Activation of the Growth-Differentiation Factor 11 Gene by the Histone Deacetylase (HDAC) Inhibitor Trichostatin A and Repression by HDAC3 (original) (raw)
Abstract
Histone deacetylase (HDAC) inhibitors inhibit the proliferation of transformed cells in vitro, restrain tumor growth in animals, and are currently being actively exploited as potential anticancer agents. To identify gene targets of the HDAC inhibitor trichostatin A (TSA), we compared the gene expression profiles of BALB/c-3T3 cells treated with or without TSA. Our results show that TSA up-regulates the expression of the gene encoding growth-differentiation factor 11 (Gdf11), a transforming growth factor β family member that inhibits cell proliferation. Detailed analyses indicated that TSA activates the gdf11 promoter through a conserved CCAAT box element. A comprehensive survey of human HDACs revealed that HDAC3 is necessary and sufficient for the repression of gdf11 promoter activity. Chromatin immunoprecipitation assays showed that treatment of cells with TSA or silencing of HDAC3 expression by small interfering RNA causes the hyperacetylation of Lys-9 in histone H3 on the gdf11 promoter. Together, our results provide a new model in which HDAC inhibitors reverse abnormal cell growth by inactivation of HDAC3, which in turn leads to the derepression of gdf11 expression.
Histone deacetylases (HDACs) are enzymes that catalyze the removal of acetyl groups from conserved lysine residues in histones' amino-terminal tails. They are found in all eukaryotic organisms and are believed to have a key role in the regulation of gene transcription. Importantly, recent studies suggest that HDACs are critically involved in cell cycle regulation, cell proliferation, differentiation, and the development of human cancer.
The availability of HDAC inhibitors accounts in large part for the initial identification of HDACs and the rapid advances in our knowledge of how HDACs repress transcription (54). Many investigators have developed and characterized a number of compounds that inhibit HDAC activity. These compounds can also cause cell growth arrest, differentiation, and/or apoptosis and can restrain tumor growth in animals, thus further implicating HDACs in these key cellular processes (36-40, 64, 68, 69). Several HDAC inhibitors are currently in clinical trials for the treatment of leukemia and solid tumors (26, 27, 37-40). Additionally, drugs that target HDACs may potentially be used against malaria and toxoplasmosis and for the treatment of Huntington's disease (6, 52).
Trichostatin A (TSA), a Streptomyces product originally discovered as a fungistatic antibiotic (55), was one of the first HDAC inhibitors identified. At nanomolar concentrations, TSA causes G1- and G2-phase cell cycle arrest along with the accumulation of hyperacetylated histones (67, 70). TSA can also induce the phenotypic reversion of _sis_-transformed fibroblast cells (70). Using differential display, Van Lint et al. found that TSA affected the expression of approximately 2% of cellular genes (8 genes out of 340 examined) (58). This finding suggests that HDAC inhibitors such as TSA do not exert global effects on gene expression. Rather, the transcriptional regulation of a highly restricted set of cellular genes is uniquely sensitive to the degree of histone acetylation in chromatin.
Although many potential TSA-responsive genes have been identified to date (8, 15, 30, 32, 58), the question of how proteins encoded by these genes mediate TSA-induced growth inhibition is far from being completely answered. For example, one of the genes most commonly induced by HDAC inhibitors including TSA is the cyclin-dependent kinase (CDK) inhibitor p21Cip1 (reviewed in reference 36). However, we and others found that mouse embryo fibroblasts from wild-type and _p21Cip1_-null mice underwent HDAC inhibitor-induced G1 arrest with a similar dose dependency (59, 64). Therefore, p21Cip1 gene expression is not necessary, at least in some situations, for TSA-induced cell cycle arrest.
In this report, we used expression profiling to identify genes whose expression is potentially regulated by TSA. Of the targets identified, the expression of the gene that encodes growth-differentiation factor 11 (Gdf11) was dramatically up-regulated by TSA, while the expression of the gene that encodes a Gdf11 antagonist, follistatin, was significantly repressed.
Gdf11 (also called Bmp11), a secreted protein in the transforming growth factor β (TGF-β) superfamily, is closely related to a negative regulator of muscle growth, Gdf8 (myostatin) (42, 43). Gdf11 binds to activin type II receptors ActRIIA and ActRIIB and induces the phosphorylation of Smad2 (45). Like other members of the Gdf family, Gdf11 is highly conserved throughout different species, with human and mouse Gdf11 sharing 99.5% identity over the entire amino acid sequence (13). Targeted deletion studies in mice reveal that Gdf11 controls anterior-posterior patterning of the axial skeleton and kidney organogenesis (9, 43). Gdf11 may also play an important role in the formation of the appendicular skeleton in the chick (12). Intriguingly, using a mouse olfactory epithelium model, Wu et al. recently showed that Gdf11 inhibits cell proliferation in vitro by inducing the expression of p27Kip1 (65). Conversely, in mice lacking follistatin, a noticeable decrease in cell proliferation was observed. It was proposed, therefore, that Gdf11 provides a general means by which tissue growth may be negatively controlled. Given this newly discovered role of Gdf11 in repressing cell growth and the growth inhibitory effects of TSA, we suggest that one of the mechanisms by which HDAC inhibitors such as TSA efficiently inhibit cell proliferation is by activation of the gdf11 gene. Here we show that activation of gdf11 results from the inhibition of HDAC3 activity on the gdf11 promoter.
MATERIALS AND METHODS
Cell culture.
BALB/c-3T3, HeLa, and Flow 2000 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml at 37°C with 5% CO2.
Microarray analysis.
Microarray analysis was done using the Affymetrix GeneChip instrument system following protocols established by Affymetrix, Inc. Total cellular RNA was prepared with TRIzol reagent (Invitrogen) and further purified using an RNeasy kit (Qiagen). RNA was converted to double-stranded cDNA using an oligo(dT)24 primer that contains a T7 RNA polymerase recognition sequence, and the resulting double-stranded cDNA was transcribed into biotin-labeled cRNA using T7 RNA polymerase. Biotinylated cRNA was hybridized to the Affymetrix murine U74Av2 array set, which contains approximately 12,000 full-length mouse genes and EST clusters. Hybridized RNA was visualized by staining the biotinylated cRNA with streptavidin-conjugated phycoerythrin. Scanned chip images were analyzed using GeneChip algorithms.
RNA isolation and Northern blot analysis.
BALB/c-3T3, HeLa, and Flow 2000 cells were cultured to 90% confluence and treated with ethanol (control) or inhibitors. Cells were collected, and total RNA was isolated using TRIzol reagent (Invitrogen). After separation on formaldehyde-agarose gels, RNA was transferred onto Biobond nylon membranes (Sigma) and cross-linked to the membranes in a Stratalinker. A 261-bp cDNA fragment encoding a portion of the mouse Gdf11 propeptide region (42) and a 1-kb human gdf11 cDNA (from IMAGE clone 4473773; Research Genetics) were labeled with [α-32P]dCTP (NEN) using a DECA prime II kit (Ambion, Inc.). Hybridizations with the radiolabeled probes were carried out overnight in PerfectHyb Plus (Sigma) at 65°C. Blots were washed twice at room temperature with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS) before exposure to X-ray film and subsequent visualization by autoradiography.
Antibodies and chemical reagents.
A peptide corresponding to amino acids 45 to 60 of human Gdf11 (GERSSRPAPSVAPEPD) coupled to keyhole limpet hemocyanin was injected subcutaneously into New Zealand White rabbits (0.1 mg of peptide per injection). The resulting anti-Gdf11 antibodies were immunoaffinity purified on an antigen peptide column.
Anti-HDAC1, anti-HDAC2, and anti-HDAC3 antibodies have previously been described (31, 63). Anti-NF-Y (A-subunit-specific) polyclonal antibody was purchased from Rockland. Anti-CDP antibody was purchased from Santa Cruz Biotechnology. Anti-acetyl histone H3 and anti-acetyl histone H4 were purchased from Upstate Biotechnology. Anti-acetyl histone H3 (Lys-9), anti-acetyl histone H3 (Lys-18), anti-acetyl histone H4 (Lys-8), and anti-acetyl histone H4 (Lys-12) were purchased from Cell Signaling Technology. Anti-β-actin antibody, TSA, and nicotinamide were purchased from Sigma.
Western blot analysis.
Proteins were separated on SDS-polyacrylamide gels and transferred onto Immobilon-P transfer membrane (Millipore). After blocking with 5% nonfat dried milk, the membranes were incubated with diluted primary antibodies and subsequently with a 1:1,000 dilution of either anti-mouse immunoglobulin G horseradish peroxidase-linked whole antibody (from sheep) or anti-rabbit immunoglobulin G horseradish peroxidase-linked whole antibody (from donkey; Amersham Biosciences). The blots were developed with SuperSignal West Pico chemiluminescent substrate (Pierce).
Plasmids.
All human gdf11 promoter fragments were derived from a BAC clone, RP11-762I7 (Research Genetics). A 2.6-kb human gdf11 promoter fragment was cloned by PCR using 5′ and 3′ primers (TGTGCTAAAGGCAATCGCTGCTCTGG and GCTGGAGGGGAGGGGAGGGAGGACTG, respectively). The 3′ end of the 2.6-kb fragment is immediately upstream of the Gdf11 translation initiation codon. The 2.6-kb PCR product was first subcloned into the TOPO TA vector (Invitrogen) and subsequently into the pGL3Basic vector at EcoRI and XhoI sites to generate pGL2628E. pGL2628r was constructed by digesting pGL2628E with EcoRI and religation. Using a similar strategy, a 781-bp human gdf11 promoter was cloned by PCR with a 5′ primer (CCCTTTCCCAGGGCACTCTTCTGTCC). The 781-bp PCR product was subcloned into the TOPO TA vector and subsequently into pGL3Basic at SacI and XhoI sites to generate pGL781SX. A HindIII/XhoI fragment was also isolated and ligated into pGL3Basic to generate pGL781XH, which contained the gdf11 upstream sequence in reverse orientation with respect to the luciferase gene. pGL669 was constructed by isolating a KpnI/XhoI fragment from pGL781 and then ligating the fragment into pGL3Basic. pGL625 was constructed by isolating a SpeI/XhoI fragment from pGL781SX and then ligating the fragment into pGL3Basic. pGL431 was generated by introducing a SacI site into pGL781SX 431 bp upstream of the translation initiation codon using a pair of primers (CTCCTTGGTCTCTCTGAGCTCGGCTCTGACTTTCCCTC and its complement) and the QuikChange site-directed mutagenesis kit (Stratagene); this construct was digested with SacI and religated. pGL288 was generated by isolating a 288-bp AfeI/XhoI fragment from pGL781 and then ligating the fragment into the pGL3Basic vector cut with SmaI and XhoI. pGL191 was generated by PCR using primers (GCGAGCTCCTCTTCATCTCTCTCTGG and GACTGAGCGGCTGGGGGGGC) and pGL781SX as the template. The PCR product was subcloned into the TOPO TA vector and ligated into pGL3Basic at SacI and XhoI sites to obtain the final product. pGL191mt, in which the upstream CCAAT box was mutated, was generated by site-directed mutagenesis using primers (CTTTTGTTGGCTCCGCAGCGATTCGCGGCCGCTGACG and its complement). Two different XmaI/XhoI fragments isolated from pGL781SX were ligated into pGL3Basic to obtain pGL214 and pGL139. Similarly, a 75-bp XmaI fragment was used to generate pGL75.
HDAC11 expression plasmid is constructed by taking a full-length HDAC11 cDNA fragment from an EST clone (IMAGE 3906049) and ligating it into the pcDNA3 vector containing an in-frame sequence for a Flag epitope. All other expression plasmids for class I and class II HDACs have been described previously (16, 17, 28, 62, 71). To generate pBS/U6-HDAC1, an 18-bp double-stranded oligodeoxynucleotide (CCGCAAGAACTCTTCCAA and its complement) was inserted into the ApaI and HindIII sites of pBS/U6 (53). A second inverted sequence was inserted into the HindIII/EcoRI sites of the intermediate plasmid to obtain the final product. Identical strategies were used to generate pBS/U6-HDAC2 and pBS/U6-HDAC3 with oligodeoxynucleotides AATACTTTCCTGGCACAG and CTATAGTTCTCCTCAGGACCC, respectively. All constructs were confirmed by DNA sequencing.
Transient transfection and luciferase assay.
All transfections were done using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For reporter assays, cells were plated in 12-well dishes at a density of 2 × 105 cells/well and incubated with Dulbecco's modified Eagle's medium containing 10% fetal calf serum overnight. TSA, nicotinamide, or 100% ethanol was added to the culture medium 18 h after transfection, and cells were incubated at 37°C for an additional 12 h before harvesting with 250 μl of passive lysis buffer (Promega). Protein concentrations of all samples were determined using Bradford reagent (Bio-Rad), and the relative light unit values were measured with firefly assay reagent (Promega) and a luminometer.
Establishment of stable cell clones.
To generate clones stably with human gdf11 promoter-luciferase, pGL781SX or pGL3Basic (control) plasmids were transfected into HeLa cells with Lipofectamine. One day later, cells were subcultured and grown in the presence of 400 μg of G418 (Invitrogen)/ml. Cells were then maintained in selection medium for about 2 weeks until G418-resistant colonies appeared. Single colonies were picked using cloning cylinders (Bellco) and transferred to 24-well plates. Individual clones were then maintained in medium containing 200 μg of G418/ml.
Core histone isolation.
Core histones were prepared as described elsewhere (5), with minor modifications. HeLa cells grown to confluency in 75-cm2 flasks were washed twice with ice-cold phosphate-buffered saline and harvested with cell scrapers. Cell pellets were washed twice with 0.5 ml of NIB buffer (1% NP-40 in IB buffer, consisting of 10 mM Tris-HCl [pH 7.4], 2 mM MgCl2, 3 mM CaCl2, 10 mM sodium butyrate, and 1 mM phenylmethylsulfonyl fluoride). Nuclei were collected by centrifugation at 500 × g and washed twice with NIB buffer and once with NIB buffer supplemented with 100 mM NaCl. The nuclei were then extracted twice with high salt (0.5 ml of 0.4 M NaCl in IB buffer) and twice with 0.25 ml of 0.2 M H2SO4 for 90 min on ice. Nuclei were collected by centrifugation at 30,000 × g for 90 min. Supernatants were dialyzed extensively at 4°C overnight in a buffer containing 10 mM HEPES (pH 7.5), 1 mM EDTA, 10 mM KCl, 0.2 mM phenylmethylsulfonyl fluoride, and 10% glycerol.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (3, 50). HeLa cells grown to a density of 5 × 106/15-cm dish were transfected with plasmids or treated with either ethanol (control) or 400 ng of TSA/ml for 18 h. Formaldehyde was then added to the cell medium to a final concentration of 1%, and the cells were incubated for 10 min at room temperature with gentle shaking; glycine was added to a final concentration of 0.125 M, and cells were shaken for another 5 min. After removal of the medium, cells were suspended in 1 ml of ice-cold phosphate-buffered saline containing a cocktail of protease inhibitors (Roche Applied Science). Cells were then spun for 4 min at 1,400 × g, and pellets were resuspended in 1 ml of SDS lysis buffer (1% SDS, 10 mM EDTA, and 50 mM Tris-HCl; pH 8.1) and incubated for 10 min on ice. The lysates were sonicated, and debris was removed by centrifugation for 10 min at 13,000 rpm at 4°C in a microcentrifuge. Supernatant fractions were diluted 10-fold in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris [pH 8.1], 16.7 mM NaCl, and protease inhibitors). To reduce nonspecific background, each chromatin solution was precleared with 80 μl of salmon sperm DNA-protein A agarose for 2 h at 4°C with agitation. After addition of antibodies, samples were incubated overnight at 4°C with rotation. Immunocomplexes were collected with 60 μl of salmon sperm DNA-protein A agarose for 2 to 4 h at 4°C with rotation. Washes were done sequentially with 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.1), and 150 mM NaCl; the same buffer containing 500 mM NaCl; 0.25 M LiCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, and 10 mM Tris-HCl (pH 8.1); and Tris-EDTA, pH 8.0 (twice). Immunocomplexes were collected by repeated elutions with 250 μl of 1% SDS in 0.1 M NaHCO3. Twenty microliters of 5 M NaCl was added to the combined eluates, and protein-DNA was reverse cross-linked at 65°C for 4 h. Ten microliters of a mixture of 0.5 M EDTA, 20 μl of 1 M Tris-HCl (pH 6.5), and 2 μl of a 10-mg/ml solution of proteinase K was added to each sample, and samples were incubated for 1 h at 45°C. DNA was recovered by phenol-chloroform extraction and ethanol precipitation using 20 μg of glycogen as carrier. Primers for PCR amplification of the gdf11 promoter DNA were CTCTTCATCTCTCTCTGGCCCTTGCTCC and CGGCTGGGGGGGCCCGAG. PCRs were carried out for 40 cycles (95°C for 1 min, 67°C for 1 min, and 72°C for 1 min), and final products were resolved on 2% agarose gels.
RESULTS
Identification of genes potentially activated or repressed by TSA in BALB/c-3T3 mouse fibroblasts.
We examined the effects of TSA on growth factor-regulated gene expression by microarray analysis. Quiescent, density-arrested BALB/c-3T3 cells were stimulated by the addition of fresh medium containing 5% calf serum and 10 ng of platelet-derived growth factor (the BB isoform)/ml in the presence or absence of 10 ng of TSA/ml. Total RNA was isolated 6 h after treatment and used to probe the mouse U74Av2 Affymetrix GeneChip expression array, which contains approximately 12,000 previously characterized mouse DNA sequences. The results of this analysis showed that in mitogenically stimulated BALB/c-3T3 cells, TSA affects the expression of about 7% of the genes (supplemental material available upon request). A representative list of genes whose mRNA abundance was increased or decreased by TSA are listed in Table 1. Of these genes, the greatest change in expression (18-fold increase) was observed for gdf11. Interestingly, the abundance of the mRNA that encodes a Gdf11 antagonist, follistatin, was reduced eightfold by TSA.
TABLE 1.
Selected list of TSA-regulated genesa
| Encoded protein | Fold activation (+) or repression (−) |
|---|---|
| Gdf11 | +18 |
| Tumor necrosis factor-induced protein 6 (TSG6) | +12 |
| Plakophilin 2A | +9 |
| Gamma interferon-induced mRNA (Mg11) | +9 |
| Follistatin | −8 |
| Mitogen-activated protein kinase kinase 5 (MEK5) | −16 |
TSA increases amounts of Gdf11 mRNA and protein.
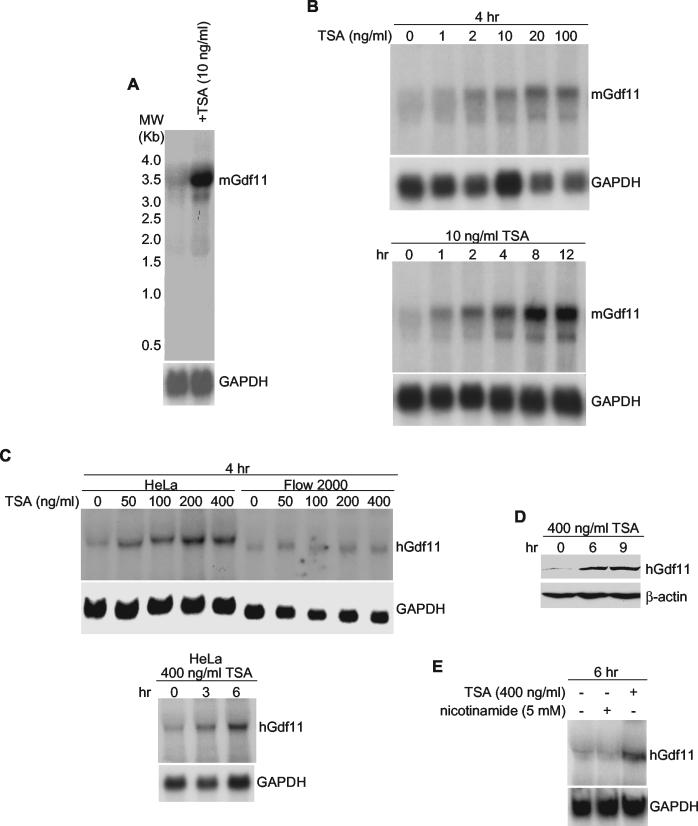
Northern blot analysis was performed to confirm that TSA treatment causes an increase in Gdf11 mRNA abundance. As shown in Fig. 1A, amounts of mouse Gdf11 mRNA were much greater in BALB/c-3T3 cells treated with TSA than in untreated cells. Unlike the expression array, these experiments were carried out with an asynchronously cycling population of cells without the addition of platelet-derived growth factor, indicating that the observed increase in Gdf11 mRNA abundance does not require additional growth factor stimulation but is solely dependent on TSA. Maximal increases in Gdf11 mRNA abundance occurred at 20 ng of TSA/ml and between 4 and 8 h after addition of TSA to BALB/c-3T3 cells (Fig. 1B).
FIG. 1.
Induction of mouse and human gdf11 by TSA. Northern blot (or Western blot [D]) assays were performed using total RNA prepared from exponentially growing cells. Each blot was stripped and rehybridized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA to standardize quality and quantity of RNA. (A) BALB/c-3T3 cells received ethanol or 10 ng of TSA/ml for 12 h. (B) BALB/c-3T3 cells were treated with the indicated concentrations of TSA for 4 h (top panel) or 10 ng of TSA/ml for the indicated times (bottom panel). (C) HeLa and Flow 2000 cells were treated with the indicated concentrations of TSA for 4 h (top panel) or 400 ng of TSA/ml for the indicated times (bottom panel). (D) HeLa cells received 400 ng of TSA/ml for the indicated times. A Western blot assay was performed using an anti-Gdf11 polyclonal antibody. The blot was stripped and reprobed with an anti-β-actin antibody to ensure equal loading and transfer of proteins. (E) HeLa cells were exposed to 400 ng of TSA/ml or 5 mM nicotinamide for 6 h.
To determine whether TSA increases amounts of Gdf11 mRNA in human cells, Northern blot assays were done using RNA prepared from TSA-treated and untreated HeLa cells. Similar to the response in BALB/c-3T3 cells, Gdf11 mRNA was much more abundant in TSA-treated than in untreated HeLa cells (Fig. 1C). However, Gdf11 induction in HeLa cells required a much greater concentration of TSA than did Gdf11 induction in BALB/c-3T3 cells. Similar to HeLa cells, TSA also induced Gdf11 mRNA expression in a normal diploid fibroblast cell line, Flow 2000, although to a lesser extent.
To determine whether increases in the abundance of Gdf11 mRNA result in increases in the abundance of Gdf11 protein, we generated a polyclonal anti-Gdf11 antibody and performed a Western blot assay using protein extracts prepared from HeLa cells treated or untreated with TSA. As shown in Fig. 1D, TSA clearly increased the amount of Gdf11 protein in HeLa cells.
Human and mouse HDACs are divided into three classes: class I (HDAC1, -2, -3, and -8), class II (HDAC4, -5, -6, -7, -9, -10), and class III (SIRT1 to -7). In addition to TSA, suberoylanilide hydroxamic acid (SAHA) and related compounds also inhibit the activities of class I and class II HDACs (36). Class III HDACs, however, are refractory to TSA and SAHA but sensitive to nicotinamide. Like TSA, SAHA greatly increases Gdf11 mRNA expression in HeLa cells as detected by Northern blot analysis (data not shown). In contrast, treatment of HeLa cells with nicotinamide, under conditions in which class III HDAC activities are inhibited, did not affect Gdf11 mRNA expression (Fig. 1E). These data strongly suggest that class I and/or class II HDACs suppress the expression of Gdf11 mRNA.
Regulation of the gdf11 promoter by TSA.
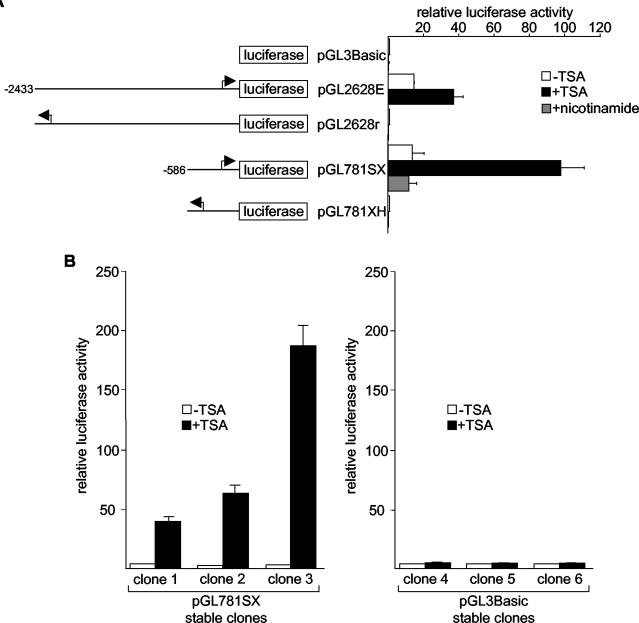
To determine whether TSA activates gdf11 transcription, we isolated and sequenced a human genomic DNA fragment containing 2,628 bp upstream of the gdf11 coding region from a bacterial artificial chromosome library. The cloned genomic DNA was inserted upstream of the luciferase reporter in pGL3Basic, and the resultant plasmid (pGL2628E) was transiently transfected into HeLa cells. As shown in Fig. 2A, pGL2628E efficiently directed the expression of the luciferase reporter (and thus increased luciferase activity) compared with pGL3Basic. Deletion of 1,847 bp of 5′ gdf11 DNA from pGL2628E (pGL781SX) had no effect on luciferase activity (i.e., amounts of activity were similar for the pGL2628E and pGL781SX constructs). In contrast, plasmids containing gdf11 DNA fragments identical to those of pGL2628E and pGL781SX but in the reverse orientation (pGL2628r and pGL781XH, respectively) produced only background levels of luciferase activity, similar to pGL3Basic. These results demonstrate the presence of a functional promoter within the 781-bp upstream of the gdf11 coding sequence.
FIG. 2.
Activation of the human gdf11 promoter by TSA. (A) Plasmids containing different portions of the human gdf11 promoter were transiently transfected into HeLa cells, and luciferase activity was determined after 12 h of treatment with ethanol (control), 400 ng of TSA/ml, or 5 mM nicotinamide. Arrows represent the putative transcription initiation site on the gdf11 promoter. (B) HeLa cells were stably transfected with either pGL781SX (left panel) or pGL3Basic (right panel). Randomly selected clones were treated with ethanol or 400 ng of TSA/ml for 12 h, and luciferase activities were determined in triplicate. Error bars show standard deviations.
TSA treatment of cells transfected with either pGL2628E or pGL781SX resulted in a considerable increase in luciferase activity (almost 10-fold for pGL781SX). This finding supports the existence of TSA response sequences within the 781-bp fragment. As expected, TSA did not activate pGL2628r or pGL781XH. Also in agreement with our Northern blot results (Fig. 1E), an inhibitor of class III HDACs, nicotinamide, had no effect on the activity of pGL781SX.
To show that TSA activates the gdf11 promoter in a chromatin environment, we cotransfected HeLa cells with a plasmid that expresses the neomycin-resistant gene (pSV2neo) and either pGL781SX or pGL3Basic and selected for cells with stably integrated reporters. Six clones, three containing integrated pGL781SX and three with pGL3Basic, were randomly chosen for analysis. As shown in Fig. 2B, TSA substantially increased luciferase activity when added to stable clones containing pGL781SX (clones 1 to 3) but not when added to stable clones containing pGL3Basic (clones 4 to 6).
Identification of a TSA response element in the human gdf11 promoter.
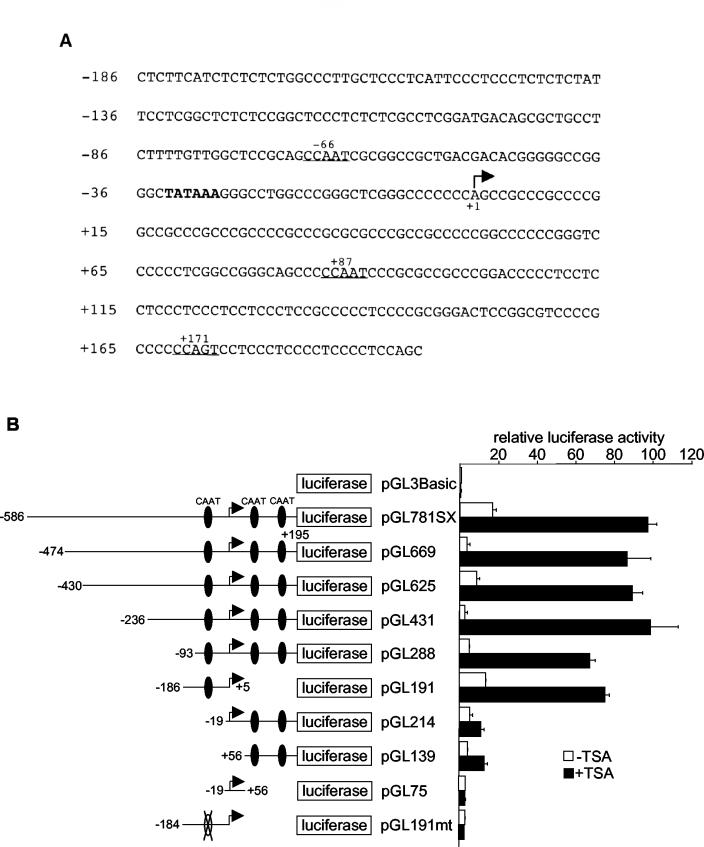
Previous studies showed that conserved cis DNA elements, particularly Sp1 and CCAAT boxes, located in the promoters of several genes are responsible for transcriptional activation by HDAC inhibitors (4, 21, 22, 25, 29, 46, 48, 49, 51, 61, 66). Although the gdf11 promoter sequence is highly GC rich (77%), a close inspection of the promoter revealed the presence of three potential CCAAT boxes but no putative Sp1 site (Fig. 3A). One of the CCAAT boxes is located 66 bp upstream from the putative transcription start site and 36 bp from the TATA box. Two additional CCAAT boxes are situated downstream from the putative transcription start site at +87 and +171.
FIG. 3.
Analysis of TSA response elements in the human gdf11 promoter. (A) DNA sequence of a 381-bp human gdf11 promoter. These sequence data have been submitted to the DDBJ, EMBL, and GenBank databases under accession no. BK001652. MatInspector software (http://www.genomatix.de) was used to identify potential TSA response elements (Sp1-binding sites and CCAAT boxes). Potential CCAAT boxes are underlined, and the TATA box is in bold. The arrow indicates the putative transcriptional initiation site. (B) Reporter plasmids containing different deletions and mutations of the gdf11 promoter were transiently transfected into HeLa cells, and luciferase activities were monitored 12 h after TSA treatment. CCAAT boxes on the promoters are illustrated by black ovals. An open oval with an X across it indicates a mutated CCAAT box. Error bars show standard deviations.
Deletion of DNA sequences upstream of the −66 CCAAT box had little or no effect on TSA-activated promoter-driven luciferase activity (Fig. 3B; compare pGL781SX to pGL669, pGL625, pGL431, and pGL288). Likewise, deletion of both CCAAT boxes downstream of the transcription start site (pGL191) had little effect on the TSA response. In contrast, deletion or a single point mutation of the CCAAT box at −66 severely reduced the abilities of the promoters to respond to TSA, regardless of the arrangements of downstream CCAAT boxes (pGL214, pGL139, pGL75, and pGL191mt). Thus, we conclude that the CCAAT box located at −66 is necessary and sufficient for TSA activation of the gdf11 promoter.
Repression of the gdf11 promoter by HDAC3.
Because TSA is a general inhibitor of class I and class II HDACs, there are many different ways in which TSA might activate gdf11. First, the gdf11 promoter is under the general repression maintenance of multiple class I and class II deacetylases, and TSA inhibits all class I and II HDAC activities exerted on the gdf11 promoter to activate gdf11. In this scenario, any or all class I and class II HDACs could repress the gdf11 promoter. Second, gdf11 may be under the selective control of either class I or class II HDACs. Third, gdf11 expression may be exquisitely regulated by a single specific HDAC belonging to either class I or II.
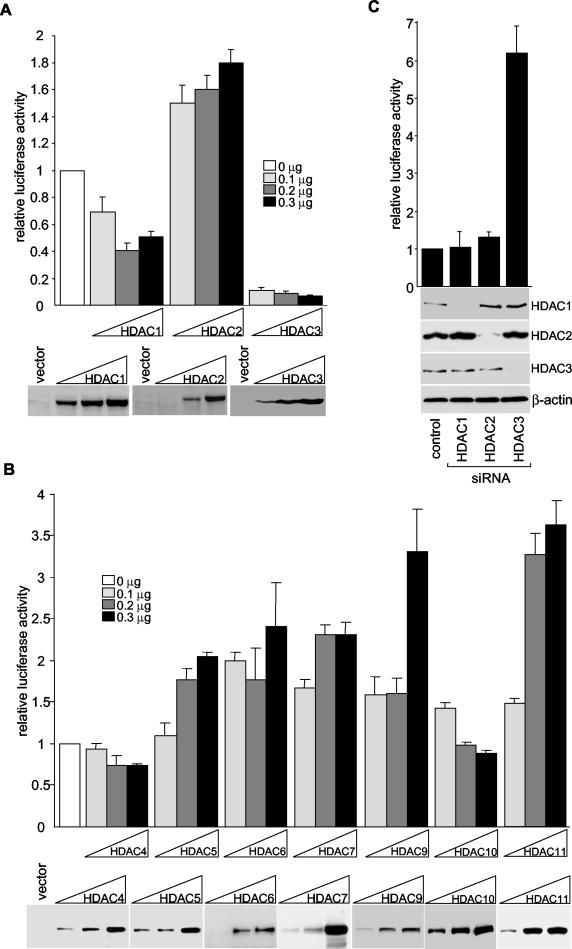
To determine which HDACs are responsible for the repression of the gdf11 promoter, we transfected HeLa cells with HDAC-expressing plasmids together with pGL191, and luciferase activity assays were performed. For the class I HDACs, overexpression of HDAC1 slightly reduced the activity of the gdf11 promoter (approximately twofold) (Fig. 4A). For unknown reasons, HDAC2 slightly increased luciferase activity from pGL191. In sharp contrast, overexpression of HDAC3 caused a significant (10-fold) repression of the gdf11 promoter. Similar to HDAC1 and HDAC2, overexpression of class II HDACs (HDAC4, -5, -6, -7, -9, or -10) and HDAC11 resulted in either an insignificant repression or a mild activation of the gdf11 promoter (Fig. 4B). These results suggest that HDAC3 is chiefly responsible for the repression of the gdf11 promoter.
FIG. 4.
Repression of human gdf11 promoter by HDAC3. (A and B) pGL191 and plasmids that express Flag-tagged HDACs were cotransfected into HeLa cells. Luciferase assays were performed 72 h after transfection, and HDAC expression levels were monitored by Western blotting with an anti-Flag antibody (representative blots are shown). All transfections were normalized to equal amounts of DNA with parental expression vectors. Results are tabulated from the average of three independent transfections ± the standard deviation. (C) The pBS/U6 vector (control) or plasmids that express siHDAC1, siHDAC2, and siHDAC3 were cotransfected with pGL191 into HeLa cells. Luciferase assays were performed, and the data shown are the averages ± standard deviations from three separate experiments. Western blotting with anti-HDAC1, anti-HDAC2, anti-HDAC3, and anti-β-actin was done to monitor protein expression (representative blots are shown).
To confirm that HDAC3 is involved in the repression of the gdf11 promoter, we used a DNA vector-based RNA interference (RNAi) method to suppress HDAC3 expression in HeLa cells. small interfering RNAs (siRNAs) targeting HDACs 1, 2, and 3 synthesized from the BS/U6 template efficiently inhibited the expression of HDAC1, -2, and -3, respectively, but not of the control protein β-actin, as monitored by immunoblotting (Fig. 4C). Transfection with the BS/U6 vector (control) had no effect on the abundance of any of the HDACs. In full agreement with the observation that HDAC3 overexpression represses the activity of the gdf11 promoter, depletion of HDAC3 but not HDAC1 or HDAC2 significantly increased the activity of the pGL191 promoter. These results strongly support the premise that HDAC3 represses transcription from the gdf11 promoter.
Deacetylation of Lys-9 of histone H3 by HDAC3 on the gdf11 promoter.
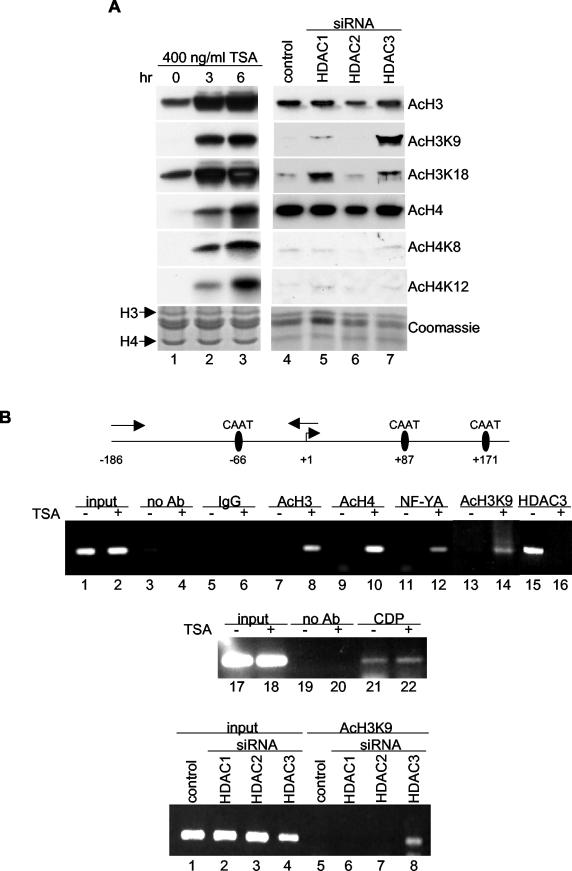
In Saccharomyces cerevisiae, HOS2, a close relative of human HDAC3, preferentially deacetylates histones H3 and H4 (60). We therefore hypothesized that TSA or HDAC3 depletion activates gdf11 transcription by promoting the hyperacetylation of histones H3 and H4 surrounding the −66 CCAAT box on the gdf11 promoter. To test this, we first performed Western blotting with different anti-acetyl-lysine antibodies on extracts prepared from HeLa cells treated with TSA or expressing siRNA to HDACs. As expected, TSA treatment increased the acetylation of histones H3 and H4 (Fig. 5A, compare lane 1 to lanes 2 and 3). TSA also increased the acetylation of Lys-9 and Lys-18 of histone H3 (H3K9 and H3K18) and Lys-8 and Lys-12 of histone H4 (H4K8 and H4K12), as determined by use of antibodies that specifically recognize these lysines when they are acetylated. This finding confirms that TSA is a general, nonspecific HDAC inhibitor.
FIG. 5.
Enhanced H3K9 acetylation on the gdf11 promoter upon TSA treatment or HDAC3 silencing. (A) Core histones prepared from HeLa cells treated with 400 ng of TSA/ml (left panels) or transfected with plasmids expressing siRNAs (right panels) were Western blotted with antibodies against acetylated H3, H3K9, H3K18, H4, H4K8, and H4K12. A Coomassie-stained gel was prepared in parallel to assess the quality of each histone preparation. (B) Schematic diagram of the human gdf11 promoter (top panel). The arrows facing in opposite directions above the promoter denote the primers used in the ChIP assays. Cross-linked chromatin prepared from HeLa cells treated with (+) or without (−) TSA (middle panels) or transfected with siRNA expression plasmids (bottom panel) was precipitated with the indicated antibodies, and PCR analysis was performed using primers specific for the gdf11 promoter region.
We next examined histone acetylation in HeLa cells depleted of HDAC1, HDAC2, or HDAC3 by RNAi. Expression of HDAC1, HDAC2, or HDAC3 siRNA did not affect the overall acetylation status of histone H3 or H4 or the acetylation of Lys-8 (H4K8) or Lys-12 (H4K12) of histone H4 (Fig. 5A, lanes 4 to 7). Expression of HDAC1 or HDAC3 siRNA (but not of HDAC2 siRNA) increased the acetylation of H3K9 and H3K18. HDAC3 siRNA increased the acetylation of H3K9 to a greater extent than did HDAC1 siRNA, whereas the reverse was true for H3K18. These data suggest that repression of gdf11 transcription results from the targeting of HDAC3 to the gdf11 promoter and the consequent deacetylation of histone H3, most notably at Lys-9.
To provide support for this model, ChIP assays were performed. HeLa cells were treated with TSA or transfected with siRNA to HDAC1, HDAC2, or HDAC3. After cross-linking, chromatin was coprecipitated with antibody to acetylated histone H3, acetylated histone H4, acetylated H3K9, NF-Y, or HDAC3. PCR was performed using primers that generated the region of the gdf11 promoter that contains the −66 CCAAT box. The results of the ChIP assays showed that more acetylated H3, H4, and H3K9 associate with the CCAAT box-containing region of the gdf11 promoter in cells treated with TSA than in untreated cells (Fig. 5B, middle panel, lanes 8, 10, and 14). TSA treatment of cells also increased occupancy by one of the CCAAT box-binding proteins, NF-YA (compare lanes 11 and 12) and decreased the binding of HDAC3 to the gdf11 promoter (compare lanes 15 and 16). In contrast, the association of another CCAAT box-binding protein, CDP, remained intact with TSA treatment (compare lanes 21 and 22). Most importantly, and consistent with a model in which HDAC3 represses gdf11 transcription by deacetylation of H3K9 on the gdf11 promoter, we found that disruption of HDAC3 expression, but not of HDAC1 or HDAC2 expression, increased H3K9 acetylation on the promoter (Fig. 5B, bottom panel, lane 8).
DISCUSSION
Using expression DNA microarrays, previous studies have identified a number of cellular genes that are induced by HDAC inhibitors (7, 15, 20, 30, 32, 36, 40, 61). Surprisingly, results from these profiles are largely nonoverlapping, suggesting that different HDAC inhibitors use distinct mechanisms to regulate gene expression. Our finding that TSA activates the gdf11 gene was unexpected, because none of the previous studies reported the induction of gdf11 by HDAC inhibitors. Although this discrepancy could be explained by differences in inhibitors, cell types, and experimental conditions (e.g., amount of inhibitors used and duration of treatment), it clearly points to the fact that the mechanisms by which HDAC inhibitors such as TSA alter gene expression may be far more complicated than initially predicted. Further work will be necessary to clarify the exact optimal physiological environment and circumstances under which HDAC inhibitors activate gdf11.
As expected, in our TSA profile TSA affected the expression of genes in addition to gdf11. Of particular interest is the eightfold decrease in amounts of the transcript that encodes follistatin, an activin-binding protein that negatively modulates the actions of several members of the TGF-β family, including Gdf11 (2, 13, 34, 41, 65). At this time, we do not know the exact mechanism by which TSA reduces the expression of follistatin. Regardless, down-regulation of follistatin represents an additional means by which TSA facilitates Gdf11-initiated events and consequent growth arrest.
In previous studies of HDAC inhibitor-induced alterations in gene transcription, issues were often raised as to whether transiently transfected DNA accurately models chromatinized DNA. An interesting outcome of our gdf11 promoter analysis is that TSA effectively activated both transiently transfected and stably integrated gdf11 promoters. This observation fits well with a recent report showing that recruitment of HDAC3 by the thyroid hormone receptor mediates thyroid hormone receptor-targeted repression of transiently transfected as well as stably integrated genes (23).
Sp1-binding sites are the target promoter DNA elements for HDAC inhibitor activation of the p21Cip1, tyrosine hydroxylase, and hTERT genes (22, 29, 48, 51, 66). In addition, Sp1 sites are enriched in promoter regions of TSA- and trapoxin-up-regulated genes identified in profiling experiments (30). Thus, Sp1-binding sites appear to be common HDAC inhibitor- and HDAC-regulated elements. However, NF-Y-binding sites (CCAAT boxes) rather than Sp1 sites mediate the activation of the genes for thioredoxin-binding protein 2, GTPase RhoB, TGF-β type II receptor, multidrug resistance 1, and GADD45 by HDAC inhibitors (4, 21, 25, 46, 61).
The human gdf11 promoter contains three potential CCAAT boxes, and our data showing that TSA activates this promoter through an upstream CCAAT box further strengthen the premise that HDAC inhibitors can potentially alter transcription through either Sp1-binding sites or CCAAT boxes. One question that remains to be addressed is how HDAC inhibitors selectively activate only a subset of the many promoters that contain Sp1 or CCAAT sites. Also unclear is how HDAC inhibitors restrictively activate only one site on a promoter that contains multiple Sp1 sites or CCAAT boxes. It is likely that the DNA sequences adjacent to Sp1 sites or CCAAT boxes and/or the location of these sites contribute significantly to a promoter's capacity to respond to HDAC inhibitors. Further experiments using additional gdf11 promoter mutants will help resolve this issue.
Using the approaches of overexpression and knock-down with siRNA, coupled with reporter assays, we have shown that HDAC3 regulates the activity of the gdf11 promoter, whereas the other HDACs tested do not. Consistent with these results, we have also demonstrated by microarray analysis that HDAC3 siRNA increases Gdf11 mRNA levels when transiently expressed in HeLa cells (X. Zhang and E. Seto, unpublished data). These are significant findings, because they argue that HDAC3 has a unique function not shared by other HDACs and, more broadly, that the biological roles of different HDACs are not redundant. To our knowledge, this is the first finding of a TSA-inducible promoter that is regulated exclusively by HDAC3.
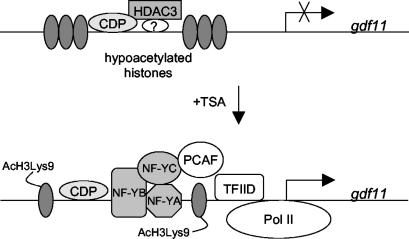
Our discovery that TSA not only inhibits the catalytic activity of HDACs but also prevents deacetylase occupancy at the promoter is not unprecedented. TSA also markedly decreases the association of HDAC1 with the metallothionein I and the p21Cip1 promoters (14, 19). Currently, we do not know the exact mechanisms by which TSA dissociates HDAC3 from the gdf11 promoter. It is conceivable that TSA may change the acetylation status of HDAC3 or of proteins in the HDAC3 complex and consequently render HDAC3 less capable of binding to the gdf11 promoter. Alternatively, changes in the acetylation states of nucleosomes in the gdf11 promoter by TSA may favor the recruitment of a transcriptional coactivator complex containing NF-Y that replaces the HDAC3 repressing complex (Fig. 6).
FIG. 6.
Model of gdf11 activation by TSA.
In addition to NF-Y, the CCAAT displacement protein (CDP) also binds specifically to and regulates promoters possessing CCAAT boxes. Interestingly, CDP is a repressor for the transcription of developmentally regulated genes (44), and it appears to compete with transcriptional activating proteins for binding to the promoter regions of various genes (35, 57). It is tempting, therefore, to speculate that HDAC3 is recruited by CDP to the upstream CCAAT box on the gdf11 promoter for repression. Upon TSA treatment, an exchange of CDP-HDAC3 for an NF-Y activator complex occurs. However, in repeated attempts, we have not detected an association of HDAC3 with CDP (data not shown). Furthermore, using ChIP assays, we found that CDP (but not HDAC3) remained bound on the gdf11 promoter in the presence of TSA. Therefore, although we cannot exclude the possibility that HDAC3 is recruited to the gdf11 promoter by a transcription factor other than NF-Y, at this time we favor a model in which HDAC3 is recruited to the promoter by a mechanism that does not involve CDP.
HDAC3 often exists in a stable large multisubunit complex that contains the nuclear receptor corepressors N-CoR/SMRT (18, 33, 56, 63). Several DNA-bound transcription factors recruit N-CoR/SMRT/HDAC3 to enzymatically modify histones and consequently repress transcription. A logical prediction, then, is that N-CoR/SMRT provides a convenient platform for HDAC3 to dock onto the gdf11 promoter. However, preliminary results from ChIP assays indicate that N-CoR/SMRT is not involved in the regulation of the gdf11 promoter (data not shown). Rather, the recruitment of HDAC3 to the gdf11 promoter involves a novel complex that requires further characterization.
Some reports suggest that HDAC3 deacetylates all four core histones at every lysine tested (reviewed in reference 36). However, interpretation of these studies is complicated by the fact that they were carried out with immunoprecipitated HDAC3 complexes, which may contain HDAC4, -5, and -7 in addition to HDAC3 (10, 16). To more conclusively determine whether HDAC3 possesses substrate specificity, we used the method of RNAi to silence the expression of HDAC3 without affecting the expression of HDAC4, -5, and -7. We found that H3K9 was especially sensitive to knock-down of HDAC3, which suggests that the overall acetylation status of H3K9 is under the tight control of HDAC3. Consistent with our finding that HDAC3 represses gdf11 gene transcription, we found that knock-down of HDAC3, but not of HDAC1 or HDAC2, caused a hyperacetylation of H3K9 on the gdf11 promoter. In the beta interferon gene, acetylation of H3K9 is critical for the recruitment of the general transcription factor TFIID (1). We propose, then, that inhibition of HDAC3 activity (by TSA for example) results in an increase in H3K9 acetylation, which promotes the binding of TFIID to the gdf11 promoter and consequently activates gdf11 transcription (Fig. 6). An alternative, though non-mutually exclusive, hypothesis is that increases in H3K9 acetylation on the gdf11 promoter affect the methylation of Lys-4 and Lys-9 and the phosphorylation of Ser-10 on histone H3 (11). This would change the overall histone modification pattern on the gdf11 promoter and perhaps ultimately alter the expression of the gdf11 gene. Finally, acetylation of H3K9 may facilitate the recruitment of NF-Y to the upstream CCATT box on the gdf11 promoter, which in turn might aid the recruitment of additional histone-modifying enzymes such as PCAF (24, 46). Our finding that the binding of NF-YA to the gdf11 promoter increases upon TSA treatment fits well with this idea.
Currently, very little is known about the mechanisms by which Gdf11 inhibits cell proliferation. In the olfactory epithelium model, Gdf11 apparently inhibits neurogenesis by inducing the expression of the CDK inhibitor p27Kip1 and thereby arresting cells in the G1 phase of the cell cycle (65). However, a separate study showed that the expression of p27Kip1 mRNA and the level of histone H4 acetylation of the p27Kip1 gene were not altered by the treatment of cells with an HDAC inhibitor (47). Also, TSA inhibited the growth of mouse embryo fibroblasts lacking both p27Kip1 and p21Cip1, suggesting that TSA modulates the expression of antiproliferative factors other than or in addition to these CDK inhibitors (64). We favor the model that Gdf11 exerts its antiproliferative effects on cells by multiple pathways, with at least one pathway independent of p27Kip1 and p21Cip1. Therefore, to fully understand how HDAC inhibitors arrest cell growth, it is imperative to elucidate intermediate signals that lead to Gdf11 activation and to identify additional downstream targets of Gdf11. Using a number of different approaches, work is now under way in our laboratory to identify potential Gdf11 targets.
Acknowledgments
We thank Ron Evans, Se-Jin Lee, Vicki Richon, Yang Shi, and Tso-Pang Yao for HDAC7 cDNA, mouse gdf11 cDNA, HDAC9 cDNA, pBS/U6, and HDAC10 cDNA, respectively; Anne Calof and Tere Antonia for discussion; and the Moffitt Cancer Center Core Facility for technical support.
This work was supported by grants to E.S. from the National Institutes of Health (GM58486 and GM64850) and the Kaul Foundation. X.Z. is a recipient of an American Cancer Society Institutional Grant.
REFERENCES
- 1.Agalioti, T., G. Chen, and D. Thanos. 2002. Deciphering the transcriptional histone acetylation code for a human gene. Cell 111**:**381-392. [DOI] [PubMed] [Google Scholar]
- 2.Armand, A. S., B. Della Gaspera, T. Launay, F. Charbonnier, C. L. Gallien, and C. Chanoine. 2003. Expression and neural control of follistatin versus myostatin genes during regeneration of mouse soleus. Dev. Dyn. 227**:**256-265. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, K. E., and P. J. Farnham. 1999. Coexamination of site-specific transcription factor binding and promoter activity in living cells. Mol. Cell. Biol. 19**:**8393-8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler, L. M., X. Zhou, W. S. Xu, H. I. Scher, R. A. Rifkind, P. A. Marks, and V. M. Richon. 2002. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc. Natl. Acad. Sci. USA 99**:**11700-11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmen, A. A., S. E. Rundlett, and M. Grunstein. 1996. HDA1 and HDA3 are components of a yeast histone deacetylase (HDA) complex. J. Biol. Chem. 271**:**15837-15844. [DOI] [PubMed] [Google Scholar]
- 6.Darkin-Rattray, S. J., A. M. Gurnett, R. W. Myers, P. M. Dulski, T. M. Crumley, J. J. Allocco, C. Cannova, P. T. Meinke, S. L. Colletti, M. A. Bednarek, S. B. Singh, M. A. Goetz, A. W. Dombrowski, J. D. Polishook, and D. M. Schmatz. 1996. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 93**:**13143-13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Della Ragione, F., V. Criniti, V. Della Pietra, A. Borriello, A. Oliva, S. Indaco, T. Yamamoto, and V. Zappia. 2001. Genes modulated by histone acetylation as new effectors of butyrate activity. FEBS Lett. 499**:**199-204. [DOI] [PubMed] [Google Scholar]
- 8.Eickhoff, B., L. Germeroth, C. Stahl, G. Kohler, S. Ruller, M. Schlaak, and J. van der Bosch. 2000. Trichostatin A-mediated regulation of gene expression and protein kinase activities: reprogramming tumor cells for ribotoxic stress-induced apoptosis. Biol. Chem. 381**:**1127-1132. [DOI] [PubMed] [Google Scholar]
- 9.Esquela, A. F., and S. J. Lee. 2003. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev. Biol. 257**:**356-370. [DOI] [PubMed] [Google Scholar]
- 10.Fischle, W., F. Dequiedt, M. Fillion, M. J. Hendzel, W. Voelter, and E. Verdin. 2001. Human HDAC7 histone deacetylase activity is associated with HDAC3 in vivo. J. Biol. Chem. 276**:**35826-35835. [DOI] [PubMed] [Google Scholar]
- 11.Fischle, W., Y. Wang, and C. D. Allis. 2003. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 15**:**172-183. [DOI] [PubMed] [Google Scholar]
- 12.Gamer, L. W., K. A. Cox, C. Small, and V. Rosen. 2001. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev. Biol. 229**:**407-420. [DOI] [PubMed] [Google Scholar]
- 13.Gamer, L. W., N. M. Wolfman, A. J. Celeste, G. Hattersley, R. Hewick, and V. Rosen. 1999. A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Dev. Biol. 208**:**222-232. [DOI] [PubMed] [Google Scholar]
- 14.Ghoshal, K., J. Datta, S. Majumder, S. Bai, X. Dong, M. Parthun, and J. T. Samson. 2002. Inhibitors of histone deacetylase and DNA methyltransferase synergistically activate the methylated metallothionein I promoter by activating the transcription factor MTF-1 and forming an open chromatin structure. Mol. Cell. Biol. 22**:**8302-8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser, K. B., M. J. Staver, J. F. Waring, J. Stender, R. G. Ulrich, and S. K. Davidsen. 2003. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol. Cancer Ther. 2**:**151-163. [PubMed] [Google Scholar]
- 16.Grozinger, C. M., C. A. Hassig, and S. L. Schreiber. 1999. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. USA 96**:**4868-4873.10220385 [Google Scholar]
- 17.Guardiola, A. R., and T. P. Yao. 2002. Molecular cloning and characterization of a novel histone deacetylase HDAC10. J. Biol. Chem. 277**:**3350-3356. [DOI] [PubMed] [Google Scholar]
- 18.Guenther, M. G., W. S. Lane, W. Fischle, E. Verdin, M. A. Lazar, and R. Shiekhattar. 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40- repeat protein linked to deafness. Genes Dev. 14**:**1048-1057. [PMC free article] [PubMed] [Google Scholar]
- 19.Gui, C. Y., L. Ngo, W. S. Xu, V. M. Richon, and P. A. Marks. 2004. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc. Natl. Acad. Sci. USA 101**:**1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, L. Z., T. Tolentino, P. Grayson, S. Zhong, R. P. Warrell, Jr., R. A. Rifkind, P. A. Marks, V. M. Richon, and P. P. Pandolfi. 2001. Histone deacetylase inhibitors induce remission in transgenic models of therapy-resistant acute promyelocytic leukemia. J. Clin. Investig. 108**:**1321-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirose, T., Y. Sowa, S. Takahashi, S. Saito, C. Yasuda, N. Shindo, K. Furuichi, and T. Sakai. 2003. p53-independent induction of Gadd45 by histone deacetylase inhibitor: coordinate regulation by transcription factors Oct-1 and NF-Y. Oncogene 22**:**7762-7773. [DOI] [PubMed] [Google Scholar]
- 22.Hou, M., X. Wang, N. Popov, A. Zhang, X. Zhao, R. Zhou, A. Zetterberg, M. Bjorkholm, M. Henriksson, A. Gruber, and D. Xu. 2002. The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp. Cell Res. 274**:**25-34. [DOI] [PubMed] [Google Scholar]
- 23.Ishizuka, T., and M. A. Lazar. 2003. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol. Cell. Biol. 23**:**5122-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin, S., and K. W. Scotto. 1998. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol. Cell. Biol. 18**:**4377-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, S., B. Gorfajn, G. Faircloth, and K. W. Scotto. 2000. Ecteinascidin 743, a transcription-targeted chemotherapeutic that inhibits MDR1 activation. Proc. Natl. Acad. Sci. USA 97**:**6775-6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnstone, R. W. 2002. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 1**:**287-299. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone, R. W., and J. D. Licht. 2003. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 4**:**13-18. [DOI] [PubMed] [Google Scholar]
- 28.Kao, H. Y., M. Downes, P. Ordentlich, and R. M. Evans. 2000. Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Genes Dev. 14**:**55-66. [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, H. S., J. S. Park, S. J. Hong, M. S. Woo, S. Y. Kim, and K. S. Kim. 2003. Regulation of the tyrosine hydroxylase gene promoter by histone deacetylase inhibitors. Biochem. Biophys. Res. Commun. 312**:**950-957. [DOI] [PubMed] [Google Scholar]
- 30.Koeller, K. M., S. J. Haggarty, B. D. Perkins, I. Leykin, J. C. Wong, M. C. Kao, and S. L. Schreiber. 2003. Chemical genetic modifier screens. Small molecule trichostatin suppressors as probes of intracellular histone and tubulin acetylation. Chem. Biol. 10**:**397-410. [DOI] [PubMed] [Google Scholar]
- 31.Laherty, C. D., W. M. Yang, J. M. Sun, J. R. Davie, E. Seto, and R. N. Eisenman. 1997. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell 89**:**349-356. [DOI] [PubMed] [Google Scholar]
- 32.Lee, H., S. Lee, M. Baek, H.-Y. Kim, and D.-I. Jeoung. 2002. Expression profile analysis of trichostatin A in human gastric cancer cells. Biotechnol. Lett. 24**:**377-381. [Google Scholar]
- 33.Li, J., J. Wang, Z. Nawaz, J. M. Liu, J. Qin, and J. Wong. 2000. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 19**:**4342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, S. Y., J. R. Morrison, D. J. Phillips, and D. M. de Kretser. 2003. Regulation of ovarian function by the TGF-beta superfamily and follistatin. Reproduction 126**:**133-148. [DOI] [PubMed] [Google Scholar]
- 35.Luo, W., and D. G. Skalnik. 1996. CCAAT displacement protein competes with multiple transcriptional activators for binding to four sites in the proximal gp91phox promoter. J. Biol. Chem. 271**:**18203-18210. [DOI] [PubMed] [Google Scholar]
- 36.Marks, P. A., T. Miller, and V. M. Richon. 2003. Histone deacetylases. Curr. Opin. Pharmacol. 3**:**344-351. [DOI] [PubMed] [Google Scholar]
- 37.Marks, P. A., V. M. Richon, R. Breslow, and R. A. Rifkind. 2001. Histone deacetylase inhibitors as new cancer drugs. Curr. Opin. Oncol. 13**:**477-483. [DOI] [PubMed] [Google Scholar]
- 38.Marks, P. A., V. M. Richon, and R. A. Rifkind. 2000. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst. 92**:**1210-1216. [DOI] [PubMed] [Google Scholar]
- 39.Marks, P. A., R. A. Rifkind, V. M. Richon, and R. Breslow. 2001. Inhibitors of histone deacetylase are potentially effective anticancer agents. Clin. Cancer Res. 7**:**759-760. [PubMed] [Google Scholar]
- 40.Marks, P. A., R. A. Rifkind, V. M. Richon, R. Breslow, T. Miller, and W. K. Kelly. 2001. Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer 1**:**194-202. [DOI] [PubMed] [Google Scholar]
- 41.Matzuk, M. M., N. Lu, H. Vogel, K. Sellheyer, D. R. Roop, and A. Bradley. 1995. Multiple defects and perinatal death in mice deficient in follistatin. Nature 374**:**360-363. [DOI] [PubMed] [Google Scholar]
- 42.McPherron, A. C., A. M. Lawler, and S. J. Lee. 1999. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 22**:**260-264. [DOI] [PubMed] [Google Scholar]
- 43.McPherron, A. C., A. M. Lawler, and S. J. Lee. 1997. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387**:**83-90. [DOI] [PubMed] [Google Scholar]
- 44.Neufeld, E. J., D. G. Skalnik, P. M. Lievens, and S. H. Orkin. 1992. Human CCAAT displacement protein is homologous to the Drosophila homeoprotein, cut. Nat. Genet. 1**:**50-55. [DOI] [PubMed] [Google Scholar]
- 45.Oh, S. P., C. Y. Yeo, Y. Lee, H. Schrewe, M. Whitman, and E. Li. 2002. Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 16**:**2749-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park, S. H., S. R. Lee, B. C. Kim, E. A. Cho, S. P. Patel, H. B. Kang, E. A. Sausville, O. Nakanishi, J. B. Trepel, B. I. Lee, and S. J. Kim. 2002. Transcriptional regulation of the transforming growth factor beta type II receptor gene by histone acetyltransferase and deacetylase is mediated by NF-Y in human breast cancer cells. J. Biol. Chem. 277**:**5168-5174. [DOI] [PubMed] [Google Scholar]
- 47.Richon, V. M., T. W. Sandhoff, R. A. Rifkind, and P. A. Marks. 2000. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. USA 97**:**10014-10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambucetti, L. C., D. D. Fischer, S. Zabludoff, P. O. Kwon, H. Chamberlin, N. Trogani, H. Xu, and D. Cohen. 1999. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J. Biol. Chem. 274**:**34940-34947. [DOI] [PubMed] [Google Scholar]
- 49.Schuettengruber, B., E. Simboeck, H. Khier, and C. Seiser. 2003. Autoregulation of mouse histone deacetylase 1 expression. Mol. Cell. Biol. 23**:**6993-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shang, Y., X. Hu, J. DiRenzo, M. A. Lazar, and M. Brown. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103**:**843-852. [DOI] [PubMed] [Google Scholar]
- 51.Sowa, Y., T. Orita, S. Minamikawa, K. Nakano, T. Mizuno, H. Nomura, and T. Sakai. 1997. Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. Biochem. Biophys. Res. Commun. 241**:**142-150. [DOI] [PubMed] [Google Scholar]
- 52.Steffan, J. S., L. Bodai, J. Pallos, M. Poelman, A. McCampbell, B. L. Apostol, A. Kazantsev, E. Schmidt, Y. Z. Zhu, M. Greenwald, R. Kurokawa, D. E. Housman, G. R. Jackson, J. L. Marsh, and L. M. Thompson. 2001. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413**:**739-743. [DOI] [PubMed] [Google Scholar]
- 53.Sui, G., C. Soohoo, B. Affar el, F. Gay, Y. Shi, W. C. Forrester, and Y. Shi. 2002. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99**:**5515-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taunton, J., C. A. Hassig, and S. L. Schreiber. 1996. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272**:**408-411. [DOI] [PubMed] [Google Scholar]
- 55.Tsuji, N., M. Kobayashi, K. Nagashima, Y. Wakisaka, and K. Koizumi. 1976. A new antifungal antibiotic, trichostatin. J. Antibiot. 29**:**1-6. [DOI] [PubMed] [Google Scholar]
- 56.Urnov, F. D., J. Yee, L. Sachs, T. N. Collingwood, A. Bauer, H. Beug, Y. B. Shi, and A. P. Wolffe. 2000. Targeting of N-CoR and histone deacetylase 3 by the oncoprotein v-erbA yields a chromatin infrastructure-dependent transcriptional repression pathway. EMBO J. 19**:**4074-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valarche, I., J. P. Tissier-Seta, M. R. Hirsch, S. Martinez, C. Goridis, and J. F. Brunet. 1993. The mouse homeodomain protein Phox2 regulates Ncam promoter activity in concert with Cux/CDP and is a putative determinant of neurotransmitter phenotype. Development 119**:**881-896. [DOI] [PubMed] [Google Scholar]
- 58.Van Lint, C., S. Emiliani, and E. Verdin. 1996. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 5**:**245-253. [PMC free article] [PubMed] [Google Scholar]
- 59.Vaziri, C., L. Stice, and D. V. Faller. 1998. Butyrate-induced G1 arrest results from p21-independent disruption of retinoblastoma protein-mediated signals. Cell Growth Differ. 9**:**465-474. [PubMed] [Google Scholar]
- 60.Wang, A., S. K. Kurdistani, and M. Grunstein. 2002. Requirement of Hos2 histone deacetylase for gene activity in yeast. Science 298**:**1412-1414. [DOI] [PubMed] [Google Scholar]
- 61.Wang, S., Y. Yan-Neale, D. Fischer, M. Zeremski, R. Cai, J. Zhu, F. Asselbergs, G. Hampton, and D. Cohen. 2003. Histone deacetylase 1 represses the small GTPase RhoB expression in human nonsmall lung carcinoma cell line. Oncogene 22**:**6204-6213. [DOI] [PubMed] [Google Scholar]
- 62.Wen, Y. D., W. D. Cress, A. L. Roy, and E. Seto. 2003. Histone deacetylase 3 binds to and regulates the multifunctional transcription factor TFII-I. J. Biol. Chem. 278**:**1841-1847. [DOI] [PubMed] [Google Scholar]
- 63.Wen, Y. D., V. Perissi, L. M. Staszewski, W. M. Yang, A. Krones, C. K. Glass, M. G. Rosenfeld, and E. Seto. 2000. The histone deacetylase-3 complex contains nuclear receptor corepressors. Proc. Natl. Acad. Sci. USA 97**:**7202-7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wharton, W., J. Savell, W. D. Cress, E. Seto, and W. J. Pledger. 2000. Inhibition of mitogenesis in Balb/c-3T3 cells by trichostatin A. Multiple alterations in the induction and activation of cyclin-cyclin-dependent kinase complexes. J. Biol. Chem. 275**:**33981-33987. [DOI] [PubMed] [Google Scholar]
- 65.Wu, H. H., S. Ivkovic, R. C. Murray, S. Jaramillo, K. M. Lyons, J. E. Johnson, and A. L. Calof. 2003. Autoregulation of neurogenesis by GDF11. Neuron 37**:**197-207. [DOI] [PubMed] [Google Scholar]
- 66.Xiao, H., T. Hasegawa, and K. Isobe. 1999. Both Sp1 and Sp3 are responsible for p21waf1 promoter activity induced by histone deacetylase inhibitor in NIH3T3 cells. J. Cell. Biochem. 73**:**291-302. [PubMed] [Google Scholar]
- 67.Yoshida, M., and T. Beppu. 1988. Reversible arrest of proliferation of rat 3Y1 fibroblasts in both the G1 and G2 phases by trichostatin A. Exp. Cell Res. 177**:**122-131. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida, M., R. Furumai, M. Nishiyama, Y. Komatsu, N. Nishino, and S. Horinouchi. 2001. Histone deacetylase as a new target for cancer chemotherapy. Cancer Chemother. Pharmacol. 48**:**S20-S26. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida, M., S. Horinouchi, and T. Beppu. 1995. Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays 17**:**423-430. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida, M., M. Kijima, M. Akita, and T. Beppu. 1990. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265**:**17174-17179. [PubMed] [Google Scholar]
- 71.Zhou, X., P. A. Marks, R. A. Rifkind, and V. M. Richon. 2001. Cloning and characterization of a histone deacetylase, HDAC9. Proc. Natl. Acad. Sci. USA 98**:**10572-10577. [DOI] [PMC free article] [PubMed] [Google Scholar]