Defective N-acetylaspartate catabolism reduces brain acetate levels and myelin lipid synthesis in Canavan's disease (original) (raw)
Abstract
Canavan's disease (CD) is a fatal, hereditary disorder of CNS development that has been linked to mutations in the gene for the enzyme aspartoacylase (ASPA) (EC 3.5.1.15). ASPA acts to hydrolyze _N_-acetylaspartate (NAA) into l-aspartate and acetate, but the connection between ASPA deficiency and the failure of proper CNS development is unclear. We hypothesize that one function of ASPA is to provide acetate for the increased lipid synthesis that occurs during postnatal CNS myelination. The gene encoding ASPA has been inactivated in the mouse model of CD, and here we show significant decreases in the synthesis of six classes of myelinassociated lipids, as well as reduced acetate levels, in the brains of these mice at the time of peak postnatal CNS myelination. Analysis of the lipid content of white matter from a human CD patient showed decreased cerebroside and sulfatide relative to normal white matter. These results demonstrate that myelin lipid synthesis is significantly compromised in CD and provide direct evidence that defective myelin synthesis, resulting from a deficiency of NAA-derived acetate, is involved in the pathogenesis of CD.
Keywords: acetyl CoA, leukodystrophy, oligodendrocytes, aspartoacylase
N-acetylaspartate (NAA) attains one of the highest concentrations of any molecule in the human CNS (1), yet the functions it serves remain controversial. NAA is synthesized from l-aspartate and acetyl CoA in neuronal mitochondria by the enzyme aspartate _N_-acetyltransferase (Asp-NAT) (EC 2.3.1.17) (2, 3). NAA is found predominantly in neurons, (4) but the catabolic enzyme aspartoacylase (ASPA) is present primarily in oligodendrocytes in the CNS (5). The high concentration of NAA in the CNS and its characteristic peak in water-suppressed proton magnetic resonance spectroscopy (MRS) permits noninvasive determinations of NAA concentrations in the human brain. MRS determinations of NAA levels are commonly used for evaluating the integrity of neurons in a number of neurological disorders, and this method has emerged as a preferred technique for following the clinical course of several CNS pathologies (6–8). MRS studies operate on the assumptions that NAA is synthesized by and accumulated in neurons and that the steady-state NAA levels in the brain can be interpreted as indicating overall neuronal health or integrity (9, 10).
Canavan's disease (CD) is a fatal, hereditary leukodystrophy that compromises normal CNS development and is caused by mutations in the gene for the enzyme ASPA (11, 12). ASPA currently is thought to function exclusively to hydrolyze NAA, a neuron-specific amino acid derivative, into l-aspartate and free acetate. However, ASPA is strongly expressed in other tissues, such as kidney, even though the only known substrate, NAA, is present predominantly in the nervous system (13). Despite the established connection between mutations in the gene for ASPA in CD and the lost capacity to deacetylate NAA, the specific connection between ASPA deficiency and the failure of proper CNS development is unclear (14). Further, the precise roles that NAA plays in CNS development and function remain a matter of inquiry.
Several hypotheses have been proposed for the roles served by NAA in the nervous system. Because of its high concentration and lack of known actions on neurons or glia, NAA has been proposed to act as an organic osmolyte that removes excess water from neurons by acting as a molecular water pump (15). In this regard, it has been proposed that excess NAA leads to osmotic dysregulation or has other cytotoxic effects that are responsible for the pathology observed in CD patients (16). NAA has also been proposed as a required substrate for the enzymatic synthesis of the neuron-specific dipeptide _N_-acetylaspartylglutamate (17).
There is more compelling evidence that NAA is essential for lipid synthesis and myelination in the CNS, in addition to the well established connection between ASPA mutations and leukodystrophy. In particular, the levels of NAA, ASPA, and Asp-NAT rise with a temporal course similar to those of myelin proteins (18, 19). Further, it has been shown that NAA contributes acetyl groups for the synthesis of lipids, which in turn are incorporated into myelin (20, 21). Finally, radiolabeled NAA is transported down optic nerve axons, and the acetate moiety is incorporated into their ensheathing myelin lipids (22). These and other observations led to our hypothesis that mutations in ASPA result in a deficiency in the supply of NAA-derived acetate, which in turn results in decreased synthesis of myelin-related fatty acids and lipids. Under our hypothesis, it is this lipogenic deficiency that compromises CNS myelination, impairs CNS development, and ultimately results in the white matter degeneration observed in CD (23, 24).
In most cell types, such as hepatocytes, the citrate–citrate lyase system provides the acetyl groups for fatty acid synthesis (25). It remains to be demonstrated that the NAA–ASPA system contributes significantly to lipid synthesis in the CNS. We addressed this issue by examining the rate of myelin lipid synthesis during the period of maximal postnatal myelination in ASPA knockout (ASPA_–/_–) mice, which were developed as a model for CD (26). These mice exhibit pathology similar to that of human CD patients. We also examined acetate levels in the brains of ASPA_–/_– mice and normal mice, as well as the lipid content in postmortem white matter samples from a 4.75-yearold CD patient compared with the lipid levels in normal human white matter.
Materials and Methods
Materials. Tritiated water [200 mCi (1 Ci = 37 GBq)] was from Amersham Biosciences. The solvents for TLC and lipid standards were from Sigma. TLC plates (UV254-sensitive, 0.25-mm silica gel-coated, aluminum-backed, 20 cm2) manufactured by Whatman were from JSP USA (Frederick, MD). Citrate synthase, acetyl CoA synthetase, CoA, malate, ATP, and NAD were from Sigma, and malate dehydrogenase was from Calbiochem.
Animals. Male mice (strain 129 Sv/Ev) that were negative for ASPA expression (ASPA_–/–) and their wild-type (ASPA+/_+) littermates were bred from heterozygous parents. Phenotypically comparable 17-day-old animals (five per group) were chosen for the experiment. Genotyping was done by PCR analysis. All animals were handled in accordance with National Institutes of Health guidelines.
Determination of ASPA Activity. ASPA activity was determined by using the radiometric assay described in ref. 27. This procedure uses _N_-acetyl-[14C] l-aspartate (specific activity = 11.63 mCi/mmol, final concentration = 1.8 mM) as substrate. Quantification of the product [14C] l-aspartate is done by phosphorimaging after separating it from the radiolabeled substrate by TLC.
Lipid Synthesis. Tritiated water of 200 mCi (1 Ci/ml) was diluted to 2.5 ml with PBS, and 0.25 ml per animal (20 mCi) was administered i.p. to the 17-day-old animals. Animals were administered with the tritiated water at 15-min intervals to facilitate dissection of brain and other organs 5 h after administration. Animals were killed after anesthetization (60 mg/kg ketamine and 10 mg/kg xylazine), and blood samples were collected to determine the specific activity of tritiated water in the serum. Brains and other dissected organs were transferred to preweighed plastic vials kept on dry ice and stored at –70°C until further analyses. Kidneys, livers, and brains were homogenized [10% (wt/vol)] by using a Polytron blender with isolation and resuspension buffer (50 mM Tris·HCl/50 mM NaCl/0.32 M sucrose/1 mM DTT) and protease inhibitor mixture (Sigma) according to the manufacturer's recommendations. The pH was adjusted to 8.0. Myelin was isolated according to the methods of Norton and Poduslo (28) on a sucrose density gradient (75,000 × g for 25 min). The myelin fraction was subjected to hypoosmotic shock, sedimented by centrifugation again (12,000 × g for 10 min), and collected as a pellet. The pellet was resuspended in 0.5 ml of resuspension buffer and repurified on a sucrose density gradient, followed by hypoosmotic shock and recentrifugation. Aliquots of samples were solubilized in 0.1 M NaOH for 3 days, and protein content was quantified by using a commercial kit (Bio-Rad). Lipid extraction was carried out by 2:1 chloroform:methanol solvent added to samples at a ratio of 1:10 (sample:solvent). The samples were thoroughly vortex-mixed, and solutes and solvents were separated by centrifugation. Clear chloroform fractions were collected for lipid analyses by TLC.
TLC. Myelin lipids were separated by TLC using 20-cm2 aluminum-backed silica gel plates (250 μm). Nonpolar lipids were separated in the first dimension with two solvent systems. The first solvent system (diethyl ether:benzene:ethanol:acetic acid = 40:50:2:0.2 by volume) was allowed to run two-thirds of the height of the TLC plate. After drying the plates under low negative pressure in a chamber, a second solvent system (diethyl ether:hexane = 6:94 by volume) was allowed to run the full course of the TLC. After drying, the TLC plate was cut along the first dimension by keeping a 2-cm margin on either side of the applied spot, excluding up to 2 cm in height from the origin. For polar lipid separation, the plate was cut again, leaving a 2-cm margin on either side of the applied spot along the second dimension and leaving behind a TLC strip 4 cm wide and 20 cm high, which was run with the second dimension solvent system (chloroform:methanol:water = 65:25:4 by volume). The TLC plates were dried under low pressure in a chamber, and the lipid spots were identified by UV-light exposure and by iodine staining. The Rf values were noted before the areas were cut into the scintillation vials for counting. The polar lipid spots were put in 0.5 ml of water and thoroughly vortex-mixed; this procedure was followed by addition of scintillation fluid and further vortex-mixing to facilitate extraction.
NAA and ASPA Immunohistochemistry. Immunohistochemical localization of NAA (4, 29) and ASPA (5) were performed as previously described. Images were acquired on a BX51 microscope (Olympus, Melville, NY) and Qcolor5 digital camera and were prepared with PC-based imaging software (Adobe Systems, San Jose, CA).
Clinical Description of CD Patient. This child was described by Manhoff et al. (30). She was a 4.75-year-old girl who had developmental delay and macrocephaly during infancy. At 8 months of age, a brain biopsy revealed spongy degeneration of the brain. She was neurologically severely impaired for the remainder of her life, required feeding gastrostomy at 2 years of age, and had severe spastic quadriparesis. Elevated NAA was found in the urine, and, at 6 weeks before death, the child developed a right third nerve palsy and emesis. A cerebral MRI at that time showed signs consistent with CD. Two weeks later, the child developed seizures, in addition to the hyperemesis and oculomotor paresis, and a cerebral computed tomography scan showed a large medullary neoplasm with leptomeningeal spread. Biopsy of the brainstem lesion revealed a primitive neuroectodermal tumor that had classic rhabdoid morphology and was immunoreactive with epithelial membrane antigen and vimentin. The cerebral white matter showed almost total depletion of myelin, and the remaining myelinated fibers had marked myelin depletion. The cortical gray matter showed vacuolation that was especially prominent in deeper layers adjacent to the white matter. There were few macrophages but numerous atypical astrocytes. Tumor cells were limited to the subarachnoid spaces of the cerebral hemispheres. The cerebellum showed marked vacuolation of the cortex, proliferation of Bergmann glia, demyelination, and vacuolated deep white matter.
Lipid Analysis of Human Samples. A frozen cerebral specimen that did not contain gross tumor was analyzed. The specimen was dissected after thawing to remove gray matter and meninges. A cerebral white matter specimen from a 76-year-old woman who had died of cardiovascular disease was used as a control. Lipids were extracted by using chloroform:methanol (2:1) and were analyzed by 1D and 2D TLC. For 1D TLC, total lipids dissolved in chloroform:methanol were spotted on high-resolution TLC silica gel plates and resolved by using CHCl3:acetone:methanol (MeOH):acetic acid (HAc):H2O = 50:20:10:10:5. For 2D TLC, the lipids were first resolved by using CHCl3:MeOH:H2O = 75:25:4 and in the second dimension were resolved by using CHCl3:acetone:MeOH:HAc:H2O = 50:20:10:10:5. Lipids were visualized by using iodine vapor.
ASPA Mutation Analysis of CD Patient. DNA was extracted from cerebral gray matter from the CD patient by using Puregene DNA isolation reagents (Gentra Systems). The common Ashkenazi mutations were screened by PCR amplification of each of the three regions most often affected in the ASPA gene, followed by diagnostic restriction digestion. The pertinent primers were 5′-ACTCT TGATGGGA AGACGATCCCA-3′ (forward primer) and 5′-GTAAGACACCGTGTAAGATGTAAG-3′ (reverse primer), and the mutation was identified after _Eag_I restriction digestion and agarose gel electrophoreses of the PCR products.
Free Acetate Determinations. A spectrophotometric procedure employing a coupled enzymatic assay was used for estimating free acetate content in tissue extracts (31, 32). The homogenates were mixed with absolute ethanol at 1:1 (vol/vol) to precipitate proteins and centrifuged at 10,000 × g for 10 min. The supernatants were lyophilized, and the residues were reconstituted in purified and deionized water. Acetate determinations were performed by the coupled enzymatic method with spectrophotometetric detection of NADH as described in ref. 32.
Results
In the present murine study, the control group consisted of homozygous wild-type (ASPA+/+) mice, and the experimental group consisted of homozygous knockout (ASPA_–/_–) mice. The animals, five in each group, were chosen from among a large litter such that they were age-matched, phenotypically similar, 17-day-old littermates. Comparison of a number of general parameters between the control and knockout mice showed that the two groups differed significantly only in two measures: brain weight and ASPA activity. Other parameters, including animal weight, liver weight, and kidney weight, were not significantly different between the groups. The brains of the mutant mice weighed significantly more than those of the normal group (461 ± 17.8 mg vs. 416 ± 22.3 mg, respectively; P < 0.05). In addition, ASPA activity was undetectable in ASPA_–/_– mice, compared with ASPA activity of 18.3 ± 5.6 nmol/h per mg of protein in control mice (P < 0.001).
The level of lipid synthesis was determined in wild-type and ASPA_–/_– mice by the method of tritiated water incorporation. Tritium incorporation into de novo synthesized lipids has previously been demonstrated to be a reliable method for determining the rate of lipid synthesis (33) and has been successfully used for determining the rate of cholesterol synthesis (34) and myelin synthesis (35). Table 1 shows the incorporation of tritium into lipids from liver, kidney, and myelin in control and ASPA_–/_– mice 5 h after i.p. administration of 20 mCi [3H]2O per animal. The specific activity of [3H]2O calculated from the serum samples of the animals at the time of death did not differ significantly (P < 0.05) in the control and ASPA_–/_– mice (36). The radioactivity (cpm) was normalized to the amount of protein in the respective tissue samples. There was no difference between the two groups with respect to lipids synthesized in liver. Total myelin lipids were decreased in ASPA_–/_– mice by ≈30% (P < 0.005). Total lipid incorporation in kidney was increased by ≈18% in the ASPA_–/_– group.
Table 1. Specific activity of the tritiated water and the values for incorporation of tritium into lipids in different tissues in control and mutant mice (n = 5).
| Lipid category | Control | _ASPA_-/- | P value |
|---|---|---|---|
| Specific activity of [3H]2O, dmp/nmol of water | 90,298 ± 6,816 | 97,290 ± 8,930 | NS |
| Total lipids, cpm/mg of protein | |||
| Liver | 60,056 ± 6,850 | 60,499 ± 4,402 | NS |
| Kidney | 95,611 ± 13,007 | 113,233 ± 8,438 | ** |
| Total myelin lipids, cpm/mg of myelin protein | 26,946 ± 3,864 | 18,991 ± 2,099 | *** |
Myelin lipids were further analyzed by 2D TLC. Table 2 shows the changes in the individual lipid classes from the myelin samples of control and ASPA_–/_– mice. Among nonpolar lipids separated on the first dimension, four spots showed significant decreases in the mutant mice, corresponding to glycerol 1-fatty acids (decreased by ≈35%), cholesterol (decreased by ≈22%), cholesteryl fatty acids (decreased by ≈35%), and glycerol trifatty acids (trimyristin, tripalmitin, trilaurin, and tristearinm, decreased by ≈21%). Glycerol 1,2-fatty acids (dimyristin, dipalmitin, dilaurin, and distearin) did not show statistically significant changes.
Table 2. Comparison of myelin lipids in control and mutant mice.
| Rf | Control | P value | _ASPA_-/- | Comigrating lipid standards |
|---|---|---|---|---|
| Nonpolar lipids* | ||||
| 0.16 ± 0.01 | 342 ± 48 | ** | 224 ± 62 | Glycerol 1-fatty acids† |
| 0.45 ± 0.02 | 3,837 ± 564 | ** | 2,989 ± 351 | Cholesterol |
| 0.53 ± 0.03 | 548 ± 208 | NS | 556 ± 98 | Glycerol 1,2-fatty acids |
| 0.61 ± 0.02 | 1,699 ± 112 | * | 1,346 ± 330 | Glycerol trifatty acids, cholesteryl fatty acids |
| 0.66 ± 0.02 | 500 ± 62 | ** | 379 ± 85 | Myristate, palmitate |
| Polar lipids | ||||
| 0.60 ± 0.06 | 821 ± 400 | NS | 788 ± 807 | Unknown |
| 0.75 ± 0.02 | 9,995 ± 2,525 | ** | 6,153 ± 1,320 | PC, PI, PG, sulfatides, PA |
| 0.88 ± 0.02 | 7,513 ± 1,114 | ** | 4,904 ± 1,157 | PE, GC, ceramide |
In the second TLC dimension for the separation of polar lipids, two of three lipid spots showed significant reductions (P < 0.05) in the ASPA_–/_– mice. The solvent system used for polar lipid separation did not yield a good resolution for the many polar lipid standards tested. The Rf of 0.75 corresponded to phospholipids and sulfatides (phosphatidylinositol, phosphatidyl choline, phosphatidyl glycerol, phosphatidic acid, and cerebroside sulfate), which were decreased by ≈38% in ASPA_–/_– mice. The Rf of 0.88 corresponded to phosphatidyl ethanolamine, galactocerebroside, and hydroxy fatty acid ceramide, which were decreased ≈35% in the experimental group.
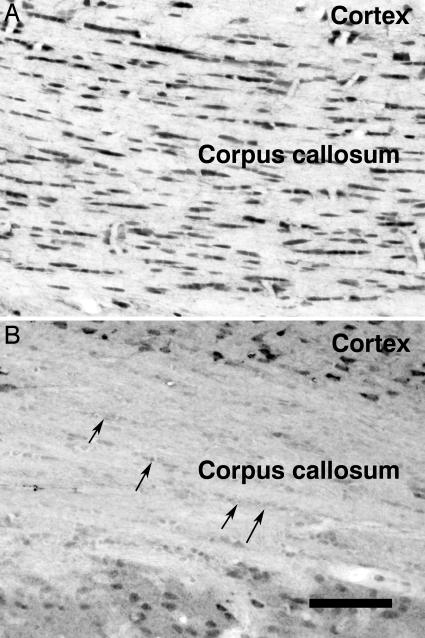
To confirm the localization of NAA relative to ASPA, immunohistochemistry was performed on rats as described in refs. 4 and 5. Fig. 1 shows the histochemical localization of NAA and ASPA in rat corpus callosum. ASPA staining was moderate to strong in oligodendrocytes throughout corpus callosum (Fig. 1 A); NAA immunoreactivity was low in oligodendrocytes (Fig. 1_B_). As reported in ref. 5, neither neurons nor astrocytes showed any staining for ASPA in the forebrain. It is not clear whether oligodendrocytes synthesize sufficient NAA for myelin synthesis (37) or whether they acquire a significant proportion of it by neuronal-glial transfer (22).
Fig. 1.
ASPA (A) and NAA (B) immunoreactivity in the rat corpus callosum. Oligodendrocytes are arranged in rows within the corpus callosum and are strongly stained for ASPA but only weakly stained for NAA (arrows in B). Neurons, such as those in the cortex, are stained much more intensely for NAA than are oligodendrocytes. (Scale bar, 120 μm.)
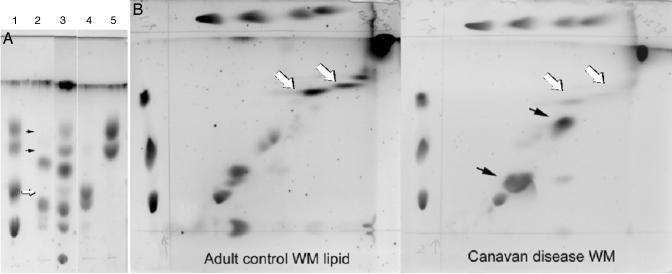
We also examined the lipid content from postmortem white matter samples from a CD patient who was determined to be homozygous for the E285A missense substitution, one of the more common ASPA gene mutations (data not shown), and compared the levels to those found in normal human white matter. On 1D TLC, the CD patient sample had a significant reduction in the amount of material comigrating with cerebroside standard and, to a lesser extent, a reduction of material comigrating with sulfatide standard, whereas the amount of material comigrating with sphingomyelin was similar to that in normal white matter (Fig. 2_A_). In addition, there were increased levels of lipids in the samples from the CD patient that did not comigrate with any of the lipid standards used (black arrows in Fig. 2_B_). These lipids remain to be characterized.
Fig. 2.
Lipid analysis in CD and normal human cerebral white matter (WM). (A) Note the relative paucity of bands comigrating with the cerebroside standards (black arrows) and relative lack of the more mobile sulfatide fraction (white arrow) in the CD patient sample compared with the control. Lane 1, standards mix; lane 2, CD patient white matter lipids; lane 3, control human white matter lipids; lane 4, sulfatides; lane 5, cerebrocides. (B) Note the prominence of the bands (comigrating with cerebroside) in the control specimen and their reduction in the CD patient specimen (white arrows). Note also the prominence of anomalous lipid bands in the CD patient (black arrows).
Free acetate determinations in normal and ASPA_–/_– mice showed that acetate levels in the brains of knockout mice were less than one-quarter of the levels found in the brains of control mice (Table 3). Acetate levels in liver and kidney were unchanged. These findings demonstrate that in the brain, NAA is a major source of free acetate and that myelin lipid synthesis in the brain derives a substantial portion of the requisite acetate (acetyl CoA) from NAA by means of ASPA-mediated catalysis.
Table 3. Acetate concentrations are decreased in brain, but not in liver or kidney, in the mutant mouse.
| Tissue | _ASPA_-/- | Control | P value |
|---|---|---|---|
| Brain, n = 5 | 0.31 ± 0.11 | 1.51 ± 0.34 | *** |
| Liver, n = 5 | 0.68 ± 0.03 | 0.81 ± 0.13 | NS |
| Kidney, n = 4 | 1.41 ± 0.11 | 1.30 ± 0.08 | NS |
Discussion
We have shown that CNS myelin lipid synthesis is decreased in the murine model of CD, whereas lipid synthesis in other organs, such as kidney and liver, is either increased or not affected. These findings indicate that lipid synthesis in the brain, in part, requires an intact ASPA enzyme, providing direct evidence for deficiency of NAA-derived acetate as an etiological mechanism of CD. Earlier studies have demonstrated that the acetate moiety from neuronally derived NAA is incorporated into myelin in the CNS (22), and the present study establishes that the acetate contribution to myelin synthesis from NAA is quantitatively sufficient to decrease myelin synthesis during the period of elevated postnatal myelination in a murine model of CD.
We have also analyzed the lipid composition of white matter from a patient with CD and found presumptive evidence for a lipid synthetic defect (Fig. 2). Because the patient we analyzed died from coincidental occurrence of a fatal brainstem neoplasm, we believe that the biochemical results are more likely representative of the true metabolic condition in white matter than would be specimens from patients who died as a consequence of end-stage CD, where the white matter is even more severely damaged. The results indicate a significant reduction in the amount of complex glycolipids in the CD patient. Specifically, reductions in lipids comigrating with cerebroside and sulfatide were observed, which are consistent with the results obtained in the model CD mice.
To confirm that defective NAA catabolism resulted in reduced acetate levels in the brains of ASPA_–/_– mice, we determined free acetate levels by using an enzymatic assay. The results showed that there was an ≈79% reduction in free acetate levels in the brains of ASPA_–/_– mice but that the levels in kidney and liver were not reduced relative to controls. The key issue with regard to acetate is the level in oligodendrocytes in CD during the period of intense postnatal axonal myelination. The current data only pertain to whole tissue levels in the developing CD mice, but, considering the predominant localization of ASPA to oligodendrocytes, it is likely that the levels of acetate in those cells would be substantially reduced in the ASPA-deficient animals.
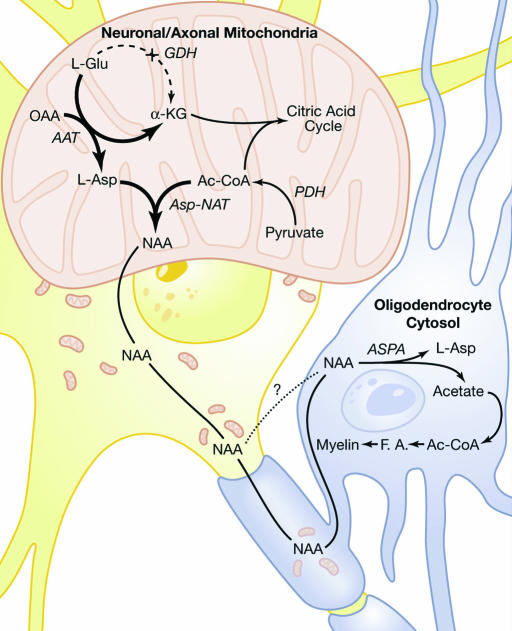
The discrepant compartmentation of the NAA synthetic enzyme Asp-NAT and the NAA-hydrolyzing enzyme ASPA into neurons and oligodendrocytes, respectively, has been proposed as a mechanism for channeling NAA-associated acetate from neurons to oligodendrocytes, wherein the myelin sheath itself could be the site of lipid synthesis (22). Based on such findings, and the results in the current and other studies, we propose an expanded model for NAA metabolism in neurons and oligodendrocytes (3) wherein NAA serves multiple roles (Fig. 3). In neurons and their axons, Asp-NAT acts as a method of removing excess aspartate from mitochondria by means of acetylation, thus favoring α-ketoglutarate formation from glutamate and energy production by means of the citric acid cycle. In this way, the extra demand for ATP in neurons is met in part by oxidation of glutamate by means of the aspartate aminotransferase pathway (38). By preferentially using the aspartate aminotransferase reaction instead of the glutamate dehydrogenase reaction to generate α-ketoglutarate, neuronal/axonal mitochondria would circumvent ammonia production and toxicity.
Fig. 3.
Proposed model for NAA synthesis and degradation. See Discussion for details. OAA, oxaloacetic acid; α-KG, α-ketoglutarate; AAT, aspartate aminotransferase; GDH, glutamate dehydrogenase; PDH, pyruvate dehydrogenase; F.A., fatty acids; Ac-CoA, acetyl CoA.
In this model, we hypothesize that NAA synthesis is intimately associated with the proper functioning of neuronal energy metabolism by means of the aspartate aminotransferase reaction in neuronal mitochondria. Further, NAA synthesized in neuronal mitochondria is transferred to oligodendrocytes, where ASPA liberates the acetate moiety to be used for myelin lipid synthesis and other metabolic functions. In this sense, NAA acts as an acetate carrier between neurons and oligodendrocytes. Although not explored in the current study, the aspartate derived from the deacetylation of NAA may enter into metabolic pathways in oligodendrocytes, as we believe occurs for the acetate moiety. In addition to participating directly in myelin protein synthesis, aspartate can enter into a variety of synthetic and bioenergetic pathways, such as for synthesis of other amino acids and the citric acid cycle, respectively. This hypothesis emphasizes the metabolic coupling of myelinated axons to oligodendrocytes, where axons provide major biochemical precursors for the demanding task of myelination during early postnatal CNS development.
Our demonstration that ASPA is primarily localized in oligodendrocytes in the CNS (5) may have important implications for gene therapy of CD. An ongoing trial using recombinant virus-transduced ASPA employs a neurotrophic viral vector for transfection (39). The rationale behind the targeting of the ASPA gene to neurons is based on the hypothesis that some or all of the pathophysiology associated with CD is attributable to either the toxicity of excess NAA or disruption of its proper regulation as a neuronal osmolyte. Under this assumption, reducing the level of NAA in the brain would prevent CD pathogenesis. However, the current data demonstrate that NAA-derived acetate is essential for normal postnatal myelin lipid synthesis, and, therefore, targeting the ASPA gene to neurons may not sufficiently correct the biochemical deficit in oligodendrocytes. But using mass action to deliver acetate to the brain by means of compounds such as glyceryl triacetate would theoretically increase acetate levels in all cell types in the brain and would provide substantially more acetate than could be achieved with low levels of neuronal ASPA expression after gene therapy.
One approach to testing whether an acetate deficiency is the etiological mechanism of CD would be to determine whether myelin synthesis can be promoted in newborn CD infants by increasing brain acetate levels through dietary supplementation. CD develops only after birth; therefore, this approach should be feasible. Orally administered acetate or an acetate precursor in a supplemented infant formula could directly provide the required substrate for the rapid myelination that takes place during early postnatal neural development. Early diagnosis of CD by using urinalysis to detect high NAA levels could be followed by immediate acetate supplementation of the diet, which could provide an extremely safe and low cost treatment for CD.
Finally, it should be noted that a recent report has described a young child with severe CNS developmental abnormalities who lacks the NAA signal in magnetic resonance spectrograms (40), most likely as a consequence of a mutation in the gene for Asp-NAT, the NAA synthetic enzyme. The pathophysiology is not identical to that of CD but involves severe developmental CNS anomalies, including hypomyelination, secondary microcephaly, and severe psychomotor dysfunction. These findings are consistent with the idea that NAA synthesis in neurons and degradation in oligodendrocytes are essential for proper CNS development and argue against NAA toxicity as being a primary etiological mechanism in the development of CD.
In conclusion, the current study demonstrates that the synthesis of myelin-associated polar and nonpolar lipids is significantly reduced in the brains of 17-day-old homozygous ASPA_–/_– mice, indicating that CNS myelin lipid synthesis during early postnatal neural development derives a significant portion of acetyl groups from the NAA–ASPA system. Acetate levels in the brains of ASPA_–/_– mice were also significantly reduced, compared with controls. Analysis of lipid content from white matter in a postmortem CD sample showed decreased levels of complex glycolipids and increased levels of unknown lipids. These findings provide direct support for the proposed etiology of CD as a deficiency of NAA/ASPA-derived acetate, resulting in reduced lipid synthesis, and a failure of proper myelination during CNS development. Based on these and previous findings, early postnatal acetate supplementation trials appear warranted in confirmed cases of CD.
Acknowledgments
We thank Drs. Robert Ledeen, Edwin Kolodny, Wayne B. Jonas, and John A. Ives for critical comments on the manuscript and Joyce Benjamins for helpful discussion on the lipid analysis of human brains. This work was supported by National Institutes of Health Grant RO1 NS39387 (to M.A.A.N.), Samueli Institute for Information Biology Grant GS170ON (to M.A.A.N.), and National Multiple Sclerosis Society Grant RG3204 (to J.G.).
Author contributions: M.A.A.N. designed research; C.N.M., P.A., J.R.M., S. Szucs, S. Surendran, R.M., J.G., D.H., A.J., W.J., and M.A.A.N. performed research; S. Szucs, S. Surendran, and R.M. contributed new reagents/analytic tools; C.N.M., P.A., J.R.M., J.G., and M.A.A.N. analyzed data; and C.N.M., J.R.M., J.G., and M.A.A.N. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ASPA, aspartoacylase; Asp-NAT, aspartate _N_-acetyltransferase; CD, Canavan's disease; NAA, _N_-acetylaspartate.
References
- 1.Frahm, J., Bruhn, H., Gyngell, M. L., Merboldt, K. D., Hanicke, W. & Sauter, R. (1989) Magn. Reson. Med. 11**,** 47–63. [DOI] [PubMed] [Google Scholar]
- 2.Patel, T. B. & Clark, J. B. (1979) Biochem. J. 184**,** 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhavarao, C. N., Chinopoulos, C., Chandrasekaran, K. & Namboodiri, M. A. (2003) J. Neurochem. 86**,** 824–835. [DOI] [PubMed] [Google Scholar]
- 4.Moffett, J. R., Namboodiri, M. A. & Neale, J. H. (1993) J. Histochem. Cytochem. 41**,** 559–570. [DOI] [PubMed] [Google Scholar]
- 5.Madhavarao, C. N., Moffett, J. R., Moore, R. A., Viola, R. E., Namboodiri, M. A. & Jacobowitz, D. M. (2004) J. Comp. Neurol. 472**,** 318–329. [DOI] [PubMed] [Google Scholar]
- 6.Klunk, W. E., Panchalingam, K., Moossy, J., McClure, R. J. & Pettegrew, J. W. (1992) Neurology 42**,** 1578–1585. [DOI] [PubMed] [Google Scholar]
- 7.Menon, D. K., Sargentoni, J., Peden, C. J., Bell, J. D., Cox, I. J., Coutts, G. A., Baudouin, C. & Newman, C. G. (1990) J. Comput. Assist. Tomogr. 14**,** 449–452. [DOI] [PubMed] [Google Scholar]
- 8.Arnold, D. L., Matthews, P. M., Francis, G. & Antel, J. (1990) Magn. Reson. Med. 14**,** 154–159. [DOI] [PubMed] [Google Scholar]
- 9.Tsai, G. & Coyle, J. T. (1995) Prog. Neurobiol. 46**,** 531–540. [DOI] [PubMed] [Google Scholar]
- 10.Clark, J. B. (1998) Dev. Neurosci. 20**,** 271–276. [DOI] [PubMed] [Google Scholar]
- 11.Kaul, R., Gao, G. P., Balamurugan, K. & Matalon, R. (1993) Nat. Genet. 5**,** 118–123. [DOI] [PubMed] [Google Scholar]
- 12.Zeng, B. J., Wang, Z. H., Ribeiro, L. A., Leone, P., De Gasperi, R., Kim, S. J., Raghavan, S., Ong, E., Pastores, G. M. & Kolodny, E. H. (2002) J. Inherited Metab. Dis. 25**,** 557–570. [DOI] [PubMed] [Google Scholar]
- 13.D'Adamo, A. F., Jr., Smith, J. C. & Woiler, C. (1973) J. Neurochem. 20**,** 1275–1278. [DOI] [PubMed] [Google Scholar]
- 14.Matalon, R., Michals, K. & Kaul, R. (1995) J. Pediatr. 127**,** 511–517. [DOI] [PubMed] [Google Scholar]
- 15.Baslow, M. H. (2002) Neurochem. Int. 40**,** 295–300. [DOI] [PubMed] [Google Scholar]
- 16.Leone, P., Janson, C. G., Bilaniuk, L., Wang, Z., Sorgi, F., Huang, L., Matalon, R., Kaul, R., Zeng, Z., Freese, A., et al. (2000) Ann. Neurol. 48**,** 27–38. [DOI] [PubMed] [Google Scholar]
- 17.Gehl, L. M., Saab, O. H., Bzdega, T., Wroblewska, B. & Neale, J. H. (2004) J. Neurochem. 90**,** 989–997. [DOI] [PubMed] [Google Scholar]
- 18.Kirmani, B. F., Jacobowitz, D. M. & Namboodiri, M. A. (2003) Brain Res. Dev. Brain Res. 140**,** 105–115. [DOI] [PubMed] [Google Scholar]
- 19.D'Adamo, A. F., Gidez, L. I. & Yatsu, F. M. (1968) Exp. Brain Res. 5**,** 267–273. [DOI] [PubMed] [Google Scholar]
- 20.D'Adamo, A. F., Jr., & Yatsu, F. M. (1966) J. Neurochem. 13**,** 961–965. [DOI] [PubMed] [Google Scholar]
- 21.Burri, R., Steffen, C. & Herschkowitz, N. (1991) Dev. Neurosci. 13**,** 403–412. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty, G., Mekala, P., Yahya, D., Wu, G. & Ledeen, R. W. (2001) J. Neurochem. 78**,** 736–745. [DOI] [PubMed] [Google Scholar]
- 23.Mehta, V. & Namboodiri, M. A. (1995) Brain Res. Mol. Brain Res. 31**,** 151–157. [DOI] [PubMed] [Google Scholar]
- 24.Kirmani, B. F., Jacobowitz, D. M., Kallarakal, A. T. & Namboodiri, M. A. (2002) Brain Res. Mol. Brain Res. 107**,** 176–182. [DOI] [PubMed] [Google Scholar]
- 25.Lowenstein, J. M. (1971) J. Biol. Chem. 246**,** 629–632. [PubMed] [Google Scholar]
- 26.Matalon, R., Rady, P. L., Platt, K. A., Skinner, H. B., Quast, M. J., Campbell, G. A., Matalon, K., Ceci, J. D., Tyring, S. K., Nehls, M., et al. (2000) J. Gene Med. 2**,** 165–175. [DOI] [PubMed] [Google Scholar]
- 27.Madhavarao, C. N., Hammer, J. A., Quarles, R. H. & Namboodiri, M. A. (2002) Anal. Biochem. 308**,** 314–319. [DOI] [PubMed] [Google Scholar]
- 28.Norton, W. T. & Poduslo, S. E. (1973) J. Neurochem. 21**,** 749–757. [DOI] [PubMed] [Google Scholar]
- 29.Moffett, J. R. & Namboodiri, M. A. (1995) J. Neurocytol. 24**,** 409–433. [DOI] [PubMed] [Google Scholar]
- 30.Manhoff, D. T., Rorke, L. B. & Yachnis, A. T. (1995) Pediatr. Neurosurg. 22**,** 214–222. [DOI] [PubMed] [Google Scholar]
- 31.Guynn, R. W. & Veech, R. L. (1974) Anal. Biochem. 61**,** 6–15. [DOI] [PubMed] [Google Scholar]
- 32.Bartelt, U. & Kattermann, R. (1985) J. Clin. Chem. Clin. Biochem. 23**,** 879–881. [PubMed] [Google Scholar]
- 33.Jungas, R. L. (1968) Biochemistry 7**,** 3708–3717. [DOI] [PubMed] [Google Scholar]
- 34.Dietschy, J. M. & Spady, D. K. (1984) J. Lipid Res. 25**,** 1469–1476. [PubMed] [Google Scholar]
- 35.Muse, E. D., Jurevics, H., Toews, A. D., Matsushima, G. K. & Morell, P. (2001) J. Neurochem. 76**,** 77–86. [DOI] [PubMed] [Google Scholar]
- 36.Jurevics, H. A. & Morell, P. (1994) J. Lipid Res. 35**,** 112–120. [PubMed] [Google Scholar]
- 37.Urenjak, J., Williams, S. R., Gadian, D. G. & Noble, M. (1992) J. Neurochem. 59**,** 55–61. [DOI] [PubMed] [Google Scholar]
- 38.Yudkoff, M., Nelson, D., Daikhin, Y. & Erecinska, M. (1994) J. Biol. Chem. 269**,** 27414–27420. [PubMed] [Google Scholar]
- 39.Janson, C., McPhee, S., Bilaniuk, L., Haselgrove, J., Testaiuti, M., Freese, A., Wang, D. J., Shera, D., Hurh, P., Rupin, J., et al. (2002) Hum. Gene Ther. 13**,** 1391–1412. [DOI] [PubMed] [Google Scholar]
- 40.Boltshauser, E., Schmitt, B., Wevers, R. A., Engelke, U., Burlina, A. B. & Burlina, A. P. (2004) Neuropediatrics 35**,** 255–258. [DOI] [PubMed] [Google Scholar]