Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform (original) (raw)
Abstract
Progesterone (P) regulates female reproduction via two nuclear receptors, PR-A and PR-B. Although both receptors display overlapping and distinct transcription regulatory properties, their individual physiological roles are unclear. To address the physiological role of PR-A, we generated a mouse model in which expression of PR-B was specifically ablated (PRBKO–/–). We show that selective activation of PR-A in PRBKO–/– mice is sufficient to elicit normal ovarian and uterine responses to P but results in reduced mammary gland morphogenesis. In the absence of PR-B, pregnancy-associated ductal sidebranching and lobuloalveolar development are markedly reduced due to decreased ductal and alveolar epithelial cell proliferation and decreased survival of alveolar epithelium. In an effort to elucidate the molecular genetic signaling pathways that are differentially regulated by PRs in the mammary gland, we have identified receptor activator of nuclear factor κB ligand (RANKL) as a paracrine mediator of P-dependent alveologenesis. Further, we demonstrate that the defects in PRBKO–/– mice are associated with an inability of PR-A to activate the RANKL signaling pathway in response to P. Our data indicate that functional interaction between PR-A and PR-B is not required for reproductive activity and that selective modulation of PR-A activity by progestin agonists may have a protective effect against both uterine and mammary gland hyperplasias.
Progesterone (P) plays a central role in establishment and maintenance of pregnancy. The physiological effects of P are mediated by interaction with intracellular P receptors (PRs) that are members of the nuclear receptor superfamily of transcription factors (1,2) and are expressed as two protein isoforms: PR-A and PR-B. Both proteins are derived from a single gene by transcription at two distinct promoters and translation initiation at two alternative AUG signals (3,4). PR-A and PR-B are structurally identical with the exception of an amino-terminal extension that is specific to the PR-B protein. This region encodes a transactivation function that contributes to differential coregulator recruitment by PR-A and PR-B (5) and to distinct cell- and promoter-specific transactivation properties of the two isoforms (6–8). The observation that binding of P results in the formation of active PR homodimers as well as heterodimers between receptor isoforms suggests that three active species of PRs, all with potentially distinct transcription activation properties, may contribute to the diverse physiological activities of P.
To examine the contribution of the individual PR isoforms to female reproduction, we generated mice lacking one or both PR proteins. Previously we demonstrated that null mutation of the PR gene results in pleiotropic female reproductive abnormalities including impaired neuroendocrine and ovarian function, uterine hyperplasia and inflammation, severely limited mammary gland development, and impaired thymic function and sexual behavior (2,9).
Both PR isoforms are often expressed in the same cells (10) in reproductive tissues, and their levels vary with developmental and hormonal status and during carcinogenesis (1,11). By selective ablation of the PR-A protein with PR-A knockout (PRAKO–/–) mice, we recently demonstrated that PR-B mediates a subset of the reproductive functions of PRs (12). Disruption of PR-A expression results in severe abnormalities in ovarian and uterine function leading to female infertility but does not affect responses of the mammary gland to P. Here we show that ablation of PR-B [PR-B knockout (PRBKO–/–)] does not affect either ovarian or uterine responses to P but results in significantly reduced mammary ductal sidebranching and alveologenesis during pregnancy. In addition, we show that mammary defects in PRBKO–/– mice are due in part to diminished PR-dependent receptor activator of nuclear factor κB ligand (RANKL) signaling in the absence of PR-B. We propose that PR-B is the primary proliferative stimulus in the mammary gland and that selective modulation of PR-A activity by progestin agonists may have a protective effect against both uterine and mammary gland hyperplasias.
Materials and Methods
Mice. To generate PRBKO–/– mice, a CRE/loxP gene-targeting strategy was used to mutate the initiating ATG for PR-B (ATGB) in exon 1 of the mouse PR gene. The tagging vector contained 3.5 kb of genomic PR DNA encoding exons 1 and 2 in the 5′ arm. The PGKneocbpa and MC1tk (neo-tk) cassette was inserted into intron 2 and flanked by loxP sites (12). The 3′ arm contained 3 kb of intron 2. The ATGB start site in exon 1 was mutated to CTG (encoding Leu), and an additional silent nucleotide substitution at the neighboring Leu-4 (CTG to CTC) eliminated an endogenous_Pst_I site in the PR gene to facilitate screening for embryonic stem (ES) cell homologous recombinants containing the mutation. The mutations were introduced by using the QuikChange mutagenesis kit (Stratagene) and the oligonucleotide 5′-GGGGAGCTTGGGTCGTCCTGACTGATCTCCAGGCAAAGGAT-3′ and verified by sequencing. Linearized vector (20 μg) was electroporated into 107 AB2.2 ES cells, and G418-resistant clones (Invitrogen) were isolated. Homologous recombinant ES cell clones, identified by Southern blot of _Hin_dIII-digested genomic DNA, were transiently transfected with a CRE recombinase expression plasmid (20 μg) and grown in the presence of 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil. ES cell mutant clones were identified by Southern blot of genomic DNA digested with _Pst_I and probed with PR cDNA fragment (nucleotides 20–452) and DNA sequencing of mutated region.
Hormone Treatments. Mice (C57BL/129SV) were ovariectomized at 6 weeks and then rested for 2 weeks. For analysis of uterine responses to estrogen (E) and P, mice (four per group) were given daily s.c. injections of sesame oil, sesame oil solution of E (100 ng), or E (100 ng) plus P (1 mg) for 4 days. Protein extracts from E-treated uteri were used for Western blot analysis. To analyze mammary gland response to E and P, mice were implanted with beeswax pellets (control) or pellets containing hormones (20 μg of E and 20 mg of P) on days 1 and 10, and tissues were collected on day 21. To induce superovulation, 21-day-old virgin females (six per genotype) were injected with 4 units of pregnant mare serum gonadotropin (Diosynth, Chicago) followed 48 h later by 5 units of human chorionic gonadotropin (Organon).
Mammary Gland Whole Mounts, Histology, and Immunodetection. Whole-mount staining and histology of mammary tissue were performed as described (12). For BrdUrd immunolabeling, mice were injected with 30 μg/g of body weight BrdUrd 2 h before being killed. Mammary tissue was fixed in Bouin's fixative, paraffin-embedded, and sectioned (5 μm). A cell-proliferation kit (Amersham Pharmacia) was used to detect BrdUrd-positive cells. For all other immunodetection, tissue sections (5 μm) were prepared from tissues fixed in 4% paraformaldehyde and paraffin-embedded. The sections were deparaffinized and subjected to antigen heat-induced retrieval (13). Rabbit polyclonal PR IgG (1:100, DAKO), goat polyclonal RANKL IgG (1: 500,R&D Systems), rabbit polyclonal cyclin D1 IgG (Lab Vision, Fremont, CA), and rabbit anti-adipophilin IgG (1:500, gift from T. W. Keenan, Virginia Tech, Blacksburg, VA) were used to detect PR, RANKL, cyclin D1, and adipophilin, respectively. To visualize immunocomplexes, we used Texas red donkey anti-rabbit (1:400) (Jackson ImmunoResearch), biotinylated horse anti-goat (1:4,000), biotinylated goat anti-rabbit (1:500), and horseradish peroxidase avidin D (1:400) (Vector Laboratories), and Alexa Fluor 488 avidin (1:1,000), FITC goat anti-mouse (1:1,000), and Alexa 568 Flvor avidin (1:1,000) (Molecular Probes). In situ DNA end labeling [terminal deoxynucleotidyltransferase-mediated dUTP end labeling (TUNEL) assay] was carried out as described (14). Images were captured by using a Zeiss Axioplan 2 and Zeiss LSM 510 confocal microscope.
RNase Protection Assay (RPA). Total RNA (10 μg) was pooled from five animals of each genotype and analyzed by using labeled [32P]UTP-antisense RNA probes and the RPA kit (Ambion, Austin, TX). Antisense RNA probes for RANKL and cyclin D1 were generated from cDNA fragments obtained from BD PharMingen (San Diego). Wnt-4 cDNA was a gift from J. M. Rosen (Baylor College of Medicine). Radioactive bands were quantified by using a Storm 860 PhosphorImager (Molecular Dynamics) standardized relative to L32 or actin and represented as an average of five experiments ± SEM.
Serum Prolactin (PRL) Levels. Serum PRL levels were determined as described (15) by using the Nb2.s rat lymphoma cell line.
Statistical Analysis. image tool software (University of Texas Health Science Center, San Antonio) was used to quantitate tertiary branch points and BrdUrd- or TUNEL-positive cells. Branch points were counted in no. 4 abdominal glands from seven to nine animals of each genotype. For BrdUrd (5 mice per group) and TUNEL (10 mice per group) analysis, four independent sections were analyzed, and 5,000–10,000 cells per animal were counted. Results are mean ± SEM and considered significant at_P_ < 0.05 by using Student's t test.
Results
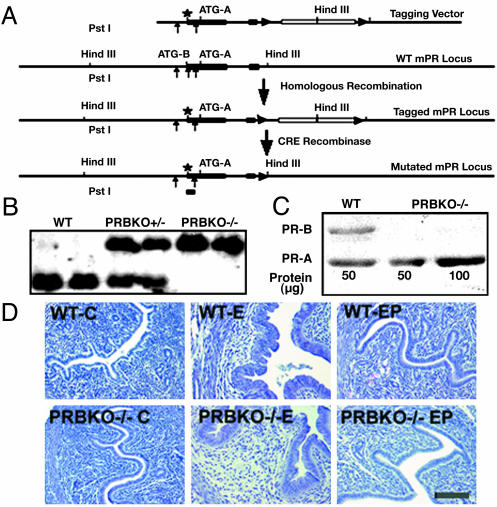
Generation of PR-B Isoform Knockout Mice. Expression of PR-B protein was ablated in PRBKO–/– mice by using CRE/loxP-based gene targeting. The mutation strategy involved a conservative amino acid substitution of Met-1 (ATGB) to Leu (CTG) and a silent nucleotide substitution at Leu-4 (CTG to CTC) to eliminate a_Pst_I restriction site and facilitate screening for the integrated mutant by Southern blot analysis (Fig. 1_A_ and B). The tagging vector contained (from the 5′-to-3′ direction) 3.5 kb of genomic PR sequence containing the ATGB mutation in exon 1, and extending from exon 2 to the second intron a neo-tk expression cassette flanked by two loxP sites (12) and an additional 3.0 kb of sequence from intron 2 of PR at the 3′ end. After CRE recombinase mediated removal of the selection cassette from homologous recombinant clones, those containing the ATGB mutation were identified by loss of the diagnostic _Pst_I site, which resulted in a 1.2-kb hybridizing band specific for the mutated allele after Southern blot analysis (Fig. 1_B_). Male chimeras obtained from two independent clones transferred the PR-B mutation to the next generation.
Fig. 1.
Generation of PRBKO–/– mutant mouse. (A) Strategy for targeted disruption of PR-B expression. Filled boxes, exons; lines, introns; star, mutated ATGB; filled triangles, loxP sites; open box, neo-tk cassette. (B) Southern blot analysis of _Pst_I-digested genomic DNA from WT, heterozygote PRBKO+/–, and homozygote PRBKO–/– mice with cDNA probe encoding nucleotides 20–452 of PR cDNA. (C) Western analysis of uterine extracts to detect PR isoform expression. (D) Hematoxylin/eosin-stained uterine sections from ovariectomized mice treated with oil (C), E, or E+P (EP). (Scale bar, 100 μm.)
The absence of PR-B in PRBKO–/– female mice was confirmed by Western blot analysis of uterine extracts from E-treated animals (Fig. 1_C_). Although a strong immunoreactive band corresponding to the PR-B protein is detected in WT animals, this band is absent from PRBKO–/– extracts, confirming exclusive expression of PR-A. Further, the level of PR-A was unaffected by inhibition of PR-B.
Litters from PRBKO+/– intercrosses were born at normal ratios of genotypes. In addition, in contrast to PRAKO–/– mice, PRBKO–/– female mice were fertile, sustained pregnancies to term, and produced viable offspring. These observations indicated that selective activation of the PR-A protein in the absence of PR-B is sufficient to mediate the normal P-dependent endocrine activity required for reproductive function.
Expression of PR-A Is Sufficient to Mediate Ovarian and Uterine Responses to P. Our previous studies with PRAKO–/– mice demonstrated that the PR-A protein is essential for normal ovulation (12). To determine whether PR-A is sufficient to induce normal ovulation, 21-day-old mice were administered pregnant mare serum gonadotropin and human chorionic gonadotropin to induce superovulation, and 24 h later oocytes were counted. The results confirmed normal superovulation in PRBKO–/– mice with numbers of oocytes (38 ± 5) comparable with those observed in WT animals (41 ± 16). Thus, selective activation of PR-A is sufficient to mediate the ovulatory activities of P, and heterodimer interaction with PR-B is not required for this event.
The ability of PRBKO–/– mice to sustain pregnancies also indicated that the neuroendocrine and uterine responses required to ensure implantation of the developing embryo are intact. In addition to its role in implantation, P acts as a potent antagonist of E-induced hyperplasia of the uterine epithelium, and the antiproliferative effects of P require the activity of uterine PR-A (12). Because both PR-A and PR-B are expressed in the uterus, we asked whether activation of PR-A alone is sufficient to elicit the antiproliferative functions of P. Ovariectomized WT and PRBKO–/– mice were injected daily with either sesame alone or with E or E+P for 4 days and examined by histology (Fig. 1_D_). Comparison of uterine sections revealed similar E-dependent hyperplasia of the luminal epithelium that was completely inhibited by P. Thus, expression of the PR-A protein is sufficient to elicit the antiproliferative properties of P previously observed in WT mice. Our data also predict that a PR-A-selective progestin agonist should mimic the protective effects of P in the uterus while protecting against potential adverse proliferative effects of P that may be mediated through the PR-B protein (12).
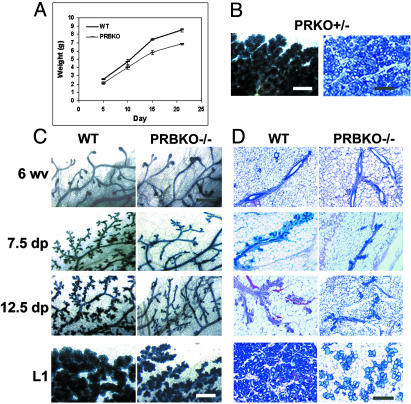
Diminished Pregnancy-Associated Ductal and Alveolar Morphogenesis in Mice Lacking the PR-B Isoform. PR-mediated P signaling is essential for tertiary sidebranching of the ductal epithelium and differentiation of alveolar lobules (2) and also plays a key role in mammary tumorigenesis (16). The ability of PRBKO–/– females to lactate indicated that the PR-A isoform is sufficient to mediate pregnancy-associated functional differentiation of the gland. However, analysis of offspring from PRBKO–/– mothers revealed a significant growth retardation relative to those nursed by WT mothers (Fig. 2_A_). The growth retardation was independent of offspring genotype and suggestive of lactational defects that were specific to the PRBKO–/– maternal genotype.
Fig. 2.
Impaired mammary gland development in mice lacking the PR-B isoform. (A) Neonatal growth curves of offspring from WT and PRBKO–/– mothers (n = 20). Error bars indicate SEM. (B_–_D) Whole-mount (C) and histological (D) analysis by hematoxylin/eosin staining of no. 4 abdominal glands of 6-week-old virgin (6 wv), 7.5 and 12.5 days pregnant (7.5 dp and 12.5 dp), and day 1 lactating (L1) WT and PRBKO–/– mice and PRKO+/– mice at L1 (B). (Scale bars: C, 500 μm; D, 100 μm.)
Both whole-mount analysis (Fig. 2_C_) and histological examination (Fig. 2_D_) of the developmental changes occurring in PRBKO–/– mammary glands throughout pregnancy revealed a marked reduction in both ductal sidebranching and development of alveolar lobules relative to WT glands. Although the virgin glands from both genotypes appeared indistinguishable after whole-mount staining, decreased pregnancy-associated morphogenesis was observed as early as 7.5 days pregnant and continued through midpregnancy (12.5 days pregnant) and end of pregnancy (day 1 lactating). Histology of the glands at day 1 lactating revealed a strikingly lower density of alveolar lobules than in corresponding WT glands (Fig. 2_D_). Despite their decreased density, however, these lobules did not appear underdeveloped. The decreased morphogenesis was specific to PRBKO–/– mice and was not due to decreased PR gene dosage, because PR knockout (PRKO+/–)mice with reduced PR gene dosage exhibited normal mammary gland development (Fig. 2_B_).
Morphogenic Defects in PRBKO Mice Are Intrinsic to the Mammary Gland. Previous studies have demonstrated that the mammary defects in PRKO–/– mice are intrinsic to the mammary gland and autonomous to the epithelium (17). These findings together with the normal female fertility in PRBKO–/– mice predicted that the defective morphogenesis during pregnancy is intrinsic to the mammary gland and is not due to abnormal systemic hormone status. Because both ovarian steroids and PRL are essential to support pregnancy-associated mammary gland morphogenesis, we next asked whether diminished serum PRL or E and P levels contribute to defective mammary gland morphogenesis in PRBKO mice. First, we compared serum PRL levels in virgin mice from each genotype and in pregnant mice from WT, PRBKO–/–, and PRKO+/– heterozygous mice. Comparison of basal PRL levels in virgin WT (10.8 ± 3 ng/ml), PRAKO–/– (10.0 ± 2 ng/ml), PRBKO–/– (10.6 ± 4 ng/ml), and PRKO–/– (17.5 ± 2 ng/ml) mice demonstrated similar levels in each genotype, with the exception of PRKO–/– mice in which serum PRL levels were moderately elevated, consistent with our previous findings (9). Importantly, comparison of serum PRL in WT (48.3 ± 1 ng/ml), PRBKO–/– (46.7 ± 11.5 ng/ml), and PRKO+/– mice (46.7 ± 10 ng/ml) at day 18 of pregnancy showed similar pregnancy-associated increases in PRL levels in all three genotypes.
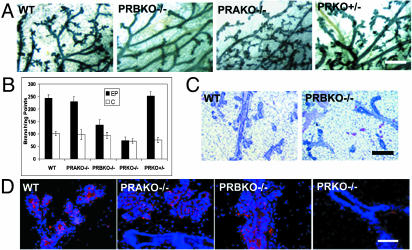
To determine whether diminished E and P levels may contribute to the phenotype, we next asked whether mammary glands from ovariectomized PRBKO–/– mice that were supplemented for 21 days with pregnancy levels of E+P displayed similar defects in mammary gland morphogenesis to those observed during pregnancy. Both whole-mount and histological comparison of WT and PRBKO–/– glands confirmed a marked decrease in ductal side-ranching and lobuloalveolar development in PRBKO–/– relative to WT glands (Fig. 3 A and_C_). The decrease was specific for PRBKO–/– glands and was not observed in PRKO+/– heterozygous glands with 50% reduced PR gene dosage or in PRAKO–/– glands (Fig. 3_A_). Quantitation of secondary and tertiary ductal branch points showed that their numbers were diminished markedly in PRBKO–/– relative to WT, PRAKO–/–, and PRKO+/– glands and only moderately increased relative to PRKO–/– mice lacking both PRs (Fig. 3_B_). However, immunohistochemical analysis with the alveolar differentiation marker, adipophilin, indicated that alveolar differentiation, although absent from PRKO–/– glands, did occur in PRAKO–/–, PRBKO–/–, and WT mice (Fig. 3_D_).
Fig. 3.
(A) Whole-mount and hematoxylin/eosin analyses of E+P-induced mammary gland morphogenesis in ovariectomized WT, PRBKO–/–, PRAKO–/–, and PRKO+/– mice. (B) Quantitation of tertiary branch points per unit area (seven to nine mice per genotype). (C) Histological staining of WT and PRBKO–/– glands. (D) Immunodetection of alveolar differentiation marker, adipophilin, with rabbit polyclonal IgG followed by Texas red-conjugated anti-rabbit IgG (red). Nuclei were counterstained with DAPI (blue). [Scale bars: A, 500 μm (whole mounts); C, 100 μm (histology); D,20 μm.]
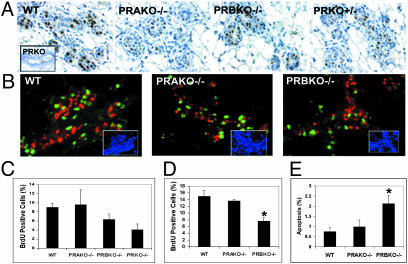
Reduced Mammary Gland Morphogenesis in PRBKO Mice Is Due to Distinct PR Isoform Expression Rather than Reduced PR Levels. PRs are expressed exclusively in the mammary epithelium in a pattern that is mostly segregated from proliferating cells (18,19) and function in a paracrine manner to regulate alveolar morphogenesis in PR-negative cells (17). To determine whether the distinct mammary gland phenotypes observed in E+P-treated PRAKO–/– and PRBKO–/– mice were due to differences in levels of PR isoforms, we compared the expression of PRs in E+P-treated ovariectomized WT, PRAKO–/–, PRBKO–/–, and PRKO+/– mice using immunohistochemistry. Because the level of expression of PRs in the mammary gland under these treatment conditions is below detection levels by Western analysis, we have chosen an immunohistochemical approach to localize and compare the levels of PR staining in each genotype at a single-cell level. The results of these analyses (Fig. 4_A_) showed a similar pattern of receptor expression in each genotype that was localized to a scattered subset of epithelial cells. Further, the specificity of antibody staining for PR was confirmed by its absence in PRKO–/– mammary epithelium (see Fig. 4_A_ Inset) and by its reduced level of expression in PRKO+/– mice with reduced_PR_ gene dosage. Significantly, we consistently observed a higher intensity of PR staining in PRBKO–/– relative to PRAKO–/– mice, consistent with previous reports that the levels of PR-A exceed those of PR-B in the virgin mouse gland (20). The observations that levels of PR-A seem to be higher than PR-B together with the lack of mammary gland defects observed in PRAKO–/– mice expressing only PR-B strongly support the conclusion that defective mammary gland development in PRBKO–/– mice is due to distinct PR isoform activity rather than reduced PR levels.
Fig. 4.
(A) Immunodetection of PRs in WT, PRAKO–/–, PRBKO–/–, and PRKO+/– glands treated with E+P. (Inset) PRKO–/– control. (B) Dual immunofluorescence staining with rabbit polyclonal antibody to PR and mouse monoclonal antibody to BrdUrd. Immunocomplexes were visualized with Texas red (PR, red) and Alexa Fluor 488 (BrdUrd, green). Nuclei were counterstained with DAPI (blue, Insets). (C and D) Cell proliferation in ductal (C) and alveolar (D) epithelium measured by BrdUrd immunohistochemistry. Five thousand cells per mouse were counted, and five mice per genotype were examined. (E) Apoptotic cells in alveolar epithelium determined by TUNEL assay. Ten thousand cells per mouse were counted, and 10 mice per genotype were examined. Statistically significant differences relative to WT are indicated by asterisks (P < 0.05). Error bars (C_–_E) indicate SEM.
Next we asked whether changes in the spatiotemporal expression of PR-A in PRBKO–/– mice may contribute to the mammary phenotype. Dual immunofluorescent staining for PR and BrdUrd (Fig. 4_B_) revealed that the PR expression pattern in each genotype retained its normal segregated relationship relative to proliferating cells.
Morphogenic Defects in PRBKO–/– Mice Are Associated with Decreased Ductal and Alveolar Epithelial Proliferation and Reduced Alveolar Epithelial Cell Survival. To determine whether the defects in PRBKO–/– mice were associated with changes in the level of proliferation or apoptosis in the glands, we examined the degrees of proliferation (Fig. 4 C and D) and apoptosis (Fig. 4_E_) in mammary epithelium of E+P-treated mice by quantitation of BrdUrd incorporation and TUNEL labeling, respectively. The proliferative and apoptotic indices were calculated as a percentage of BrdUrd- or TUNEL-positive cells in the ductal or alveolar regions per total number of epithelial cells. In the absence of PRs (PRKO–/–), the number of BrdUrd-positive cells was reduced by >50% (4.08 ± 1.26%) in the ductal epithelium (Fig. 4_C_), whereas a 30% decrease in proliferation was observed in PRBKO–/– (6.32 ± 1.23%) relative to WT (9.0 ± 0.86%) ductal epithelium. In contrast, the ductal epithelium of PRAKO–/– glands showed comparable numbers of proliferating cells (9.61 ± 3.26) relative to WT (Fig. 4_C_). The proliferative index of the alveolar epithelium (Fig. 4_D_) of PRBKO–/– mice (7.8 ± 0.98%) was also reduced markedly relative to WT (15.0 ± 1.61%) and PRAKO–/– (13.71 ± 0.26%) epithelium. Finally, a significant and selective increase in the number of apoptotic cells was observed in the alveolar epithelium of PRKBO–/– (2.13 ± 0.41%) relative to WT (0.74 ± 0.20%) and PRAKO–/– (0.98 ± 0.32%) epithelium (Fig. 4_E_). The ductal epithelium obtained from all genotypes lacked detectable TUNEL-positive cells (data not shown). Taken together, these data indicate that the PR-B protein is required to elicit normal ductal epithelial proliferation as well as proliferative expansion and survival of alveolar epithelial cells during P-induced mammary gland morphogenesis.
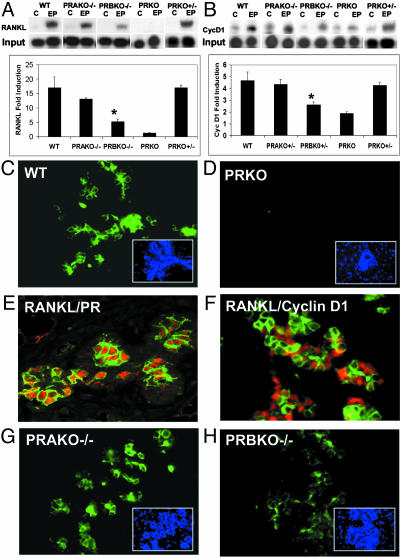
Mammary Gland Defects in PRBKO–/– Mice Are Associated with PR Isoform-Selective Regulation of RANKL Signaling Pathways. Recent studies have uncovered a critical role for RANKL signaling in the development of alveolar lobules during pregnancy (21). Interaction of RANKL with its mammary epithelial receptor RANK results in nuclear factor κB-dependent transcriptional up-regulation of cyclin D1 (22). Ablation of RANKL or components of this signaling pathway (21,22) result in defects in alveologenesis that are secondary to reduced proliferative expansion of alveolar buds and enhanced apoptosis of alveolar epithelial cells. The striking overlap between this phenotype and that observed in PRBKO–/– mice prompted us to determine whether PR-dependent alveologenesis is mediated through RANKL and whether the defects observed in PRBKO–/– mice may be associated with PR isoform-selective activation of RANKL signaling. The expression of RANKL mRNA transcripts and those of its downstream target, cyclin D1, were compared in WT, PRBKO–/–, PRAKO–/–, and PRKO–/– mice and PRKO+/– heterozygous ovariectomized mice treated with either control pellets or E+P for 21 days. Treatment of WT mice with E+P resulted in >16-fold up-regulation of RANKL relative to that observed in control mice (Fig. 5_A_). This up-regulation was clearly PR-dependent and inhibited in PRKO–/– mice. Remarkably, the induction of RANKL was also reduced dramatically in PRBKO–/– mice, consistent with the alveolar defects observed in these animals, but was not altered significantly in either PRKO+/– heterozygotes or PRAKO–/– mice. The changes observed in RANKL expression were also reflected in significantly reduced expression of its downstream target, cyclin D1, selectively in PRKO–/– and PRBKO–/– mice (Fig. 5_B_) but not in either PRKO+/– or PRAKO–/– mice. Taken together, these data support the conclusion that impaired activation of the RANKL signaling pathway in PRBKO–/– mice is a consequence of selective loss of expression of the PR-B isoform rather than a reduction in total PR levels.
Fig. 5.
Decreased activation of RANKL signaling in PRBKO–/– mice. Levels of mRNA transcript for RANKL (A) and cyclin D1 (B) in mammary gland of control and E+P-treated mice were analyzed by RPAs. Values are representative of five independent experiments. Error bars indicate SEM. Statistically significant differences observed when the PRBKO–/– group was compared with WT, PRAKO–/–, or PRKO+/– groups for both RANKL and cyclin D1 (P < 0.05) are indicated by asterisks. (C and_D_) Immunofluorescence staining for RANKL was performed in mammary tissues from E+P-treated WT (C) and PRKO (D) mice. (E and F) Double immunostaining for RANKL and PR (E) and RANKL and cyclin D1 (F) in mammary epithelial cells. Positive cells were visualized with Texas red (PR and cyclin D1, red) and Alexa Fluor 488 (RANKL, green). (G and H) Immunofluorescence staining for RANKL was performed in mammary tissues from E+P-treated PRAKO–/– (G) and PRBKO–/– (H) mice. (C, D, G, and H Insets) Nuclei of the same field counterstained with DAPI.
To confirm dependence of RANKL expression on PR and to determine whether RANKL may be a paracrine mediator of PR-dependent alveologenesis, we examined its expression relative to PRs in the mammary epithelium from E+P-treated WT and PRKO–/– mice using immunofluorescence assays and 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (Fig. 5_C_–E). Comparison of RANKL and DAPI staining in WT mice showed that a subset of mammary epithelial cells expresses RANKL protein (Fig. 5_C_). This expression depended on PRs as demonstrated by its absence in PRKO mice (Fig. 5_D_). Further, comparison of the expression pattern with that of PRs (Fig. 5_E_) demonstrated that RANKL is localized to PR-positive epithelial cells and supports a direct role of PRs in induction of RANKL expression in these cells. In contrast, the expression of its downstream target, cyclin D1, is observed in segregated but neighboring cells relative to RANKL (Fig. 5_F_). These data clearly demonstrate that the expression of cyclin D1 in the normal mammary gland, although PR-dependent, is not directly regulated by P in PR-positive cells. Next we examined the expression of RANKL in E+P-treated PRAKO–/– (Fig. 5_G_) and PRBKO–/– (Fig. 5_H_) mice. Although the expression of RANKL in PRAKO–/– epithelium was comparable to that observed in WT (Fig. 5_C_) mice, its level was reduced significantly in PRBKO–/– epithelium (Fig. 5_H_). Taken together, our data suggest that RANKL may be a key mediator of the paracrine signaling pathways that lead to P-dependent cyclin D1 activation and alveologenesis, that induction of RANKL is PR isoform-selective, and that PR-B is the primary contributor to this response.
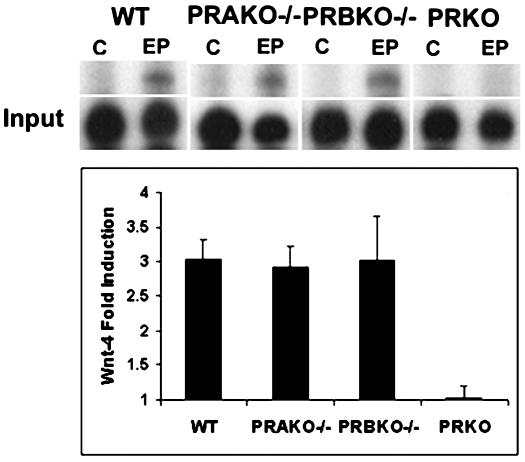
The early onset of developmental defects in the mammary glands of PRBKO–/– mice during pregnancy (7.5 days pregnant) together with the observed decrease in ductal branching and proliferation indicate that diminished RANKL-dependent signaling alone is insufficient to account for all the observed defects in these glands. We therefore analyzed the expression of wnt-4, a potential mediator of P-dependent ductal branching (23), to determine whether its regulation was affected by the absence of PR-B. Surprisingly, induction of wnt-4 transcripts was similar in WT, PRAKO–/–, and PRBKO–/– mammary glands despite its inhibition in PRKO–/– mice (Fig. 6). Thus, PR-A retains the ability to mediate P-dependent regulation of wnt-4, and alternative as-yet-unidentified PR-B-dependent signaling pathways are likely to contribute to ductal morphogenesis in the WT mammary epithelium. Similarly, we found that P-dependent induction of PRL receptors is not diminished in PRBKO–/– mice (data not shown). These results emphasize the signaling pathway selectivity of PR-A isoform activity in the mammary gland.
Fig. 6.
RPA analysis of wnt-4 expression in E+P-treated glands of WT, PRAKO–/–, PRBKO–/–, and PRKO–/– mice relative to L32 control (C). Values are representative of five independent experiments. Error bars indicate SEM.
Discussion
Using a mouse model in which expression of the PR-B isoform is selectively ablated, we have demonstrated that PR-A and PR-B mediate mostly distinct but partially overlapping responses to P in the female reproductive tract and mammary gland. Analysis of the reproductive phenotypes of PRBKO–/– mice indicates that selective activation of PR-A in the absence of PR-B is sufficient to elicit normal ovarian and uterine responses to P but results in reduced pregnancy-associated mammary gland morphogenesis. The activities of PR-A observed in these mice are strikingly different from those previously observed after selective activation of PR-B in PRAKO–/– mice (12). In the latter case, PR-B activation was sufficient to elicit normal mammary gland responses to P but failed to induce ovulatory or uterine implantation responses to the hormone. The PR-B-independent reproductive activities of the PR-A isoform observed in this study reveal a surprising lack of requirement for PR heterodimer activity in mediating most of the reproductive activities of P despite the apparent coexpression of both isoforms in cells of the reproductive tract (10).
We have demonstrated that selective activation of PR-A in the mammary glands of PRBKO–/– mice results in impaired P-dependent ductal branching and alveolar morphogenesis, a defect that is a consequence of reduced PR-dependent ductal and alveolar epithelial proliferation and decreased alveolar epithelial cell survival. Second, we have shown that this phenotype is PR-A isoform-specific rather than a consequence of reduced total PR levels. The phenotype is not observed after reduction of total PR levels in PRKO+/– mice or after selective activation of PR-B in PRAKO–/– mice. Despite lower levels of expression of PR-B in mammary glands of PRAKO–/– mice relative to PR-A in PRBKO–/– mammary glands, PRAKO–/– mice exhibit normal PR-dependent mammary morphogenesis that is indistinguishable from WT mice. Third, we have shown that the mammary gland defects in PRBKO–/– mice are intrinsic to the mammary gland and are not due to decreased systemic PRL or steroid hormones required for this response. Fourth, we have demonstrated that the defects are associated with distinct PR isoform-dependent activation of P-responsive signaling pathways rather than reduced or abnormal spatiotemporal expression of the PR-A isoform. Although mammary glands of both PRBKO–/– and PRAKO–/– mice exhibit normal PR-dependent up-regulation of wnt4, P-dependent activation of RANKL signaling pathways is selectively impaired in PRBKO–/– mammary glands.
The activation of both RANKL and cyclin D1 is essential for alveologenesis during pregnancy, and the combined action of both PRL and P is required for this morphogenic response. Although expression of cyclin D1 is induced by PRL in addition to P (24), the participation of RANKL activation in mediating cyclin D1 induction is specific to P, because RANKL expression was shown recently to be unaffected in mammary epithelial cells by PRL signaling (24). Our data clearly demonstrate that expression of cyclin D1 in mammary epithelium is segregated from PR/RANKL-positive cells but induced by PRs in a PR isoform-selective manner via a paracrine regulatory mechanism involving PR-B-dependent induction of RANKL. This paracrine mechanism and PR isoform selectivity of regulation of cyclin D1 in the normal mammary gland is clearly at odds with that observed in breast cancer cells in which P-dependent up-regulation of cyclin D1 has been observed in response to both PR isoforms in PR-positive cells (25). Because both PRs and cyclin D1 play a critical role in mammary tumorigenesis (16,26) and overexpression of cyclin D1 is associated with breast tumors (27), the disruption of normal paracrine regulation of cyclin D1 by PRs may represent one mechanism by which PRs can contribute to cyclin D1 overexpression in breast tumor cells.
The mammary gland defects in PRBKO–/– mice do not phenocopy the responses previously observed when disruption of PR isoform ratios was achieved by transgenic expression of PR-A in mice by using the cytomegalovirus promoter (28). As a consequence of PR-A overexpression, mammary glands exhibited increased ductal branching and hyperplasia (28). Given the segregated expression pattern of PR isoforms relative to proliferating cells in the normal gland, the striking differences in defects observed in PR-A transgenic relative to PRBKO–/– mice could be explained by inappropriate targeting in transgenic mice of PR-A expression to epithelial subtypes that normally would not express PR but may be competent to proliferate. Such targeting would breach the cellular segregation rules that apply to normal P-dependent epithelial cell growth, resulting in a scenario reminiscent of the inappropriate colocalization of steroid receptor expression and proliferation observed in mammary glands that have been exposed to carcinogen (19) and in cells of breast tumors (11). Thus, disruption of the normal spatiotemporal expression pattern of PR-A may lead to aberrant regulation of proliferative target genes in the mammary gland.
Finally, our observations that the tissue-selective functions of PR-A and PR-B are mostly distinct may have important clinical implications with regard to the development of improved tissue-selective progestins for reproductive management and hormone-replacement therapy. The ability of PR-A to inhibit hyperplasia of the uterine epithelium together with the decreased proliferative activity of the mammary gland in response to PR-A indicate that selective activation of PR-A by using isoform-selective agonists is likely to have a protective effect against both uterine and mammary gland hyperplasias.
Acknowledgments
We thank L. H. Nguyen, R. A. Mullinax, and Dr. D. Stenoien for technical assistance and Drs. D. Medina and J. M. Rosen for helpful discussions. This work was supported by National Institutes of Health Grant HD32007 (to O.M.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: P, progesterone; PR, P receptor; PRAKO, PR-A knockout; PRBKO, PR-B knockout; RANKL, receptor activator of nuclear factor κB ligand; E, estrogen; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP end labeling; RPA, RNase protection assay; PRL, prolactin; PRKO, PR knockout; DAPI, 4′,6-diamidino-2-phenylindole.
References
- 1.Conneely, O. M., Mulac-Jericevic, B., DeMayo, F., Lydon, J. P. & O'Malley, B. W. (2002) Recent Prog. Horm. Res. 57**,** 339–355. [DOI] [PubMed] [Google Scholar]
- 2.Lydon, J. P., DeMayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A., Jr., Shyamala, G., Conneely, O. M. & O'Malley, B. W. (1995) Genes Dev. 9**,** 2266–2278. [DOI] [PubMed] [Google Scholar]
- 3.Conneely, O. M., Kettelberger, D. M., Tsai, M.-J., Schrader, W. T. & O'Malley, B. W. (1989) J. Biol. Chem. 264**,** 14062–14064. [PubMed] [Google Scholar]
- 4.Kastner, P., Krust, A., Turcotte, B., Strupp, U., Tora, L., Gronemeyer, H. & Chambon, P. (1990) EMBO J. 9**,** 1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giangrande, P. H., Kimbrel, E. A., Edwards, D. P. & McDonnell, D. P. (2000) Mol. Cell. Biol. 20**,** 3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tora, L., Gronemeyer, H., Turcotte, B., Gaub, M. P. & Chambon, P. (1988) Nature 333**,** 185–188. [DOI] [PubMed] [Google Scholar]
- 7.Hovland, A. R., Powell, R. L., Takimoto, G. S., Tung, L. & Horwitz, K. B. (1998) J. Biol. Chem. 273**,** 5455–5460. [DOI] [PubMed] [Google Scholar]
- 8.Giangrande, P. H. & McDonnell, D. P. (1999) Recent Prog. Horm. Res. 54**,** 291–313. [PubMed] [Google Scholar]
- 9.Chappell, P. E., Lydon, J. P., Conneely, O. M., O'Malley, B. W. & Levine, J. E. (1997) Endocrinology 138**,** 4147–4152. [DOI] [PubMed] [Google Scholar]
- 10.Graham, J. D. & Clarke, C. L. (2002) Breast Cancer Res. 4**,** 187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham, J. D. & Clarke, C. L. (1997) Endocr. Rev. 18**,** 502–519. [DOI] [PubMed] [Google Scholar]
- 12.Mulac-Jericevic, B., Mullinax, R. A., DeMayo, F. J., Lydon, J. P. & Conneely, O. M. (2000) Science 289**,** 1751–1754. [DOI] [PubMed] [Google Scholar]
- 13.Katoh, A. K., Stemmler, N., Specht, S. & D'Amico, F. (1997) Biotech. Histochem. 72**,** 291–298. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys, R. C., Krajewska, M., Krnacik, S., Jaeger, R., Weiher, H., Krajewski, S., Reed, J. C. & Rosen, J. M. (1996) Development (Cambridge, U.K.) 122**,** 4013–4022. [DOI] [PubMed] [Google Scholar]
- 15.Fantl, V., Edwards, P. A. W., Steel, J. H., Vonderhaar, B. K. & Dickson, C. (1999) Dev. Biol. 212**,** 1–11 [DOI] [PubMed] [Google Scholar]
- 16.Lydon, J. P., Ge, G., Kittrell, F. S., Medina, D. & O'Malley, B. W. (1999) Cancer Res. 59**,** 4276–4284. [PubMed] [Google Scholar]
- 17.Brisken, C., Park, S., Vass, T., Lydon, J. P., O'Malley, B. W. & Weinberg, R. A. (1998) Proc. Natl. Acad. Sci. USA 95**,** 5076–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seagroves, T. N., Lydon, J. P., Hovey, R. C., Vonderhaar, B. K. & Rosen, J. M. (2000) Mol. Endocrinol. 14**,** 359–368. [DOI] [PubMed] [Google Scholar]
- 19.Sivaraman, L., Hilsenbeck, S. G., Zhong, L., Gay, J., Conneely, O. M., Medina, D. & O'Malley, B. W. (2001) J. Endocrinol. 171**,** 75–83. [DOI] [PubMed] [Google Scholar]
- 20.Shyamala, G., Schneider, W. & Schott, D. (1990) Endocrinology 126**,** 2882–2889. [DOI] [PubMed] [Google Scholar]
- 21.Fata, J. E., Kong, Y. Y., Li, J., Sasaki, T., Irie-Sasaki, J., Moorehead, R. A., Elliott, R., Scully, S., Voura, E. B., Lacey, D. L., et al. (2000) Cell 103**,** 41–50. [DOI] [PubMed] [Google Scholar]
- 22.Cao, Y., Bonizzi, G., Seagroves, T. N., Greten, F. R., Johnson, R., Schmidt, E. V. & Karin, M. (2001) Cell 107**,** 763–775. [DOI] [PubMed] [Google Scholar]
- 23.Brisken, C., Heineman, A., Chavarria, T., Elenbaas, B., Tan, J., Dey, S. K., McMahon, J. A., McMahon, A. P. & Weinberg, R. A. (2000) Genes Dev. 14**,** 650–654. [PMC free article] [PubMed] [Google Scholar]
- 24.Brisken, C., Ayyannan, A., Nguyen, C., Heineman, A., Reinhardt, F., Jan, T., Dey, S. K., Dotto, G. P. & Weinberg, R. A. (2002) Dev. Cell 3**,** 877–887. [DOI] [PubMed] [Google Scholar]
- 25.Richer, J. K., Jacobsen, B. M., Manning, N. G., Abel, M. G., Wolf, D. M. & Horwitz, K. B. (2002) J. Biol. Chem. 277**,** 5209–5218. [DOI] [PubMed] [Google Scholar]
- 26.Yu, Q., Geng, Y. & Sicinski, P. (2001) Nature 411**,** 1017–1021. [DOI] [PubMed] [Google Scholar]
- 27.Weinstat-Saslow, D., Merino, M. J., Manrow, R. E., Lawrence, J. A., Bluth, R. F., Wittenbel, K. D., Simpson, J. F., Page, D. L. & Steeg, P. S. (1995) Nat. Med. 1**,** 1257–1260. [DOI] [PubMed] [Google Scholar]
- 28.Shyamala, G., Yang, X., Silberstein, G., Barcellos-Hoff, M. H. & Dale, E. (1998) Proc. Natl. Acad. Sci. USA 95**,** 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]