Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures (original) (raw)
. Author manuscript; available in PMC: 2006 Dec 1.
Abstract
Granule cell (GC) neurogenesis increases following seizures, and some newborn GCs develop in abnormal locations within the hilus. These ectopic GCs (EGCs) display robust spontaneous and evoked excitatory activity. However, the pattern of afferent input they receive has not been fully defined. This study used electron microscopic immunolabeling to quantitatively evaluate mossy fiber (MF) input to EGCs since MFs densely innervate the hilus normally and undergo sprouting in many animal models of epilepsy. EGC dendrites were examined in tissue from epileptic rats that had initially been treated with pilocarpine to induce status epilepticus and subsequently had spontaneous seizures. MF terminals were labeled with a zinc transporter-3 antibody, and calbindin immunoreactivity was used to label hilar EGCs and GC layer GCs. The pattern of input provided by sprouted MF terminals to EGC dendrites was then compared to the pattern of MF input to GC dendrites in the inner molecular layer (IML), where most sprouted fibers are thought to project. Analysis of EGC dendrites demonstrated that MF terminals represented their predominant source of afferent input: they comprised 63% of all terminals and, on average, occupied 40% and 29% of the dendritic surface in the dorsal and ventral dentate gyrus, respectively, forming frequent synapses. These measures of connectivity were significantly greater than comparable values for MF innervation of GC dendrites located in the IML of the same tissue sections. Thus, EGCs develop a pattern of synaptic connections that could help explain their previously identified predisposition to discharge in epileptiform bursts and suggest that they play an important role in the generation of seizure activity in the dentate gyrus.
Keywords: Mossy fiber, Granule cell, Dentate gyrus, Pilocarpine, Hippocampus, Epilepsy, Synapse, Active zone, Axon terminal, Hilus
Introduction
Both brief and prolonged periods of seizures increase granule cell (GC) neurogenesis (for a review, see Parent and Lowenstein, 2002; Scharfman, 2004), a process that normally occurs throughout life (Gage et al., 1998; Gould and Cameron, 1996). Some newly generated GCs appear to develop at abnormal locations within the hilus (Dashtipour et al., 2001; Parent et al., 1997; Ribak et al., 2000; Scharfman et al., 2000). These ectopic GCs (EGCs) can be anatomically subdivided into two groups: those with somata near the GC layer (subgranular EGCs) and dendrites which extend into and branch within the molecular layer (ML) (Dashtipour et al., 2001; Scharfman et al., 2003) and those that are located deep in the hilus with dendrites that appear to be almost exclusively restricted to this region (Scharfman et al., 2000, 2003). This division also has a physiological basis, reflected by the latency to onset of the synaptic potential evoked by perforant path stimulation (Scharfman et al., 2003). Perforant path stimulation evokes synaptic potentials of equivalent amplitude in both populations, but EGCs without ML dendrites have significantly longer latencies, suggesting that polysynaptic pathways are involved in their responses.
A pathway that could explain these longer latencies would be disynaptic activation by the perforant path via GCs, whose axons (mossy fibers (MFs)) have been observed contacting subgranular EGCs (Dashtipour et al., 2001). Additional support for the involvement of MFs comes from a large body of evidence showing that seizures can induce MFs to sprout new collaterals, which then innervate both the inner ML (IML) (Dudek et al., 1994; Nadler et al., 1980; Okazaki et al., 1995; Represa et al., 1994; Sutula et al., 1992) and hilus (Buckmaster and Dudek, 1999; Sutula et al., 1998). Finally, MF input to EGCs could originate from other EGCs since the axons of EGCs develop complex arborizations that project both locally and to CA3 (Scharfman et al., 2000).
Preembedding dual electron microscopic (EM) immunolabeling techniques were therefore employed in this study to determine if MF terminals represent a major source of synaptic input to hilar EGC dendrites. To label MF terminals selectively, immunoperoxidase labeling was employed using an antibody to one of the zinc transporters (ZnT-3; Palmiter et al., 1996; Wenzel et al., 1997). Immunogold labeling was then used in conjunction with an antibody to calbindin D28K (CaBP) to selectively label EGCs. It has been shown that mature GC layer GCs (Rami et al., 1987; Sloviter, 1989) and EGCs (Scharfman et al., 2000, 2002) are well-labeled by antibodies to CaBP. Although some labeling of interneurons occurs in the hippocampus, such neurons are rare in the dentate gyrus (Gulyas et al., 1991; Scharfman et al., 2000), so CaBP was used as a preferential marker of GCs in this region. This approach thus allowed EGC dendrites to be characterized in terms of the extent of MF synaptic coverage and compared to the pattern of sprouted MF coverage of GC dendrites in the IML of the same sections. Such a comparison is important since it has been assumed that MFs sprout primarily to GCs in the GC layer, forming recurrent connections that are thought to contribute to the development of hyperexcitability. The findings of this study suggest that MF input to EGCs may play a role as well.
Methods
Seizure induction and animal care
Adult male Sprague–Dawley rats (180 to 240 g, approximately 42 days old) were injected with atropine methylbromide (1 mg/kg s.c.) and 30 min later with pilocarpine hydrochloride (380 mg/kg i.p.). The onset of status epilepticus was defined as the first stage 5 seizure (Racine, 1972) that did not abate after several minutes. Diazepam (5 mg/kg i.p., Wyeth-Ayerst) was injected 1 h after the onset of status. After approximately 5 h, animals were injected with 2.5 ml 5% dextrose in lactate-Ringer's (s.c.). An apple was cut in half and laid at the bottom of the cage each day for approximately 7 days. Rats were then periodically observed to confirm the development of spontaneous stage 5 seizures. Saline controls (the same age as the drug-treated rats) received identical treatment (atropine, diazepam, apple) but were injected with saline instead of convulsant. Animal care and use followed the guidelines set by the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize both the number of animals used and any discomfort to the animal.
Tissue processing
Several months after seizure induction, animals were overdosed with pentobarbital (150 mg/kg i.p.) and perfused through the aortic arch sequentially with: (a) 15 ml of normal saline (0.9%) containing 1000 units/ml of heparin; (b) 50 ml of 3.75% acrolein and 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB); and (c) 200 ml of 2% paraformaldehyde in PB. The brains were removed, placed in a coronal brain mold (Activational Systems Inc., Detroit, MI), cut into 5 mm blocks, and postfixed in 2% paraformaldehyde in PB for 30 min. Brain sections (40 μm) through the hippocampal formation were then cut on a Leica Vibratome VT1000S (Leica Instruments GmbH, Nussloch, Germany) into cold PB, transferred to a storage solution (30% sucrose and 10% ethylene glycol in 0.1 M PB), and refrigerated at −25°C.
Immunohistochemistry
For each animal, a random systematic series of sections was processed simultaneously to concurrently label ZnT-3 and CaBP using dual labeling immunohistochemical techniques. A rabbit antibody to ZnT-3 was kindly provided by Dr. Richard Palmiter (University of Washington, Seattle, WA). It was raised to the C terminus portion of ZnT-3, had been affinity-purified, and had been used to detect ZnT-3 protein in zinc-containing neurons throughout the brain (Palmiter et al., 1996), producing a pattern of staining identical to that obtained with Timm's stain for histochemically reactive zinc (Wenzel et al., 1997). A mouse monoclonal antibody (clone CB-955) to CaBPD28K purchased from Sigma-Aldrich Inc. (St. Louis, MI) was also used, whose specificity has been extensively tested (it does not display cross-reactivity with related proteins such as calretinin or parvalbumin). All tissue sections examined in this study were processed simultaneously, so that they would be exposed to exactly the same concentrations for exactly the same periods of time.
Sections were first incubated in 1% sodium borohydride in PB to reduce reactive aldehydes (Eldred et al., 1983) and then briefly frozen using a freeze–thaw technique (Descarries et al., 1992) to increase the extent of antibody penetration. After being transferred to a Tris–saline solution (TS; 0.9% NaCl in 0.1 M Tris, pH 7.6), they then passed through a series of incubations to label ZnT-3 with immunoperoxidase, using the avidin–biotin–peroxidase complex (ABC) method (Hsu et al., 1981). This involved the following steps, separated by TS washes (×3, 10 min each): sequential incubation in (a) a 0.5% bovine serum albumin (BSA) solution in TS for 30 min; (b) an antibody cocktail of 1:100 rabbit anti-ZnT-3 and 1:200 mouse anti-CaBP in 0.1% BSA/TS for 24 h at room temperature; (c) a 1:400 dilution of goat anti-rabbit biotinylated-IgG in 0.1% BSA/TS for 30 min (Jackson Immunoresearch, West Grove, PA), (d) a 1:100 dilution of ABC in 0.05% BSA/TS (Vectastain Elite Kit, Vector Laboratories, Burlingame, CA) for 30 min; (e) a solution of 0.022% 3,3′-diaminobenzidine (DAB) and 0.003% H2O2 in TS for 8 min, and (f) a 10 min wash in PB.
Following the immunoperoxidase labeling procedure, sections were further processed to label CaBP with immunogold, as follows: (a) rinse in PB saline (PBS, 0.9% NaCl in 0.01 M PB, pH 7.4) for 10 min, (b) incubation in blocking buffer (0.8% BSA and 0.1% gelatin in PBS) for 10 min, (c) incubation in 1 nm gold particle-conjugated goat anti-mouse IgG (Amersham, Arlington Heights, IL) in blocking buffer for 18 h; (d) wash in blocking buffer for 5 min, (e) PBS rinses (3×, 5 min each) and postfixation in 2% glutaraldehyde for 10 min, (f) PBS rinses (3×, 5 min each), (g) wash in citrate buffer (0.2 M, pH 7.4) for 10 min, (h) silver intensification for 7 min using an IntenSE-M Kit (Amersham, Arlington Heights, IL), and (i) wash in citrate buffer for 10 min. Some experimental and control sections were processed to only label CaBP, with immunoperoxidase, using an incubation in a 1:400 dilution of the mouse anti-CaBP antibody and the ABC method described above.
Electron microscopic processing
Sections were then prepared for EM examination, as follows: (a) wash in PB for 5 min, (b) postfixation in 2% osmium for 1 h, (c) dehydration in a series of graded alcohols (5 min each: 30%, 50%, 70%, 95%, then ×2 in 100% for 10 min each) and 100% propylene oxide (×2, 10 min each), (d) overnight incubation in 1:1 Embed 812 (Electron Microscopy Sciences Inc., Hatfield, PA) and propylene oxide, (e) incubation in Embed 812 for 2 h, (f) flat-embedding between two sheets of Aclar plastic film (Allied Signal, Pottsville, PA), and (g) polymerization at 60°C for 72 h. Select portions of both the dorsal (from the section nearest −3.5 mm from Bregma; Paxinos and Watson, 1986) and ventral (−6.0 mm) dentate gyrus containing both hilar and IML subfields were excised, glued onto plastic blocks, and trimmed in preparation for thin sectioning on an ultratome (RMC, Tucson, AZ). Ultrathin sections (65 nm) were cut from the block face with a diamond knife (Diatome, Fort Washington, PA), collected on copper mesh grids, and counterstained with uranyl acetate (20 min) and Reynolds lead citrate (5 min) (Reynolds, 1963) prior to examination on a Technai transmission electron microscope (FEI Company, Hillsboro, OR).
Captured light microscopic (LM) images of the block face were then compared to captured low magnification (169Χ)EM images of thin sections in a given series to both determine the position of hilar and IML boundaries and identify the surface of the tissue (the plastic/tissue interface), within a given section. To obtain optimal labeling and control for the effect of penetration (allowing comparison across sections), only ultra-structural fields adjacent to the plastic/tissue interface were examined (Auchus and Pickel, 1992). Sampling within the hilus and IML was thus guided by mapping the intersection of the interface with the borders of these subregions, for a given randomly selected thin section. Within the hilus, grid squares adjacent to the GC layer were excluded to reduce the possibility of sampling both GC basal dendrites and the dendrites of CaBP-positive interneurons. CaBP-positive interneurons were infrequently observed, usually near the GC layer (see CaBP and ZnT-3 light microscopy). All dendritic profiles containing two or more gold particles (CaBP labeling) in portions of interface-adjacent grid squares that displayed no tissue damage were identified, and digital images captured for later analysis (at 9300Χ to 30,000Χ depending on process size) using an AMT Advantage HR/HR-B CCD Camera System (Advanced Microscopy Techniques, Danvers, MA). Digital images were printed using a Tektronix phaser 860 printer.
Electron microscopic analysis
Each micrograph was then carefully examined to characterize the microenvironment of the labeled dendrite in that plane of section, microenvironment being defined as the types of processes in contact with the dendrite, and the presence or absence of specialized associations, such as active zones. All process profiles in direct apposition to a labeled dendritic profile were categorized as either terminals, unmyelinated axons, myelinated axons, dendrites, spines, somata, astrocytic processes, or undistinguishable processes, based on the criteria of Peters et al. (1991). Briefly, terminals contained numerous clear vesicles, unmyelinated axons were <0.1 μm in diameter and contained few clear vesicles, myelinated axons were wrapped in myelin, dendrites were postsynaptic to terminals, spines were small and usually contained tubulovesicular processes and an absence of mitochondria, somata contained a nucleus and Golgi apparatus, and astrocytic processes were usually thin, conformed to the shape of other processes, and contained glial filaments. Terminals were subdivided by the presence or absence of peroxidase reaction product (ZnT-3 labeling) and whether they formed a synapse with the labeled dendrite in the plane of section examined. If a synapse was formed, the terminal was further categorized on the basis of whether the active zone was asymmetric (type 1; Gray, 1959), with a distinct postsynaptic density, or symmetric (type 2; Gray, 1959), with a postsynaptic density that was virtually absent or limited to small patches (and which could be best identified by the presence of filamentous material in the synaptic cleft and presynaptic vesicles) (Peters et al., 1991) (see also Fig. 5). Puncta adhaerentia, although very rarely observed, were distinguished from active zones by the presence of a thick presynaptic density and lack of presynaptic vesicles (Peters et al., 1991). The analysis used was thus based on the two-dimensional sampling of dendritic surfaces, which provides an estimate of the average relative frequency of various types of associations formed by a population of dendrites (see below), when averaged across multiple samples.
Fig. 5.
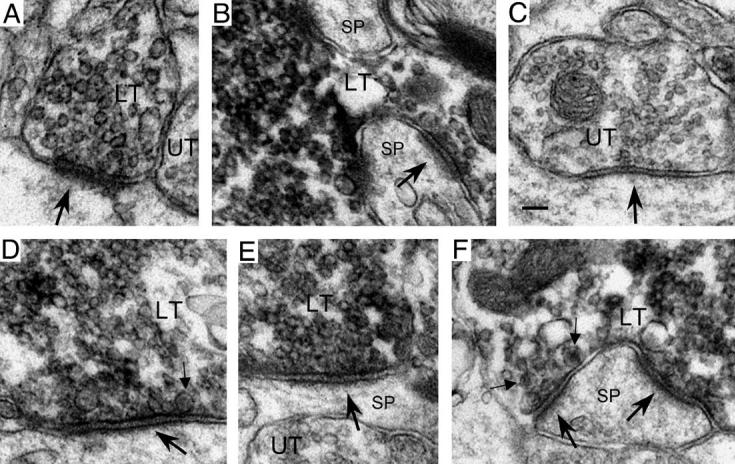
Synapses (large arrows) formed by ZnT-3-labeled (LT) and unlabeled (UT) terminals in pilocarpine-treated animals and saline-injected controls. (A and B) In the hilus of saline-injected controls, asymmetric synapses with thick postsynaptic densities were formed by both small (A) and large (B) ZnT-3-labeled MF terminals (in panel B, the MF terminal partially envelops two spines (SP)). (C) An unlabeled hilar terminal from a pilocarpine-treated animal that formed a symmetric synapse in which the postsynaptic density is virtually absent (note however the presence of filamentous material within the synaptic cleft, providing evidence that an active zone was present). (D, E, and F) In the hilus (D, E) and IML (F) of pilocarpine-treated animals, ZnT-3-labeled MF terminals formed asymmetric synapses with both dendritic shafts (D) and spines (E, F) that often had less pronounced postsynaptic densities (IML MF synapses tended to have relatively thicker densities (F)). Small arrows indicate dense core vesicles in MF terminals. Scale bar = 0.1 μm.
For each dendritic profile, the contact length of every process profile apposed to the perimeter of the dendritic profile was measured, and these lengths were summed by the category of process profile (as defined above: labeled terminal profile, unlabeled terminal profile, unmyelinated axon profile, glial profile, etc.). These sums were then used to calculate the percentage of the dendritic profile perimeter apposed to each category of process profile, referred to as ‘%coverage’ in the text and tables (% coverage = (sum of contact lengths for a given category of process profile / dendritic profile perimeter length) * 100). When averaged across the dendritic profiles examined, these values represent a measure of the average percentage of the dendritic surface covered by that category of process (in a manner analogous to the Delesse principle, _A_A = _V_V, where the average of the percentage of area occupied by a type of particle (_A_A) in random sections represents a measure of the average percentage of volume (_V_V) occupied by that type of particle (Pierce and Milner, 2001; Weibel, 1979)).
Comparably, determining how frequently synaptic specializations are present in two-dimensional sections through the apposed surface of terminals contacting dendrites provides an estimate of the average relative proportion of apposed surface area that is occupied by active zones for the type of terminal examined but does not estimate average synapse number (which would require serial analysis). Additionally, dendritic cross-sectional diameter was measured, and the number of spines on the dendrite in the plane of section was counted.
Data were assembled in Excel X (Microsoft, Redmond, WA) spreadsheets and analyzed statistically using StatView 5.0 (SAS Institute Inc., Cary, NC). Data are presented as mean ± SEM, n representing the number of profiles examined for a given measure. When counts were analyzed, like the number of terminal profiles labeled by immunoperoxidase or displaying a synapse in the examined plane of section, data were summed by subregion (hilus or IML) and hippocampal level (dorsal or ventral). When measurements were analyzed, like dendritic diameter or perimeter percentages, data were averaged by subregion and hippocampal level. These values, summed or averaged per animal, were then analyzed statistically, significance being determined using unpaired t tests with P < 0.05. Data were first analyzed by level, and, if significant differences were not observed, the data were then pooled (see Table 1). All figures were assembled using Photoshop 7.0 (Adobe Inc., San Jose, CA).
Table 1.
Ultrastructural characteristics of CaBP-labeled GC dendrites in the IML and EGC dendrites in the hilus
| IML | Hilus | ||||||
|---|---|---|---|---|---|---|---|
| D | V | Pooled | D | V | Pooled | ||
| 1 | Gold particle # | 3.3 ± 0.3 (85) | 3.2 ± 0.2 (103) | 3.2 ± 0.3 (188) | 3.3 ± 0.2 (225) | 3.2 ± 0.2 (243) | 3.3 ± 0.1 (468) |
| 2 | Dendritic profile diameter (μm) | 1.3 ± 0.1 (85) | 1.2 ± 0.1 (103) | 1.3 ± 0.1 (188) | 1.4 ± 0.1 (225) | 1.4 ± 0.1 (243) | 1.4 ± 0.1 (468) |
| 3 | % MF terminal coverage | 10 ± 2 (85) | 12 ± 1 (103) | 11 ± 1a (188) | 40 ± 2a,c (225) | 29 ± 1a,c (243) | – |
| 4 | % of MF terminal profiles with an active zone (AZ) | 13 ± 3 (129) | 10 ± 1 (203) | 12 ± 1a (332) | 49 ± 2 (1350) | 48 ± 3 (1128) | 48 ± 1a (2478) |
| 5 | % of MF terminal profile AZs formed with labeled spines | 30 ± 10 (19) | 50 ± 20 (22) | 40 ± 10b (41) | 7 ± 2 (669) | 13 ± 3 (555) | 9 ± 2b (1224) |
| 6 | % of MF terminal profile AZs formed with all spines (labeled and unlabeled) | 87 ± 2 (44) | 94 ± 2 (65) | 92 ± 1 (109) | – | – | – |
| 7 | % terminal coverage (all terminals) | 23 ± 2 (85) | 25 ± 1 (103) | 24 ± 1a (188) | 57 ± 2a,c (225) | 46 ± 2a,c (243) | – |
| 8 | % of all terminal profiles that were MF terminal profiles | 42 ± 6 (296) | 51 ± 4 (396) | 46 ± 4c (692) | 67 ± 3 (2002) | 59 ± 2 (1918) | 63 ± 2c (3920) |
| 9 | % of all AZs that were asymmetric | 80 ± 10 (54) | 81 ± 5 (67) | 81 ± 5 (121) | 92 ± 2 (895) | 91 ± 1 (844) | 91 ± 1 (1739) |
| 10 | % of all AZs that were symmetric | 20 ± 10 (54) | 19 ± 5 (67) | 19 ± 5 (121) | 8 ± 2 (895) | 9 ± 1 (844) | 9 ± 1 (1739) |
| 11 | % of all terminal profiles with asymmetric AZs that were MFs | 40 ± 10 (44) | 39 ± 5 (53) | 40 ± 5a (97) | 80 ± 3 (828) | 73 ± 3 (770) | 77 ± 2a (1598) |
| 12 | % dendritic coverage | 21 ± 3 (85) | 19 ± 1 (103) | 20 ± 1a (188) | 0.9 ± 0.3 (225) | 2.5 ± 0.8 (243) | 1.9 ± 0.5a (468) |
| 13 | % unmyelinated axon coverage | 28 ± 1 (85) | 26 ± 2 (103) | 27 ± 1c (188) | 18 ± 2 (225) | 22 ± 2 (243) | 20 ± 1c (468) |
| 14 | % glial coverage | 18 ± 2 (85) | 21 ± 2 (103) | 20 ± 1 (188) | 18 ± 1 (225) | 21 ± 2 (243) | 19 ± 1 (468) |
| 15 | % spine coverage | 6 ± 1 (85) | 7 ± 1 (103) | 6.1 ± 0.4 (188) | 5 ± 1 (225) | 6 ± 1 (243) | 5.7 ± 0.8 (468) |
| 16 | % somal coverage | 0.6 ± 0.3 (85) | 0.5 ± 0.4 (103) | 0.6 ± 0.3 (188) | 0.1 ± 0.1 (225) | 0.3 ± 0.2 (243) | 0.2 ± 0.1 (468) |
| 17 | % myelinated axon coverage | 0.6 ± 0.3 (85) | 0.7 ± 0.4 (103) | 0.6 ± 0.3 (188) | 0.3 ± 0.1 (225) | 0.5 ± 0.4 (243) | 0.3 ± 0.2 (468) |
| 18 | % unclassifiable coverage | 2.8 ± 0.6b (85) | 0.8 ± 0.3b (103) | – | 0.7 ± 0.2b (225) | 1.1 ± 0.3 (243) | 1.0 ± 0.2 (468) |
Results and discussion
Experimental groups and sampling scheme
Tissue from eight pilocarpine-treated rats was examined in this study, all of which displayed multiple spontaneous stage 5 seizures in the months following status epilepticus. They were killed 8 ± 2 months after pilocarpine injection (range: 2.5 to 13.5). To examine the effect of survival time on data, animals were also subdivided into two groups: a relatively young group (5 animals, average survival time: 4.5 ± 0.6 months, range: 2.5 to 6.5 months) and an older group (3 animals, average survival time: 13.2 ± 0.3 months, range: 12.5 to 13.5 months).
A random systematic series of sections (1 in 12) through the entire hippocampal formation of each animal was then concurrently processed immunohistochemically. From each series, one dorsal section and one ventral section was selected for ultrastructural analysis (the sections from the series closest to −3.5 mm and −6.0 mm from Bregma, respectively; Paxinos and Watson, 1986), yielding 16 blocks. Hilar and IML fields were examined in the same 2 to 4 thin sections, per block. Digital images of 225 dorsal and 243 ventral hilar CaBP-labeled dendritic profiles and 85 dorsal and 103 ventral IML CaBP-labeled dendritic profiles were captured and analyzed (although one cannot exclude the possibility that some interneuron and GC basal dendrites were included in the analysis, not sampling fields adjacent to the GC layer would presumably limit them to a very small portion of the total examined; see Electron microscopic processing). These dendritic profiles were contacted by 2002 dorsal and 1918 ventral hilar terminal profiles and 296 dorsal and 396 ventral IML terminal profiles, respectively. Additionally, tissue from five saline-treated control animals was processed and examined concurrently by LM and EM.
The pattern of CaBP and ZnT-3 labeling
CaBP and ZnT-3 light microscopy
In agreement with previous findings (Scharfman et al., 2000; 2002), immunoperoxidase labeling for CaBP revealed numerous darkly stained cell bodies and dendrites in the hilus of pilocarpine-treated animals, which at times formed dense clusters near CA3c (Fig. 1). CaBP labeling was also apparent within the GC layer of pilocarpine-treated animals, although, in accord with other studies (Shetty and Turner, 1995; Sloviter et al., 1991; Tonder et al., 1994), the labeling was lighter and less uniform in pilocarpine-treated animals relative to saline-injected controls. This is consistent with reports of a reduction in CaBP expression in some GCs after seizures (Baimbridge et al., 1985; Tonder et al., 1994).
Fig. 1.
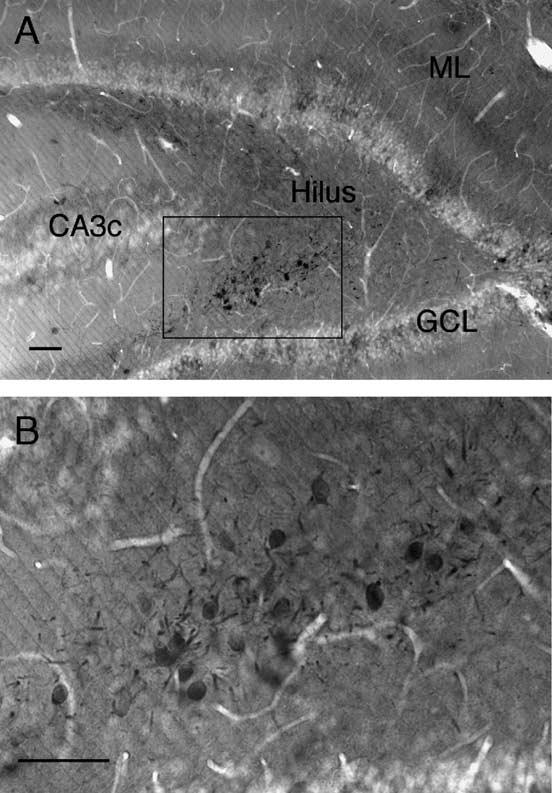
CaBP immunoperoxidase-labeled cell bodies and dendrites within the hilus of a pilocarpine-treated animal, clustered near CA3c. Panel (B) is an enlargement of the region marked by the box in panel (A). ML, molecular layer; GCL, granule cell layer. Scale bar = 50 μm.
Immunoperoxidase labeling for ZnT-3 in tissue from saline-injected controls produced a pattern of labeling similar to that observed with Timm's staining, darkly filling the entire MF pathway, as previously reported for mouse and monkey tissue (Wenzel et al., 1997). Tissue from all pilocarpine-treated animals also displayed a dense band of labeling within the IML (Fig. 2), presumably corresponding to sprouted MFs. Additionally, in accord with Wenzel et al. (1997), saline-injected controls displayed weak labeling of the outer ML and even lighter diffuse labeling of the IML. Labeling within the IML was so light that it would not be apparent ultrastructurally (although a few darkly labeled individual fibers were observed, usually at ventral levels, probably representing a sparse MF projection to the IML that has been reported in normal tissue (Cavazos et al., 1992; Gaarskjaer, 1978; Molnár and Nadler, 1999) (see CaBP and ZnT-3 electron microscopy).
Fig. 2.
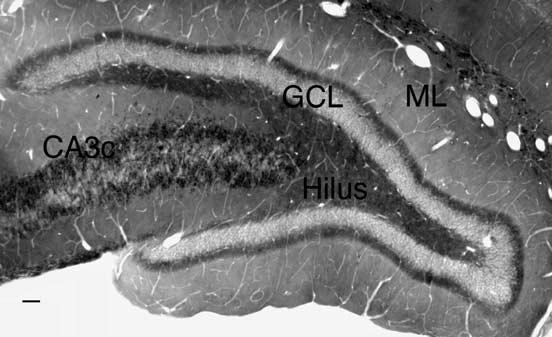
Immunoperoxidase labeling for ZnT-3 in a pilocarpine-treated animal visualized both the normal MF pathway and a dense region of sprouted MFs in the IML. ML, molecular layer; GCL, granule cell layer. Scale bar = 50 μm.
In dual-labeled tissue, the density of ZnT-3 labeling at the LM level generally obscured the immunogold CaBP labeling, using conventional illumination. However, oblique illumination, which reflected light from immunogold particles, revealed a distribution of CaBP-labeled cell bodies and dendrites in the hilus that matched that observed when CaBP immunoperoxidase labeling was used. These patterns of labeling appeared comparable along the dorsoventral axis, with the exception that, as previously reported (Buckmaster and Dudek, 1997; Cronin and Dudek, 1988), the density of MF labeling in the ventral IML was higher.
Dense clusters of CaBP-labeled cells were not observed in saline-treated control animals, where at most one or two labeled hilar cells per 40 μm section could be observed, usually within 100 μm of the GC layer. These cells presumably represent either CaBP-positive interneurons (Baimbridge and Miller, 1982; Gulyas et al., 1991, or EGCs, since EGCs have been observed in the normal hilus, albeit rarely (Gaarskjaer and Laurberg, 1983; Marti-Subirana et al., 1986; Scharfman et al., 2003; Sloviter, 1989). The incidence of control CaBP-labeled hilar cells is somewhat higher than previously reported (Scharfman et al., 2000), probably reflecting the use of a different antibody and immunolabeling protocol.
CaBP and ZnT-3 electron microscopy
At the ultrastructural level, dendritic profiles containing CaBP immunogold labeling were relatively common in the IML (Fig. 3) and hilus (Fig. 4) of tissue from pilocarpine-treated animals at dorsal and ventral hippocampal levels. Both the pattern of labeling and general structure of dendritic profiles in both regions was similar (see Table 1). Dendritic profiles in the IML contained multiple immunogold particles (on average 3.2 ± 0.3, n = 188), tended to be oriented perpendicular to the GC layer, and had a mean diameter of 1.3 ± 0.1 μm. In the hilus, CaBP-labeled dendritic profiles had 3.3 ± 0.1 (n = 468) immunogold particles, displayed no apparent consistent orientation to the GC layer, and had a mean diameter of 1.4 ± 0.1 μm. Interestingly, the diameter of hilar CaBP-labeled dendritic profiles changed significantly in relation to survival time (see Table 2): older animals had larger dendritic profiles (1.6 ± 0.1 μm, n = 186) on average than younger animals (1.3 ± 0.3 μm, P = 0.01, n = 282), possibly reflecting a restructuring of EGC dendritic arbors over time. These factors did not vary significantly along the dorsoventral axis (see Table 1).
Fig. 3.
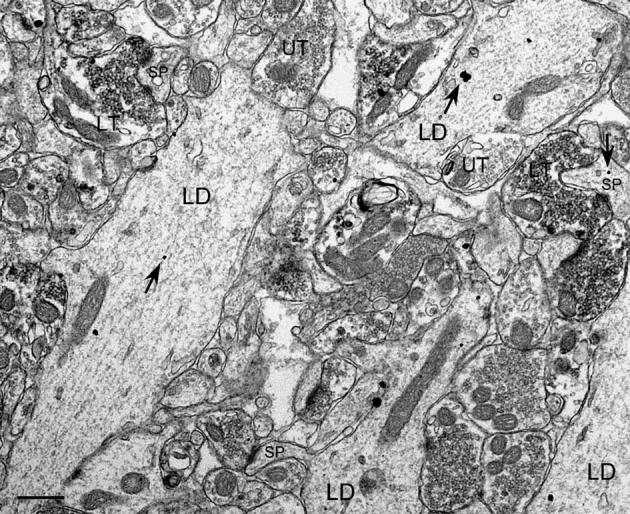
CaBP immunogold-labeled GC dendrites and ZnT-3 immunoperoxidase-labeled MF terminals in the IML of a pilocarpine-treated animal. LD, labeled dendrites; LT, representative labeled terminals; UT, representative unlabeled terminals; SP, spines. Arrows indicate representative immunogold particles. Scale bar = 0.5 μm.
Fig. 4.
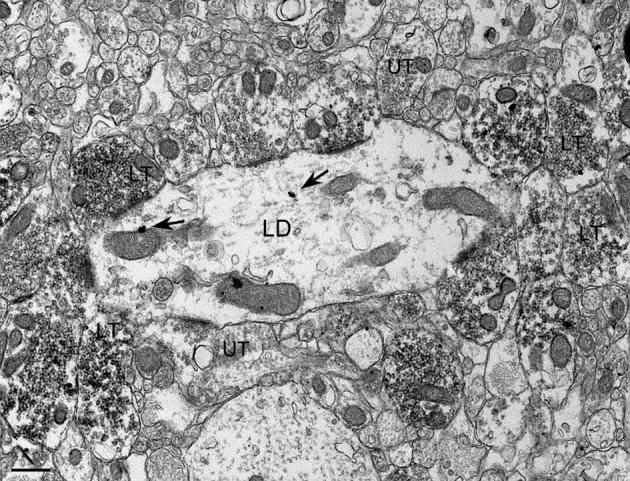
CaBP immunogold-labeled GC dendrites and ZnT-3 immunoperoxidase-labeled MF terminals in the hilus of a pilocarpine-treated animal. Labeled as in Fig. 3. Scale bar = 0.5 μm.
Table 2.
Ultrastructural characteristics of CaBP-labeled GC dendrites in the IML, and EGC dendrites in the hilus, subdivided by survival time after pilocarpine administration into two groups: a young group (Y, 5 animals, average survival time: 4.5 ± 0.6 months, range: 2.5 to 6.5 months) and an older group (O, 3 animals, average survival time: 13.2 ± 0.3 months, range: 12.5 to 13.5 months)
| IML | Hilus | ||||
|---|---|---|---|---|---|
| Y | O | Y | O | ||
| 1 | Gold particle # | 3.4 ± 0.4 (129) | 2.8 ± 0.2 (59) | 3.3 ± 0.2 (282) | 3.2 ± 0.2 (186) |
| 2 | Dendritic profile diameter (Am) | 1.28 ± 0.6 (129) | 1.27 ± 0.6 (59) | 1.3 ± 0.3* (282) | 1.6 ± 0.1* (186) |
| 3 | % MF terminal coverage (D) | 11 ± 3 (53) | 9 ± 1 (32) | 39 ± 1 (137) | 42 ± 4 (84) |
| 3 | % MF terminal coverage (V) | 14 ± 2 (76) | 10 ± 1 (27) | 27.9 ± 0.6 (145) | 31 ± 3 (102) |
| 4 | % of MF terminal profiles with an active zone (AZ) | 12 ± 1 (254) | 10 ± 3 (78) | 47 ± 2 (1333) | 51 ± 2 (1145) |
| 5 | % of MF terminal profile AZs formed with labeled spines | 45 ± 10 (33) | 40 ± 20 (8) | 9 ± 4 (629) | 10 ± 3 (595) |
| 6 | % of MF terminal profile AZs formed with all spines (labeled and unlabeled) | 91 ± 2 (84) | 92 ± 2 (25) | – | – |
| 7 | % terminal coverage (D) (all terminals) | 25 ± 2 (53) | 21 ± 2 (32) | 55 ± 3 (137) | 60.6 ± 0.6 (84) |
| 7 | % terminal coverage (V) (all terminals) | 25 ± 2 (76) | 24 ± 2 (27) | 44 ± 2 (145) | 51 ± 3 (102) |
| 8 | % of all terminal profiles that were MF terminal profiles | 49 ± 5 (511) | 42 ± 3 (181) | 64 ± 3 (2097) | 62 ± 4 (1823) |
| 9 | % of all AZs that were asymmetric | 79 ± 6 (99) | 86 ± 10 (22) | 91 ± 2 (869) | 92 ± 3 (870) |
| 10 | % of all AZs that were symmetric | 21 ± 6 (99) | 14 ± 10 (22) | 9 ± 2 (869) | 8 ± 3 (870) |
| 11 | % of all terminal profiles with asymmetric AZs that were MFs | 41 ± 7 (78) | 38 ± 7 (19) | 79 ± 2 (791) | 72 ± 3 (807) |
| 12 | % dendritic coverage | 20 ± 2 (129) | 20 ± 2 (59) | 1.7 ± 0.3 (282) | 2 ± 1 (186) |
| 13 | % unmyelinated axon coverage | 27 ± 1 (129) | 27 ± 2 (59) | 22 ± 1 (282) | 18 ± 1 (186) |
| 14 | % glial coverage | 19 ± 2 (129) | 21 ± 2 (59) | 21 ± 1 (282) | 16 ± 2 (186) |
| 15 | % spine coverage | 6.4 ± 0.5 (129) | 5.5 ± 0.4 (59) | 5 ± 1 (282) | 6 ± 2 (186) |
| 16 | % somal coverage | 0.4 ± 0.3 (129) | 1.2 ± 0.3 (59) | 0.1 ± 0.1 (282) | 0.3 ± 0.3 (186) |
| 17 | % myelinated axon coverage | 0.8 ± 0.5 (129) | 0.4 ± 0.1 (59) | 0.1 ± 0.1 (282) | 0.7 ± 0.4 (186) |
| 18 | % unclassifiable coverage | 1.2 ± 0.3 (129) | 2.4 ± 0.6 (59) | 1.2 ± 0.3 (282) | 0.6 ± 0.2 (186) |
While CaBP immunogold-labeled dendritic profiles appeared to be equally frequent in the IML of tissue from saline-treated animals, they were quite difficult to locate in random hilar fields, as one would expect given that saline controls have so few CaBP-positive cells. ZnT-3-labeled terminal profiles were very common in the hilus of both pilocarpine and saline-treated animals and displayed features characteristic of MF terminals (Amaral, 1979; Blackstad and Kjaerheim, 1961; Claiborne et al., 1986), such as the frequent presence of dense core vesicles (Fig. 5). CaBP immunogold labeling was also often present in MF terminal and unmyelinated axon profiles, of course, since they are GC axons. As previously observed (Wenzel et al., 1997), ZnT-3 peroxidase labeling was relatively evenly distributed within a given terminal profile, although the absolute intensity of labeling did vary between profiles. ZnT-3-labeled terminal profiles were also frequently observed in the IML of pilocar-pine-treated animals, at both dorsal and ventral levels (see Table 1). In contrast, ZnT-3-labeled terminal profiles were only rarely present in the IML of saline-injected animals, usually at ventral levels, where a sparse MF projection to the IML is known to be concentrated (Cavazos et al., 1992; Gaarskjaer, 1978; Molnár and Nadler, 1999).
Synaptic input to GC dendrites in the IML
CaBP-labeled GC dendrites in the IML were often contacted by ZnT-3-labeled MF terminals (see Table 1). Measurements of the amount of contact between profiles of these processes indicate that, on average, 11 ± 1% (n = 188) of the surface of GC IML dendrites was occupied by MF terminals (this includes the surface of both the dendritic shaft and any spines observed directly emanating from it). Additionally, 12 ± 1% of the MF terminals (n = 332) formed an asymmetric synaptic contact with the dendrite in the examined plane of section. Of these synapses, 40 ± 10% were formed with spines, while 60 ± 10% were formed with the dendritic shaft (n = 41). It should be noted that this probably represents an underestimate of the relative proportion of synaptic input to spines versus dendritic shafts because CaBP immunogold labeling did not fill the entire postsynaptic process (unlike ZnT-3 peroxidase labeling, which filled terminals). Thus, the heads of spines that were not in direct contact with the dendritic shaft in the plane of section could not be identified as part of a labeled dendrite (a few spine head profiles did contain a single gold particle, however; Figs. 3, 6). Labeled MF terminal profiles in apposition to labeled dendritic profiles were frequently observed only forming synapses with small isolated spine head profiles in the surrounding neuropil (Figs. 3, 6). If these spine head synapses were included in the analysis, then 92 ± 1% of synapses were formed with spines versus 8 ± 1% with the dendritic shaft (n = 109). This is comparable to values reported by Buckmaster et al. (2002), who examined intracellularly labeled MFs of GC layer GCs that projected to the IML of pilocarpine-treated animals (93% of synapses were formed with spines, and 7% were formed with dendritic shafts, a portion being interneuron dendrites based on postembedding GABA immunohistochemistry). It also closely parallels the findings of Zhang and Houser (1999), who examined dynorphin-immunolabeled MF terminals in the IML of tissue from patients with intractable temporal lobe epilepsy (TLE): 93.6% of the synaptic contacts examined were axospinous and 6.4% were axodendritic. It is less comparable to the findings of Cavazos et al. (2003), who used Timm's histochemistry to identify IML MF terminals of kainic-acid-treated animals (34% of the synapses formed were axospinous, 22% were axodendritic, and 4% were axosomatic), possibly due to differences in the animal model and methods. However, Cavazos et al. (2003) did observe some phenomena similar to those in the present study, such as clustering of IML MF terminals on the surface of dendritic shafts (Fig. 7).
Fig. 6.
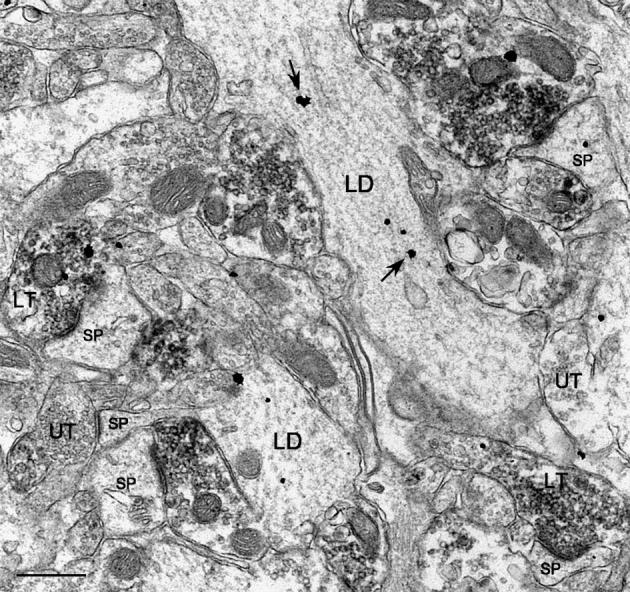
Although labeled MF terminals could often be observed in apposition to labeled GC dendrites in the IML, they most frequently formed synapses with small spines, some of which were also immunogold-labeled for CaBP. Labeled as in Fig. 3. Scale bar = 0.5 μm.
Fig. 7.
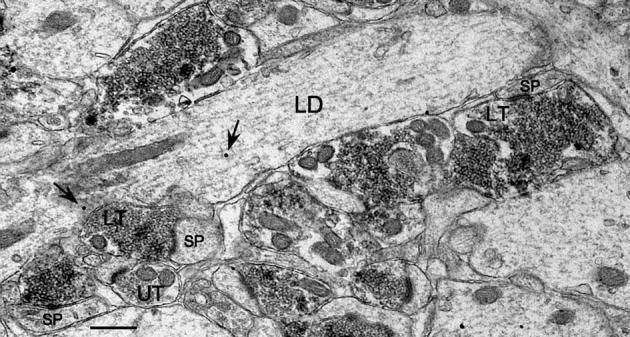
MF terminals were at times observed clustered along the shafts of GC dendrites in the IML. Labeled as in Fig. 3. Scale bar = 0.5 μm.
Axon terminals of all types covered, on average, about a quarter (24 ± 1%, n = 188) of the surface of GC dendrites in the IML (and 46 ± 4% (n = 692) of the terminal profiles examined were MF terminal profiles). Of the terminals with a synapse in the observed plane of section, 81 ± 5% were asymmetric and thus presumably excitatory, while 19 ± 5% were symmetric and thus presumably inhibitory (n = 121) (Peters et al., 1991). MF terminals comprised 40 ± 5% of the terminal profiles displaying asymmetric contacts (n = 97). None of these measures varied significantly between dorsal and ventral portions of the dentate gyrus, so the data were pooled (see Table 1).
Synaptic input to EGC dendrites in the hilus
The most striking feature of CaBP-labeled dendrites in the hilus was the extent to which they were contacted by MF terminals (Table 1, see also Figs. 4, 8-10). Measurements of the amount of apposition between these processes indicate that 40 ± 2% (n = 225) of the surface of EGC dendrites in the dorsal hilus, and 29 ± 1% (n = 243) of the surface of EGC dendrites in the ventral hilus, were occupied by MF terminals (this dorsoventral difference was significant, P < 0.0001). The pattern of MF coverage in the hilus was also significantly denser than that observed at comparable levels in the IML (10 ± 2% dorsally, 12 ± 1% ventrally, both P < 0.0001). Hilar MF terminal profiles could be observed forming synapses with both sides of a spine, just after the spine base extended out from an EGC dendritic shaft (Fig. 9). MF terminals in the hilus also appeared to form a particularly strong synaptic linkage relative to the IML since a synaptic contact was seen on approximately one half (48 ± 1%; n = 2478) of the MF terminals in the plane of section examined (significantly greater than the 12 ± 1% observed in the IML, P < 0.0001). This indicates that, on average, a larger portion of terminal surface area apposed to labeled dendrites in the hilus was composed of active zones.
Fig. 8.
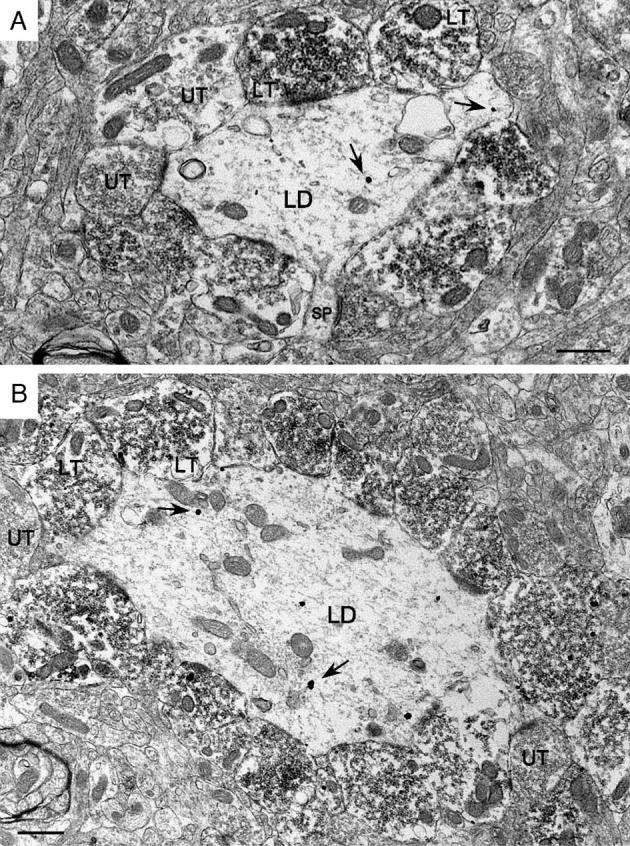
The most prominent characteristic of CaBP-labeled dendrites (A, B) in the hilus was the extent to which they were contacted by MF terminals. Labeled as in Fig. 3. Scale bar = 0.5 μm.
Fig. 10.
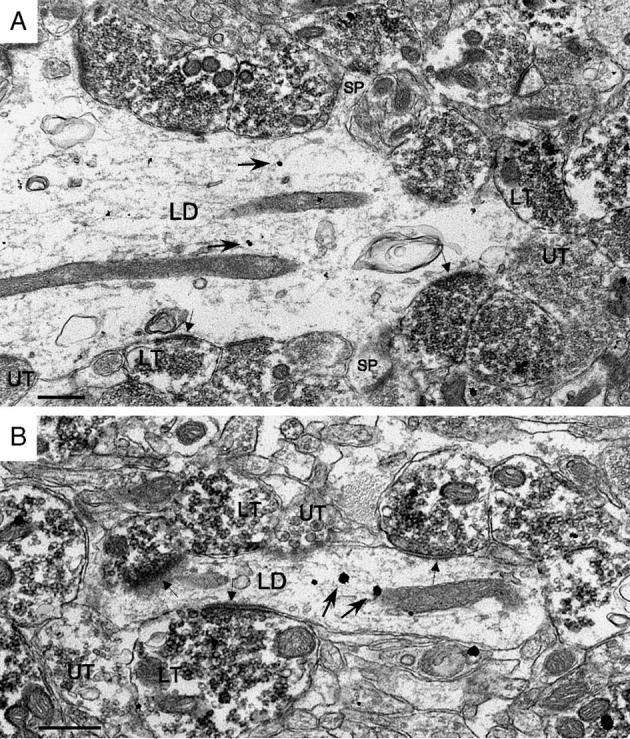
Hilar MF terminals frequently formed axodendritic synapses with both large (A) and small (B) diameter EGC dendrites (small arrows indicate synaptic specializations formed by MF terminals). Labeled as in Fig. 3. Scale bar = 0.5 μm.
Fig. 9.
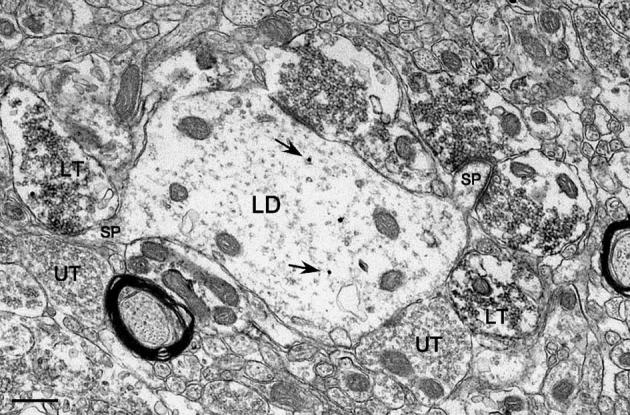
MF terminals within the hilus could be observed forming synapses with spines as they emerged from the dendritic shaft. Labeled as in Fig. 3. Arrows indicate representative immunogold particles. Scale bar = 0.5 μm.
Surprisingly, the postsynaptic densities of synapses on EGC dendrites, although distinct, were often thinner than what one would observe with an MF terminal in control tissue (Fig. 5). As a result, their identification as excitatory ‘asymmetric’ MF terminal connections also relied on other factors, such as the frequent presence of dense core vesicles within the terminal profile (Fig. 5) and the specificity of the ZnT-3 antibody (which has been shown to only label glutamatergic terminals within the hilus with MF characteristics, using dual labeling techniques for ZnT-3 and glutamate; Wenzel et al., 1997). In the present study, the presence of a thin postsynaptic density appeared relatively characteristic of MF terminal synapses on the shafts and spines of hilar EGC dendrites (Figs. 4, 8-10) and could reflect an aspect of a projection that has reorganized to synapse upon an abnormal postsynaptic target.
Axospinous MF synapses comprised 9 ± 2% (n = 1224) of the synapses, significantly less than in the IML (40 ± 10%, P = 0.01). As discussed above (in relation to IML dendrites), this presumably represents an underestimate of the proportion of MF terminals contacting EGC spines. LM examination of the dendrites of intracellularly labeled hilar EGCs indicates that they can be quite spiny (Scharfman et al., 2000; Scharfman et al., 2003). The low percentage of observed axospinous synapses also presumably reflects the increased frequency with which MF terminals formed synapses directly on the shafts of EGC dendrites (Fig. 10).
MF synaptic input also represented the predominant source of afferent input to EGC dendrites. Axon terminals of all types covered 57 ± 2% (n = 225) of the surface of EGC dendrites in the dorsal hilus and 46 ± 2% (n = 243) of the surface in the ventral hilus (this dorsoventral difference was significant, P = 0.0009). The majority of the terminal profiles (63 ± 2%; n = 3920) examined could be identified as MF terminal profiles, a significantly greater proportion ( P = 0.001) than in the IML (46 ± 4%; n = 692). Examining all terminals, of those with synapses in the plane of section, 91 ± 1% of the synapses were asymmetric and 9 ± 1% were symmetric (n = 1739) (suggesting that EGC dendrites might receive higher levels of excitatory input than GC dendrites in the IML). Additionally, of the terminal profiles forming asymmetric synapses, 77 ± 2% (n = 1598) were MF terminal profiles, a significantly greater proportion than in the IML (40 ± 5%, P < 0.0001).
As mentioned above, to examine the effect of survival time, animals were subdivided into two groups (see Table 2). Except for the change in hilar dendritic profile diameter already described, there were no significant differences in values generated from relatively young (average survival time: 4.5 ± 0.6 months) and older (average survival time: 13.2 ± 0.3 months) animals, suggesting that these measures of synaptic connectivity did not change systematically over time.
Differences in the microenvironment of EGC dendrites in the hilus and GC dendrites in the IML
These measures of synaptic connectivity strongly suggest that the pattern of synaptic input that develops on EGC dendrites as they mature within the hilus is significantly different from the pattern that develops on GC dendrites in the IML. As mentioned, overall, approximately half (57 ± 2% dorsally, n = 225; 46 ± 2% ventrally, n = 243) of the surface of EGC dendrites was exposed to terminals (of all types), roughly twice what was observed for GC dendrites at comparable levels of the IML (23 ± 2% dorsally, 25 ± 1% ventrally, both P < 0.0001). Other differences in the microenvironment of these dendrites in the hilus and IML could, at least in part, help to explain why contrasting patterns of synaptic input develop. For example, the dense packing of GC layer GCs and the associated array of dendrites extending into the ML could obstruct access, particularly in the IML, where dendrites were often in contact with one another. Thus, 20 ± 1% (n = 188) of the surface of CaBP-labeled dendrites in the IML was apposed to other dendrites (Fig. 11). In contrast, only 1.9 ± 0.5% (n = 468) of the surface of EGC dendrites in the hilus was in contact with other dendrites (P < 0.0001). An apparent difference in the density of unmyelinated axons might also be a factor since GC dendrites in the IML had 27 ± 1% of their surface in contact with unmyelinated axons, a significantly higher level than observed for EGC dendrites in the hilus (20 ± 1%, P < 0.0003). The levels of coverage for all other types of processes did not differ significantly between the IML and hilus (see Table 1). For example, glial processes covered 20 ± 1% of the surface of GC IML dendrites and 19 ± 1% of EGC dendrites. Finally, it should be noted that all comparisons between EGC dendrites in the hilus and GC layer GC dendrites in the IML might be limited by the fact that the analyzed dendritic segments were probably not entirely comparable in terms of distance from the cell body. While most of the IML dendritic profiles were presumably relatively proximal, EGC dendritic profiles probably represented samples from many points of the dendritic tree.
Fig. 11.
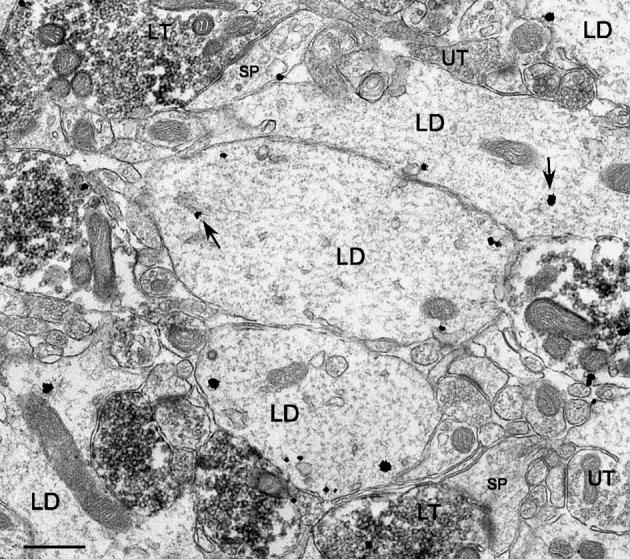
Labeled GC dendrites in the IML were often seen in direct apposition with each other. Labeled as in Fig. 3. Scale bar = 0.5 μm.
EGCs and hilar circuitry
The results described above suggest that, when newborn GCs mature ectopically in the hilus after pilocarpine-induced status epilepticus, their dendrites develop in a microenvironment that allows a much greater exposure of the dendritic surface to afferent input. The frequency with which synaptic connections are observed on these dendrites implies that EGCs are able to insert themselves into hilar circuitry, as previously suggested (Dashtipour et al., 2001; Nadler, 2003; Ribak et al., 2000; Scharfman et al., 2002, 2003). In these circuits, MFs seem to play a critical role in providing afferent input to EGCs since roughly three quarters of all excitatory synapses on EGC dendrites were formed by MF terminals. These terminals could originate from the sprouting of local axon collaterals of either GC layer GCs (Buckmaster and Dudek, 1999; Sutula et al., 1998) or EGCs which develop arborizations that project locally, to CA3, and even back to the IML (Scharfman et al., 2000).
The synaptic linkage formed between MF terminals and the dendritic shafts and spines of EGC dendrites appears to be particularly strong relative to GC layer GCs. ZnT-3-labeled MF terminals displayed a synapse in the examined plane of section approximately half of the time, indicating that a relatively large portion of the apposed surface was composed of active zones. Both the size and number of active zones have been correlated with quantal release characteristics. Using the binomial model (Katz, 1969), active zone number is thought to correspond to n, the number of release sites, and active zone size is thought to correspond to p, the probability of release from a given site, based on findings from a wide variety of synapse types (Propst and Ko, 1987; Schikorski and Stevens, 1997; Walmsley, 1991).
In addition to the surprisingly strong pattern of MF innervation of EGCs, synapses were more frequently formed with the dendritic shafts of EGCs, compared to GC layer GCs. These synapses also often displayed an unusual morphology, with thinner postsynaptic densities than one would observe with MF terminals in the IML, or in control tissue. MF terminals that innervate GC layer GCs preferentially terminate on spines (Buckmaster et al., 2002; Cavazos et al., 2003; Zhang and Houser, 1999). In the hilus of the normal rodent, the local collaterals of MFs produce large terminals that form synapses which envelop mossy cell spines and small terminals that synapse on the spines and dendritic shafts of interneurons (Acsády et al., 1998; Blackstad and Kjaerheim, 1961; Claiborne et al., 1986; Pierce et al., 1999). The hilar MF innervation pattern observed in the present study suggests that factors which normally control the destination of MFs are disrupted after pilocarpine-induced status epilepticus and chronic seizures. Alternately, developing GCs might express different patterns of surface adhesion molecules, or differentially release trophic factors relative to normal GCs, and this could have an effect on synapse formation.
Recurrent excitatory connections, EGCs, and epilepsy
Since the initial studies of MF sprouting (Nadler et al., 1980; Tauck and Nadler, 1985), many laboratories have provided evidence that abnormal GC to GC connections, which develop as a result of sprouting, could create an excitatory feedback pathway that could be relevant to the pathophysiology of TLE (Dudek et al., 1994; Nadler, 2003; Sutula et al., 1992). MF sprouting has been observed using a wide variety of TLE models, including kainic acid (Buckmaster and Dudek, 1997; Cronin and Dudek, 1988; Tauck and Nadler, 1985), kindling (Represa et al., 1993; Sutula et al., 1988), pilocarpine (Mello et al., 1993; Okazaki et al., 1995), pentylenetetrazol (Golarai et al., 1992), and head trauma (Golarai et al., 1992), and has also be described in tissue from TLE patients (de Lanerolle et al., 1989; Houser et al., 1990; Sutula et al., 1989). Analysis of MF terminals in the IML at the EM level has demonstrated that the preponderance of synapses represents recurrent connections with GC dendrites (Buckmaster et al., 2002; Cavazos et al., 2003; Frotscher and Zimmer, 1983), although there is also evidence that sprouted MFs innervate GABAergic neurons (Kotti et al., 1997; Sloviter, 1992). MF sprouting is associated with an increase in seizure frequency and duration (Gorter et al., 2001; Zhang et al., 2002), and modeling suggests that even relatively sparse levels of sprouting could significantly influence the spread of epileptiform activity (Santhakumar et al., 2005). However, it has also been argued that MF sprouting can be dissociated from the development of recurrent seizures (Armitage et al., 1998), and can lead to increased inhibition (Sloviter, 1992). The factors involved in determining whether MF sprouting does or does not contribute to increased excitability remain unclear.
Recent studies indicate that additional recurrent excitatory GC to GC pathways, beyond those postulated above (GC layer GC to other GC layer GC IML dendrites), can develop following the induction of seizures. Some GCs develop basal dendrites that extend into the hilus and can receive MF synaptic input (Buckmaster and Dudek, 1999; Ribak et al., 2000; Spigelman et al., 1998). MF contacts have also been observed on the proximal dendrites of EGCs near the GC layer (Dashtipour et al., 2001). This pattern of restructuring suggests that an interconnected GC network can evolve which could potentially support protracted epileptiform activity within the dentate gyrus (Nadler, 2003).
The current findings indicate that EGCs should now be considered a potentially critical component of this recurrent excitatory network. Hilar EGCs are activated when spontaneous recurrent seizures occur after pilocarpine-induced status epilepticus (Scharfman et al., 2002), and they can be readily activated by perforant path stimulation, displaying robust responses to stimulus levels that also activate GC layer GCs (Scharfman et al., 2003). The present study demonstrates that MFs represent the predominant source of afferent input to EGC dendrites throughout the hilus. Comprising 70% of all terminals and 77% of all excitatory terminals that formed synapses with these dendrites, they occupied on average a full third of the dendritic surface. The synapses were formed with both dendritic spines and shafts, reflecting an apparently strong pattern of input. In addition, all of these measures of MF connectivity were significantly greater than the comparable values measured for sprouted MFs that innervate GC layer GCs in the IML within the same tissue sections, underscoring the relative importance of recurrent excitatory pathways involving EGCs. These anatomical characteristics indicate that EGCs that mature in the hilus develop synaptic connections that could both predispose them to being unusually excitable and provide a path through which that activity could spread. As mentioned, a portion of the MFs contacting EGCs presumably originates from other EGCs, which have local axonal arbors within the hilus (Scharfman et al., 2000). Thus, by becoming integrated into an evolving network of recurrent excitatory connections, EGCs are critically positioned to play a role in epileptiform activity and, possibly, the generation of seizure activity. Indeed, a recent study indicates that EGCs may enhance the frequency and duration of recurrent seizures after status epilepticus induced by lithium and pilocarpine (Jung et al., 2004).
In summary, this study provides further support for the hypothesis that seizure-induced neurogenesis, and specifically the development of EGCs in the hilus, disrupts normal hippocampal circuitry. The finding that EGCs receive a strong pattern of MF afferent input, apparently even stronger than the pattern observed with MF input to GC layer GCs, suggests that recurrent excitatory circuits that develop after pilocarpine-induced status epilepticus are more complex than previously considered. Potentially, inhibitory networks are as well, and future studies will be required to define these and other changes in the dentate gyrus network. However, it is clear that a complex network of excitatory connections develops, involving not only GCs in the GC layer innervating each other, but also novel EGCs in the hilus, and these new circuits may contribute to epileptogenesis in the pilocarpine model of TLE.
Acknowledgments
This research was supported by NIH NS 41490, the Epilepsy Foundation, and the Helen Hayes Hospital Foundation. We greatly appreciate Dr. Richard Palmiter's generous gift of zinc transporter-3 antibody, without which this project could not have been completed.
References
- Acsády L, Kamondi A, Sik A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. Synaptic extensions from the mossy fibers of the fascia dentata. Anat. Embryol. 1979;155:241–251. doi: 10.1007/BF00317638. [DOI] [PubMed] [Google Scholar]
- Armitage LL, Mohapel P, Jenkins EM, Hannesson DK, Corcoran ME. Dissociation between mossy fiber sprouting and rapid kindling with low-frequency stimulation of the amygdala. Brain Res. 1998;781:37–44. doi: 10.1016/s0006-8993(97)01218-3. [DOI] [PubMed] [Google Scholar]
- Auchus AP, Pickel VM. Quantitative light microscopic demonstration of increased pallidal and striatal Met5-enkephalin like immunoreactivity in rats following chronic haloperidol but not with clozapine. Exp. Neurol. 1992;117:17–27. doi: 10.1016/0014-4886(92)90106-z. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium binding protein in the cerebellum, hippocampal formation, and olfactory bulb of the rat. Brain Res. 1982;245:223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Mody I, Miller JJ. Reduction of rat hippocampal calcium-binding protein following commissural, amygdala, septal, perforant path, and olfactory bulb kindling. Epilepsia. 1985;26:460–465. doi: 10.1111/j.1528-1157.1985.tb05681.x. [DOI] [PubMed] [Google Scholar]
- Blackstad TW, Kjaerheim A. Special axo-dendritic synapses in the hippocampal cortex. Electron and light microscopic studies on the layer of mossy fibers. J. Comp. Neurol. 1961;117:133–159. doi: 10.1002/cne.901170202. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuronal loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J. Comp. Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. J. Neurophysiol. 1999;81:712–721. doi: 10.1152/jn.1999.81.2.712. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J. Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Golarai G, Sutula TP. Septohippocampal variation of the supragranular projection of the mossy fiber pathway in the dentate gyrus of normal and kindled rats. Hippocampus. 1992;2:363–372. doi: 10.1002/hipo.450020404. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Zhang P, Qazi R, Sutula TP. Ultrastructural features of sprouted mossy fiber synapses in kindled and kainic acid-treated rats. J. Comp. Neurol. 2003;458:272–292. doi: 10.1002/cne.10581. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. The light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Cronin J, Dudek FE. Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res. 1988;474:181–184. doi: 10.1016/0006-8993(88)90681-6. [DOI] [PubMed] [Google Scholar]
- Dashtipour K, Tran PH, Okazaki MM, Nadler JV, Ribak CE. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res. 2001;890:261–271. doi: 10.1016/s0006-8993(00)03119-x. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Descarries L, Soghomonian J-J, Garcia S, Doucet G, Bruno JP. Ultrastructural analysis of the seritonin hyperinnervation in adult rat neostriatum following neonatal dopamine denervation with 6-hydroxydopamine. Brain Res. 1992;569:1–13. doi: 10.1016/0006-8993(92)90363-e. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Obenaus A, Schweitzer JS, Wuarin JP. Functional significance of hippocampal plasticity in epileptic brain: electrophysiological changes of the dentate granule cells associated with mossy fiber sprouting. Hippocampus. 1994;4:259–265. doi: 10.1002/hipo.450040306. [DOI] [PubMed] [Google Scholar]
- Eldred WD, Zucker C, Karten HJ, Yazulla S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J. Histochem. Cytochem. 1983;31:285–292. doi: 10.1177/31.2.6339606. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Zimmer J. Lesion-induced mossy fibers to the molecular layer of the rat fascia dentata: identification of postsynaptic granule cells by the Golgi-EM technique. J. Comp. Neurol. 1983;215:209–311. doi: 10.1002/cne.902150306. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB. Organization of the mossy fiber system of the rat studied in extended hippocampi: I. Terminal area related to number of granule and pyramidal cells. J. Comp. Neurol. 1978;178:49–72. doi: 10.1002/cne.901780104. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB, Laurberg S. Ectopic granule cells of hilus fasciae dentate projecting to the ipsilateral regio inferior of the rat hippocampus. Brain Res. 1983;274:11–16. doi: 10.1016/0006-8993(83)90516-4. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Golarai G, Cavazos JE, Sutula TP. Activation of the dentate gyrus by pentylenetetrazol evoked seizures induces mossy fiber synaptic reorganization. Brain Res. 1992;593:257–264. doi: 10.1016/0006-8993(92)91316-7. [DOI] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur. J. Neurosci. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA. Regulation of neuronal birth, migration, and death in the rat dentate gyrus. Dev. Neurosci. 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J. Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Toth K, Danos P, Freund TF. Subpopulations of GABAergic neurons containing parvalbumin, calbindin D28k, and cholecystokinin in the rat hippocampus. J. Comp. Neurol. 1991;312:371–378. doi: 10.1002/cne.903120305. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J. Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981;29:557–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK. Continuous cytosine-b-d-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur. J. Neurosci. 2004;19:3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Liverpool University; Liverpool: 1969. [Google Scholar]
- Kotti T, Riekkinen PJ, Miettinen R. Characterization of target cells for aberrant mossy fiber collaterals in the dentate gyrus of epileptic rat. Exp. Neurol. 1997;146:323–330. doi: 10.1006/exnr.1997.6553. [DOI] [PubMed] [Google Scholar]
- Marti-Subirana A, Soriano E, Garcia-Verdugo JM. Morphological aspects of the ectopic granule-like cellular populations in the albino rat hippocampal formation: a Golgi study. J. Anat. 1986;144:31–47. [PMC free article] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Babb TL, Kupfer WR, Pretorius JK, Tan AM, Finch DM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Molnár P, Nadler JV. Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J. Neurophysiol. 1999;82:1883–1894. doi: 10.1152/jn.1999.82.4.1883. [DOI] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem. Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Cotman CW. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3–CA4 afferents with kainic acid. Brain Res. 1980;182:1–9. doi: 10.1016/0006-8993(80)90825-2. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Evenson DA, Nadler JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J. Comp. Neurol. 1995;352:515–534. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Lowenstein DH. Seizure-induced neurogenesis: are more new neurons good for an adult brain? Prog. Brain Res. 2002;135:121–131. doi: 10.1016/S0079-6123(02)35012-X. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geshwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the rat. J. Neurosci. 1997;17:3727–3728. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Peters A, Palay SL, Webster H. Oxford Univ. Press; New York: 1991. The Fine Structure of the Nervous System. [Google Scholar]
- Pierce JP, Milner TA. Parallel increases in the synaptic and surface areas of mossy fiber terminals following seizure induction. Synapse. 2001;39:249–256. doi: 10.1002/1098-2396(20010301)39:3<249::AID-SYN1006>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Propst JW, Ko C-P. Correlations between active zone ultrastructure and synaptic function studied with freeze-fracture of physiologically identified neuromuscular junctions. J. Neurosci. 1987;7:3654–3664. doi: 10.1523/JNEUROSCI.07-11-03654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RC. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rami A, Brehier A, Thomasset M, Rabie A. The comparative immunocytochemical distribution of 28 kDa cholecalcin (CaBP) in the hippocampus of rat, guinea pig and hedgehog. Brain Res. 1987:422–153. doi: 10.1016/0006-8993(87)90549-x. [DOI] [PubMed] [Google Scholar]
- Represa A, Jorquera I, Le Gal La Salle G, Ben-Ari Y. Epilepsy induced collateral sprouting of hippocampal mossy fibers: does it induce the development of ectopic synapses with granule cell dendrites? Hippocampus. 1993;3:257–268. doi: 10.1002/hipo.450030303. [DOI] [PubMed] [Google Scholar]
- Represa A, Niquet J, Pollard H, Khrestchatisky M, Ben-Ari Y. From seizures to neo-synaptogenesis: intrinsic and extrinsic determinants of mossy fiber sprouting in the adult hippocampus. Hippocampus. 1994;4:270–274. doi: 10.1002/hipo.450040308. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J. Comp. Neurol. 2000;428:240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: a network model of the dentate gyrus incorporating cell types and axonal topography. J. Neurophysiol. 2005;93:437–453. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Functional implications of seizure-induced neurogenesis. Adv. Exp. Med. Biol. 2004;548:192–212. doi: 10.1007/978-1-4757-6376-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J. Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AE, Berger RE, Goodman JH, Pierce JP. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience. 2003;121:1017–1029. doi: 10.1016/s0306-4522(03)00481-0. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Intracerebroventricular kainic acid administration in adult rat alters hippocampal calbindin and non-phosphorylated neurofilament expression. J. Comp. Neurol. 1995;363:581–599. doi: 10.1002/cne.903630406. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry: localization in the rat hippocampus with special reference to the selective vulnerability of hippocampal neurons to seizure activity. J. Comp. Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci. Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Sollas AL, Barbaro NM, Laxer KD. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J. Comp. Neurol. 1991;308:381–396. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Yan X-X, Obenaus A, Lee Y-S, Wasterlain CG, Ribak CE. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86:109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Sutula T, Xiao-Xian H, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann. Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Golarai G, Cavazos J. Assessing the functional significance of mossy fiber sprouting. Epilepsy Res. 1992;(Suppl 7):251–259. [PubMed] [Google Scholar]
- Sutula T, Zhang P, Lynch M, Sayin U, Golarai G, Rod R. Synaptic and axonal remodeling of mossy fibers in the hilus and supragranular region of the of the dentate gyrus in kainate-treated rats. J. Comp. Neurol. 1998;390:578–594. doi: 10.1002/(sici)1096-9861(19980126)390:4<578::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J. Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonder N, Kragh J, Bolwig T, Zimmer J. Transient decrease in calbindin immunoreactivity of the rat fascia dentata granule cells after repeated electroconvulsive shocks. Hippocampus. 1994;4:79–83. doi: 10.1002/hipo.450040110. [DOI] [PubMed] [Google Scholar]
- Walmsley B. Central synaptic transmission: studies at the connection between primary afferent fibres and dorsal spinocerebellar tract (DSCT) neurones in Clarke's column of the spinal cord. Prog. Neurobiol. 1991;36:391–423. doi: 10.1016/0301-0082(91)90017-u. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Stereological Methods. Academic Press; London: 1979. [Google Scholar]
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Houser CR. Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: a study of reorganized mossy fiber synapses. J. Comp. Neurol. 1999;405:472–490. [PubMed] [Google Scholar]
- Zhang X, Cui SS, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, Honer WG, Corcoran ME. Relations between brain pathology and temporal lobe epilepsy. J. Neurosci. 2002;22:6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]