Matrix-Induced Inhibition of Membrane Binding Contributes to Human Immunodeficiency Virus Type 1 Particle Assembly Defects in Murine Cells (original) (raw)
Abstract
Defective human immunodeficiency virus type 1 (HIV-1) assembly in murine cells is accompanied by poor plasma membrane binding and proteolytic processing of the HIV-1 Gag precursor. Here, we show that such defects are induced by the propensity of the HIV-1 MA globular head to inhibit membrane binding and particle assembly, particularly at the low expression levels observed in murine cells. Simple additions to or deletion of the MA globular head can improve the yield of infectious virions from murine cells by >50-fold. Expression level and autoinhibition can be important confounding variables in studies of HIV-1 assembly and contribute to defects encountered in murine cells.
The human immunodeficiency virus type 1 (HIV-1) replication cycle is blocked at several stages in murine cells, including at binding and entry (11), postentry (1, 6), transcription (14, 21), late gene expression (2, 9, 25), and assembly (2, 12). Expression of required human cofactors can overcome some defects (4, 11, 26), and certain murine cells can be engineered to support a partial HIV-1 replication cycle, up to and including early gene expression (2, 5, 12). Defects in the late stages of HIV-1 replication are substantially rescued by fusion with human cells (2, 12, 25), suggesting that an additional required factor(s) that act by unknown mechanisms is (are) absent or nonfunctional in mice. In addition to low levels of late gene expression in HIV-1-infected murine cells, defects in assembly accompanied by reduced plasma membrane binding and proteolytic processing of the HIV-1 Gag precursor (Pr55Gag) are evident (2, 3, 9, 13). Pr55Gag-membrane interaction, processing, and particle release are enhanced by replacing the HIV-1 matrix (MA) domain with that of murine leukemia virus (MLV) (3, 18), and this has been interpreted to suggest that a Pr55Gag-membrane targeting cofactor is defective in murine cells. Conversely, recent studies have suggested that defects are programmed by the Rev-dependent nuclear export pathway taken by the HIV-1 Gag-Pol mRNA and can be corrected by inducing it to follow the TAP/NFX1-p15-dependent export pathway taken by conventional mRNAs (23). Other studies have indicated that expression of p32, a factor that at least partly corrects the tendency of the HIV-1 genome to be spliced too efficiently in murine cells, can partly restore infectious HIV-1 production therein (27).
Effects of MA manipulation on Pr55Gag-membrane binding in murine cells.
We recently found that membrane binding by Pr55Gag is highly cooperative, i.e., dependent on its expression level (17). Cooperativity is conferred by Gag oligomerization and the propensity of the HIV-1 MA globular head to inhibit membrane binding, particularly at low Pr55Gag expression levels (17). This is likely a functional manifestation of the “myristoyl switch” (15, 16, 22, 28), whereby the Pr55Gag N-terminal myristate is largely sequestered within the MA globular head when Pr55Gag is monomeric but becomes exposed upon oligomerization (20, 24). Membrane binding is, therefore, likely driven by Pr55Gag concentration-dependent oligomerization.
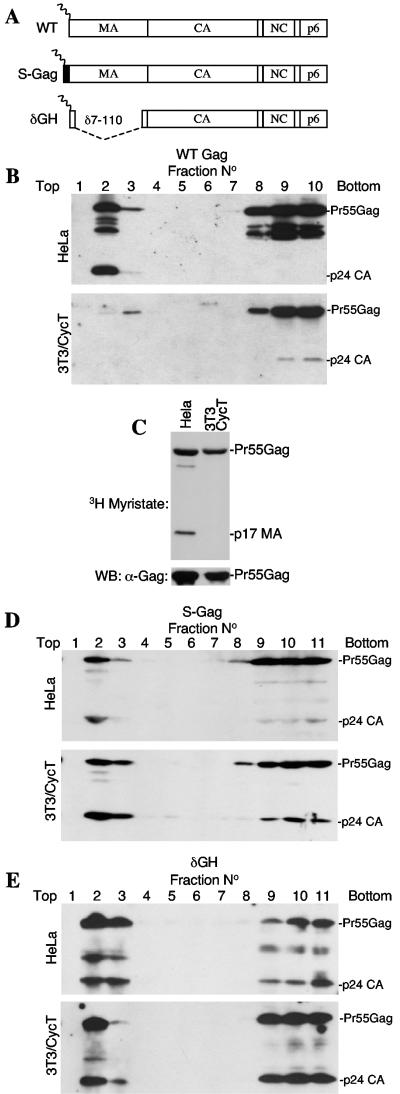
To determine whether such mechanisms, specifically a failure to trigger the myristoyl switch, could underlie the defect in HIV-1 assembly observed in murine cells, we engineered Pr55Gag proteins with modified MA domains (Fig. 1A). Specifically, the construct termed S-Gag expresses a full-length Pr55Gag protein, appended at its N terminus by a 10-residue myristoylated peptide (MGSSKSKPKD) from c-Src. Conversely, the δGH construct retains the authentic HIV-1 myristoylation sequence but has a deletion (amino acids 7 to 110) which removes the MA globular head (Fig. 1A). Similarly large deletions in MA have previously been shown to be compatible with HIV-1 replication, at least in some contexts (19). These modified constructs and an unmodified wild-type (WT) construct were generated in the context of a HIV-1 proviral plasmid (R7/NL/δEnv/GFP) (8) in which the env gene is defective, and the green fluorescent protein (GFP) gene replaces nef. Upon transfection of 293T cells with the proviral plasmids and a vesicular stomatitis virus G protein (VSV-G) envelope expression plasmid, each construct yielded highly infectious virions (see below) which were used to inoculate human (HeLa) and humanized cyclin T-expressing murine NIH 3T3 (3T3/CycT) cell lines (2), such that approximately 50% of the cells became infected. Forty-eight hours after infection, cell lysates were subjected to membrane flotation analysis by using previously described procedures (16, 17, 22). As has been reported, a substantial fraction of WT HIV-1 Gag was processed and membrane associated when expressed in human HeLa cells (16) (Fig. 1B). Conversely, Pr55Gag largely remained cytosolic and unprocessed in murine 3T3/CycT cells. This was not a consequence of defective Pr55Gag myristoylation, because immunoprecipitation analysis of metabolically labeled cells showed that the level of [3H]myristate incorporated into WT HIV-1 Gag was indistinguishable in human and murine cells (Fig. 1C), consistent with previous studies (9, 23). Nonetheless, appending the HIV-1 Gag protein with a myristoylated c-Src peptide or simply deleting the MA globular head dramatically increased the proportion of HIV-1 Gag that was associated with murine cell membranes (Fig. 1D and E). Indeed, the Gag proteins expressed by the S-Gag and δGH constructs bound membranes as efficiently in NIH 3T3 cells as they did in HeLa cells. Thus, simple manipulations of Pr55Gag, in particular removing the inhibitory influence on membrane binding imparted by the MA globular head, can rescue membrane binding and processing defects in murine cells.
FIG. 1.
Simple modifications of HIV-1 MA markedly improve membrane binding in murine cells. (A) Schematic representation of the constructs used in this study. WT is wild-type unmodified Gag with intact MA capsid (CA), nucleocapsid (NC), and p6 domains. S-Gag is appended with a 10-residue peptide MGSSKSKPKD from c-Src, while δGH lacks MA residues 7 to 110. (B) Flotation analysis of Gag membrane interactions. Clarified homogenates of WT-infected HeLa (upper panel) and 3T3/CycT (lower panel) cells were mixed with 80% sucrose and overlaid with 65% and 10% sucrose prior to ultracentrifugation. A Western blot analysis using an α-CA antibody (183-H12-5C) of gradient fractions is shown. Membrane-associated proteins appear in the top three fractions, while cytosolic proteins appear in the bottom three fractions. Note that a longer exposure of the 3T3/CycT-derived blot is shown. (C) Myristoylation of Pr55Gag in both human and murine cells. [3H]myristate-labeled Gag proteins from WT HIV-1-infected HeLa and 3T3/CycT cells were immunoprecipitated by using serum from an HIV-1-infected individual and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography (upper panel). The lower pane shows the same immunoprecipitates analyzed for Pr55Gag content by Western blotting with the 183-H12-5C antibody (lower panels). (D and E) Modifications of Pr55Gag improve membrane binding and processing. Analyses were carried out as described for panel B except that the S-Gag and δGH viruses were used to infect HeLa cells.
Enhanced virion production resulting from MA modification.
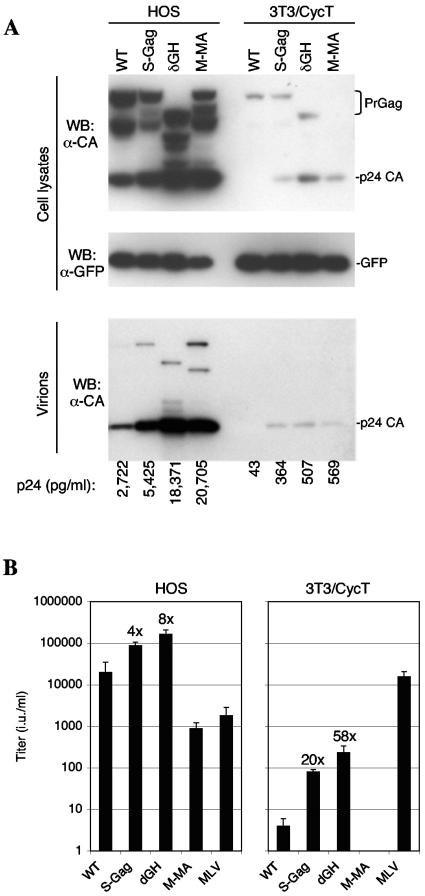
Previous studies have shown that replacement of the HIV-1 MA domain with that of MLV MA can improve HIV-1 assembly in murine cells (3, 18). However, such chimeric viruses are poorly infectious and no manipulation has yet succeeded in improving the yield of infectious HIV-1 virions from murine cells. We next compared the properties of the S-Gag and δGH proviral constructs with a counterpart, termed M-MA, in which the MA domain of MLV Gag replaced HIV-1 MA. To facilitate comparisons of assembly in 3T3/CycT cells with that in human cells, we identified a human cell line (HOS) that could be transfected with virtually equivalent efficiency (by using Lipofectamine Plus; Invitrogen). Cells were transfected with VSV-G expression plasmids along with either R7/NL/δEnv/GFP-derived proviral plasmids or, as a control, previously described MLV-based packaging and GFP-expressing vector plasmids (7). The levels of early gene expression (GFP) in HOS and 3T3/CycT cells transfected with the various R7/NL/δEnv/GFP constructs were approximately equivalent (Fig. 2A). However, as has previously been reported, late gene expression (Gag) was markedly lower in 3T3/CycT cells than in HOS cells (Fig. 2A) (2). This is likely due to the low level of unspliced HIV-1 mRNA that is present therein (2, 25, 27). Western blot and p24 enzyme-linked immunosorbent assay analyses showed that the S-Gag, δGH, and M-MA constructs generated higher yields of extracellular particles in both cell lines (Fig. 2A). However, the effects of MA manipulation were greater in 3T3/CycT cells, where the amounts of virion-associated p24 generated by S-Gag, δGH, and M-MA were 9- to 13-fold higher than WT levels (Fig. 2A). The effects of the S-Gag and δGH manipulations were even more dramatic when infectious particles were measured (Fig. 2B). Indeed, the δGH proviral plasmid yielded 50- to 60-fold more infectious virions than the WT equivalent in 3T3/CycT cells, while in HOS cells, δGH yielded eightfold more infectious virions than the WT.
FIG. 2.
MA modifications enhance infectious virion release in HOS and 3T3/CycT cells. (A) Western blot analysis of HOS cells (left) and 3T3/CycT cells (right) using antibodies specific for late gene expression (Gag, upper panel) and early gene expression (GFP, center panel). The lower panel shows Western blot analysis of Gag pelleted through a 20% sucrose cushion following filtration (0.2 μm) of culture supernatants. Also shown below the blots are the concentrations of p24 capsid (CA) (pg/ml) measured in the culture supernatants by enzyme-linked immunosorbent assay. (B) Infectious virus yield from transfected HOS and 3T3/CycT cells. The VSV-G-pseudotyped virions were used to inoculate HOS cells, and the GFP-positive target cells were enumerated 48 h later by fluorescence-activated cell sorter analysis, as previously described. Titers are given as infectious units (i.u.) per ml of supernatant. Figures above the bars represent the increase (_n_-fold) in titer over that of the WT construct.
Low expression level enhances MA-induced inhibition of processing and virus-like particle (VLP) release.
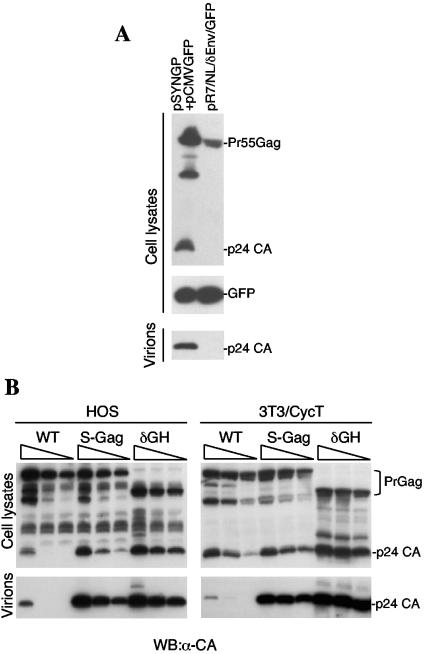
Analysis of HIV-1 assembly defects in murine cells is complicated by the aforementioned cooperativity in Pr55Gag-membrane binding (17), coupled with the low Pr55Gag expression levels observed when proviral constructs are used in murine cells (Fig. 2A) (2, 9, 25, 27). Therefore, we compared the assembly properties of Gag-Pol expressed in human and murine cells by a plasmid carrying a synthetic codon-optimized gag-pol gene (pSYNGP) (10). In this case, Gag-Pol expression is not dependent on Rev or regulated splicing of the HIV-1 genome and achieved similar levels in murine 3T3/CycT and human HOS cells (Fig. 3). Under these conditions, pSYNGP-derived Pr55Gag was processed and released as particles, even in 3T3/CycT cells (Fig. 3A), as has been previously reported (23). Importantly, however, when the levels of Pr55Gag expression were decreased by transfection with smaller amounts of pSYNGP, Pr55Gag processing and particle release defects became evident. Notably, these effects were similar in HOS cells and 3T3/CycT cells (Fig. 3B). Thus, Gag-Pol expression levels can significantly influence Pr55Gag processing and release, and low-level expression by Rev-independent constructs (Fig. 3B) could recapitulate the processing and release defects encountered upon expression of proviral constructs in rodent cells (Fig. 2A) (2, 13).
FIG. 3.
Efficiently expressed, codon-optimized Gag-Pol constructs yield HIV-1 virus-like particles in murine cells, but MA-induced assembly defects can be recapitulated by expression at lower levels. (A) Western blot analysis of 3T3/CycT cell lysates (upper and middle panels) and virions or VLPs pelleted through 20% sucrose (lower panels) following transfection with 1 μg pSYNGP (cytomegalovirus promoter) plus 1 μg pCMV/GFP (left lane) or 2 μg of the R7/NL/δEnv/GFP proviral plasmid (right lane). Western blots were probed as described in the legend to Fig. 1. (B) Western blot analysis using an α-capsid (α-CA) antibody (183-H12-5C) of cell lysates (upper panels) and VLPs (lower panels) following transfection of HOS cells (left panels) or 3T3/CycT cells (right panels) with pSYNGP-derived WT, S-Gag, or δGH Gag-Pol expression vector. Each group of three lanes shows cells transfected with decreasing amounts (from left to right, 1 μg, 0.5 μg, and 0.25 μg) of each pSYNGP-derived construct.
Upon the introduction of the S-Gag and δGH modifications into pSYNGP, Gag processing and particle release were dramatically enhanced in both 3T3 and HOS cells (Fig. 3B). The effects of these modifications were especially evident at lower levels of Gag-Pol expression, at which very low levels of WT VLPs were released, while S-Gag and δGH VLP release was extremely efficient. Again, effects in HOS and 3T3/CycT cells were similar (Fig. 3B), suggesting that the enhancing effects of MA modifications on HIV-1 particle release are dependent on expression level rather than host cell species.
Conclusions.
These studies do not refute the notion that the nuclear export pathway taken by HIV-1 mRNA influences assembly or that required HIV-1 assembly cofactors are nonfunctional in murine cells. However, they do show that expression level can be an important confounding variable in studies of HIV-1 assembly and at least contribute to the assembly defect encountered in murine cells. In particular, HIV-1 assembly can be quite markedly inhibited by the MA globular head, in both human and murine cells, and this effect is especially pronounced at low Gag-Pol expression levels. At present, the role of concentration-dependent autoinhibition of membrane binding in the viral replication cycle is unclear, but it may provide a means by which HIV-1 assembly is temporally or spatially regulated.
Acknowledgments
We thank Simone Cowan for technical assistance and Kyriacos Mitrophanous for reagents.
This work was supported by a grant from the NIH (R01AI50111) P.D.B. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Baumann, J. G., D. Unutmaz, M. D. Miller, S. K. Breun, S. M. Grill, J. Mirro, D. R. Littman, A. Rein, and V. N. KewalRamani. 2004. Murine T cells potently restrict human immunodeficiency virus infection. J. Virol. 78**:**12537-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieniasz, P. D., and B. R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74**:**9868-9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, B. K., I. Rousso, S. Shim, and P. S. Kim. 2001. Efficient assembly of an HIV-1/MLV Gag-chimeric virus in murine cells. Proc. Natl. Acad. Sci. USA 98**:**15239-15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272**:**872-877. [DOI] [PubMed] [Google Scholar]
- 5.Garber, M. E., P. Wei, V. N. KewalRamani, T. P. Mayall, C. H. Herrmann, A. P. Rice, D. R. Littman, and K. A. Jones. 1998. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 12**:**3512-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatziioannou, T., S. Cowan, and P. D. Bieniasz. 2004. Capsid-dependent and -independent postentry restriction of primate lentivirus tropism in rodent cells. J. Virol. 78**:**1006-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. J. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22**:**385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatziioannou, T., S. Cowan, U. K. Von Schwedler, W. I. Sundquist, and P. D. Bieniasz. 2004. Species-specific tropism determinants in the human immunodeficiency virus type 1 capsid. J. Virol. 78**:**6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koito, A., H. Shigekane, and S. Matsushita. 2003. Ability of small animal cells to support the postintegration phase of human immunodeficiency virus type-1 replication. Virology 305**:**181-191. [DOI] [PubMed] [Google Scholar]
- 10.Kotsopoulou, E., V. N. Kim, A. J. Kingsman, S. M. Kingsman, and K. A. Mitrophanous. 2000. A Rev-independent human immunodeficiency virus type 1 (HIV-1)-based vector that exploits a codon-optimized HIV-1 _gag_-pol gene. J. Virol. 74**:**4839-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maddon, P. J., A. G. Dalgleish, J. S. McDougal, P. R. Clapham, R. A. Weiss, and R. Axel. 1986. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47**:**333-348. [DOI] [PubMed] [Google Scholar]
- 12.Mariani, R., B. A. Rasala, G. Rutter, K. Wiegers, S. M. Brandt, H. G. Krausslich, and N. R. Landau. 2001. Mouse-human heterokaryons support efficient human immunodeficiency virus type 1 assembly. J. Virol. 75**:**3141-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariani, R., G. Rutter, M. E. Harris, T. J. Hope, H. G. Krausslich, and N. R. Landau. 2000. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74**:**3859-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newstein, M., E. J. Stanbridge, G. Casey, and P. R. Shank. 1990. Human chromosome 12 encodes a species-specific factor which increases human immunodeficiency virus type 1 _tat_-mediated trans activation in rodent cells. J. Virol. 64**:**4565-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono, A., and E. O. Freed. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 73**:**4136-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paillart, J. C., and H. G. Gottlinger. 1999. Opposing effects of human immunodeficiency virus type 1 matrix mutations support a myristyl switch model of gag membrane targeting. J. Virol. 73**:**2604-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Caballero, D., T. Hatziioannou, J. Martin-Serrano, and P. D. Bieniasz. 2004. Human immunodeficiency virus type 1 matrix inhibits and confers cooperativity on gag precursor-membrane interactions. J. Virol. 78**:**9560-9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed, M., R. Mariani, L. Sheppard, K. Pekrun, N. R. Landau, and N. W. Soong. 2002. Chimeric human immunodeficiency virus type 1 containing murine leukemia virus matrix assembles in murine cells. J. Virol. 76**:**436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reil, H., A. A. Bukovsky, H. R. Gelderblom, and H. G. Gottlinger. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17**:**2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resh, M. D. 2004. A myristoyl switch regulates membrane binding of HIV-1 Gag. Proc. Natl. Acad. Sci. USA 101**:**417-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seigel, L. J., L. Ratner, S. F. Josephs, D. Derse, M. B. Feinberg, G. R. Reyes, S. J. O'Brien, and F. Wong-Staal. 1986. Transactivation induced by human T-lymphotropic virus type III (HTLV III) maps to a viral sequence encoding 58 amino acids and lacks tissue specificity. Virology 148**:**226-231. [DOI] [PubMed] [Google Scholar]
- 22.Spearman, P., R. Horton, L. Ratner, and I. Kuli-Zade. 1997. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 71**:**6582-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson, C. M., B. A. Puffer, K. M. Ahmad, R. W. Doms, and M. H. Malim. 2004. Retroviral mRNA nuclear export elements regulate protein function and virion assembly. EMBO J. 23**:**2632-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang, C., E. Loeliger, P. Luncsford, I. Kinde, D. Beckett, and M. F. Summers. 2004. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. USA 101**:**517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trono, D., and D. Baltimore. 1990. A human cell factor is essential for HIV-1 Rev. action. EMBO J. 9**:**4155-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92**:**451-462. [DOI] [PubMed] [Google Scholar]
- 27.Zheng, Y. H., H. F. Yu, and B. M. Peterlin. 2003. Human p32 protein relieves a post-transcriptional block to HIV replication in murine cells. Nat. Cell. Biol. 5**:**611-618. [DOI] [PubMed] [Google Scholar]
- 28.Zhou, W., and M. D. Resh. 1996. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J. Virol. 70**:**8540-8548. [DOI] [PMC free article] [PubMed] [Google Scholar]