Residual Antigen Presentation after Influenza Virus Infection Affects CD8 T Cell Activation and Migration (original) (raw)
. Author manuscript; available in PMC: 2010 Apr 29.
Summary
Activated virus-specific CD8 T cells remain in the lung airways for several months after influenza virus infection. We show that maintenance of this cell population is dependent upon the route of infection and prolonged presentation of viral antigen in the draining lymph nodes (DLN) of the respiratory tract. The local effects on T cell migration have been examined. We show retention of virus-specific CD8 T cells in the mediastinal lymph node (MLN) and continuing recruitment of blood-borne migrants into the lung airways during antigen presentation. These data show that antigen that is retained after pulmonary influenza virus infection controls the migratory pattern and activation state of virus-specific CD8 T cells near the site of virus amplification.
Introduction
Cytotoxic T cells (CTL) play an important role in clearance of influenza and other respiratory virus infections and can provide short-term protective cellular immunity against recurrent illness with a new serotype (Nguyen et al., 1999, 2001; Topham et al., 1997; Webby et al., 2003). Curiously, this heterosubtypic protection declines over the course of a few months (Liang et al., 1994) even though large numbers of virus-specific CD8 T cells continue to circulate through the lungs and other organs of the infected animals for at least 2 years (Doherty et al., 1996). The reason for the rapid decline in cellular immunity is unclear, although the disappearance of antigen-specific CD8 T cells from the lung airways is believed to contribute (Hogan et al., 2001a; Ostler et al., 2001; Woodland et al., 2002; Ray et al., 2004). It has been established that T cells that are transferred into the lung airways can promote viral clearance (Hogan et al., 2001b). In another study, protection declined when CD8T cell migration to the airways was inhibited after viral infection (Ray et al., 2004).
The mechanisms that control T cell migration to the lungs are poorly defined; however, it is clear that some level of T cell activation and/or inflammation of the lungs is essential for virus-specific CD8 T cells to remain in the airways by a process that is assisted by integrin VLA-1 (Ray et al., 2004). Small numbers of bystander CD8 T cells also enter the lung airways during heterologous respiratory infections and other conditions of nonspecific inflammation (Ely et al., 2003a; Stephens et al., 2002), but whether these T cell populations are maintained or replenished later in the response has not been investigated. The virus-specific CD8 T cells that can be isolated from the lung airways after influenza virus infection are distinct from the virus-specific memory CD8 T cells in other tissues, including the lung parenchyma. A large percentage of these T cells express markers that are characteristically found on recently activated effector T cells, such as CD69 and CD25 (Marshall et al., 2001; Hogan et al., 2001a; Ostler et al., 2001). Our studies show that these T cells also lack IL-7R expression, which is another indication of recent antigen stimulation (Lang et al., 2005; Schluns et al., 2000). Together, these data suggest that a prolonged effector T cell response may be responsible for the chronic activation of virus-specific CD8 T cells in the lung airways. A recent study showed that influenza antigens were presented to CD4 T cells up to 4 weeks after infection (Jelly-Gibbs et al., 2005). A small number of the responding CD4 T cells survived to become resting memory T cells. The possibility that persistent antigen presentation is required for sustained cellular immunity has been suggested in other viral models (Gray, 2002; Kundig et al., 1996), but a link between the activated phenotype of memory T cells in the airways and the mechanisms that control local T cell migration have yet to be defined. Here, we have investigated the influence of antigen presentation on the migration of virus-specific memory CD8 T cells to the lungs and DLN of the respiratory tract during declining cellular immunity. Our studies show that influenza virus antigens, which are retained in the DLN of the respiratory tract, are presented to CD8 T cells for at least 2 months after infection. At the same time, the CD8 effector T cell population in the lung airways continues to be replenished by migrating T cells in the circulation. These data show that prolonged antigen presentation in the MLN helps to maintain activated CD8 T cells near the site of virus amplification in the lungs, where they can respond rapidly to secondary viral challenge.
Results
The Route of Infection Controls T Cell Activation in the Lung Airways
Virus-specific CD8 T cells that express CD69 have been detected in the lung airways for up to 3 months after influenza or other respiratory virus infections (Hogan et al., 2001a; Ostler et al., 2001; Marshall et al., 2001). In these studies, the CD69+ phenotype was stable, but the numbers of virus-specific CD8 T cells in the lung airways decreased with time. CD69 is typically induced by TcR signaling, although some cytokines can also upregulate CD69 expression on T cells (Kranzer et al., 2000; Sun and Sprent, 2000). As an additional indicator of TcR-mediated activation, we analyzed IL-7R (CD127) expression, because this receptor is expressed by naive T cells and resting memory T cells but is downregulated after activation (Schluns et al., 2000; Kaech et al., 2003; Huster et al., 2004; Lang et al., 2005). Virus-specific CD8 T cells were identified with the NP366–374/Db tetramer (Flynn et al., 1998) after i.n. HKx31 infection (Townsend and Skehel, 1984). Bronchoalveolar lavage (BAL) cells were collected on days 40 and 70 after infection, and lymphocytes were isolated from the perfused lung tissues (lung parenchyma) by collagenase digestion. On day 40 after viral infection, ∼15% of the BAL and ∼4% of the lung parenchyma CD8+ T cells were tetramer positive, which included 62% and 18% CD69+ IL-7R− cells, respectively, indicating the presence of activated T cells (Figure 1A). A large percentage of virus-specific CD8 T cells that remained in the lung airways after Sendai virus infection was recently shown to express CD11a at low levels (Ely et al., 2003b; Masopust et al., 2004). We found similarly low levels of CD11a expression on a large percentage of the virus-specific T cells in the BAL so that by day 44 after HKx31 infection only 10% of the NP366–374/Db-specific cells expressed CD11a at the levels found on antigen-experienced T cells in other tissues (Figure S1 available in the Supplemental Data with this article online). By day 70, the size of the NP366–374/Db-specific CD8 T cell population in the BAL had greatly decreased, but 46% of the cells remained CD69+ and IL-7R−. These cells were no longer detectable in BAL at 4 months after infection (data not shown).
Figure 1. Route of Infection Controls T Cell Activation in the Lung Airways.
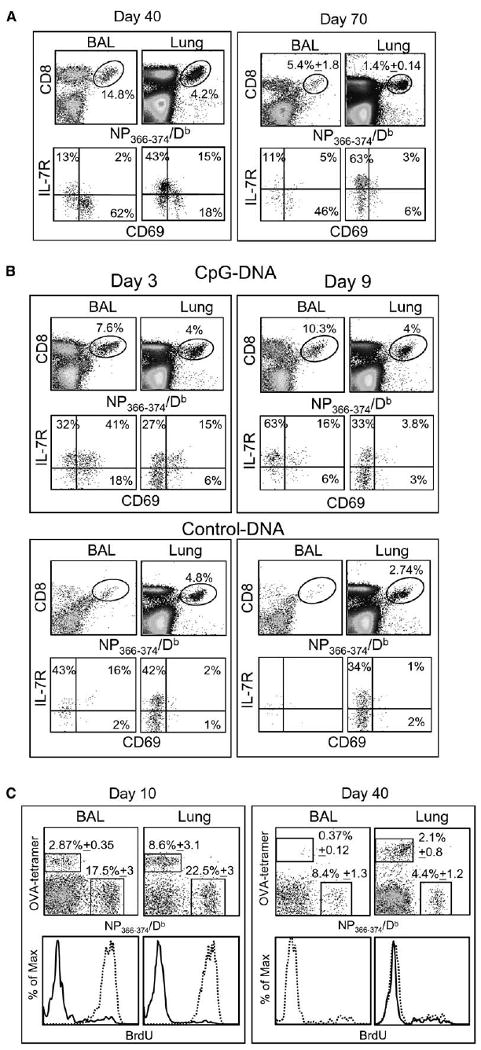
(A) C57BL/6 mice were given 300 EID50 HKx31 influenza virus by i.n. infection. On days 40 and 70 after infection, NP366–374/Db-specific CD8 T cells in the BAL and parenchyma (Lung) were counted and analyzed for CD69 and IL-7R expression. Percentages of NP366–374/Db-specific cells within the total CD8 T cell populations are shown.
(B) Mice were given 5000 EID50 HKx31 influenza virus by i.p. injection. One month later, the mice were treated with 15 μg CpG or control ODN-DNA in the lungs. Gated populations of NP366–374/Db-specific CD8 T cells were analyzed for CD69 and IL-7R expression on days 3 (left) and 9 (right) after ODN-DNA treatment.
(C) Mice were primed with LM-OVA i.v. and 1 month later infected with HKx31. Groups of five mice were fed with BrdU water between days 0 and 10 or 30 and 40 after infection. Lymphocytes from the BAL and lung parenchyma were analyzed for NP366–374/Db- and OVA-specific CD8 T cells at the end of the BrdU treatment. Overlaid histograms show BrdU content in gated populations of NP366–374/Db- (dashed lines) and OVA-specific (continuous lines) CD8 T cells. Error bars represent the SEM from five mice.
In order to determine if a direct infection of the lungs was required to generate activated T cells in the lung airways, mice were infected with HKx31 i.p. and analyzed 30 days later. Substantial numbers of virus-specific CD8 T cells were detected in the spleens and the lung parenchyma (data not shown), but not the airways without further treatment (Figure 1B, control). To analyze the effect of inflammatory signals on the recruitment and activation of virus-specific CD8 T cells in the airways, we used oligodeoxynucleotide (ODN)-DNA (Chang and Braciale, 2002). Mice that had been infected with HKx31 i.p. were treated 30 days later with CpG or control ODN-DNA in the lungs. Three days after CpG ODN-DNA treatment, large numbers of CD8 T cells, were detected in the BAL, including NP366–374/Db-specific cells (Figure 1B), which were mostly CD69+ and IL-7R+. Elevated numbers of NP366–374/Db-specific CD8 T cells were also detected in the lung airways on day 9 after treatment, as compared to the control mice, but these cells had lost CD69 expression and remained IL7R+, indicating that they were resting memory cells (Figure 1B). This showed that the lung environment was not sufficient for chronic T cell activation. In another model, OVA-specific memory CD8 T cells that were generated during a systemic infection with a recombinant Listeria monocytogenes expressing chicken ovalbumin (LM-OVA) entered the lung airways after treatment with CpG-ODN DNA. These cells also transiently expressed CD69 but did not down-regulate IL-7R expression (data not shown). It is likely that the indirect effects of cytokines (type 1 interferons) that are released during CpG ODN-DNA treatment (Sun and Sprent, 2000; Kranzer et al., 2000) were responsible for the transient CD69 expression in these experiments. These results showed that nonspecific inflammation of the lungs was not sufficient to maintain the CD69+ IL-7R− phenotype of the influenza virus-specific CD8 T cells in the airways.
We used a dual infection model to determine whether OVA-specific CD8 T cells could persist in the airways of influenza virus-infected lungs after nonspecific recruitment. Mice that had been infected with LM-OVA 30 days earlier were given a pulmonary HKx31 infection. During the recovery phase of the response to the influenza infection, the mice were divided into two groups and maintained on BrdU water either 0–10 or 30–40 days after infection. Each group of mice was analyzed for OVA and NP366–374/Db-specific CD8 T cells at the end of the BrdU treatment (Figure 1C).
Mice that have been infected systemically with LM-OVA typically have less than about 104 lymphocytes in the lung airways, which include only very small numbers of antigen-specific CD8 T cells (Figure S2C). By the peak of the response to HKx31 infection (day 10), there were large numbers of NP366–374/Db-specific CD8 T cells in the BAL, as well as some OVA-specific cells, that were recruited by nonspecific inflammation (Ely et al., 2003a; Stephens et al., 2002). A majority of the NP366–374/Db-specific CD8 T cells were activated (Figure S2A) and BrdU+ (Figure 1C), showing that they had undergone cell division in response to viral infection (left panels). In contrast, most of the OVA-specific cells were CD69− IL-7R+ (Figure S2B) and BrdU−, indicating substantial bystander recruitment to the lung airways but very little proliferation. There were still large numbers of NP366–374/Db-specific CD8 T cells in the lung airways on day 40, but the frequency of OVA-specific T cells had decreased almost 10-fold, leaving insufficient numbers of cells for BrdU analysis (right panels). These data showed that although bystander memory CD8 T cells were recruited into the lung airways during acute influenza virus infection, they were not maintained later in the response.
Blood-Borne Migrants Replenish Activated Airway T Cell Populations after Influenza Virus Infection
The data shown in Figure 1 indicated that antigen-specific memory CD8 T cells did not enter the lung airways without local infection or inflammation (Figure 1). To determine whether pulmonary influenza virus infection promoted continuing recruitment of antigen-specific CD8 T cells into the airways after viral clearance, we utilized parabiotic mice to analyze CD8 T cell migration (Donskoy and Goldschneider, 1992; Klonowski et al., 2004).
CD45.2+ C57BL/6 mice were infected i.n. with HKx31 influenza virus. One month later, when the response to acute viral infection had resolved, each mouse was joined to an uninfected congenic CD45.1+ partner as described previously (Donskoy and Goldschneider, 1992; Klonowski et al., 2004). The joined pairs were maintained for 1–3 weeks and then analyzed for NP366–374/Db-specific CD8 T cells in the lymphoid and nonlymphoid tissues. Others have shown that joined mice develop a shared blood supply in a matter of days, allowing circulating lymphocytes to pass freely in the blood between the infected and uninfected partners (Klonowski et al., 2004). By day 8 after surgery, the NP366–374/Db-specific CD8 T cells had completely equilibrated in the spleens (Figure 2A) and most other lymphoid organs (data not shown) of the infected and uninfected partners. In contrast, much slower rates of T cell equilibration were detected in the lung parenchyma, where on day 15 there were only half as many NP366–374/Db-specific CD8 T cells in the uninfected mice, as compared to the influenza virus-infected partners (Figure 2B). Complete mixing was not observed until at least 3 weeks after surgery (Figure 2B). Very similar results were obtained when both partners were infected with HKx31 influenza virus (Figure S3A). There was also a small difference in the ratio of the total donor and recipient CD8 T cell populations in the lungs (Figure S3B), indicating that other virus-specific CD8 T cells, in addition to the NP366–374/Db-specific cells, were in disequilibrium on day 15 after surgery. These results were in contrast to the migration kinetics of virus-specific CD8 memory T cells after systemic infection with vesicular stomatitis virus (VSV), where the antigen-specific cells equilibrated in the lungs and spleens by day 8 after surgery (Klonowski et al., 2004).
Figure 2. Different Rates of T Cell Equilibration in Lymphoid and Nonlymphoid Tissues.
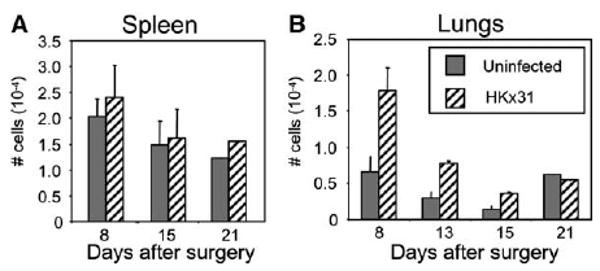
C57BL/6 mice were given 300 EID50 HKx31 i.n. and 1 month later were joined to uninfected CD45.1+ partner animals. The total number of NP366–374/Db-specific CD8 T cells in the (A) spleens and (B) lungs are shown with standard errors from five pairs of mice.
We used different combinations of parabiotic mice to determine what conditions were necessary for virus-specific CD8 memory T cells to reach the lung airways. First, CD45.2+ influenza (day 30) memory mice were joined to uninfected CD45.1+ partners and analyzed 15 days later. Even though substantial numbers of CD45.2+ NP366–374/Db-specific CD8 T cells reached the lung parenchyma of the uninfected mice, very few entered the airways (Figure 3A). Other CD45.2+ influenza memory mice (day 30) were joined to CD45.1+ mice that had also been infected with HKx31 i.n. 30 days previously. There were large numbers of NP366–374/Db-specific CD8 T cells in the BAL of all the animals on day 15 after surgery, of which 19% were partner-derived T cells (Figure 3B), directly showing that a prior pulmonary influenza virus infection was sufficient to promote continuing recruitment of small numbers of virus-specific CD8 T cells into the airways. The percentage of partner-derived NP366–374/Db-specific CD8 T cells that expressed CD11a at high levels in the BAL was considerably larger than the percentage of self-derived cells that expressed this marker (Figure 3C). Evidence suggests that CD11a expression declines soon after T cells arrive in the lung airways (Ely et al., 2006), indicating that some self-derived T cells arrived in the airways before surgery and persisted for the duration of the experiment.
Figure 3. Influenza Virus-Specific, but Not Bystander, CD8 Memory T Cells Continuously Migrate to the Lung Airways.
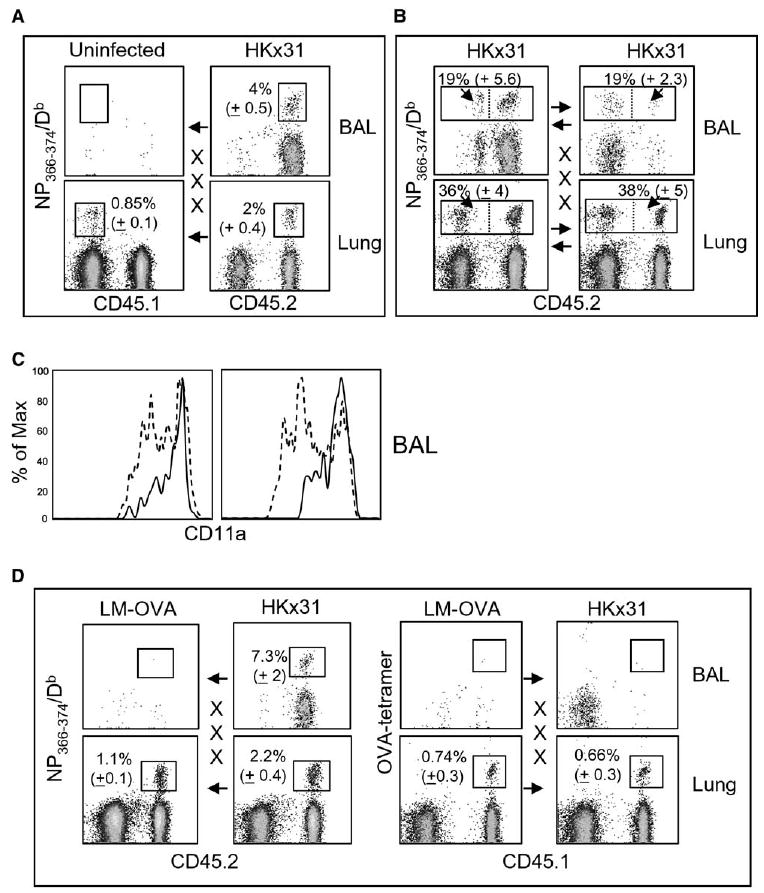
(A and B) C57BL/6 mice were infected with HKx31 and 1 month later were joined to congenic CD45.1+ partner animals that were either (A) uninfected or (B) primed with HKx31 i.n. On day 15 after surgery, five pairs of mice were analyzed for self- and partner-derived NP366–374/Db-specific CD8 T cells in the BAL and lung parenchyma. (A) and (B) show CD8 gate showing percentages of NP366–374/Db-specific cells from the partner animals. The arrows denote the direction of T cell migration.
(C) Joined mice that were both infected with HKx31 were analyzed for CD11a expression on NP366–374/Db-specific cells in the lung airways. The CD45.1 marker was used to distinguish partner (continuous line) and self-derived (dashed line) NP366–374/Db-specific CD8 T cells in each animal. In (A), (B), and (C), error bars represent the SEM from five pairs of mice.
(D) LM-OVA-infected mice were joined to HKx31-infected partners. Each animal was analyzed for OVA- (right) and NP366–374/Db-specific (left) CD8 T cells in the BAL and lung parenchyma.
To further analyze the specificity of the recruitment to the lung airways, CD45.2+ influenza memory mice were joined to CD45.1+ mice that had been infected with LM-OVA 1 month previously. Fifteen days after surgery, each partner was analyzed for NP366–374/Db-specific and OVA-specific CD8 T cells in the lungs (Figure 3D). Both partners had large numbers of OVA-specific CD8 T cells in the lung parenchyma, which had reached equilibrium by day 15 after surgery. The influenza-specific T cells were much slower to reach equilibrium, with half as many NP366–374/Db-specific CD8 T cells in the lung parenchyma of the LM-OVA-infected mice on day 15, as compared to the HKx31 infected donors. This was very similar to the pattern seen when HKx31-infected mice were joined to uninfected partners in Figure 3A and again suggested that some virus-specific T cells were retained in the parenchyma after influenza virus infection. Although large numbers of OVA-specific CD8 T cells entered the lung parenchyma of the partner mice (Figure 3D, right panels), very few cells reached the lung airways, similar to Figure 1C. Together, these data indicated that continuing recruitment and retention of virus-specific CD8 T cells into the lung airways was dependent on pulmonary viral infection and appeared to be antigen specific.
Processed Viral Antigens Persist In Vivo after Pulmonary Influenza Virus Infection
Despite the fact that influenza virus is considered to be an acute infection, the CD69+ IL-7R− phenotype (Figure 1) and selective recruitment of virus-specific CD8 T cells into the lung airways (Figure 3) were suggestive of a continuing response to residual viral antigens. To directly assay for processed T cell antigens in vivo, we transferred CFSE-labeled CD45.1+ RAG−/− TcR transgenic CD8 T cells that are specific for the NP366–374/Db epitope (F5) (Moskophidis and Kioussis, 1998) to CD45.2+ mice that had previously been infected with the E61-13-H17 influenza virus (Townsend and Skehel, 1984). The E61-13-H17 virus was used because it differs from HKx31 by two amino acids at residues 372 and 373 in the NP epitope (Townsend et al., 1986), which are essential for its recognition by F5 CD8 T cells. All the other components of the two viruses are identical. Eight days after transfer, CD45.1+ T cells were analyzed for cell division by CFSE-dilution (Figure 4). Control mice were uninfected or infected with HKx31.
Figure 4. Residual Viral Antigens Persist In Vivo for at Least 2 Months after Influenza Virus Infection.
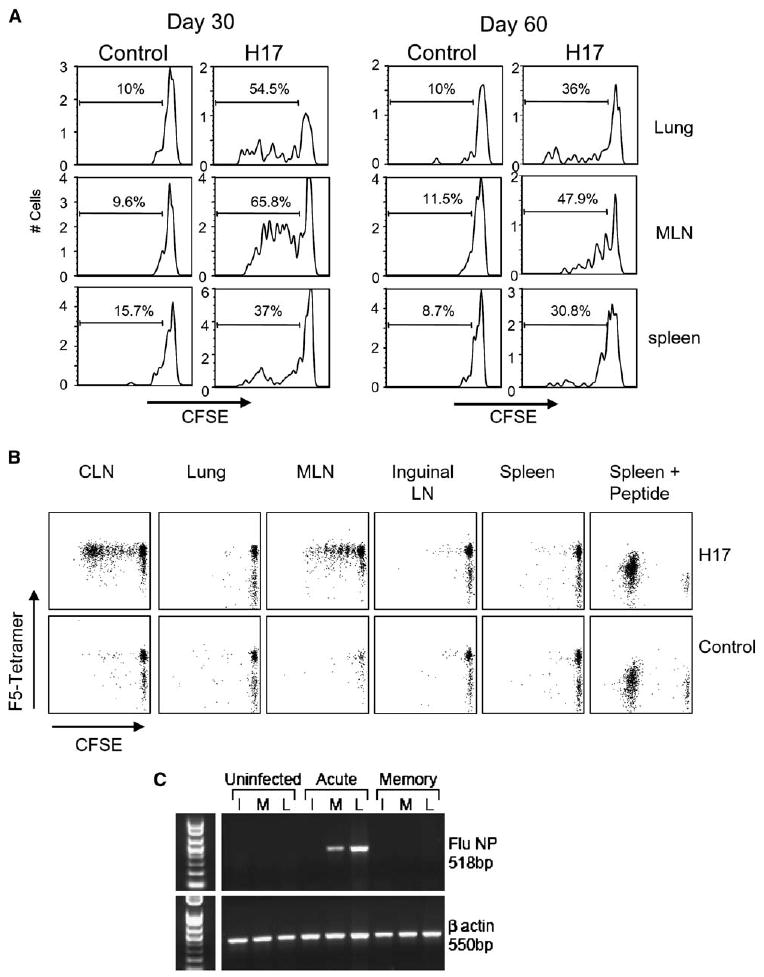
(A) E61-13-H17-infected and control mice received 2 × 3 106 CFSE-labeled CD45.1+ CD8 T cells from the F5-RAG−/− mice by i.v. injection on day 30 (left) or 60 (right) after infection. Eight days after transfer, the NP366–374/Db-specific CD8 T cells in the lungs, MLN, and spleens were analyzed for CD45.1 expression and CFSE intensity. Gated populations of CD45.1+ NP366–374/Db-specific CD8 T cells are shown. Marked regions indicate the percentages of F5 cells that underwent two or more cell divisions after transfer.
(B) C57BL/6 mice were infected with E61-13-H17 and 1 month later received 2 × 3 106 CFSE-labeled CD45.1+ RAG−/− F5 cells by i.v. injection. Lymphocytes were isolated from the recipient lungs, inguinal LN, CLN, MLN, and spleens 20 hr after transfer. Lymphocytes were cultured for 3 days in IL-2-supplemented media (20 U/ml). Gated population of CD45.1+ CD8 T cells are shown. Peptide NP366–374 was used as a positive control for cell proliferation.
(C) RNA was extracted from the MLN (M), lungs (L), and inguinal lymph nodes (I) from uninfected and E61-13-H17-infected mice 6 (acute) or 33 (memory) days previously. NP and β-actin fragments were amplified from cDNA by PCR. NP expression was detected in the lungs and MLN of acutely infected mice, but not the uninfected controls or memory mice. Multiple experiments gave similar results.
FACS analysis showed large numbers of transgenic CD8 T cells that had initiated proliferation in the lungs and MLN of the E61-13-H17-infected recipient mice, but not the control animals (Figure 4A). Some CFSE-dull cells were also present in the spleens but may have been the progeny of cells that divided before migrating to this tissue. Other F5 T cells were transferred to mice 60 days after E61-13-H17 infection and also initiated cell division in the lungs and MLN (Figure 4A). These data indicated that processed T cell antigens were still present in the tissues of the influenza virus-infected recipient mice 2 months after transfer. In both experiments, the highest frequencies of proliferating CD8 T cells were detected in the MLN, suggesting that this may be an important site of antigen presentation. To determine exactly where T cells were activated, CFSE-labeled T cells from F5 mice were used in a transient transfer system using E61-13-H17-infected CD45.2+ recipient mice as in the previous experiment (Figure 4B). Twenty hours after transfer, lymphocytes were recovered from the tissues of the recipient mice and placed in culture for 3 days at 37°C with r-IL2. This time interval was selected to allow T cell activation in situ, without subsequent migration. After culture, relatively small numbers of undivided F5 T cells were detected in the samples from the control mice, as well as the lungs, pLN, and spleens of the infected animals. However, greatly expanded populations of F5 T cells were detected in the CLN and MLN. This indicated that the DLN were the primary site of sustained antigen presentation. Very similar results were obtained when CFSE-labeled T cells from OTI mice (Hogquist et al., 1994) were transferred to recipient animals that had been infected with WSN-OVA1 influenza virus (data not shown), which encodes the SIINFELK epitope in the neuraminidase stalk influenza virus (Topham et al., 2001).
A potential explanation for the persistence of processed T cell antigens in vivo was a chronic viral infection. To investigate this possibility, we used RT-PCR to assay for viral RNA on days 6 (acute) and 30 (chronic) postinfection. Viral RNA that encoded the NP epitope was detected in the lungs and MLN on day 6 after viral infection but had dropped below the level of detection by day 30 (Figure 4C). Very similar results were obtained by using primers for viral RNA encoding the Matrix protein (data not shown). The lower intensity of the NP fragment in the MLN on day 6 most likely reflects the requirement for host protease for virus amplification. Because this protease is only present in epithelial cells of the respiratory tract, influenza infections are largely restricted to the lungs where virus amplification takes place. Together, these results suggest that viral antigens were retained in the tissues for an extended period of time without virus amplification, although it is also possible that very tiny quantities of virus, which were below of detection by PCR, may have been retained in some tissues.
Virus-Specific CD8 T Cells Are Retained in the MLN after Influenza Virus Infection
Our data showed that the MLN and CLN were important sites of antigen presentation during the chronic stage of the response to pulmonary viral infection. We have found that the MLN of influenza virus-infected mice remain detectably enlarged for at least 6 months after infection (data not shown). We therefore wished to determine whether the presence of processed viral antigens in the MLN affected the migration of virus-specific CD8 T cells in the circulation during parabiosis. CD45.1+ and CD45.2+ congenic partner mice were infected with HKx31 influenza virus and joined 1 month later. Fifteen days after surgery (i.e., 45 days after viral infection), the numbers of self- and partner-derived NP366–374/Db-specific CD8 T cells had reached equilibrium in the pLN (Figures 5A and 5B) and spleens of the joined mice (data not shown), which was consistent with the migration kinetics of resting memory CD8 T cells in an earlier study (Klonowski et al., 2004). In contrast, there was significant disequilibrium in the numbers of NP366–374/Db-specific CD8 T cells in the MLN, where 80%–90% of the NP366–374/Db-specific cells were self-derived CD8 T cells that had not entered the circulation (Figures 5A and 5B). This disequilibrium only applied to the virus-specific CD8 T cells because the total numbers of donor and self-derived CD8 T cells in the MLN of each of the partnered mice were approximately equal (Figure S3). These findings demonstrated selective retention of virus-specific CD8 T cells in the MLN during antigen presentation.
Figure 5. Virus-Specific CD8 T Cells Are Retained in the MLN after Influenza Virus Infection.
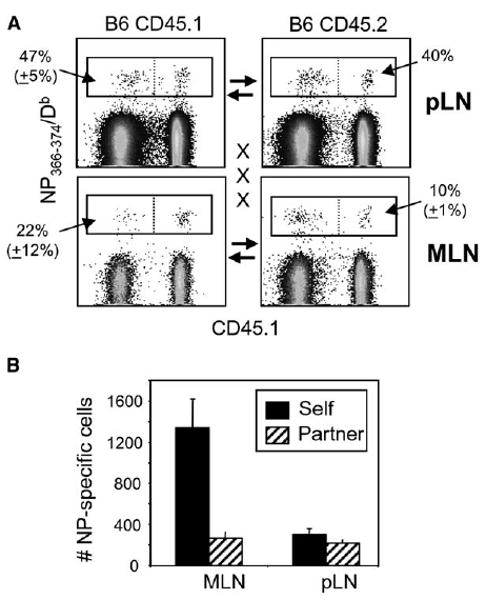
CD45.2+ C57BL/6 and congenic CD45.1+ partner animals were infected with HKx31 influenza virus and joined 1 month later. On day 15 after surgery, five pairs of mice were analyzed for partner and self-derived NP366–374/Db-specific CD8 T cells in the MLN and other pLN with the CD45.1 and CD45.2 markers.
(A) Gated populations of CD8 T cells with percentages of NP366–374/Db -specific cells from the partner animals are shown.
(B) The numbers of self- and partner-derived NP366–374/Db-specific CD8 T cells in the MLN and other pLN with SE from five pairs of mice. Error bars represent the SEM from five pairs of mice.
Resting Memory Cells Comprise the Majority of Migrating CD8 T Cells
Our parabiosis studies showed continuing recruitment of virus-specific CD8 T cells into the lung airways after infection, but it was unclear whether these blood-borne migrants included activated T cells or whether the newly arrived cells became activated upon entry to the lungs. To test this, we employed two recombinant influenza viruses that differ at a single T cell epitope. The WSN-OVA1 virus expresses the SIINFEKL epitope in the stalk of the neuraminidase protein (Topham et al., 2001). A variant of this virus, WSN-OVA0, has a point mutation in one of the peptide anchor residues of the OVA epitope (J.A. Hollenbaugh and R.W. Dutton, unpublished data) that prevents an OVA-specific CD8 T cell response.
CD45.2+ mice were infected with WSN-OVA1 and 1 month later joined to CD45.1+ partners that had been infected with either WSN-OVA0 or WSN-OVA1 (Figure 6). Three weeks after surgery, OVA-specific CD8 T cells in the lungs and MLN of the donor and recipient mice were analyzed for CD69 and IL-7R expression. Between 50% and 60% of self-derived OVA-specific cells in the lungs of WSN-OVA1-infected mice were CD69+ and included 25% with reduced IL-7R expression (Figure 6A, left). Less than 5% of the OVA-specific cells that migrated to the lungs and MLN of the WSN-OVA0-infected partners expressed CD69, with a majority expressing a resting CD69− IL-7R+ memory phenotype (Figures 6A and 6B, right panels). Activated self-derived NP366–374/Db-specific cells were detected in all the animals, showing that a productive infection had occurred (data not shown).
Figure 6. Pulmonary Influenza Virus Infection Is Insufficient for Sustained CD8 T Cell Activation in the Lungs and MLN.
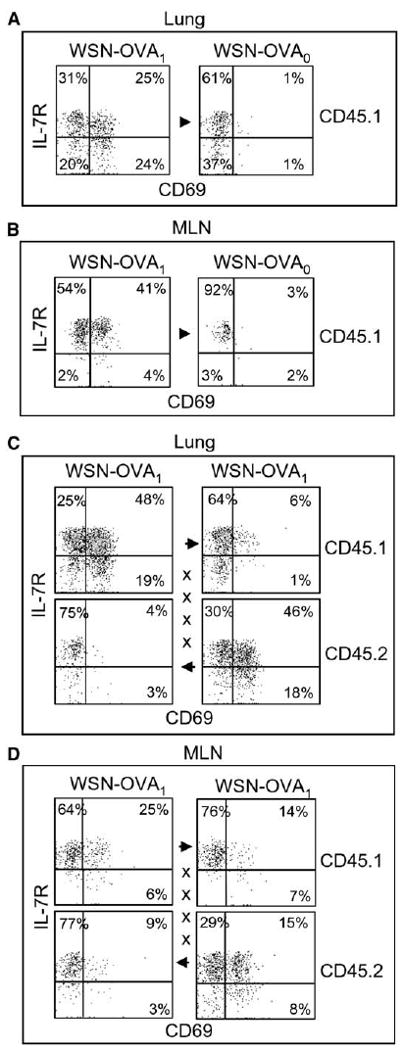
WSN-OVA1-infected mice were joined to congenic CD45.1+ partners that had been infected with (A and B) WSN-OVA0 or (C and D) WSN-OVA1. Three weeks after surgery, the lungs (A and C) and MLN (B and D) were analyzed for OVA-specific CD8 T cells that expressed CD69 and IL-7R. The CD45.1 marker was used to distinguish T cells from each animal. Gated populations of CD45.1+ or CD45.2+ OVA-specific CD8 T cells are shown as indicated. Suture marks and arrows denote the direction of T cell migration.
When both partners were infected with the WSN-OVA1, only a small percentage of OVA-specific CD8 T cells expressed CD69 in the lungs after migration (∼7%), even though both partners expressed the SIINFEKL epitope (Figure 6C). Analysis of the NP and PA epitopes gave almost identical results (data not shown). This frequency of activated T cells was only slightly higher than the percentages of cells that expressed CD69 after migration the lungs of the WSN-OVA0-infected partners (Figure 6A) which expressed the mutant SIINLEKL epitope (J.A. Hollenbaugh and R.W. Dutton, unpublished data). The percentage of OVA-specific CD8 T cells that expressed CD69 after migration into the MLN of the WSN-OVA1-infected mice was substantially higher (12%–21%) and included some IL-7Rlow cells (Figure 6D). These data were consistent with our earlier conclusion that the MLN was a primary site of antigen presentation during the chronic stage of the response.
Discussion
The possibility that low levels of antigen could be important for sustaining protective immunity has been suggested for several chronic and acute viral infections (Zinkernagel, 2002; Gray, 2002; Kundig et al., 1996). In this paper, we have defined a role for residual viral antigens in tissue-specific T cell migration to the lungs after pulmonary influenza virus infection. We demonstrate that processed viral antigens that are retained in the DLN continue to be presented to CD8 T cells for at least 2 months after infection. We show that these antigens control the migratory pattern and activation state of virus-specific CD8 T cells near the site of virus amplification, where effector T cells can respond rapidly to secondary viral challenge. Transfer experiments were recently used to analyze the development of CD4 memory T cells after influenza virus infection (Jelly-Gibbs et al., 2005). This study showed that viral antigens were presented to naive CD4 T cells up to 4 weeks after infection. Although T cell activation and migration to the lungs were not analyzed, a small number of the transferred CD4 T cells survived to become memory T cells after antigen stimulation, demonstrating that MHC class II+ antigen presenting cells participated in T cell stimulation and memory formation. We have extended these studies to analyze the impact of chronic antigen stimulation on the migration of virus-specific CD8 T cells near the site of infection during the months of declining cellular immunity (Liang et al., 1994). We show that the reservoir of antigen-specific CD8 T cells in the lung airways is replenished by blood-borne migrants through a dynamic process that continues for many weeks after infection. Replenishment of this cell population is virus specific and dependent on the route of infection. In contrast, bystander CD8 memory T cells, which were recruited to the lungs during acute influenza virus infection, were not activated and were not retained later in the response. We also show that the MLN and CLN were the primary sites of antigen presentation during the chronic stage of the response. This conclusion was supported by the parabiosis studies, which showed that some virus-specific CD8 T cells upregulated CD69 when they reached the MLN of the influenza virus-infected partners. Other migrating CD8 T cells that entered the lungs of the same animals remained CD69, indicating that they were not responding to antigens in the lung tissues.
It is possible that the slow rate of equilibration by virus-specific CD8 T cells in the lungs of the influenza virus-infected mice during parabiosis was due, in part, to some preferential trafficking between the MLN and lungs of the donor animals (Figures 2 and 3A). A model of the proposed pathway of T cell migration is shown in Figure 7. It is known that activated CD8 T cells leave the MLN of the infected mice in the efferent lymph, which joins the venous blood supply at the thoracic duct. After the first pass through the heart, the migrating T cells reach the lungs of the donor animal in the pulmonary circulation. Blood that returns to the heart from the pulmonary circulation can then join the general circulation and thus gain passage to the recipient partner. We suggest that this indirect route of T cell migration leads to preferential retention of activated CD8 T cells in the lungs of the influenza virus-infected donor mice. The precise mechanism of this retention is currently under investigation. In contrast, less-activated T cells pass through the lungs of the infected mice more easily and have an increased probability of reaching the recipient lungs. This model is compatible with the data that are shown in Figure 4, which show that processed viral antigens are not accessible to naive T cells in the lung tissues. We find no evidence that activated CD8T cells that leave the MLN must first pass through the spleen before reaching the lungs; however, the transfer studies in Figure 4 showed that some proliferating CD8 T cells ultimately reached the spleen, which is known to be a collection point for effector CD8 T cells. In future studies, we will use parabiosis to analyze the impact of residual antigen presentation on T cell migration after the pathogen has been administered by different routes of infection. Based on the data described here, we believe that if activated T cells were generated in another part of the body they would not collect in the lung airways without the residual inflammation of a prior pulmonary virus infection. Migration studies will show whether antigen-specific T cells are retained in other DLN when the infection is administered by these alternative routes.
Figure 7. Proposed Pathway of CD8 T Cell Migration in Joined Mice.
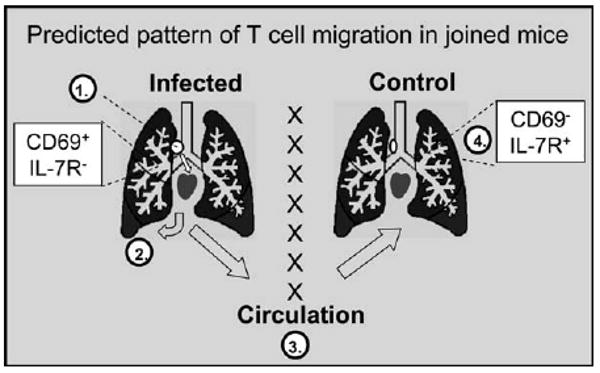
Virus-specific CD8 T cells that respond to residual viral antigens in the MLN (1) of the influenza virus-infected mice preferentially migrate to the lungs of the donor animals (2) via the pulmonary circulation. Activated (CD69+ IL-R−) CD8 T cells are preferentially retained in the lung tissues of the infected mice, whereas less activated cells pass through and rejoin the circulation (3) where they can gain access to the lungs of the recipient animals (4).
The different rates of T cell equilibration in the lung parenchyma and BAL after parabiosis indicate that distinct mechanisms of recruitment were responsible for maintaining the T cell populations at each location. By 2 weeks after surgery, 19% of the NP366–374/Db-specific CD8 T cells that were in the BAL were derived from the partner animals. If we assume that self-derived NP366–374/Db-specific CD8 T cells are also recruited to the BAL at a similar rate (which is probably a conservative estimate), then we might expect that the entire BAL population would be replaced within 4–5 weeks. This is considerably slower than the 10 day replacement period that was recently calculated by using changing CD11a expression as an indirect measure of T cell migration after Sendai virus infection (Ely et al., 2006). We also find some CD11a bright cells in the lung airways after influenza virus infection (∼10% of the NP366–374/Db-specific cells on day 44 after infection), but not as many as the 30%–50% reported in the Sendai virus model. Different proportions of CD11a bright cells within the partner and self-derived NP366–374/Db-specific CD8 T cells in the BAL after parabiosis also indicated that some cells entered the airways before surgery and persisted until the end of the experiment. The different rates of T cell recruitment to the lung airways in these studies indicate that there may be important differences between the Sendai and influenza viruses, leading to an emphasis of different mechanisms during recruitment. Our studies indicate that T cell recruitment to the lung airways stops within a few months, when residual antigens disappear from the DLN (data not shown). The potential influence of residual antigen presentation on T cell activation could not be evaluated in the Sendai virus model because TcR transgenic mice are not available. Transferred spleen cells only reached the lung airways of the Sendai virus-infected mice after CpG-DNA treatment, suggesting that resting memory T cells were probably not the primary source of cells that were recruited to the BAL (Ely et al., 2006). Others have used parabiosis to analyze CD8 T cell migration after systemic infections (Klonowski et al., 2004). In these studies, VSV and LM-OVA-specific CD8 T cells equilibrated quickly in most tissues, including the lungs, but migration to the intestinal lamina propria was severely limited. Together, these data indicate that T cell migration to mucosal tissues is regulated by distinct mechanisms that are not present in other tissues. The reduction in CD11a expression may prevent virus-specific CD8 T cells that enter the lung airways during respiratory virus infections from returning to the circulation (Ely et al., 2003b; Masopust et al., 2004).
In some situations, chronic presentation of T cell antigens can be deleterious to immunity and result in the development of dysfunctional memory CD8 T cell populations with poor survival characteristics (Redmond and Sherman, 2005). However, not all chronic infections are linked to memory T cell defects in vivo. For example, virus-specific CD8 T cells that accumulated in the trigeminal ganglia after herpes simplex virus infection maintained CD69 expression for several months in vivo without a detectable loss of effector functions (Khanna et al., 2003). Similarly, it has been established that virus-specific CD8 T cells that remain in the airways after pulmonary influenza virus infection are not anergic or terminally differentiated (end-stage) effector T cells, because these cells can proliferate and produce cytokines in vitro and initiate recall responses after transfer to naive mice (Hogan et al., 2001a; Ostler and Ehl, 2002; Ely et al., 2003b).
In a recently published study, we showed that dendritic cells (DC) were required to maximize the response to secondary challenge, using three different microbial infections, including influenza virus (Zammit et al., 2005). Here, we have shown that naive CD8 T cells proliferated in response to the antigens that were retained in the DLN, which strongly suggests that DC play a role in persistent antigen presentation. Infectious influenza virus can be detected in the lungs of mice for only about 10–14 days after inoculation (Liang et al., 1994). Although long-term infection of the CNS has been reported in immunocompromised mice (Aronsson et al., 2001, 2002), viral RNA had dropped below the level of detection by day 30 in wild-type animals in this and other published studies (Hamilton-Easton and Eichelberger, 1995; Jelly-Gibbs et al., 2005). Together, these data suggest that protein antigens are retained in vivo without virus amplification, although we cannot rule out the possibility that very low levels of viral RNA persist in some tissues. In support of this suggestion, a population of antigen-bearing DC was found in the airways of mice more than 8 weeks after aerosol inoculation with the Leishmania LACK antigen (Julia et al., 2002). Others have shown long-term effects of influenza virus infection on the activation markers of DC in the lungs (Dahl et al., 2004; Yamamoto et al., 2000).
Our data indicate that sustained antigen presentation is required for activated CD8 T cells to be retained near the site of infection, where they can mount a rapid response to secondary viral challenge during heterosubtypic infection. A detailed analysis of the antigen presentation pathway that leads to chronic T cell activation in the MLN and CLN will be required to determine which antigen presenting cells are involved and whether cytosolic or endosomal antigen processing pathways are required. These data hold important implications for understanding pulmonary immunity and thus will impact the design of vaccines for protection against influenza virus and other airborne infectious agents (Yewdell and Haeryfar, 2005).
Experimental Procedures
Mice and Reagents
Female C57BL/6J and congenic C57BL/6-Ly5.2 mice were purchased from Charles River through the NCI animal program. The OTI mouse line (Hogquist et al., 1994) was generously provided by Dr. W.R. Heath (WEHI, Melbourne, Australia) and Dr. F. Carbone (University of Melbourne, Melbourne, Australia). Female mice at 8–12 weeks of age, were anesthetized by i.p. injection with avertin (2,2,2-tribromoethanol) before i.n. infection with 300 50% egg infectious doses (EID50) of HKx31 or E61-13-H17 influenza virus or 103 pfu WSN-OVA. Virus stocks were grown in chicken eggs, titered, and stored as described previously (Daly et al., 1995). Alternatively, mice were given 103 CFU recombinant LM-OVA by i.v. injection and boosted 1 month later with 104 CFU (Pope et al., 2001). ODN-DNA was synthesized with modified phosphate bonds to prevent degradation by nuclease activity (Midland Reagent Co. TX). The stimulatory sequence includes two embedded CpG-motifs that are marked in bold (5′-ATAATCGACGTTCAAGCAAG-3′) (Schwartz et al., 1997). Substitutions in the control sequence (5′-ATAATAGAGCTTCAAG CAAG-3′) are also marked in bold. Anesthetized mice were treated with 15 μg of ODN-DNA (in 30 μl PBS) by passive inhalation.
Sample Preparation for Flow Cytometry
Lymphocytes were collected from the lungs by lavage five times in Hank's balanced saline solution (HBSS). The mice were perfused with PBS 75 U/ml heparin until the lungs were white in color. Lymphocytes were released from the chopped lung tissues by digestion with 150 U/ml collagenase (Life Technologies, Rockville, MD) in RPMI, 1 mM MgCl2, 1 mM CaCl2, and 5% FCS at 37°C for 1 hr. Cells were mashed through strainers and suspended in 44% isotonic Percoll, underlaid with 67% isotonic Percoll, and centrifuged at 400 × 3 g for 20 min. Lymphocytes were recovered from the interface and washed with HBSS. Tetramers that are specific for the influenza virus NP366–374/Db epitope have been described previously (Flynn et al., 1998). Tetramers were supplied by the NIAID (Emory University Vaccine Center at Yerkes, Atlanta, GA). Lymphocytes were stained with APC-conjugated NP366–374/Db for 1 hr at room temperature. All other markers were stained at 4°C with antibodies to CD45.1, CD45.2, CD69, CD11a, and CD127 (BD Pharmingen, San Diego, CA). Fixed samples were analyzed on a Becton-Dickenson FACSCa-libur flow cytometer and analyzed by using FlowJo software (Tree Star Inc.). CD8 T cells were labeled with 5 μM CFSE-dye at 37°C for 10 min (Lyons and Parish, 1994).
Analysis of Viral RNA by Reverse-Transcriptase Polymerase Chain Reaction
LNs and lung tissues were ground up in 1 ml Tri Reagent (Sigma, St Louis, MO) by using a dounce homogenizer. RNA was prepared by using Bromochloropropane (Sigma, St Louis MO) phase separation and precipitated with isopropanol. cDNA was prepared by using RTII superscript and random primers (Invitrogen, Carlsbad, CA). PCR amplification of first-strand cDNA was performed for 35 cycles with the following primers: influenza NP (5′-TGATCGGAACTTCTGGAGGG-3′ and 5′-TGGCCCAGTACCTGCTTCTC-3′) and β-actin (5′-ATGGATGACGATATCGCTG-3′ and 5′-ATGAGGTAGTCTGTCAGGT-3′).
Parabiosis Surgery
Parabiosis surgery was carried out as described previously (Donskoy and Goldschneider, 1992; Klonowski et al., 2004) in accordance with the University of Connecticut Institutional Animal Care Committee. Incisions were made in the skin on the opposing flanks of the donor and recipient animals. Surgical sutures were used to bring the body walls of the two mice into direct physical contact. The outer skin was then attached with surgical staples. Joined animals were maintained for 15–21 days after surgery.
Supplementary Material
Supplementary Figures
Acknowledgments
The authors thank Dr. David Topham (University of Rochester) for the WSN-OVA1 and WSN-OVA0 viruses, Dr. Jonathan Yewdell (National Institutes of Health) for supplying the E61-13-H17 virus, and Dr. Ulrich von Andrian (Harvard) for helpful discussion regarding the transfer studies. In addition, we thank the National Institute of Allergy and Infectious Disease tetramer facility for the antigen-specific staining reagents and Quynh-Mai Pham for assistance with the parabiosis surgery. This work was supported by the American Lung Association RG1119 (L.S.C.), National Institutes of Health grants AI41576, AI0515 83, DK45260 (L.L.), and by National Institutes of Health postdoctoral Fellowship AI053970 (K.D.K.).
Footnotes
References
- Aronsson F, Karlsson H, Ljunggren HG, Kristensson K. Persistence of the influenza A/WSN/33 virus RNA at midbrain levels of immunodefective mice. J Neurovirol. 2001;7:117–124. doi: 10.1080/13550280152058771. [DOI] [PubMed] [Google Scholar]
- Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlsson H. Persistence of viral RNA in the brain of off-spring to mice infected with influenza A/WSN/33 virus during pregnancy. J Neurovirol. 2002;8:353–357. doi: 10.1080/13550280290100480. [DOI] [PubMed] [Google Scholar]
- Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat Immunol. 2004;5:337–343. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- Daly K, Nguyen P, Woodland DL, Blackman MA. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J Virol. 1995;69:7416–7422. doi: 10.1128/jvi.69.12.7416-7422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty PC, Topham DJ, Tripp RA. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8(+) T cells to the lung airways during respiratory virus infections. J Immunol. 2003a;170:1423–1429. doi: 10.4049/jimmunol.170.3.1423. [DOI] [PubMed] [Google Scholar]
- Ely KH, Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J Immunol. 2003b;171:3338–3342. doi: 10.4049/jimmunol.171.7.3338. [DOI] [PubMed] [Google Scholar]
- Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8(+) T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- Gray D. A role for antigen in the maintenance of immunological memory. Nat Rev Immunol. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- Hamilton-Easton A, Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001a;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- Hogan RJ, Zhong WM, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001b;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelly-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- Kranzer K, Bauer M, Lipford GB, Heeg K, Wagner H, Lang R. CpG-oligodeoxynucleotides enhance T-cell receptor-triggered interferon-gamma production and up-regulation of CD69 via induction of antigen-presenting cell-derived interferon type I and interleukin-12. Immunology. 2000;99:170–178. doi: 10.1046/j.1365-2567.2000.00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig TM, Bachmann MF, Oehen S, Hoffmann UW, Simard JJ, Kalberer CP, Pircher H, Ohashi PS, Hengartner H, Zinkernagel RM. On the role of antigen in maintaining cytotoxic T-cell memory. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang KS, Recher M, Navarini AA, Harris NL, Lohning M, Junt T, Probst HC, Hengartner H, Zinkernagel RM. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188:223–232. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HH, Moldoveanu Z, Novak MJ, van Ginkel FW, Ban E, Kiyono H, Mcghee JR, Mestecky J. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology. 1999;254:50–60. doi: 10.1006/viro.1998.9521. [DOI] [PubMed] [Google Scholar]
- Nguyen HH, van Ginkel FW, Vu HL, Mcghee JR, Mestecky J. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J Infect Dis. 2001;183:368–376. doi: 10.1086/318084. [DOI] [PubMed] [Google Scholar]
- Ostler T, Ehl S. Pulmonary T cells induced by respiratory syncytial virus are functional and can make an important contribution to long-lived protective immunity. Eur J Immunol. 2002;32:2562–2569. doi: 10.1002/1521-4141(200209)32:9<2562::AID-IMMU2562>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ostler T, Hussell T, Surh CD, Openshaw P, Ehl S. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur J Immunol. 2001;31:2574–2582. doi: 10.1002/1521-4141(200109)31:9<2574::aid-immu2574>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, De Fougerolles AR, Topham DJ. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Quinn TJ, Thorne PS, Sayeed S, Yi AK, Krieg AM. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. 1997;100:68–73. doi: 10.1172/JCI119523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Randolph DA, Huang G, Holtzman MJ, Chaplin DD. Antigen-nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J Immunol. 2002;169:5458–5467. doi: 10.4049/jimmunol.169.10.5458. [DOI] [PubMed] [Google Scholar]
- Sun S, Sprent J. Role of type I interferons in T cell activation induced by CpG DNA. Curr Top Microbiol Immunol. 2000;247:107–117. doi: 10.1007/978-3-642-59672-8_7. [DOI] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- Townsend AR, Skehel JJ. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J Exp Med. 1984;160:552–563. doi: 10.1084/jem.160.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend AR, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Webby RJ, Andreansky S, Stambas J, Rehg JE, Webster RG, Doherty PC, Turner SJ. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc Natl Acad Sci USA. 2003;100:7235–7240. doi: 10.1073/pnas.1232449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland DL, Ely KH, Crowe SR, Tighe M, Brennan JW, Harmsen AG, Cauley LS. Antiviral memory T-cell responses in the lung. Microbes Infect. 2002;4:1091–1098. doi: 10.1016/s1286-4579(02)01633-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Suzuki S, Shirai A, Suzuki M, Nakazawa M, Nagashima Y, Okubo T. Dendritic cells are associated with augmentation of antigen sensitization by influenza A virus infection in mice. Eur J Immunol. 2000;30:316–326. doi: 10.1002/1521-4141(200001)30:1<316::AID-IMMU316>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Haeryfar SM. Understanding presentation of viral antigens to CD8+ T cells in vivo: the key to rational vaccine design. Annu Rev Immunol. 2005;23:651–682. doi: 10.1146/annurev.immunol.23.021704.115702. [DOI] [PubMed] [Google Scholar]
- Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel RM. On differences between immunity and immunological memory. Curr Opin Immunol. 2002;14:523–536. doi: 10.1016/s0952-7915(02)00367-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures