The Human Immunodeficiency Virus Type 1 Ribosomal Frameshifting Site Is an Invariant Sequence Determinant and an Important Target for Antiviral Therapy (original) (raw)
Abstract
Human immunodeficiency virus type 1 (HIV-1) utilizes a distinctive form of gene regulation as part of its life cycle, termed programmed −1 ribosomal frameshifting, to produce the required ratio of the Gag and Gag-Pol polyproteins. We carried out a sequence comparison of 1,000 HIV-1 sequences at the slippery site (UUUUUUA) and found that the site is invariant, which is somewhat surprising for a virus known for its variability. This prompted us to prepare a series of mutations to examine their effect upon frameshifting and viral infectivity. Among the series of mutations were changes of the HIV-1 slippery site to those effectively utilized by other viruses, because such mutations would be anticipated to have a relatively mild effect upon frameshifting. The results demonstrate that any change to the slippery site reduced frameshifting levels and also dramatically inhibited infectivity. Because ribosomal frameshifting is essential for HIV-1 replication and it is surprisingly resistant to mutation, modulation of HIV-1 frameshifting efficiency potentially represents an important target for the development of novel antiviral therapeutics.
Due to their compact genomes, viruses often develop novel modes of gene expression in order to synthesize multiple proteins from a single RNA species. For example, human immunodeficiency virus type 1 (HIV-1) uses a unique mode of gene regulation termed programmed −1 ribosomal frameshifting. The structural and enzymatic components of the virus are synthesized as polyproteins with a common N terminus. The open reading frame (ORF) encoding the structural 55-kDa Gag protein is located at the 5′ end of the mRNA. The 160-kDa Gag-Pol polyprotein is translated only as a result of a programmed −1 ribosomal frameshift event (Fig. 1A) (17). The pol ORF, which encodes the enzymatic proteins, including viral protease (PR), reverse transcriptase, and integrase, is 3′ to the gag gene and out of reading frame with respect to the gag ORF. Programmed −1 ribosomal frameshifting allows synthesis of the required ratio of Gag to Gag-Pol, which is highly regulated in retroviruses, with frameshifting occurring at levels of 5 to 10% for HIV-1 (17, 30). These polyprotein precursors are incorporated into viral particles and are subsequently cleaved during viral maturation by the viral PR.
FIG. 1.
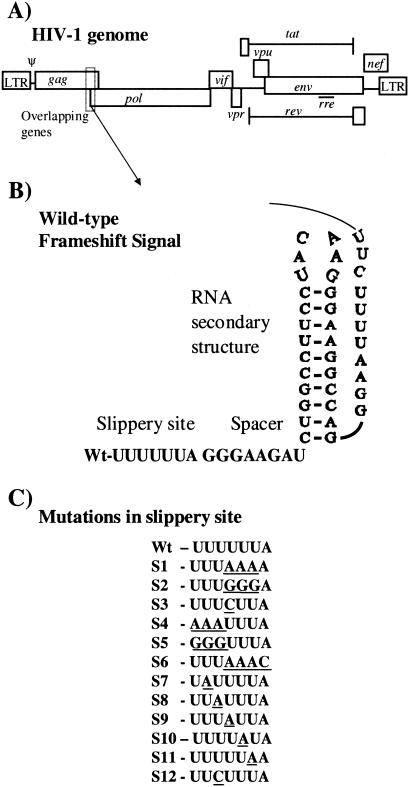
HIV-1 genome and mutations in the slippery site. (A) HIV-1 genome with the nine major ORFs. Ψ indicates sequences important for genomic RNA packaging. The overlapping region of gag and pol genes contains the −1 ribosomal frameshift signal. LTR, long terminal repeat. (B) Nucleotide sequence of wild-type HIV-1 (strain HXB2) frameshift signal with the slippery site, the spacer, and the putative RNA secondary structure. (C) Sequences of mutations in the slippery site used in the frameshift and infectivity assays. Wt, wild type.
In vitro and in vivo studies demonstrate that there are two _cis_-acting elements located within the overlapping region of the HIV-1 gag and pol genes that are critical for translational frameshifting to occur (Fig. 1B). The first is the heptamer UUUUUUA, where frameshifting occurs and which computer analysis indicates is invariant (see Results). The second is a structural RNA motif downstream of the heptamer that assumes a stem-loop and possibly a pseudoknot structure (9, 11, 12, 26). An 8-nucleotide spacer separates these two regions. Other viruses as well as additional retroviruses also employ ribosomal frameshifting during viral gene expression (3, 12). The consensus viral slippery sequence is X XXY YYZ, where spaces indicate the zero-frame codons before shifting. In some cases X can equal Y, as in HIV-1 (17). The simultaneous slippage model proposes that the mRNA translocation machinery encounters a barrier when it reaches the slippery site and pauses. The likely function of the secondary structure is to impede the progressing ribosomes (16, 17) such that the two ribosome-bound tRNAs in the P and A sites slip backwards in the 5′ direction simultaneously from their initial positions in the zero frame (17). In HIV-1, following the −1 frameshift event but before the peptidyl transfer and translocation, the leucyl tRNA is edited off the A site, allowing the UUU triplet to be decoded by a phenylalanyl tRNA. Similar observations were made for Rous sarcoma virus and mouse mammary tumor virus (5, 16, 18). In HIV-1 all three codon-anticodon interactions in the P site and two out of three in the A site are maintained (16). Following the slip backwards, the ribosome continues reading in the new −1 frame.
Highly active antiretroviral therapy has had an important impact upon AIDS-related morbidity and mortality. However, therapy is hampered by the emergence of drug-resistant mutants and by drug toxicity, making continued drug development an important priority. Studies of the HIV-1 life cycle at the molecular level have played a key role in identifying targets for the development of therapeutic antiviral agents (29).
In this study, mutations were engineered into the slippery site, including mutations that function effectively in other viruses. Frameshifting efficiency and viral propagation were then monitored, and it was found that any change to the slippery site dramatically reduced frameshifting efficiency and inhibited viral replication. Defects in viral infectivity were due to reduced levels of Gag-Pol polyprotein expression. Thus, the frameshifting site is a well-conserved region in the HIV-1 genome and is resistant to mutation, so it represents a novel target for antiviral drug development.
MATERIALS AND METHODS
Database analysis.
For analyzing the sequence similarity in the slippery site region, we used the GenBank nucleotide sequence database at the National Center for Biotechnology Information (NCBI). The search was done on the website of the NCBI-developed Entrez retrieval system (http://www.ncbi.nih.gov/Entrez/). We extracted the slippery site sequence TTTTTTA along with 10 bases before and after the slippery site from one of the HIV-1 isolates (NCBI accession number AF212297) and searched for similarity by using a BLAST search in the NCBI database. Subsequently the output file containing 1,000 sequence hits was analyzed manually for variations in the heptanucleotide slippery site sequence.
Construction of plasmids.
General DNA manipulations were carried out using standard protocols (2). For the frameshifting assay in HeLa cells, the dual luciferase reporter plasmid system was used (14). The HIV-1 frameshift signal was positioned between rluc and fluc, where fluc is in the −1 frame with regard to rluc (Fig. 2A). We used the following control constructs that were previously described: (i) p2luci, where fluc is in the same frame as rluc (14), serves to generate a zero-frame baseline of translation of the second ORF and (ii) p2luc, where fluc is in the −1 frame with respect to rluc with no intervening sequences, serves as a negative control (14). We constructed mutants in the frameshifting signal by using PCR fragments of 70 nucleotides that were generated between the _Bam_HI and _Sac_I sites of the dual luciferase reporter construct. The synthetic oligonucleotides (Integrated DNA Technologies, Coralville, Iowa) were annealed, digested with _Bam_HI and _Sac_I, and cloned into similarly digested p2luc such that fluc is in the −1 frame compared to rluc ORF. The forward primer for the slippery site mutants HIVlucS1/pPB101 to HIVlucS12/pPB112 was 5′-CCCCGGATCCNNNNNNNGGGAAG-3′, where N represents mutations shown in Fig. 1C. All of these PCR fragments for the mutants were generated using a common reverse primer, 5′-CTTAAAAGAAGGCTCGAGCCCC-3′.
FIG. 2.
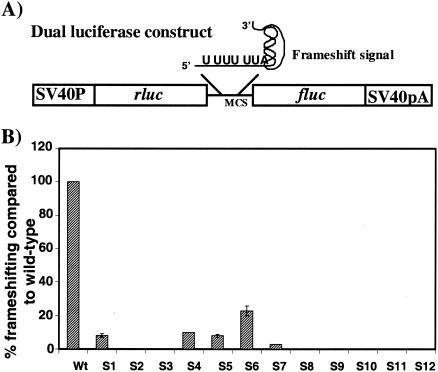
Mutations in the HIV-1 slippery site effect programmed −1 ribosomal frameshifting. (A) The dual luciferase construct contains wild-type or mutant HIV-1 frameshift signal inserted into the multiple cloning site (MCS), between the Renilla luciferase (rluc) and the firefly luciferase (fluc) genes. It has simian virus 40 promoter (SV40P) and polyadenylation (SV40pA) sequences. (B) The ratios of expression of two luciferase genes were measured for wild type and mutants and compared to the luciferase activity for the p2luci vector to obtain percent frameshifting. In the wild type, the level of frameshifting is approximately 4 to 6%. In the graph, wild-type levels are set at 100% and compared to those of the slippery site mutants. These are average values from three experiments done in duplicate.
The HIV-1 based gag-pol vector was constructed as follows. (i) First, plasmids pPB125 through pPB130 were generated by the following method. We generated plasmid pPB113 by deleting Bgl_II/Bgl_II sequences in pSPUTK (Stratagene). Into the _Nhe_I site of plasmid pPB113 we inserted the 3.3-kb _Nhe_I fragment containing HIV-1 sequence from plasmid JS (25) to obtain pPB114. At position 2081 a conservative C-to-G change deleted a single _Hin_cII site. This change in the context of a gag-pol vector did not have any effect in HIV-1 infectivity assays (data not shown). PCR fragments of 500 bp were generated for slippery site mutations by using a forward primer spanning the _Apa_I site, 5′-CCAGAAATTGCAGGGCCCCTAGGAAAAAGGGCTGTTGG-3′, and a reverse primer spanning the _Bgl_II site, 5′-GACTCTGTCCGATTNNNNNNNCCCTTCTAGACCGG-3′, where N contains mutations as shown in Fig. 1C. Then the oligonucleotides were annealed, digested with _Apa_I and _Bgl_II, and cloned into similarly digested pPB114, resulting in plasmids pPB125 to pPB130. (ii) Second, we generated pPB115 by inserting the _Nhe_I-_Pme_I HIV-1 fragment from pJS into _Nhe_I-_Pme_I-digested pcDNA3.1hygro (Invitrogen). (iii) Final gag-pol vector constructs were made by inserting the 3.3-kb _Nhe_I fragment from the wild type (pPB114) or mutants (pPB125-pPB130) into _Nhe_I-cut pPB115, resulting in the following vector constructs: wild-type HIVgpwt/pPB122 and slippery site mutants HIVgpS1/pPB137 to HIVgpS6/pPB142. The mutations in the gag-pol vector constructs S1 to S6 are listed in Fig. 1C. The vesicular stomatitis virus (VSV) G expression vector pMD.G (24) and the lentivirus vector HSGIP (21) used in infectivity assays were previously described.
Cell culture and frameshift assays.
HeLa cells and 293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U of penicillin G/ml, and 100 μg of streptomycin/ml. Frameshifting in HeLa cells was monitored using the dual luciferase vector constructs by transient transfections in six-well plates with Fugene 6 reagent (Roche) per the manufacturer's instructions. Briefly, 1 μg of reporter constructs was transiently transfected into 4 × 105 HeLa cells and cultured for 24 h. Cells were lysed using the lysis buffer provided by the manufacturer, and the activities of Renilla luciferase (first ORF) versus firefly luciferase were determined using a dual luciferase assay kit (Promega) and a Turner 20/20 luminometer (Palo Alto, Calif.). The fluc gene is in the −1 frame with regard to rluc; therefore, firefly luciferase can be produced only as a consequence of a programmed −1 ribosomal frameshift event (9). The vector construct p2luc served as a control for undirected frameshift events, as fluc is out of frame with regard to rluc with no intervening sequences (14). Conversely, in the construct p2luci, fluc is in the same frame as rluc and serves as a zero-frame baseline for translation of the second luciferase gene. The ratio of firefly and Renilla luciferase activities provided the normalized measurement of translation of the second luciferase gene (14). Moreover, comparison of this ratio to controls gave a measure for the percentage of frameshifting in the wild-type or mutant test constructs.
Virus production and infection.
For generating infectious vector virus 293T cells were transiently transfected by a modified calcium phosphate precipitation method (6). Cotransfections were carried out in duplicate with 5 to 8 μg each of gag-pol vector construct (wild type or mutant), pMD.G (containing VSV-G env), and the transducing vector HSGIP (containing green fluorescent protein [GFP] gene [_gfp_] and puromycin resistance gene [_puro_] markers) on 2.5 × 106 293T cells in 100-mm-diameter tissue culture plates, for 24 h at 35°C and 3% CO2. After transfection, the cells were washed in phosphate-buffered saline, refed with fresh medium, and maintained at 37°C and 5% CO2. Viral titers were determined by the infection of target HeLa cells that were seeded at a density of 2.5 × 105 cells 1 day before. Cells were maintained at 37°C and 5% CO2 for the duration of the experiment. Seventy-two hours posttransfection supernatant containing virus particles was filtered to eliminate cellular debris, serially diluted, supplemented with Polybrene (8 μg/ml), and added to HeLa cell monolayers in six-well plates. Medium containing virus was aspirated, and fresh medium was added after 6 h. Twenty-four hours after infection, the target HeLa cells were subjected to puromycin (Sigma) selection at 1 μg/ml. The viral genomic DNA that integrates into the host cell renders infected cells GFP positive and puromycin resistant. Infectious units (IU) were measured by counting GFP-positive colonies visualized with a fluorescence microscope (Zeiss). For the infectivity assay, infections were done in duplicate for each dilution of viral supernatant.
Determination of Gag versus Gag-Pol protein ratios with in vitro TNT-coupled reticulocyte lysate systems.
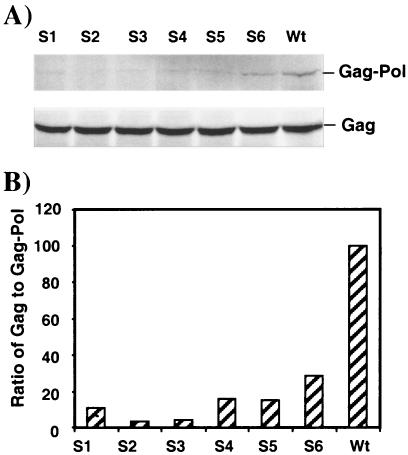
The TNT-coupled reticulocyte lysate kit (Promega) was used to analyze the ratio of Gag to Gag-Pol. In this system we tested the gag-pol vector constructs, wild type or mutant, by labeling the proteins with [35S]methionine per the manufacturer's instructions. Starting with circular plasmid DNA, the in vitro reaction incorporates the steps of transcription directly in the translation mix to produce proteins. Following a 90-min reaction protein samples were separated on a sodium dodecyl sulfate-8% polyacrylamide gel, dried, and quantitated with a PhosphorImager (Typhoon) using ImageQuant software (Molecular Dynamics PSI-PC, Sunnyvale, Calif.). The gel depicted in Fig. 4A is representative of four separate analyses.
FIG. 4.
Correlation of frameshifting levels with Gag and Gag-Pol polyprotein ratios. The ratios of Gag to Gag-Pol proteins in the wild type and the mutants were assayed using a coupled in vitro transcription and translation (TNT) reticulocyte lysate kit. Please note that the figure depicts one experiment representative of four performed. (A) The sodium dodecyl sulfate-polyacrylamide gel shows the wild type and mutants with each lane marked accordingly. The mutants S1 through S6 are defined in Fig. 1. The positions of the 55-kDa Gag and 160-kDa Gag-Pol polyproteins are indicated. (B) Gag and Gag-Pol protein ratios were calculated from the intensities of protein bands on the gel by densitometry and compared to the wild-type ratio, which was set at 100%. Wt, wild type.
RESULTS
The HIV-1 slippery site is essentially invariant.
Initially, we were interested in determining the degree of variation found at the slippery site of reported HIV-1 sequences. With the slippery site and 10 nucleotides on each side of the slippery site, a BLAST search was performed using the NCBI GenBank nucleotide sequence database. Of the 1,000 hits obtained, the TTTTTTA sequence was identical in all cases. Next, the HIV-1 sequence compendium from Los Alamos National Laboratory was visually inspected, and it was found that of the 190 unique isolates in this database all except one were identical at the slippery site (22). As data described below show, it is not likely that the single divergent isolate with TTCTTTA at the slippery site is replication competent, because it results in a severe inhibition of ribosomal frameshifting. This strong sequence conservation at the slippery site suggests that this sequence is indispensable for viral replication.
Slippery site mutations.
To explain further the importance of the TTTTTTA sequence at the slippery site, a cell-based dual luciferase reporter system was utilized to examine the effect of mutations in the frameshift signal on programmed −1 ribosomal frameshifting efficiencies (14). The reporter contained the HIV-1 frameshifting signal spanning the slippery site and the RNA secondary structure, placed between Renilla luciferase (rluc) and firefly luciferase (fluc) genes. The firefly luciferase protein can be produced only as a consequence of a programmed −1 ribosomal frameshifting event (Fig. 2A). We constructed a series of mutations in the slippery site (Fig. 1C) and inserted them between the two reporter genes according to the following criteria: (i) triple nucleotide changes, some based on the slippery site signal of other viruses, and (ii) disruption of the homopolymeric nature of the slippery site. These reporter constructs were transiently transfected into HeLa cells, and the programmed −1 ribosomal frameshifting efficiencies were determined by measuring the activities of both luciferase proteins (Fig. 2B). The ratio of luciferase activities of the wild type or the mutants was compared to the control construct p2luci vector to obtain the percentage of frameshifting. In vector p2luci fluc is in the same frame as rluc (14) and serves to generate a zero-frame baseline of translation of the second ORF. In this system the wild-type HIV-1 signal exhibited 4 to 6% levels of programmed −1 ribosomal frameshifting. Frameshifting levels of the wild-type dual luciferase vector were set at 100% and were used to calculate the relative frameshifting level of the mutants. Slippery site mutations in HIVlucS1, HIVlucS4, and HIVlucS5 reduced frameshifting efficiency to approximately 8 to 10% of what was observed in the wild-type frameshift reporter. The HIVlucS2 and HIVlucS3 mutant frameshift signals completely abolished any observable frameshifting (Fig. 2B). The HIVlucS6 frameshift mutation reduced the frameshifting efficiency to 23% of the wild-type level. In addition, point mutations in the slippery site contained in mutants HIVlucS7 through HIVlucS12 dramatically reduced or abolished any observable level of frameshifting.
Previous studies have shown that the XXXYYYZ configuration in other viruses promotes efficient frameshifting (13). Triplet changes in other viruses such as yeast L-A virus or human coronavirus slightly reduced or, in some cases, even increased frameshifting efficiency (4, 10). The tRNA on the P site is paired with XXY and with YYZ on the A site and shifts back 1 nucleotide during frameshifting to pair again at XXX and YYY, respectively, while maintaining the correct pairing of their nonwobble bases. In mutants HIVlucS4, HIVlucS5, and HIVlucS6 the triple nucleotide changes are based on the normal slippery site sequences found in Rous sarcoma virus (18), yeast L-A virus (8), and human coronavirus (4), respectively. The other two mutants with three nucleotide changes are HIVlucS1 and HIVlucS2. Surprisingly, in HIV-1 any kind of triplet change, whether in the P site or the A site, greatly reduces the frameshifting efficiency. It has been observed that point mutations which reduce the homopolymeric nature of the slippery site greatly diminish or abolish frameshifting (4, 8, 16, 17). The single nucleotide change from U to C in HIVlucS3 disrupted the homopolymeric stretch and interfered with the frameshift event as expected. Similarly, the single nucleotide changes from U to A in HIVlucS7 through HIVlucS12 spanning the heptameric slippery site showed dramatic reduction in frameshift efficiencies when tested in HeLa cells by a dual luciferase cell-based assay. The point mutation in HIVlucS12 from U to C was identical to that found in the single divergent isolate reported in the HIV-1 sequence compendium (22). This mutation dramatically inhibited frameshifting, indicating that the isolate with this mutation would not be replication competent (Fig. 2B). Our results clearly demonstrate that any change to the HIV-1 slippery site markedly reduces programmed −1 ribosomal frameshifting efficiency.
Correlation of frameshifting levels with HIV-1 infectivity and propagation.
Next we tested the ability of the frameshift mutants to propagate HIV-1 by a cell-based infectivity assay. In the HIV-1 life cycle, the level and activity of enzymatic proteins that are produced as a consequence of frameshifting are crucial at the stage of viral assembly and maturation (20). Following translation, the viral proteins are assembled at the cell membrane and the virions bud as immature particles. Subsequent to budding, the HIV-1 PR cleaves the Gag-Pol polyprotein into its individual components, rendering the viral particle mature and infectious. The amount of PR is critical for efficient production of infectious virus. Over- or underexpression of the PR leads to aberrant rates of processing and decreased infectivity (23).
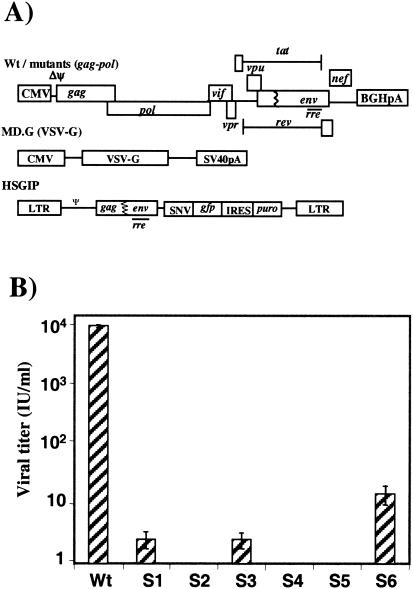
We used an HIV-1 single cycle infectivity assay to examine the effect of frameshift mutations on the efficiency of virus propagation. Our assay system consists of three components (Fig. 3A): (i) the gag-pol vector that provides the Gag and Gag-Pol polyproteins as well as all of the HIV-1 accessory proteins; (ii) the pMD.G plasmid (24), which encodes VSV G envelope protein (VSV-G); and (iii) the transducing vector HSGIP (21), which contains _cis_-acting signals for vector propagation, including the long terminal repeats and the packaging signal, as well as the marker genes (GFP gene [_gfp_] and puromycin resistance gene [_puro_]) for the assay. Following cotransfection of the three vector constructs, pseudotyped HIV-1 vector particles are generated. While viral particles containing HIV envelope proteins require the presence of a CD4 receptor on target cells for optimal infectivity, HIV-1 particles pseudotyped with VSV-G do not (1). The viral RNA packaged into the virion encodes the marker genes (gfp and puro) as well as the _cis_-acting elements needed for replication; however, it lacks the messages for production of any HIV-1 structural or functional proteins, ensuring that the pseudotyped virus cannot propagate itself further. Taken together, the steps involving generation of viral particles from cotransfection through infection of a target cell and integration of proviral DNA represent a single cycle of virus replication. Viral titers can then be measured as IU, by counting the puromycin-resistant colonies of target cells expressing GFP. We chose mutations in the slippery site that altered frameshifting efficiency in the dual luciferase assays (Fig. 2) and included them in the context of the gag-pol vector construct used in the infectivity assay (Fig. 3A). Following cotransfection of the vector constructs we harvested viral supernatant and used it to infect target HeLa cells. The cells were then placed under puromycin selection, and we scored the HIV-1 IU by counting GFP-positive, puromycin-resistant colonies (Fig. 3B).
FIG. 3.
HIV-1 infectivity affected by changes in the slippery site. (A) Schematic representation of the vector constructs used for the HIV-1 infectivity assay. We used wild-type HIVgpwt or the mutant gag-pol constructs HIVgpS1 to HIVgpS6. Boxes interrupted with a jagged line symbolize partial deletions; ΔΨ signifies a 33-bp deletion in the encapsidation signal downstream of the splice donor site; CMV, immediate-early promoter from human cytomegalovirus; BGHpA, bovine growth hormone polyadenylation signal; VSV-G, coding sequence for the G envelope protein from VSV; SV40pA, simian virus 40 polyadenylation signal; SNV, spleen necrosis virus U3 promoter; gfp, GFP gene; IRES, internal ribosomal entry site from encephalomyocarditis virus 5′ untranslated region; puro, puromycin resistance gene; LTR, long terminal repeat. (B) Results from viral infectivity assays testing the slippery site mutants in comparison to the wild type. Results were scored as GFP-positive and puromycin-resistant colonies of infected target HeLa cells. Results of viral infectivity assays of slippery site mutants are expressed in IU per milliliter of viral supernatant. These values are averages of four independent transfections. Wt, wild type.
We found that changes in frameshifting efficiency led to a marked decrease in the levels of virus production. The wild-type gag-pol vector yielded approximately 104 IU/ml, while the mutants HIVgpS2, HIVgpS4, and HIVgpS5 did not produce any detectable infectious virus and mutants HIVgpS1 and HIVgpS3 had titers of only 2 to 4 IU/ml. The slippery site mutant HIVgpS6 showed a 100-fold reduction in viral titer compared to that of the wild type. When results were correlated with those of the frameshift assay with HeLa cells performed earlier, a reduction in frameshifting levels to around 25% greatly disabled virus production, whereas frameshifting below 10% completely abolished virus production. These results establish that altering the slippery site results in a reduced level of frameshifting that subsequently leads to a reduction in infectious viral particles.
Reduced frameshifting as reflected by ratio of Gag to Gag-Pol polyproteins.
The mutations in the slippery site region showed a reduction in both frameshifting efficiency and viral infectivity. We next assessed whether the infectivity defect in the gag-pol vector was manifested as a change in the Gag/Gag-Pol ratio by using [35S]methionine-labeled proteins generated by a coupled in vitro transcription and translation (TNT) system (Promega) (Fig. 4). We compared the ratios of p55 Gag and gp160 Gag-Pol polyproteins of the mutants to those of the wild type (Fig. 4). The results clearly demonstrate that the slippery site mutants undergo a reduced level of frameshifting and therefore produce less of the Gag-Pol polyprotein. The observed reduction in viral titers (Fig. 3B) is likely a consequence of reduced frameshifting efficiency, leading to lower amounts of the Gag-Pol polyprotein that is essential for viral maturation and propagation.
A comparison of the results for frameshifting, viral infectivity, and Gag/Gag-Pol ratio for the six slippery site mutations is depicted in Table 1. Our analysis clearly indicates that the maintenance of the slippery site, the _cis_-acting signal for frameshifting, is very important for the process of frameshifting that is also necessary to maintain the correct ratio of Gag to Gag-Pol and therefore is critical for viral infectivity.
TABLE 1.
Comparison of slippery site mutants of HIV-1 to wild typea
| Mutation | Frameshifting efficiency (%) | Viral infectivity (%) | Ratio of Gag to Gag-Pol (%) |
|---|---|---|---|
| S1 | 8.1 ± 1.01 | 0.03 ± 0.01 | 10.8 |
| S2 | 0 | <0.008 | 3.9 |
| S3 | 0 | 0.028 ± 0.01 | 4.0 |
| S4 | 10 | <0.008 | 16.0 |
| S5 | 8 ± 1.0 | <0.008 | 14.6 |
| S6 | 23 ± 2.9 | 1.06 ± 0.15 | 28.4 |
DISCUSSION
The results presented here point to an invariant slippery site in the HIV-1 genome, and changes to this site alter frameshifting efficiency. Although triple nucleotide changes in the slippery sites of other viruses have been shown to be nearly optimal for frameshifting (4, 10), our results indicate that, for HIV-1, any of the triple nucleotide substitutions, even those shown to have little effect in other viral systems, did not rescue HIV-1 frameshifting levels. In addition, substitute sequences that are normally used by other viruses as slippery sites did not function in the HIV-1 frameshift assay. For example, slippery sites from the yeast L-A virus (GGGUUUA) (8); Rous sarcoma virus (AAAUUUA) (18); and mouse hepatitis virus, human coronavirus, or Berne virus (UUUAAAC) (reviewed in reference 3) did not function effectively. Moreover, we found that point mutations disrupting the homopolymeric stretch of the slippery site also had a detrimental effect on frameshifting efficiency. Thus, any alterations that were made had an inhibitory affect upon the level of programmed −1 ribosomal frameshifting.
It is clear from these experiments that a reduction in frameshifting due to mutations in the HIV-1 slippery site had a dramatic effect upon virus propagation (summarized in Table 1). The mutations, which reduced the efficiency of frameshifting as monitored by the dual luciferase reporter assay, also altered the ratio of Gag to Gag-Pol (Fig. 2 and 4). Reducing the levels of the Gag-Pol polyprotein decreased the amounts of the enzymatic components needed for maturation, reverse transcription, and integration. Consistent with this decrease, all of the slippery site mutants examined demonstrated a reduction in the level of infectious virus. In the case of mutant HIVlucS6/HIVgpS6 (Table 1), it exhibited a frameshifting efficiency of 23% relative to the wild type as well as an altered ratio of Gag to Gag-Pol protein, and this fourfold decrease translated into a 100-fold reduction in viral infectivity, suggesting that a relatively subtle modulation of frameshifting can have an even more profound effect upon infectivity.
The nucleotide changes in the slippery site mutants also resulted in some changes to the peptides of the Gag and Gag-Pol polyprotein regions. In the gag ORF, the frameshifting signal encodes a portion of p1, a 16-amino-acid protein located between nucleocapsid (NC) and p6, the last peptide of the Gag polyprotein (15). Recent results suggest that the proline residues at positions 7 and 13 of the p1 protein are important for the HIV-1 genomic RNA dimer stability (28). Other than these residues there is no known function ascribed to the N terminus of the p1 protein encoded by the slippery site of the frameshift signal. In the gag-pol ORF, the frameshifting signal is a part of the N-terminal region of the 68-amino-acid transframe protein p6 that is directly upstream of PR. Only four amino acids in the C-terminal region of the p6 protein are suggested to be involved in regulating HIV-1 PR activity during viral replication (27). Our results indicate that the mutations in the slippery site encompass only two amino acids at the N terminus of p6 protein. Moreover, by comparing the ratios of Gag to Gag-Pol polyproteins we show that these mutants are defective in producing Gag-Pol, the product of frameshifting. Taken together, these results indicate that the observed virus production defects were due to an alteration of frameshifting efficiency that caused a change in the levels of enzymatic components. However, it cannot be formally excluded that the effects upon replication were amplified by the amino acid changes.
To date, conventional antiviral strategies have targeted the virus-specific proteins. The most commonly used classes of anti-HIV agents are nucleoside analogs and PR inhibitors, which inhibit reverse transcriptase and viral PR, respectively (reviewed in reference 7). However, this therapeutic strategy exerts a selective pressure on the virus, resulting in the growth of drug-resistant variants. The results described here characterize a novel target for anti-HIV drug discovery. It has been demonstrated that an alteration in frameshifting efficiency reduces viral titers. Viral programmed −1 ribosomal frameshifting is dependent on the cellular translational machinery; therefore, compounds that alter programmed −1 ribosomal frameshifting might affect the cellular translational machinery. This may be an advantage, because selective pressure on the cellular translational machinery would have to occur on the host evolutionary time scale. In addition, viral variants that might overcome the effects of the drugs by restoring the wild-type efficiency of programmed −1 ribosomal frameshifting would likely involve multiple mutations in the slippery site and/or the RNA secondary structure (5, 19). Thus, targeting the machinery that governs viral frameshifting might make it more difficult for drug-resistant mutants to arise, so it should represent an excellent target for the development of novel antiviral agents.
Acknowledgments
We thank members of the Peltz laboratory for helpful discussions and reading of the manuscript. Also, we thank Jon Dinman for providing us with plasmids pHIVlucwt, p2luc, and p2luci.
J.P.D. and S.W.P. are supported by grants from the National Institutes of Health (AI43886, AI51910, and CA5077).
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71**:**5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Current protocols in molecular biology, vol. 2. John Wiley & Sons, Inc., New York, N.Y.
- 3.Brierley, I. 1995. Ribosomal frameshifting viral RNAs. J. Gen. Virol. 76**:**1885-1892. [DOI] [PubMed] [Google Scholar]
- 4.Brierley, I., A. J. Jenner, and S. C. Inglis. 1992. Mutational analysis of the “slippery-sequence” component of a coronavirus ribosomal frameshifting signal. J. Mol. Biol. 227**:**463-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamorro, M., N. Parkin, and H. E. Varmus. 1992. An RNA pseudoknot and an optimal heptameric shift site are required for highly efficient ribosomal frameshifting on a retroviral messenger RNA. Proc. Natl. Acad. Sci. USA 89**:**713-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, C., and H. Okayama. 1998. Calcium phosphate-mediated gene transfer: a highly efficient system for stably transforming cells with plasmid DNA. BioTechniques 6**:**632-638. [PubMed] [Google Scholar]
- 7.Cohen, J. 2001. AIDS research. Debate begins over new vaccine trials. Science 293**:**1973. [DOI] [PubMed] [Google Scholar]
- 8.Dinman, J. D., T. Icho, and R. B. Wickner. 1991. A −1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc. Natl. Acad. Sci. USA 88**:**174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinman, J. D., S. Richter, E. P. Plant, R. C. Taylor, A. B. Hammell, and T. M. Rana. 2002. The frameshift signal of HIV-1 involves a potential intramolecular triplex RNA structure. Proc. Natl. Acad. Sci. USA 99**:**5331-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinman, J. D., and R. B. Wickner. 1992. Ribosomal frameshifting efficiency and gag/_gag_-pol ratio are critical for yeast M1 double-stranded RNA virus propagation. J. Virol. 66**:**3669-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulude, D., M. Baril, and L. Brakier-Gingras. 2002. Characterization of the frameshift stimulatory signal controlling a programmed −1 ribosomal frameshift in the human immunodeficiency virus type 1. Nucleic Acids Res. 30**:**5094-5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farabaugh, P. J. 1996. Programmed translational frameshifting. Annu. Rev. Genet. 30**:**507-528. [DOI] [PubMed] [Google Scholar]
- 13.Gesteland, R. F., and J. F. Atkins. 1996. Recoding: dynamic reprogramming of translation. Annu. Rev. Biochem. 65**:**741-768. [DOI] [PubMed] [Google Scholar]
- 14.Grentzmann, G., J. A. Ingram, P. J. Kelly, R. F. Gesteland, and J. F. Atkins. 1998. A dual-luciferase reporter system for studying recoding signals. RNA 4**:**479-486. [PMC free article] [PubMed] [Google Scholar]
- 15.Hill, M. K., M. Shehu-Xhilaga, S. M. Crowe, and J. Mak. 2002. Proline residues within spacer peptide p1 are important for human immunodeficiency virus type 1 infectivity, protein processing, and genomic RNA dimer stability. J. Virol. 76**:**11245-11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacks, T., H. D. Madhani, F. R. Masiarz, and H. E. Varmus. 1988. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell 55**:**447-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacks, T., M. D. Power, F. R. Masiarz, P. A. Luciw, P. J. Barr, and H. E. Varmus. 1988. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature 331**:**280-283. [DOI] [PubMed] [Google Scholar]
- 18.Jacks, T., and H. E. Varmus. 1985. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science 230**:**1237-1242. [DOI] [PubMed] [Google Scholar]
- 19.Kang, H. 1998. Direct structural evidence for formation of a stem-loop structure involved in ribosomal frameshifting in human immunodeficiency virus type 1. Biochim. Biophys. Acta 1397**:**73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan, A. H., J. A. Zack, M. Knigge, D. A. Paul, D. J. Kempf, D. W. Norbeck, and R. Swanstrom. 1993. Partial inhibition of the human immunodeficiency virus type 1 protease results in aberrant virus assembly and the formation of noninfectious particles. J. Virol. 67**:**4050-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaul, M., H. Yu, Y. Ron, and J. P. Dougherty. 1998. Regulated lentiviral packaging cell line devoid of most viral cis-acting sequences. Virology 249**:**167-174. [DOI] [PubMed] [Google Scholar]
- 22.Kuiken, C., B. Foley, B. Hahn, P. Marx, F. McCutchan, J. Mellors, S. Wolinsky, and B. E. Korber. 2001. HIV-1 sequence compendium 2001. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 23.Luukkonen, B. G., W. Tan, and S. Schwartz. 1995. Efficiency of reinitiation of translation on human immunodeficiency virus type 1 mRNAs is determined by the length of the upstream open reading frame and by intercistronic distance. J. Virol. 69**:**4086-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272**:**263-267. [DOI] [PubMed] [Google Scholar]
- 25.Pacchia, A. L., M. E. Adelson, M. Kaul, Y. Ron, and J. P. Dougherty. 2001. An inducible packaging cell system for safe, efficient lentiviral vector production in the absence of HIV-1 accessory proteins. Virology 282**:**77-86. [DOI] [PubMed] [Google Scholar]
- 26.Parkin, N. T., M. Chamorro, and H. E. Varmus. 1992. Human immunodeficiency virus type 1 _gag_-pol frameshifting is dependent on downstream mRNA secondary structure: demonstration by expression in vivo. J. Virol. 66**:**5147-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulus, C., S. Hellebrand, U. Tessmer, H. Wolf, H. G. Krausslich, and R. Wagner. 1999. Competitive inhibition of human immunodeficiency virus type-1 protease by the Gag-Pol transframe protein. J. Biol. Chem. 274**:**21539-21543. [DOI] [PubMed] [Google Scholar]
- 28.Pettit, S. C., G. J. Henderson, C. A. Schiffer, and R. Swanstrom. 2002. Replacement of the P1 amino acid of human immunodeficiency virus type 1 Gag processing sites can inhibit or enhance the rate of cleavage by the viral protease. J. Virol. 76**:**10226-10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richman, D. D. 2002. HIV chemotherapy. Nature 410**:**995-1001. [DOI] [PubMed] [Google Scholar]
- 30.Vaishnav, Y. N., and F. Wong-Staal. 1991. The biochemistry of AIDS. Annu. Rev. Biochem. 60**:**577-630. [DOI] [PubMed] [Google Scholar]