The axonal transport of mitochondria (original) (raw)
. Author manuscript; available in PMC: 2006 Aug 8.
Published in final edited form as: J Cell Sci. 2005 Dec 1;118(Pt 23):5411–5419. doi: 10.1242/jcs.02745
Summary
Organelle transport is vital for the development and maintenance of axons, in which the distances between sites of organelle biogenesis, function, and recycling or degradation can be vast. Movement of mitochondria in axons can serve as a general model for how all organelles move: mitochondria are easy to identify, they move along both microtubule and actin tracks, they pause and change direction, and their transport is modulated in response to physiological signals. However, they can be distinguished from other axonal organelles by the complexity of their movement and their unique functions in aerobic metabolism, calcium homeostasis and cell death. Mitochondria are thus of special interest in relating defects in axonal transport to neuropathies and degenerative diseases of the nervous system. Studies of mitochondrial transport in axons are beginning to illuminate fundamental aspects of the distribution mechanism. They use motors of one or more kinesin families, along with cytoplasmic dynein, to translocate along microtubules, and bidirectional movement may be coordinated through interaction between dynein and kinesin-1. Translocation along actin filaments is probably driven by myosin V, but the protein(s) that mediate docking with actin filaments remain unknown. Signaling through the PI 3-kinase pathway has been implicated in regulation of mitochondrial movement and docking in the axon, and additional mitochondrial linker and regulatory proteins, such as Milton and Miro, have recently been described.
Keywords: Mitochondria, Axonal transport, Kinesin, Myosin, Dynein
Introduction
Nerve axons are the mother of all cellular processes, usually containing the majority – often the vast majority – of the cell cytoplasm, much of it at an enormous distance from the nucleus, ER and Golgi. This far-flung biomass is supported in part by fast axonal transport, a steady stream of organelles moving from their major sites of biogenesis in the cell body to the distant reaches of the axon at rates of micrometers per second, hundreds of centimeters per day. A similar transport system runs in the opposite direction, moving organelles, including those of the endocytic and autophagic pathways, from the distal axon back to the cell body. Mitochondria are prominent members of the cast of axonally transported organelles. They are essential for the function of all aerobic cells, including neurons. They produce ATP, buffer cytosolic calcium and sequester apoptotic factors. But when things go awry, mitochondria can also be a nexus for many of the neuron's woes: excitotoxicity, metabolic insufficiency, cell death and oxidative damage that contribute to severe pathologies of the nervous system (Heales et al., 1999; Kosel et al., 1999; Nicholls et al., 1999; Tatton and Olanow, 1999; Sawa, 2001; Swerdlow and Kish, 2002; Krieger and Duchen, 2002; Nieminen, 2003; Rego and Oliveira, 2003). Recent work has directed our attention not just to the functions of mitochondria but also to their axonal transport as a potential contributor to diseases of the nervous system (Guzik and Goldstein, 2004; Hirokawa and Takemura, 2004).
Like many other neuronal organelles, mitochondria are thought to arise mainly in the neuronal cell body, but their transport is distinctive. They are not only delivered to but also retrieved from the axon, and their average velocity falls between that of fast-moving small vesicles and slow-moving cytoskeletal proteins (Grafstein and Forman, 1980; Vallee and Bloom, 1991). This is because individual mitochondria spend a significant part of their time stationary. Furthermore, mitochondrial starts, stops and redistributions can be regulated by physiological events and intracellular signals (Morris and Hollenbeck, 1993; Chada and Hollenbeck, 2003; Ruthel and Hollenbeck, 2003; Chada and Hollenbeck, 2004; Miller and Sheetz, 2004; Hollenbeck, 1996). Thus, the axonal traffic of mitochondria serves not just to deliver them and then reverse gears and retrieve them but also to continually position and reposition them along the axon.
Indeed, mitochondria, like many other organelles, must be positioned properly to serve the needs of the cell. For example, even small cuboidal epithelial cells such as those in the proximal convoluted tubules of the kidney, despite their modest dimensions, position their mitochondria immediately adjacent to their basolateral membrane ATPases. It is reasonable to expect that the importance of positioning looms much larger in axons, and a large body of data supports this idea. Mitochondria are delivered to and remain in areas of the axon where metabolic demand is high, such as synapses (e.g. Palay, 1956; Treeck and Pirsig, 1979; Gotow et al., 1991; Bogan and Cabot, 1991; Peters et al., 1991), active growth cones and branches (Povlishock, 1976; Morris and Hollenbeck, 1993; Ruthel and Hollenbeck, 2003), nodes of Ranvier (Fabricius et al., 1993), distal initial segments (Li et al., 2004), myelination boundaries (Bristow et al., 2002) and regions of demyelination (Mutsaers and Carroll, 1998) or axonal protein synthesis (Martin et al., 1998). In addition, mitochondria in cultured neurons actively space themselves evenly along undistinguished regions of axon (Miller and Sheetz, 2004). Furthermore, comparative studies show that axonal regions that have higher levels of activity have higher mitochondrial densities and/or activities, as seen in comparisons of tonic and phasic synapses (King et al., 1996; Nguyen et al., 1997; Brodin et al., 1999), and in relatively active tracts of the nervous system (Kageyama and Wong-Riley, 1982; Price, 1985). Finally, when the activity of a growth cone or axonal tract decreases, so does the density of mitochondria (Wong-Riley and Welt, 1980; Marciniak, 1983; Wong-Riley and Carroll, 1984; Carroll and Wong-Riley, 1984; Kageyama and Wong-Riley, 1985; Morris and Hollenbeck, 1993; Nie and Wong-Riley, 1996a; Nie and Wong-Riley, 1996b).
How do mitochondria achieve these distributions in the axon? Here, we survey recent progress in our understanding of how mitochondria move and dock, how their behavior is regulated in axons and how their functions and pattern of transport are related.
How do mitochondria move in the axon?
Mitochondria apparently retain their identity as discrete organelles through a long transit that involves frequent stops and re-starts, some changes in direction and, after some time spent residing in the axon, a return to the cell body, possibly in a senescent state (Fahim et al., 1985). Their movement is, to varying degrees, saltatory and bidirectional, and this sets them apart from small vesicles, endosomes and most other axonally transported organelles (Forman et al., 1987; Morris and Hollenbeck, 1993; Overly et al., 1996; Hollenbeck, 1996; Ligon and Steward, 2000a). Throughout their traffic in the axon, mitochondria can quickly switch between anterograde and retrograde movement, and their net direction has been shown in isolated neurons to result primarily from modulation of the fraction of time spent moving anterogradely (Morris and Hollenbeck, 1993). In addition, they can be shifted between moving and stationary states by changes in axonal growth or intracellular signaling (Morris and Hollenbeck, 1993; Hollenbeck, 1996; Chada and Hollenbeck, 2004). Hence, the machinery on the mitochondrial membrane must include motors, anchoring components and sensors.
Long-distance fast axonal transport of mitochondria requires microtubules (MTs) (Grafstein and Forman, 1980; Hollenbeck, 1996). The simple organization of axonal MTs – parallel polymers that have virtually all of their plus ends directed towards the nerve terminal (Heidemann, 1996; Baas, 2002) – and the patterns of mitochondrial movement implicate motor proteins of the kinesin superfamily in anterograde organelle transport and those of the dynein family in retrograde transport (Hollenbeck, 1996). Mitochondria, in common with other axonally transported organelles, can also move along actin filaments in axons (Kuznetsov et al., 1992; Morris and Hollenbeck, 1995; Ligon and Steward, 2000b; Langford, 2002; Bridgman, 2004). This implicates myosin motors, but the role of this short-range movement is less clear (discussed below). The complex, coordinated movement of mitochondria argues that they have a larger toolkit of proteins for regulated transport than other axonal organelles. A higher-order analysis of the axonal transport and distribution of mitochondria will require that we understand not only what movements mitochondria undergo but also which motor proteins move them, which proteins anchor them, which outer membrane proteins bind the motors and anchors, and how the activities of all of these interacting proteins are regulated.
Different motors for different cargos?
Different classes of organelle show distinct transport behaviors in the axon. One critical question is which machinery for organelle movement – the motor proteins, motor-organelle linkers, static docking proteins, regulatory factors, signaling pathways – is general and which is specific to each class of organelle. Although the diversity of organelles in the axon has been apparent for decades, an accurate description of the remarkable diversity of potential organelle motors is more recent (Miki et al., 2001; Lawrence et al., 2004; Susalka and Pfister, 2000; Asai and Wilkes, 2004; Berg et al., 2001; Thompson and Langford, 2002). This motor diversity has stimulated the hypothesis that movements of different organelles are driven by different motor proteins, perhaps involving different motor-organelle linkers (e.g. Guzik and Goldstein, 2004; Hirokawa and Takemura, 2005). However, many studies of motor proteins in axonal transport have not distinguished motor protein family members, their specific associations with organelles or their influence over specific organelle movements. Thus a major effort currently is to assign specific transport machinery to specific organelle types. In this context, it is important to note that the transport toolkit for any class of organelle may vary in different cell or tissue types, and also between species. In particular, species that have simpler nervous systems may accomplish their necessary mitochondrial transport tasks with simpler machinery.
Kinesin motors
There is abundant evidence, direct and indirect, that plus-end-directed kinesins provide force for anterograde axonal transport (Brady et al., 1990; Saxton et al., 1991; Gho et al., 1992; Amaratunga et al., 1993; Amaratunga et al., 1995; Hurd and Saxton, 1996; Stenoien and Brady, 1997; Gindhart et al., 1998; Martin et al., 1999b; Pilling, 2005). However, the diversity of kinesins in the nervous system presents many choices (Martin et al., 1999b; Hirokawa and Takemura, 2004; Hirokawa and Takemura, 2005; Lawrence et al., 2004). Members of six kinesin families – 1, 2, 3, 4, 13 and 14 – have been implicated in axonal organelle transport, and two of these in mitochondrial movement in particular. The widely expressed, ‘conventional’ kinesins of the kinesin-1 family associate with mitochondria (Jellali et al., 1994; Khodjakov et al., 1998; Leopold et al., 1992) as well as with other axonal organelles, such as vesicles containing ApoER2, JNK scaffolding proteins or amyloid precursor protein (APP) (Kamal et al., 2000; Kamal et al., 2001; Verhey et al., 2001; Matsuda et al., 2003; Inomata et al., 2003; Horiuchi et al., 2005). Furthermore, specific inhibition of kinesin-1 stops most mitochondrial movement in Drosophila melanogaster motor axons (Pilling, 2005) and can substantially shift the distribution of mitochondria in non-neuronal cells towards the cell center (Tanaka et al., 1998; Wu et al., 1998). Thus, it is reasonable to conclude that kinesin-1 is a motor for anterograde mitochondrial transport in the axon as well as for other organelles. A member of the kinesin-3 family (KIF1Bβ) has been more specifically associated with mitochondrial transport in mice. It is enriched in neurons, where it colocalizes and physically associates with mitochondria. It also supports the movement of mitochondria along MTs in an in vitro assay at rates similar to those of mitochondria in the axon (Nangaku et al., 1994). Other members of the kinesin-3 family probably transport synaptic vesicles but not mitochondria (Hall and Hedgecock, 1991; Okada et al., 1995; Yonekawa et al., 1998; Zhao et al., 2001).
Dynein motors
A variety of past studies have implicated dyneins non-specifically in retrograde axonal transport (e.g. Goldberg, 1982; Ekstrom and Kanje, 1984; Forman et al., 1983; Schnapp and Reese, 1989; Wang et al., 1995). The only alternative appears to be the minus-end-directed kinesin-14 motors. One of them, KIFC2, has been reported to be present in mammalian axons, but gene disruption caused no detectable defects in mice (Hanlon et al., 1997; Yang et al., 2001). The low velocities of kinesin 14 proteins also argue against major contributions to fast retrograde mitochondrial transport. Thus, cytoplasmic dynein seems likely to be the primary retrograde motor.
There are only two or three non-axonemal cytoplasmic dynein heavy chains in vertebrates and just one each in Drosophila and Caenorhabditis elegans (Asai and Wilkes, 2004; Vaisberg et al., 1996). However, this rather spartan kit for heavy chains is augmented by a variety of accessory (non-motor) proteins (Susalka and Pfister, 2000) that may provide great diversity, both for linkage with different cargo types and for regulation. Dynein heavy chain and accessory proteins have been shown to associate with mitochondria and many other organelle types (Haberman et al., 2001). Inhibition of dynein proteins can alter many cellular processes, including the movement and distribution of axonal mitochondria (Bowman et al., 1999; Koushika et al., 2004; LaMonte et al., 2002; Martin et al., 1999a; Waterman-Storer et al., 1997). Moreover, in Drosophila nervous systems, mutations in the gene that encodes cytoplasmic dynein heavy chain have strong effects on specific mitochondrial transport parameters in axons, including retrograde run velocity, run length, and duty cycle (Pilling, 2005).
One particularly interesting aspect of dynein-driven retrograde axonal transport is that it is dependent on kinesin-1. It was first reported that monovalent antibodies specific for kinesin-1, when perfused into squid axoplasm, halt organelle transport in both directions (Brady et al., 1990). Later, genetic interaction tests in Drosophila indicated a functional interdependence of kinesin 1 and dynein (Martin et al., 1999b). Most recently, time-lapse imaging of GFP-tagged mitochondria in Drosophila axons has shown that kinesin-1 mutations cause a profound reduction in the retrograde transport of mitochondria (Pilling, 2005). Although a complex containing both cytoplasmic dynein and kinesin-1 heavy chains has not been isolated (e.g. Martin et al., 1999b), the intermediate chain of cytoplasmic dynein has been shown to bind to the light chains of kinesin-1 (Ligon et al., 2004). Thus, there may be a direct physical or regulatory linkage between the two motors that is critical for controlling the transport and distribution of axonal mitochondria.
Myosin motors
Robust, bidirectional axonal transport of mitochondria along actin filaments (Morris and Hollenbeck, 1995) indicates that they, like other organelles (Kuznetsov et al., 1992; Kuznetsov et al., 1994), use myosin motors. The best candidates for these in neurons are members of the myosin I, II, V and VI families (Berg et al., 2001; Bridgman, 2004). Although it has not been reported to be associated with mitochondria, the monomeric motor myosin I has been implicated in organelle transport (Barylko et al., 2000). Myosin I is present in neurons, in which it has a punctate and cortical distribution (Wagner et al., 1992; Bahler et al., 1994; Lewis and Bridgman, 1996). In axons much of it is distributed in the cortex, where it may drive vesicle–plasma-membrane fusion or plasma-membrane–actin interactions (Lewis and Bridgman, 1996), perhaps during slow axonal transport (Lund et al., 2005). Myosin II, a dimeric motor, has also been reported to associate with vesicular organelles in axoplasm (DeGiorgis et al., 2002), but its role in axonal transport is unclear.
Another dimeric motor, myosin V, is associated with a variety of organelles in neurons (Prekeris and Terrian, 1997; Evans et al., 1998; Tabb et al., 1998; Miller and Sheetz, 2000) and moves with small vesicles (Bridgman, 1999). Although it has not been reported to associate with mitochondria, myosin V supports movement in vitro at rates similar to axonal transport of mitochondria on actin filaments (Morris and Hollenbeck, 1995) and slower than axonal transport of vesicles (Cheney et al., 1993; Wolenski et al., 1995; Evans et al., 1998). In addition, disruption of myosin V activity alters the distribution and transport behavior of axonal vesicles in vivo (Bridgman, 1999) and in vitro (Brown et al., 2004), the effect being similar to that of elimination of actin filaments from the axon (Morris and Hollenbeck, 1995). Thus, myosin V is a good candidate for the modulatory, actin-based motor underlying certain aspects of mitochondrial transport in the axon (Hollenbeck, 1996).
Gross et al. have shown that myosin V can regulate organelle transport by MT motors (Gross et al., 2002). Pigment granules (melanosomes) in Xenopus leavis melanophores carry myosin V, dynein and kinesin-2 (Nascimento et al., 2003). High-resolution organelle tracking and disruption-of-function approaches suggest that kinesin-2 drives granules away from the cell center and that dynein drives them toward the cell center. The two motors alternate rather than competing in a tug-of-war. However, myosin V can influence the net direction of transport by competing with and shortening dynein-driven minus-end runs. Although this might be peculiar to melanocytes, myosin V could serve a similar direction-modulating function in axons. Direct tests of myosin V in axons will be required to address this possibility.
Myosin VI, which drives movement toward the opposite (minus) end of the actin filament from other myosins, is also present in axons (Suter et al., 2000). Although it has a punctate distribution characteristic of vesicular organelles rather than of mitochondria, its potential to produce minus-end-directed movement is attractive because it would explain the bidirectional movement of mitochondria on actin filaments (Morris and Hollenbeck, 1995). Alternatively, a single plus-end-directed myosin could operate if actin filaments in the axon have mixed polarity. It is notable that vesicles from squid axoplasm undergo in vitro movement only toward the plus ends of defined actin arrays (Langford et al., 1994).
Given the characteristics of mitochondrial movement in axons and what we know about movement of other organelles, it seems likely that kinesin(s), cytoplasmic dynein and myosin(s) cooperate, perhaps through direct interactions, to give rise to the complex movement of mitochondria. Any mechanism proposed must account for smooth transitions between MT- and actin-based movement (Langford, 2002), for the short-range movement of mitochondria into regions that have few or no MTs (Bridgman, 1999) and, most importantly, for regulation of mitochondrial position by both the modulation of MT-based long-range transport and of mitochondrion-specific anchorage systems (Hollenbeck, 1996).
Linkers and anchors
Motor receptors
Organelle surface receptors for motor proteins are thought to target the correct motility machinery to the correct organelle type (Guzik and Goldstein, 2004; Hirokawa and Takemura, 2005). Several potential motor protein receptors and adaptors have been identified for axonal vesicles, including the membrane or membrane-associated proteins APP (Kamal et al., 2000; Kamal et al., 2001; Gunawardena and Goldstein, 2001) (see Lazarov et al., 2005), ApoER2 (Verhey et al., 2001), fodrin (Takeda et al., 2000), and two different types of JNK-interacting scaffolding protein (JIP) (Bowman et al., 2000; Verhey et al., 2001; Inomata et al., 2003; Matsuda et al., 2003, Horiuchi et al., 2005). Although no evidence links any of these to mitochondria, recent studies in Drosophila have revealed a mitochondrion-specific motor protein linker that is needed for movement of mitochondria into the axon. This protein, Milton, was identified in a screen for genes required for synaptic transmission in photoreceptors. Milton mutants form normal synapses, but their axons and terminals are devoid of mitochondria (Stowers et al., 2002; Gorska-Andrzejak et al., 2003). Milton colocalizes and co-purifies with mitochondria but not other organelles. It interacts indirectly with kinesin-1 and is probably not an outer membrane protein. Thus, it is likely to be part of a larger complex that anchors kinesin-1 to the surface of mitochondria, allowing their axonal transport and delivery to the presynaptic region (Stowers et al., 2002).
Some motors bind to axonal organelles by direct interaction with specific membrane lipids. Unc104, a kinesin-3 motor, binds through its pleckstrin homology (PH) domain to phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)_P_2] in the membranes of vesicles, and this interaction is essential for their anterograde axonal transport in C. elegans (Klopfenstein et al., 2002; Klopfenstein et al., 2004). A similar mechanism might operate on mitochondria. In addition, there is evidence that the chaperone protein Hsc70 can catalyze ATP-dependent release of kinesin-1 from organelle surfaces (Tsai et al., 2000). This raises the possibility that a regulated balance of motor binding and release contributes to the pattern of mitochondrial movement in the axon.
‘Static’ anchors
As discussed above, a distinguishing feature of mitochondria in the axon is that once delivered to a region by motor proteins, they often reside there for an extended period. This indicates that there might be an apparatus for specific mitochondrial docking. Although most of the cross-bridges observed between mitochondria and the axonal cytoskeleton in vivo (Smith et al., 1977; Hirokawa, 1982; Benshalom and Reese, 1985; Pannese et al., 1986; Hirokawa and Yorifuji, 1986; Price et al., 1991) now seem to be dynamic (perhaps motor proteins themselves), others are likely to represent more static links. Indeed, the specialized presynaptic region of the calyx of Held contains a mitochondrion-associated adherens complex, a clearly observable physical substrate for holding and organizing the mitochondria (Rowland et al., 2000). Other regions in which mitochondria reside show less obvious morphological links, but biochemical studies have suggested possibilities. Isolated mitochondria bind MT-associated proteins and show apparent links to both MTs and neurofilaments in vitro (Linden et al., 1989a; Linden et al., 1989b; Jung et al., 1993; Leterrier et al., 1994a; Leterrier et al., 1994b), and they also show direct interactions in vitro with the high molecular weight neurofilament (NF) subunit (Wagner et al., 2003). However, in vivo data favor the actin cytoskeleton as the docking substrate. It has long been clear that some of the regions in which mitochondria accumulate, such as presynaptic zones and growth cones, are actin-rich and MT- and NF-poor (Peters et al., 1991). Disruption of actin filaments in cultured neurons increases the velocity and maximum excursion length of mitochondrial movement (Morris and Hollenbeck, 1995). Finally, as discussed below, the ability of local nerve growth factor (NGF)/phosphoinositide 3-kinase (PI3K) signaling to halt mitochondrial movement depends on the presence of actin filaments (Chada and Hollenbeck, 2004). Whether mitochondrial docking on actin filaments requires a myosin motor or another actin-binding protein(s) is unclear.
Cellular signals that control mitochondrial traffic
Although the molecular parts list for mitochondrial axonal transport remains incomplete, a number of recent studies have begun to elucidate a higher-order question: what intracellular signals modulate the motor, linker, and anchoring proteins? There is evidence for several signals that affect most or all axonal organelle traffic; although these cannot explain the unique transport behavior of mitochondria, they may nonetheless play a role in controlling their movement. First, biochemical and in vitro motility studies in axoplasm indicate that total anterograde organelle traffic in axoplasm can be reduced by inhibition of cyclin-dependent kinase 5 (CDK5) and by activation of glycogen synthase kinase 3 (GSK3) (Ratner et al., 1998; Morfini et al., 2002; Morfini et al., 2004). CDK5 activity is thought to induce the activity of GSK3, which in turn phosphorylates KLC, causing the release of kinesin-1 from the organelle surface. Recent biochemical and genetic work in Drosophila indicates that Ena/VASP, controlled by Abl tyrosine kinase, can bind kinesin-1 heavy chain and can reduce its axonal transport activity, raising the possibility of control by signaling pathways that use Abl (Martin et al., 2005). Kinesin-based mitochondrial movement in non-neuronal cells and in vitro motility assays can also be regulated specifically by a cytokine-stimulated pathway that alters the activity of kinesin while it remains on the mitochondrion surface (De Vos et al., 1998; De Vos et al., 2000). In addition, axonal organelle transport can be blocked by inhibition of the GTPase cycle of monomeric G proteins (Bloom et al., 1993). This finding is of interest because of recent evidence that the GTPase Miro is involved in transport of mitochondria to the synapse (Gou et al., 2005). The inhibition of protein kinase A activity has also been reported to cause a general inhibition of anterograde vesicle transport but has no effect on mitochondrial transport (Okada et al., 1995a).
Mitochondrion-specific regulation of axonal transport has also been reported. Several perturbations of the neuron can halt mitochondrial movement throughout the axon, including treatment with drugs that uncouple the proton gradient across the inner membrane and that inhibit ATP synthase (but not by inhibiting electron transport at complex III) (Rintoul et al., 2003; Miller and Sheetz, 2004). In addition, two neurotoxic treatments can inhibit mitochondrial movement in cultured neurons. Glutamate treatment, by triggering Ca2+ influx through the NMDA receptor, decreases mitochondrial movement as well as size (Rintoul et al., 2003), and elevation of intracellular [Zn2+] halts mitochondrial movement at concentrations that do not affect transmembrane potential (Malaiyanda et al., 2005). Both of these ionic effects are mediated through additional cytoplasmic effectors; the Zn2+ effect in particular depends upon PI3K activity (Malaiyanda et al., 2005). Although global downregulation of mitochondrial transport is potentially important, local inhibition of movement is more directly relevant for explaining mitochondrial localization. As reviewed above, the non-uniform distribution of mitochondria indicates that they halt in specific regions of the axon, can space out evenly along the axon shaft (Miller and Sheetz, 2004) and also halt near active growth cones. Significantly, this localization ability is diminished by anaerobic conditions (Morris and Hollenbeck, 1993).
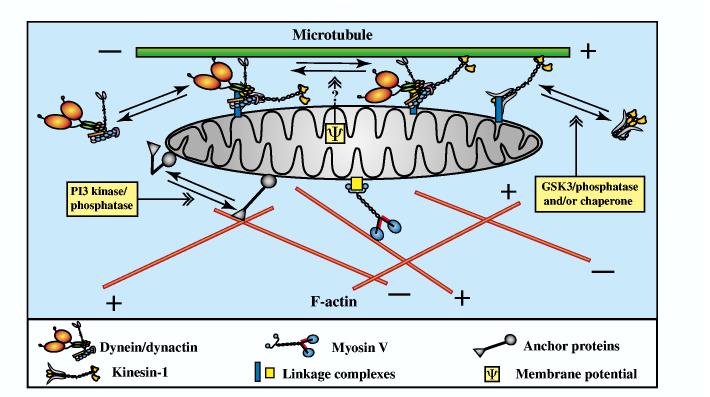
Recent work has implicated specific signaling pathways in mitochondrial docking. Focal stimulation of axon shafts with NGF causes a local accumulation of mitochondria that relies on PI3K activity and the integrity of the actin cytoskeleton. This suggests that the mitochondria are docked to actin (Chada and Hollenbeck, 2003; Chada and Hollenbeck, 2004). In addition, the effect involves both preferential entry of mitochondria into the region and their subsequent halting there. Preferential entry into and escape from a region of NGF stimulation both show directional specificity (Chada and Hollenbeck, 2004). Data from neuroblastoma cells have also implicated PI-dependent signaling in the control of mitochondrial direction: the expression of PtdIns(4,5)_P_2− specific PH domains increases anterograde mitochondrial movement while decreasing retrograde movement, without altering other aspects of mitochondrial motility (De Vos et al., 2003). As discussed above, many different kinds of subcellular positioning of mitochondria occur in axons, and each must rely on regulated motility, direction and docking. Thus, mitochondrial localization in neurons probably relies on more than one signaling pathway and responds to more than one physiological stimulus (see Fig. 1).
Fig. 1.
A summary framework for considering mechanisms that contribute to the transport and localization of mitochondria in axons. All three motor-protein families are likely to participate in moving axonal mitochondria. Kinesins and dynein, perhaps bound in the same motor complex or perhaps independently associated with mitochondria, drive long-range anterograde and retrograde transport. Mechanisms that coordinate those opposing motors to produce net transport in one direction are largely unknown, although the dynactin complex could be an important factor. Myosins drive short-range movements along F-actin, they may modulate long-range transport by pulling mitochondria away from microtubules, and they might facilitate anchorage of mitochondria to F-actin by unknown actin-mitochondrion crosslinkers. Possible control mechanisms that could regulate transport and docking are numerous and remain largely speculative. However, it is relatively certain that mitochondrial motors and anchors are controlled by phosphorylation/dephosphorylation and perhaps by other regulatory schemes. The regulatory pathways may include, but are certainly not limited to, CDK5/GSK3, NGF/PI3K, Abl/Ena/VASP, mitochondrial inner membrane potential and the levels of Ca2+ and Zn2+.
Conclusion and perspectives
It is still early days in the elucidation of the molecular machinery that drives and regulates the axonal transport of mitochondria, or any other organelle. Given the power of available genetic techniques, we can expect the full array of mitochondrial motor, linker and docking proteins to be identified in the near future. This should point to possible targets for regulatory events such as kinase and phosphatase action, and bring us closer to understanding how cell signaling guides mitochondrial movement, and how mitochondrial location and function are related. But a full understanding of the transport of mitochondria in neurons will require that we consider a larger issue: the life cycle of mitochondria in a very large and long-lived cell. For instance, the movement of mitochondria into regions of the axon where they are needed might be supplemented by an increase in their activity once they arrive. But what of mitochondrial biogenesis – are distant regions of the axon also supported by local mitochondrial replication? Data from PC12 cells suggests that most if not all mitochondrial biogenesis occurs close to the cell body (Davis and Clayton, 1996). However, in trying to understand the biology of the axon we have been fooled before by negative evidence. This is perhaps best exemplified by recent work demonstrating that neuronal protein synthesis, long thought to be restricted to the somatodendritic compartment, has an essential component that occurs in the distal axon (Piper and Holt, 2004). Recent studies show that Drosophila mutants lacking the dynamin-related protein involved in mitochondrial fission fail to populate the distal axon with mitochondria (Verstreken et al., 2005), reminding us that the relationship between transport, location and biogenesis bears closer study.
References
- Amaratunga A, Morin PJ, Kosik KS, Fine RE. Inhibition of kinesin synthesis and rapid anterograde axonal transport in vivo by an antisense oligonucleotide. J. Biol. Chem. 1993;268:17427–17430. [PubMed] [Google Scholar]
- Amaratunga A, Leeman SE, Kosik KS, Fine RE. Inhibition of kinesin synthesis in vivo inhibits the rapid transport of representative proteins for three transport vesicle classes into the axon. J. Neurochem. 1995;64:2374–2376. doi: 10.1046/j.1471-4159.1995.64052374.x. [DOI] [PubMed] [Google Scholar]
- Asai DJ, Wilkes DE. The dynein heavy chain family. J. Eukaryot. Microbiol. 2004;51:23–29. doi: 10.1111/j.1550-7408.2004.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Baas PW. Microtubule transport in the axon. Int. Rev. Cytol. 2002;212:41–62. doi: 10.1016/s0074-7696(01)12003-6. [DOI] [PubMed] [Google Scholar]
- Bahler M, Kroschewski R, Stoffler HE, Behrmann T. Rat myr 4 defines a novel subclass of myosin I: identification, distribution, localization, and mapping of calmodulin-binding sites with differential calcium sensitivity. J. Cell Biol. 1994;126:375–389. doi: 10.1083/jcb.126.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barylko B, Binns DD, Albanesi JP. Regulation of the enzymatic and motor activities of myosin I. Biochim. Biophys. Acta. 2000;1496:23–35. doi: 10.1016/s0167-4889(00)00006-9. [DOI] [PubMed] [Google Scholar]
- Benshalom G, Reese TS. Ultrastructural observations on the cytoarchitecture of axons processed by rapid-freezing and freeze-substitution. J. Neurocytol. 1985;14:943–960. doi: 10.1007/BF01224806. [DOI] [PubMed] [Google Scholar]
- Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol. Biol. Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom GS, Richards BW, Leopold PL, Ritchey DM, Brady ST. GTP gamma S inhibits organelle transport along axonal microtubules. J. Cell Biol. 1993;120:467–476. doi: 10.1083/jcb.120.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan N, Cabot JB. Light and electron microscopic analyses of intraspinal axon collaterals of sympathetic preganglionic neurons. Brain Res. 1991;541:241–251. doi: 10.1016/0006-8993(91)91024-u. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Patel-King RS, Benashski SE, McCaffery JM, Goldstein LS, King SM. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J. Cell Biol. 1999;146:165–180. [PMC free article] [PubMed] [Google Scholar]
- Bowman AB, Kamal A, Ritchings BW, Philp AV, McGrail M, Gindhart JG, Goldstein LS. Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell. 2000;103:583–594. doi: 10.1016/s0092-8674(00)00162-8. [DOI] [PubMed] [Google Scholar]
- Brady ST, Pfister KK, Bloom GS. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc. Natl. Acad. Sci. USA. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J. Cell Biol. 1999;146:1045–1060. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC. Myosin-dependent transport in neurons. J. Neurobiol. 2004;58:164–174. doi: 10.1002/neu.10320. [DOI] [PubMed] [Google Scholar]
- Bristow EA, Griffiths PG, Andrews RM, Johnson MA, Turnbull DM. The distribution of mitochondrial activity in relation to optic nerve structure. Arch. Ophthalmol. 2002;120:791–796. doi: 10.1001/archopht.120.6.791. [DOI] [PubMed] [Google Scholar]
- Brodin L, Bakeeva L, Shupliakov O. Presynaptic mitochondria and the temporal pattern of neurotransmitter release. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1999;354:365–372. doi: 10.1098/rstb.1999.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR, Stafford P, Langford GM. Short-range axonal/dendritic transport by myosin-V: A model for vesicle delivery to the synapse. J. Neurobiol. 2004;58:175–188. doi: 10.1002/neu.10317. [DOI] [PubMed] [Google Scholar]
- Carroll EW, Wong-Riley MT. Quantitative light and electron microscopic analysis of cytochrome oxidase-rich zones in the striate cortex of the squirrel monkey. J. Comp. Neurol. 1984;222:1–17. doi: 10.1002/cne.902220102. [DOI] [PubMed] [Google Scholar]
- Chada SR, Hollenbeck PJ. Mitochondrial movement and positioning in axons: the role of growth factor signaling. J. Exp. Biol. 2003;206:1985–1992. doi: 10.1242/jeb.00263. [DOI] [PubMed] [Google Scholar]
- Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr. Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Cheney RE, O'Shea MK, Heuser JE, Coelho MV, Wolenski JS, Espreafico EM, Forscher P, Larson RE, Mooseker MS. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Davis AF, Clayton DA. In situ localization of mitochondrial DNA replication in intact mammalian cells. J Cell. Biol. 1996;135:883–893. doi: 10.1083/jcb.135.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgis JA, Reese TS, Bearer EL. Association of a nonmuscle myosin II with axoplasmic organelles. Mol. Biol. Cell. 2002;13:1046–1057. doi: 10.1091/mbc.01-06-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos K, Goossens V, Boone E, Vercammen D, Vancompernolle K, Vandenabeele P, Haegeman G, Fiers W, Grooten J. The 55-kDa tumor necrosis factor receptor induces clustering of mitochondria through its membrane-proximal region. J. Biol. Chem. 1998;273:9673–9680. doi: 10.1074/jbc.273.16.9673. [DOI] [PubMed] [Google Scholar]
- De Vos K, Severin F, Van Herreweghe F, Vancompernolle K, Goossens V, Hyman A, Grooten J. Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. J. Cell Biol. 2000;149:1207–1214. doi: 10.1083/jcb.149.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom P, Kanje M. Inhibition of fast axonal transport by erythro-9-[3-(2-hydroxynonyl)]adenine. J. Neurochem. 1984;43:1342–1345. doi: 10.1111/j.1471-4159.1984.tb05392.x. [DOI] [PubMed] [Google Scholar]
- Evans LL, Lee AJ, Bridgman PC, Mooseker MS. Vesicle-associated brain myosin-V can be activated to catalyze actin-based transport. J. Cell Sci. 1998;111:2055–2066. doi: 10.1242/jcs.111.14.2055. [DOI] [PubMed] [Google Scholar]
- Fabricius C, Berthold CH, Rydmark M. Axoplasmic organelles at nodes of Ranvier. II. Occurrence and distribution in large myelinated spinal cord axons of the adult cat. J. Neurocytol. 1993;22:941–954. doi: 10.1007/BF01218352. [DOI] [PubMed] [Google Scholar]
- Fahim MA, Lasek RJ, Brady ST, Hodge AJ. AVEC-DIC and electron microscopic analyses of axonally transported particles in cold-blocked squid giant axons. J. Neurocytol. 1985;14:689–704. doi: 10.1007/BF01170822. [DOI] [PubMed] [Google Scholar]
- Forman DS, Brown KJ, Promersberger ME. Selective inhibition of retrograde axonal transport by erythro-9-[3-(2-hydroxynonyl)]adenine. Brain. Res. 1983;272:194–197. doi: 10.1016/0006-8993(83)90381-5. [DOI] [PubMed] [Google Scholar]
- Forman DS, Lynch KJ, Smith RS. Organelle dynamics in lobster axons: anterograde, retrograde and stationary mitochondria. Brain. Res. 1987;412:96–106. doi: 10.1016/0006-8993(87)91443-0. [DOI] [PubMed] [Google Scholar]
- Gho M, McDonald K, Ganetzky B, Saxton WM. Effects of kinesin mutations on neuronal functions. Science. 1992;258:313–316. doi: 10.1126/science.1384131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart JG, Jr, Desai CJ, Beushausen S, Zinn K, Goldstein LS. Kinesin light chains are essential for axonal transport in Drosophila. J. Cell Biol. 1998;141:443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DJ. Microinjection into an identified axon to study the mechanism of fast axonal transport. Proc. Natl. Acad. Sci. USA. 1982;79:4818–4822. doi: 10.1073/pnas.79.15.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorska-Andrzejak J, Stowers RS, Borycz J, Kostyleva R, Schwarz TL, Meinertzhagen IA. Mitochondria are redistributed in Drosophila photoreceptors lacking milton, a kinesin-associated protein. J. Comp. Neurol. 2003;463:372–388. doi: 10.1002/cne.10750. [DOI] [PubMed] [Google Scholar]
- Gotow T, Miyaguchi K, Hashimoto PH. Cytoplasmic architecture of the axon terminal: filamentous strands specifically associated with synaptic vesicles. Neuroscience. 1991;40:587–598. doi: 10.1016/0306-4522(91)90143-c. [DOI] [PubMed] [Google Scholar]
- Grafstein B, Forman DS. Intracellular transport in neurons. Physiol. Rev. 1980;60:1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/s0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Guzik BW, Goldstein LS. Microtubule-dependent transport in neurons: steps towards an understanding of regulation, function and dysfunction. Curr. Opin. Cell Biol. 2004;16:443–450. doi: 10.1016/j.ceb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Habermann A, Schroer TA, Griffiths G, Burkhardt JK. Immunolocalization of cytoplasmic dynein and dynactin subunits in cultured macrophages: enrichment on early endocytic organelles. J. Cell Sci. 2001;114:229–240. doi: 10.1242/jcs.114.1.229. [DOI] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hanlon DW, Yang Z, Goldstein LS. Characterization of KIFC2, a neuronal kinesin superfamily member in mouse. Neuron. 1997;18:439–451. doi: 10.1016/s0896-6273(00)81244-1. [DOI] [PubMed] [Google Scholar]
- Heales SJ, Bolanos JP, Stewart VC, Brookes PS, Land JM, Clark JB. Nitric oxide, mitochondria and neurological disease. Biochim. Biophys. Acta. 1999;1410:215–228. doi: 10.1016/s0005-2728(98)00168-6. [DOI] [PubMed] [Google Scholar]
- Heidemann SR. Cytoplasmic mechanisms of axonal and dendritic growth in neurons. Int. Rev. Cytol. 1996;165:235–296. doi: 10.1016/s0074-7696(08)62224-x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J. Cell Biol. 1982;94:129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Yorifuji H. Cytoskeletal architecture of reactivated crayfish axons, with special reference to crossbridges among microtubules and between microtubules and membrane organelles. Cell Motil. Cytoskeleton. 1986;6:458–468. [Google Scholar]
- Hirokawa N, Takemura R. Kinesin superfamily proteins and their various functions and dynamics. Exp. Cell Res. 2004;301:50–59. doi: 10.1016/j.yexcr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front. Biosci. 1996;1:91–102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- Horiuchi D, Barkus RV, Pilling AD, Gassman A, Saxton WM. APLIP1, a kinesin-binding JNK scaffold protein, influences bidirectional transport of vesicles and retrograde transport of mitochondria in. Drosophila axons. Curr. Biol. 2005 doi: 10.1016/j.cub.2005.10.047. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata H, Nakamura Y, Hayakawa A, Takata H, Suzuki T, Miyazawa K, Kitamura N. A scaffold protein JIP-1b enhances amyloid precursor protein phosphorylation by JNK and its association with kinesin light chain 1. J. Biol. Chem. 2003;278:22946–22955. doi: 10.1074/jbc.M212160200. [DOI] [PubMed] [Google Scholar]
- Jellali A, Metz-Boutigue MH, Surgucheva I, Jancsik V, Schwartz C, Filliol D, Gelfand VI, Rendon A. Structural and biochemical properties of kinesin heavy chain associated with rat brain mitochondria. Cell Motil. Cytoskeleton. 1994;28:79–93. doi: 10.1002/cm.970280108. [DOI] [PubMed] [Google Scholar]
- Jung D, Filliol D, Miehe M, Rendon A. Interaction of brain mitochondria with microtubules reconstituted from brain tubulin and MAP2 or TAU. Cell Motil. Cytoskeleton. 1993;24:245–255. doi: 10.1002/cm.970240405. [DOI] [PubMed] [Google Scholar]
- Kageyama GH, Wong-Riley M. An analysis of the cellular localization of cytochrome oxidase in the lateral geniculate nucleus of the adult cat. J. Comp. Neurol. 1985;242:338–357. doi: 10.1002/cne.902420304. [DOI] [PubMed] [Google Scholar]
- Kageyama GH, Wong-Riley MT. Histochemical localization of cytochrome oxidase in the hippocampus: correlation with specific neuronal types and afferent pathways. Neuroscience. 1982;7:2337–2361. doi: 10.1016/0306-4522(82)90199-3. [DOI] [PubMed] [Google Scholar]
- Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Lizunova EM, Minin AA, Koonce MP, Gyoeva FK. A specific light chain of kinesin associates with mitochondria in cultured cells. Mol. Biol. Cell. 1998;9:333–343. doi: 10.1091/mbc.9.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MJ, Atwood HL, Govind CK. Structural features of crayfish phasic and tonic neuromuscular terminals. J. Comp. Neurol. 1996;372:618–626. doi: 10.1002/(SICI)1096-9861(19960902)372:4<618::AID-CNE9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Klopfenstein DR, Vale RD. The lipid binding pleckstrin homology domain in UNC-104 kinesin is necessary for synaptic vesicle transport in Caenorhabditis elegans. Mol. Biol. Cell. 2004;15:3729–3739. doi: 10.1091/mbc.E04-04-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Tomishige M, Stuurman N, Vale RD. Role of phosphatidylinositol(4,5)bisphosphate organization in membrane transport by the Unc104 kinesin motor. Cell. 2002;109:347–358. doi: 10.1016/s0092-8674(02)00708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosel S, Hofhaus G, Maassen A, Vieregge P, Graeber MB. Role of mitochondria in Parkinson disease. Biol. Chem. 1999;380:865–870. doi: 10.1515/BC.1999.106. [DOI] [PubMed] [Google Scholar]
- Koushika SP, Schaefer AM, Vincent R, Willis JH, Bowerman B, Nonet ML. Mutations in Caenorhabditis. elegans cytoplasmic dynein components reveal specificity of neuronal retrograde cargo. J. Neurosci. 2004;24:3907–3916. doi: 10.1523/JNEUROSCI.5039-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger C, Duchen MR. Mitochondria, Ca2+ and neurodegenerative disease. Eur. J. Pharmacol. 2002;447:177–188. doi: 10.1016/s0014-2999(02)01842-3. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Langford GM, Weiss DG. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Rivera DT, Severin FF, Weiss DG, Langford GM. Movement of axoplasmic organelles on actin filaments from skeletal muscle. Cell Motil. Cytoskeleton. 1994;28:231–242. doi: 10.1002/cm.970280306. [DOI] [PubMed] [Google Scholar]
- LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascano J, Tokito M, Van Winkle T, Howland DS, Holzbaur EL. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- Langford GM. Myosin-V, a versatile motor for short-range vesicle transport. Traffic. 2002;3:859–865. doi: 10.1034/j.1600-0854.2002.31202.x. [DOI] [PubMed] [Google Scholar]
- Langford GM, Kuznetsov SA, Johnson D, Cohen DL, Weiss DG. Movement of axoplasmic organelles on actin filaments assembled on acrosomal processes: evidence for a barbed-end-directed organelle motor. J. Cell Sci. 1994;107:2291–2298. doi: 10.1242/jcs.107.8.2291. [DOI] [PubMed] [Google Scholar]
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, et al. A standardized kinesin nomenclature. J. Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Morfini GA, Lee EB, Farah MH, Szodorai A, DeBoer SR, Koliatsos VE, Kins S, Lee VM, Wong PC, et al. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J. Neurosci. 2005;25:2386–2395. doi: 10.1523/JNEUROSCI.3089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold PL, McDowall AW, Pfister KK, Bloom GS, Brady ST. Association of kinesin with characterized membrane-bounded organelles. Cell Motil. Cytoskeleton. 1992;23:19–33. doi: 10.1002/cm.970230104. [DOI] [PubMed] [Google Scholar]
- Leterrier JF, Rusakov DA, Linden M. Statistical analysis of the surface distribution of microtubule-associated proteins (MAPs) bound in vitro to rat brain mitochondria and labelled by 10 nm gold-coupled antibodies. Bull. Assoc. Anat. (Nancy) 1994a;78:47–51. [PubMed] [Google Scholar]
- Leterrier JF, Rusakov DA, Nelson BD, Linden M. Interactions between brain mitochondria and cytoskeleton: evidence for specialized outer membrane domains involved in the association of cytoskeleton-associated proteins to mitochondria in situ and in vitro. Microsc. Res. Tech. 1994b;27:233–261. doi: 10.1002/jemt.1070270305. [DOI] [PubMed] [Google Scholar]
- Lewis AK, Bridgman PC. Mammalian myosin I alpha is concentrated near the plasma membrane in nerve growth cones. Cell Motil. Cytoskeleton. 1996;33:130–150. doi: 10.1002/(SICI)1097-0169(1996)33:2<130::AID-CM5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Li YC, Zhai XY, Ohsato K, Futamata H, Shimada O, Atsumi S. Mitochondrial accumulation in the distal part of the initial segment of chicken spinal motoneurons. Brain. Res. 2004;1026:235–243. doi: 10.1016/j.brainres.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J. Comp. Neurol. 2000a;427:340–350. doi: 10.1002/1096-9861(20001120)427:3<340::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J. Comp. Neurol. 2000b;427:351–361. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Tokito M, Finkelstein JM, Grossman FE, Holzbaur EL. A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J. Biol. Chem. 2004;279:19201–19208. doi: 10.1074/jbc.M313472200. [DOI] [PubMed] [Google Scholar]
- Linden M, Nelson BD, Leterrier JF. The specific binding of the microtubule-associated protein 2 (MAP2) to the outer membrane of rat brain mitochondria. Biochem. J. 1989a;261:167–173. doi: 10.1042/bj2610167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden M, Nelson BD, Loncar D, Leterrier JF. Studies on the interaction between mitochondria and the cytoskeleton. J. Bioenerg. Biomembr. 1989b;21:507–518. doi: 10.1007/BF00762522. [DOI] [PubMed] [Google Scholar]
- Lund LM, Machado VM, McQuarrie IG. Axonal isoforms of myosin-I. Biochem. Biophys. Res. Commun. 2005;330:857–864. doi: 10.1016/j.bbrc.2005.02.187. [DOI] [PubMed] [Google Scholar]
- Malaiyandi L, Honick A, Rintoul G, Wang Q, Reynolds I. ZN2+ inhibits mitochondrial movement in neurons by PI-3 kinase activation. J. Neurosci. 2005;25:9507–9514. doi: 10.1523/JNEUROSCI.0868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik R, Gross SP. Molecular motors: strategies to get along. Curr. Biol. 2004;14:971–982. doi: 10.1016/j.cub.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Marciniak M. Morphometric ultrastructural evaluation of the axonal endings in the neuromuscular junctions of pigeons after long lasting limitation of movement. Exp. Pathol. 1983;23:27–34. doi: 10.1016/s0232-1513(83)80038-3. [DOI] [PubMed] [Google Scholar]
- Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Jr, Hays TS, Saxton WM. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell. 1999a;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MA, Hurd DD, Saxton WM. Kinesins in the nervous system. Cell Mol. Life Sci. 1999b;56:200–216. doi: 10.1007/s000180050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MA, Ahern-Djamali SM, Hoffmann FM, Saxton WM. Abl tyrosine kinase and its substrate Ena/VASP have functional interactions with kinesin-1. Mol. Biol. Cell. 2005;16:4225–4230. doi: 10.1091/mbc.E05-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Vaida B, Bleher R, Crispino M, Giuditta A. Protein synthesizing units in presynaptic and postsynaptic domains of squid neurons. J. Cell Sci. 1998;111:3157–3166. doi: 10.1242/jcs.111.21.3157. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Matsuda Y, D'Adamio L. Amyloid beta protein precursor (AbetaPP), but not AbetaPP-like protein 2, is bridged to the kinesin light chain by the scaffold protein JNK-interacting protein 1. J. Biol. Chem. 2003;278:38601–38606. doi: 10.1074/jbc.M304379200. [DOI] [PubMed] [Google Scholar]
- Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc. Natl. Acad. Sci. USA. 2001;98:7004–7011. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Characterization of myosin V binding to brain vesicles. J. Biol. Chem. 2000;275:2598–2606. doi: 10.1074/jbc.275.4.2598. [DOI] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, Beffert U, Brady ST. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J. Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J. Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaers SE, Carroll WM. Focal accumulation of intraaxonal mitochondria in demyelination of the cat optic nerve. Acta. Neuropathol. (Berl.) 1998;96:139–143. doi: 10.1007/s004010050873. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nascimento AA, Roland JT, Gelfand VI. Pigment cells: a model for the study of organelle transport. Annu. Rev. Cell Dev. Biol. 2003;19:469–491. doi: 10.1146/annurev.cellbio.19.111401.092937. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Marin L, Atwood HL. Synaptic physiology and mitochondrial function in crayfish tonic and phasic motor neurons. J. Neurophysiol. 1997;78:281–294. doi: 10.1152/jn.1997.78.1.281. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL, Castilho RF, Ward MW. Glutamate excitotoxicity and neuronal energy metabolism. Ann. N. Y. Acad. Sci. 1999;893:1–12. doi: 10.1111/j.1749-6632.1999.tb07813.x. [DOI] [PubMed] [Google Scholar]
- Nie F, Wong-Riley MT. Differential glutamatergic innervation in cytochrome oxidase-rich and -poor regions of the macaque striate cortex: quantitative EM analysis of neurons and neuropil. J. Comp. Neurol. 1996a;369:571–590. doi: 10.1002/(SICI)1096-9861(19960610)369:4<571::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Nie F, Wong-Riley MT. Metabolic and neurochemical plasticity of gamma-aminobutyric acid-immunoreactive neurons in the adult macaque striate cortex following monocular impulse blockade: quantitative electron microscopic analysis. J. Comp. Neurol. 1996b;370:350–366. doi: 10.1002/(SICI)1096-9861(19960701)370:3<350::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Nieminen AL. Apoptosis and necrosis in health and disease: role of mitochondria. Int. Rev. Cytol. 2003;224:29–55. doi: 10.1016/s0074-7696(05)24002-0. [DOI] [PubMed] [Google Scholar]
- Okada Y, Sato-Yoshitake R, Hirokawa N. The activation of protein kinase A pathway selectively inhibits anterograde axonal transport of vesicles but not mitochondria transport or retrograde transport in vivo. J. Neurosci. 1995a;15:3053–3064. doi: 10.1523/JNEUROSCI.15-04-03053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995b;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- Overly CC, Rieff HI, Hollenbeck PJ. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J. Cell Sci. 1996;109:971–980. doi: 10.1242/jcs.109.5.971. [DOI] [PubMed] [Google Scholar]
- Palay S. Synapses in the central nervous system. J. Biophys. Biochem. Cytol. 1956;2(suppl):193–202. doi: 10.1083/jcb.2.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E, Procacci P, Ledda M, Arcidiacono G, Frattola D, Rigamonti L. Association between microtubules and mitochondria in myelinated axons of Lacerta muralis. A quantitative analysis. Cell Tissue Res. 1986;245:1–8. doi: 10.1007/BF00218080. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay S, Webster H. The Fine Structure of the Nervous System: The Neurons and Supporting Cells. Oxford University Press; New York: 1991. [Google Scholar]
- Pilling A. Analysis of the Role of Kinesin-1 and Cytoplasmic Dynein in Axonal Organelle Transport in Drosophila melanogaster. Indiana University; Bloomington: 2005. Ph.D. Thesis. [Google Scholar]
- Piper M, Holt C. RNA translation in axons. Annu. Rev. Cell Dev. Biol. 2004;20:505–523. doi: 10.1146/annurev.cellbio.20.010403.111746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT. The fine structure of the axons and growth cones of the human fetal cerebral cortex. Brain Res. 1976;114:379–389. doi: 10.1016/0006-8993(76)90961-6. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Terrian DM. Brain myosin V is a synaptic vesicle-associated motor protein: evidence for a Ca2+-dependent interaction with the synaptobrevin-synaptophysin complex. J. Cell Biol. 1997;137:1589–1601. doi: 10.1083/jcb.137.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DJ. Patterns of cytochrome oxidase activity in areas 17, 18 and 19 of the visual cortex of cats and kittens. Exp. Brain Res. 1985;58:125–133. doi: 10.1007/BF00238960. [DOI] [PubMed] [Google Scholar]
- Price RL, Lasek RJ, Katz MJ. Microtubules have special physical associations with smooth endoplasmic reticula and mitochondria in axons. Brain Res. 1991;540:209–216. doi: 10.1016/0006-8993(91)90509-t. [DOI] [PubMed] [Google Scholar]
- Ratner N, Bloom GS, Brady ST. A role for cyclin-dependent kinase(s) in the modulation of fast anterograde axonal transport: effects defined by olomoucine and the APC tumor suppressor protein. J. Neurosci. 1998;18:7717–7726. doi: 10.1523/JNEUROSCI.18-19-07717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2003;28:1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J. Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J. Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J. Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa A. Mechanisms for neuronal cell death and dysfunction in Huntington's disease: pathological cross-talk between the nucleus and the mitochondria? J. Mol. Med. 2001;79:375–381. doi: 10.1007/s001090100223. [DOI] [PubMed] [Google Scholar]
- Saxton WM, Hicks J, Goldstein LS, Raff EC. Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell. 1991;64:1093–1102. doi: 10.1016/0092-8674(91)90264-y. [DOI] [PubMed] [Google Scholar]
- Schnapp BJ, Reese TS. Dynein is the motor for retrograde axonal transport of organelles. Proc. Natl. Acad. Sci. USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Jarlfors U, Cayer ML. Structural cross-bridges between microtubules and mitochondria in central axons of an insect (Periplaneta americana) J. Cell Sci. 1977;27:255–272. doi: 10.1242/jcs.27.1.255. [DOI] [PubMed] [Google Scholar]
- Stenoien DL, Brady ST. Immunochemical analysis of kinesin light chain function. Mol. Biol. Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Susalka SJ, Pfister KK. Cytoplasmic dynein subunit heterogeneity: implications for axonal transport. J. Neurocytol. 2000;29:819–829. doi: 10.1023/a:1010995408343. [DOI] [PubMed] [Google Scholar]
- Susalka SJ, Hancock WO, Pfister KK. Distinct cytoplasmic dynein complexes are transported by different mechanisms in axons. Biochim. Biophys. Acta. 2000;1496:76–88. doi: 10.1016/s0167-4889(00)00010-0. [DOI] [PubMed] [Google Scholar]
- Suter DM, Espindola FS, Lin CH, Forscher P, Mooseker MS. Localization of unconventional myosins V and VI in neuronal growth cones. J. Neurobiol. 2000;42:370–382. [PubMed] [Google Scholar]
- Swerdlow RH, Kish SJ. Mitochondria in Alzheimer's disease. Int. Rev. Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin V. J. Cell Sci. 1998;111:3221–3234. doi: 10.1242/jcs.111.21.3221. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yamazaki H, Seog DH, Kanai Y, Terada S, Hirokawa N. Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J. Cell Biol. 2000;148:1255–1265. doi: 10.1083/jcb.148.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Olanow CW. Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim. Biophys. Acta. 1999;1410:195–213. doi: 10.1016/s0005-2728(98)00167-4. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Langford GM. Myosin superfamily evolutionary history. Anat. Rec. 2002;268:276–289. doi: 10.1002/ar.10160. [DOI] [PubMed] [Google Scholar]
- Treeck HH, Pirsig W. Differentiation of nerve endings in the cochlear nucleus on morphological and experimental basis. Acta. Otolaryngol. 1979;87:47–60. doi: 10.3109/00016487909126386. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Morfini G, Szebenyi G, Brady ST. Release of kinesin from vesicles by hsc70 and regulation of fast axonal transport. Mol. Biol. Cell. 2000;11:2161–2173. doi: 10.1091/mbc.11.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR. Mammalian cells express three distinct dynein heavy chains that are localized to different cytoplasmic organelles. J. Cell Biol. 1996;133:831–842. doi: 10.1083/jcb.133.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Bloom GS. Mechanisms of fast and slow axonal transport. Annu. Rev. Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJT, Koh T-W, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wagner MC, Barylko B, Albanesi JP. Tissue distribution and subcellular localization of mammalian myosin I. J. Cell Biol. 1992;119:163–170. doi: 10.1083/jcb.119.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner OI, Lifshitz J, Janmey PA, Linden M, McIntosh TK, Leterrier JF. Mechanisms of mitochondria-neurofilament interactions. J. Neurosci. 2003;23:9046–9058. doi: 10.1523/JNEUROSCI.23-27-09046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Asai DJ, Robinson KR. Retrograde but not anterograde bead movement in intact axons requires dynein. J. Neurobiol. 1995;27:216–226. doi: 10.1002/neu.480270208. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki SB, Kuznetsov SA, Tabb JS, Weiss DG, Langford GM, Holzbaur EL. The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport. Proc. Natl. Acad. Sci. USA. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolenski JS, Cheney RE, Mooseker MS, Forscher P. In vitro motility of immunoadsorbed brain myosin-V using a Limulus acrosomal process and optical tweezer-based assay. J. Cell Sci. 1995;108:1489–1496. doi: 10.1242/jcs.108.4.1489. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc. Natl. Acad. Sci. USA. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M, Carroll EW. Effect of impulse blockage on cytochrome oxidase activity in monkey visual system. Nature. 1984;307:262–264. doi: 10.1038/307262a0. [DOI] [PubMed] [Google Scholar]
- Wu Q, Sandrock TM, Turgeon BG, Yoder OC, Wirsel SG, Aist JR. A fungal kinesin required for organelle motility, hyphal growth, and morphogenesis. Mol. Biol. Cell. 1998;9:89–101. doi: 10.1091/mbc.9.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Roberts EA, Goldstein LS. Functional analysis of mouse C-terminal kinesin motor KifC2. Mol. Cell. Biol. 2001;21:2463–2466. doi: 10.1128/MCB.21.7.2463-2466.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa Y, Harada A, Okada Y, Funakoshi T, Kanai Y, Takei Y, Terada S, Noda T, Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J. Cell Biol. 1998;141:431–441. doi: 10.1083/jcb.141.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Takita J, Tanaka Y, Setou M, Nakagawa T, Takeda S, Yang HW, Terada S, Nakata T, Takei Y, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]