Parallel somatic and synaptic processing in the induction of intermediate-term and long-term synaptic facilitation in Aplysia (original) (raw)
Abstract
The induction of different phases of memory depends on the amount and patterning of training, raising the question of whether specific training patterns engage different cellular mechanisms and whether these mechanisms operate in series or in parallel. We examined these questions by using a cellular model of memory formation: facilitation of the tail sensory neuron-motor neuron synapses by serotonin (5-hydroxytryptamine, 5-HT) in the CNS of Aplysia. We studied facilitation in two temporal domains: intermediate-term facilitation (1.5-3 h) and long-term facilitation (LTF, >24 h). Both forms can be induced by using several different temporal and spatial patterns of 5-HT, including (i) repeated, temporally spaced pulses of 5-HT to both the sensory neuron soma and the sensory neuron-motor neuron synapse, and (ii) temporally asymmetric exposure of 5-HT to the soma and synapse under conditions in which neither exposure alone induces LTF. We first examined the protein and RNA synthesis requirements for LTF induced by these two patterns and found that asymmetric (but not repeated) 5-HT application induced LTF that required postsynaptic protein and RNA synthesis. We next focused on the patterning and protein synthesis requirements for intermediate-term facilitation. We found that intermediate-term facilitation (i) is induced locally at the synapse, (ii) requires multiple pulses of 5-HT, and (iii) requires synaptic protein synthesis. Our findings show that different temporal and spatial patterns of 5-HT induce specific temporal phases of long-lasting facilitation in parallel by engaging different cellular and molecular mechanisms.
It has long been appreciated that memories can persist in dramatically different time frames, from seconds and minutes up to a lifetime (1, 2). A major question now concerns the relationship between memories that exist in different temporal domains. One possibility, for which there is considerable empirical support (see ref. 1), is that memories are processed in series. However, there is a growing body of evidence showing that, at least under certain circumstances, memories can also be processed in parallel (3-7).
Like memory, neuronal plasticity can exist in several temporal domains. This feature of plasticity has been revealed in many experimental contexts, including three model systems that have been especially useful for studying the synaptic basis for learning and memory: the mammalian hippocampus (8-11), the photo-receptor neurons in Hermissenda (12, 13), and the sensory neuron-motor neuron (SN-MN) synapses of Aplysia. In Aplysia, the tail-elicited siphon-withdrawal reflex has been useful for examining the temporal relationships between synaptic plasticity and memory. Memory for sensitization induced by repeated tail shocks can exist in at least three temporal domains: short-term memory (<25 min), intermediate-term memory (≈90 min), and long-term memory (>24 h) (14-16). Because tail shock releases serotonin (5-hydroxytryptamine, 5-HT) systemically (17) and in regions of the CNS containing SN and MN neurites (ref. 18; S. Marinesco, K. E. Kolkman, and T.J.C., unpublished data), direct application of 5-HT to the CNS can serve as a proxy for tail shock in experiments examining synaptic facilitation underlying memory formation. A single exposure to 5-HT induces short-term facilitation (STF, <25 min; refs. 19 and 20), whereas repeated exposures induce both intermediate-term facilitation (ITF; refs. 21 and 22), which lasts up to 90 min, and long-term facilitation (LTF), which begins to be expressed at ≈10 h and lasts >24 h (23).
On the synaptic level, ITF exists in multiple forms that can be distinguished mechanistically (22, 24). In addition, many forms of LTF have been described. At the tail SN-MN synapses in the pleural-pedal ganglia, LTF can be induced by (i) five spaced pulses of 5-HT to both ganglia (23, 25, 26), (ii) a prolonged (90-min) continuous application of 5-HT to either the SN somata alone or to the synapses alone (27, 28), and (iii) 5 min of 5-HT at the synapse coincident with 25 min of 5-HT at the SN soma (29). In this latter form, temporally asymmetric LTF (referred to as coincident LTF in ref. 29), neither somatic nor synaptic 5-HT alone induces LTF. Asymmetric LTF requires a coordinated cellular response to the combination of 5-HT in the two cellular compartments.
The general observation that various patterns of 5-HT, which differ in their temporal and spatial characteristics, can induce intermediate-term and long-term forms of plasticity raises the question of whether the various forms of ITF and LTF engage cellular processes that converge on a common set of signaling mechanisms or whether there are multiple parallel pathways that ultimately give rise to each form of plasticity. In the present study, we examined different temporal phases of synaptic facilitation induced by two patterns of 5-HT exposure, repeated 5-HT and asymmetric 5-HT, focusing specifically on their temporal and spatial requirements for translation and transcription. Our results show that different temporal and spatial patterns of 5-HT engage parallel and independent somatic and synaptic mechanisms for the induction of ITF and LTF.
Methods
Animals and Dissection. Wild-caught Aplysia californica were obtained from Marinus (Long Beach, CA) or Marine Specimens Unlimited (Pacific Palisades, CA) and kept in seawater aquaria at 17°C. Animals were anesthetized by using isotonic MgCl2. One pair of pleural-pedal ganglia was removed and pinned in a Sylgard-lined recording dish filled with 1:1 artificial seawater (ASW)/MgCl2, and the cell clusters containing tail SNs and MNs were desheathed. For experiments in which the SN soma and synapse were differentially exposed to 5-HT and/or other drugs, the ganglia were pinned in a two-chamber recording dish, as has been described (27, 29). Each chamber held a volume of ≈2.5 ml. Briefly, the pedal ganglion, containing the cluster of MN somata and tail SN-MN synapses, was pinned on one side of a plastic barrier (synaptic compartment). The pleural ganglion, containing the SN somata, was pinned on the other side (somatic compartment). The connective between the two ganglia lay a notch in the barrier that was sealed with Vaseline. The integrity of the barrier was tested (i) by placing a few drops of Fast Green dye in one chamber and looking for leakage into the other, and (ii) by perfusing 5-HT onto the pleural ganglion alone; because somatic 5-HT does not induce STF, its induction would indicate a leak. Preparations were perfused with ASW for 15 min before recording to wash out the MgCl2 and throughout the experiment (except where noted). Intracellular recordings were made from SNs and MNs.
In all experiments, three pretests were taken (at 15-min intertrial intervals) to ensure a stable baseline excitatory postsynaptic potential (EPSP) amplitude. Synapses in which pretests showed a deviation of >20% from their mean amplitude were discarded (≈15% of preparations). After completing all necessary short-term and intermediate-term tests, SNs and MNs were marked by filling the surrounding neurons with 5% Fast Green dye for identification in the long-term test (27, 29). Preparations were incubated overnight at 15°C. Preparations were removed from the incubator 20-24 h later, washed for 15 min with ASW at room temperature, and tested for LTF.
Drugs. All drugs were obtained from Sigma. We bath-perfused 5-HT (50 μM, in ASW), Emetine (100 μM, dissolved in water and diluted 1:2000 in ASW), and 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB, 100 μM, dissolved in DMSO, final concentration 0.6%, and diluted in ASW) to one or both chambers. Actinomycin D (40 μM, dissolved in water and diluted 1:10 in ASW) was applied in static bath for 1 h and then washed out before initial recording. Gelonin (dissolved in PBS and diluted in microelectrode buffer, resulting in final concentrations of 25 μM gelonin, 350 mM KCl, and 50 mM Tris) was pressure-injected into MNs by using a picospritzer.
Data Analysis. EPSP amplitudes were normalized to the average pretest. For most experiments, data were analyzed by SPSS by using unpaired t tests for between-group comparisons and difference scores for within-group comparisons. In cases in which more than two groups were tested, data were first subjected to ANOVA, followed by a least significant difference test.
Results
Multiple Forms of LTF Have Different Translational Requirements. LTF induced by repeated 5-HT requires both protein and RNA synthesis (25, 26), and in cultured SN-MN synapses, the protein synthesis requirement is solely presynaptic (25). We have shown (29) that LTF induced by asymmetric 5-HT requires protein synthesis both in the SN soma (i.e., presynaptic) as well as in the region of the SN-MN synapse. However, our synaptic experiments were done by blocking protein synthesis in the synaptic compartment, which contains both presynaptic SN terminals and the postsynaptic MN soma and neurites. Thus, in those experiments we could not determine whether the protein synthesis requirement for asymmetric LTF in the synaptic compartment might also have a postsynaptic component.
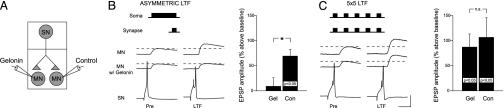
To examine the role of postsynaptic protein synthesis in the induction of asymmetric LTF, we injected the ribosomal blocker gelonin (see ref. 30) into a single MN before 5-HT exposure. Microelectrode recordings were made from one SN and two follower MNs (Fig. 1_A_). Gelonin was injected into one MN, and control buffer was injected into the other, followed by asymmetric 5-HT treatment: 25 min of 5-HT to the soma and 5 min of 5-HT at the synapse, coincident with the final 5 min of somatic exposure (29). We found that LTF was induced by asymmetric 5-HT only at the control synapse (EPSP amplitude 20-24 h after 5-HT: +69.8 ± 13%, _t_8 = 5.26, P < 0.05; Fig. 1_B_) and not at the synapse injected with gelonin (EPSP amplitude +9.3 ± 17%, _t_8 = 0.56, not significant). There was a significant difference between these two groups (_t_16 = 2.84, P < 0.05). In contrast, there was no significant difference between STF induced in gelonin-treated and control groups (n = 7 for each group, _t_12 = 0.143, not significant, data not shown). Thus, the induction of asymmetric LTF, but not STF, has a postsynaptic requirement for protein synthesis.
Fig. 1.
LTF induced by asymmetric 5-HT requires postsynaptic protein synthesis, whereas LTF induced by repeated 5-HT does not. (A) Diagram of recordings in the two-compartment chamber. Protein synthesis was blocked in one MN by injection of gelonin; the other MN received a control injection. (B and C) Gelonin blocked induction of LTF by asymmetric 5-HT (B, n = 9) but not by repeated pulses of 5-HT (C, n = 8). [Scale bar, 20 ms, 10 mV (MNs), 36 mV (SN).] *, P < 0.05.
These results differ from those of Martin et al. (25) in cultured Aplysia SN-MN synapses, in which gelonin was injected into either an MN or an SN before induction of LTF by five pulses of 5-HT. They found that gelonin blocked LTF induction when injected into the SN but had no effect when injected into the MN. The disparity between these two findings could be due to differences in the experimental systems or could be the result of the different patterns of 5-HT used to induce LTF. To distinguish between these possibilities, we induced LTF in our system (Fig. 1 A) by using repeated 5-HT (five pulses, 5 min each, with a 15-min intertrial interval) delivered to both somatic and synaptic compartments (Fig. 1_C_). We found, similar to Martin et al. (25), that significant LTF was induced in both the gelonin (+87.6 ± 26%) and control (+107.1 ± 39%) groups [_t_7 = 3.40 (gelonin) and 2.75 (control), P < 0.05 for each group], and there was no significant difference between the groups (_t_14 = 0.418, not significant). These results show that two different patterns of 5-HT exposure, asymmetric and repeated pulses, both induce LTF, but the protein synthesis requirements for each form are different: repeated 5-HT induces LTF, which has only a presynaptic translational requirement, whereas asymmetric LTF has both a presynaptic and a postsynaptic translation requirement.
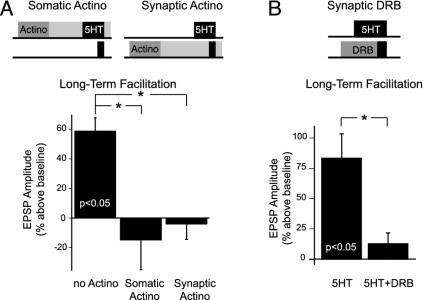
Multiple Forms of LTF Have Different Transcriptional Requirements. LTF induced by repeated 5-HT also requires transcription (25, 26). We next asked whether transcription was required for the induction of asymmetric LTF and, if so, whether it was required in the SN (somatic compartment), in the MN (synaptic compartment), or in both. We preincubated either the somatic or synaptic compartment with the irreversible transcriptional blocker actinomycin D for 1 h before asymmetric 5-HT exposure (see Methods). We found that induction of asymmetric LTF was blocked in both cases (Fig. 2_A_; ANOVA _F_2,6 = 9.07, P < 0.05). EPSP amplitude at the LT time point (20-24 h after 5-HT) was reduced significantly (P < 0.05) after actinomycin treatment in either compartment (-15.3 ± 20% somatic actinomycin and -4.3 ± 10% synaptic actinomycin), compared with noactinomycin controls, which exhibited LTF (+59.0 ± 5%).
Fig. 2.
LTF induced by symmetric 5-HT requires both presynaptic and postsynaptic gene expression. (A) Treatment of either the somatic or synaptic compartment with the transcription blocker actinomycin D blocked the induction of LTF (n = 3 in each group). (B) LTF was also blocked when transcription in the synaptic compartment was reversibly blocked with DRB (5-HT + DRB, n = 5) compared with 5-HT alone (5-HT, n = 6). *, P < 0.05.
To ensure that the block of LTF induced by asymmetric 5-HT was due to the prevention of RNA synthesis induced normally by 5-HT and not to nonspecific effects due to the irreversible transcriptional block, we used another transcriptional blocker that was reversible. DRB, which blocks RNA polymerase II-mediated transcription by inhibiting casein kinase II, has been shown (31, 32) to block transcription reversibly in Aplysia. We perfused DRB into the synaptic compartment 30 min before and during the 5 min of 5-HT in the synaptic compartment. We found that DRB also blocked induction of LTF (Fig. 2_B_; ANOVA _F_2,14 = 6.93, P < 0.05). EPSP amplitudes at the LT time point were reduced significantly in preparations that received synaptic DRB relative to those preparations that received no DRB (+12.8 ± 9% versus +83.5 ± 20%, P < 0.05). Controls with DRB alone showed no change in baseline transmission at the LT time point (+9.8 ± 15%, n = 6, data not shown).
These results indicate that, whereas 5 min of 5-HT at the synapse alone does not induce LTF, the postsynaptic gene products generated by this brief 5-HT exposure can interact with the presynaptic gene products resulting from the 25-min somatic 5-HT exposure in the induction of LTF (29). Furthermore, because studies have found (31) that the effects of DRB can be reversed in <2 h, our data show that the requirement for transcription in the synaptic compartment occurs either during or shortly after 5-HT exposure. The dependence of LTF induced by asymmetric 5-HT on postsynaptic transcription and translation shows that inductions of LTF by asymmetric 5-HT and by repeated 5-HT have different molecular requirements.
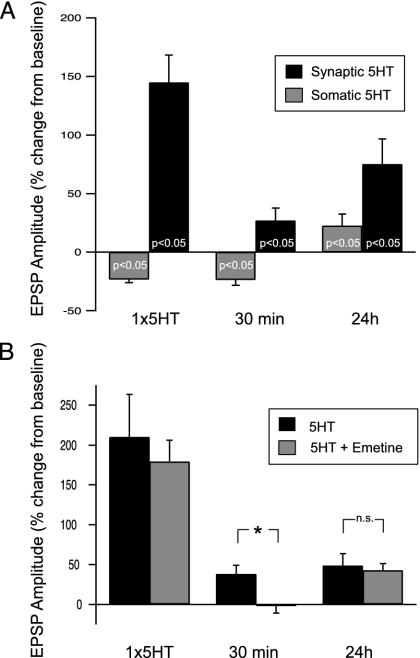
Repeated 5-HT at the Synapse Induces ITF. In addition to LTF, repeated pulses of 5-HT also induce ITF that is expressed within 20 min after the final 5-HT exposure (21-23). EPSP amplitude then returns to baseline by about 90 min after 5-HT and remains unchanged until the onset of LTF at about 10 h after 5-HT (22, 23). We next examined the site of induction of ITF. Five repeated pulses of 5-HT were delivered to either the synaptic or the somatic compartment (Fig. 3_A_). We found that synaptic 5-HT induced both ITF and LTF (EPSP at 30 min = +26.9 ± 10.7%, _t_7 = 2.52; EPSP at 24 h = +75.0 ± 21.7%, _t_4 = 3.46; P < 0.05 in each case). In contrast, 5-HT in the somatic compartment did not induce ITF (EPSP at 30 min = -23.6 ± 4.6%, _t_6 = 5.09); the ITF (30 min) time point was significantly different between the somatic and synaptic groups (_t_13 = 4.13, P < 0.05). Somatic 5-HT did induce significant LTF, but it was modest compared with synaptically induced LTF (EPSP = +22.5 ± 10.2%, _t_5 = 2.21, P < 0.05). Finally, confirming previous results (27, 28), synaptic 5-HT also induced STF exclusively at the synapse (EPSP after 1× 5-HT = +144.8 ± 23.4%, _t_6 = 6.189, P < 0.05). Not only did somatic 5-HT fail to induce STF (Fig. 3_A_, and ref. 27), but after the first pulse of 5-HT, the EPSP amplitude was modestly but significantly depressed below baseline (EPSP after 1× 5-HT = -22.8 ± 2.5%, _t_6 = -8.42, P < 0.05; see below).
Fig. 3.
ITF induced at the synapse requires local protein synthesis. (A) Both STF (n = 7) and ITF (n = 8) were induced by repeated synaptic 5-HT, whereas neither was induced by repeated somatic 5-HT (n = 7 in each group). LTF was induced by both synaptic 5-HT (n = 5) and somatic 5-HT (n = 6). (B) ITF induced by repeated 5-HT (n = 5) was blocked by emetine at the synapse (n = 6). STF was induced in both groups (5-HT, n = 5; 5-HT + emetine, n = 6), and LTF (5-HT, n = 5; 5-HT + emetine, n = 3) was unaffected. *, P < 0.05.
Studies have shown (21, 22) that the induction of ITF requires protein synthesis. However, it is not known whether this requirement is cell-wide or restricted to the synapse. We next asked whether induction of ITF requires protein synthesis in the synaptic compartment. The translational blocker emetine was perfused into the synaptic compartment for 30 min before and for the duration of repeated 5-HT exposure to both compartments. We found that emetine in the synaptic compartment blocked the induction of ITF (Fig. 3_B_; EPSP amplitude at 30 min = -2.3 ± 8.7%, _t_5 = -0.26, not significant), whereas the controls exhibited significant ITF (+38.7 ± 10.6% in no-emetine controls; _t_9 = 3.65, P < 0.05). Interestingly, both groups exhibited significant LTF [EPSP =+43.1 ± 8.3%, _t_2 = 5.19 (emetine), +48.9 ± 15.0%, _t_4 = 3.27 (no-emetine control), P < 0.05 in each case]; there was no significant difference between preparations treated with emetine and controls at the LT test (_t_6 = 0.69, not significant). Presumably, protein synthesis carried out in the somatic compartment was sufficient for the induction of LTF (recall that this pattern of 5-HT exposure gave rise to LTF (Fig. 3_A_). These data show that repeated pulses of 5-HT induce ITF that requires protein synthesis in the synaptic compartment.
Short-Term and Intermediate-Term Depression After Somatic 5-HT. An interesting and unexpected result was the small but significant decrease in EPSP amplitude immediately after the first pulse of 5-HT exclusively to the soma and also 30 min after the final pulse of 5-HT, when 5-HT was given only to the somatic compartment (Fig. 3_A_). It is possible that 5-HT might activate a secondary, inhibitory pathway in the somatic compartment. Inhibitory 5-HT responses have been observed at Aplysia synapses, including other SN to MN synapses (33) and interneuron to MN synapses (34, 35), and 5-HT receptors that are negatively coupled to adenylate cyclase have been cloned from Aplysia (36, 37). Our observations of somatic 5-HT inducing transient inhibition of the tail SN-MN synapse extend these findings by showing that 5-HT can have differential facilitatory and inhibitory effects at different sites of the same neuron.
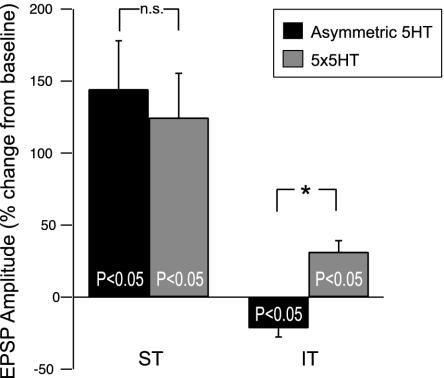
Asymmetric 5-HT Induces LTF Without ITF. Last, we asked whether asymmetric 5-HT induces ITF, and if so, whether it also requires protein synthesis? Two groups were explored: one group received asymmetric exposure of 5-HT and the other group received repeated pulses of 5-HT. Whereas repeated 5-HT induced ITF as expected (Fig. 4), we found no evidence of ITF after asymmetric exposure of 5-HT; in fact, EPSP amplitudes were significantly below baseline at 15 and 30 min [Fig. 4; EPSP at 30 min (IT) = -21.5 ± 6.0, _t_9 = 3.56, P < 0.05]. Thus, asymmetric 5-HT does not induce ITF, even though it does induce LTF that has a requirement for translation in the postsynaptic cell (Fig. 1). Moreover, asymmetric 5-HT can induce LTF in the absence of ITF, demonstrating that different phases of synaptic facilitation can be induced in parallel.
Fig. 4.
Asymmetric 5-HT does not induce ITF. Asymmetric 5-HT (n = 13) and repeated-pulse 5-HT (n = 11) induced similar STF, but only repeated-pulse 5-HT (n = 9) induced ITF. Asymmetric 5-HT (n = 10) actually induced depression. *, P < 0.05.
Discussion
Several temporal and spatial patterns of 5-HT have been used to induce LTF at Aplysia synapses in both the CNS and SN-MN cocultures. LTF has commonly been induced by repeated pulses of 5-HT (typically 5 × 5 min) to both the somatic and synaptic regions (23, 25, 26). Other patterns include (i) repeated pulses of 5-HT to either the soma or the synapse alone (25, 28, 38); (ii) a single, prolonged 5-HT exposure to the somatic and/or synaptic compartment (27, 39); (iii) pairing 5-HT with SN activation (40, 41); and (iv) asymmetric exposure of the soma and synapse to 5-HT, under conditions in which neither exposure alone induces LTF (29, see also ref. 38).
The temporal patterning of 5-HT exposure is important for the induction of synaptic plasticity at Aplysia SN-MN synapses. For example, five 5-min pulses of 5-HT at a 15-min intertrial interval induces LTF, whereas an equivalent net exposure (e.g., a single 25-min application) does not reliably induce LTF (39). Activation of signaling cascades that are thought to contribute to changes in synaptic strength are also sensitive to patterning; repeated pulses of 5-HT lead to a persistent activation of PKA in the intermediate-term time range, whereas a single, prolonged application does not (42).
In addition to temporal patterning, spatial patterning also affects the induction of facilitation, as reflected by the differential induction of cell-wide and synapse-specific LTF. Five repeated pulses of 5-HT at the soma induces cell-wide LTF, which does not require cell growth and lasts about 24 h (27, 28, 38). In contrast, synapse-specific LTF is induced by repeated pulses of 5-HT at the synapse, requires cell growth, and lasts up to 72 h (25, 27, 28).
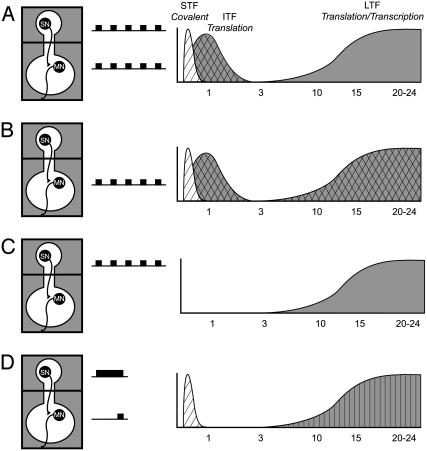
That several patterns of 5-HT have different effects on the induction of various forms of facilitation suggests that these patterns might activate different cellular mechanisms of synaptic plasticity. In the present article, we examined the possibility that four different patterns of 5-HT might differ (i) in their ability to initiate protein synthesis, (ii) in the cellular location where they might activate protein synthesis, and (iii) in the phases of synaptic facilitation that they induce. As shown in Fig. 5, three of these patterns have the same temporal characteristics (five repeated pulses) but differ in their spatial location. Specifically, repeated pulses were given (i) to the soma and synapse simultaneously (Fig. 5_A_), (ii) to the synapse alone (Fig. 5_B_), or (iii) to the soma alone (Fig. 5_C_). The fourth pattern of 5-HT was asymmetric (Fig. 5_D_). We found that the spatial location of 5-HT plays a critical role in the induction of ITF and LTF and that different patterns of 5-HT induce LTF with different requirements for protein synthesis.
Fig. 5.
Four different spatial patterns of 5-HT produce distinct temporal phases of synaptic facilitation. (A and B) Repeated pulses of 5-HT to both the somatic and synaptic compartments (A) or to only the synaptic compartment (B) induce all three phases of facilitation: STF, ITF, and LTF. (C) Repeated pulses of 5-HT only to the soma induce LTF in the absence of STF and ITF. (D) Asymmetric 5-HT induces STF and LTF but not ITF. Hatched shading, local induction at the synapse; cross-hatched shading, protein synthesis-dependent local induction at the synapse; vertical stripes, protein and RNA synthesis-dependent local induction at the synapse.
Repeated Pulses of 5-HT. By restricting 5-HT to either the synaptic or somatic compartment, we could determine the contribution of each region in the induction of ITF and LTF. It is well established that repeated pulses of 5-HT to the soma and synapse together induce STF, ITF, and LTF at Aplysia SN-MN synapses (22-26). Induction of ITF by repeated pulses of 5-HT in the pleural-pedal ganglia requires five pulses of 5-HT (23) and depends on protein synthesis (21, 22). In this article, we show that ITF resulting from repeated 5-HT is induced in the synaptic compartment, independent of the SN soma. Mechanisms underlying the local induction of ITF include the possibility that the protein synthesis machinery and/or the signaling pathways required for ITF induction are localized to the synaptic compartment. Our results showing that induction of ITF depends on protein synthesis in the synaptic compartment support the notion of a requirement for local synaptic protein synthesis for this form of plasticity (43).
In contrast to ITF, which is induced only synaptically, LTF can be induced in either the synapse or the soma. For example, Emptage and T.J.C. (27) found that prolonged (90-min) 5-HT exposure in either the somatic or the synaptic compartment induced LTF at tail SN-MN synapses. Clark and Kandel (28) obtained similar results for repeated pulses of 5-HT at Aplysia siphon SN-MN synapses. Moreover, in SN-MN cocultures, repeated pulses of 5-HT induce cell-wide LTF when presented to the SN soma and synapse-specific LTF when presented to the synapse (25, 38). Both cell-wide and synapse-specific LTF require presynaptic, but not postsynaptic, protein synthesis (25, 38). By using the two-compartment preparation, we found that repeated pulses of 5-HT induced LTF when presented either to the somatic or the synaptic compartment, confirming and extending the results obtained in cultured Aplysia synapses. Comparable with cultured synapses (25), we found also that induction of LTF requires protein synthesis only presynaptically; neither postsynaptic injection of gelonin nor synaptic emetine blocked the induction of LTF when it is induced by repeated pulses of 5-HT to both compartments.
Asymmetric 5-HT. In contrast to repeated pulses of 5-HT, asymmetric presentation of 5-HT to the somatic and synaptic compartments did not induce ITF (Fig. 5_D_). These data are consistent with the results described above, in which ITF is induced only locally at the synapse by multiple exposures of 5-HT. As shown in Fig. 5, the only patterns of 5-HT that induced ITF were those that included multiple 5-HT exposures at the synapse.
We also found that, in contrast to repeated-pulse LTF, LTF induced by asymmetric 5-HT required postsynaptic protein synthesis. Postsynaptic injections of gelonin directly into the MN blocked this form of LTF. Reports (30) have described long-term postsynaptic effects of 5-HT at Aplysia SN-MN synapses; 24 h after repeated 5-HT, MNs have an increased depolarizing response to glutamate, which has been hypothesized to be due to an increase in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor insertion (44). The present study identifies a requirement for postsynaptic protein synthesis in the induction of LTF. We also determined that, surprisingly, transcription in the synaptic compartment was required for induction of LTF by asymmetric 5-HT. We suggest that the most likely explanation is that postsynaptic gene expression is required in the MN. However, we cannot rule out the possibility that the transcriptional blockers may have also blocked mitochondrial transcription (e.g., in the SN presynaptic neurites in the pedal ganglion) or that nuclear transcription in cell bodies of other neurons in the synaptic compartment can exert polysynaptic effects on the SN-MN synapses.
An interesting feature of our results is that induction of LTF induced by asymmetric 5-HT, in which the postsynaptic cell receives only a single 5-min pulse of 5-HT, requires postsynaptic protein synthesis, whereas the induction of LTF by repeated pulses of 5-HT, in which the MN receives five times the 5-HT exposure, does not. One explanation is that the pattern of repeated 5-HT pulses might so strongly activate the presynaptic molecular pathways involved in LTF that protein synthesis-dependent postsynaptic effects, although still present, are not critical for LTF induction. In contrast, asymmetric 5-HT may just exceed the threshold for LTF induction, in which case the presynaptic protein synthesis induced by the single 25-min somatic 5-HT exposure is insufficient by itself for the induction of LTF. The postsynaptic translational events, which are not essential in the case of repeated pulses of 5-HT, now play a critical role in the induction of LTF.
Parallel Versus Serial Processing of Different Phases of Facilitation. Two models for the relationship between the different temporal phases of synaptic plasticity (and by extension, memory) are that they are induced in series (as discussed in ref. 1) or in parallel (3-6). If serial processing were operative at the SN-MN synapse, brief activation of the signaling pathways, as would occur with a single pulse of 5-HT, would induce STF, which would serve as a necessary step in the induction of longer-lasting forms of facilitation, first ITF and then LTF. In this model, ITF and LTF would not be induced if the induction of STF was blocked.
In the case of parallel processing, the different phases of facilitation could be activated independently. Thus, blocking the induction of one temporal phase (i.e., STF) need not affect the subsequent expression of other forms; an initial pulse of 5-HT would activate different (parallel) signaling pathways required for different phases of facilitation. In this model, blocking STF would not prevent the induction of ITF and LTF. Evidence from this study and from ref. 27 shows that parallel processing can occur at tail SN-MN synapses. Specifically, LTF can be induced in the absence of STF. Our present results demonstrate further that asymmetric 5-HT induces LTF in the absence of ITF (Fig. 5_D_), and repeated somatic 5-HT induces LTF without prior expression of STF or ITF (Fig. 5_C_).
It is interesting to consider the difference in the phases of facilitation induced by different patterns of 5-HT. When the 5-HT exposure is predominantly somatic (Fig. 5 C and D), LTF is induced independently of ITF (and of STF in the case of repeated somatic 5-HT). In contrast, when 5-HT exposure is mainly synaptic, as with repeated synaptic 5-HT (Fig. 5_B_), all three temporal phases of facilitation are induced. In this latter case, it is possible that synaptic 5-HT is activating the individual signaling pathways for each phase independently and in parallel, but it is equally possible that the molecular route to LTF activated by 5-HT at the synapse is one that induces STF, ITF, and LTF in series. Further studies are required to inform this question.
Implications of Multiple Processing Pathways for Synaptic Facilitation and Memory Formation. We have shown here that four different temporal and spatial patterns of 5-HT can induce LTF (Fig. 5). Studies have revealed (27, 39-41) that there are even more patterns that can be effective. These different patterns might differentially activate various signaling pathways to induce different combinations of facilitatory phases. The feature of multiple induction pathways is not unique to LTF; multiple forms of both STF (24, 45) and ITF (22, 24, 46) have been described in Aplysia as well. Finally, in other systems, synaptic plasticity in a specific time domain can be induced by multiple patterns of activation. For example, hippocampal long-term potentiation can be induced by high-frequency stimulation, by theta-burst stimulation, or by brain-derived neurotrophic factor (47-49), and in each case, different molecular mechanisms are required for induction.
As is the case with synaptic facilitation, behavioral studies in Drosophila (3, 50) and in rodents (51-53) have shown that different forms of mechanistically distinct memory can be induced by different patterns of training. In Aplysia, it is now possible to begin to forge links between multiple forms of synaptic plasticity and multiple forms of memory. For example, in the intermediate time domain, activity-dependent and activity-independent forms of ITF directly predicted the existence of two forms of intermediate-term memory (7, 16, 54). It will now be interesting to determine the possible behavioral differences that might correspond to the different forms of LTF that we have explored. Particularly intriguing is the asymmetric pattern of 5-HT, which generates a mechanistically unique form of LTF (Fig. 5). Does the tail-elicited siphon-withdrawal reflex circuit actually have the capacity to generate a comparable form of long-term memory? We already know that tail nerve shock causes release of 5-HT in the vicinity of the tail SN cell bodies and in the synaptic neuropil containing the SN-MN synapses (18). We also have recently identified several serotonergic cells and cell clusters that are activated by tail stimulation (S. Marinesco, K. E. Kolkman, and T.J.C., personal communication). Thus, at least the possibility exists for differential activation of 5-HT input to SN cell bodies and synapses using behaviorally relevant stimuli. We can now ask whether there are specific patterns of behavioral activation that give rise to a form of long-term memory that shares the mechanistic features of LTF induced by asymmetric 5-HT. These and related experiments may help to further elucidate the roles of signaling pathways engaged by specific spatial and temporal patterns of activation during memory formation.
Acknowledgments
We thank Kate Reissner, Joanna Schaffhausen, Shiv Sharma, and Justin Shobe for their helpful comments. This work was supported by National Science Foundation Grant IBN-0049013 and National Institute of Mental Health Grant R01-MH-14-1083 (to T.J.C.).
Abbreviations: 5-HT, 5-hydroxytryptamine; SN, sensory neuron; MN, motor neuron; STF, short-term facilitation; ITF, intermediate-term facilitation; LTF, long-term facilitation; DRB, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole; ASW, artificial seawater; EPSP, excitatory postsynaptic potential.
References
- 1.Squire, L. R. (1987) Memory and Brain (Oxford Univ. Press, Oxford).
- 2.James, W. (1890) The Principles of Psychology (Henry Holt, New York).
- 3.Tully, T., Preat, T., Boynton, S. C. & Del Vecchio, M. (1994) Cell 79**,** 35-47. [DOI] [PubMed] [Google Scholar]
- 4.Dezazzo, J. & Tully, T. (1995) Trends Neurosci. 18**,** 212-218. [DOI] [PubMed] [Google Scholar]
- 5.Izquierdo, I. (1995) Cienc. Cult. (São Paulo) 47**,** 217-218. [Google Scholar]
- 6.Muller, U. (1996) Neuron 16**,** 541-549. [DOI] [PubMed] [Google Scholar]
- 7.Sutton, M. A., Masters, S. E., Bagnall, M. W. & Carew, T. J. (2001) Neuron 31**,** 143-154. [DOI] [PubMed] [Google Scholar]
- 8.Frey, U., Huang, Y. Y. & Kandel, E. R. (1993) Science 260**,** 1661-1664. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen, P. V., Abel, T. & Kandel, E. R. (1994) Science 265**,** 1104-1107. [DOI] [PubMed] [Google Scholar]
- 10.Huang, Y.-Y. & Kandel, E. R. (1994) Learn. Mem. 1**,** 74-81. [PubMed] [Google Scholar]
- 11.Winder, D. G., Mansuy, I. M., Osman, M., Moallem, T. M. & Kandel, E. R. (1998) Cell 92**,** 25-37. [DOI] [PubMed] [Google Scholar]
- 12.Crow, T., Siddiqi, V. & Dash, P. K. (1997) Neurobiol. Learn. Mem. 68**,** 340-347. [DOI] [PubMed] [Google Scholar]
- 13.Crow, T., Xue-Bian, J.-J. & Siddiqi, V. (1999) J. Neurophysiol. 82**,** 495-500. [DOI] [PubMed] [Google Scholar]
- 14.Carew, T. J., Castellucci, V. F. & Kandel, E. R. (1971) Int. J. Neurosci. 2**,** 79-98. [DOI] [PubMed] [Google Scholar]
- 15.Pinsker, H., Carew, T. J., Hening, W. & Kandel, E. R. (1973) Science 182**,** 1039-1042. [DOI] [PubMed] [Google Scholar]
- 16.Sutton, M. A. & Carew, T. J. (2002) Int. Comp. Biol. 42**,** 725-735. [DOI] [PubMed] [Google Scholar]
- 17.Levenson, J., Byrne, J. & Eskin, A. (1999) J. Neurosci. 19**,** 8094-8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinesco, S. & Carew, T. J. (2002) J. Neurosci. 22**,** 2299-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walters, E. T., Byrne, J. H., Carew, T. J. & Kandel, E. R. (1983) J. Neurophysiol. 50**,** 1543-1559. [DOI] [PubMed] [Google Scholar]
- 20.Mercer, A. R., Emptage, N. J. & Carew, T. J. (1991) Science 254**,** 1811-1813. [DOI] [PubMed] [Google Scholar]
- 21.Ghirardi, M., Montarolo, P. G. & Kandel, E. R. (1995) Neuron 14**,** 413-420. [DOI] [PubMed] [Google Scholar]
- 22.Sutton, M. A. & Carew, T. J. (2000) Neuron 26**,** 219-231. [DOI] [PubMed] [Google Scholar]
- 23.Mauelshagen, J., Parker, G. R. & Carew, T. J. (1996) J. Neurosci. 16**,** 7099-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghirardi, M., Braha, O., Hochner, B., Montarolo, P. G., Kandel, E. R. & Dale, N. (1992) Neuron 9**,** 479-489. [DOI] [PubMed] [Google Scholar]
- 25.Martin, K. C., Casadio, A., Zhu, H., Yaping, E., Rose, J. C., Chen, M., Bailey, C. H. & Kandel, E. R. (1997) Cell 91**,** 927-938. [DOI] [PubMed] [Google Scholar]
- 26.Montarolo, P. G., Goelet, P., Castellucci, V. F., Morgan, J., Kandel, E. R. & Schacher, S. (1986) Science 234**,** 1249-1254. [DOI] [PubMed] [Google Scholar]
- 27.Emptage, N. J. & Carew, T. J. (1993) Science 262**,** 253-256. [DOI] [PubMed] [Google Scholar]
- 28.Clark, G. A. & Kandel, E. R. (1993) Proc. Natl. Acad. Sci. USA 90**,** 11411-11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherff, C. M. & Carew, T. J. (1999) Science 285**,** 1911-1914. [DOI] [PubMed] [Google Scholar]
- 30.Trudeau, L.-E. & Castellucci, V. F. (1995) J. Neurosci. 15**,** 1275-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koumenis, C., Tran, Q. & Eskin, A. (1996) J. Biol. Rhythms 11**,** 45-56. [DOI] [PubMed] [Google Scholar]
- 32.Raju, U., Koumenis, C., Nunez-Regueiro, M. & Eskin, A. (1991) Science 253**,** 673-675. [DOI] [PubMed] [Google Scholar]
- 33.Storozhuk, M. V. & Castellucci, V. F. (1999) J. Exp. Biol. 202**,** 115-120. [DOI] [PubMed] [Google Scholar]
- 34.Bristol, A. S., Fischer, T. M. & Carew, T. J. (2001) J. Neurosci. 21**,** 8990-9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trudeau, L. E. & Castellucci, V. F. (1993) J. Neurophysiol. 70**,** 1210-1220. [DOI] [PubMed] [Google Scholar]
- 36.Barbas, D., Zappulla, J. P., Angers, S., Bouvier, M., Castellucci, V. F. & DesGroseillers, L. (2002) J. Neurochem. 80**,** 335-345. [DOI] [PubMed] [Google Scholar]
- 37.Angers, A., Storozhuk, M. V., Duchaine, T., Castellucci, V. F. & Desgroseillers, L. (1998) J. Neurosci. 18**,** 5586-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casadio, A., Martin, K. C., Giustetto, M., Zhu, H., Chen, M., Bartsch, D., Bailey, C. H. & Kandel, E. R. (1999) Cell 99**,** 221-237. [DOI] [PubMed] [Google Scholar]
- 39.Mauelshagen, J., Sherff, C. M. & Carew, T. J. (1998) Learn. Mem. 5**,** 246-256. [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey, C. H., Giustetto, M., Zhu, H., Chen, M. & Kandel, E. R. (2000) Proc. Natl. Acad. Sci. USA 97**,** 11581-11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, Z.-Y. & Schacher, S. (1998) J. Neurosci. 18**,** 3991-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller, U. & Carew, T. J. (1998) Neuron 21**,** 1423-1434. [DOI] [PubMed] [Google Scholar]
- 43.Kim, J. H., Udo, H., Li, H. L., Youn, T. Y., Chen, M., Kandel, E. R. & Bailey, C. H. (2003) Neuron 40**,** 151-165. [DOI] [PubMed] [Google Scholar]
- 44.Chitwood, R. A., Li, Q. & Glanzman, D. L. (2001) J. Physiol. (London) 534**,** 501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrne, J. H. & Kandel, E. R. (1996) J. Neurosci. 16**,** 425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanow, S. K., Manseau, F., Hislop, J., Castellucci, V. F. & Sossin, W. S. (1998) J. Neurochem. 70**,** 572-583. [DOI] [PubMed] [Google Scholar]
- 47.Kang, H. & Schuman, E. M. (1996) Science 273**,** 1402-1406. [DOI] [PubMed] [Google Scholar]
- 48.Kang, H. & Schuman, E. M. (1995) Science 267**,** 1658-1662. [DOI] [PubMed] [Google Scholar]
- 49.Larson, J., Wong, D. & Lynch, G. (1986) Brain Res. 368**,** 347-350. [DOI] [PubMed] [Google Scholar]
- 50.Yin, J. C. P., Wallach, J. S., Del Vecchio, M., Wilder, E. L., Zhou, H., Quinn, W. G. & Tully, T. (1994) Cell 79**,** 49-58. [DOI] [PubMed] [Google Scholar]
- 51.Berman, D. E., Hazvi, S., Stehberg, J., Bahar, A. & Dudai, Y. (2003) Learn. Mem. 10**,** 16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berman, D. E. & Dudai, Y. (2001) Science 291**,** 2417-2419. [DOI] [PubMed] [Google Scholar]
- 53.Cain, C. K., Blouin, A. M. & Barad, M. (2003) J. Exp. Psychol. Anim. Behav. Processes 29**,** 323-333. [DOI] [PubMed] [Google Scholar]
- 54.Sutton, M. A., Bagnall, M. W., Sharma, S. K., Shobe, J. & Carew, T. J. (2004) J. Neurosci. 24**,** 3600-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]