Comprehensive Structure-Function Analysis of the Core Domain of Human Telomerase RNA (original) (raw)
Abstract
Telomerase is a cellular reverse transcriptase that uses part of its integral RNA (called TER) as the template to synthesize telomeric DNA repeats. Vertebrate TERs are thought to share a conserved, highly structured core domain that includes the templating sequence and a pseudoknot, but not all features of the predicted core structure have been verified directly or shown to affect telomerase enzymatic activity. Here, we report a systematic mutational analysis of the core domain (residues 1 to 210) of human telomerase RNA (hTER). Our data confirm that optimal hTER activity requires the integrity of four short helices (P2a.1, P2a, P2b, and P3) which create the proposed pseudoknot and that features of both the primary sequence and secondary structure in P2b and P3 contribute to optimal function. At least part of the long-range P1 pairing is also required, despite the lack of a known P1 counterpart in rodent TERs. Among the predicted single-stranded regions, we found that J2b/3, portions of J2a/3, and residues in and around the template make sequence-specific contributions to telomerase function. Additionally, we provide evidence that naturally occurring hTER sequence polymorphisms found in some patients with aplastic anemia can inhibit telomerase activity by disrupting critical structures within the hTER core domain.
The conventional DNA replication machinery of the cell is predicted to be unable to replicate the extreme 3′ ends of linear chromosomes, which would result in chromosomal shortening at each round of cell division (21, 28). To circumvent this problem, eukaryotic cells possess an additional DNA polymerase complex, called telomerase, which adds tandem repeats of a short telomeric DNA repeat unit sequence to the chromosomal 3′ termini (reviewed in reference 7). While telomerase activity is virtually undetectable in most adult human somatic cells, this activity is upregulated in stem cells and mitogen-stimulated mature T and B cells of the immune system (16). Telomerase is also activated in the majority (>85%) of human cancers (24).
The telomerase holoenzyme is a ribonucleoprotein (RNP) complex with two core components: a protein (called TERT) with RNA-dependent DNA polymerase (i.e., reverse transcriptase) catalytic activity and an associated RNA called TER (reviewed in reference 7). During telomere synthesis, a short portion of TER, called the templating sequence, is used by the TERT protein for copying into telomeric DNA repeats (14). Vertebrate TERs are roughly 400 to 500 bases long, and their sequences differ among species, but phylogenetic comparisons suggest that they share a highly conserved secondary structure (9). This proposed structure was deduced by sequence covariation analysis and is viewed as comprising four conformational domains called the core (or pseudoknot), CR4-CR5, box H/ACA, and CR7 domains, respectively. Though experimental evidence indicates that all four domains contribute to telomerase function in vivo (19), human telomerase catalytic activity in vitro requires only the core and CR4-CR5 domains, each of which can bind independently to the TERT protein (20).
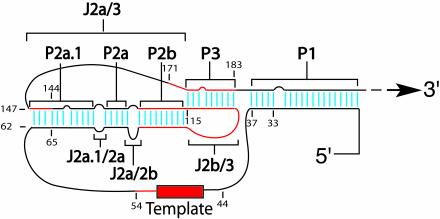
The 210-base core domain of human TER (hTER) corresponds roughly to the 5′ half of the RNA molecule and thus includes the 11-base templating sequence (Fig. 1A) (10). The deduced vertebrate consensus structure for this core domain (9) encompasses five short, helically paired (P) regions designated P1, P2a.1, P2a, P2b, and P3, as well as multiple single-stranded junctional (J) regions. Three of the paired sequences (P2a.1, P2a, and P2b) together form the stem of a hairpin, a portion of whose loop can base pair with sequences downstream to form the P3 helix, creating a potential pseudoknot adjacent to the templating sequence. In both human and murine TERs, mutations predicted to disrupt P3 base pairing reduce or abolish telomerase activity, whereas compensatory mutations generally restore it, providing evidence that the pseudoknot forms and that it is important for TER function (2, 10, 18, 19). However, chemical and enzymatic accessibility mapping (1) and biophysical studies (12, 26) suggest that the P3 region may also adopt alternative conformations. By contrast, accessibility mapping has in general supported most of the other predicted structures within the hTER core, including the four remaining helices and the single-stranded J regions that separate them. The templating sequence, in particular, appears single-stranded by criteria of accessibility (1).
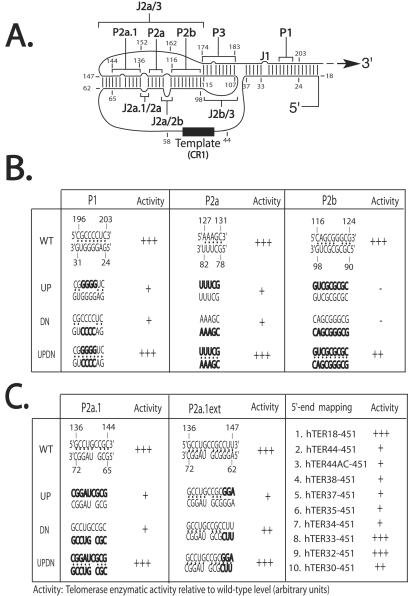
FIG. 1.
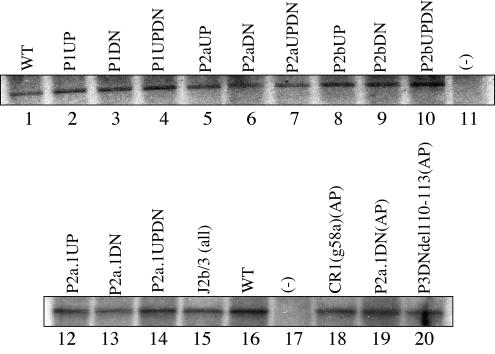
(A) Schematic view of the consensus secondary structure of the core 210-nucleotide domain of hTER RNA as proposed by Chen et al. (9). The putative P and J regions are indicated by brackets, and the templating sequence is shown as a solid rectangle. Residues are numbered with respect to the transcriptional start site of the hTER gene. (B and C) Summary results of semiquantitative (TRAP) analyses of telomerase enzymatic activity in reconstituted VA13 cells for various mutations (indicated in boldface) targeting individual paired regions P1, P2, P2a, P2b, P2a.1 and P2a.1ext (B) and sequential deletions of the 5′ terminus (C). The telomerase enzymatic activity of each mutant is expressed in comparison to that of the wild type (+++, 20 to 100%; ++, 2 to 20%; +, 1 to 2%; −, undetectable), based on at least two or three independent determinations that each involved at least three sequential dilutions of extract from the transfected cells.
Apart from the template and the P3 helix, most proposed substructures in the hTER core have not yet been subjected to extensive mutagenesis, so genetic evidence for their existence is lacking and their functional significance remains unproven. We therefore undertook a comprehensive structure-function analysis using targeted point and compensatory mutations to map critical sequences and substructures within the core domain. With this collection of defined hTER mutants, we also attempted to map features required for hTERT binding to the hTER core in vitro. In the process, we have tested for the first time the functional significance of certain naturally occurring hTER sequence polymorphisms that have been associated with aplastic anemia, a human degenerative bone marrow disorder (27). Our results verify both the general validity of the predicted hTER structure and its relevance to human disease.
MATERIALS AND METHODS
Expression constructs.
Sequences encoding hTERT protein with a FLAG epitope appended to its N terminus were cloned into the eukaryotic expression plasmid pcI-Neo (Promega) under the control of a cytomegalovirus immediate-early promoter, creating pcI-FLAG-hTERT. pcDNA3-hTER, which expresses full-length hTER using a cytomegalovirus immediate-early promoter, was previously described (18); mutations were introduced into this plasmid using the Quik Change site-directed mutagenesis kit (Stratagene) and verified by DNA sequencing.
In vivo reconstitution of telomerase activity.
The VA13 cell line (American Type Culture Collection), a human lung fibroblast line transformed by simian virus 40 large T antigen that expresses neither the hTERT nor the hTER component of the human telomerase complex (8), was maintained in Dulbecco's modified Eagle's medium with 4.5 g of glucose/liter and 10% (vol/vol) bovine calf serum. Wild-type or mutant pcDNA3-hTER DNAs (6 μg) were cotransfected with pcI-hTERT-FLAG (6 μg) into VA13 cells (at ∼60% confluency) in a 100-mm-diameter polystyrene dish using SuperFect transfection reagent (Qiagen). Transfection efficiency was monitored by visual scoring of green fluorescent protein expression in parallel transfections of the vector pEGFP (Stratagene) alone. Approximately 48 h after transfection, the cells were scraped from the dish into 3 ml of cold phosphate-buffered saline, pelleted by centrifugation, and then lysed by resuspension in CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} lysis buffer (Intergen). Telomerase activity was assayed using lysate from 2 × 104 cells in the TRAPeze Telomerase Detection kit (Intergen) according to the manufacturer's directions, except that the PCR was done using preincubation at 95°C for 2 min, followed by 25 cycles of 94°C for 10 s, 50°C for 30 s, and 72°C for 30 s, and then by a final incubation at 72°C for 5 min. Reaction products comprising telomeric DNA repeats were then separated on a 12% polyacrylamide gel and quantified by phosphorimaging (Molecular Dynamics).
Northern blotting analysis.
Wild-type or mutant pcDNA3-hTER (6 μg) was cotransfected together with pcI-hTERT-FLAG (6 μg) into VA13 cells as described above. Approximately 48 h after transfection, total cellular RNA was extracted using Trizol reagent (Invitrogen). hTER expression was then assayed by Northern blotting using a riboprobe complementary to the full-length hTER coding sequence, transcribed from pcDNA3-hTER with SP6 RNA polymerase (Promega). After the phosphorimaging, the blot was stripped of hTER riboprobe by overnight incubation at 75°C in 10 mM Tris- 0.2% sodium dodecyl sulfate and then rehybridized with an antisense riboprobe specific for β-actin mRNA as an internal control.
Immunoprecipitation-Northern blotting analysis.
FLAG-tagged hTERT protein was expressed in vitro from the pcI-FLAG-hTERT vector (see above) using the TnT quick-coupled transcription-translation system (Promega) in the presence of 200 ng of in vitro-transcribed, gel-purified hTER core 210-nucleotide fragment at 37°C for 2 h. The assembled telomerase complex was affinity enriched on anti-FLAG agarose beads (Sigma). To detect hTERT-bound telomerase RNAs, Northern blotting was performed on the immunoprecipitated telomerase preparations as follows. First, the telomerase complex was washed extensively (four or five times) with 1× CHAPS buffer (Intergen). The immunoprecipitated hTER was extracted with acid phenol-chloroform at 50°C for 5 min, followed by another extraction at room temperature. The extracted RNA was ethanol precipitated and resuspended in 15 μl of diethylpyrocarbonate-treated water; 37.5 μl of an RNA denaturation cocktail (0.27% glyoxal, 1.4% dimethyl sulfoxide, 0.03% NaPO4) was then added to each RNA sample, and the mixture was incubated at 50°C for 1 h. RNA loading buffer (20% sucrose, 25 mM NaPO4, 0.1% xylene cyanol, and 0.1% bromphenol blue) was added to each sample before loading it into a 1.5% agarose gel. The samples were subjected to electrophoresis at 100 V in 10 mM NaPO4 buffer for 3 to 4 h. RNA was then transferred and UV cross-linked onto nitrocellulose membranes (Schleicher & Schuell). hTER probes were generated using the Random Prime Probe system (Gibco-BRL) and were hybridized to the membrane at 65°C overnight. The membrane was washed with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate at 65°C for 15 min and exposed directly on a PhosphorImager screen.
RESULTS
We carried out scanning mutagenesis of the core domain by introducing defined substitutions or deletions into a eukaryotic expression plasmid encoding full-length (451-base) hTER. Each mutation targeted 2 to 9 bases (usually consecutive) within a single P or J region of the structure as proposed (9). Substitution mutations typically involved replacing bases with their complements and were named to indicate the region targeted and, in the case of P regions, whether the mutation altered the upper (up) or lower (dn) strand of the helix, as depicted in Fig. 1A and elsewhere. As the up and dn mutations in a given P region were usually complementary, we also combined them in cis to create a compensatory mutant (updn) designed to restore a putative helix while altering its sequence.
The biological effects of these mutations were determined by cotransfecting each mutant hTER vector into human VA13 cells along with a second vector that encoded a FLAG-tagged version of the hTERT protein. VA13 cells lack endogenous hTER and hTERT but can assemble active telomerase when both components are provided exogenously (5, 18, 29). Approximately 48 h after transfection, the cells were lysed, and the telomerase catalytic activity of the lysates was assayed semiquantitatively using a PCR-based telomeric repeat amplification protocol (TRAP) assay, which measured the ability to add telomeric DNA repeats to a synthetic DNA primer. The steady-state levels of informative hTER mutants in the lysates of the transfected cells were verified by Northern blotting analysis.
Requirement for partial helical character in the P1 subregion.
We began by examining whether the P1 helix, which is absent from rodent TER but conserved among other vertebrates, is important for the function of hTER. In the human RNA, P1 is a potential bulged 19-bp helix; our substitution mutations targeted a 4-bp cluster near its center, as indicated in Fig. 1A and B. We found that the P1up and P1dn mutations, each designed to disrupt this short portion of the helix, significantly reduced telomerase enzymatic activity when tested individually (Fig. 1B, left, and 2A, lanes 23 to 28). Combining these in a compensatory (updn) mutant fully restored activity (Fig. 2A, lanes 29 to 31), suggesting that some portion of the P1 helix forms as predicted and contributes to hTER function. Because rodent TERs lack any counterparts to residues 1 to 42 of hTER, including the entire lower strand of P1, and have instead only two apparently unpaired bases upstream of the template sequence, we next asked what minimal 5′ sequences are required for hTER activity. As summarized in Fig. 1C, right, activity as measured by the TRAP assay was not affected when the first 17, presumably single-stranded residues were deleted (hTER18-451) but was dramatically reduced when all 43 residues upstream of the template were removed (hTER44-451), in agreement with previous reports (2, 3, 25, 29). Activity was not detectably restored when we replaced those 43 residues with the dinucleotide AC to mimic the 5 ′ ends of murine TERs (Fig. 1C, line 3). As these results implied that features critical for hTER function mapped within positions 18 to 44, we then tested the effects of progressive truncations through this range. Whereas deleting residues 1 to 31 had minimal effect, deletions beyond residue 33 substantially reduced telomerase activity, again consistent with previous reports (2, 3, 25, 29) (Fig. 1C, lines 4 to 10, and 2B, lanes 11 to 18). With reference to the proposed structure, this suggests that at least four paired bases in the P1 region most proximal to the template might be required to create a minimal helix that is essential in hTER but not in murine TERs.
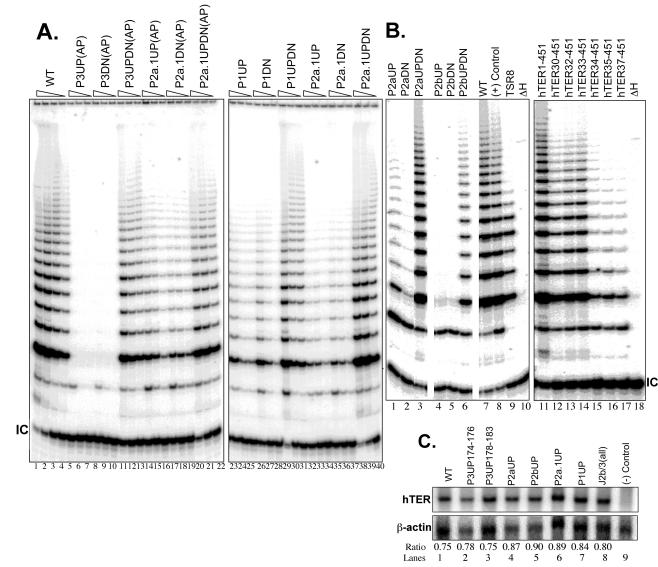
FIG. 2.
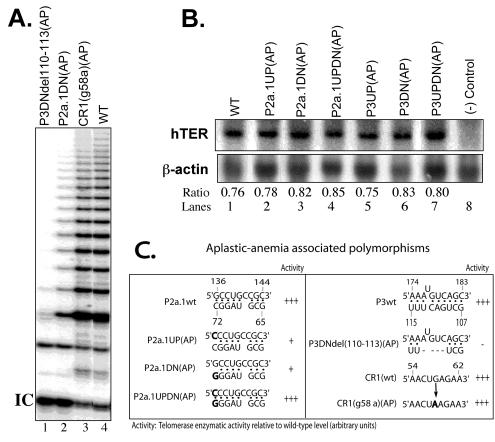
Representative TRAP gels illustrating the relative telomerase activities obtained from representative substitution and compensatory mutations in the hTER core domain. The triangles above the lanes indicate serial fivefold dilutions of the cell lysates, with the first lanes of each sample showing telomerase enzymatic activities of approximately equal concentrations of extract made from 2 × 104 cells. WT, wild-type; IC, internal controls for normalization of PCRs. (A) Lanes 5 to 22, aplastic-anemia (AP)-associated polymorphisms in the P3 and P2a.1 stems. (B) Lanes 11 to 18, 5′ deletion mapping of hTER RNA; lanes 8, 9, and 10, control 293T cell lysate, standard telomeric repeats amplified from control TSR8 DNA templates, and heat-inactivated wild-type cell lysate, respectively. All lanes are from the same gel and autoradiogram. (C) Northern blotting analysis of informative hTERs expressed in transfected VA13 cell extracts. The level of hTER RNA was quantified by PhosphorImager image analysis software and is expressed as a ratio of hTER RNA to cellular β-actin mRNA. Lane 9, negative control cell lysate transfected only with hTERT expression construct.
Requirement for all three helical segments of the pseudoknot stem.
One prominent feature of the hTER core domain is the proposed stem-loop, whose stem is composed of helices P2a.1, P2a, and P2b and whose loop forms part of the P3 helix that creates the pseudoknot fold. While published genetic evidence supports the existence of the P3 helix, the P2a.1, P2a, and P2b structures have not previously been verified through targeted mutagenesis. Using the approach outlined above, we individually mutated the upper and lower strands in the P2a and P2b helices (Fig. 1B, center and right) and found that disrupting either helix reduced or completely abolished hTER function (Fig. 2B). Interestingly, whereas restoration of P2a by compensatory mutation fully restored function, only partial activity was regained in the P2b compensatory mutant (Fig. 2B, lanes 1 to 6), suggesting that whereas the primary sequence in the P2a region may be inconsequential, both the sequence and structure of P2b contribute to telomerase function.
Like P1, the putative stem helix P2a.1 is not universally conserved among vertebrate TERs. Instead, this third helical segment has been observed only among mammalian TERs, whose stem is correspondingly longer than those from other vertebrates. Even among mammals, phylogenetic conservation of P2a.1 appears less stringent than that of P2a or P2b. Nevertheless, we found that substitution mutations expected to prevent formation of this stem also abolished hTER function and that function could be restored fully through compensatory mutation (Fig. 1C, left). This implies that the P2a.1 stem indeed forms as predicted in hTER and that its structure, but not its primary sequence, is critical for biological activity. In addition, because recent evidence suggests that the P2a.1 stem might be somewhat longer than originally predicted by phylogenetic analysis (1, 9), we designed a pair of mutants (designated P2a.1ext-up and P2a.1ext-dn) targeting nucleotides 145 to 147 and 62 to 64, respectively, and tested for telomerase enzymatic activity. As summarized in Fig. 1C (center), these mutations individually reduced telomerase activity to various degrees. This activity was, however, fully restored in the compensatory mutant P2a.1ext-updn, providing further evidence that the P2a.1 helix is more extensive than previously predicted.
To determine whether the functional changes described above might be due to differences in the stabilities of the hTER mutants, we probed for hTER expression by Northern blotting in lysates of the transfected VA13 cells. As shown in Fig. 2C (lanes 4 to 7), we found no evidence that any informative P1, P2a.1, P2a, or P2b mutation appreciably altered the steady-state level of exogenous hTER expression in these cells.
Evidence for essential sequence and structure in the P3 helix.
The P3 helix in hTER is proposed to form by pairing between residues 107 to 115 and 174 to 183, creating a 9-bp helix with a single unpaired (bulged) U residue at position 177 in the upper strand (Fig. 3, top). The primary sequences of the upper and lower P3 strands have been highly conserved during vertebrate evolution, and although variant bases have become fixed at a few positions in some species, complementarity has been preserved. The existence of P3 is supported by the results of systematic mutagenesis in TER from the mouse and limited mutagenesis in hTER (2, 12, 18, 19), but chemical and enzymatic accessibility mapping has failed to confirm stable P3 base pairing in the human RNA either in vitro or in vivo (1). To address this disparity, we carried out an extensive mutational analysis of the P3 region of hTER, searching for features of structure or sequence that contributed to catalytic activity.
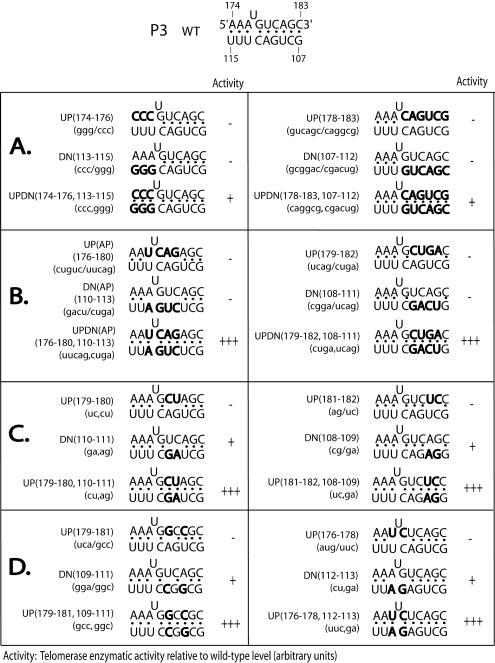
FIG. 3.
Summary of semiquantitative TRAP analyses for mutations in the P3 stem. The introduced mutations are highlighted in boldface. The telomerase enzymatic activity of each mutant relative to that of the wild-type hTER is indicated as described in the legend to Fig. 1. (A) Three- and 6-nucleotide substitutions near the 5′ and 3′ termini of the P3 stem. (B) Four-nucleotide substitutions in the center of the P3 stem. (C and D) Dinucleotide substitutions throughout the P3 stem.
As previously described, simultaneously mutating all residues in either the upper or lower P3 strand completely abolished hTER function (18). In the present study, we introduced a series of shorter point mutations and found that activity was likewise markedly impaired when as few as 3 or 4 bases were changed in either strand and at either end of the helix (Fig. 3A and B). Surprisingly, while compensatory mutations of the 4-base substitutions completely restored activity (Fig. 3B), compensation of 3-base mutations located at residues 113 to 115 and 174 to 176 only minimally restored the wild-type activity (Fig. 3A, left), suggesting that both the helical structure of P3 and the specific identities of certain residues within it might be functionally important. To explore this possibility further, we scanned across the P3 region with a series of 2-base substitutions and found that while even these relatively small mutations adversely affected activity, each could be fully restored by compensatory mutations (Fig. 3C and D). Curiously, we consistently observed that mutations in the lower strand of P3 had lesser effects than comparable mutations in the upper strand. Northern blotting analysis of the transfected cells confirmed that the functional effects we did observe were not due to differences in the steady-state level or stability among the hTER mutants (Fig. 2C, lanes 2 and 3).
Essential sequences in the J regions.
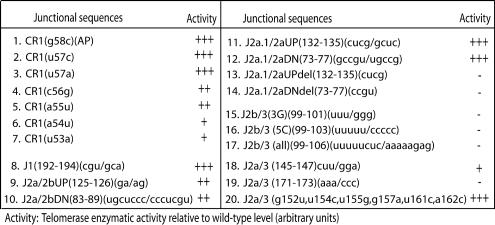
Five proposed single-stranded regions of variable length that separate helical regions of the core domain have been designated J regions (Fig. 1A). In our series of mutants, deletion of entire individual J regions reduced telomerase activity, presumably by causing nonspecific distortions of local conformation (e.g., Fig. 4, lines 13 and 14). In contrast, substitution mutations within most J regions produced little or no functional deficit (Fig. 4, lines 8 to 12). The exceptions were the J2a/3, J2b/3, and templating (CR1) regions. Replacing as few as 3 of the 8 bases of J2b/3 (residues 99 to 106) completely abolished hTER function (Fig. 4, lines 15 to 17), consistent with recent nuclear magnetic resonance evidence suggesting possible alternative base pairings between these bases and the lower strand of the P3 helix (12, 26). Trinucleotide mutation at either the 5′ or 3′ end of the 29-base J2a/3 region also reduced enzymatic activity (Fig. 4, lines 18 and 19). In contrast, simultaneous substitutions for six noncontiguous bases within the middle of this region produced no detectable effect (Fig. 4, line 20), which is consistent with a previous report (10). Whereas residues 145 to 147 at the extreme 5′ end of J2a/3 may form part of the proximal P2a.1 stem (Fig. 1C, middle), the sequence-specific functional requirement for nucleotides 171 to 173 suggested that they might be involved in the structural and/or functional integrity of the adjacent P3 helix. In addition, we confirmed that mutations of the presumably single-stranded template sequence and of some, but not all, sequences immediately adjacent to it adversely affected telomerase enzymatic activity (Fig. 4, lines 1 to 7), reemphasizing the critical importance of this region (17). As before, Northern blotting analysis confirmed that the stability of the informative J-region mutants was unimpaired (e.g., Fig. 2C, lane 8). These results suggest that while the primary sequences of most of the junctional regions are nonessential, those that flank the functional stem and the template sequence are specifically required for telomerase activity, possibly helping to ensure proper RNA folding and/or interaction with the catalytic hTERT subunit.
FIG. 4.
Summary results of semiquantitative (TRAP) analysis of telomerase enzymatic activities in reconstituted VA13 cells for various substitution mutations within the indicated J regions. The telomerase enzymatic activity of each mutant is expressed in comparison to that of the wild type (+++, 20 to 100%; ++, 2 to 20%; +, 1 to 2%; −, undetectable), based on at least two or three independent determinations that each involved at least three sequential dilutions of extract from the transfected cells.
Sequence polymorphisms from patients with aplastic anemia disrupt hTER function in vitro.
Aplastic anemia is a rare but serious disorder of the bone marrow whose cause is often unidentifiable but which sometimes occurs in heritable form. In one recent survey (27) of 44 patients with this disorder, 5 were found to carry hemizygous sequence polymorphisms in one of three distinct locations within the core domain, whereas no such gene polymorphisms were observed among over 200 normal individuals. This study also reported that average telomeric lengths were reduced among patients with aplastic anemia compared to controls, but it provided no genetic evidence linking these hTER mutations directly to any defect in telomerase function or to the disease phenotype. The frequency of such polymorphisms among patients with aplastic anemia has, moreover, since been questioned (R. T. Calado, M. C. Pintao, W. A. Silva, Jr., R. P. Falcao, and M. A. Zago, Letter, Lancet **360:**1608, 2002). To help clarify any possible link between these hTER polymorphisms and dysfunction, we tested the effects of three such candidate disease alleles on hTER function in VA13 cells, using the assays described above.
As shown in Fig. 5A, we found that one of the three reported polymorphisms, the CR1(g58a)(AP) variant templating sequence, showed no discernible effect on telomerase activity (Fig. 5A, lane 3, and C, right). In contrast, point substitution of G for C at residue 72 [called the P2a.1DN(AP) variant] or deletion of GACU at residues 110 to 113 [called the P3DNdel110-113(AP) variant] reduced catalytic activity to low or undetectable levels (Fig. 5A, lanes 1 and 2, and C). Since the last two deleterious substitutions mapped to the essential P2a.1 and P3 helices, respectively, we asked whether their effects could be attributed to disruption of these stems per se. In both cases, we found that mutations targeting only the complementary bases on the opposite strand had deleterious effects on function and that compensatory mutations designed to reestablish the stems fully restored function (Fig. 5C, left; 3B, left; and 2A, lanes 5 to 22). These effects, moreover, could not be explained by differences in expression or stability of the mutant RNAs in transfected VA13 cells, since all had similar steady-state levels (Fig. 5B). Taken together, these data indicate that at least two out of three hTER polymorphisms identified in a subset of patients with idiopathic aplastic anemia are likely to perturb hTER function in vivo and that they do so by perturbing critical secondary structures within the core domain.
FIG. 5.
(A) Representative TRAP gel of three aplastic-anemia-associated hTER polymorphisms. WT, wild-type; IC, internal controls for normalization of PCRs. (B) Northern blot analysis of the representative aplastic anemia-associated hTER RNAs. The level of hTER RNA was quantified by PhosphorImager image analysis software and is expressed as a ratio of hTER RNA to cellular β-actin mRNA. (C) Telomerase enzymatic activities of the aplastic-anemia-associated hTER polymorphisms, which are highlighted in boldface. The activity of each mutant was assayed by TRAP and is expressed as described in the legend to Fig. 1. Deletions are indicated by dashed lines.
Mutations of the hTER core domain do not grossly disrupt the hTERT-hTER interaction in vitro.
As suggested by earlier studies (4, 11, 20, 25), the catalytic hTERT protein interacts with two separable regions of hTER RNA, and interactions at both regions are necessary to reconstitute in vitro telomerase enzymatic activity. One of the hTERT binding sites has been mapped specifically to the CR4-CR5 domain at nucleotides 254 to 266 (11), whereas the second site of contact has been postulated to reside somewhere within the core domain (20). Here, we attempted to map the latter hTERT binding site more precisely by assaying the efficiency with which hTER could be coimmunoprecipitated along with hTERT from rabbit reticulocyte lysates containing a FLAG-tagged hTERT protein and various core hTER mutants (i.e., RNAs encompassing only residues 1 to 210). Surprisingly, as illustrated in Fig. 6, Northern blotting analysis showed that none of the core RNA mutants tested, including the aplastic-anemia-associated mutants, showed any defect in hTERT binding. Similar results were obtained with similar core mutations in the context of the full-length 451-nucleotide fragments (data not shown). These results raise the possibility that hTERT binding might result from the cumulative contributions of binding to multiple sites within this core RNA domain, so that disrupting any one region has minimal or no effect. Alternatively, hTER-hTERT association might be mediated through another protein intermediate(s) present in the rabbit reticulocyte lysate.
FIG. 6.
Coimmunoprecipitation of wild-type (WT) and mutant hTERs with epitope-tagged hTERT protein expressed in a rabbit reticulocyte lysate. RNA-protein complexes were precipitated with a FLAG-specific antibody and then analyzed by polyacrylamide gel electrophoresis and probed for hTER sequences. The negative control (−) was a lysate that did not contain the hTERT expression vector.
DISCUSSION
This study provides direct genetic evidence that each of the double-stranded regions which have been postulated to exist in the hTER core domain (9) does indeed form and contributes to telomerase catalytic function in vivo (Fig. 7). Together with earlier reports from other laboratories (2, 12, 18, 19), our results lend further support to the proposed core RNA structure that was originally inferred from phylogenetic comparative sequence analysis (9) and later validated in many respects by chemical and nuclease accessibility mapping (1). This highly structured core domain, which we have somewhat arbitrarily defined as residues 1 to 210 of the RNA, includes at least one binding site for the hTERT protein, as well as the templating sequence, the pseudoknot, and additional _cis_-acting sequences required for alignment of the template and for processive DNA repeat synthesis (10). Hence, it is perhaps not surprising that deleting this domain eliminates hTER activity or that, at least in some systems, the core domain alone can substitute for full-length hTER in reconstituting telomerase catalytic function in cell lysates (2, 6, 25).
FIG. 7.
Functional topology of the hTER core domain. In this schematic view of the 210-base RNA core, the indicated regions of primary sequence (red) and base-pairing interactions (light blue) were shown in the present study to contribute to optimal telomerase function in cells. The core conformation depicted is identical to that proposed by Chen et al. (9), except that the P2a.1 helix has been extended proximally by 3 bp, as indicated by the results of the present study (Fig. 1C) and others (1). Mutagenesis of the hTER template and its flanking sequences has been reported elsewhere (10, 17). The resolution of the present analysis was limited by the sizes of the mutations used, which generally altered 2 to 9 bases simultaneously; hence, not every indicated base or base pair may be individually required for optimal function. As few as 4 bp in the long-range P1 helix can support wild-type telomerase activity (Fig. 1C and 2B).
To map more precisely the topography of the hTER core, we constructed a large series of targeted deletion, substitution, and compensatory mutant RNAs; transfected them transiently into hTER-deficient human VA13 cells; and assayed lysates of these cells for telomerase activity. Northern blotting confirmed that all of the mutants were expressed at comparable levels in the lysates, implying that none of the effects we observed was attributable to differences in hTER stability. One set of mutants targeted the putative P1 stem, a helix that has no known counterpart in rodent TERs but is believed to be present in most, if not all, of the ∼30 other vertebrate TERs sequenced to date (9). Sequences near the 5′ terminus, which has been rigorously mapped by RNase protection and primer extension assays of both human and mouse TERs, form the lower strand of P1 in hTER (15). The human TER extends 45 bases upstream of its template sequence, and 19 of those bases (residues 18 to 37) are postulated to form base pairs in P1. The corresponding region of rodent TERs, by contrast, consists of only 2 bases that have no obvious potential to form a stable helix. Nevertheless, our compensatory mutational analysis indicated that at least some base pairing in the P1 region was required for optimal hTER function (Fig. 1B, left). By progressively truncating the 5′ end, we found that deleting residue 33 or beyond drastically reduced hTER activity (Fig. 2B, lanes 11 to 18) but that residues 1 to 32 were dispensable for full telomerase activity. This is in accord with data from Tesmer et al., who found that hTER residues 33 to 325 were minimally required to reconstitute active telomerase in rabbit reticulocyte lysates or in human cell extracts (25). Given these findings, it was unexpected to find that disrupting the center of the portion of the P1 helix involving residues 26 to 29 and their complements was as deleterious to activity as simply deleting residues 1 to 32, even though both types of mutations leave the minimal P1 helix (residues 33 to 37 and 184 to 188) intact.
In regard to the other helical structures (P2a.1, P2a, P2b, and P3) of the core domain, our data suggested that they form essentially as predicted by previous phylogenic comparative sequence analysis (9). Consistent with our findings, previously reported deletions that encompassed the corresponding stem structures in the mouse RNA (i.e., nucleotides 23 to 102 of mTER) were found to effectively abolish telomerase enzymatic activity (19). In addition, we have provided, for the first time, genetic evidence for the formation of an extended P2a.1 stem that had been implicated in previous chemical and nuclease probing analyses (1). More importantly, our data suggest that both the primary sequence and secondary structure of the P2b and P3 stems are relevant in functional reconstitution of the human telomerase complex (Fig. 1B, right, and 3).
Accessibility mapping analysis of hTER has failed to confirm P3 stem formation in hTER (1), suggesting that P3 base pairing either does not occur or is not static. Similarly, recent nuclear magnetic resonance structural analyses of oligonucleotides that mimic P3 suggest that this region of hTER can adopt at least two different conformations (12, 26). These data together suggest that P3 may have a dynamic structure, perhaps adopting different conformations during various stages of telomere repeat synthesis or in different physiologic states of the cell. More recently, we have shown that P3 can also mediate homodimerization of hTER both in vivo and in vitro and that such P3-dependent dimer formation is essential for activity of the telomerase RNP complex (18). This is consistent with data obtained from the biochemical purification of an active human telomerase complex (30) and from genetic and biochemical analyses of active yeast telomerase complexes (22, 23), all of which suggest that the functional telomerase complex includes at least two copies of TER.
Mutagenesis of the putatively single-stranded J regions located between the core helices revealed that, in most instances, the primary sequences of these so-called J regions are not specifically required for function. Two notable exceptions were the J2a/3 region and certain residues (i.e., positions 38 to 44, 53, and 54) immediately flanking the templating sequence. The precise function of the J2a/3 sequence remains to be explored, but it may relate to the as yet ill-defined functions of the pseudoknot region itself. Recent evidence from Chen and Greider, by contrast, suggests that residues 53 to 56 make sequence-specific contributions to the in vitro processivity and overall enzymatic activity of the human telomerase complex (10). Whereas these authors attributed the functional defects of certain hTER core mutants to an inability to assemble properly with hTERT, our results failed to identify any discrete sequence-specific hTERT-binding locus within the core domain, suggesting perhaps that direct contacts with this protein are distributed over an extensive region of the RNA.
Another novel finding from our study is the demonstration that certain hTER sequence polymorphisms observed in patients with aplastic anemia cause severe defects in telomerase activity and that these defects result from perturbations of the normal secondary structure of hTER (Fig. 5). One striking example is the replacement of a single residue within the P2a.1 helix (i.e., G replacing C at position 72), which markedly impairs telomerase activity but which can be fully complemented by a compensatory mutation designed to restore the normal pattern of P2a.1 base pairing (Fig. 5C, left). Comparable results were also obtained for a different naturally occurring polymorphism that disrupts the P3 stem (Fig. 3B, left). While it is not yet proven, these results suggest that the hTER polymorphisms may contribute to the observed shortening of telomeric DNAs in patients with aplastic anemia (27). Our studies thus indicate a critical role for the conserved hTER core structure in telomerase biologic function and its relevance to human disease.
ADDENDUM
While this paper was being reviewed, a paper by Fu and Collins appeared (13), which corroborated our results for the functional analysis of the aplastic-anemia-associated polymorphisms in the hTER gene.
Acknowledgments
We thank Lifeng Xu for technical advice and protocols and Arif Hussain for excellent technical assistance.
This work was supported by NIH grant GM26259 and funds from the Steven and Michelle Kirsch Foundation (to E.H.B.) and by NIH grants AI36636 and AI40317 (to T.G.P.). H.L. was supported in part by NIH postdoctoral training grant AI07395 and by a Special Fellowship from the Leukemia and Lymphoma Society of America.
REFERENCES
- 1.Antal, M., E. Boros, F. Solymosy, and T. Kiss. 2002. Analysis of the structure of human telomerase RNA in vivo. Nucleic Acids Res. 30**:**912-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autexier, C., R. Pruzan, W. D. Funk, and C. W. Greider. 1996. Reconstitution of human telomerase activity and identification of a minimal functional region of the human telomerase RNA. EMBO J. 15**:**5928-5935. [PMC free article] [PubMed] [Google Scholar]
- 3.Bachand, F., and C. Autexier. 2001. Functional regions of human telomerase reverse transcriptase and human telomerase RNA required for telomerase activity and RNA-protein interactions. Mol. Cell. Biol. 21**:**1888-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachand, F., I. Triki, and C. Autexier. 2001. Human telomerase RNA-protein interactions. Nucleic Acids Res. 29**:**3385-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie, T. L., W. Zhou, M. O. Robinson, and L. Harrington. 2001. Functional multimerization of the human telomerase reverse transcriptase. Mol. Cell. Biol. 21**:**6151-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beattie, T. L., W. Zhou, M. O. Robinson, and L. Harrington. 1998. Reconstitution of human telomerase activity in vitro. Curr. Biol. 8**:**177-180. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106**:**661-673. [DOI] [PubMed] [Google Scholar]
- 8.Bryan, T. M., A. Englezou, L. Dalla-Pozza, M. A. Dunham, and R. R. Reddel. 1997. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 3**:**1271-1274. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J. L., M. A. Blasco, and C. W. Greider. 2000. Secondary structure of vertebrate telomerase RNA. Cell 100**:**503-514. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J. L., and C. W. Greider. 2003. Determinants in mammalian telomerase RNA that mediate enzyme processivity and cross-species incompatibility. EMBO J. 22**:**304-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, J. L., K. K. Opperman, and C. W. Greider. 2002. A critical stem-loop structure in the CR4-CR5 domain of mammalian telomerase RNA. Nucleic Acids Res. 30**:**592-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comolli, L. R., I. Smirnov, L. Xu, E. H. Blackburn, and T. L. James. 2002. A molecular switch underlies a human telomerase disease. Proc. Natl. Acad. Sci. USA 99**:**16998-17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu, D., and K. Collins. 2003. Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol. Cell 11**:**1361-1372. [DOI] [PubMed] [Google Scholar]
- 14.Greider, C. W., and E. H. Blackburn. 1989. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337**:**331-337. [DOI] [PubMed] [Google Scholar]
- 15.Hinkley, C. S., M. A. Blasco, W. D. Funk, J. Feng, B. Villeponteau, C. W. Greider, and W. Herr. 1998. The mouse telomerase RNA 5′-end lies just upstream of the telomerase template sequence. Nucleic Acids Res. 26**:**532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiyama, K., Y. Hirai, S. Kyoizumi, M. Akiyama, E. Hiyama, M. A. Piatyszek, J. W. Shay, S. Ishioka, and M. Yamakido. 1995. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 155**:**3711-3715. [PubMed] [Google Scholar]
- 17.Kim, M. M., M. A. Rivera, I. L. Botchkina, R. Shalaby, A. D. Thor, and E. H. Blackburn. 2001. A low threshold level of expression of mutant-template telomerase RNA inhibits human tumor cell proliferation. Proc. Natl. Acad. Sci. USA 98**:**7982-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ly, H., L. Xu, M. A. Rivera, T. G. Parslow, and E. H. Blackburn. 2003. A role for a novel “trans-pseudoknot” RNA-RNA interaction in the functional dimerization of human telomerase. Genes Dev. 17**:**1078-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Rivera, L., and M. A. Blasco. 2001. Identification of functional domains and dominant negative mutations in vertebrate telomerase RNA using an in vivo reconstitution system. J. Biol. Chem. 276**:**5856-5865. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell, J. R., and K. Collins. 2000. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol. Cell 6**:**361-371. [DOI] [PubMed] [Google Scholar]
- 21.Olovnikov, A. M. 1973. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 41**:**181-190. [DOI] [PubMed] [Google Scholar]
- 22.Prescott, J., and E. H. Blackburn. 1997. Functionally interacting telomerase RNAs in the yeast telomerase complex. Genes Dev. 11**:**2790-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prescott, J., and E. H. Blackburn. 1997. Telomerase RNA mutations in Saccharomyces cerevisiae alter telomerase action and reveal nonprocessivity in vivo and in vitro. Genes Dev. 11**:**528-540. [DOI] [PubMed] [Google Scholar]
- 24.Shay, J. W., and S. Bacchetti. 1997. A survey of telomerase activity in human cancer. Eur. J. Cancer 33**:**787-791. [DOI] [PubMed] [Google Scholar]
- 25.Tesmer, V. M., L. P. Ford, S. E. Holt, B. C. Frank, X. Yi, D. L. Aisner, M. Ouellette, J. W. Shay, and W. E. Wright. 1999. Two inactive fragments of the integral RNA cooperate to assemble active telomerase with the human protein catalytic subunit (hTERT) in vitro. Mol. Cell. Biol. 19**:**6207-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theimer, C. A., L. D. Finger, L. Trantirek, and J. Feigon. 2003. Mutations linked to dyskeratosis congenita cause changes in the structural equilibrium in telomerase RNA. Proc. Natl. Acad. Sci. USA 100**:**449-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vulliamy, T., A. Marrone, I. Dokal, and P. J. Mason. 2002. Association between aplastic anaemia and mutations in telomerase RNA. Lancet 359**:**2168-2170. [DOI] [PubMed] [Google Scholar]
- 28.Watson, J. D. 1972. Origin of concatemeric T7 DNA. Nat. New Biol. 239**:**197-201. [DOI] [PubMed] [Google Scholar]
- 29.Weinrich, S. L., R. Pruzan, L. Ma, M. Ouellette, V. M. Tesmer, S. E. Holt, A. G. Bodnar, S. Lichtsteiner, N. W. Kim, J. B. Trager, R. D. Taylor, R. Carlos, W. H. Andrews, W. E. Wright, J. W. Shay, C. B. Harley, and G. B. Morin. 1997. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat. Genet. 17**:**498-502. [DOI] [PubMed] [Google Scholar]
- 30.Wenz, C., B. Enenkel, M. Amacker, C. Kelleher, K. Damm, and J. Lingner. 2001. Human telomerase contains two cooperating telomerase RNA molecules. EMBO J. 20**:**3526-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]