Characterization of the Proteasome Accessory Factor (paf) Operon in Mycobacterium tuberculosis (original) (raw)
Abstract
In a previous screen for Mycobacterium tuberculosis mutants that are hypersusceptible to reactive nitrogen intermediates (RNI), two genes associated with the M. tuberculosis proteasome were identified. One of these genes, pafA (_p_roteasome _a_ccessory _f_actor A), encodes a protein of unknown function. In this work, we determined that pafA is in an operon with two additional genes, pafB and pafC. In order to assess the contribution of these genes to RNI resistance, we isolated mutants with transposon insertions in pafB and pafC. In contrast to the pafA mutant, the pafB and pafC mutants were not severely sensitized to RNI, but pafB and pafC were nonetheless required for full RNI resistance. We also found that PafB and PafC interact with each other and that each is likely required for the stability of the other protein in M. tuberculosis. Finally, we show that the presence of PafA, but not PafB or PafC, regulates the steady-state levels of three proteasome substrates. Taken together, these data demonstrate that PafA, but not PafB or PafC, is critical for maintaining the steady-state levels of known proteasome substrates, whereas all three proteins appear to play a role in RNI resistance.
Mycobacterium tuberculosis is a successful pathogen that persists in nearly one-third of the Earth's population (11). Infection occurs by the inhalation of aerosolized droplets containing M. tuberculosis bacilli into the lungs, where alveolar macrophages and other phagocytic cell types then engulf the bacteria. Within these cells, M. tuberculosis must face various host defenses (26). One of the major protective defenses used by macrophages to contain M. tuberculosis infection is nitric oxide (NO), which is produced by the inducible nitric oxide synthase (19). NO and other reactive nitrogen intermediates (RNI) can damage DNA, lipids, and proteins and interfere with various cellular processes (23, 30, 36). As a result, mice that are deficient in inducible nitric oxide synthase are highly susceptible to M. tuberculosis infection (19). However, despite the presence of RNI, M. tuberculosis is able to persist within the host.
Two genes, mpa (_Mycobacterium p_roteasomal _A_TPase) and pafA (_p_roteasome _a_ccessory _f_actor), were previously identified to be important for the ability of M. tuberculosis to survive exposure to RNI in vitro and cause disease in vivo (6). mpa and pafA were predicted to encode proteins involved in proteasome function in bacteria (10, 22). Proteasomes are barrel-shaped proteases consisting of 14 α subunits and 14 β subunits (“20S core”) (1, 18). In eukaryotes, a 19S cap complex associates with the 20S core particle. The base of the cap consists of six AAA (_A_TPase _a_ssociated with various cellular _a_ctivities) ATPases, while the lid proteins recognize ubiquitinated substrates targeted for degradation (4, 9, 25, 34). Mpa is similar to ATPases found in the eukaryotic proteasome base (7), and chemical inhibition of the M. tuberculosis proteasome protease activity sensitizes wild-type (WT) M. tuberculosis to RNI to a degree similar to that of the mpa or pafA mutants (6). The strongest evidence connecting Mpa, PafA, and the proteasome protease is the observation that all three are required for the apparent degradation of three M. tuberculosis proteasome substrates (24).
Although Mpa resembles proteasome-associated ATPases, PafA shares no homology with any protein of known function. In this work, we determined that pafA is in an operon with genes encoding two conserved proteins, Rv2096c (PafB) and Rv2095c (PafC). We also looked for interactions between proteins encoded by the pafABC operon and proteins involved in proteasome function. Finally, we investigated the role of each gene with respect to RNI resistance and substrate degradation by the M. tuberculosis proteasome. Taken together, this work represents the first study to examine the function of the previously uncharacterized pafABC operon.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are described in Table 1. Escherichia coli strains were grown in Luria-Bertani (LB) Miller broth (Difco) at 37°C with aeration on an orbital shaker. E. coli strains were chemically transformed as previously described (28). MacConkey plates with 1% maltose were prepared for use in the bacterial two-hybrid (BTH) experiments (16). For T7 promoter induction, isopropylthio-β-d-galactoside (IPTG) was added to a final concentration of 0.1 mM.
TABLE 1.
Strains, plasmids, and primers used in this work
| Strain, plasmid, or primer | Genotype or sequence | Source or reference |
|---|---|---|
| M. tuberculosis strains | ||
| H37Rv | WT | ATCC 25618 |
| MHD2 | pafA::ΦMycoMarT7 (282) | 6 |
| MHD18 | WT, pMV-306 | 6 |
| MHD62 | pafA::ΦMycoMarT7, pMV306 | This work |
| MHD63 | pafA::ΦMycoMarT7, pMV-pafA | This work |
| MHD64 | pafA::ΦMycoMarT7, pMV-pafABC | This work |
| MHD75 | pafB::ΦMycoMarT7 (769) | This work |
| MHD76 | pafC::ΦMycoMarT7 (466) | This work |
| MHD77 | pafC::ΦMycoMarT7 (767) | This work |
| MHD79 | WT, pMN-FLAG-_fabD_-His6 | 24 |
| MHD78 | WT, pMN-FLAG-_panB_-His6 | 24 |
| MHD82 | pafA::ΦMycoMarT7, pMN-FLAG-_panB_-His6 | 24 |
| MHD83 | pafA::ΦMycoMarT7, pMN-FLAG-_fabD_-His6 | 24 |
| MHD98 | WT, pMV-pafC | This work |
| MHD99 | pafC::ΦMycoMarT7 (466), pMV-pafC | This work |
| MHD100 | pafC::ΦMycoMarT7 (767), pMV-pafC | This work |
| MHD102 | pafC::ΦMycoMarT7 (466), pMN-FLAG-_panB_-His6 | This work |
| MHD112 | pafC::ΦMycoMarT7 (466), pMN-FLAG-_fabD_-His6 | This work |
| MHD117 | pafB::ΦMycoMarT7, pMN-FLAG-_fabD_-His6 | This work |
| MHD118 | pafB::ΦMycoMarT7, pMN-FLAG-_panB_-His6 | This work |
| E. coli strains | ||
| DH5α | F− φ80d_lacZ_ΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | Gibco, BRL |
| BTH101 | F−cya-99 araD139 galE15 galK16 rpsL1 (Strr) hsdR2 mcrA1 mcrB1 | 16 |
| ER2566 | F− λ−fhuA2 [lon] ompT lacZ::T7 geneI gal sulA11 Δ(mcrC-mrr)114::IS_10_ R(mcr-73::miniTn10)2 R(zgb-210::Tn10)1 (Tets) endA1 [dcm] | 3 |
| Plasmids | ||
| pMV306 | Hygr; integrates in single copy on the chromosome | 31 |
| pMV-pafA | Hygr; for complementation of the pafA mutant | This work |
| pMV-pafABC | Hygr; for complementation of the pafA mutant | This work |
| pMV-pafC | Hygr; for complementation of the pafC mutants | This work |
| pET24b(+) | Kanr; for gene overexpression | Novagen |
| pET24b+pafA | Kanr; for overexpression of pafA | This work |
| pET24b+pafB | Kanr; for overexpression of pafB | This work |
| pET24b+pafC | Kanr; for overexpression of pafC | This work |
| pET24b+pafABC | Kanr; for overexpression of pafABC | This work |
| pUT18C_pafC_ | Ampr; pafC cloned into pUT18C with XbaI and KpnI, translationally fused to the C terminus of the T18 domain | This work |
| pUT18C_pafB_ | Ampr; pafB cloned into pUT18C with XbaI and KpnI, translationally fused to the C terminus of the T18 domain | This work |
| pKT25_pafC_ | Kanr; pafC cloned into pKT25 with XbaI and KpnI, translationally fused to the C terminus of the T25 domain | This work |
| pKT25_pafB_ | Kanr; pafB cloned into pKT25 with XbaI and KpnI, translationally fused to the C terminus of the T25 domain | This work |
| pMN-FLAG-_fabD_-His6 | Hygr; pMN402 with green fluorescent protein replaced by FLAG-_fabD-_His6 | 24 |
| pMN-FLAG-_panB_-His6 | Hygr; pMN402 with green fluorescent protein replaced by FLAG-_panB_-His6 | 24 |
| pMN-FLAG-_dlaT_-His6 | Hygr; pMN402 with green fluorescent protein replaced by FLAG-_dlaT_-His6 | 24 |
| pKD13 | Kanr; used for Southern analysis | 8 |
| Primers | ||
| Rv2098c-f1 | GCCTGTTCCAGGTAGGTAGT | |
| Rv2097c-rt | GTCATGGGTGACCAGCTGC | |
| Rv2098c-r1 | GCCGGACGGGCCGGGGAC | |
| tatA-f1 | GTGGGCAGTCTGAGTCCGTGGC | |
| tatA-rt | GCCGGCCGAGCCTCGGTGC | |
| Rv2095-f1 | GGTCGCGTGAGGCCGAAGG | |
| tatC-rev1 | ATGCGAATACCAGACGAACC | |
| pafoperon-f1 | GTCACCACCGACGAAGAAAT | |
| pafoperon-r1 | ATCCACAGCTGGTTGAGGTC | |
| pafB-end-BTH | GCGGTACCTCATGCCAGTGCTCCGGCTT | |
| pafB-start-BTH | GCTCTAGAGATGGCGACCTCGAAAGTCGAAC | |
| pafC-start-BTH | GCTCTAGAGATGAGCGCCCTGTCCACCCG | |
| pafC-end-BTH | GCGGTACCTCACGGCGGCGCAGCTGC | |
| pafC-rev4-HindIII | CGAAGCTTTCACGGCGGCGCAGCTGC | |
| pafC-rev4-HindIII-NO STOP | CGAAGCTTCGGCGGCGCAGCTGCCTGGTA | |
| NdeI-pafA | GGAATTCCATATGCAGCGTCGAATCATGGGC | |
| pafB-for1-NdeI | GGAATTCCATATGGCGACCTCGAAAGTCGAACG | |
| pafB-rev1-HindIII | GTCAAGCTTTGCCAGTGCTCCGGCTTGCGC | |
| pafC-for1-NdeI | GGAATTCCATATGAGCGCCCTGTCCACCCGG | |
| pafA-rev1-HindIII | GTCAAGCTTCATGCTCGCGATCAGCCGCTTAAC | |
| pafA-RT-for | GATCAGCCCCCACAGACC | |
| pafA-RT-rev | GCTTAACCCGCTCATCGAC | |
| pafB-RT-for | GTGTGGACCTACGCAGCAT | |
| pafB-RT-rev | TCGAATCTCAAGCTCGATCA | |
| pafC-RT-for | GCTGTTCGACGGTGACCTAT | |
| pafC-RT-rev | ATCCAATCCTCAGAGGCGTA |
All M. tuberculosis strains used are derivatives of H37Rv (Table 1). M. tuberculosis strains were grown in Middlebrook 7H9 with ADN (0.5% bovine serum albumin [Roche], 0.2% dextrose, 0.085% sodium chloride) without shaking in 75-cm2 vented flasks (Corning). Middlebrook 7H11 plates enriched with BBL Middlebrook oleic acid-albumin-dextrose-catalase were used to grow M. tuberculosis on solid media. Nitrite killing assays are described elsewhere (6).
The final concentrations of antibiotics used for E. coli were as follows: ampicillin, 200 μg/ml; hygromycin, 150 μg/ml; and kanamycin, 100 μg/ml. For M. tuberculosis, both hygromycin and kanamycin were used at a concentration of 50 μg/ml.
Plasmids.
All plasmids and primers are listed in Table 1. pMV-pafABC was made in several cloning steps that resulted in a plasmid with a 3.5-kb fragment including 208 bp upstream of the predicted start codon of pafA to the stop codon of pafC (GenBank accession number for pafABC, DQ990836). pMV-pafA was created by digesting pMV-pafABC with ClaI, which deleted pafC and 486 bp of pafB. pMV-pafC was constructed as follows: a KpnI-PstI fragment containing 208 bp upstream of the pafA start codon (containing the presumed native promoter) and 477 bp of pafA sequence was cloned upstream of a PstI-NcoI fragment containing the last 63 bp of pafB and the entire pafC coding sequence. This cloning resulted in a fusion of part of pafA and part of pafB; however, polyclonal antibodies against PafA and PafB were unable to detect this hypothetical fusion protein.
pET24b(+) was used to express pafABC in E. coli for in vitro interaction studies. pET24b+pafABC and pET24b+_pafABC-_His6 were constructed using primers specific to the start of pafA (the GTG start site was changed to ATG for optimal expression in E. coli) and the end of pafC. pET24b+_pafABC-_His6 does not have a stop codon, which allowed inclusion of the His6 tag encoded in the vector. Primers included restriction sites that allowed the PCR products to be cloned into the NdeI and HindIII sites of pET24b(+).
pET24b(+) was also used for the construction of plasmids to overexpress pafA, pafB, and pafC individually for antibody production. These constructs were made using primers with restriction sites for NdeI and HindIII (Table 1).
All PCR-generated plasmids were sequenced by either the New York University School of Medicine DNA Sequencing Facility or Genewiz, Inc. (New Brunswick, NJ).
Mutant mining.
A PCR-based approach was used to identify pafB and pafC transposon insertion mutants in a previously assembled H37Rv ΦMycoMarT7 library (6). A similar technique is described elsewhere (12, 17). The ∼10,100 mutants in the library were pooled into groups of 60, and chromosomal DNA was isolated from each pool. Each pool was screened using primers specific to the Himar sequence of ΦMycoMarT7 (5′-AGACCGGGGACTTATCAGCCAACCTG-3′) (29) and the 3′ end of pafC (5′-CGCAGCTGCCTGGTATGCATCCAG-3′). Amplified products of the predicted molecular weight were gel purified and sequenced using the Himar primer (New York University School of Medicine DNA Sequencing Facility). Once pools with pafB or pafC mutants were identified, each mutant within a pool was separately grown in 1 ml of 7H9 plus ADN with 50 μg/ml kanamycin in 96-well plates with 2-ml wells (Nunc). After 2 weeks, chromosomal DNA was purified from individual mutants (Ultra Clean DNA purification kit; MoBio), and PCR was used as described above to identify mutants within the pool. Once identified, each mutant was single-colony purified by passing a mid- to late-log-phase culture through a 5.0-μm filter (Millipore) by gravity flow. The resulting cell suspension was inoculated onto solid medium and incubated for 2 to 3 weeks. The presence of a single transposon insertion in each mutant was confirmed by Southern blotting. Genomic DNA was digested with BamHI and transferred to a nylon membrane (Hybond-XL; Amersham Biosciences). To probe for the presence of the transposon insertion on the chromosome, we used the entire pKD13 plasmid digested with HindIII to probe for the neomycin (kanamycin resistance) cassette encoded on the transposon. Detection was performed using the DIG High Prime DNA labeling and detection starter kit I (Roche).
RNA isolation, reverse transcriptase PCR (RT-PCR), and qRT-PCR.
RNA was extracted from M. tuberculosis cultures grown in 7H9 plus ADN to an optical density at 580 nm (OD580) of 1.0. An equal volume of 4 M guanidinium isothiocyanate-0.5% sodium _N_-lauryl sarcosine-25 mM trisodium citrate was added to cultures to arrest transcription, and cells were pelleted at 2,885 × g. Bacterial pellets were resuspended in 1 ml TRIzol reagent (Invitrogen) and bead beaten with zirconia silica beads (BioSpec Products) in a BioSpec Mini Bead Beater two times for 30 s, with cooling of the samples on ice in between. Preparation of the RNA was performed as described by the manufacturer. RNA extraction was repeated two more times to ensure removal of all genomic DNA. RNA was stored in aliquots at −80°C. cDNA was synthesized using the Reverse Transcriptase System (Promega) with 100 ng of total M. tuberculosis RNA and random hexamers (Amersham Biosciences). For quantitative real-time PCR (qRT-PCR), we used Platinum SYBR green PCR SuperMix UDG (Invitrogen) in a Bio-Rad MyiQ real-time PCR system.
BTH analysis.
BTH assays were performed as described previously (16). Protein fusions were constructed using pUT18C or pKT25 plasmids, and the primers used to make fusions in this study are listed in Table 1. BTH101 cells were transformed with both plasmids and inoculated onto MacConkey agar supplemented with 1% maltose. Colonies were colony purified on the same medium. β-Galactosidase assays were performed as previously described to quantify the interactions between two fusion proteins (21).
Affinity chromatography.
Plasmids used for the production of His6-tagged Paf proteins are listed in Table 1. One-hundred-milliliter cultures of E. coli strains containing plasmids carrying pafABC or _pafABC-_His6 were induced with IPTG at an OD600 of 0.6 for 5 h at 26°C. Cell lysates were prepared exactly as described in The QIAexpressionist manual. To examine protein-protein interactions, proteins were purified under native conditions. Lysate (750 μl) was then added to 30 μl of Ni-nitrilotriacetic acid (Ni-NTA) agarose (QIAGEN) and incubated with agitation for 1 h at 4°C. Ni-NTA agarose was pelleted and washed with 750 μl wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole) three times. The agarose was resuspended in 200 μl of elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole) and collected by centrifugation, and the supernatant was saved (“elution”). This was repeated four times to obtain four elutions. Samples were boiled for 5 min, and PafA, PafB, and PafC were detected by immunoblotting.
Antibodies and immunoblotting.
Purification of proteins for antibody production was performed under denaturing conditions according to the manufacturer's specifications (QIAGEN). Polyclonal rabbit antibodies were raised against PafA, PafB, and PafC with C-terminal His6 tags, each expressed individually in E. coli. Antibodies were produced using Freund's incomplete adjuvant by Sigma-Genosys (St. Louis, MO). Mpa and DlaT antibodies were described previously (7, 32). For immunoblotting analysis, cell numbers equivalent to 10 OD580 units were harvested. Bacteria were washed once in an equal volume of phosphate-buffered saline-0.05% Tween 20 and were resuspended in 350 μl of lysis buffer (100 mM Tris-Cl, 100 mM KCl, 1 mM EDTA, 5 mM MgCl2, pH 8). Cells were lysed by bead beating with zirconia beads three times for 30 s. Total cell lysate (150 μl) was mixed with 50 μl of 4× sodium dodecyl sulfate sample buffer and boiled for 10 min. Immunoblotting was performed as previously described (14). Antibodies to His6-tagged PafA, PafB, and PafC antibodies were affinity purified as described elsewhere (7). Anti-PafA was used at a dilution of 1:1,000, and antibodies to PafB and PafC were used at a dilution of 1:100. FLAG antibodies were purchased from Sigma (St. Louis, MO). Horseradish peroxidase-conjugated goat anti-rabbit antibodies (Amersham Biosciences) were used for chemiluminescent detection (SuperSignal West Pico or Femto chemiluminescent substrate; Pierce). Antibodies to DlaT (dihydrolipoamide acyltransferase) were a kind gift from Ruslana Bryk and Carl Nathan.
RESULTS
Complementation of the pafA transposon mutation.
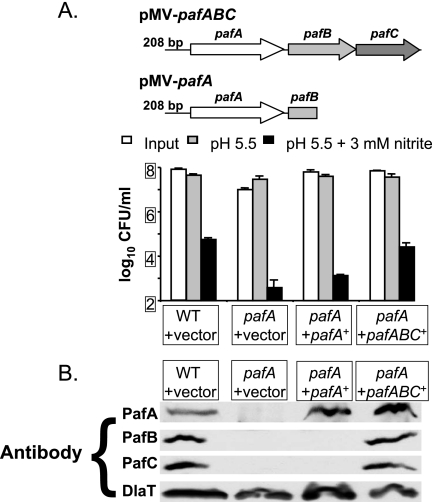
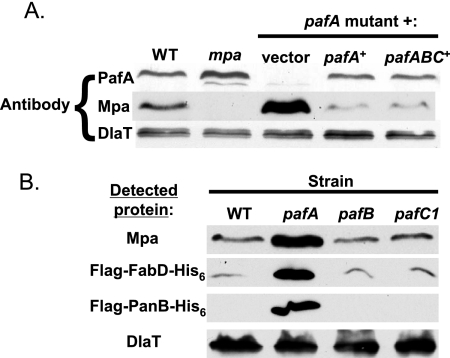
pafA transposon mutants are severely sensitive to RNI in vitro (6). We attempted to complement the pafA transposon mutation by introducing pafA in single copy into the M. tuberculosis chromosome at the attB site. When exposed to 3 mM sodium nitrite in medium at pH 5.5 for 6 days, the pafA mutation was partially complemented for the RNI-sensitive phenotype (Fig. 1A). To determine if the entire pafABC operon could fully complement the pafA mutation, we introduced pMV-pafABC into the pafA mutant. Survival after RNI treatment was restored to WT levels (Fig. 1A). These data suggested that the sensitivity to RNI of the pafA mutant was partly due to the polarity of this mutation on pafB and/or pafC. Consistent with this hypothesis, PafB and PafC proteins were undetectable in the pafA mutant (Fig. 1B). Furthermore, RT-PCR revealed that there was no pafB or pafC transcript in the pafA mutant (data not shown). Thus, the pafA transposon mutation was polar on pafBC expression.
FIG. 1.
Complementation of a pafA transposon mutation. (A) Top, schematic of the pMV-pafABC and pMV-pafA complementation plasmids. Bottom, assay for M. tuberculosis RNI resistance in vitro, showing CFU/ml of WT M. tuberculosis containing pMV306 (vector), the pafA mutant with pMV306, and the pafA mutant with pMV-pafA or pMV-pafABC after exposure to acidified medium (pH 5.5) (gray bars) or acidified medium with 3 mM nitrite (black bars) for 6 days. White bars indicate starting CFU/ml. One experiment representative of three independent experiments, each done in triplicate, is shown. Error bars indicate standard deviations. (B) Immunoblot analysis of PafA, PafB, and PafC in total cell lysates without exposure to RNI. DlaT (dihydrolipoamide acyltransferase) was used as a loading control.
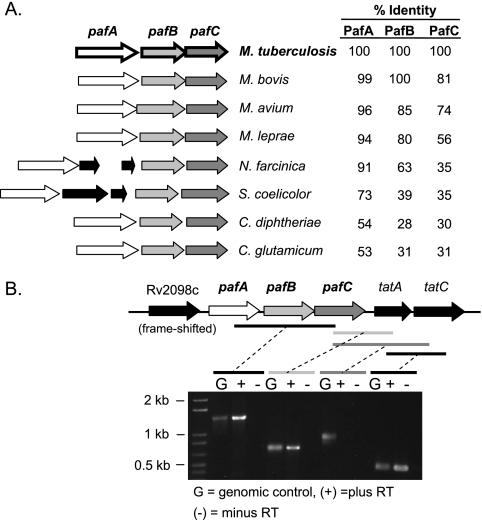
Examination of the DNA sequence around pafA suggested that pafA forms an operon with the two downstream genes, pafB and pafC. In M. tuberculosis, pafA and pafB are separated by eight nucleotides, while the stop codon of pafB overlaps the pafC start codon. The pafABC operon structure is conserved, and the predicted gene products are homologous only between other Actinomycetales (Fig. 2A). At 65 bp downstream of pafC is tatA, which is involved in the twin-arginine translocation (Tat) pathway. The Tat pathway translocates folded proteins across the cell membranes in numerous gram-positive and gram-negative bacteria (2), as well as in Mycobacterium smegmatis (20). This gene is not always located downstream of the pafABC operon in other Actinomycetales. Rv2098c is 52 base pairs upstream of the predicted start codon of pafA and encodes a hypothetical protein, PE_PGRS36, which contains a frameshift mutation towards the 5′ region of the open reading frame. An Rv2098c transcript was not detected by RT-PCR (data not shown). Additionally, it does not appear that Rv2098c is cotranscribed with pafA, because we could not detect a transcript between Rv2098c and pafA (data not shown).
FIG. 2.
Organization of the pafABC operon in the Actinomycetales. (A) Schematic showing the organization of the pafABC operon in selected Actinomycetales. The percent identity of each protein orthologue to the M. tuberculosis protein is noted. Between pafA and pafB, Nocardia farcinica encodes a hypothetical protein and a putative transcriptional regulator and Streptomyces coelicolor encodes a peptidyl-prolyl cis-trans isomerase (fkb) and a hypothetical protein. (B) PCR analysis of cDNA made from WT M. tuberculosis RNA. The genetic organization of this region is shown above. Black and gray bars indicate the amplified regions.
Our complementation data suggested that the pafA mutation was polar on the expression of pafB and pafC, based on the absence of pafBC message and PafB and PafC proteins in the pafA mutant. We performed RT-PCR analysis to determine if pafABC was transcribed as a single message. Primers spanning from the middle of pafA to the middle of pafC amplified an approximately 1.4-kb product (Fig. 2B). DNA sequence analysis of this product confirmed that pafA, pafB, and pafC are indeed cotranscribed in WT M. tuberculosis. In addition, it appeared that tatA, which is predicted to be essential (29), is cotranscribed with pafC (Fig. 2B). In contrast, tatC, which is immediately downstream of tatA, does not appear to be cotranscribed with pafC. However, tatC is cotranscribed with tatA (Fig. 2B) (27). Therefore, it appears that there is readthrough transcription from the pafA operon to tatA, as well as expression of tatAC from a _tat_-specific promoter. qRT-PCR showed no change in tatA transcript levels between WT M. tuberculosis and the pafA transposon mutant strains (data not shown), suggesting that the pafC-tatA readthrough transcript is a minor product. Importantly, tatA does not appear to affect RNI resistance in the pafA transposon mutant, as pafABC was sufficient to restore RNI resistance to the pafA mutant.
pafB and pafC mutants have a subtle RNI-sensitive phenotype.
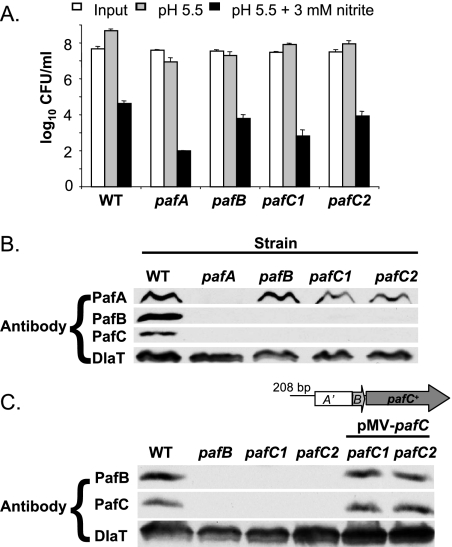
pafB and pafC in addition to pafA are required to fully complement the pafA mutation, so we wanted to determine the individual contributions of these genes to RNI sensitivity. We isolated pafB and pafC mutants from a library of 10,100 transposon mutants (6). We identified mutants with transposon insertions at nucleotide 769 of pafB and at nucleotides 466 and 767 of pafC.
The pafB and pafC mutants were tested for sensitivity to RNI. Neither mutant was as susceptible to RNI as the pafA mutant; however, the pafB and pafC mutants were more susceptible than WT M. tuberculosis to RNI-induced killing (Fig. 3A). The pafB and pafC mutants were consistently killed between 5- and 65-fold more than WT M. tuberculosis; however, the statistical significance of these results varied across experiments (not shown). Figure 3A represents one experiment where the difference in the degree of RNI-induced killing between the WT and the pafB or pafC mutants had a P value of ≤0.05. Compared to the pafA mutant (P < 0.004), these mutants had a much more subtle phenotype. This likely explains why we did not identify the pafB and pafC mutants, which were present in the screened library, in the previous screen for RNI-sensitive mutants (6). Nonetheless, these data showed for the first time that pafB and pafC were playing at least a small role in resistance to RNI in vitro.
FIG. 3.
pafB and pafC mutants are susceptible to RNI. (A) RNI survival assay, as described for Fig. 1A, of a pafB mutant and two pafC mutants. This experiment represents one of three independent experiments, each done in triplicate. Error bars indicate standard deviations. (B) Total cell lysates of WT, pafA, pafB, and two pafC strains were tested for the presence of PafA, PafB, PafC, and DlaT by immunoblotting. (C) Detection of PafB and PafC in WT, pafB, pafC, and _pafC_-complemented strains. Antibodies against DlaT were used for the loading control. A schematic of the pMV-pafB complementation plasmid is also shown.
We examined Paf protein levels in the pafB and pafC mutants. Although PafA was present at similar levels in the pafB and pafC mutants, both PafB and PafC were absent from either the pafB or pafC mutants (Fig. 3B). We checked for the presence of pafB or pafC mRNA in the pafC or pafB mutant, respectively, using RT-PCR and found that pafB mRNA was present in the pafC mutant and that pafC mRNA was present in the pafB mutant (data not shown). Because PafB and PafC were both absent in either the pafB or the pafC mutant, despite the presence of the respective mRNA, we hypothesized that each protein was necessary for the stability of the other. To test this, we complemented the pafB mutation with pMV-pafB and the pafC mutation by using pMV-pafC. Complementation of the pafB mutation with pafB did not restore steady-state PafC protein levels, despite restoring PafB protein levels (data not shown). This was not surprising, because the transposon insertion that introduced at least one stop codon in pafB most likely uncoupled translation from transcription of pafC. In addition, although we could qualitatively observe pafC transcript in the pafB mutant, it is possible that the pafC transcript was less abundant than in WT M. tuberculosis, further reducing PafC protein levels. Although PafB protein could be restored in the absence of PafC, this may be due to increased expression or translation of pafB expressed from the complementation plasmid.
In contrast to the case for the pafB insertion mutation, we found that complementation of the pafC mutation restored WT levels of both PafB and PafC (Fig. 3C). This suggested that PafC was required for the stability of PafB (Fig. 3C) and supported the hypothesis that PafB and PafC could interact.
PafB and PafC interact.
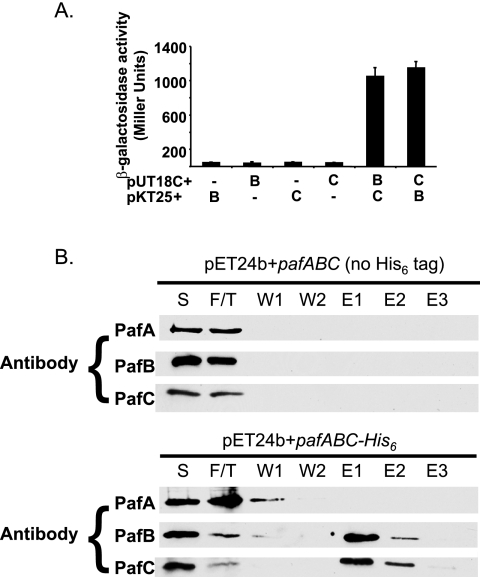
PafB levels were affected by the absence of PafC; therefore, we hypothesized that these two proteins interacted with each other. We used a BTH approach to look for the interactions between PafB and PafC. This assay utilizes two domains of the adenylate cyclase from Bordetella pertussis (T25 and T18), each encoded on a separate plasmid (16). These plasmids are introduced into an adenylate cyclase (cya) mutant of E. coli that cannot use several carbon sources, including maltose and lactose. If two proteins of interest interact, they will bring the two Cya domains together, resulting in the production of cyclic AMP. This complements the cya mutation and allows the metabolism of maltose or lactose, which can be quantified by β-galactosidase assays. BTH analysis revealed that PafB and PafC interact strongly (Fig. 4A). This result was comparable to those for previously tested interactions between other proteasome-associated components (i.e., PrcA with PrcB and Mpa with Mpa) with well-established interactions (6, 7, 15). Neither fusion measured β-galactosidase activity above background with any other proteins tested, including PafA, Mpa, and PrcA (the α subunit of the M. tuberculosis proteasome) (data not shown). However, this does not rule out possible interactions with these or other proteins; for example, the Cya domains may sterically hinder some protein-protein interactions.
FIG. 4.
PafB and PafC interact. (A) BTH interactions were quantified by β-galactosidase assay. Constructs used are denoted beneath the bars, where pafB (“B”) or pafC (“C”) was fused to the T18 or T25 domain of Cya in pUT18C or pKT25, respectively. Each assay was done in triplicate using three independent samples per assay that were then averaged. These results are representative of two independent experiments. Error bars indicate standard deviations. (B) PafB coelutes with PafC-His6 from nickel-agarose. Immunoblot analysis was performed on the soluble lysates (“S”), flowthrough (“F/T”), two washes (“W”), and the first three elutions (“E”) using polyclonal antibodies to PafA, PafB, and PafC. Paf proteins were not detected in the fourth elution (not shown).
The interaction observed between PafB and PafC in the BTH system was validated using Ni-NTA affinity chromatography. PafA, PafB, and PafC were produced in E. coli by using two pafABC constructs: one with a C-terminal His6 epitope tag encoded at the end of pafC and the other without a His6 tag. After incubation of lysates made from these strains with Ni-NTA beads and subsequent washing, immunoblot analysis of the eluted proteins showed that PafB specifically eluted with PafC-His6 (Fig. 4B). PafA did not elute with PafBC but was found in the flowthrough and wash fractions (Fig. 4B). Importantly, PafB did not bind to the Ni-NTA agarose nonspecifically (Fig. 4B). Taken together, these data show that PafB and PafC interact, and this interaction may explain why PafC is necessary for the steady-state stability of PafB in M. tuberculosis.
Mutations in pafB and pafC do not affect the stability of proteasome substrates in M. tuberculosis.
We recently determined that Mpa and PafA are required for the apparent degradation of three proteins: FabD (malonyl coenzyme A acyl carrier protein transacylase), PanB (ketopantoate hydroxymethyltransferase), and Mpa itself (24). In this work, we show that there is no PafB or PafC in the pafA mutant; thus, it was possible that PafB and PafC were also important for the stability of these proteins. Immunoblot analysis of total M. tuberculosis cell lysates showed that Mpa levels were dramatically increased in the pafA mutant compared to WT M. tuberculosis (Fig. 5A) (24). Complementation of the pafA mutation with pafA or pafABC restored Mpa to WT levels (Fig. 5A). In contrast to the case for the pafA mutant, Mpa levels appeared similar to those seen in WT M. tuberculosis in both the pafB and pafC mutants, suggesting that PafB and PafC do not regulate Mpa levels (Fig. 5B).
FIG. 5.
PafA, but not PafB or PafC, is required for maintaining WT steady-state levels of M. tuberculosis proteasome substrates. (A) Immunoblot analysis of Mpa in the WT and a pafA mutant complemented with empty vector and in the pafA mutant with pMV-pafA or pMV-pafABC. (B) Immunoblot analysis of Mpa, FLAG-FabD-His6, and FLAG-PanB-His6 in WT, pafA, pafB, and pafC strains. Proteins were detected using antibodies to Mpa or the FLAG epitope. Antibodies to DlaT were used for the loading control.
We then examined the steady-state levels of FabD and PanB in the pafB and pafC mutants. FLAG-_fabD_-His6 and FLAG-_panB_-His6 were expressed in M. tuberculosis under the control of a heterologous mycobacterial hsp60 promoter and an E. coli ribosome-binding site (24). In contrast to the mpa and pafA mutants (24), epitope-tagged FabD and PanB did not accumulate in the pafB and pafC mutants (Fig. 5B; data not shown for pafC2). Thus, these data suggest that PafB and PafC are not necessary for the degradation of these substrates by the M. tuberculosis proteasome under the conditions tested.
DISCUSSION
This work has furthered our understanding of the previously uncharacterized pafABC operon. We have determined that pafA is cotranscribed with two additional genes, pafB and pafC. We showed that the RNI-sensitive phenotype caused by the transposon insertion in pafA was due to a lack of expression of the pafABC operon and not just pafA. While these data suggested that pafB and pafC have a role in RNI resistance, pafB and pafC transposon mutants were not as sensitive to RNI as pafA mutants. Our work also demonstrated that PafB and PafC interact, suggesting that PafB-PafC complex formation is required for their function. Finally, we showed that presumptive proteasome substrates did not accumulate in the pafB or pafC mutants, suggesting that PafB and PafC do not play as important a role in proteasome function as PafA and Mpa.
The pafABC operon is conserved in several other Actinomycetales. When comparing this operon to other species in the genus Mycobacterium, PafA is the most highly conserved protein, with >94% identity, while PafB and PafC are not as conserved (Fig. 2A). Interestingly, Mycobacterium leprae, the obligate host-associated bacterium that causes leprosy, maintains an intact pafABC operon despite having undergone massive genome decay (5, 33). pafA and the proteasome protease genes (prcBA) are expressed in M. leprae based on microarray analysis (Ric Slayden and Diana Williams, personal communication) (35). If M. leprae has conserved a minimal number of genes necessary for survival in vivo, this suggests that the paf operon plays an integral role during infection.
Outside of the genus Mycobacterium, the homology between PafB or PafC and its orthologues sharply declines, perhaps suggesting a less important role for these proteins than for PafA. Representative species from the genera Nocardia and Streptomyces are exceptional in that they contain genes in between pafA and pafB (Fig. 2A). However, these genes appear to be unrelated to the paf genes as well as to each other. Corynebacterium species do not appear to encode proteasome protease subunits, perhaps explaining why the PafABC proteins are the most degenerate compared to the other species. It is possible that the functions of these proteins are used differently in Corynebacterium species or are involved with another protease system.
Currently, very little is known about proteasome biology in prokaryotes. In the eukaryotic proteasome system, the 19S complex that associates with the proteasome core consists roughly of two parts, the base, which binds to the protease core, and the lid (34). This 19S complex consists of six ATPases as well as non-ATPase subunits (34). Due to the lack of ubiquitin and homologous 19S cap structures in bacteria, it is likely that the M. tuberculosis proteasome uses a different system for targeting proteins for degradation. PafA appears to be required for protein degradation by the M. tuberculosis proteasome (Fig. 5B) (24), perhaps having a function similar to that of the non-ATPase subunits of the eukaryotic 19S regulatory complex. This may include the binding and recognition of substrates targeted for proteolysis.
Although PafB and PafC do not appear to be required for the degradation of known substrates, it is possible that they are involved in the degradation of other, unidentified substrates. There is precedence for the presence of different “adaptor” requirements for protein degradation. For example, the sigma factor RpoS, but not the lambda O protein, requires RssB for degradation by ClpXP in E. coli (37). Other adaptors have been found to be involved in selectively targeting proteins to proteases in both gram-negative and gram-positive bacteria (13). Clp proteases are biochemically different from the proteasome, but the idea that proteins are selectively degraded by different targeting mechanisms is likely to be a conserved theme. Future studies will test this hypothesis. Importantly, these studies will be critical as we design experiments to reconstitute proteasome activity in vitro. This work shows that PafA is an integral part of protein degradation by the proteasome, whereas PafB and PafC appear to be less important for proteasome function under the conditions tested.
Acknowledgments
We thank Susan Butler-Wu, Andrew Darwin, Catherine Potenski, and Naoko Tanese for critically reviewing the manuscript. We thank Diana Williams and Ric Slayden for sharing unpublished results.
M.J.P. was supported by grant 5T32 AI07189-25. This work was supported by a Center for AIDS Research (CFAR) Pilot Project grant (NIH S P30 A1027742-17) awarded to K.H.D.
Footnotes
▿
Published ahead of print on 2 February 2007.
REFERENCES
- 1.Baumeister, W., J. Walz, F. Zühl, and E. Seemüller. 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92**:**367-380. [DOI] [PubMed] [Google Scholar]
- 2.Berks, B. C., T. Palmer, and F. Sargent. 2005. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr. Opin. Microbiol. 8**:**174-181. [DOI] [PubMed] [Google Scholar]
- 3.Chong, S., and G. A. Garcia. 1994. A versatile and general prokaryotic expression vector, pLACT7. BioTechniques 17**:**686, 688, 690-691. [PubMed] [Google Scholar]
- 4.Chu-Ping, M., J. H. Vu, R. J. Proske, C. A. Slaughter, and G. N. DeMartino. 1994. Identification, purification, and characterization of a high molecular weight, ATP-dependent activator (PA700) of the 20 S proteasome. J. Biol. Chem. 269**:**3539-3547. [PubMed] [Google Scholar]
- 5.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honoré, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Conner, R. M. Davies, K. Devlin, K. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Mclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Science 409**:**1007-1011. [DOI] [PubMed] [Google Scholar]
- 6.Darwin, K. H., S. Ehrt, N. Weich, J.-C. Gutierrez-Ramos, and C. F. Nathan. 2003. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302**:**1963-1966. [DOI] [PubMed] [Google Scholar]
- 7.Darwin, K. H., G. Lin, Z. Chen, H. Li, and C. Nathan. 2005. Characterization of a Mycobacterium tuberculosis proteasomal ATPase homologue. Mol. Microbiol. 55**:**561-571. [DOI] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97**:**6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMartino, G. N., C. R. Moomaw, O. P. Zagnitko, R. J. Proske, M. Chu-Ping, S. J. Afendis, J. C. Swaffield, and C. A. Slaughter. 1994. PA700, an ATP-dependent activator of the 20 S proteasome, is an ATPase containing multiple members of a nucleotide-binding protein family. J. Biol. Chem. 269**:**20878-20884. [PubMed] [Google Scholar]
- 10.De Mot, R., I. Nagy, J. Walz, and W. Baumeister. 1999. Proteasomes and other self-compartmentalizing proteases in prokaryotes. Trends Microbiol. 7**:**88-92. [DOI] [PubMed] [Google Scholar]
- 11.Dye, C., S. Scheele, P. Dolin, V. Pathania, M. C. Raviglione, et al. 1999. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 282**:**677-686. [DOI] [PubMed] [Google Scholar]
- 12.Ewann, F., M. Jackson, K. Pethe, A. Cooper, N. Mielcarek, D. Ensergueix, B. Gicquel, C. Locht, and P. Supply. 2002. Transient requirement of the PrrA-PrrB two-component system for early intracellular multiplication of Mycobacterium tuberculosis. Infect. Immun. 70**:**2256-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottesman, S. 2003. Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 19**:**565-587. [DOI] [PubMed] [Google Scholar]
- 14.Harlow, E., and D. P. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 15.Hu, G., G. Lin, M. Wang, L. Dick, R.-M. Xu, C. Nathan, and H. Li. 2006. Structure of the Mycobacterium tuberculosis proteasome and mechanism of inhibition by a peptidyl boronate. Mol. Microbiol. 59**:**1417-1428. [DOI] [PubMed] [Google Scholar]
- 16.Karimova, G., A. Ullmann, and D. Ladant. 2000. A bacterial two-hybrid system that exploits a cAMP signaling cascade in Escherichia coli. Methods Enzymol. 328**:**59-73. [DOI] [PubMed] [Google Scholar]
- 17.Lane, J. M., and E. J. Rubin. 2006. Scaling down: a PCR-based method to efficiently screen for desired knockouts in a high density Mycobacterium tuberculosis picked mutant library. Tuberculosis 86**:**310-313. [DOI] [PubMed] [Google Scholar]
- 18.Lin, G., G. Hu, C. Tsu, Y. Z. Kunes, H. Li, L. Dick, T. Parsons, P. Li, Z. Chen, P. Zwickl, N. Weich, and C. Nathan. 2006. Mycobacterium tuberculosis prcBA genes encode a gated proteasome. Mol. Microbiol. 59**:**1405-1416. [DOI] [PubMed] [Google Scholar]
- 19.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94**:**5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonough, J. A., K. E. Hacker, A. R. Flores, M. S. Pavelka, Jr., and M. Braunstein. 2005. The twin-arginine translocation pathway of Mycobacterium smegmatis is functional and required for the export of mycobacterial beta-lactamases. J. Bacteriol. 187**:**7667-7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 22.Nagy, I., S. Geert, J. Vanderleyden, and R. De Mot. 1997. Further sequence analysis of the DNA regions with the Rhodococcus 20S proteasome structural genes reveals extensive homolgy with Mycobacterium leprae. DNA Seq. 7**:**225-228. [DOI] [PubMed] [Google Scholar]
- 23.Nathan, C. F., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97**:**8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearce, M. J., P. Arora, R. A. Festa, S. M. Butler-Wu, R. S. Gokhale, and K. H. Darwin. 2006. Identification of substrates of the Mycobacterium tuberculosis proteasome. EMBO J. 25**:**5423-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickart, C. M., and R. E. Cohen. 2004. Proteasomes and their kin: proteases in the machine age. Nat. Rev. Mol. Cell. Biol. 5**:**177-187. [DOI] [PubMed] [Google Scholar]
- 26.Russell, D. R. 2001. Mycobacterium tuberculosis: here today, and here tomorrow. Nat. Rev. 2**:**1-9. [DOI] [PubMed] [Google Scholar]
- 27.Saint-Joanis, B., C. Demangel, M. Jackson, P. Brodin, L. Marsollier, H. Boshoff, and S. T. Cole. 2006. Inactivation of Rv2525c, a substrate of the twin-arginine translocation (Tat) system of Mycobacterium tuberculosis, increases beta-lactam susceptibility and virulence. J. Bacteriol. 188**:**6669-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., T. Maniatis, and E. Fritsch. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2001. Comprehensive identification of conditionally essential genes in mycobacteria. Proc. Natl. Acad. Sci. USA 98**:**12712-12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiloh, M. U., and C. F. Nathan. 2000. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr. Opin. Microbiol. 3**:**35-42. [DOI] [PubMed] [Google Scholar]
- 31.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351**:**456-460. [DOI] [PubMed] [Google Scholar]
- 32.Tian, J., R. Bryk, M. Itoh, M. Suematsu, and C. Nathan. 2005. Variant tricarboxylic acid cycle in Mycobacterium tuberculosis: identification of alpha-ketoglutarate decarboxylase. Proc. Natl. Acad. Sci. USA 102**:**10670-10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vissa, V. D., and P. J. Brennan. 2001. The genome of Mycobacterium leprae: a minimal mycobacterial gene set. Genome Biol. 2**:**REVIEWS1023. [DOI] [PMC free article] [PubMed]
- 34.Voges, D., P. Zwickl, and W. Baumeister. 1999. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rev. Biochem. 68**:**1015-1068. [DOI] [PubMed] [Google Scholar]
- 35.Williams, D. L., M. Torrero, P. R. Wheeler, R. W. Truman, M. Yoder, N. Morrison, W. R. Bishai, and T. P. Gillis. 2004. Biological implications of Mycobacterium leprae gene expression during infection. J. Mol. Microbiol. Biotechnol. 8**:**58-72. [DOI] [PubMed] [Google Scholar]
- 36.Zahrt, T. C., and V. Deretic. 2002. Reactive nitrogen and oxygen intermediates and bacterial defenses: unusual adaptations in Mycobacterium tuberculosis. Antioxid. Redox Signal. 4**:**141-159. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, Y., and S. Gottesman. 1998. Regulation of proteolysis of the stationary-phase sigma factor RpoS. J. Bacteriol. 180**:**1154-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]