Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration (original) (raw)
. Author manuscript; available in PMC: 2007 Jun 20.
Abstract
People can consciously re-experience past events and pre-experience possible future events. This fMRI study examined the neural regions mediating the construction and elaboration of past and future events. Participants were cued with a noun for 20 seconds and instructed to construct a past or future event within a specified time period (week, year, 5–20 years). Once participants had the event in mind, they made a button press and for the remainder of the 20 seconds elaborated on the event. Importantly, all events generated were episodic and did not differ on a number of phenomenological qualities (detail, emotionality, personal significance, field/observer perspective). Conjunction analyses indicated the left hippocampus was commonly engaged by past and future event construction, along with posterior visuospatial regions, but considerable neural differentiation was also observed during the construction phase. Future events recruited regions involved in prospective thinking and generation processes, specifically right frontopolar cortex and left ventrolateral prefrontal cortex, respectively. Furthermore, future event construction uniquely engaged the right hippocampus, possibly as a response to the novelty of these events. In contrast to the construction phase, elaboration was characterized by remarkable overlap in regions comprising the autobiographical memory retrieval network, attributable to the common processes engaged during elaboration, including self-referential processing, contextual and episodic imagery. This striking neural overlap is consistent with findings that amnesic patients exhibit deficits in both past and future thinking, and confirms that the episodic system contributes importantly to imagining the future.
Keywords: episodic, autobiographical memory, fMRI, hippocampus, frontopolar
Episodic memory allows individuals to project themselves backward in time and recollect many aspects of their previous experiences (Tulving, 1983). Numerous cognitive and neuroimaging studies have attempted to delineate the psychological and biological properties of episodic memory. One common assumption in such studies is that episodic memory is primarily or entirely concerned with the past. However, a growing number of investigators have begun to approach episodic memory in a broader context, one that emphasizes both the ability of individuals to re-experience episodes from the past and also imagine or pre-experience episodes that may occur in the future (Atance & O’Neill, 2001, 2005; Buckner & Carroll, in press; D’Argembeau & Van der Linden, 2004; Gilbert, 2006; Hancock, 2005; Klein & Loftus, 2002; Schacter & Addis, in press; Suddendorf & Busby, 2005; Tulving, 1983, 2002; Williams et al., 1996). From this perspective, both past and future event representations can be episodic in nature, containing rich contextual details about events that are specific in time and place.
Some evidence for this close linkage of past and future events comes from studies of patients with episodic memory deficits. For example, Tulving (1985) reported that patient K.C., a patient who suffered from total loss of episodic memory as a result of head injury that produced damage to the medial temporal and frontal lobes. Consequently, he, was unable to imagine specific events in his personal future (Tulving, 1985) despite no loss in general imagery abilities (Rosenbaum, McKinnon, Levine, & Moscovitch, 2004). A more systematic investigation in another amnesic patient, D.B. (Klein & Loftus, 2002) revealed that he, too, exhibited deficits in both retrieving past events and imagining future events. Interestingly, this deficit in imagining the future was specific to D.B.’s personal future; he could still imagine possible future events in the public domain (e.g., political events and issues). Taken together, the pattern of deficits in these patients suggest there may be something unique about imagining personal future events above and beyond the general processes involved in constructing non-personal events and generating images.
Another population exhibiting episodic memory impairments -- suicidally-depressed individuals -- show reduced specificity of both past and future autobiographical events, and notably, the reduction in specificity of past and future events was significantly correlated (Williams et al., 1996). Moreover, Williams and colleagues demonstrated that in healthy individuals, manipulations that reduced the specificity of past events (e.g., instructions or cues which induce a general retrieval style) also reduced the specificity of subsequently generated future events. Furthermore, factors that influence the phenomenology of past events also influence future events in the same way. For example, D’Argembeau and Van der Linden (2004) investigated how event valence and temporal distance from the present affects phenomenological qualities of past and future events. Positive events were associated with subjective ratings of greater re-experiencing and pre-experiencing than negative events, and temporally close events comprised more sensory and contextual details than temporally distant events.
These converging lines of evidence suggest a great deal of overlap between the retrieval of past events and the imagining of future events. What cognitive mechanisms and neural substrates underlie such overlap? When remembering the past and imagining the future, one must draw upon similar types of information. Events in one’s past and future are inherently personal and thus should be comprised of autobiographical information. Furthermore, both tasks involve the construction of an event representation, and thus should include conceptual and visuospatial information known to comprise event representations (e.g., Greenberg & Rubin, 2003). Conceptual and semantic information about the self and one’s life (e.g., familiar people, common activities) is thought to be mediated by anterior temporal regions (Addis, McIntosh, Moscovitch, Crawley, & McAndrews, 2004; Fink et al., 1996; Graham, Lee, Brett, & Patterson, 2003). Episodic and contextual imagery should feature in both types of event, thus requiring activation of precuneus (Fletcher et al., 1995) and parahippocampal/retrosplenial cortices (Bar & Aminoff, 2003), respectively. Finally, both retrieving past events and imagining future events requires the binding of details into a coherent event: either the reintegration of a memory trace, or the novel integration of disparate details into a coherent future event. Given the known role of the hippocampus in relational processing in memory (Cohen & Eichenbaum, 1993; Eichenbaum, 2001) and specifically, the reintegration of recollective details in autobiographical memories (Addis, Moscovitch, Crawley, & McAndrews, 2004), it is likely this structure will also bind event details for novel future scenarios. Finally, the personal nature of both past and future events should engage regions mediating self-referential processing (e.g., left medial PFC, Craik et al., 1999; Gilboa, 2004; Gusnard, Akbudak, Shulman, & Raichle, 2001). Consistent with these suppositions, the one neuroimaging study that has compared directly the neural correlates of past and future events found common engagement of bilateral medial PFC, hippocampus and parahippocampus and the left precuneus (Okuda et al., 2003).
Remembering the past and imagining the future differ, at least with respect to temporal orientation, and thus some unique cognitive processes and neural regions should be associated with each. The retrieval of past events is known to activate right lateral prefrontal regions supporting memory search and post-retrieval processing (Fletcher & Dolan, 1999; Fletcher & Henson, 2001; Rugg, Otten, & Henson, 2002; Tulving, Kapur, Craik, Moscovitch, & Houle, 1994), as well as lateral parietal cortex, whose function in memory retrieval may involve orienting attention to internal representations (Wagner, Shannon, Kahn, & Buckner, 2005). In contrast, future events are expected to engage generative processing mediated by left lateral prefrontal cortex (Poldrack et al., 1999) to support the creation of novel events, and frontopolar cortex mediating prospective thinking and future planning (Burgess, Quayle, & Frith, 2001; Burgess, Veitch, de Lacy Costello, & Shallice, 2000; Okuda et al., 1998). Damage to this latter region has been associated with deficits in advantageous decision making and awareness of future consequences (Bechara, Damasio, Damasio, & Anderson, 1994). Okuda and colleagues (2003) report that right anteromedial frontal pole (BA 10) was more active for future than past events, and that activity in this region correlated with the number of references to intentions.
Notably, however, Okuda et al. (2003) used a blocked design that did not allow a direct linkage between specific events and neural activity; participants were instructed to talk freely regarding events in certain time periods, and it is unclear whether the events were truly episodic (i.e., specific in time and place). Previous research has shown that specificity of past autobiographical events can influence regions engaged during retrieval (Addis, McIntosh et al., 2004; Graham et al., 2003). Moreover, it is possible that in the study by Okuda and colleagues, the phenomenological qualities of past and future events differed, particularly in light of behavioral evidence demonstrating that past events are typically more detailed and more strongly re-experienced than future events (D’Argembeau & Van der Linden, 2004). Importantly, neuroimaging findings indicate that these qualities can modulate activity in regions supporting autobiographical memory retrieval (Addis, Moscovitch et al., 2004).
The present study used event-related functional magnetic resonance imaging (fMRI) to examine the neural correlates of past and future events that are truly episodic in nature (i.e., specific in time and place) and of equivalent phenomenology. To this end, we employed an objective rating for the episodic specificity of events generated during scanning, and collected subjective ratings of the level of detail, emotionality, personal significance and field/observer perspective. Furthermore, we exploit the advantages of event-related fMRI to examine patterns of neural activity associated with the construction (i.e., the search and reconstruction of a past event or the creation of a future event) and subsequent elaboration (i.e., retrieving or imagining supplementary details) of past and future events. It is hypothesized that past and future events will be maximally differentiated during the construction phase, when cognitive processes specific to each event type should be engaged. Specifically, past events are predicted to activate regions supporting a strategic memory search, including cue specification processes (e.g., ventrolateral PFC, BA 47, Fletcher, Shallice, Frith, Frackowiak, & Dolan, 1998; Moscovitch & Winocur, 2002), and orienting attention to internal memorial representations (e.g., lateral parietal cortex, Wagner et al., 2005). In contrast, future events are expected to recruit regions related to generative processing and prospective thinking, namely the left lateral PFC (Poldrack et al., 1999) and right frontal polar cortex (Okuda et al., 2003), respectively.
Patterns of neural activity common to past and future events are expected at both the construction and elaboration phases. For instance, self-referential processing and associated left medial PFC activity should be sustained throughout both phases. Even so, we predict that overlap will be maximal during the elaboration phase. At this point, episodic and contextual imagery processes should be fully engaged for both event types, drawing on the resources of precuneus, retrosplenial and parahippocampal cortices. Further, the hippocampus should bind details retrieved or imagined during the elaboration phase, irrespective of whether the event is located in the past or future.
While nothing is known about the neural processes underlying the construction and elaboration of future events, very little is known about past event construction versus elaboration. The designs of neuroimaging studies examining retrieval of past events have typically precluded the analysis of construction and elaboration phases. Most often studies are designed to allow participants to gain access directly to personal memories without a retrieval search (Addis, Moscovitch et al., 2004; Gilboa, Winocur, Grady, Hevenor, & Moscovitch, 2004; Maguire, Mummery, & Buchel, 2000; Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003; Ryan et al., 2001; Steinvorth, Corkin, & Halgren, 2006). Those studies that do invoke a retrieval search have used blocked designs, thus collapsing across the construction and elaboration phases (Conway et al., 1999; Graham et al., 2003; Rekkas & Constable, 2005). Two previous event-related studies that explored the construction and elaboration of past events utilized electroencephalography (Conway, Pleydell-Pearce, & Whitecross, 2001; Conway, Pleydell-Pearce, Whitecross, & Sharpe, 2003). While construction and elaboration were differentiated electrophysiologically, with the former engaging left PFC and the latter activating bilateral posterior cortices, these studies failed to detect hippocampal activity at either stage. We expect that direct comparisons of event construction and elaboration will reveal a similar pattern of cortical activation, but that with use of fMRI, we will also be able to characterize hippocampal engagement during these phases.
Methods
Participants
Sixteen healthy, right-handed adults (seven male; mean age, 23 years; range, 18–33 years) with no prior history of neurological or psychiatric impairment participated in the study. Two participants were excluded due to an insufficient number of responses during the scan and post-scan interview. All participants gave informed written consent in a manner approved by the Harvard and Massachusetts General Hospital Institutional Review Boards.
Stimuli
Ninety-six nouns were selected from the Clark and Pavio extended norms (Clark & Paivio, 2004) for use as cue-words in this study. All were high in Thorndike-Lorge frequency (M = 1.66, sd = .290), imageability (M = 5.85, sd = .330) and concreteness (M = 6.83, sd = .342) in order to increase the likelihood that an event could be retrieved or imagined, and also so that each word could be used in all conditions in a fully counterbalanced design (i.e., only imageable words can be used in the visual imagery task; see below). The cue-words were divided into four lists of twenty-four and Analyses of Variance (ANOVA) confirmed the lists did not differ significantly with respect to frequency [F(3,92) = .842, p = .940], imageability [F(3,92) = .133, p = .940] or concreteness [F(3,92) = .951, p = .419]. The word lists cycled through conditions in a fully counterbalanced design, and each participant was randomly assigned to a counterbalanced version.
Scanning
Immediately prior to scanning, the experimental tasks were explained to participants and they completed two practice trials for each condition (eight in total). The contents of the all events retrieved or imagined during this practice session were then probed to confirm that participants understood the instructions (e.g., that events generated were specific in time and place). Participants were aware that following the scan they would be required to describe the events generated in response to each cue word presented during scanning.
In the MRI environment, participants completed six runs of functional neuroimaging, each ten minutes and twenty-four seconds in duration. Within each run, 16 trials were randomly presented; this number comprised 4 trials from each condition (past event, future event, semantic retrieval, and visual imagery). Each trial consisted of a construction and elaboration phase (20 seconds) and three rating scales (5 seconds each). Trials were separated by a rest period during which a fixation cross was presented for a mean duration of four seconds (jittered between two and six seconds). All stimuli were presented in black text on a white background and projected on a screen viewed by participants on a mirror incorporated into the head-coil. E-Prime software (Psychology Software Tools, Inc., Pittsburgh, P.A.) was used for the presentation and timing of stimuli and collection of reaction times and response data. Responses were made on an MR-compatible five-button response box.
Event Tasks
Twenty-four past and twenty-four future event trials were presented randomly across the entire scanning session. Each trial was 35 seconds in duration and began with a 20-second construction and elaboration phase, during which a modified version of the Crovitz cueing procedure (Crovitz & Schiffman, 1974) was used. A cueing slide was presented for the duration of this phase and comprised three lines: (1) task instructions (“recall past event” or “envisage future event”); (2) the timeframe for the event (“last week” or “next week”; “last year” or “next year”; or “last 5–20 years” or “next 5–20 years”); and, (3) a cue word.
On presentation of this cueing slide, participants were required to recall a past event that occurred during the specified timeframe or imagine a future event that could occur within the timeframe. The event did not have to strictly involve the object named by the cue. Participants were encouraged to freely associate so that they were successful in generating an event. Events were, however, required to be temporally and contextually specific, occurring over minutes or hours, but not more than one day (i.e., episodic events). Examples were provided to illustrate this requirement (e.g., remembering a 3-week trip to France versus remembering visiting the Eiffel Tower on one specific day; imagining one’s future child versus imagining the birth of one’s future child). Future events had to be novel (i.e., not been previously experienced by the participant) and plausible given the participant’s plans for the future, to ensure the projection of the self over time (e.g., if one is not planning to have children, they should not imagine giving birth). Further, participants were instructed to experience events from a field perspective (i.e., seeing the event from the perspective of being there) rather than from an observer perspective (i.e., observing the self from an external vantage point). Once participants had the event in mind (i.e., an event had been retrieved or imagined), they pressed a button on the response box. This response time was recorded and marked the end of event construction and the beginning of elaboration. Participants were instructed prior to scanning that once they made this response, they were then to elaborate, that is, expand the event representation by retrieving or generating as much detail as possible until the end of the phase (i.e., until the rating task appears). The cueing slide remained onscreen for the entire 20 second duration, irrespective of when the response was made. If no response was made within the 20 seconds, the next phase of the trial (rating tasks) began. Note that all elaboration of detail was completed silently.
During the ratings phase of each event trial, participants rated the contents of the event. Three rating scales were presented, each for five seconds: (1) a five-point of the amount of detail they retrieved or imagined (1 = vague with no/few details; 5 = vivid and highly detailed); (2) a five-point scale of the intensity of emotion experienced upon retrieving or imagining this event (1=detachment; 5=highly emotional); and (3) a binary scale regarding whether the event was experienced primarily from a field or observer perspective (1 = saw event through my own eyes; 5 = saw myself from an external perspective). These particular scales were presented during scanning as the ratings depended directly on the phenomenology of the event generated during the preceding construction and elaboration phase and could potentially change if made after scanning.
Control tasks
Twenty-four semantic memory and twenty-four visual imagery trials, each 35 seconds in duration, were randomly interspersed through the scanning session. These tasks followed the same sequence as the event tasks and thus began with a 20-second construction and elaboration phase, during which a cueing slide was presented. The instruction line described the task (i.e., “words – sentence/define” or “objects – triangle/imagine” for the semantic and visual imagery tasks, respectively). For the semantic task, the second line specified that “2 related words” (i.e., related to the cue word) be generated; for the imagery task, the size of the 2 objects to be imagined was specified in relation to the cue object (i.e., “bigger/smaller”). In both tasks, the words or objects generated were required to be semantically related to the cue word, to prevent participants from simply using the same words or objects for each trial. Finally, a cue word was presented.
For the semantic retrieval task, participants were required to retrieve two words semantically related to the cue word, and then arrange all three words (i.e., cue word and two retrieved words) into a sentence. Thus, this control task construction phase controlled for both the generation and integration of information, processes which feature in the construction phase of the past and future event tasks. Once a sentence was devised, participants made a button-press, marking the end of construction and the beginning of elaboration. For the remainder of the 20-second cue presentation, participants generated as much detail as possible about the semantic meaning of each of the three words. For the visual imagery task, participants were required to imagine two objects related to the cue word, one bigger and one smaller than the object named by the cue word (i.e., a size comparison task). All three objects (i.e., the two generated objects and the cue object) were then imagined simultaneously in a triangular arrangement, and thus this task also controlled for the generation and integration of information. Once the triangular arrangement was constructed, participants made a button press and for the remainder of the 20-second cue-presentation, elaboration ensued and participants were required to generate as much detail as possible about the imagined objects. Requiring the generation of as much detail as possible meant the control elaboration phase was goal-directed in the same way as past and future elaboration.
By this design, the control tasks contained processes similar to those recruited during the event tasks: one must first retrieve information (words or objects) and integrate these together (i.e., into a sentence or a triangular arrangement), then decide that the construction phase is over and make a button press, and finally generate as much semantic or visuospatial details as possible for the remainder of the elaboration phase.
During the rating phase, three scales were presented, each for five seconds, to control for the rating scales used in the past and future event tasks: (1) a five-point scale for the average amount of detail generated during the elaboration of word meanings or visual object images (1 = no/few details; 5 = highly detailed); a five-point scale for how semantically related, on average, the two words or objects they generated were to the cue word (1 = semantically unrelated; 5 = highly semantically related); and (3) a binary scale for task difficulty (1 = easy; 5 = difficult).
Post-scan interview
Immediately following scanning, participants completed an interview in which they were prompted with each cue shown in the past and future event conditions. They were required to think back to the event they retrieved or imagined in the scanner, and to describe the event to the experimenter. Pilot testing demonstrated that participants were able to reflect back on events retrieved or generated during the experiment with acceptable reliability. The episodic specificity of the event (i.e., whether it was specific in time and place) was determined by the experimenter according to a three-point episodic specificity scale (Williams, Healy, & Ellis, 1999): an event specific in both time and place received a score of three; events specific in time or place received a score of two; and events general in time and place (e.g., personal semantics) received a score of one. Only those events receiving an episodic specificity score of three were included in analyses. Participants rated each event for personal significance on a five-point scale (1 = insignificant, did not change my life; 5 = personally significant and life-changing event), and provided their age (or predicted age) at the time of the event for those events in the 5–20 year timeframe. Collection of these data, in conjunction with ratings of detail, emotionality and field/observer perspective collected during scanning, allowed us to ensure that past and future events were episodic and did not differ in terms of phenomenological qualities and temporal distance. While these data may provide further insight into the nature of activations associated with past and future events (e.g., neural responses to these variables may differ according to whether the event is past or future in orientation), the focus of this paper is on construction and elaboration of events and thus imaging analyses utilizing these phenomenological data will be presented in a separate report.
Data acquisition
Images were acquired on a 3 Tesla Siemens Sonata MRI scanner. Detailed anatomical data were collected using a multiplanar rapidly acquired gradient echo (MP-RAGE) sequence. Functional images were acquired using a T2*-weighted echo planar imaging (EPI) sequence (TR= 2000 ms, TE = 23 ms, FOV = 200mm, flip angle = 90°). Twenty-five coronal oblique slices (5 mm thick) were acquired at an angle perpendicular to the long axis of the hippocampus in an interleaved fashion.
Data processing and statistical analyses
All pre-processing and analyses of imaging data was performed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Standard pre-processing of functional images was performed, including discarding the first four functional images to allow scanner equilibrium effects, rigid-body motion correction and unwarping, slice timing correction, spatial normalization to the Montreal Neurological Institute (MNI) template (resampled at 2 × 2 × 2 mm3 voxels) and spatial smoothing (using an 8mm full-width half maximum isotropic Gaussian kernel). Data were high-pass filtered to account for low-frequency drifts; a cut-off value of 128 was used.
Each event was modeled by SPM2’s canonical hemodynamic response function (hrf). Note that for each trial, two cognitive events were modeled: (1) the construction phase, and (2) the elaboration phase. As the start of the elaboration phase was based on response times, the amount of time separating the start of the construction phase and the start of the elaboration phase was random, highly variable (M = 7470.12 ms, sd = 2212.83 ms) and thus, effectively jittered. For the construction phase, the hrf was applied after reading of the cue was completed (1.8 seconds after task onset for past, future and semantic tasks, and 2 seconds after task onset for the imagery task, as determined through behavioral piloting of 5 participants), ensuring that the cognitive process being sampled is indeed construction rather than reading of the cue. With respect to the elaboration phase, the canonical hrf was applied one second before the response time on each trial, based on electrophysiological evidence indicating that neural changes associated with the formation of an autobiographical memory begin typically 800 to 1000 ms before a manual response is made (Conway et al., 2001). Thus it should coincide with the decision marking the end of the construction phase and the beginning of the elaboration phase, that is, the decision that a past or future event or control task items had been retrieved or generated. Neural activity related to the construction and elaboration of events was modeled at the onset of these respective phases rather than across the entire phase (i.e., as an extended event of variable duration) to reduce contamination by other cognitive processes including the possible onset of elaboration-related processes prior to the button press in the construction phase and potential decreases in effort and participant engagement across the duration of elaboration phase.
The fixed-effects model for each subject comprised eight event types corresponding to the construction and elaboration of past events, future events, semantic retrieval and visual imagery. In order to identify regions differentially engaged by past and future events, direct contrast analyses were used for both the construction and elaboration phases. Thus, four contrasts were computed for each subject: (1) past event construction > future event construction; (2) future event construction > past event construction; (3) past event elaboration > future event elaboration; and (4) future event elaboration > past event elaboration. Furthermore, contrasts of the main effect of construction and elaboration, collapsed across past and future, were also computed: (1) construction > elaboration; and (2) elaboration > construction. Similarly, contrasts of the interaction of temporal orientation (past or future) and the task phase (construction or elaboration) were also computed: (1) (past event construction > past event elaboration) > (future event construction > future event elaboration); and (2) (future event construction > future event elaboration) > (past event construction > past event elaboration). The contrast images for the various comparisons were subsequently entered into random-effects one-sample _t_-tests. A threshold of p < .001, uncorrected was employed for these contrasts (e.g., Maguire & Frith, 2003; Maguire, Frith, Rudge, & Cipolotti, 2005), with an extent threshold of 5 contiguously activated voxels (2 × 2 × 2 mm). However, in two a priori regions of interest, the bilateral hippocampus and the right frontal pole (Okuda et al., 2003), the height threshold was set at p < .005, uncorrected.
Conjunction analyses were used to examine regions shared between past and future events, both at the construction and elaboration phases. To begin, four contrasts were performed at the fixed-effects level: (1) past event construction > control (imagery and semantic) task construction; (2) future event construction > control (imagery and semantic) task construction; (3) past event elaboration > control (imagery and semantic) task elaboration; and (4) future event elaboration > control (imagery and semantic) task elaboration. At the random-effects level, these contrasts were used for two conjunction analyses: (1) the conjunction of event construction tasks (i.e., [past event construction > control (imagery and semantic) task construction AND future event construction > control (imagery and semantic) task construction]; and (2) the conjunction of event elaboration tasks [past event elaboration > control (imagery and semantic) elaboration AND future event elaboration > control (imagery and semantic) elaboration]). This involved using the masking function of SPM2 to select voxels to include or exclude. Thus, a one-sample _t_-test for one contrast of interest was computed, and the activated voxels from this analysis were used to form a mask. A second one-sample _t_-test for the other contrast of interest was computed, and the mask from the first analysis was applied, such that the resulting conjunction revealed regions active in both contrasts of interest. The individual one-sample _t_-tests were thresholded at p < .01, such that the conjoint probability of the conjunction analysis, estimated using Fisher’s method (Fisher, 1950; Lazar, Luna, Sweeney, & Eddy, 2002), was p < .001. To examine activity in our two a priori regions of interest (bilateral hippocampus and right frontal pole; Okuda et al., 2003), the conjoint probability was set at p < .005, uncorrected. In all regions, an extent threshold of 5 contiguously activated voxels (2 × 2 × 2 mm) was applied. For all analyses, the peak MNI co-ordinates of active regions were converted to Talairach space, and regions of activations were localized in reference to a standard stereotaxic atlas (Talairach & Tournoux, 1988). Percent signal change was extracted from activations of interest for past, future and control (collapsed across imagery and semantic tasks) construction and elaboration conditions using MarsBar toolbox for SPM (Brett, Anton, Valabregue, & Poline, 2002).
Results
Behavioral results
Participants were successfully able to construct an event during scanning and describe the event in the post-scan interview for an average of 21.64 past (sd = 2.17) and 22.29 future (sd = 1.90) event tasks (out of a maximum of 24 of each event type). These events were then rated objectively for episodic specificity and only events with a score of 3 (i.e., specific in time and place) were included in subsequent analyses (examples of specific past and future events are provided in Appendix 1). Accordingly, 11 past and 32 future events from 10 participants were excluded. Any events for which reaction time (6 past and 3 future events from 6 participants) and field/observer perspective (4 past and 8 future events from 7 participants) data were missing were also excluded. In accordance with instructions, participants experienced significantly more events from a field rather than an observer perspective as confirmed by Mann Whitney U tests (U < .001, p < .001; see Table 1). A chi-square test indicated that the frequencies of field and observer ratings did not significantly differ in frequency between past and future events (χ2 = 2.33, p = .127). Thus, the event types were considered matched for perspective, and all events from both perspectives were included in all subsequent analyses. Each participant contributed an average of 20.24 past (sd = 2.61) and 19.14 future events (sd = 2.66). Non-parametric Mann-Whitney U tests confirmed the final set of past and future events did not differ significantly with respect to ratings of detail (U = 68.50, p = .174), emotionality (U = 67.00, p = .154) and personal significance (U = 84.00, p = .520; see Table 1). Similarly, parametric _t_-tests demonstrated the event types did not differ significantly in event construction reaction times (t = −.56, p = .579) or temporal distance (years) (t = −.350, p = .729).
Table 1.
Mean reaction times, phenomenological ratings and temporal distance of past and future events.
| Past Events | Future Events | |||
|---|---|---|---|---|
| mean | sd | mean | sd | |
| Reaction time (event construction; ms) | 7232.22 | 2260.80 | 7708.03 | 2221.89 |
| Detail | 3.97 | .46 | 3.73 | .57 |
| Emotionality | 1.49 | .73 | 1.84 | .53 |
| Personal significance | 1.78 | .44 | 1.93 | .50 |
| Field perspective (frequency) | 17.00 | 3.70 | 18.71 | 4.05 |
| Observer perspective (frequency) | 2.14 | 2.11 | 1.50 | 1.79 |
| Temporal distance (years) | 3.57 | .90 | 3.68 | .73 |
Regions commonly engaged by past and future events
In order to examine shared regions of activity for past and future events, conjunction analyses at both the construction and elaboration phases were conducted (Table 2 and Figures 1 and 2). The conjunction analysis of past and future event construction revealed that a number of regions were commonly recruited, including left hippocampus, right inferior parietal lobule (BA 39/40), left superior occipital gyrus/cuneus (BA 18) and right middle occipital gyrus (BA 19; Figure 1a). Percent signal change in these regions confirmed that the left hippocampus and right middle occipital gyrus were significantly activated for both past and future events. However, the significant conjunction for right inferior parietal and left superior occipital cortex reflected significantly less deactivation associated with both past and future events relative to the control tasks. In all regions significant in this conjunction analysis, the control tasks were associated with deactivation or minimal activation.
Table 2.
Regions commonly recruited during the construction and elaboration of past and future events.
| Co-ordinates | ||||
|---|---|---|---|---|
| Brain Region | x | y | z | _Z_-score |
| Event construction > control construction | ||||
| L. Hippocampus* | −22 | −20 | −12 | 2.02 |
| R. Postcentral gyrus (BA 2) | 55 | −27 | 38 | 2.48 |
| R. Inferior parietal lobule (BA 39/40) | 55 | −43 | 41 | 2.71 |
| L. Superior occipital gyrus/cuneus (BA 18) | −14 | −70 | 33 | 2.85 |
| R. Middle occipital gyrus (BA 19) | 36 | −78 | 4 | 2.58 |
| Eevent elaboration > control elaboration | ||||
| L. Frontal pole (BA 10) | −2 | 62 | 4 | 4.59 |
| L. Superior frontal gyrus (BA 9) | −6 | 48 | 33 | 3.97 |
| R. Superior/middle frontal gyrus (BA 8) | 22 | 33 | 43 | 2.96 |
| L. Inferior medial prefrontal cortex (BA 11) | −4 | 40 | −12 | 3.40 |
| L. Anterior cingulate cortex (BA 32) | −4 | 46 | −4 | 3.79 |
| L. Anterior cingulate cortex (BA 25) | −4 | 9 | −6 | 4.37 |
| R. Anterior cingulate cortex (BA 24) | 2 | 33 | −3 | 3.82 |
| L. Cingulate cortex (BA 24) | −4 | −14 | 39 | 2.65 |
| L. Hippocampus | −22 | −18 | −13 | 2.83 |
| L. Parahippocampal gyrus (BA 30) | −18 | −35 | −3 | 3.39 |
| R. Parahippocampal gyrus (BA 37) | 28 | −35 | −3 | 2.63 |
| L. Superior temporal gyrus (BA 38) | −34 | 14 | −26 | 3.91 |
| L. Middle temporal gyrus (BA 38) | −53 | −9 | −15 | 4.27 |
| L. Middle temporal gyrus (BA 20/21) | 67 | −8 | −13 | 3.17 |
| L. Posterior cingulate/retrosplenial cortex (BA 29/30/31) | −12 | −41 | 39 | 5.14 |
| R. Posterior cingulate/retrosplenial cortex (BA 30/31) | 2 | −50 | 17 | 4.20 |
| L. Posterior cingulate (BA 23) | −8 | −51 | 25 | 3.73 |
| L. Precuneus (BA 7) | −12 | −53 | 36 | 4.37 |
| L. Inferior parietal lobule/angular gyrus (BA 39) | −46 | −55 | 34 | 4.21 |
| R. Inferior parietal lobule (BA 39) | 46 | −54 | 40 | 4.64 |
| L. Cuneus (BA 18) | −26 | −95 | 5 | 2.72 |
| L. Cerebellum | −4 | −52 | −34 | 3.45 |
| R. Cerebellum | 10 | −50 | −36 | 3.40 |
Figure 1.
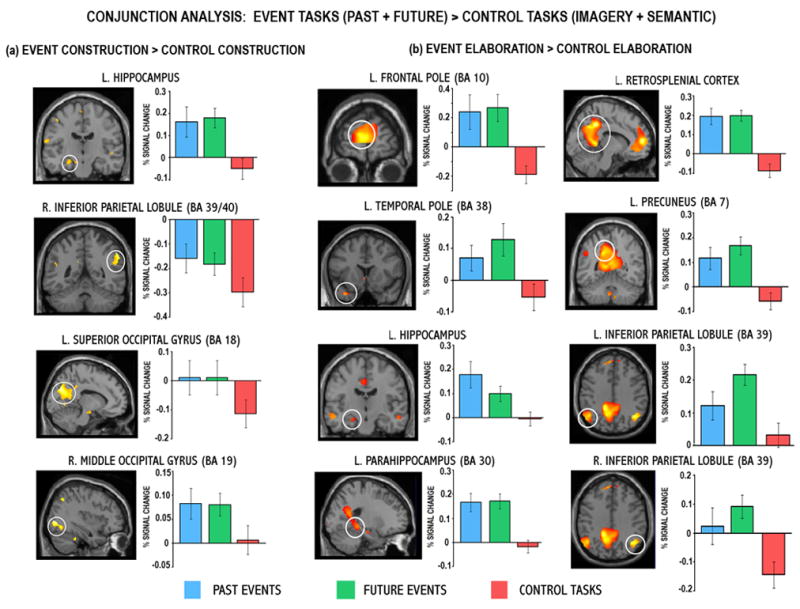
Neural regions commonly engaged during the (a) construction and (b) elaboration of past and future events relative to the control task. For all regions significant in these conjunction analyses, past and future events were engaged more than the control task at a threshold of p < .001 uncorrected (p < .005 for hippocampal regions of interest). Percent signal change data associated with each of these conditions was extracted from the peak voxels of these clusters (see Table 2 for coordinates) and are plotted. Note that future events activated left inferior parietal lobule significantly more than past events (p = .045), and there was a trend towards the left hippocampus being significantly more active during past relative to future events (p = .058). BA = Brodmann area.
Figure 2.
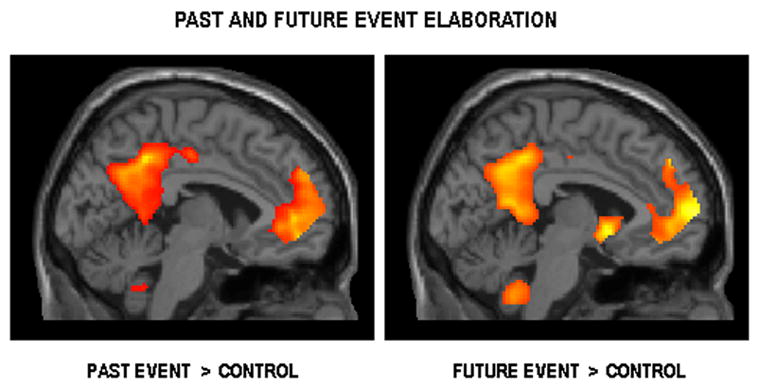
Sagittal slice (x = −4) illustrating the striking commonalities in medial left prefrontal and parietal activity during the elaboration of (a) past and (b) future events (relative to the control tasks) at a threshold of p < .001, uncorrected (shown at p < .005, uncorrected).
The conjunction of past and future event elaboration revealed extensive overlap in neural activity (Figures 1b and 2). Common activity was evident in many regions of the autobiographical memory network, notably the frontopolar (BA 10) and inferior (BA 11) aspects of the left medial PFC, left temporal pole (BA 38) and middle temporal gyrus (BA 20/21), left hippocampus and bilateral parahippocampal gyrus, bilateral posterior cingulate/retrosplenial regions (BA 29/30/31), left precuneus (BA 7), bilateral inferior parietal lobule (BA 39) and cerebellum. Percent signal change in these regions confirmed significant activations for both past and future elaboration relative to the control tasks, which were either associated with deactivations or with minimal activity, not reliably different from zero. The only exception was in the right inferior parietal lobule and although this region exhibited significantly greater activity during past and future elaboration relative to control tasks, activity for past events did not appear to be reliably different from zero. The left hippocampus was significantly activated by both past and future events relative to the control tasks, and a paired _t_-test on data extracted from this peak voxel indicated a trend towards past event elaboration eliciting a significantly higher level of activity (p = .058) than that associated with future elaboration. Finally, although this conjunction analysis did not reveal shared past-future activity in right hippocampus, this structure was engaged by both tasks (x = 36, y = −18, z = −8, Z = 2.54), albeit below the extent threshold.
Regions differentially engaged by past and future events
The question of whether past and future events engage distinct neural regions was examined at both the construction and elaboration phases (Table 2 and Figure 3; note percent signal change data for control tasks are also provided for descriptive purposes). This analysis did not reveal any regions during either phase that were engaged more by past than future events. In contrast, future events differentially recruited many regions during event construction relative to past events, including both a priori regions of interest - the frontopolar aspect of right medial PFC (BA 10) and the right hippocampus. Thus, while the left hippocampus was activated commonly by past and future event construction, the right hippocampus was engaged only during the creation of future events, albeit to a lower magnitude than left hippocampal activity (Figures 1a and 3a). In fact, there was a trend towards a significant deactivation in right hippocampus during past event construction. Additional regions of activity during future event construction included bilateral middle (BA 9/10/46) and inferior (BA 44/45/46/47) frontal gyri, bilateral fusiform gyrus (BA 19/37), left superior (BA 19) and inferior (BA 18) occipital gyrus and left cerebellum. There was also evidence of a significant difference between future and past event construction in the right middle temporal gyrus (BA 21), but this outcome reflected the fact that future events were significantly less deactivated than past events. As in the conjunction analyses, the control tasks were associated predominantly with deactivations or minimal activations in these regions. However, in the right inferior frontal gyrus the control tasks were associated with activation, and further, while this region was activated by the control tasks, the right frontal pole was deactivated (p = .004), suggesting a possible fractionation of right PFC function with respect to the control tasks. Finally, note that although the right precuneus (BA 7) was significantly more engaged by future than past event construction, this region exhibited even more activity during the control tasks and thus this past-future difference cannot be interpreted as unique to future event construction.
Figure 3.
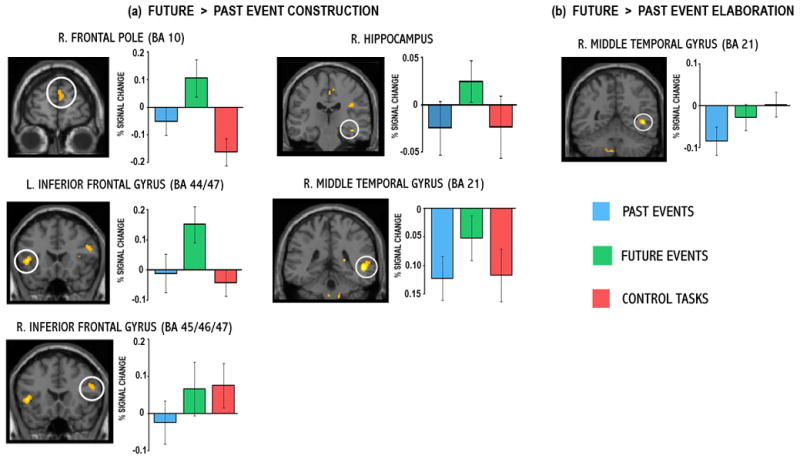
Neural regions showing significant increases in activity during the (a) construction and (b) elaboration of future relative to that of past events. All regions were significant at p < .001, uncorrected (p < .005, uncorrected, for hippocampal and right frontopolar regions of interest). Percent signal change data associated with each of these conditions was extracted from the peak voxels of these clusters (see Table 3 for coordinates) and are plotted. Data from control (imagery and semantic) tasks is also provided. BA = Brodmann area.
In contrast to the extensive past-future differences evident during the construction phase, few regions showed differential activity for one event type during elaboration. Increased activity associated with future events was observed in right middle temporal gyrus (BA 21; Figure 3b), however, percent signal change suggests that this past-future difference reflects significantly more deactivation during past event elaboration. Finally, signal extracted from left inferior parietal lobule (BA 39) indicated that this region was significantly more active during the elaboration of future events relative to both the past and control tasks (p = .045; Figure 1b).
Neural correlates of event construction and elaboration
To determine whether different regions were engaged during event construction and elaboration, irrespective of past versus future orientation, neural activity during these phases was contrasted directly (Table 4 and Figure 4). Event construction was associated with extensive activity in posterior regions, including inferior temporal/fusiform cortex (BA 37), right superior and inferior parietal lobule (BA 7), left lingual gyrus (BA 17/18) and cuneus (BA 19), right superior occipital gyrus and bilateral middle and inferior occipital gyri (BA 18/19; Figure 4a). Examination of signal extracted from the peak voxels in left and right fusiform cortex show that these regions become deactivated during elaboration. Event elaboration, relative to construction, engaged left superior and middle frontal gyri (BA 9/10), right inferior frontal gyrus (BA 47), left precuneus (BA 7), left supramarginal gyrus (BA 40) and bilateral cerebellum (Figure 4b). The contrasts for an interaction of temporal orientation (past and future) and task phase (construction and elaboration) did not reveal any significant regions of activity. This suggests that the differences between construction and elaboration do not change according to whether the event is located in the past or the future, likely a reflection of the remarkable similarity of neural activity underpinning past and future elaboration.
Table 4.
Regions differentially engaged by the construction and elaboration of events (collapsed across past and future).
| Co-ordinates | ||||
|---|---|---|---|---|
| Brain Region | x | y | z | _Z_-score |
| Construction > Elaboration | ||||
| L. Fusiform gyrus (BA 37) | −38 | −51 | −11 | 3.26 |
| R. Inferior temporal gyrus (BA 37) | 38 | −74 | 2 | 4.63 |
| R. Inferior parietal lobule (BA 7) | 22 | −60 | 45 | 3.35 |
| R. Superior parietal lobule (BA 7) | 20 | −54 | 54 | 3.14 |
| R. Superior occipital gyrus (BA 19) | 26 | −66 | 35 | 3.38 |
| L. Middle occipital gyrus (BA 18/19) | −30 | −81 | 4 | 3.89 |
| R. Middle occipital gyrus (BA 19) | 38 | −64 | −5 | 3.97 |
| L. Inferior occipital gyrus (BA 18) | −32 | −86 | −1 | 4.09 |
| R. Inferior occipital gyrus (BA 18) | 32 | −82 | −1 | 4.47 |
| L. Lingual gyrus (BA 17/18) | −16 | −84 | −3 | 3.35 |
| L. Cuneus (BA 19) | −14 | −84 | 23 | 3.26 |
| Elaboration > Construction | ||||
| L. Superior frontal gyrus (BA 10) | −22 | 59 | 6 | 3.90 |
| L. Middle frontal gyrus (BA 9) | −34 | 25 | 37 | 3.28 |
| L. Middle frontal gyrus (BA 10) | −34 | 50 | −1 | 3.54 |
| R. Inferior frontal gyrus (BA 47) | 53 | 27 | −5 | 4.24 |
| L. Precuneus (BA 7) | −8 | −48 | 48 | 4.17 |
| L. Inferior parietal lobule (BA 40) | −55 | −55 | 34 | 3.29 |
| L. Cerebellum | −32 | −56 | −36 | 3.18 |
| R. Cerebellum | 36 | −49 | −40 | 3.96 |
Figure 4.
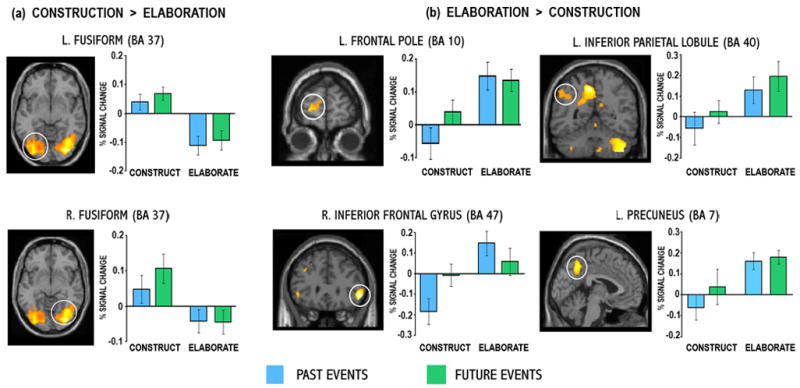
Activations associated with the (a) construction of past and future events relative to their elaboration, and conversely the (b) elaboration of past and future events relative to their construction. All regions were significant at p < .001, uncorrected. Percent signal change data associated with each of these conditions was extracted from the peak voxels of these clusters (see Table 4 for coordinates) and are plotted. BA = Brodmann area.
Discussion
Our data support the hypothesis that both common and distinct neural substrates mediate past and future events, consistent with the findings of the one previous neuroimaging study that examined this question (Okuda et al., 2003). In the present study, however, we teased apart neural processes contributing to event construction and elaboration, demonstrating that neural differentiation of past and future events was maximal during construction while overlap was most striking during elaboration. Moreover, the finding that numerous regions, including bilateral frontal pole and right hippocampus, demonstrated opposite patterns of activations and deactivations during construction and elaboration suggests that collapsing across these phases in a block design (Conway et al., 1999; Graham et al., 2003; Okuda et al., 2003; Rekkas & Constable, 2005) may obscure important patterns of activity. Finally, the matching of event types for phenomenological and episodic qualities in this study enables the interpretation of past-future differences as reflecting differences in temporal orientation and engagement of task-specific processes (e.g., prospective thinking, retrieval processes).
Neural overlap during past and future construction
Commonalities between the neural substrates of past and future events were evident during the event construction phase, though certainly, this was not as extensive as the overlap evident during elaboration. The conjunction of past and future event construction relative to the control task construction phase revealed significant overlap in visuospatial regions, as did the direct comparison of event construction to elaboration. In posterior regions, ROI analyses revealed that occipital and lateral parietal cortex were significantly activated relative to the control tasks, but even so, these regions exhibited minimal or negative percent signal change. In contrast, bilateral inferior temporal and fusiform cortices (BA 37) were significantly activated by event construction, reflecting higher level visuospatial processing and recognition of the objects named in the cue words. These findings are contrary to electrophysiological data from Conway and colleagues (Conway et al., 2001; Conway et al., 2003) demonstrating that the reconstruction phase of past event retrieval is dominated by strong left prefrontal activity and that widespread posterior cortical activity is not evident until elaboration. It is possible that posterior activity in these electrophysiological studies actually reflects extensive parietal activity, akin to that evident during event elaboration in the present study.
The conjunction of past and future event construction also revealed significant overlap in the left hippocampus. This finding demonstrates, for the first time, that the hippocampus is engaged very early on in the construction of an event, possibly before even prefrontal mechanisms are recruited. The early engagement may reflect the first interactions between event cues and hippocampally-mediated memory traces (i.e., ecphory, Tulving, 1983) in order to retrieve content from autobiographical memory needed to complete the past or future task. It is thought that cue information, either externally provided or internally generated, is conveyed to the hippocampus where it interacts with an index to a relevant memory trace, resulting in the elicitation of that memory (Moscovitch, 1992; Moscovitch & Winocur, 2002). A personalized cue can directly evoke a specific past event, while impersonal cues such as those used in this study, typically do not. If anything is retrieved, it is likely to be a semantic or general autobiographical memory, and more elaborate cue-specification and further retrieval attempts ensue (i.e., generative retrieval, Conway & Pleydell-Pearce, 2000; also termed strategic retrieval, Moscovitch, 1992; Moscovitch & Melo, 1997; Moscovitch & Winocur, 2002). Most studies of autobiographical memory use a direct cueing approach with personalized cues (e.g., Addis, Moscovitch et al., 2004; Gilboa et al., 2004; Maguire & Frith, 2003; Ryan et al., 2001; Steinvorth et al., 2006) and therefore cannot speak to this issue. Others who have used impersonal cues to engage a generative retrieval search have, on the whole, failed to show hippocampal activity, due to use of block designs averaging across search and elaboration (Conway et al., 1999; Graham et al., 2003; but see Rekkas & Constable, 2005) or electrophysiological techniques lacking adequate spatial resolution (Conway et al., 2001; Conway et al., 2003).
Neural differentiation of past and future event construction
We confirmed the hypothesis of maximal differentiation of past and future events during the construction phase, and in all instances, this reflected significantly more activity during future relative to past event construction. In contrast to common past-future activity in the left hippocampus, the right hippocampus was differentially recruited by future event construction. This finding is notable, not only because others report right hippocampal activity to be common to both past and future events (Okuda et al., 2003) but also because it is surprising that future events engage a structure more than the very task it is thought to be crucial for: retrieval of past autobiographical events (e.g., Nadel & Moscovitch, 1997; R. S. Rosenbaum et al., 2005; Scoville & Milner, 1957; Steinvorth, Levine, & Corkin, 2005; Viskontas, McAndrews, & Moscovitch, 2000). However, given that we do find hippocampal activity associated with past events, in the left hippocampus during past event construction and in both hippocampi during elaboration (albeit, subthreshold for the right hippocampus), the overall pattern of hippocampal activity is consistent with the literature.
It is interesting to consider the role the hippocampus plays in future events, particularly with respect to the unique engagement of the right hippocampus. We expected hippocampal activity to be common to both event types, based on previous findings (Okuda et al., 2003) and the assumption that both tasks require retrieval and integration of event details. With respect to past events, the hippocampus serves to index, reactivate and reintegrate the various aspects of a memory trace it bound together during encoding (Moscovitch, 1992). With future events, however, we hypothesized that the hippocampus would be involved in the retrieval and novel integration of disparate event details into future events (Cohen et al., 1999; Eichenbaum, 2001). The use of a relatively uncontrolled paradigm in the present study makes it difficult to determine which attributes of future events preferentially engage the right hippocampus. One possibility is novelty: the future events in this study were, by definition, novel events, even if certain details comprising the future events were not entirely novel. There may also be an interaction between novelty and relational processing; a recent study reported that the right hippocampus is responsive to novel relational information but not novel items (Kohler, Danckert, Gati, & Menon, 2005). While it is possible that this hippocampal effect reflects a difference in the amount of detail that is integrated when retrieving or generating event representations, this possibility is unlikely considering that the level of detail of past and future events was not significantly different. However, additional relational processing and hippocampal resources may be required to successfully bind event details into a coherent event when these details are disparate, as would likely occur during future event construction. This processing could reflect a neural difference between the reintegration of previously bound information (i.e., the memory trace) during past event retrieval, and the novel integration of information during future event construction. If right hippocampal activity does reflect novel integration, one would expect the control tasks, which also involve a component of novel integration, to also engage this structure. Although we did not find evidence in support of this idea, it is likely that the integration in the control tasks reflects a simpler type or lesser degree of relational processing relative to the complex integration of various types of contextual, conceptual and imagery-based information required during event construction. However, consistent with a neural response related to novelty processing, activity in the right hippocampus attenuates over the duration of the trial to a subthreshold level by the time of elaboration. Even so, further research manipulating various aspects of future events, including novelty and relational processing is needed to better understand the precise role played by the hippocampus in future event construction.
The right frontopolar cortex (BA 10) was also uniquely recruited by future events, a finding in line with those of Okuda et al (2003), but here we demonstrate that this activity is associated specifically with event construction. Both neuroimaging and lesion evidence suggest right frontopolar cortex plays an important role in prospective thinking, such as the representation of intentions. Okuda and colleagues (2003) demonstrated this region was responsive to the amount of intentional information comprising the future events. Studies of prospective memory, the ability to form and hold an intention to remember, have also found activity in the anterior aspects of the left (Okuda et al., 1998) and right (Burgess et al., 2001) frontal pole (BA 10). Moreover, lesion studies demonstrate that frontopolar damage is associated with impaired prospective memory (Burgess et al., 2000), as well as deficient anticipatory processing and insensitivity to future consequences when making decisions (Bechara et al., 1994; Bechara, Damasio, & Damasio, 2000).
Our contrast analysis also confirmed the hypothesis that the generative nature of future event construction would engage left ventrolateral PFC, a region typically associated with semantic generation (Fletcher, Shallice, & Dolan, 2000; Poldrack et al., 1999). Constructing imagined future events necessitates retrieval of semantic information regarding typical life events (e.g., moving into one’s first home), as well as more elaborate cue-specification strategies and increased speculation, and others have reported activity in this region when constructing imagined events (Conway et al., 2003). With similar reasoning, Okuda et al. (2003) also expected this finding, but found no evidence of differential left PFC activity, likely as a consequence of their block design that collapsed construction with elaboration, at which point we did not find this regions to be engaged.
There was no evidence of any regions engaged uniquely by past events, not only in the PFC but across the entire brain. This outcome was unexpected in light of previous results (Okuda et al., 2003). Moreover, regions mediating retrieval processes (e.g., cue-specification, Fletcher et al., 1998) such right ventrolateral PFC (e.g., BA 47) should be engaged by a pure retrieval task (i.e., past events) more than a generation task (i.e., future events). More surprising was the finding that right BA 47 showed more activity for future than past events, and that past events did not engage this region significantly more than control tasks. However, even though the past event task is a retrieval task, most comparisons of past autobiographical retrieval to semantic retrieval reveal that PFC activity is characteristically limited to left medial regions (see Gilboa, 2004, for a review; but also see Maguire, Henson, Mummery, & Frith, 2001). What role, then, might the right ventrolateral PFC play in future event construction? Based on lesion evidence, Burgess and colleagues (2000) suggest this region supports future planning. However, while we see differential engagement for future relative to past events, the fact that this region is also engaged by the control task suggests this activation is not necessarily specific to prospective thinking.
Common network mediating the elaboration of past and future events
The elaboration phase was characterized by extensive overlap between past and future events. One striking example was the common activity observed in left medial PFC, a region known to respond to self-referential information (Craik et al., 1999; Gusnard et al., 2001; Johnson et al., 2002) including autobiographical memories (Gilboa, 2004; Maguire, 2001) and personal future events (Okuda et al., 2003, though note, the foci in their were more lateral and inferior in location to those reported here). This finding is consistent with the instruction for both past and future tasks to generate only events that were personal in nature. Although it was expected that left medial PFC would be active throughout event construction and elaboration, the impersonal nature of the event cues may have delayed its engagement until some amount of autobiographical information had been retrieved and a personal event constructed.
In medial posterior regions, there was also an extensive swath of overlapping activity that extended from bilateral parahippocampal cortex into the retrosplenial cortex, posterior cingulate and precuneus. This pattern of activity is ubiquitous in studies examining autobiographical memory retrieval (Maguire, 2001), and its association with future events is not surprising given that the cognitive processes these regions are thought to mediate would be central to event elaboration irrespective of temporal orientation. For instance, the parahippocampal and retrosplenial cortex support contextual processing (Bar & Aminoff, 2003), and as predicted, these cortices were only engaged when instructed to generate as much detail about a past or future event (i.e., during the elaboration phase). The posterior cingulate is thought to play a role in self-reflection (Johnson et al., 2002; Northoff & Bermpohl, 2004) and the integration of emotion and memory during autobiographical memory retrieval of past events (Maddock, 1999; Maddock, Garrett, & Buonocore, 2001). These processes may be especially prominent during the elaborative processing of a personal event. However, whether the activity observed here contributed to the emotional or self-reflective elements of events remains unclear. The precuneus, which supports episodic imagery (Fletcher et al., 1995), was also expected to be active primarily during elaboration, and indeed the left precuneus exhibited this pattern of responsiveness. Although Okuda and colleagues (2003) report common past-future activity in bilateral parahippocampal cortex and left precuneus, they found no evidence of retrosplenial or posterior cingulate activity.
The personal nature of both the past and future event tasks implicates the retrieval of personal semantic information during both tasks, and thus the engagement of associated anterior and lateral temporal cortex. Indeed, the elaboration of both event types resulted in significant activation of left temporal pole and middle temporal gyrus. The absence of activity in the right temporal pole was unexpected, given the proposed role of this region in conceptual representations about self (Fink et al., 1996), general personal events (Addis, McIntosh et al., 2004) and familiar people (Graham et al., 2003; Thompson et al., 2004).
Distinct regions mediating past and future elaboration
Neural differentiation of past and future events was evident in only two regions during the elaboration phase, which contrasts with the extensive past-future differences evident during event construction. First, the posterior right middle temporal gyrus was uniquely engaged by future event elaboration, and as evident during future event construction, this result reflected less deactivation during the future event task relative to past event task. The aspects of future thinking that this pattern of deactivation reflects, however, remain unclear. Second, the left inferior parietal lobule exhibited significantly more activity during the elaboration of future relative to past events. Several theories regarding the role this region plays in episodic memory have been advanced, including that its activity is associated with the perception or awareness of ‘pastness’, (i.e., that a memory is old, Wheeler & Buckner, 2004). Although completion of both the past and future task presumably involves the retrieval of information from memory, it seems unlikely that the level of ‘pastness’ experienced would be substantially higher during the future task. More recently it has been suggested that this region is involved in recollective orienting, particularly when a task requires selective retrieval of event details (Dobbins, Rice, Wagner, & Schacter, 2003; Wagner et al., 2005). It is plausible that such a mechanism could be recruited differentially by future events, particularly as this task requires the retrieval of event details from numerous distinct memory traces in order to obtain material to recombine into a coherent novel event.
The adaptive significance of past and future episodic thinking
The neural overlap of past and future event representations was extensive, particularly during elaboration. In fact, every region engaged by the construction and elaboration of past events was also engaged by future events either to a similar or significantly higher level, in addition to regions specific to future events. As a consequence of this extensive overlap, the common network active during elaboration strongly resembled the network consistently documented in studies of past autobiographical event retrieval (Maguire, 2001, Figure 2). These findings are consistent with the pattern of episodic deficits in amnesic patients, who exhibit significant impairments in not only past, but also future episodic thinking (Klein & Loftus, 2002; Tulving, 1985). Furthermore, these results raise some questions about the adaptive significance of the episodic system. Although the function of the episodic system is typically conceived of as retrieval of past events, as demonstrated by the abundance of research on episodic memory, it is possible that the primary role of this system is not reminiscence, but rather, future thinking. As such, the ability to retrieve episodic information would exist primarily for the purpose of simulating possible future scenarios and outcomes, and anticipating future needs. Indeed, there is no adaptive advantage conferred by simply remembering, if such recollection does not provide one with information to evaluate future outcomes (Suddendorf & Busby, 2005). Not only does the episodic system permit one to retrieve past episodes for evaluation regarding future approach or avoidance of similar scenarios, it also allows for the simulation of novel events in considerable detail, at least in as much detail as past events, as we have shown here. Such detailed simulation of possible outcomes enables one to consider whether a particular situation would be approached or avoided if encountered. Moreover, simulating of future events can help one to anticipate future goals and needs, and such simulation is evolutionarily advantageous if one modifies current behavior with the aim of satisfying these future needs (Suddendorf & Busby, 2005). If, indeed, the primary function of the neurocognitive system commonly referred to as episodic memory centers on simulation of future events, we might even need to re-think whether the familiar term “episodic memory” is the most appropriate descriptor for this system. An emphasis on future event simulation as a primary function of the episodic system is, however, highly consistent with the general conception of episodic memory as a constructive activity rather than a passive replay of the past, a perspective that has been embraced in both cognitive psychology and cognitive neuroscience (e.g., Conway & Pleydell-Pearce, 2000; Johnson et al., 2002; Moscovitch & Melo, 1997; Neisser, 1967; Schacter, Norman, & Koutstaal, 1998; Tulving, 1983). Indeed, we have suggested (Schacter & Addis, in press) that simulation of future episodes requires a system that draws on the past in a manner that flexibly extracts and re-combines elements of previous experiences. According to this constructive episodic simulation hypothesis, some of the vulnerabilities of episodic memory, such as memory distortions and illusions, may be attributable to the role of the episodic system in allowing us to mentally simulate our personal futures by flexibly drawing on elements of the past (for further discussion, see Schacter & Addis, in press).
In summary, this study confirms that the representations of past and future events are mediated by both common and distinct neural substrates. All regions active during the construction and elaboration of past events were also active during future event construction and elaboration. Importantly, we demonstrate that the neural correlates of past and future events were maximally differentiated during event construction, despite the fact that these event types were matched on a variety of episodic and phenomenological qualities. The left hippocampus was commonly engaged by past and future event construction, as were posterior visuospatial regions, possibly reflecting the first stages of ecphory, that is, interaction between external cues and the hippocampal system. Notably, this process occurred even prior to the engagement of prefrontal retrieval mechanisms. In comparison to the construction of past events, future events recruited a number of additional regions thought to be involved in prospective thinking and generation, such as the right frontopolar cortex and left ventrolateral PFC, respectively. Furthermore, future event construction uniquely engaged the right hippocampus, possibly as a response to the novelty of these combinations of event details. Event elaboration was characterized by a remarkable overlap of activity in regions comprising the autobiographical memory retrieval network, attributable to the common processes engaged during this phase, including self-referential processing, contextual and episodic imagery. This striking overlap suggests that episodic future thinking is indeed an important, if not the primary, function of the episodic system.
Table 3.
Regions differentially engaged by future events during construction and elaboration.
| Co-ordinates | ||||
|---|---|---|---|---|
| Brain Region | x | y | z | _Z_-score |
| Future event construction > Past event construction | ||||
| R. Frontal pole (BA 10)* | 4 | 57 | 21 | 2.86 |
| L. Middle frontal gyrus (BA 9) | −42 | 6 | 35 | 3.51 |
| R. Middle frontal gyrus (BA 46) | 51 | 34 | 15 | 3.56 |
| R. Middle frontal gyrus (BA 10) | 36 | 49 | 5 | 3.35 |
| R. Middle frontal gyrus (BA 9) | 48 | 15 | 32 | 3.22 |
| L. Inferior frontal gyrus (BA 44) | −40 | 3 | 29 | 3.72 |
| L. Inferior frontal gyrus (BA 47) | −22 | 13 | −14 | 3.50 |
| R. Inferior frontal gyrus (BA 45/46) | 50 | 36 | 13 | 3.55 |
| R. Inferior frontal gyrus (BA 47) | 57 | 29 | −5 | 3.37 |
| L. Anterior cingulated cortex (BA 32) | −18 | 45 | 5 | 4.02 |
| R. Hippocampus* | 40 | −22 | −11 | 3.05 |
| R. Middle temporal gyrus (BA 21) | 51 | −37 | 0 | 3.73 |
| R. Precuneus (BA 7) | 4 | −54 | 51 | 3.89 |
| L. Fusiform gyrus (BA 37) | −40 | −57 | −16 | 3.40 |
| R. Fusiform gyrus (BA 19/37) | 34 | −54 | 1 | 3.53 |
| L. Superior occipital gyrus (BA 19) | −32 | −76 | 24 | 3.19 |
| L. Lingual gyrus (BA 18) | −14 | −86 | −9 | 3.21 |
| L. Cerebellum | −16 | −70 | −10 | 3.81 |
| Future event elaboration > Past event elaboration | ||||
| R. Middle temporal gyrus (BA 21) | 44 | −50 | 1 | 3.59 |
Acknowledgments
We thank W. Dale Stevens for assistance with data collection, Kelly S. Giovanello, Angela Gutchess, Elizabeth Kensinger and Itamar Kahn for assistance with statistical analyses, and anonymous reviewers for helpful comments. This research was supported by National Institute of Mental Health (NIMH) grant MH060941 and National Institute on Aging (NIA) grant AG08441, awarded to DLS.
Appendix 1. Examples of specific past and future events generated by a pilot participant
Past event (5 years ago; cue = star)
It was my birthday and I was about to leave for a trip with my family … And so my friend, he has just gotten his license, and he said, okay, you know, I’ll take you out for your birthday before you leave … so we went to this place in Berkeley … famous for its deep dish pizzas. He had just gotten his license, I’m a kind of oblivious [of this] … so when I got in the car I immediately started talking to him, and he’s, um, okay I cant talk right now … We had the pizza and he took me to this place called Indian Rock in Berkeley and it was a very interesting place, and I had always heard of it but you need a car to get there, so perfect timing, so we walked up with the pizzas and it’s this big rock on the top of this kind of hill at Berkeley. And when you’re up at the top you can see the whole bay and you can see San Francisco … the view was gorgeous, and the sun was setting.
Future event (in 5 years; cue = dress)
My sister will be finishing … her undergraduate education, I imagine some neat place, Ivy league private school … it would be a very nice spring day and my mom and my dad will be there, my dad with the camcorder as usual, and my mom with the camera as usual. My sister will be in the crowd and they’d be calling everyone’s name … I can see her having a different hair style by then, maybe instead of straight, very curly with lots of volume. She would be wearing contacts by then and heels of course. And I can see myself sitting in some kind of sundress, like yellow, and under some trees … the reception either before or after and it would be really nice summer food, like salads and fruits, and maybe some sweets, and cold drinks that are chilled but have no ice. And my sister would be sitting off with her friends, you know, talking with them about graduating, and they’d probably get emotional.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Atance CM, O’Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5(12):533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Atance CM, O’Neill DK. The emergence of episodic future thinking in humans. Learning and Motivation. 2005;36:126–144. [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage; Presented at the 8th International Conference on Functional Mapping of the Human Brain; 2002. [abstract] [Google Scholar]
- Buckner RL, Carroll DC. Common brain networks for envisioning the future and related forms of self-projection. Trends Cogn Sci. XX:xxx–xxx. in press. [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Clark JM, Paivio A. Extensions of the Paivio, Yuille, and Madigan (1968) norms. Behav Res Methods Instrum Comput. 2004;36:371–383. doi: 10.3758/bf03195584. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, M.A: The MIT Press; 1993. [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE. The neuroanatomy of autobiographical memory: A slow cortical potential study of autobiographical memory retrieval. Journal of Memory & Language. 2001;45:493–524. [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE, Sharpe H. Neurophysiological correlates of memory for experienced and imagined events. Neuropsychologia. 2003;41:334–340. doi: 10.1016/s0028-3932(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Conway MA, Turk DJ, Miller SL, Logan J, Nebes RD, Meltzer CC, et al. A positron emission tomography (PET) study of autobiographical memory retrieval. Memory. 1999;7:679–702. doi: 10.1080/096582199387805. [DOI] [PubMed] [Google Scholar]
- Craik FI, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In search of the self: A positron emission tomography study. Psychol Sci. 1999;10:26–34. [Google Scholar]
- D’Argembeau A, Van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Conscious Cogn. 2004;13:844–858. doi: 10.1016/j.concog.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41(3):318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss W. Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. J Neurosci. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. London: Oliver & Boyd; 1950. [Google Scholar]
- Fletcher P, Dolan RJ. Right prefrontal cortex responds to item familiarity during a memory encoding task. Memory. 1999;7(5–6):703–713. doi: 10.1080/096582199387814. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Frith C, Baker SC, Shallice T, Frackowiak RS, Dolan R. The mind’s eye - precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Henson RN. Frontal lobes and human memory: Insights from neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Shallice T, Dolan RJ. “Sculpting the response space”--an account of left prefrontal activation at encoding. Neuroimage. 2000;12(4):404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Shallice T, Frith C, Frackowiak RS, Dolan R. The functional roles of the prefrontal cortex in episodic memory II. Retrieval. Brain. 1998;121:1249–1256. doi: 10.1093/brain/121.7.1249. [DOI] [PubMed] [Google Scholar]
- Gilbert DT. Stumbling on Happiness. New York: Knopf; 2006. [Google Scholar]
- Gilboa A. Autobiographical and episodic memory--one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Graham KS, Lee AC, Brett M, Patterson K. The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cogn Affect Behav Neurosci. 2003;3:234–254. doi: 10.3758/cabn.3.3.234. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rubin DC. The neuropsychology of autobiographical memory. Cortex. 2003;39:687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock PA. Time and the privileged observer. Kronoscope. 2005;5:176–191. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Klein SB, Loftus J. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Social Cognition. 2002;20:353–379. [Google Scholar]
- Kohler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: a comparison based on event-related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: a survey of methods for statistical pooling of information. Neuroimage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. Retrosplenial cortex and emotion: New insights from functional imaging studies of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003;23:5302–5307. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Rudge P, Cipolotti L. The effect of adult-acquired hippocampal damage on memory retrieval: An fMRI study. Neuroimage. 2005;27:146–152. doi: 10.1016/j.neuroimage.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Henson RN, Mummery CJ, Frith CD. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport. 2001;12:441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ, Buchel C. Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus. 2000;10:475–482. doi: 10.1002/1098-1063(2000)10:4<475::AID-HIPO14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: a component process model based on modules and central systems. J Cogn Neurosci. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Melo B. Strategic retrieval and the frontal lobes: Evidence from confabulation and amnesia. Neuropsychologia. 1997;35:1017–1034. doi: 10.1016/s0028-3932(97)00028-6. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. 2002. pp. 188–209. [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Neisser U. Cognitive Psychology. New York: Appleton-Century-Crofts; 1967. [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, et al. Thinking of the future and the past: The roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Yamadori A, Kawashima R, Tsukiura T, Fukatsu R, et al. Participation of the prefrontal cortices in prospective memory: evidence from a PET study in humans. Neurosci Lett. 1998;253:127–130. doi: 10.1016/s0304-3940(98)00628-4. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Poldrack R, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Rekkas PV, Constable RT. Autobiographical memory retrieval does not become independent of the hippocampus: An fMRI study contrasting very recent with remote events. J Cogn Neurosci. 2005;17:1950–1961. doi: 10.1162/089892905775008652. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Köhler S, Schacter DL, Moscovitch M, Westmacott R, Black SE, et al. The case of K.C.: contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, McKinnon MC, Levine B, Moscovitch M. Visual imagery deficits, impaired strategic retrieval, or memory loss: disentangling the nature of an amnesic person’s autobiographical memory deficit. Neuropsychologia. 2004;42(12):1619–1635. doi: 10.1016/j.neuropsychologia.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357(1424):1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Nadel L, Keil K, Putnam K, Schnyer D, Trouard T, et al. Hippocampal complex and retrieval of recent and very remote autobiographical memories: evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11:707–714. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. XX:xxx–xxx. doi: 10.1098/rstb.2007.2087. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Norman KA, Koutstaal W. The cognitive neuroscience of constructive memory. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage. 2006;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia. 2005;43:479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Busby J. Making decisions with the future in mind: Developmental and comparative identification of mental time travel. Learning and Motivation. 2005;36:110–125. [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York, N.Y: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Thompson SA, Graham KS, Williams G, Patterson K, Kapur N, Hodges JR. Dissociating person-specific from general semantic knowledge: roles of the left and right temporal lobes. Neuropsychologia. 2004;42(3):359–370. doi: 10.1016/j.neuropsychologia.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory. Vol. 2. New York, N.Y: Oxford University Press; 1983. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychologist. 1985;25:1–12. [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, Moscovitch M. Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. J Neurosci. 2000;20:5853–5857. doi: 10.1523/JNEUROSCI.20-15-05853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler M, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ellis NC, Tyers C, Healy H, Rose G, MacLeod AK. The specificity of autobiographical memory and imageability of the future. Mem Cognit. 1996;24(1):116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]
- Williams JM, Healy HG, Ellis NC. The effect of imageability and predictability of cues in autobiographical memory. Q J Exp Psychol A. 1999;52(3):555–579. doi: 10.1080/713755828. [DOI] [PubMed] [Google Scholar]