Human Immunodeficiency Virus Type 1 Envelope Glycoproteins That Lack Cytoplasmic Domain Cysteines: Impact on Association with Membrane Lipid Rafts and Incorporation onto Budding Virus Particles (original) (raw)
Abstract
The human immunodeficiency virus type 1 (HIV-1) envelope comprises a surface gp120 and a transmembrane gp41. The cytoplasmic domain of gp41 contains cysteine residues (C764 and C837) which are targets for palmitoylation and were reported to be required for envelope association with lipid rafts and assembly on budding virions (I. Rousso, M. B. Mixon, B. K. Chen, and P. S. Kim, Proc. Natl. Acad. Sci. USA **97:**13523-13525, 2000). Several infectious HIV-1 clones contain envelopes that have no gp41 cytoplasmic cysteines. Since no other gp41 amino acid is a target for palmitoylation, these clones imply that palmitoylation is not essential for envelope trafficking and assembly. Here, we show that HIV-1 envelope mutants that lack gp41 cytoplasmic cysteines are excluded from light lipid rafts. Envelopes that contained residues with bulky hydrophobic side chains instead of cysteines retained their association with heavy rafts and were nearly fully functional for incorporation into virions and infectivity. Substitution of cysteines with alanines or serines eliminated raft association and more severely reduced envelope incorporation onto virions and their infectivity. Nevertheless, the A764/A837 mutant envelope retained nearly 40% infectivity compared to the wild type, even though this envelope was excluded from lipid rafts. Our results demonstrate that gp41 cytoplasmic cysteines that are targets for palmitoylation and are required for envelope trafficking to classical lipid rafts are not essential for HIV-1 replication.
The covalent attachment of fatty acid groups, e.g., myristate and palmitate, to proteins plays an important role in their structure and function. Such fatty acid groups usually facilitate the interaction of proteins with lipid membranes. The cytoplasmic regions of diverse viral envelope glycoproteins contain cysteines that are targets for palmitoylation. Such viruses include orthomyxoviruses (influenza virus) (28), alphaviruses (Sindbis virus) (9), retroviruses (Rous sarcoma virus) (17), and lentiviruses (human immunodeficiency virus [HIV]) (21, 26). Although the exact function of palmitoylation is not known, it has been proposed to target viral glycoproteins into the lipid rafts (20) of the membranes for incorporation onto newly budding virions (21). However, Dolganiuc et al. recently reported that the addition of palmitate to the cytoplasmic domain of Newcastle disease virus was not required for lipid raft association (7).
Lipid rafts (also known as detergent-resistant glycosphingolipid glycosylphosphatidylinositol [GPI]-enriched membranes or detergent-resistant membrane fragments [DRMs]) are discrete microdomains in cell membranes that are enriched in specific types of lipids and proteins and insoluble in cold nonionic detergents (3, 14, 20). Lipid rafts are rich in sphingolipids and cholesterol and contain GPI-linked as well as palmitoylated proteins (14, 20, 22). This combination of lipids may support the efficient budding of newly synthesized virions (18). Purified HIV particles thus contain high concentrations of cholesterol, sphingomyelin, and GPI-anchored glycoproteins, consistent with lipid rafts as the sites of budding (2). Recently, the definition of lipid rafts has been refined to distinguish light and heavy lipid rafts (16). In sucrose gradients, classical rafts are light and are present in fractions with densities between 1.09 and 1.13 g/ml. Nebl et al. described a heavier raft that was present in fractions with densities between 1.16 and 1.2 g/ml. These heavy rafts were termed DRM-H to distinguish them from the lighter DRM-L rafts (16).
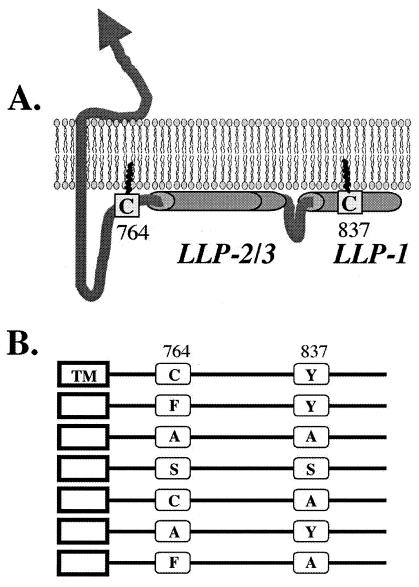
HIV envelopes usually contain two cysteines (C764 and C837) in the cytoplasmic domain (Fig. 1A) that have been shown to be palmitoylated (26). The gp41 cytoplasmic domain is a complex region of about 150 amino acids. Several gp41 regions form α-helices that interact with lipid bilayers (4). Three such α-helices were described as lentivirus lytic peptides (LLPs) (Fig. 1). C764 is located immediately upstream of LLP-2 and may therefore help anchor this region to the cell membrane. C837 is located in LLP-1. The precise functions of the gp41 cytoplasmic domain are not clear. This region has been shown to interact with several cellular proteins (8, 11, 24, 27) and contains determinants for binding to the matrix domain of the p55_gag_ precursor (10, 15).
FIG. 1.
The cytoplasmic domain of gp41. (A) LLP-1, LLP-2, and LLP-3 are putative α-helices that are proposed to interact with lipid bilayers. Palmitate groups covalently attached to C764 and C837 may insert into the lipid bilayer and anchor these regions to the plasma membrane. Palmitoylation may target gp41 into lipid rafts (21). (B) Amino acid substitutions introduced into the gp41 cytoplasmic domain.
Mutations that replaced both gp41 cytoplasmic cysteines with serines were shown to completely eliminate palmitoylation (26). These substitutions also resulted in decreased association of envelopes with lipid rafts, decreased incorporation onto virions, and a severe reduction in infectivity (21). Nevertheless, 5% of envelope sequences in the HIV sequence database (http://hiv-web.lanl.gov) do not contain cysteines at positions 764 and 837. A widely used molecular clone of HIV type 1 (HIV-1), pJRCSF, contains no cysteines in the gp41 cytoplasmic domain yet is fully replication competent and fusigenic, thus implying that palmitoylation of gp41 is not essential for HIV-1 replication. Here, we have directly tested whether gp41 cytoplasmic cysteines can be replaced with amino acids carrying hydrophobic side chains, the residues that most commonly substitute for C764 and C837. We showed that an NL43 envelope lacking gp41 cytoplasmic cysteines and with phenylalanine at position 764 is efficiently assembled on virions and confers near-wild-type levels of infectivity. This envelope was excluded from DRM-L but retained its association with DRM-H.
Construction of NL43 gp41 mutants.
C764 and C837 are usually the only cysteine residues present in the gp41 transmembrane and cytoplasmic regions. C764 is highly conserved. A cysteine is present at position 764 in over 93% of the sequences of 291 aligned envelopes in the HIV sequence database (http://hiv-web.lanl.gov). Phenylalanine is the most common substitute for cysteine at residue 764. Moreover, three infectious HIV-1 molecular clones which lack cytoplasmic cysteines (BORI [F764/Y837], JRCSF [F764/Y837], and DH123 [F764/G837]) each contain phenylalanine at position 764. Only about 40% of the HIV-1 strains carry a cysteine at position 837, while 37% have a residue with a bulky hydrophobic side chain including tyrosine, phenylalanine, and tryptophan. The cytoplasmic domain of NL43 gp41 contains only one cysteine (C764) and has a tyrosine at position 837 (Y837). Thus, only one amino acid substitution is required to completely eliminate cysteines from the cytoplasmic region of NL43 gp41. We first substituted phenylalanine for cysteine at residue 764 to construct an F764/Y837 NL43 envelope. We also introduced alanines at residues 764 and 837, since this residue is frequently used in mutagenesis studies. Additionally, we constructed an S764/S837 mutant, since this combination was previously reported to abrogate envelope incorporation onto virions and infectivity (21). These and other envelope mutants constructed are shown in Fig. 1B. Mutations were introduced by PCR into the NL43 envelope sequence contained in the pSVIIIenv expression vector. The presence of mutations was confirmed by sequencing.
Mutant NL43 envelopes lacking cytoplasmic cysteines are expressed and functional.
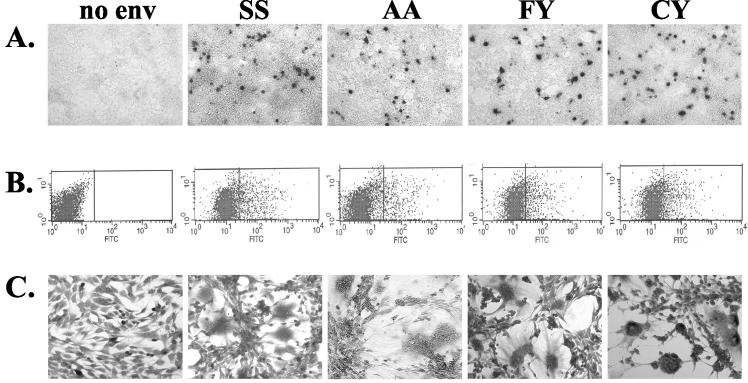
We first sought to evaluate whether mutant envelopes were expressed in 293T cells. Each pSVIIIenv construct was cotransfected into 293T cells with a pNL43env− construct. pNL43env− expresses all HIV proteins (except envelope) required for particle formation. pNL43env− also expresses TAT, which is required for efficient envelope expression from pSVIIIenv. Forty-eight hours after transfection, 293T cells were permeabilized with methanol-acetone (1:1) and immunostained with Chessie 8, an anti-gp41 monoclonal antibody (MAb) (5). Positively stained envelope-expressing cells were clearly visible for each mutant envelope construct (Fig. 2A). We next tested whether each mutated envelope was expressed on the cell surface. Transfected 293T cells were removed by treatment with EDTA 48 h after transfection, and envelope expression was tested by flow cytometry with gp120-specific MAb 902 (5, 19). Figure 2B shows that 293T cells transfected with each mutant envelope were positive for cell surface expression of gp120. These results indicate that palmitoylated cysteines in gp41 are not essential for the expression or cell surface trafficking of the HIV-1 envelope and confirm previously reported observations for S764/S837 HIV-1 envelope mutants (21, 26).
FIG. 2.
Envelope expression and function. (A) 293T cells were cotransfected with pNL43env− and pSVIIIenv containing gp41 envelope mutants (C764/Y837 [CY], F764/Y837 [FY], A764/A837 [AA], and S764/S837 [SS]) and were immunostained for envelope. Cells were fixed and permeabilized with methanol-acetone (1:1) 48 h after transfection before immunostaining with anti-gp41 MAb Chessie 8 (1). (B) Cell surface envelope expression. 293T cells (transfected as described for panel A) were stained for surface envelope expression by using anti-gp120 MAb 902 (5, 19) and examined by flow cytometry. (C) Cell-cell fusion induced by NL43 mutant envelopes. 293T cells (transfected as described for panel A) were cocultivated with NP2/CD4/CXCR4. Cells were fixed and stained with 1% methylene blue-0.25% basic fuchsin in methanol.
The ability of HIV-1 gp120 to mediate cell-cell fusion is frequently used to assess envelope function as well as receptor-coreceptor utilization. Transfected 293T cells expressing mutant envelopes were tested for their capacity to induce syncytia in CD4+, GHOST/CXCR4 and NP2/CD4/CXCR4 cells. Figure 2C shows syncytia induced following cocultivation of NP2/CD4/CXCR4 cells (23) with 293T cells cotransfected with pSVIIIenv and pNL43env−. Each mutant envelope was functional for cell-cell fusion and induced levels of syncytia comparable to those of the NL43 wild-type envelope. Syncytia induced by each mutant envelope became apparent in the culture at the same time (between 5 and 6 h) after env+ 293T cells and NP2/CD4/CXCR4 were mixed.
Effect of gp41 mutations on infectivity.
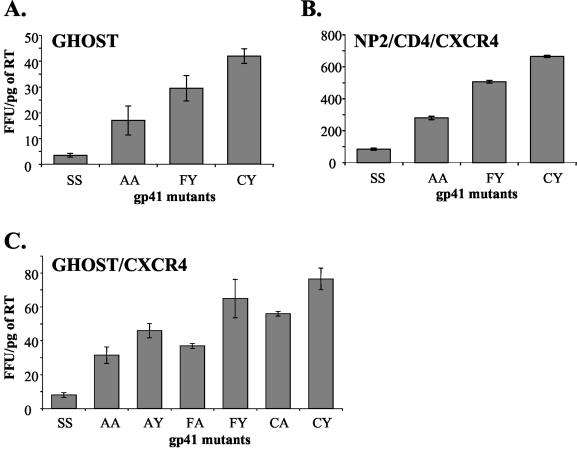
Each env+ pSVIIIenv plasmid was cotransfected with pNL43env− into 293T cells. Pseudotype viruses were harvested 48 h posttransfection and measured for reverse transcriptase (RT) activity with an RT-enzyme-linked immunosorbent assay (ELISA) kit (Cavidi Tech Inc., Uppsala, Sweden). Pseudotype viruses carrying wild-type and mutant envelopes were tested in single-round infectivity assays with CD4+ CCR5+ GHOST and NP2/CD4/CXCR4 cells. Cells were challenged with pseudotype viruses containing equal amounts of RT activity. Infectivity was reduced by replacement of C764 with other residues (Fig. 3A). The replacement of C764/Y837 with S764/S837 resulted in approximately 90% reduction in infectivity, consistent with previous reports (21). The infectivity of the A764/A837 mutant was also reduced; however, this mutant consistently retained up to 40% infectivity compared to the NL43 wild-type envelope. In contrast, the F764/Y837 mutant envelope retained over 80% infectivity compared to the wild type and this envelope was therefore nearly fully functional. Figure 3C shows the infectivity conferred by other envelope mutants lacking cytoplasmic cysteines, including F764/A837 and A764/Y837 as well as C764/A837. These results show a range of infectivities from 40 to 95% of the infectivity measured for the wild-type envelope.
FIG. 3.
Infectivity conferred by HIV-1 NL43 envelopes lacking gp41 cytoplasmic cysteines. Infectivity was tested with CD4+ indicator cell lines as shown. (A) GHOST parental cells that express endogenous CXCR4; (B) NP2/CD4/CXCR4 cells; (C) GHOST/CXCR4 cells. Cells were fixed 72 h after infection and immunostained for p24. Infectivity titers were then scored as focus-forming units (FFU) per picogram of RT in virus supernatants as measured by ELISA.
Effect of gp41 mutations on envelope incorporation onto virions.
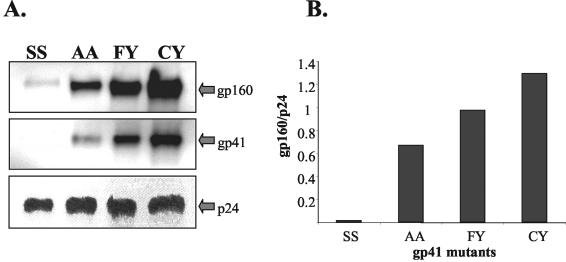
We next investigated how the gp41 substitutions affected incorporation of the envelope onto virus particles. 293T cells were cotransfected with pSVIIIenv containing either S764/S837, A764/A837, F764/Y837, or C764/Y837 mutant envelope together with pNL43env−. The envelope-positive pseudotype viruses were harvested at 48 h, centrifuged at low speed, and passed through 0.45-μm-pore-size syringe filters to remove cell debris and residual 293T cells. Virus particles were pelleted at 100,000 × g for 2 h at 4°C, and the pellets were reconstituted in phosphate-buffered saline. The RT activity of each reconstituted pellet was measured by RT-ELISA, and equal amounts of RT activity were then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; then, the pellet was blotted and probed for gp160/gp41 with the Chessie 8 MAb and for p24 with 183-H12-5C (25). Figure 4 shows the level of each NL43 gp41 mutant envelope on virions. As previously reported (21), the S764/S837 mutant envelope was assembled very inefficiently on virions. The assembly of the A764/A837 envelope was also reduced compared to that of the wild-type envelope, while only a modest reduction was observed for F764/Y837. These results show that the replacement of NL43 gp41 cytoplasmic cysteines consistently reduced the amount of envelope incorporated onto virions. Substitutions with amino acids that carried bulky hydrophobic side chains conferred only a minimal reduction of envelope on virions, while substitutions with serine residues resulted in a severe decrease of virion-associated envelope.
FIG. 4.
Incorporation of mutant NL43 envelopes onto virus particles. 293T cells were cotransfected with pNL43env− and pSVIIIenv containing gp41 envelope mutants (C764/Y837 [CY], F764/Y837 [FY], A764/A837 [AA], and S764/S837 [SS]). Supernatants harvested 48 h after transfection were spun at low speed and filtered through a 0.45-μm-pore-size syringe filter. The supernatants were then ultracentrifuged at 100,000 × g for 2 h at 4°C to pellet virus particles. The pelleted virions were resuspended and measured for RT activity. Equivalent amounts of RT activity were then resolved on a sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis gel followed by Western blotting with anti-gp41 Chessie 8 or anti-p24 183-H12-5C as primary antibodies. (A) Western blot analysis of envelope on virions; (B) ratios of envelope to p24 gag concentration evaluated by densitometry of Western blot bands.
Association of envelope mutants with lipid rafts.
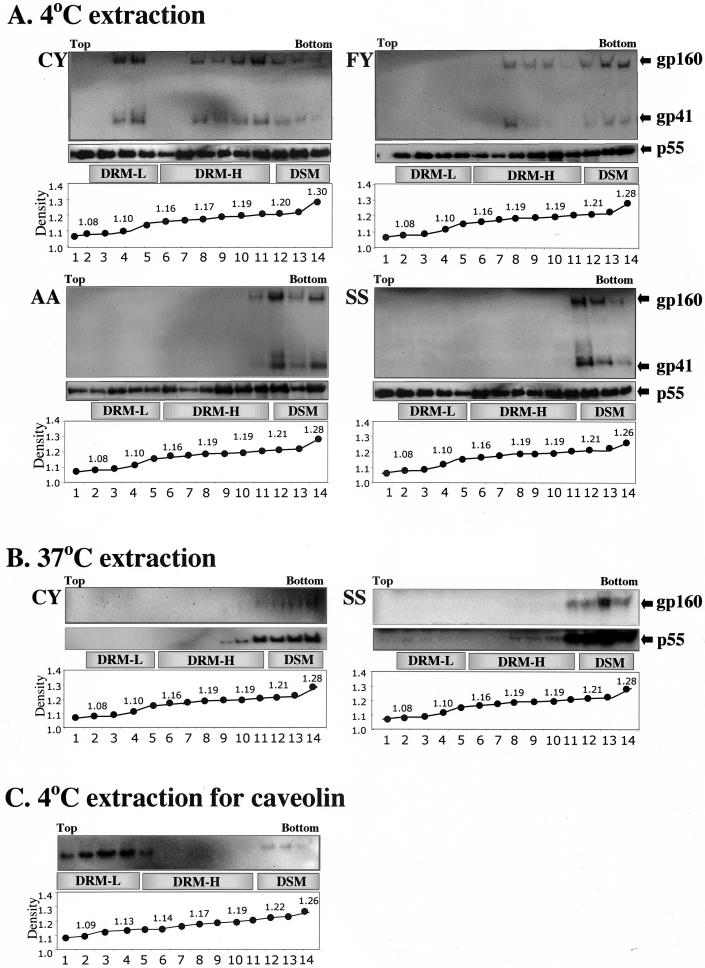
Finally, we tested whether envelope mutants that lacked cysteines in the cytoplasmic domain were associated with lipid rafts. The procedures used to analyze lipid rafts were based on those described previously by others (7, 16). 293T cells (1.2 × 106 cells) were transfected with pNL43env− and pSVIIIenv constructs carrying each envelope mutant. Transfected cells were lysed with Triton X-100 at a final concentration of 0.5% in TNE (10 mM Tris, 100 mM sodium chloride, 10 mM EGTA [pH 7.5], protease inhibitor cocktail [Sigma Inc.]) for 30 min. Lysates were homogenized and centrifuged at low speed to remove nuclei. Supernatants were adjusted to 60% sucrose in a 1-ml volume and loaded on top of 250 μl of 80% sucrose. The sucrose gradient was completed by layering 1 ml of 50% sucrose, 3 ml of 38% sucrose, and 750 μl of 10% sucrose on top of the lysate sample. These steps were carried out with the sample either on ice or at a temperature below 4°C. Sucrose gradients were centrifuged for 18 h at 100,000 × g (4°C) in an SW 50.1 Ti rotor. Fourteen fractions were collected, and each fraction was immunoprecipitated with HIV-1+ human serum (1:1,000). Immunoprecipitated proteins were analyzed by polyacrylamide gel electrophoresis followed by Western blotting. Blots were probed with anti-p55_gag_/p24_gag_ MAb 183-H12-5C (6) and anti-gp41 MAb Chessie 8 (1) (Fig. 5A). DRM-L and DRM-H float at densities of 1.09 to 1.13 and 1.16 to 1.2 g/ml, respectively. The p55_gag_ precursor was detected throughout the gradient but was concentrated in both DRM-H and DRM-L, consistent with previously reported observations (18). The envelope precursor gp160 and the processed gp41 were consistently both located in the same fractions. The wild-type C764/Y837 envelope was detected in fractions with densities consistent with both DRM-L and DRM-H lipid rafts. In contrast, envelopes that lacked gp41 cytoplasmic cysteines were not associated with DRM-L fractions. However, the F764/Y837 envelope was detected in fractions with densities consistent with DRM-H, indicating that association with these heavier rafts was retained.
FIG.5.
Association of mutant NL43 envelopes with lipid rafts. 293T cells were cotransfected with pNL43env− and pSVIIIenv containing gp41 envelope mutants. Cells were lysed with 0.5% Triton X-100 in TNE buffer on ice. Lysates were homogenized, cleared of nuclei, adjusted to 60% sucrose, and applied to the bottom of a sucrose gradient (see text). Gradient fractions were Western blotted and probed for envelope and gag with mouse MAbs. (A) Sucrose gradient fractionation of cold Triton X-100 extracts from 293T cells cotransfected with pNL43env− and pSVIIIenv encoding the NL43 envelope mutants C764/Y837 (CY), F764/Y837 (FY), A764/A837 (AA), and S764/S837 (SS). Note that the NL43 CY envelope associates with both the DRM-L and the DRM-H fractions. FY is excluded from DRM-L but retains an association with DRM-H. The AA and SS envelopes are completely excluded from rafts. (B) Sucrose gradient fractionations, as described for panel A except that Triton X-100 extractions were carried out at 37°C to solubilize lipid rafts. Note that all the gag precursor and envelope detected is present in the fractions representing soluble membranes and not at densities that correspond to lipid rafts. (C) Sucrose gradient of 293T cold Triton X-100 extracts, blotted and probed with a rabbit polyclonal antibody to caveolin (molecular mass, 20 to 22 kDa; DRM-L marker) (BD Biosciences Transduction Laboratories Inc.).
Envelopes that did not contain gp41 cytoplasmic cysteines or residues with bulky hydrophobic side chains at positions 764/837 (A764/A837 and S764/S837) were almost completely excluded from both DRM-L and DRM-H. Treatment of Triton X-100 cell lysates at 37°C disrupted the DRM relocating envelope and the p55_gag_ precursor into the soluble fraction at the bottom of the gradient (Fig. 5B). Western blots of cold Triton X-100 extracts of 293T cells, fractionated on sucrose gradients (prepared as described for Fig. 5A), indicated that caveolin (a marker for lipid rafts) was contained almost exclusively in fractions with densities consistent with DRM-L.
These results show that (i) gp41 cytoplasmic cysteines, which are targets for palmitoylation, are required for the association of the envelope with DRM-L; (ii) envelopes that contained residues with bulky hydrophobic side chains (instead of cysteines) retained their association with DRM-H and were efficiently assembled on virions; and (iii) envelopes with C764A/C837A and C764S/C837S substitutions were excluded from lipid rafts yet were incorporated onto virions with efficiencies of up to 40% compared to wild-type envelopes.
Previously, Yang et al. (26) reported that gp41 cytoplasmic cysteines (C764 and C837) were palmitoylated and that the replacement of these residues completely abolished palmitoylation. Subsequently, Rousso et al. showed that HIV-1 envelopes carrying double C764S/C837S substitutions lost their association with lipid rafts, were inefficiently incorporated onto budding viruses, and conferred only low levels of infectivity (21). Nevertheless, several infectious molecular clones of HIV-1, e.g., JRCSF, lack cysteines in the cytoplasmic domain of gp41 and yet are apparently fully functional and replication competent. Since no other gp41 amino acid has been shown to be a substrate for palmitic acid addition (26), this observation implied that palmitoylation of gp41 was not essential for envelope assembly on virions or infectivity.
Palmitoylation occurs almost exclusively via the covalent attachment of palmitate groups to cysteine residues via thioester bonds (12, 20). A single exception was recently reported by Kleuss and Krause (12), who demonstrated that an N-terminal glycine of the G-protein α subunit Gsα was palmitoylated via an amide linkage. The mechanism of glycine palmitoylation is unclear, although it is possible that the palmitate group is transferred from an adjacent palmitoylated cysteine in Gsα (13). Here, we further examined the role of gp41 cytoplasmic cysteine residues in envelope assembly. Using HIV-1 NL43, which has only a single gp41 cytoplasmic cysteine at position 764 (C764), we showed that gp41 cytoplasmic cysteines are not required for HIV-1 envelope incorporation onto virions and infectivity. The replacement of C764 and C837 with residues carrying bulky hydrophobic side chains may therefore compensate for the lack of a palmitate group. Here, we showed that cytoplasmic cysteines which are targets for palmitoylation are required for the localization of envelope glycoproteins to light lipid rafts (DRM-L). The replacement of cysteines with residues that carry bulky hydrophobic side chains resulted in the retention of the association with heavy lipid rafts (DRM-H) and conferred near-wild-type levels of envelope assembly on virions. The replacement of cysteines with alanines or serines eliminated raft association and reduced envelope incorporation onto virions and the infectivity of virions. Nevertheless, the A764/A837 mutant envelope retained nearly 40% infectivity compared to the wild type, even though this envelope was excluded from lipid rafts. In summary, our data show that the presence of cysteine residues in the gp41 cytoplasmic domain is not essential for envelope incorporation onto virions or the infectivity of virions.
Acknowledgments
We thank Trudy Morrison (University of Massachusetts Medical School) for expert advice and very helpful comments on the lipid raft experiments. We also thank Lori McGinnes (University of Massachusetts Medical School) for help in running lipid raft gradients and advice on Western blotting of gradient fractions. We appreciate expert help from Jim Coderre and Bruce Blais (University of Massachusetts Medical School) with Western blotting and flow cytometry, respectively. We are highly appreciative of the NIH AIDS Research and Reference Reagent Program and the United Kingdom Centralized Facility for AIDS Reagents, which provided reagents for the work presented.
This work was supported by University of Massachusetts Medical School Center for AIDS Research developmental grants and by NIH grant R01MH64408-01. P.R.C. is an Elizabeth Glaser Pediatric AIDS Foundation scientist.
REFERENCES
- 1.Abacioglu, Y. H., T. R. Fouts, J. D. Laman, E. Claassen, S. H. Pincus, J. P. Moore, C. A. Roby, R. Kamin-Lewis, and G. K. Lewis. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res. Hum. Retrovir. 10**:**371-381. [DOI] [PubMed] [Google Scholar]
- 2.Aloia, R. C., H. Tian, and F. C. Jensen. 1993. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. USA 90**:**5181-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, D. 2002. Structure and function of membrane rafts. Int. J. Med. Microbiol. 291**:**433-437. [DOI] [PubMed] [Google Scholar]
- 4.Chen, S. S., S. F. Lee, and C. T. Wang. 2001. Cellular membrane-binding ability of the C-terminal cytoplasmic domain of human immunodeficiency virus type 1 envelope transmembrane protein gp41. J. Virol. 75**:**9925-9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesebro, B., and K. Wehrly. 1988. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J. Virol. 62**:**3779-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesebro, B., K. Wehrly, J. Nishio, and S. Perryman. 1992. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J. Virol. 66**:**6547-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolganiuc, V., L. McGinnes, E. J. Luna, and T. G. Morrison. 2003. Role of the cytoplasmic domain of the Newcastle disease virus fusion protein in association with lipid rafts. J. Virol. 77**:**12968-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, D. T., K. C. Tillman, and R. C. Desrosiers. 2002. Envelope glycoprotein cytoplasmic domains from diverse lentiviruses interact with the prenylated Rab acceptor. J. Virol. 76**:**327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanova, L., and M. J. Schlesinger. 1993. Site-directed mutations in the Sindbis virus E2 glycoprotein identify palmitoylation sites and affect virus budding. J. Virol. 67**:**2546-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalia, V., S. Sarkar, P. Gupta, and R. C. Montelaro. 2003. Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J. Virol. 77**:**3634-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, J. T., E. M. Kim, K. H. Lee, J. E. Choi, B. H. Jhun, and J. W. Kim. 2002. Leucine zipper domain of HIV-1 gp41 interacted specifically with alpha-catenin. Biochem. Biophys. Res. Commun. 291**:**1239-1244. [DOI] [PubMed] [Google Scholar]
- 12.Kleuss, C., and E. Krause. 2003. Galpha(s) is palmitoylated at the N-terminal glycine. EMBO J. 22**:**826-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linder, M. E., and R. J. Deschenes. 2003. New insights into the mechanisms of protein palmitoylation. Biochemistry 42**:**4311-4320. [DOI] [PubMed] [Google Scholar]
- 14.Melkonian, K. A., A. G. Ostermeyer, J. Z. Chen, M. G. Roth, and D. A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274**:**3910-3917. [DOI] [PubMed] [Google Scholar]
- 15.Murakami, T., and E. O. Freed. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74**:**3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nebl, T., K. N. Pestonjamasp, J. D. Leszyk, J. L. Crowley, S. W. Oh, and E. J. Luna. 2002. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem. 277**:**43399-43409. [DOI] [PubMed] [Google Scholar]
- 17.Ochsenbauer-Jambor, C., D. C. Miller, C. R. Roberts, S. S. Rhee, and E. Hunter. 2001. Palmitoylation of the Rous sarcoma virus transmembrane glycoprotein is required for protein stability and virus infectivity. J. Virol. 75**:**11544-11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98**:**13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pincus, S. H., K. Wehrly, and B. Chesebro. 1989. Treatment of HIV tissue culture infection with monoclonal antibody-ricin A chain conjugates. J. Immunol. 142**:**3070-3075. [PubMed] [Google Scholar]
- 20.Resh, M. D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451**:**1-16. [DOI] [PubMed] [Google Scholar]
- 21.Rousso, I., M. B. Mixon, B. K. Chen, and P. S. Kim. 2000. Palmitoylation of the HIV-1 envelope glycoprotein is critical for viral infectivity. Proc. Natl. Acad. Sci. USA 97**:**13523-13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder, R. J., S. N. Ahmed, Y. Zhu, E. London, and D. A. Brown. 1998. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J. Biol. Chem. 273**:**1150-1157. [DOI] [PubMed] [Google Scholar]
- 23.Soda, Y., N. Shimizu, A. Jinno, H. Y. Liu, K. Kanbe, T. Kitamura, and H. Hoshino. 1999. Establishment of a new system for determination of coreceptor usages of HIV based on the human glioma NP-2 cell line. Biochem. Biophys. Res. Commun. 258**:**313-321. [DOI] [PubMed] [Google Scholar]
- 24.Tencza, S. B., T. A. Mietzner, and R. C. Montelaro. 1997. Calmodulin-binding function of LLP segments from the HIV type 1 transmembrane protein is conserved among natural sequence variants. AIDS Res. Hum. Retrovir. 13**:**263-269. [DOI] [PubMed] [Google Scholar]
- 25.Toohey, K., K. Wehrly, J. Nishio, S. Perryman, and B. Chesebro. 1995. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology 213**:**70-79. [DOI] [PubMed] [Google Scholar]
- 26.Yang, C., C. P. Spies, and R. W. Compans. 1995. The human and simian immunodeficiency virus envelope glycoprotein transmembrane subunits are palmitoylated. Proc. Natl. Acad. Sci. USA 92**:**9871-9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, H., L. Wang, S. Kao, I. P. Whitehead, M. J. Hart, B. Liu, K. Duus, K. Burridge, C. J. Der, and L. Su. 1999. Functional interaction between the cytoplasmic leucine-zipper domain of HIV-1 gp41 and p115-RhoGEF. Curr. Biol. 9**:**1271-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zurcher, T., G. Luo, and P. Palese. 1994. Mutations at palmitylation sites of the influenza virus hemagglutinin affect virus formation. J. Virol. 68**:**5748-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]