GABAergic control of the ascending input from the median raphe nucleus to the limbic system (original) (raw)
. Author manuscript; available in PMC: 2006 Oct 1.
Published in final edited form as: J Neurophysiol. 2005 Jun 8;94(4):2561–2574. doi: 10.1152/jn.00379.2005
Abstract
The median raphe nucleus (MRN) is the primary source of serotonergic afferents to the limbic system which are generally considered to suppress hippocampal theta oscillations. GABA receptors are expressed in the MRN by serotonergic and non-serotonergic cells, including GABAergic and glutamatergic neurons. This study investigated the mechanisms by which the fluctuating GABA tone in the MRN leads to induction or suppression of hippocampal theta rhythm. 1. We found that MRN application of the GABAA agonist muscimol (0.05–1.0 mM) or GABAB agonist baclofen (0.2 mM) by reverse microdialysis had strong theta promoting effects. 2. The GABAA antagonist bicuculline infused in low concentrations (0.1, 0.2 mM) eliminated theta rhythm. A short period of theta activity of higher than normal frequency preceded hippocampal desynchronization in 46% of rats. 3. Bicuculline in larger concentrations (0.5, 1.0, 2.0 mM) resulted in a biphasic response of an initial short (<10 min) hippocampal desynchronization followed by stable theta rhythm which lasted as long as the infusion continued. The frequency and amplitude of theta waves were higher than in control recordings and the oscillations showed a conspicuous intermittent character. 4. Hippocampal theta rhythm elicited by MRN administration of bicuculline could be completely (0.5 mM bicuculline) or partially (1.0 mM bicuculline) blocked by simultaneous infusion of the GABAB antagonist CGP35348. Our findings suggest that the GABAergic network may have two opposing functions in the MRN: relieving the theta-generators from serotonergic inhibition and regulating the activity of a theta-promoting circuitry by the fluctuating GABA tone. The two mechanisms may be involved in different functions.
Keywords: hippocampus, theta oscillations, median raphe nucleus, rat
Introduction
The role of the hippocampus in learning and memory is well established and the importance of subcortical networks controlling the electrical activity of the hippocampus has been recognized for decades. Hippocampal network activity exhibits two behavior-dependent patterns associated with different states of hippocampal information processing: large amplitude irregular activity with sharp waves and theta rhythm (Buzsaki et al. 1983; O’Keefe and Nadel 1978; Vanderwolf 1969). The ascending serotonergic system has a fundamental role in switching between the two states of hippocampal activity (Vertes and Kocsis 1997). The main source of the serotonergic input to the hippocampus is the median raphe nucleus (MRN, (Freund et al. 1990). Stimulation of the MRN blocks theta rhythm by desynchronizing the hippocampal EEG (Vertes 1981) while lesioning or pharmacological suppression of the MRN produces long-lasting, uninterrupted theta activity in the hippocampus (Assaf and Miller 1978; Vertes et al. 1994). The latter effect can be elicited by the selective blockade of 5-HT neurons in the MRN (Vertes et al. 1994).
Besides the serotonergic population a large number of GABAergic neurons can be found in the midbrain raphe nuclei, including both dorsal (DRN) and MRN, which innervate serotonergic (5-HT) cells (Forchetti and Meek 1981; Maloney et al. 1999b; Tao et al. 1996; Varga et al. 2001; Wang et al. 1992). Several lines of evidence indicate that GABAergic mechanisms participate in the behavioral state-dependent regulation of 5-HT cell firing (Gervasoni et al. 2000). Excitatory pathways from limbic cortical and diencephalic structures target GABAergic neurons in the raphe nuclei; and forebrain regions exert inhibitory influence on 5-HT cells via local GABAergic neurons (Ferraro et al. 1996; Varga et al. 2003; Varga et al. 2001). These cells fire at higher frequency (Allers and Sharp 2003) and in some cases phase-locked to the hippocampal theta rhythm (Kocsis and Vertes 1992, 1996; Viana Di Prisco et al. 2002). Both GABAA and GABAB receptors (GABA-Rs) are expressed in the MRN, the latter almost exclusively by 5-HT neurons (Gao et al. 1993; Serrats et al. 2003; Varga et al. 2002). Local application of GABAA- and GABAB-R agonists in the MRN reduces the 5-HT tone in forebrain regions including the hippocampus (Forchetti and Meek 1981; Shim et al. 1997; Tao et al. 1996). Furthermore, intra-raphe injection of the GABAA antagonist bicuculline results in an elevation of forebrain 5-HT level, which suggests that GABAA-Rs are tonically active in both DRN (Tao et al. 1996) and MRN (Forchetti and Meek 1981). GABAB-R antagonist failed to increase forebrain 5-HT level unless it was coadministered with GABAA-R antagonist (Tao et al. 1996).
Serotonergic neurons play a prominent role in brain state regulation (Hobson et al. 1975). The activity of serotonergic neurons of the DRN changes according to vigilance states; i.e. their average firing rate decreases from waking to slow wave sleep to rapid eye movement (REM) sleep (McGinty and Harper 1976). Recent c-fos studies indicated that the MRN have a similar pattern of discharge (Maloney et al. 1999a; Yamuy et al. 1995). It has been demonstrated that during REM sleep when 5-HT neurons are virtually silent extracellular GABA level increased in the DRN (Nitz and Siegel 1997) and GABAergic cells were activated in both DRN and MRN (Maloney et al. 1999b). The suppressed firing of 5-HT neurons could be restored to waking level by blocking GABAA-Rs.
Under urethane anesthesia, hippocampal activity spontaneously alternates between slow theta (4–5 Hz) and non-theta patterns whereas faster theta oscillations (up to 8 Hz) can be elicited for relatively short periods by sensory stimulation. It was reported that in anesthetized rats a transition from non-theta to a theta state in the hippocampus could be induced by either GABAA- or GABAB-R activation in the MRN and that the effect of the GABAB agonist was mediated by 5-HT neurons (Kinney et al. 1995; Varga et al. 2002). Unexpectedly, the blockade of GABAA-Rs by high dose of GABAA antagonist bicuculline produced the same effect as GABAA-R activation i.e. long-lasting theta in the hippocampus (Thinschmidt et al. 1995).
The aim of this study was to unravel the specific role of GABAA- and GABAB-Rs in the regulation of MRN-mediated state transitions of the hippocampal EEG. We hypothesized that fluctuations in a GABA tone in the MRN modulates the ascending raphe-limbic input by maintaining a delicate balance of activity of several types of local and projecting MRN neurons involved in the regulation of theta and non-theta states in the hippocampus. There are at least three neuronal targets of GABA in the MRN the activation of which would have an impact on hippocampal EEG: 5-HT cells (GABAA- and GABAB-Rs), local GABA interneurons (GABAA-Rs), and ascending glutamatergic neurons (GABAA-R). In principle, GABA in the MRN may act either to decrease or increase the ascending 5-HT output through direct inhibition of 5-HT cells or by suppressing local GABAergic interneurons, respectively. The hippocampal theta generators would be suppressed or activated, accordingly.
For investigating the effect of GABA tone in the MRN on the occurrence and characteristics of spontaneous, i.e. state-dependent, theta oscillations we chose the in vivo microdialysis technique because it allows maintaining stable drug concentrations in the MRN for extended periods of time. Assuming that the intensities of GABA action at different concentrations on the three MRN targets are uneven we hypothesized that after altering the level of sustained MRN GABA tone the changes in hippocampal EEG pattern would not be limited to gradual shifts in the theta parameters. Instead, switch from a stable theta to a lasting non-theta state would be expected if the primary target of GABA at a certain concentration shifted from the receptors on 5-HT cells to the receptors on interneurons. Thus the present experiments were designed to answer the following questions. First we tested the effect of direct suppression of the MRN output by GABAA- and GABAB-R agonists to confirm earlier findings (Kinney et al. 1995; Varga et al. 2002) in the experimental settings of this study. Second, we tested whether different levels of GABAA-R antagonists would lead to opposite patterns of hippocampal EEG. Third, we tested the hypothesis that complete GABA blockade i.e. including GABAA- GABAB-Rs will desynchronize hippocampal EEG even when GABAA-Rs in other MRN neurons are blocked, too (e.g. at high bicuculline concentrations). Forth, since GABA was shown acting on glutamatergic neurons in the raphe (Tao and Auerbach 2003). and there is a massive glutamatergic input from the MRN to the limbic theta oscillators (Kiss et al. 2002), we also hypothesized that GABA-R blockade will activate this pathway. The character of theta rhythm would be different however, as glutamatergic activation is involved in generation of fast theta episodes rather than lasting state-dependent, slow theta oscillations.
Methods
Experiments were performed on 67 male Sprague–Dawley rats (220–420g, Charles River Laboratories, MA) treated in accordance with NIH guidelines. All procedures were approved by the Institutional Animal Care and Use Committees of Harvard Medical School and Beth Israel Deaconess Medical Center. All effort was made to minimize both the suffering and the number of animals used. Rats were allowed food and water ad libitum prior to the beginning of the experiments.
Electrophysiological recordings
Surgery and electrophysiological recordings were performed under urethane anesthesia (i.p. 1.2–1.5 g/kg). Hippocampal field activity was recorded with insulated stainless steel electrodes positioned in the dorsal hippocampi on both sides. With the rats mounted in a stereotaxic frame, two pairs of twisted wires (diameter: 125 jm) separated by 1 mm at their tips were implanted (AP: −3.7, L: +/−2.2, H: −3.5), one in the CA1 region and the other below the hippocampal fissure, verified by the out-of-phase rhythmicity in the two recordings, and fixed with dental cement. Hippocampal EEG was amplified, filtered (0.1–70 Hz) and stored on a computer (sampling rate: 256/s; Daq/216B, IOTech Inc., Cleveland, OH). The traces of hippocampal EEG along with their spectra and the power within the theta range (2–8 Hz) was continuously monitored during the experiment (DasyLab).
Drugs and drug administration by microdialysis
The following drugs were applied (see Table 1): Muscimol, Bicuculline, Gabazine, Baclofen, CGP35348, and Atropine from Sigma (St. Louis, MO). Atropine was injected intraperitoneally in a dose of 50 mg/kg. All other drugs, administered intracerebrally were dissolved in artificial cerebrospinal fluid (ACSF: NaCl: 147 mM, KCl: 2.7 mM, CaCl2: 1.2 mM, MgCl2: 0.85 mM, pH = 7.4 CMA/Microdialysis AB, Solna, Sweden). For drug administration concentric microdialysis probes mounted in a guide cannula were used (cuprophane membrane with an outside diameter of 0.24 mm and length of 1 or 3 mm, mol. wt. Cutoff: 6 kDa; CMA/Microdialysis AB, Solna, Sweden). The probes were perfused (perfusion rate: 80 jl/h; sp101 syringe pump, WPI Inc., Sarasota, FL) with ACSF. They were placed in the brainstem in the sagital plane under a 24 deg angle in a caudo-rostral direction so the dialysis membrane was entirely in the MRN (Fig 1). The dialysis probe was lowered in 0.5 mm steps made every 2–5 min for a total of ~1 hr to reach the target and was left in its final position for one additional hour before starting experiments. The probe was lowered at a slow speed in order to reduce tissue damage. After halting the probe in the target area we waited an hour to allow consolidation of the surrounding tissue. Following this "recovery period" no overt changes can be detected as indicated by stable amino acid and nucleoside levels from the end of this period (Robinson and Jusctice 1991).
Table 1.
Drugs and concentrations used
| GABAA-R | GABAB-Rs | |
|---|---|---|
| Agonists | Muscimol | Baclofen* |
| 0.05, 0.1, 0.5, 1.0 mM | 0.2 mM | |
| Antagonists | Bicuculline | CGP35348# |
| 0.01, 0.1, 0.2, 0.5, 1.0, 2.0 mM | 2.5, 5, 18, 50 mM | |
| Gabazine | ||
| 0.04, 0.5 mM |
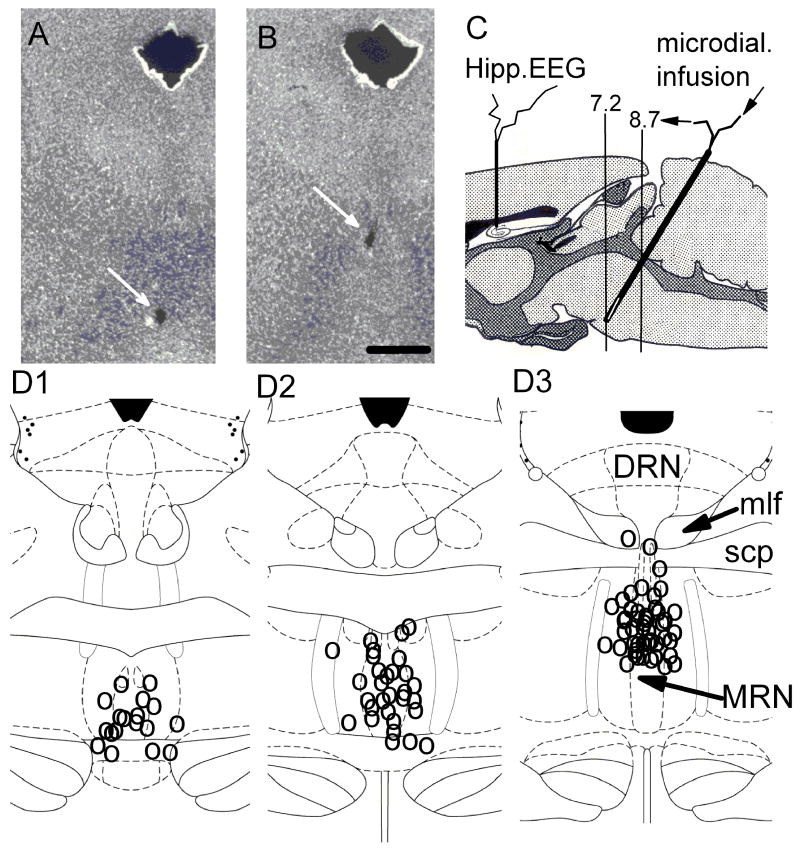
Figure 1.
Location of the microdialysis probe in the median raphe nucleus. A and B. Microphotographs show the track of the probe in 2 coronal section at separated by 0.6 mm. C. Schematic drawing of the location of the microdialysis probe inserted into the median raphe nucleus under a 24 deg angle in dorsocaudal to ventrolateral direction, in the sagital plane. Vertical markers at −7.2 and −8.7 indicate the borders of the MRN. The dialysis membrane was in the anterior, “bulky” part of the MRN. D. Location of the probes at coronal section −7.9 mm from Bregma (D3) (Paxinos and Watson 2005) and the tip of the probes in different experiments (D1 and D2). Calibration in A and B: 1 mm.
Data analysis
Power spectra were calculated using Fast Fourier Transform on 4s windows as described previously in detail (Kocsis and Vertes 1994). The spectral peaks corresponding to theta oscillations (frequency between 3 and 10 Hz, power at least 4 times larger than background) were identified and subjected to the following statistical analyses: peak frequency and power of EEG segments recorded during control and drug administrations (time windows of equal lengths) were compared using two-way analysis of variance with drug concentrations and time points (before, 20–30 min and 60 min after drug administration) as classification variables with post-hoc Bonferroni comparisons where significance was found. Differences were considered significant if p<0.05. Values are expressed as mean±S.E.M.
Histology
At the end of experiments the rats were deeply anesthetized and perfused through the aortic arch with 0.9% NaCl followed by a fixative solution containing 4% paraformaldehyde (Sigma-Aldrich, Germany), and 15% (V/V%) saturated picric acid (Sigma-Aldrich, Germany) in 0.1M phosphate buffer (PB, pH 7.4, 0.1M). After perfusion, brains were removed, coronal blocks were dissected containing the raphe nuclei, then 60 μm thin sections were cut from the blocks with a vibrotome, (Leica, VT 1000S, Germany); washed extensively in phosphate buffer (pH 7.4, 0.1 M) containing 0.1 % Triton X (Sigma-Aldrich, Germany). Sections were incubated in 4′,6-Diamidino-2-phenylindole (DAPI, 300nM, Molecular Probes, USA) for 10 minutes in the dark, washed, mounted, and coverslipped using Moviol antifade medium. Specimens were examined using fluorescent light microscopes (Olympus Provis AX70) and photographs were taken by a Spot digital camera.
There are numerous methods to unspecifically stain cells’ nuclei which are used for identifying brain areas. Conventional histochemical procedures involve air-drying of sections, whereas fluorescent labeling, like DAPI, use hydrated sections. We have shown previously, that air-dried sections shrink ~75% in Z direction (and about 25% in X and Y axes). Using hydrated tissue prevents this problem (Pyapali et al. 1998). Because the collapse of the tissue makes the probe localization inaccurate (i.e. difficult to find the extreme tip of the probe’s track) or in some cases even impossible, we used the DAPI labeling that is a widely used and accepted method. As the figure illustrates the location of the probe is very well visible in hydrated DAPI labeled sections.
Results
The effect of GABA-R agonist administration in the MRN on hippocampal activity
Strong, lasting activation of hippocampal theta rhythm after injection of GABAA or GABAB agonists in the median raphe nucleus was reported previously (Kinney et al. 1995; Varga et al. 2002). Similar experiments in the present study were thus performed primarily to verify and extend these findings with two major modifications in the experimental procedure. First, drugs in this study were injected using the reverse microdialysis technique which, unlike pressure injection used earlier (Kinney et al. 1995), allowed administration of muscimol by maintaining prolonged stable concentrations in the target tissue (see Methods). Second, unlike in previous studies (Varga et al. 2002), the microdialysis probe was inserted in a way which allowed avoiding the DRN (see Methods) and to manipulate GABAergic mechanisms only in the median raphe with primary projection to the hippocampus and related limbic structures (Fig 1).
Figure 2 shows two representative experiments in which the GABAA agonist, muscimol, or GABAB agonist, baclofen, was administered into the MRN for one hour. In control recordings hippocampal EEG alternated between two patterns of activity, theta rhythm and irregular non-rhythmic activity. The average time spent in the theta state was 40–60% for different groups with large individual variations (Fig. 4A; note large standard errors). After switching the infusion from ACSF to muscimol, theta rhythm became dominant (theta percentage ~95%, note small standard errors, Fig 4A) and ran uninterrupted for 30–40 min. After ~40 min theta completely disappeared, as reported previously (Kinney et al. 1995), and did not return even after infusion of ACSF for up to 3 h. Concentrations of muscimol above 0.05 mM (0.05, 0.1, 0.5, and 1.0 mM n=2, 2, 5, and 5, respectively) induced similar changes in the hippocampus; lasting theta occurred in all experiments. The same effect was also observed in five rats in which 1.0 mM muscimol was perfused for a shorter period of time (15 min). Muscimol administered in a concentration of 0.001 mM was ineffective. Muscimol was also ineffective when infused in the adjacent reticular formation (0.5 mM, n=3, Fig. 5A; see also in (Kinney et al. 1995)).
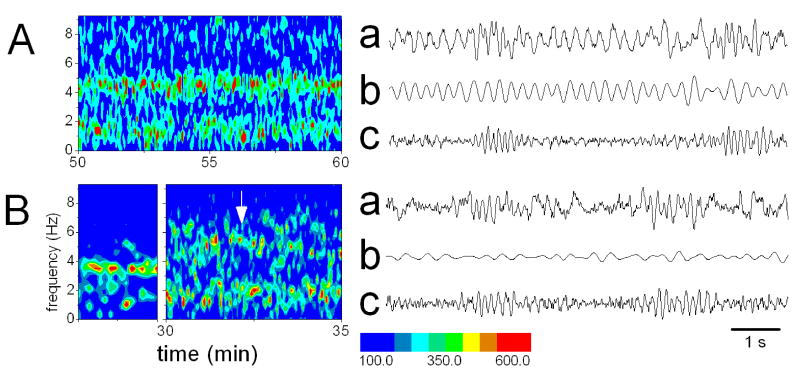
Figure 2.
Effect of injections of GABA receptor agonists in the median raphe nucleus. A. Sample hippocampal EEG recordings during control non-theta (a); and control theta epochs (b); during muscimol (0.1mM) injection (c); and 20 min after perfusion with muscimol halted (d). B. Power spectra of hippocampal EEG (4 s segments) during perfusion of GABAA agonist (a, Muscimol 0.1 mM, red line) and GABAB agonist (b, Baclofen 0.2 mM, red line). Black lines show control ACSF perfusion. C. time-frequency contour plot of hippocampal EEG power spectra before and during drug administration (a, Muscimol 0.1 mM and b, Baclofen 0.2 mM). White arrow indicates start of drug perfusion. Calibration: 2 mV, 1 sec (A).
Figure 4.
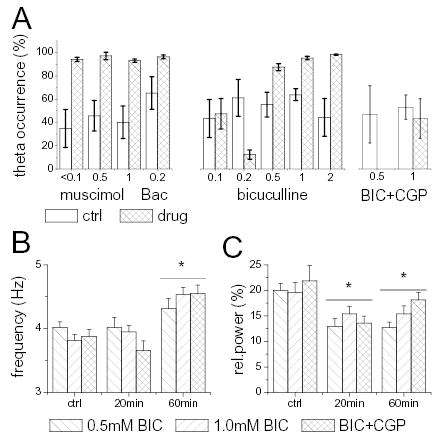
Effect of GABA receptor blockade in the MRN on the occurrence of hippocampal theta rhythm, and its frequency and amplitude. A. Percent time spent in the theta state during 20 min segments immediately before and 20 min after the start of drug injection. Note large decrease in standard error after Muscimol, Baclofen, and BIC injections. B. Theta frequency before, during (20 min), and at the end (60 min) of infusion of GABA antagonists. C. Theta power before, during (20 min), and at the end (60 min) of infusion of GABA antagonists. Error bars mark standard error of the mean.
Figure 5.
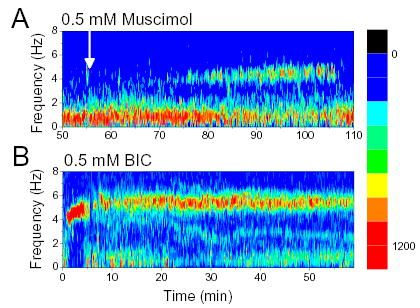
Effect of GABAA receptor agonists (A) and antagonists (B) administered in brainstem structures adjacent to MRN. Time-frequency contour plot of hippocampal EEG spectra before and during microdialysis perfusion of 0.5 mM Muscimol (A, start marked by white arrow) and 0.5 mM Bicuculline (B, start at 0 min).
Similarly, the GABAB-R agonist baclofen (0.2 mM, n=6) elicited continuous hippocampal theta rhythm (Fig. 2, Table 2, Fig 4A), which outlasted the 1 hr infusion and after which the usual, alternating theta/non-theta EEG pattern returned. These findings were consistent with those reported earlier (Varga et al. 2002) indicating that baclofen can modulate theta activity via mechanisms in the MRN even without the involvement of DRN (see location of the microdialysis probe in Fig 1). Similar to muscimol, continuous baclofen administration can also lead to hippocampal desynchronization after 40–60 min but only in concentrations >0.5 mM which were not tested in this study (Varga et al. 2002).
Table 2.
Theta peak frequency and peak power
| Theta peak frequency (Hz) | Theta peak power (% of RMS) | ||||||
|---|---|---|---|---|---|---|---|
| Drugs concentrations | N | Control | 20–30 min | 60 min | control | 20–30 min | 60 min |
| Muscimol | |||||||
| 0.05–0.1 mM | 4 | 3.97±0.06 | 3.79±0.07 | 5.2±1.0 | 17.09±2.3 | ||
| 0.5 mM | 5 | 4.10±0.14 | 4.10±0.15 | 10.5±3.5 | 13.19±2.8 | ||
| 1.0 mM | 5 | 4.15±0.05 | 4.10±0.08 | 11.3±2.3 | 12.55±1.5 | ||
| Baclofen | |||||||
| 0.2 mM | 6 | 3.74±0.11 | 3.70±0.08 | 12.3±1.8 | 18.56±2.4 | ||
| Bicuculline | |||||||
| 0.1 mM | 3+ | 4.07±0.16 | 4.15±0.14 | 4.07+0.08 | 17.5±1.8 | 13.80±0.4 | 17.1±4.4 |
| 0.2 mM | 2+ | 4.03±0.36 | 4.03±0.36 | 4.03+0.12 | 14.1±3.2 | 5.54±1.2 | 12.6±1.3 |
| 0.5 mM | 9 | 4.01±0.09 | 4.01±0.16 | 4.31+0.16 | 20.0±1.3 | 12.97±1.5 | 12.7±1.1 |
| 1.0 mM | 15 | 3.82±0.09 | 3.95±0.09 | 4.53+0.11 | 19.6±1.9 | 15.41±1.4 | 15.4±1.65 |
| 2.0 mM | 5 | 3.66±0.27 | 5.08±0.41 | 6.34+0.56 | 8.6±1.5 | 5.74.6±1.0 | 5.8±1.0 |
| SR95531 (Gabazine) | |||||||
| 0.04 mM | 5 | 3.89±0.19 | no theta | no theta | |||
| 0.5 mM | 4 | 3.91±0.15 | 4.58±0.21 | 15.3±3.0 | 14.30±1.88 | ||
| CGP35348+Bicuculline | |||||||
| 0.5mM BIC | 2 | 3.91±0.24 | no theta | no theta | 20.4±2.8 | no theta | no theta |
| 1.0mM BIC | 8 | 3.88±0.11 | 3.66±0.15# | 4.55±0.13 | 21.9±2.9 | 13.6±1.4 | 18.1±1.4 |
Hippocampal activity after GABAA-R blockade of MRN neurons
GABAA-R blockade was achieved by either one of two different antagonists, bicuculline (BIC) and Gabazine, administered in the MRN using the reverse microdialysis technique for 1 hr. After the probe was placed in the MRN, hippocampal EEG was monitored at least one hour before the perfusion was switched from ACSF to BIC or Gabazine. One or two concentrations were used in each rat with at least one hour wash out between two drug perfusions.
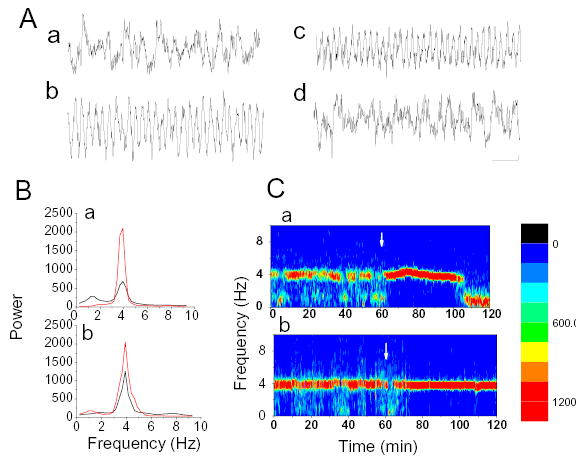
Bicuculline was applied in concentrations of 0.05, 0.1, 0.2, 0.5, 1.0 and 2.0 mM. A typical example of these experiments is shown in Figure 3. In control recordings, i.e. during ACSF perfusion, hippocampal EEG irregularly switched between theta rhythm with high concentration of the signal power within a narrow range around 4 Hz (see red zones in Fig. 3C) and non-theta periods when the EEG power was distributed over a wide frequency band with relatively low peaks below 2 Hz (Fig 3B and 3C, top). Ten minutes after switching to 0.2 mM BIC the amplitude of theta abruptly decreased and a few minutes later completely disappeared (Fig 3C, middle) and only returned 20 min after switching back to ACSF (not shown). BIC administration in higher concentrations exerted a different effect. Theta suppression by 1.0 mM BIC in the same rat only lasted 7 minutes after which theta returned with gradually increasing amplitude and continued until the end of infusion (Fig 3C, bottom).
Figure 3.
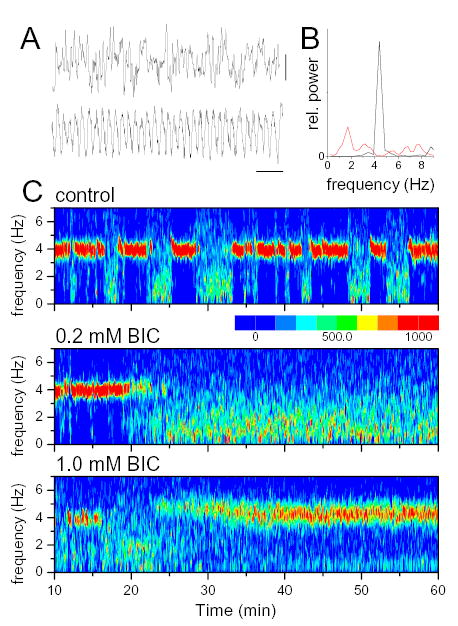
Effect of GABAA receptor antagonist injection in median raphe nucleus. A. Specimen recordings of hippocampal non-theta (upper trace) and theta (lower trace) EEG. B. power spectra of 4 s theta and non-theta EEG segments. C. Time-frequency contour plot of hippocampal EEG power spectra before (control) and during microdialysis perfusion of 0.2 mM and 1.0 mM Bicuculline (start at 10 min).
The effect of GABAA-R blockade was tested in 48 rats. In control recordings, hippocampal EEG alternated between theta and non-theta, irregularly. The average proportion of theta segments in the last 20 min of control was between 43.3% and 61.2% in different dose-groups with large standard deviations (19.7%–36.2%) due to differences from experiment to experiment (Fig. 4A). Depending on the concentration, BIC either elicited lasting theta suppression or a biphasic response composed of a short period of desynchronized activity followed by lasting theta facilitation. These effects were extremely robust and independent of hippocampal activity before drug administration. The latency and duration of hippocampal desynchronization are summarized in Table 3. BIC had no effect when it was given in 0.05 mM concentration (n=3). After 0.1 mM BIC injection, theta disappeared in 50% of experiments (3 out of 6 rats) with latencies between 15 and 35 min (average 25.0 min) and did not return until perfusion switched back to ACSF. During administration of 0.2 mM BIC, theta was eliminated in most (8 of 10) experiments at a shorter average latency of 11.2 min. On average, percent theta occurrence dropped to 12.4 with a dramatic decrease in standard deviation to 5.6 (Fig. 4A).
Table 3.
Latency and duration of suppression of hippocampal theta elicited by GABAA receptor blockade in the MRN
| Concentration | N# | Latency of theta suppression | Duration (min) |
|---|---|---|---|
| BIC | |||
| 0.1 mM | 3 of 6 | 25.0+5.0 | >50+0.0 |
| 0.2 mM | 9 of 10 | 11.2+2.0 | >50+0.0 |
| 0.5 mM | 9 of 11 | 4.3+1.5 | 9.7+1.0 |
| 1.0 mM | 15 of 15 | 3.3+1.0 | 7.5+2.8 |
| 2.0 mM | 5 of 5 | 2.6+0.2 | 5.8+1.1 |
| Gabazine | |||
| 0.04mM | 5 of 5 | 10.0+1.8 | >50+0.0 |
| 0.5mM | 4 of 4 | 4.2+0.7 | 7.5+1.2 |
| BIC + CGP 35348 | |||
| 0.5mM BIC+ | 2 | 8.0+2.0 | >50+0.0 |
| 1.0mM BIC* | 8 | 2.7+1.5 | 25.1+5.5 |
Biphasic effect was found after using the threshold concentration of 0.5 mM or higher (see example of 1.0 mM BIC in Fig. 3C). At these concentrations hippocampal desynchronization was observed from 3.3 to 9.7 min (group averages for 0.5 mM; 4.3 and 7.5 min for 1.0 mM concentration; see Table 1) after which theta activity was maintained until the end of drug administration. Theta segments occupied 87.6 to 98.3% of the 20 min test period in different concentration-groups (Fig. 4A). The robustness of the effect is indicated by a remarkable decrease in standard deviations (i.e. compare with theta/non-theta alternation before injections). BIC-elicited theta was atropine sensitive as it was prevented or eliminated by systemic application of high doses of atropine (50 mg/kg i.p.) given either before (n=2) or 15 min after switching to BIC perfusion (n=6). The effect of BIC was reversible, normal hippocampal activity returned 20–30 min after cessation of drug infusion. The effect was identical when a probe with 3 mm membrane was used (n=5; 1.0 mM BIC).
The changes in hippocampal EEG were also tested after injection of another GABAA antagonist, gabazine. This test was necessary because, the salts of bicuculline, i.e. the water soluble forms of BIC used in pharmacological experiments, in addition to GABAA-Rs, exert a direct effect on Ca++ activated K+ channels and the functional consequences of the resulting blockade of afterhyperpolarization of the target neurons was reported to be similar to that of their disinhibition (Seutin and Johnson 1999). Gabazine was infused in concentrations of 0.04 and 0.5 mM in 5 rats using the same experimental protocol. The results were similar to those observed after BIC administration (Tables 2 and 3). In low concentration, gabazine lead to hippocampal desynchronization at latency of 10±1.8 min. Increase in the concentration to 0.5 mM changed the response to lasting theta which followed a short (11.7+1.0 min) period of desynchronized EEG. The effect was reversible and could be repeated after 1 hr wash with ACSF.
In 9 rats, the microdialysis probe was placed outside the MRN (2 mm lateral from midline, tilt varied between 24–70 degrees) and BIC was administered in 0.1 or 0.5 mM concentrations. Theta suppression was not observed in any of these experiments. Strong, lasting theta oscillation was elicited in most experiments (Fig. 5B). The dynamics of this rhythm was different from that after MRN injection; there was a rapid rise in theta frequency from the onset reaching 6–8 Hz which is similar to the theta frequency elicited by electrical stimulation of the reticular formation (Green and Arduini 1954; Kocsis and Li 2004; Petsche et al. 1962; Vertes 1981).
Characterization of the theta pattern elicited by GABAA-R blockade in the MRN
The frequency of theta elicited by infusion of 0.5 or 1.0 mM BIC into the MRN was generally maintained near the control level (~4 Hz, Table 2) with a steady slow rise (Fig. 6B) resulting in a mild but significant theta acceleration by the end of the 60 min injections (Table 2 and Fig. 4B). 2.0 mM BIC or 0.5 mM of Gabazine elicited a larger (25%) increase in theta at shorter latencies (8.4+1.2 min). In addition to this “late” theta acceleration, in many experiments there was a rapid “early” increase in frequency which appeared right after the start of drug administration but tended to last only a few minutes before the onset of hippocampal desynchronization (Fig. 6A). Early theta acceleration could develop more easily at low concentrations of BIC when theta suppression arrived at longer latencies, i.e. in 1 of 3 experiments with 0.1, and in 5 of 10 experiments with 0.2 mM BIC infusion, and only in 3 and 4 rats (<30%) with 0.5 and 1.0 mM, respectively. In this latter group, however elevated theta frequency was observed immediately after the initial non-theta phase, in another 5 rats (see Fig. 3C for example).
Figure 6.
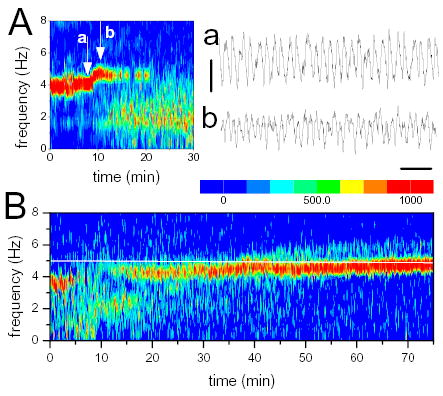
Theta acceleration during infusion of GABAA receptor blockers in the MRN. A. Early theta acceleration in a rat receiving 0.2 mM BIC (injection started at 0 min). Note rapid change in theta frequency at the beginning of infusion. a and b. specimen EEG segments before and after theta acceleration. B. Time-frequency contour plot of EEG power spectra during infusion of 1.0 mM BIC. Note rapid increase of theta frequency at the beginning and slow increment after 30–40 min of drug administration.
The amplitude of theta oscillations were generally reduced as indicated by a significant decrease in the relative power at theta frequency (spectral peak) during perfusion of BIC in concentrations higher than 0.2 mM (two-way ANOVA, Source: BIC main effect, F=4.35844, df=2, p=0.016; Table 2 and Fig 4B). Furthermore, the amplitude was much more variable during drug administration than in control recordings and the rhythm in most cases exhibited a remarkable intermittent character. Figure 7 shows two examples in which theta was strongly modulated either by regularly occurring disruptions of the oscillations or by periodic modulation of its amplitude. The frequency of the intermittency was below 2 Hz and in many experiments was strong enough to generate a spectral peak comparable to the peak at theta frequency (Fig. 7A).
Figure 7.
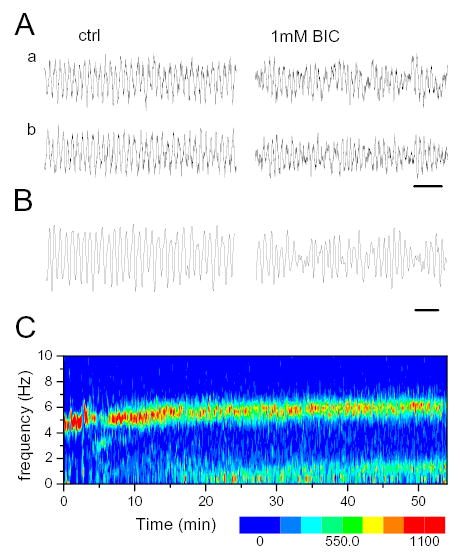
Modulation of theta amplitude during infusion of GABAA receptor blocker in the MRN. A. EEG traces before (ctrl) and during BIC infusion. Traces in b were passed through a digital high pass filter (Butterworth, order 10) to eliminate slow baseline fluctuations. B. Strong amplitude modulation in another experiment with slow theta (theta frequency: 4.2 Hz, modulation frequency: 1.8 Hz). C. Time-frequency contour plot of hippocampal EEG. Note increase in theta frequency from 5Hz in control to 5.8 Hz 15–20 min after start of drug administration. A low frequency component (~1.1 Hz) also appeared in the power spectra about the same time.
Another form of intermittent hippocampal oscillations introduced by BIC administration in the MRN in concentrations of 1.0 mM or higher was represented by well separated theta bursts lasting ~1 s each and periodically repeated every 2–7 s. The intraburst frequency of these oscillations (6–8 Hz) was considerably higher than the regular theta rhythm (~4 Hz). The bursts seemed independent of the ongoing slow hippocampal EEG as they could develop either on the background of theta (Fig. 8A) or non-theta (Fig. 8B) activity.
Figure 8.
Fast theta bursts in the hippocampus during infusion of GABAA receptor blocker in the MRN. A. Time frequency contour plot shows three frequency components in an experiment at the end of the 1 hr long injection of 1.0 mM BIC. Note a fast ~8 Hz component in addition to theta (4–5 Hz) and delta (1–2 Hz) peaks. Traces on the right show a segment of the original EEG (a; 1–70 Hz); and its low-pass (b; <5Hz) and high-pass filtered (c; >5 Hz) components. B. Regular ~4Hz theta rhythm during control (left panel) and Fast theta bursts (~6 Hz; right panel) in a different rat receiving 0.5 mM BIC. Contour plot shows regular (~4 Hz) theta in control; a, b, and c as above.
The effect of combined GABAA- and GABAB-R blockade of MRN neurons on hippocampal activity
It was demonstrated earlier that serotonergic neurons in the MRN, in addition to GABAA-Rs express GABAB-Rs, on their soma and dendrites. Thus to achieve total GABA-R blockade a mixture of BIC and GABAB-R antagonist CGP35348 was infused in the MRN in 10 rats, in various proportions. Theta rhythm elicited by 0.5 mM BIC was completely eliminated by coadministration of 2.5 mM CGP35348 (n=2) (see example in Fig 9A). When the concentration of BIC was increased to 1.0 mM, coadministration of the GABAB-R antagonist in proportions 1:5, 1:18, or 1:50 was effective in blocking theta rhythm during the first 40 min in 4 of 8 experiments. In three of the four remaining experiments lasting theta appeared 12, 22, or 27 min after the start of infusion and in one rat theta and non-theta alternated (this experiments is shown in Fig. 9B). The group average of the duration of theta suppression was still significantly higher (25.1+5.5 min) than during administration of BIC alone (Table 3). In addition, the frequency of theta rhythm was also reduced and was significantly different than during control or BIC. Late theta acceleration was similar to that after administration of BIC alone (Table 2 and Fig. 4C) whereas early theta acceleration was not observed in any of these experiments.
Figure 9.
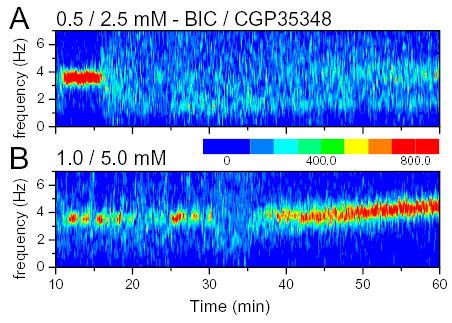
Effect of total GABA blockade (GABAA and GABAB) of the MRN on hippocampal theta rhythm. A. Time frequency contour plot of hippocampal EEG before and during coadministration of 0.5 mM BIC and 2.5 mM CGP35348. B. Same for another rat receiving 1 mM BIC and 5 mM CGP35348. The occurrence of theta segments in the EEG was highest in this experiment. Note lack of constant theta observed during 1 mM BIC (compare with Figs 3C, 4B, 5A).
Discussion
This study concerned the role of GABAergic mechanisms in MRN in the control of hippocampal activity. MRN is the primary source of serotonergic afferents to the limbic system which are generally considered to have a suppressing effect on hippocampal theta oscillations (Vertes and Kocsis 1997). Thus, GABAergic inhibition or disinhibition of 5-HT neurons in the MRN is expected to facilitate or inhibit, respectively, the theta rhythm. GABA-Rs are also expressed, however, on other types of MRN neurons, including local GABAergic interneurons and possibly long-projecting GABAergic (Jankowski and Sesack 2002; Puig et al. 2004) and glutamatergic cells (Tao and Auerbach 2003). The action of GABAergic substances at these sites can be opposite to those on 5-HT neurons which might explain earlier findings of similar actions of muscimol (Kinney et al. 1995) and BIC (Thinschmidt et al. 1995) injected into the same structure. The present report offers several important observations to clarify this issue. First, we confirmed and extended previous findings (Kinney et al. 1995; Varga et al. 2002) regarding the theta-promoting effects of MRN application of the GABAA agonist muscimol and GABAB agonist baclofen. Second, we demonstrated that the GABAA antagonist bicuculline infused in low concentrations (0.1, 0.2 mM) into the MRN eliminated theta rhythm. Lasting hippocampal desynchronization followed a short period of theta activity of higher than normal frequency in 46% of rats. Third, we found that MRN administration of bicuculline in larger concentrations (0.5, 1.0, 2.0 mM) resulted in a biphasic response. In these experiments the period of hippocampal desynchronization was shorter than 10 min and was followed by lasting theta rhythm as long as the perfusion continued. The frequency and amplitude of theta waves were significantly higher than in control recordings and the oscillations showed a conspicuous intermittent character. Fourth, hippocampal theta rhythm elicited by MRN administration of bicuculline could be completely (BIC in 0.5 mM) or partially (BIC in 1.0 mM) blocked by simultaneous infusion of GABAB antagonist CGP35348.
Advantages and limitations of the methodology
Administration of drugs using reverse microdialysis has several advantages over pressure microinjections. After microinjection, the drug concentration at the cannula, at the moment of injection is relatively high which represents a powerful drive for diffusion. The drug diffuses to a certain distance and gets eliminated by different mechanisms. The affected area first rapidly expands and then shrinks at a lower pace. The concentration is therefore never stable at any point in the structure, at any time and the affected area constantly changes. Microdialysis can deliver the same amount of drug stretched out in time. The concentration in the tissue starts from low values and rises continuously until it reaches equilibrium. From that moment it stays relatively stable at any location for as long as the experiment continues. The affected area also remains steady. From this there are two important practical conclusion: 1. that the latencies are longer i.e. the latency is not the time necessary for diffusion (as in microinjection) rather the time necessary to equilibrate the concentrations in different probe and tissue compartments; 2. The distance of the diffusion is much more limited due to the lack of the initial big pressure inherent for microinjection. Modeling and experimental investigations (Boehnke and Rasmusson 2001; Dykstra et al. 1992; Robinson and Jusctice 1991) have shown that once the equilibrium is established the distance of the diffusion and thus the affected area no longer increases - no matter how long the dialysis lasts.
Although the specifics regarding the effective spread of bicuculline in the brain tissue are not known, the diffusion of neuroactive compounds are limited by a number of factors, including binding to receptors and removal into the microcirculation. Earlier dialysis studies indicated for example, that bicuculline, muscimol or baclofen infused in the MRN did not diffuse as far as the nearby DRN and vice versa (Tao and Auerbach 2003; Tao et al. 1996). Furthermore, mathematical modeling and in vivo measurements of various substances (e.g. sucrose, mannitol) indicated that the concentration outside of a 1 mm radius around the probe was very low (Dykstra et al. 1992; Höistad et al. 2002). Yet in another study, significant physiological effect of lidocaine and tetrodotoxin could be detected as far as 1.5–2 mm, after 15–20 min dialysis perfusion (Boehnke and Rasmusson 2001). Although this issue remains unresolved, the possibility of the buildup of an effective concentration of bicuculline outside the MRN cannot be excluded. It could have played a role in promoting theta when applied in high concentrations (1.0 mM and higher), especially during the second half of the 1 hr perfusion period. It could also contribute to the steady increase in theta frequency in the second half of the perfusion; “late” theta acceleration usually started after 40 min; see Figs. 3B, 4B, and 8B).
GABAergic suppression of 5-HT neurons drives hippocampal theta rhythm
There is ample evidence indicating that the 5-HT input originating in the MRN suppresses theta bursts in the medial septum and theta rhythm in the hippocampus (Vertes and Kocsis 1997). Electrical stimulation of the MRN desynchronizes the hippocampal EEG in freely behaving as well as in anesthetized rodents (Assaf and Miller 1978; Kitchigina et al. 1999; Macadar et al. 1974; Vertes 1981; Yamamoto et al. 1979) while MRN lesions (Kitchigina et al. 1999; Maru et al. 1979; Vinogradova et al. 1999; Yamamoto et al. 1979), 5-HT depletion (Mushiake et al. 1988) or selective inhibition of MRN serotonergic cells through 5-HT1A autoreceptors (Vertes et al. 1994) produce continuous theta rhythm. [PARAGRAPH SHORTENED]
The median raphe contains a large population of GABAergic interneurons (Jacobs and Azmitia 1992; Maloney et al. 1999b; Mugnaini and Oertel 1985) and receives GABAergic input from numerous distant regions from the forebrain to the medulla (Gervasoni et al. 2000). The schematics in Figure 10 summarizes existing data on the complex interactions between GABAergic and serotonergic neuron populations. GABAergic neurons are excited by locally released 5-HT via 5-HT2-Rs and, in turn, inhibit serotonergic neurons via GABAA and GABAB-Rs (Bowery et al. 1987; Chu et al. 1990; Gao et al. 1993; Serrats et al. 2003; Varga et al. 2002). 5-HT neuron firing was suppressed by stimulation of GABAA (Gallager and Aghajanian 1976a) and GABAB-Rs (Abellan et al. 2000; Innis and Aghajanian 1988), and MRN injection of GABA or muscimol was followed by a significant decrease in 5-HT concentration within the raphe (Tao et al. 1996) and in the hippocampus (Forchetti and Meek 1981; Wirtshafter et al. 1987b). Thus the strong theta rhythm observed after injection of GABA-R agonists (Kinney et al. 1995; Varga et al. 2002) was most likely due to direct inhibition of the ascending serotonergic pathway. In this study we found that both muscimol and baclofen were effective in eliciting theta when injected in the MRN in small concentrations. Continuous hippocampal theta was observed in all experiments during infusion of muscimol in concentrations as low as 50 jM while the threshold for baclofen was found higher, at 200 jM (Varga et al. 2002). Considering a significant, i.e. up to 90% (Dykstra et al. 1992), drop in drug concentrations across the dialysis membrane, these findings were in agreement with results of direct examination of serotonergic neurons in raphe slices. In vitro, 10 jM muscimol effectively decreased local serotonin release (Bagdy et al. 2000) and the firing rate (Gallager and Aghajanian 1976b). Baclofen induced marked hyperpolarization of 5-HT neurons and completely inhibited their firing at concentrations between 10 and 50 jM (Innis and Aghajanian 1988, 1987).
Figure 10.
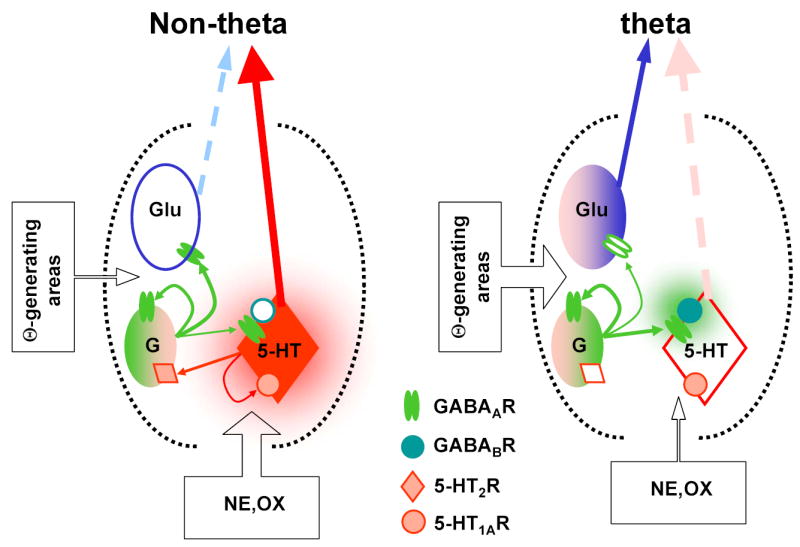
Minimal circuit model of the GABAergic control of MRN limbic forebrain projections involved in the control of hippocampal theta rhythm. During non-theta states the MRN output is dominated by serotonergic activity. At the onset of theta, incoming excitatory drive increases GABAergic tone and suppresses serotonergic cells via GABAA- and GABAB-Rs (Varga et al. 2002) releasing the theta-generating circuitry from the serotonergic inhibition. The MRN 5-HT-GABA system is stabilized by feedback through 5-HT1A autoreceptors and 5-HT2-Rs on GABAergic neurons (Liu et al. 2000). GABAB-Rs are recruited by increased GABAergic tone (Nitz and Siegel 1997) and synchronized firing of GABAergic cells (Kocsis and Vertes 1992). Besides the inhibition of serotonergic neurons putative glutamatergic (Kiss et al. 2002), theta-promoting circuits are activated which can also be the targets of GABAergic inhibition. Thus, the GABAergic network may have two seemingly opposing function in the MRN: relieving the theta-generators from the serotonergic inhibition and regulating the activity in the theta-promoting circuitry by the fluctuating GABAergic tone.
After approximately 40 min of Muscimol application theta activity disappeared and did not recover until the end of the experiments. This delayed effect of Muscimol can be due either to local mechanisms or to suppression of theta-inducing circuitry outside the median raphe nucleus, or both. In the first case prolonged administration of the agonist can induce adaptive changes in GABAA-R function. Desensitization followed by the upregulation of agonist binding sites has recently been reported (Pericic et al. 2003). These changes can be subunit specific affecting various GABAA-R subunits to different extent. Thus, prolonged GABAA-R activation can result in a disinhibition of the MRN neuronal network leading to an increased serotonergic outflow and desynchronization of the hippocampal EEG. The return of theta can take several hours beyond the length of the experiments.
The effect of GABA-R antagonism in the MRN on hippocampal theta rhythm
Depending on the concentration and the length of exposure, perfusion of GABAA-R antagonist BIC in the MRN consistently elicited an orderly combination of the following three patterns of hippocampal activity:
Lasting hippocampal desynchronization appeared at decreasing latencies as the BIC concentration increased. Anatomical distribution of GABAA-Rs suggests that in the 5-HT-GABA network of the raphe, BIC can lead to activation of both neuron populations resulting in a rapid elevation in both GABA and 5-HT levels (see Fig. 10). This balance, however, can eventually shift to either GABA or 5-HT dominance. Elevation of local serotonin concentration in MRN will, through 5-HT2-R activation, further enhance GABA tone which now can reach extrasynaptic GABAB-Rs on 5-HT cells and together with 5-HT1A-R mediated autoregulation suppresses serotonergic cells. This can in turn lead to disfacilitation of GABA neurons, an ensuing disinhibition of 5-HT, and so on. The source of asymmetry in this feedback system is the postsynaptic GABAB-Rs which in the raphe are primarily expressed by 5-HT neurons (Varga et al. 2002) and might thus play a significant role in switching between states depending on the level of local GABA tone. Due to their extrasynaptic location these receptors have a higher threshold (Scanziani 2000) and can be activated by fast or synchronized firing of GABAergic interneurons as found in unanesthetized rats during REM sleep (Kocsis and Vertes 1992; Maloney et al. 2000; Nitz and Siegel 1997; Torterolo et al. 2000). Accordingly, in the present experiments lasting desynchronization of hippocampal EEG only appeared at moderate (0.1–0.2 mM) concentrations of BIC disinhibiting both 5-HT and GABA neurons. Suppression of hippocampal theta rhythm indicates that at this level the elevated GABA input to serotonergic cells was subthreshold for GABAB-Rs resulting in a net increase in 5-HT output. When the probe was outside the MRN suppression of theta was not observed.
Sustained slow (4–5 Hz) theta rhythm observed during high concentration BIC administration can be the result of two, synergistically acting mechanisms. First, it could be elicited by GABAB-R-mediated blockade of 5-HT cells as discussed earlier (Fig. 10). The role of GABAB-receptors in the suppression of serotonergic activity was strongly supported by the observation that combined blockade of GABAA-and GABAB-Rs eliminated or attenuated this type of hippocampal theta activity (see Fig. 9). A second mechanism which may have played an increasing role toward the end of the 1 hr-long infusion in many experiments, was a lateral diffusion of BIC into adjacent areas surrounding the MRN also known as the theta induction zone (Green and Arduini 1954; Kocsis and Li 2004; Vertes 1981). In control experiments when the probe was located in or near this area theta rhythm was elicited by 0.5 mM BIC and the frequency of this rhythm was fast rising from the onset of infusion (Fig. 5B). The distance of diffusion from the probe is very limited but for certain drugs can be > 1 mm (Boehnke and Rasmusson 2001). A cylinder with a radius of 1 – 2 mm around the probe membrane partially overlaps with the area of the theta induction zone (see Fig. 1). Activation of extra-raphe mechanisms would also explain the late acceleration of theta oscillations which does not appear even after total blockade of 5-HT cells (Kinney et al. 1995; Varga et al. 2002; Vertes et al. 1994). [PARAGRAPH SHORTENED]
Fast (6–8 Hz) intermittent theta oscillations
Immediately following the onset of BIC infusion in low concentrations a transient, short lasting, fast theta rhythm dominated the hippocampal EEG. Its frequency significantly surpassed the maximum observed during non-selective or selective suppression of the serotonergic output of the MRN (e.g. Fig. 2, see also in (Kinney et al. 1995; Varga et al. 2002; Vertes et al. 1994). This indicates that the initial transient fast theta during the injection of BIC in < 0.5 mM and the short theta bursts which appeared at later stages on the background of either stationary theta or non-theta EEG could be primarily caused by the activation of non-serotonergic, theta-inducing circuits within the MRN (Fig. 10, right). MRN has a massive nonserotonergic projection to the limbic system including glutamatergic neurons targeting the supramammillary nucleus (SUM) (Kiss et al. 2002). This latter is directly involved in theta generation (Kirk and McNaughton 1991; Kocsis and Vertes 1994), in particular during episodes of theta acceleration (Kaminski and Kocsis 2003). Furthermore, changes in glutamate concentration in the MRN also suggested that the mechanisms involved in the MRN control of lasting slow theta rhythm and those driving fast theta episodes were different. In a microdialysis study Varga et al. (Varga et al. 1998) found that spontaneous slow theta was coupled with a decrease in MRN glutamate levels in keeping with the concept of theta due to disfacilitation of 5-HT neurons, whereas the opposite was true for fast theta episodes elicited by sensory stimulation. Nonserotonergic MRN efferents and their GABAergic control have also been implicated in behavioral regulation (Tao and Auerbach 2003; Wirtshafter et al. 1987a; Wirtshafter et al. 1989).
GABAergic control of serotonergic and non-serotonergic elements of the raphe
In principle, the GABAA-R antagonist BIC could influence two pathways ascending from MRN which have opposite effects on hippocampal theta rhythm. The major serotonergic MRN input desynchronizes hippocampal EEG whereas the recently described glutamatergic input to SUM (Kiss et al. 2002) most likely drives theta rhythm. In addition, the two pathways also differ in the function and the dynamics of the regulation they provide. 5-HT is a state-controlling modulatory input i.e. its suppression alone is sufficient to switch the hippocampus from desynchronization back to the theta state (4–5 Hz under urethane). In contrast, the MRN glutamatergic neurons along with the reticular input, SUM, and others are rapid-action elements generating fast theta oscillations (6–8 Hz). GABAA-Rs are expressed by serotonergic and non-serotonergic cells which include GABAergic and glutamatergic neurons whereas postsynaptic GABAB-Rs in the MRN are predominantly located on 5-HT neurons (Serrats et al. 2003; Varga et al. 2002). 5-HT cells are also known to be embedded in multiple feedback loops through 5-HT1A autoreceptors and through 5-HT2 activation of local GABAergic neurons (Glass et al. 2004; Liu et al. 2000). Thus, BIC could act as a theta-suppressing factor through direct effect on 5-HT cells as well as a pro-theta agent by acting on GABAergic or glutamatergic units.
Figure 10 shows a simplified wiring model of GABAergic regulation of the serotonergic output of the MRN: during non-theta states the MRN output is dominated by serotonergic activity. At the onset of theta, incoming excitatory drive increases GABAergic tone and suppresses serotonergic cells via GABAA- and GABAB-Rs thus releasing the theta-generating circuitry from serotonergic inhibition. The MRN 5-HT-GABA system is stabilized by feedback through 5-HT1A autoreceptors and 5-HT2-Rs on GABAergic neurons (Liu et al. 2000). The recruitment of GABAB-Rs requires increased GABAergic tone (Nitz and Siegel 1997) and synchronized firing of GABAergic cells (Kocsis and Vertes 1992). Besides the inhibition of serotonergic neurons putative glutamatergic, theta-promoting circuits are activated. The latter neurons can also be the targets of GABAergic inhibition.
Thus, the GABAergic network may have two seemingly opposing functions in the MRN: relieving the theta-generators from serotonergic inhibition and regulating the activity in the theta-promoting circuitry by the fluctuating GABAergic tone. The resulting hippocampal oscillations would however show different characteristics i.e. slow and on-going theta rhythm due to 5-HT withdrawal and fast intermittent theta bursts due to glutamatergic activation or disinhibition. The two mechanisms may be involved in different functions and they may show species differences in their development and activation. In the rat, REM sleep is characterized by on-going theta rhythm in the hippocampus which however is not stationary and includes rapid bursts of high frequency theta oscillations. It was shown that these episodes of fast theta are not distributed randomly but follow a sequence predictable by the animal’s previous learning experience (Louie and Wilson 2001). In human, REM sleep-associated hippocampal theta appears in the form of short (1–2s) theta bursts of 4–7 Hz repeated regularly every ~6s on the background of non-theta activity (Cantero et al. 2003), and similar theta bursts in waking were shown to be related to spatial learning (Kahana et al. 1999). Under urethane anesthesia spontaneous theta is usually slow; fast theta requires electrical stimulation of rapid-action elements in the pons (Kocsis and Li 2004; Vertes 1981). The present experiments demonstrated an extended capacity of intrinsic raphe circuits even in this reduced preparation to force the hippocampal theta generator into various modes of activity producing patterns closely reminiscent of those seen in REM sleep, by changing the MRN GABA tone.
Acknowledgments
Present address of Viktor Varga: Institute of Experimental Medicine, Hungarian Academy of Sciences, Budapest, Hungary. This study was supported by the National Institute of Health (MH062525), the Hungarian Science Foundation (OTKA 34344), and the Canadian Institutes of Health Research. Attila Sik is a Fonds de Recherche et Sante du Quebec (FSRQ) Scholar.
References
- Abellan MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABAB receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Assaf SY, Miller JJ. The role of a raphe-serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience. 1978;3:539–550. doi: 10.1016/0306-4522(78)90018-0. [DOI] [PubMed] [Google Scholar]
- Bagdy E, Kiraly I, Harsing L. Reciprocal innervation between serotonergic and GABAergic neurons in raphe nuclei of the rat. Neurochem Res. 2000;25:1465–1473. doi: 10.1023/a:1007672008297. [DOI] [PubMed] [Google Scholar]
- Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: ene electrophysiological study in cerebral cortex. J Neurosci Methods. 2001;105:133–141. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung LS, Vanderwolf CH. Cellular basis of hippocampal EEG in the behaving rat. Brain Research Review. 1983;6:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Kahana M, Madsen J, Stickgold R, Kocsis B. REM-sleep dependent theta waves in the human hippocampus and neocortex. J Neurosci. 2003;34:10897–10903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DC, Albin RL, Young AB, Penney JB. Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience. 1990;34:341–357. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- Dykstra KH, Hsiao JK, Morrison PF, Bungay PM, Mefford IN, Scully MM, Dedrick RL. Quantitative examination of tissue concentration profiles associated with microdialysis. J Neurochem. 1992;58:931–940. doi: 10.1111/j.1471-4159.1992.tb09346.x. [DOI] [PubMed] [Google Scholar]
- Ferraro G, Montalbano ME, Sardo P, La Grutta V. Lateral habenular influence on dorsal raphe neurons. Brain Res Bull. 1996;41:47–52. doi: 10.1016/0361-9230(96)00170-0. [DOI] [PubMed] [Google Scholar]
- Forchetti CM, Meek JL. Evidence for a tonic GABAergic control of serotonin neurons in the median raphe nucleus. Brain Res. 1981;206:208–212. doi: 10.1016/0006-8993(81)90118-9. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas A, I, Acsady L, Gorcs T, Toth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proceedings of the National Academy of Sciences of the USA. 1990;87:8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallager DW, Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphe cells. II. Reversal by picrotoxin. European Journal of Pharmacology. 1976a;39:357–364. doi: 10.1016/0014-2999(76)90145-x. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphe neurons. II. Reversal by picrotoxin. Eur J Pharmacol. 1976b;39:357–364. doi: 10.1016/0014-2999(76)90145-x. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Benke D, Mohler H. Neuron-specific expression of GABAA-receptor subtypes: differential association of the alpha 1- and alpha 3-subunits with serotonergic and GABAergic neurons. Neuroscience. 1993;54:881–892. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ, Grossman GH, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J Neurosci. 2004;23:7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD, Arduini A. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinsky PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Höistad M, Chen KC, Nicholson C, Fuxe K, Kehr J. Quantitative dual-probe analysis: evaluation of [3H]mannitol diffusion in agr and rat striatum. J Neurochem. 2002;81:80–93. doi: 10.1046/j.1471-4159.2002.00791.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Evidence of G protein mediation of serotonin- and GABAB-induced hyperpolarization of rat dorsal raphe neurons. Brain Res. 1988;459:27–36. doi: 10.1016/0006-8993(88)90282-x. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur J Pharmacol. 1987;143:195–204. doi: 10.1016/0014-2999(87)90533-4. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Electron microscopic analysis of the GABA projection from the dorsal raphe nucleus to prefrontal cortex in the rat. Neurosci Abstr. 2002;587:8. [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kaminski M, Kocsis B. Direction of the theta rhythmic drive between neurons in the supramammillary nucleus and the septohippocampal system in urethane anesthetized rats. Neurosci Abstr. 2003;29:000. [Google Scholar]
- Kinney GG, Kocsis B, Vertes RP. Injections of muscimol into the median raphe nucleus produce hippocampal theta rhythm in the urethane anesthetized rat. Psychopharmacology (Berl) 1995;120:244–248. doi: 10.1007/BF02311170. [DOI] [PubMed] [Google Scholar]
- Kirk IJ, McNaughton N. Supramammillary cell firing and hippocampal rhythmical slow activity. Neuroreport. 1991;2:723–725. doi: 10.1097/00001756-199111000-00023. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csaki A, Kocsis K, Kocsis B. Glutamatergic/aspartatergic projections to the supramammillary nucleus and their origins in the rat studied by the selective [3H]D-aspartate labelling and immunocytochemistry. Neuroscience. 2002;111:671–691. doi: 10.1016/s0306-4522(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Kitchigina VF, Kudina TA, Kutyreva EV, Vinogradova OS. Neuronal activity of the septal pacemaker of theta rhythm under the influence of stimulation and blockade of the median raphe nucleus in the awake rabbit. Neuroscience. 1999;94:453–463. doi: 10.1016/s0306-4522(99)00258-4. [DOI] [PubMed] [Google Scholar]
- Kocsis B and Li S In vivo contribution of h-channels in the septal pacemaker to the generation of theta rhythm in the rat. Eur J Neurosci in press, 2004. [DOI] [PubMed]
- Kocsis B, Vertes RP. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. J Neurosci. 1994;14:7040–7052. doi: 10.1523/JNEUROSCI.14-11-07040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Dorsal raphe neurons: synchronous discharge with theta rhythm of the hippocampus in the freely moving rat. J Neurophysiol. 1992;68:1463–1467. doi: 10.1152/jn.1992.68.4.1463. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Midbrain raphe cell firing and hippocampal theta rhythm in urethane anesthetized rats. Neuroreport. 1996;7:2867–2872. doi: 10.1097/00001756-199611250-00012. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian GK. Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Macadar AW, Chalupa LM, Lindsley DB. Differentiation of brainstem loci which affect hippocampal and neocortical electrical activity. Exp Neurol. 1974;43:499–514. doi: 10.1016/0014-4886(74)90190-3. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. c-fos expression in GABAergic, serotonergic, and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;20:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, and Jones BE Differential c-fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci 19, 1999a. [DOI] [PMC free article] [PubMed]
- Maloney KJ, Mainville L, Jones BE. Differential c-fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. Journal of Neuroscience. 1999b;19:3057–3072. doi: 10.1523/JNEUROSCI.19-08-03057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru E, Takahashi LK, Iwahara S. Effects of median raphe nucleus lesions on hippocampal EEG in the freely moving rat. Brain Res. 1979;163:223–234. doi: 10.1016/0006-8993(79)90351-2. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Mugnaini E and Oertel WH An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunochemistry. In: Handbook of Chemical Neuroanatomy: GABA and neuropeptides in the CNS, edited by Bjorklund A and Hoekfelt T. New York: Elsevier, 1985, p. 436–608.
- Mushiake H, Kodama T, Shima K, Yamamoto M, Nakahama H. Fluctuations in spontaneous discharge of hippocampal theta cells during sleep-waking states and PCPA-induced insomnia. J Neurophysiol. 1988;60:925–939. doi: 10.1152/jn.1988.60.3.925. [DOI] [PubMed] [Google Scholar]
- Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997;273:R451–R455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J and Nadel L The Hippocampus as a Cognitive Map. Oxford: Clarendon, 1978.
- Paxinos G and Watson C The Rat Brain in Stereotaxic Coordinates. Amsterdam: Elsevier Academic Press, 2005.
- Pericic D, Strac DS, Jembreck MB, Rajcan I. Prolonged exposure to gamma-aminobutyric acid up-regulates stably expressed recombinant alpha 1 beta 2 gamma 2s GABAA receptors. Eur J Pharmacol. 2003;482:117–125. doi: 10.1016/j.ejphar.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Petsche H, Stumpf CH, Gogolak G. The significance of the rabbit’s septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Puig MV, Artigas F, and Celada P Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: invovement of serotonin and GABA. Cerebral Cortex in press, 2004. [DOI] [PubMed]
- Pyapali GK, Sik A, Penttonen M, Buzsaki G, Turner DA. Dendritic properties of hippocampal CA1 pyramidal neurons in the rat: intracellular staining in vivo and in vitro. J Comp Neurol. 1998;391:335–352. doi: 10.1002/(sici)1096-9861(19980216)391:3<335::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE and Jusctice JB Microdialysis in Neuroscience New York: Elsevier, 1991.
- Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Serrats J, Artigas F, Mengod G, Cortes R. GABAB receptor mRNA in the raphe nuclei: co-expression with serotonin transporter and glutamic acid decarboxylase. J Neurochem. 2003;84:743–752. doi: 10.1046/j.1471-4159.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of the quaternary salts of bicuculline. Trends Pharmacol Sci. 1999;20:268–270. doi: 10.1016/s0165-6147(99)01334-6. [DOI] [PubMed] [Google Scholar]
- Shim I, Javaid J, Wirtshafter D. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Behavioral Brain Research. 1997;89:191–198. [Google Scholar]
- Tao R, Auerbach SB. Influence of inhibitory and excitatory inputs on serotonin efflux differs in the dorsal and median raphe nuclei. Brain Res. 2003;961:109–120. doi: 10.1016/s0006-8993(02)03851-9. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br J Pharmacol. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinschmidt JS, Kocsis B, Kinney GG. Similar effects on the hippocampal EEG following injection of GABA agonists and antagonists into the median raphe nucleus. Neurosci Abstr. 1995;21:1205. [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. GABAergic neurons of the cat dorsal raphe nucleus express c-fos during carbachol-induced active sleep. Brain Res. 2000;884:68–76. doi: 10.1016/s0006-8993(00)02891-2. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Varga V, Kekesi A, Juhasz G, Kocsis B. Reduction of the extracellular level of glutamate in the median raphe nucleus associated with hippocampal theta activity in the anesthetized rat. Neuroscience. 1998;84:49–57. doi: 10.1016/s0306-4522(97)00489-2. [DOI] [PubMed] [Google Scholar]
- Varga V, Kocsis B, Sharp T. Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex (mPFC) and lateral habenula (lHb) on single neurones in the midbrain raphe nuclei. Eur J Neurosci. 2003;17:280–286. doi: 10.1046/j.1460-9568.2003.02465.x. [DOI] [PubMed] [Google Scholar]
- Varga V, Sik A, Fritschy JM, Freund TM, Kocsis B. GABAB receptors in the median raphe nucleus: distribution and role in the serotonergic control of hippocampal activity. Neuroscience. 2002;109:119–132. doi: 10.1016/s0306-4522(01)00448-1. [DOI] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csilleg A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP. An analysis of ascending brain stem systems involved in hippocampal synchronization and desynchronization. J Neurophysiol. 1981;46:1140–1159. doi: 10.1152/jn.1981.46.5.1140. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kinney GG, Kocsis B, Fortin WJ. Pharmacological supression of the median raphe nucleus with 5-HT1A agonists, 8-OH-DPAT and Buspirone, produces hippocampal theta rhythm in the rat. Neuroscience. 1994;60:441–451. doi: 10.1016/0306-4522(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Albo Z, Vertes RP, Kocsis B. Discharge properties of neurons of the median raphe nucleus during the hippocampal theta rhythm in the rat. Experimental Brain Research. 2002;145:383–394. doi: 10.1007/s00221-002-1123-8. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS, Kitchigina VF, Kudina TA, Zenchenko KI. Spontaneous activity and sensory responses of hippocampal neurons during persistent theta-rhythm evoked by median raphe nucleus blockade in the rabbit. Neuroscience. 1999;94:745–753. doi: 10.1016/s0306-4522(99)00253-5. [DOI] [PubMed] [Google Scholar]
- Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29:943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Klitenick MA, Asin KE. Evidence against serotonin involvement in the hyperactivity produced by injections of muscimol into the median raphe nucleus. Pharmacol Biochem Behav. 1987a;27:45–52. doi: 10.1016/0091-3057(87)90475-8. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Klitenick MA, Asin KE. Is dopamine involved in the hyperactivity produced by injections of muscimol into the median raphe nucleus? Pharmacology Biochemistry & Behavior. 1987b;30:577–583. doi: 10.1016/0091-3057(88)90068-8. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Trifunovic R, Krabs JC. Behavioral and biochemical evidence for a functional role of excitatory amino acids in the median raphe nucleus. Brain Res. 1989;482:225–234. doi: 10.1016/0006-8993(89)91185-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Watanabe S, Oishi R, Ueki S. Effects of midbrain raphe stimulation and lesion on EEG activity in rats. Brain Res Bull. 1979;4:491–495. doi: 10.1016/0361-9230(79)90033-9. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Sampogna S, Lopez-Rodrigez F, Luppi PH, Morales FR, Chase MH. Fos and serotonin immunoreactivity in the raphe nuclei of the cat during carbachol-induced active sleep: A double-labeling study. Neuroscience. 1995;67:211–223. doi: 10.1016/0306-4522(94)00633-g. [DOI] [PubMed] [Google Scholar]