Proc Natl Acad Sci USA. 2001;98:2115–2116. doi: 10.1073/pnas.051000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridges C B, Fukuda K, Cox N J, Singleton J A. Morbid Mortal Wkly Rep. 2001;50:1–44. [PubMed] [Google Scholar]
- 19.Meindl P, Bodo G, Palese P, Schulman J, Tuppy H. Virology. 1974;58:457–463. doi: 10.1016/0042-6822(74)90080-4. [DOI] [PubMed] [Google Scholar]
- 20.Palese P, Compans R W. J Gen Virol. 1976;33:2142–2146. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 21.Palese P, Tobita K, Ueda M, Compans R W. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Watanabe S, Ito H, Kida H, Kawaoka Y. J Virol. 2001;75:5656–5662. doi: 10.1128/JVI.75.12.5656-5662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda M, Pekosz A, Shuck K, Pinto L H, Lamb R A. J Virol. 2002;76:1391–1399. doi: 10.1128/JVI.76.3.1391-1399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couch R B. N Engl J Med. 2000;343:1778–1787. doi: 10.1056/NEJM200012143432407. [DOI] [PubMed] [Google Scholar]
- 25.Gubareva L V, Kaiser L, Matrosovich M N, Soo-Hoo Y, Hayden F G. J Infect Dis. 2001;183:523–531. doi: 10.1086/318537. [DOI] [PubMed] [Google Scholar]
- 26.Blick T J, Sahasrabudhe A, McDonald M, Owens I J, Morley P J, Fenton R J, McKimm-Breschkin J L. Virology. 1998;246:95–103. doi: 10.1006/viro.1998.9194. [DOI] [PubMed] [Google Scholar]
- 27.Barbeito M S, Abraham G, Best M, Cairns P, Langevin P, Sterritt W G, Barr D, Meulepas W, Sanchez-Vizcaino J M, Saraza M, et al. Rev Sci Tech. 1995;14:873–887. doi: 10.20506/rst.14.3.880. [DOI] [PubMed] [Google Scholar]
- 28.Basler C F, García-Sastre A, Palese P. J Virol. 1999;73:8095–8103. doi: 10.1128/jvi.73.10.8095-8103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayden F G, Cote K M, Douglas R G., Jr Antimicrob Agents Chemother. 1980;17:865–870. doi: 10.1128/aac.17.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohnishi H, Yamaguchi K, Shimada S, Himuro S, Suzuki Y. Antimicrob Agents Chemother. 1982;22:250–254. doi: 10.1128/aac.22.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pleschka S, Jaskunas R, Engelhardt O G, Zurcher T, Palese P, García-Sastre A. J Virol. 1996;70:4188–4192. doi: 10.1128/jvi.70.6.4188-4192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohuchi M, Orlich M, Ohuchi R, Simpson B E, Garten W, Klenk H D, Rott R. Virology. 1989;168:274–280. doi: 10.1016/0042-6822(89)90267-5. [DOI] [PubMed] [Google Scholar]
- 33.Horimoto T, Kawaoka Y. J Virol. 1994;68:3120–3128. doi: 10.1128/jvi.68.5.3120-3128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goto H, Wells K, Takada A, Kawaoka Y. J Virol. 2001;75:9297–9301. doi: 10.1128/JVI.75.19.9297-9301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Schulman J, Itamura S, Palese P. J Virol. 1993;67:6667–6673. doi: 10.1128/jvi.67.11.6667-6673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatta M, Gao P, Halfmann P, Kawaoka Y. Science. 2001;293:1840–1842. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- 37.Govorkova E A, Leneva I A, Goloubeva O G, Bush K, Webster R G. Antimicrob Agents Chemother. 2001;45:2723–2732. doi: 10.1128/AAC.45.10.2723-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubareva L V, McCullers J A, Bethell R C, Webster R G. J Infect Dis. 1998;178:1592–1596. doi: 10.1086/314515. [DOI] [PubMed] [Google Scholar]
- 39.Mendel D B, Tai C Y, Escarpe P A, Li W, Sidwell R W, Huffman J H, Sweet C, Jakeman K J, Merson J, Lacy S A, et al. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephen E L, Dominik J W, Moe J B, Spertzel R O, Walker J S. Antimicrob Agents Chemother. 1975;8:154–158. doi: 10.1128/aac.8.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy B R, Buckler-White A J, London W T, Snyder M H. Vaccine. 1989;7:557–561. doi: 10.1016/0264-410x(89)90283-1. [DOI] [PubMed] [Google Scholar]
- 42.Subbarao E K, Perkins M, Treanor J J, Murphy B R. Virus Res. 1992;25:37–50. doi: 10.1016/0168-1702(92)90098-t. [DOI] [PubMed] [Google Scholar]
- 43.Hayden F G. Philos Trans R Soc London B. 2001;356:1877–1884. doi: 10.1098/rstb.2001.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laver G, Garman E. Science. 2001;293:1776–1777. doi: 10.1126/science.1063817. [DOI] [PubMed] [Google Scholar]
- 45.Hayden F G, Treanor J J, Fritz R S, Lobo M, Betts R F, Miller M, Kinnersley N, Mills R G, Ward P, Straus S E. J Am Med Assoc. 1999;282:1240–1246. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 46.Monto A S, Robinson D P, Herlocher M L, Hinson J M, Jr, Elliott M J, Crisp A. J Am Med Assoc. 1999;282:31–35. doi: 10.1001/jama.282.1.31. [DOI] [PubMed] [Google Scholar]
- 47.Hayden F G, Gubareva L V, Monto A S, Klein T C, Elliot M J, Hammond J M, Sharp S J, Ossi M J. N Engl J Med. 2000;343:1282–1289. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 48.Peters P H, Jr, Gravenstein S, Norwood P, De Bock V, Van Couter A, Gibbens M, von Planta T A, Ward P. J Am Geriatr Soc. 2001;49:1025–1031. doi: 10.1046/j.1532-5415.2001.49204.x. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler T, Hemphill M L, Ziegler M L, Perez-Oronoz G, Klimov A I, Hampson A W, Regnery H L, Cox N J. J Infect Dis. 1999;180:935–939. doi: 10.1086/314994. [DOI] [PubMed] [Google Scholar]
- 50.Hay A J. In: Antiviral Drug Resistance. Richman D D, editor. Chichester, U.K.: Wiley; 1996. [Google Scholar]
- 51.Munoz F M, Galasso G J, Gwaltney J M, Jr, Hayden F G, Murphy B, Webster R, Wright P, Couch R B. Antiviral Res. 2000;46:91–124. doi: 10.1016/S0166-3542(00)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai C Y, Escarpe P A, Sidwell R W, Williams M A, Lew W, Wu H, Kim C U, Mendel D B. Antimicrob Agents Chemother. 1998;42:3234–3241. doi: 10.1128/aac.42.12.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gubareva L V, Kaiser L, Hayden F G. Lancet. 2000;355:827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- 54.Lu X, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J M. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dybing J K, Schultz-Cherry S, Swayne D E, Suarez D L, Perdue M L. J Virol. 2000;74:1443–1450. doi: 10.1128/jvi.74.3.1443-1450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley A J, Kawaoka Y. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, et al. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 58.Claas E C, Osterhaus A D, van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]