Proteomic analysis of in vivo-assembled pre-mRNA splicing complexes expands the catalog of participating factors (original) (raw)
Abstract
Previous compositional studies of pre-mRNA processing complexes have been performed in vitro on synthetic pre-mRNAs containing a single intron. To provide a more comprehensive list of polypeptides associated with the pre-mRNA splicing apparatus, we have determined the composition of the bulk pre-mRNA processing machinery in living cells. We purified endogenous nuclear pre-mRNA processing complexes from human and chicken cells comprising the massive (>200S) supraspliceosomes (a.k.a. polyspliceosomes). As expected, RNA components include a heterogeneous mixture of pre-mRNAs and the five spliceosomal snRNAs. In addition to known pre-mRNA splicing factors, 5′ end binding factors, 3′ end processing factors, mRNA export factors, hnRNPs and other RNA binding proteins, the protein components identified by mass spectrometry include RNA adenosine deaminases and several novel factors. Intriguingly, our purified supraspliceosomes also contain a number of structural proteins, nucleoporins, chromatin remodeling factors and several novel proteins that were absent from splicing complexes assembled in vitro. These in vivo analyses bring the total number of factors associated with pre-mRNA to well over 300, and represent the most comprehensive analysis of the pre-mRNA processing machinery to date.
INTRODUCTION
Eukaryotic RNA polymerase II (RNA Pol II) transcripts are matured through a highly coordinated program of processing steps prior to export from the nucleus to the cytoplasm where they are translated into protein by the ribosome (1–5). These pre-mRNA processing events include 5′ end modification by 7-methyl-guanosine cap addition and binding of the nuclear cap binding complex, intron removal by the spliceosome, 3′ end cleavage and poly-adenosine tail addition, transcript-specific modifications such as adenosine deamination and binding of specific protein factors to regulate and promote mature mRNA export from the nucleus. Coordination of these events involves interaction between the machineries involved in each process. For example, the RNA Pol II transcription complex communicates and interacts extensively with the 5′ end capping, pre-mRNA splicing and 3′ end processing machineries (1).
While native pre-mRNAs contain multiple, often extremely large introns, in vitro pre-mRNA splicing reactions are carried out using synthetic pre-mRNA fragments containing a single, efficiently spliced intron of a size compatible with acrylamide gel electrophoresis analysis. Although the core pre-mRNA processing machinery will likely be very similar between different transcripts as well as for the multiple introns contained within a single transcript, the bulk pre-mRNA processing machinery purified from its native context is likely to contain a more comprehensive sample of the polypeptides required for or participating in the splicing of pre-mRNA in vertebrate cells.
Several groups have purified and characterized spliceosomes formed on model vertebrate pre-mRNAs in vitro (6–8) and shown that they contain a remarkably large number of associated polypeptides. Nevertheless, as these synthetic precursors have generally been modified from their natural state by internal deletions within the intron and truncations of the exons, the pattern of associated proteins is inevitably less complex than on the full-length, generally multi-intronic precursors that exist in vivo. In addition, the spliceosomes purified from in vitro reactions were assembled on pre-mRNAs derived from either the adenovirus major late or β-globin loci. Thus, it is likely that there exist a number of factors that are required for or participate in pre-mRNA processing in vivo, yet are not present in previously purified splicing complexes because they are specific to one or more of the thousands of other pre-mRNAs present in metazoan cells. Finally, the pathway by which pre-mRNA processing complexes are assembled in vitro using salt-extracted nuclear fractions most likely bypasses many interactions relevant to this process in vivo. Thus spliceosomes purified following in vivo assembly are expected to contain additional components that reflect the native pathway, but are not required to effect model intron removal in vitro. Additionally, factors that assist in inter-spliceosome interactions in multi-intron substrates will be absent from mono-spliceosome purifications, and should be present in _in vivo_-purified complexes.
Consistent with this view, other investigators have shown that endogenous pre-mRNA is processed in extremely large ribonucleoprotein particles, called supraspliceosomes (9–11) or polyspliceosomes (12). Biochemical and structural analyses of these complexes have demonstrated the presence of RNA Pol II transcripts (13,14) and the pre-mRNA splicing machinery components (15,16) as well as functional interactions that mirror those in active splicing complexes assembled in vitro (12). The higher order particles formed in vivo partly reflect the presence of multiple introns, an average of eight per pre-mRNA (17) with some transcripts possessing as many as 147 introns [Nebulin (18)], that need to be faithfully removed prior to nuclear export. In Figure 1, we present a schematic model of the pre-mRNA processing pathway in vivo that encompasses the concept of the supra/polyspliceosome. Whether the individual ‘spliceosome’ moieties are formed via stepwise snRNP assembly on individual introns (2) or via pre-formed penta-snRNPs (19) in vertebrates is still a matter of considerable debate, although recent chromatin immunoprecipitation experiments in human cells provide support for the penta-snRNP model (20).
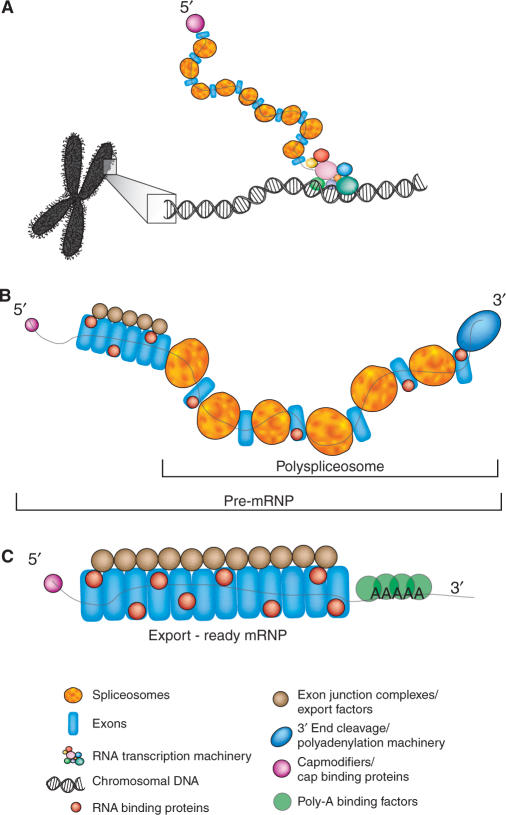
Figure 1.
Model describing the role of vertebrate supraspliceosomes in gene expression. (A) Co-transcriptional assembly of spliceosomes, 5′ end modification machinery and other pre-mRNA binding factors on RNA polymerase II transcripts. (B) The released transcript is partially spliced and bound by numerous spliceosome moieties as well as the 5′ cap-binding complex and 3′ end processing factors. (C) The mature mRNA is associated in the nucleus with RNA binding proteins, 5′- and 3′-end stabilizing factors (the CBP heterodimer and poly(A)-binding protein), and proteins that promote export to the cytoplasm.
With the goal of expanding our understanding of pre-mRNA splicing as it occurs in intact cells, we have purified the endogenous pre-mRNA processing machines from HeLa cells and from chicken DT40 pre-B cells (21) on a preparative scale and have defined their RNA and polypeptide compositions. We have chosen the chicken DT40 system to compare with the HeLa system for a number of reasons. First, we have shown that working with this rapidly growing cell type, which possesses high rates of homologous recombination, allows for downstream experimental flexibility in epitope-tagging of other genes (22). The evolutionary distance between human and chicken will also allow us to assess the evolutionary conservation of the machinery as well as validating novel co-purifying factors. We show that these pre-mRNA processing complexes contain spliced and unspliced mRNAs, all five spliceosomal snRNAs and polypeptides involved in all aspects of pre-mRNA processing from transcription to nuclear export.
Although our strategy may not be sensitive enough to identify very low abundance pre-mRNA-specific factors, it has allowed us to probe more deeply into the general pre-mRNA processing machinery present in vertebrate cells. Indeed, when combined with the data from _in vitro_-assembled spliceosome characterization and known splicing factors not detected in any complexes previously purified, we show there are at least 305 polypeptides involved in or present during the processing of nuclear pre-mRNA.
MATERIALS AND METHODS
Purification of HeLa supraspliceosomes
Ten liters of HeLa cells (purchased from the National Cell Culture Center) were processed essentially as described (11). Briefly, cells were washed in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) and disrupted by mechanical breakage in a glass dounce (20 strokes, pestle ‘B’) in a hypotonic solution (30 mM Tris–Cl pH 7.5, 10 mM KCl, 5 mM MgCl2, 10 mM 2-mercaptoethanol) at 4°C. Nuclei were pelleted at 1000 × g at 4°C for 5 min through the hypotonic buffer containing 25% glycerol. The nuclei were washed three times in the hypotonic buffer containing 0.5% Triton X-100 and once with detergent-free hypotonic buffer. Nuclei were re-suspended in a low-salt buffer (LS+; 10 mM Tris–Cl pH 7.5, 100 mM KCl, 2 mM MgCl2, 10 mM 2-mercaptoethanol, 0.15 mM spermine, 0.05 mM spermidine) and sonicated twice for 20 s at the maximum microtip setting. The resulting nuclear debris was pelleted at 14 000 × g for 10 s, and the supernatant was layered onto a 15–45% glycerol gradient (11ml Beckman SW41) made isotonic to LS- buffer (LS buffer without polyamines) and sedimented at 40 000 × g for 90 min. Fractions (420 μl) were collected from the top. Protein and nucleic acid were separated by phenol/chloroform extraction and precipitation with acetone (23) (protein) or ethanol (nucleic acid). Fractions corresponding to the supraspliceosomes were pooled from six velocity gradients run in parallel fashion, diluted to ∼8% glycerol with LS- buffer and incubated with 20 mg Y12 antibody which had been covalently attached to 1 g CnBr-sepharose (GE Biosciences) according to the manufacturer's instructions. After incubation with rotation for 2 h at 4°C, the sepharose matrix was washed with 200 ml LS- buffer by gravity flow in a column and supraspliceosome material was eluted with 0.2 M glycine. Protein and nucleic acids were separated by phenol/chloroform extraction as described above.
Purification of chicken supraspliceosomes
Six liters of SmD3-TAP DT40 cells (22) were grown in Dulbecco's modified Eagle media supplemented with 5% chicken serum and 2.5% Fetalplex (Gemini Bio-Products) to a density of 7.5 × 105 cells/ml for TAP purification. Cells were harvested by centrifugation (1000 x g for 5 min), washed twice with ice-cold PBS, allowed to swell in 10 ml of TM buffer (10 mM Tris–Cl pH 7.5, 3 mM MgCl2) with 0.2 mM PMSF, 1 µg/ml leupeptin, and 1 µg/ml pepstatin for 10 min ice, and lysed with 25 strokes of a Dounce homogenizer at 4°C. The nuclei were pelleted and washed twice with 10 ml of TM buffer containing 0.1% NP40, re-suspended in 5 ml of low salt buffer (30 mM Tris–Cl, 125 mM KCl, 5 mM MgCl2, 0.5% Triton-X100), and sonicated at the maximum output, twice for 20 s on ice with 1 min in ice between sonications. The sonicated mixture was centrifuged at 14 000 x g for 1 min and the supernatant was used for TAP purification. TAP-tagged protein material for SmD3-TAP DT40 cells was affinity purified by the TAP procedure (24). The TEV eluate was layered onto glycerol gradients and fractionated as described above for the human supraspliceosomes.
Immunoprecipitations
Polyclonal antisera directed against the carboxyl-terminal 15 amino acids of KIAA0332 and NP_035897 (NCBI accession numbers) were produced by Genemed Synthesis and the IgG fraction was partially purified by ammonium sulfate precipitation at 50% saturation. Antiserum or non-immune serum was incubated for 1 h at 4°C with the sample(s) of interest prior to addition of 50 µl Protein-A agarose beads. This mixture was incubated one further hour with rotation at 4°C prior to washing with 4 × 15 ml IPP150. Proteins and nucleic acids were released from the matrix by incubation in IPP150 at 100°C for 5 min. The supernatant was collected and phenol extracted as described above to harvest, separate and precipitate the nucleic acids and proteins.
Northern blot analysis
Nucleic acids were transferred to Brightstar membranes (Ambion) and hybridized with snRNA probes consisting of antisense chicken snRNAs transcribed with α32P-GTP using T7 RNA polymerase (U5) or SP6 RNA polymerase (U1, U2, U4, U6 snRNAs) from plasmids containing cDNA versions of the chicken snRNAs.
Western blot analysis
Polypeptides were resolved in 10% polyacrylamide gels (25), transferred to nitrocellulose membranes (Biorad) and blotted with antiserum as described in the text. The secondary antibody used was horseradish peroxidase-conjugated goat anti-rabbit IgG (Rockland) and the signal was detected by enhanced chemiluminescence (Perkin Elmer).
Mass spectrometry peptide identification
Pooled supraspliceosome protein fractions were separated by polyacrylamide gel electrophoresis and stained with Coomassie Blue G-250 (26). Discrete gel slices were dissected from the top of the gel lane to the bottom and all regions were subjected to trypsin digestion. Mass spectrometry and database searching was performed as previously described (27–29).
RESULTS AND DISCUSSION
Purification of endogenous human pre-mRNA processing complexes
We discovered that in gently sonicated nuclei treated with low salt (11), the majority of the snRNA, as judged by visual inspection of ethidium bromide stained gels (Figure 2A), was engaged in very large (>80S) ribonucleoprotein complexes that closely resemble supraspliceosomes in sedimentation values and other properties (9,10,30). These particles may also be related to the polyspliceosomes described in salt-extracted nuclei, which sedimented as complexes slightly smaller than our supra/polyspliceosome, likely reflecting salt-induced factor loss during nuclear extraction (12).
Figure 2.
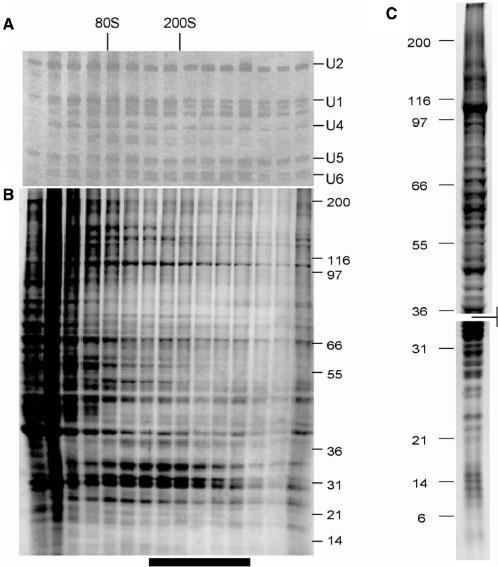
Human supraspliceosome-associated polypeptides and snRNAs. RNA (A) and protein (B) were extracted from preparative glycerol gradient fractions and electrophoretically resolved through urea-PAGE (A) or SDS-PAGE (B) gels stained with silver (RNA) or coomassie blue (protein). Bar below B represents the fractions of the material pooled for immunopurification with Y12 antibody. (C) Affinity-purified supraspliceosomal proteins run under two SDS-PAGE conditions to resolve either large or small polypeptides. Gels were aligned to show all polypeptides in the affinity-purified fractions and are delineated by the marking between them. The entire gel lanes shown from the two gels in (C) were dissected and each gel slice was subjected to mass spectrometry protein identification. The proteins identified are reported under the Hs PS column in Tables 1–3 and in Supplemental Table S1.
For purification of the human supraspliceosomes, we gently sonicated nuclei in a buffered low-salt solution and purified the material in the ∼200S region (Figures 2A and B) as previously described (11). This material was immunopurified using the antibody Y12 on a solid matrix. Remarkably, this treatment nearly quantitatively retained the detectable material from this region of the glycerol gradient as judged by coomassie gel staining, even after extensive washing, indicating that the majority of the nuclear contents of this size are Sm-antigen-containing complexes. The lack of polyribosomes in the rapidly sedimenting material (as judged by the absence of 5S and 5.8S rRNAs and ribosomal proteins by mass spectrometry) indicates that the nuclei we prepare are not contaminated with cytoplasm. The material purified on a preparative scale was separated by SDS-PAGE gels of two compositions to provide optimal resolution of the large number of polypeptides present (Figure 2C). To demonstrate the specificity of these purifications, gradient-separated supraspliceosomes were subjected to affinity chromatography using identical beads and identical washing and elution conditions, but lacking the Y12 antibody. In Figure S1, we show that from the mock purification, there is no detectable coomassie-stained material in the resulting protein gel (panel A) and no snRNAs present (panel B).
Purification of endogenous chicken pre-mRNA processing complexes
Using our recently developed CLEP tagging procedure (22), we tagged the SmD3 polypeptide in chicken DT40 cells by introducing a TAP tag (24) at the native genomic locus. For each experiment, 6 l of SmD3-TAP-DT40 cells were harvested and processed as described for purification of supraspliceosomes from HeLa cells. Affinity chromatography was performed according to the TAP procedure (24) and the TEV eluate was sedimented through a glycerol gradient. The material corresponding to the supraspliceosomes was isolated; proteins and nucleic acids are shown in Figures 3A and B, respectively. In Figure S1, we show the proteins (panel C) and RNA (panel D) resulting from an identical affinity purification procedure performed using extracts from untagged DT40 cells. The absence of proteins, beyond the contaminating TEV protease, and the absence of snRNAs indicates that the purification is specific and the proteins identified by mass spectrometry are likely to be bona fide supraspliceosome components. Additional confidence is provided in that there is size-selection as well as one or two steps of affinity chromatography.
Figure 3.
Chicken supraspliceosome-associated polypeptides and snRNAs. Fractions corresponding to the CLEP-tag purified, glycerol gradient-sedimented chicken supraspliceosomes were separated into protein (A) and RNA (B) fractions and electrophoresed through SDS-PAGE (A) or urea-PAGE (B) gels and stained with coomassie blue (protein) or ethidium bromide (RNA). The identities of the snRNAs are indicated on the right of panel B. The entire gel lane from (A) was dissected and each gel slice was subjected to mass spectrometry for protein identification. The proteins identified are reported under the Gg PS column in Tables 1–3 and in Supplemental Table S1.
RNA content of the supraspliceosomes
RNAs corresponding to the supraspliceosome fractions from human and chicken cells are shown in Figures 2B and 3B, respectively. Identities of the RNAs were confirmed by northern blotting (data not shown). The presence of all five spliceosomal snRNAs in this material indicated that it contained a mixture of pre-mRNA splicing complexes in varying stages of assembly and activity, as both U1 and U4 snRNAs have been shown to be released from the spliceosome before the first catalytic step of the splicing reaction in vitro (31–33). Alternatively, the presence of both U1 and U4 may reflect functional differences between our preparations and the spliceosomes assembled in vitro; for example, it is possible that the U1 and U4 snRNAs do not completely dissociate in conjunction with catalytic activation in vivo, but are only destabilized and maintained locally. The presence of all five snRNAs in roughly equivalent amounts also lends experimental evidence to the participation of the penta-snRNP in these functional complexes (19).
Mass spectrometry analysis of supraspliceosome-associated polypeptides
Polyacrylamide gel lanes from the entire human and chicken supraspliceosome fraction were dissected and the material analyzed by tandem mass spectrometry (27–29). Though a wide variety of polypeptides were identified, it is notable that we detected very little background contamination of factors known to be unrelated to gene expression. In Tables 1–4, we categorize the identified polypeptides according to function. Remarkably, we detected 222 distinct polypeptides in the chicken supraspliceosomes, and 177 distinct polypeptides in the HeLa supraspliceosomes. These numbers are significantly higher than the number of polypeptides detected in any one of the three previously published spliceosome purifications (6–8).
Table 1.
Comparisons of snRNP, snRNP biogenesis and known spliceosome associated proteins (SAPs) profiles from supraspliceosomes purified from human or chicken cells and mono-spliceosomes purified from three _in vitro_-assembled preparations
| ENSEMBL accession #a | HGNCb | Polypeptidec | Gg PSd | Hs PSe | Nf | Rg | Zh |
|---|---|---|---|---|---|---|---|
| U1 snRNP | |||||||
| ENSG00000104852 | SNRP70 | U1–70K | • | • | • | • | • |
| ENSG00000077312 | SNRPA | U1A | • | • | • | • | |
| ENSG00000124562 | SNRPC | U1-C | • | • | |||
| U2 snRNP | |||||||
| ENSGALG00000008038 | SF3B1 | SF3b155 | • | • | • | • | |
| ENSG00000087365 | SF3B2 | SF3b145 | • | • | • | • | • |
| ENSGALG00000002531 | SF3B3 | SF3b130 | • | • | • | • | • |
| ENSGALG00000000581 | DDX42 | SF3b125 | • | • | |||
| ENSGALG00000007937 | SF3A1 | SF3a120 | • | • | • | • | |
| ENSGALG00000021679 | SF3A2 | SF3a66 | • | • | • | • | • |
| ENSGALG00000001540 | SF3A3 | SF3a60 | • | • | • | • | • |
| ENSGALG00000013352 | SF3B4 | SF3b49 | • | • | • | • | |
| ENSGALG00000008729 | SNRPB2 | U2B′′ | • | • | • | • | • |
| ENSGALG00000007170 | SNRPA1 | U2A′ | • | • | • | • | • |
| ENSGALG00000016501 | – | SF3b14 | • | • | |||
| ENSGALG00000020000 | SF3B5 | SF3b10 | • | • | |||
| U2-snRNP associated | |||||||
| ENSGALG00000014395 | DHX15 | PRP43/DDX15 | • | • | • | • | |
| ENSGALG00000002612 | – | SR140 | • | • | • | • | |
| ENSGALG00000006332 | RBM17 | SPF45 | • | • | • | • | |
| ENSGALG00000008561 | SMNDC1 | SPF30 | • | • | • | • | |
| ENSGALG00000003824 | CHERP | CHERP | • | • | • | ||
| U5, U4/U6 & U4/U6•U5 snRNP | |||||||
| ENSGALG00000002943 | PRPF8 | U5–220K | • | • | • | • | |
| ENSGALG00000003477 | ASCC3L1 | U5–200K | • | • | • | • | |
| ENSGALG00000000988 | EFTUD2 | U5–116K | • | • | • | • | |
| ENSG00000175467 | SART1 | U4/U6•U5–110K | • | • | • | • | |
| ENSGALG00000006001 | C20ORF14 | U5–102K | • | • | • | • | |
| ENSG00000174243 | DDX23 | U5–100K | • | • | • | • | • |
| ENSGALG00000000465 | PRPF3 | U4/U6–90K | • | • | • | • | • |
| ENSG00000168883 | USP39 | U4/U6•U5–65K | • | • | • | • | |
| ENSG00000105618 | PRPF31 | U4/U6•U5–61K | • | • | • | • | |
| ENSGALG00000008857 | PRPF4 | U4/U6–60K | • | • | • | • | • |
| ENSGALG00000004874 | PPIH | USA–CYP | • | • | |||
| ENSGALG00000000615 | WDR57 | U5–40K | • | • | • | ||
| ENSGALG00000011931 | NHP2L1 | U4/U6•U5–15.5k | • | • | • | ||
| ENSGALG00000017396 | TXNL4A | U5–15K | • | • | |||
| AT/AC | |||||||
| ENSGALG00000005162 | RNPC3 | U11/U12–65K | • | ||||
| ENSGALG00000013005 | C6ORF151 | U11/U12–48K | • | ||||
| Sm/LSM | |||||||
| ENSGALG00000007250 | SNRPB | SmB/B' | • | • | • | • | • |
| ENSGALG00000011842 | SNRPD1 | SmD1 | • | • | • | • | |
| ENSG00000125743 | SNRPD2 | SmD2 | • | • | • | • | |
| ENSGALG00000006596 | SNRPD3 | SmD3 | • | • | • | • | |
| ENSGALG00000000137 | SNRPE | SmE | • | • | • | • | |
| ENSGALG00000011409 | SNRPF | SmF | • | • | • | • | |
| ENSG00000143977 | SNRPG | SmG | • | • | • | • | |
| ENSG00000111987 | LSM2 | LSM2 | • | • | • | ||
| ENSG00000170860 | LSM3 | LSM3 | • | • | |||
| ENSGALG00000003385 | LSM4 | LSM4 | • | • | • | ||
| ENSG00000106355 | LSM5 | LSM5 | |||||
| ENSGALG00000009985 | LSM6 | LSM6 | • | • | • | • | |
| ENSG00000130332 | LSM7 | LSM7 | • | • | |||
| ENSGALP00000014820 | LSM8 | LSM8 | • | • | • | ||
| snRNP biogenesis | |||||||
| ENSGALG00000010154 | SIP1 | SIP1 | • | ||||
| ENSG00000119953 | SMNDC1 | SMNrp30 | • | ||||
| ENSGALG00000003158 | COIL | Coilin | • | ||||
| SAPs | |||||||
| ENSGALG00000002514 | SFPQ | PSF | • | • | • | ||
| ENSGALG00000002060 | FUS | TLS/FUS | • | • | • | • | |
| ENSGALG00000012468 | PRPF39 | PRP39 | • | ||||
| ENSGALG00000010501 | SNW1 | SKIP/PRP45 | • | • | • | • | • |
| ENSGALG00000016704 | CDC5L | CDC5 | • | • | • | • | |
| ENSGALG00000005012 | RAB43 | ISY1 | • | • | • | ||
| ENSGALG00000008429 | CRNKL1 | CRN1 | • | • | • | • | • |
| ENSGALG00000009257 | PRLG1 | Prp46/PRL1 | • | • | • | ||
| ENSGALG00000015061 | CDC40 | CDC40/PRP17 | • | • | • | ||
| ENSGALG00000002002 | BCAS2 | SPF27 | • | • | |||
| ENSGALG00000013919 | PRPF19 | PRP19 | • | • | • | • | • |
| ENSGALG00000010627 | PRPF38A | PRP38 | • | • | |||
| ENSGALG00000005507 | NONO | p54nrb | • | • | |||
| ENSGALG00000004555 | RBM22 | ECM2/RBM22 | • | • | • | ||
| ENSGALG00000001247 | SYF2 | SYF2 | • | • | • | ||
| ENSGALG00000000726 | ELAVL1 | ELAV/Hu | • | • | • | • | |
| ENSGALG00000009001 | – | CWC22 | • | • | • | ||
| ENSGALG00000008149 | EWSR1 | EWSR1 (RBP) | • | • | • | ||
| ENSGALG00000004705 | BUD31 | BUD31 | • | • | • | ||
| ENSG00000076924 | XAB2 | SYF1 | • | • | • | • | |
| ENSGALG00000001962 | PTBP1 | PTB | • | • | • | ||
| ENSGALG00000011857 | LUC7L2 | LUC7/CROP | • | • | |||
| ENSG00000196504 | PRPF40A | PRP40 | • | • | • | ||
| ENSGALG00000010167 | PNN | Pinin | • | • | |||
| ENSGALG00000012813 | PRPF4B | Prp4K | • | • | • | • | |
| ENSGALG00000000833 | IK | RED | • | • | • | • | |
| ENSGALG00000001500 | – | SLU7 | • | • | • | ||
| ENSG00000063244 | U2AF2 | U2AF65 | • | • | • | • | • |
| ENSGALG00000016198 | U2AF1 | U2AF35 | • | • | • | • | • |
| ENSG00000168066 | SF1 | SF1 | • | • | • | ||
| ENSGALG00000001034 | C20ORF4 | AAR2 | • | ||||
| ENSGALG00000005525 | SFRS1 | SF2/ASF | • | • | • | • | |
| ENSG00000102241 | HTATSF1 | TAT-SF1 | • | ||||
| ENSGALG00000002087 | NCBP1 | CBC80 | • | • | • | • | |
| ENSGALG00000006843 | NCBP2 | CBC20 | • | • | • | • | |
| ENSG00000087087 | – | ASR2B | • | • | |||
| ENSG00000100296 | THOC5 | KIAA0983 | • | • | • | • | |
| ENSG00000159086 | C21ORF66 | C21ORF66 | • | • | |||
| ENSG00000126803 | HSPA2 | HSP70-2 | • | • | • | • | |
| ENSGALG00000006512 | HSPA8 | HSP71 | • | • | • | • | |
| ENSGALG00000009838 | AQR | Aquarius | • | • | • | • | |
| ENSGALG00000002014 | SMU1 | SMU1 | • | • | • | • | |
| ENSGALG00000005623 | TFIP11 | SPP382 | • | • | • | • | |
| ENSG00000137656 | – | CWC26 | • | • | |||
| ENSG00000126698 | DNAJC8 | SPF31 | • | • | • | ||
| ENSG00000105705 | SF4 | SF4 | • | • | |||
| ENSG00000113649 | TCERG1 | CA150 | • | • | • | • | |
| ENSGALG00000004626 | RBM5 | E1B-AP5 | • | • | • | ||
| ENSG00000100056 | DGCR14 | DGCR14 | • | • | |||
| ENSG00000105298 | C19ORF29 | C19ORF29 | • | • | |||
| ENSG00000109536 | FRG1 | FRG1 | • | ||||
| ENSG00000171824 | EXOSC10 | RRP6 | • | ||||
| ENSG00000160799 | CCDC12 | CCDC12/CWF18 | • | ||||
| ENSGALG00000011678 | DNAJC13 | DnaJ | • | • | • | ||
| ENSG00000100813 | ACIN1 | Acinus | • | • | • | ||
| ENSG00000131051 | RNPC2 | HCC | • | • | • | ||
| ENSG00000084463 | WBP11 | WBP11 | • | • | |||
| ENSG00000196419 | XRCC6 | Ku70 | • | • |
Table 2.
Polypeptides demonstrated or predicted by sequence homology to interact with the pre-mRNA, mRNA or the spliceosome and comparisons of those identified in the supraspliceosomes with those of spliceosomes formed in vitro
| ENSEMBL accession #a | HGNCb | Polypeptidec | Gg PSd | Hs PSe | Nf | Rg | Zh |
|---|---|---|---|---|---|---|---|
| RNA Helicase-like | |||||||
| ENSGALG00000016461 | DDX1 | DDX1 | • | • | |||
| ENSGALG00000016231 | DDX3X | DDX3 | • | • | • | ||
| ENSGALG00000003532 | DDX5 | DDX5/p68 | • | • | • | • | |
| ENSGALG00000012247 | DDX17 | DDX17/p72 | • | • | • | • | |
| ENSGALG00000012147 | DDX18 | DDX18 | • | ||||
| ENSG00000102786 | DDX26 | DDX26/HDB | • | ||||
| ENSGALG00000006974 | DDX27 | DDX27 | • | ||||
| ENSGALG00000003030 | DDX41 | DDX41/ABSTRAKT | • | • | • | ||
| ENSG00000123136 | DDX39 | DDX39 | • | ||||
| ENSG00000145833 | DDX46 | DDX46/PRP5 | • | • | • | ||
| ENSGALG00000008530 | DDX48 | DDX48 | • | • | • | • | |
| ENSGALG00000004144 | DDX50 | DDX50/Gu-ß | • | • | |||
| ENSGALG00000001186 | DHX8 | DHX8/PRP22 | • | • | • | • | |
| ENSG00000135829 | DHX9 | DHX9/HELICASEA | • | • | • | • | |
| ENSG00000137333 | DHX16 | DHX16/PRP2 | • | • | |||
| ENSGALG00000005027 | DHX30 | DHX30 | • | • | |||
| ENSGALG00000003658 | DHX35 | DHX35 | • | ||||
| ENSG00000174953 | DHX36 | DHX36 | • | ||||
| ENSGALG00000014709 | SKIV2L2 | SKIV2L2 | • | • | • | • | |
| ENSG00000198563 | BAT1 | UAP56 | • | • | • | ||
| hnRNP | |||||||
| ENSGALG00000006160 | HNRNPA0 | hnRNPA0 | • | • | |||
| ENSGALG00000011036 | HNRNPA2B1 | hnRNPA2/B1 | • | • | • | • | • |
| ENSG00000135486 | HNRPA1 | hnRNPA1 | • | • | • | • | • |
| ENSGALG00000009250 | HNRNPA3 | hnRNPA3 | • | • | • | • | |
| ENSGALG00000014381 | HNRNPAB | hnRNPAB | • | • | |||
| ENSG00000092199 | HNRPC | hnRNPC1/C2 | • | • | • | • | • |
| ENSG00000138668 | HNRNPD | hnRNPD0/AUF1 | • | • | |||
| ENSGALG00000011184 | HNRNPDL | hnRNPD0 | • | • | • | • | |
| ENSG00000169813 | HNRPF | hnRNPF | • | ||||
| ENSGALG00000006457 | RBMX | hnRNPG | • | • | • | ||
| ENSGALG00000005955 | HNRNPH1 | hnRNPH1 | • | • | • | ||
| ENSGALG00000003947 | HNRNPH3 | hnRNPH3 | • | • | |||
| ENSGALG00000012591 | HNRNPK | hnRNPK | • | • | • | • | |
| ENSG00000104824 | HNRNPL | hnRNPL | • | • | • | • | |
| ENSGALG00000000377 | HNRNPM | hnRNPM | • | • | • | • | |
| ENSGALG00000015830 | SYNCRIP | hnRNPQ | • | • | • | • | |
| ENSGALG00000000814 | HNRNPR | hnRNPR | • | • | • | • | |
| ENSGALG00000010671 | HNRNPU | hnRNPU | • | • | • | ||
| ENSGALG00000018665 | – | hnRNP novel | • | ||||
| ENSG00000126457 | HRMT1L2 | HRMT1L2 | • | ||||
| SR family | |||||||
| ENSG00000133226 | SRRM1 | SRm160 | • | • | |||
| ENSG00000167978 | SRRM2 | SRm300 | • | • | |||
| ENSGALG00000005525 | SFRS1 | SF2p33 | • | ||||
| ENSG00000161547 | SFRS2 | SC35 | • | • | |||
| ENSGALG00000000533 | SFRS3 | SFRS3SRp20 | • | • | • | • | |
| ENSG00000116350 | SFRS4 | SRp75 | • | ||||
| ENSGALG00000009484 | SFRS5 | SRp40 | • | • | |||
| ENSGALG00000000990 | SFRS6 | SRp55 | • | • | • | • | |
| ENSGALG00000013825 | SFRS7 | 9G8 | • | • | • | • | |
| ENSGALG00000002487 | SFRS8 | SFRS8 | • | ||||
| ENSG00000111786 | SFRS9 | SRp30 | • | • | • | ||
| ENSGALG00000006531 | SFRS10 | SFRS10 | • | • | • | ||
| ENSG00000116754 | SFRS11 | SRp54 | • | • | |||
| ENSG00000153914 | SFRS12 | SFRS12 | • | ||||
| ENSGALG00000004133 | FUSIP1 | SRrp35 | • | • | |||
| Cyclophillins | |||||||
| ENSGALG00000013383 | PPIE | CYP-E | • | • | • | ||
| ENSGALG00000004874 | PPIH | USA-CYP | • | ||||
| ENSGALG00000014747 | SDCCAG10 | CYP16 | • | • | |||
| ENSG00000137168 | PPIL1 | PPIL1/CWF27 | • | • | • | ||
| ENSG00000100023 | PPIL2 | PPIL2/CYP60 | • | • | • | ||
| ENSG00000115934 | PPIL3 | PPIL3B | • | • | |||
| ENSG00000113593 | PPWD1 | PPWD1 | • | • | • | ||
| RBP | |||||||
| ENSG00000102317 | RBM3 | RBM3 | • | ||||
| ENSGALG00000018992 | RBM4B | RBM4B/Lark | • | ||||
| ENSGALG00000004626 | RBM5 | RBM5 | • | • | |||
| ENSGALG00000007017 | RBM7 | RBM7 | • | • | |||
| ENSG00000131795 | RBM8A | RBM8B | • | • | |||
| ENSGALG00000013411 | RBM14 | RBM14 | • | • | |||
| ENSG00000162775 | RBM15 | RBM15 | • | • | • | ||
| ENSGALG00000002372 | RBM15B | RBM15B | • | • | |||
| ENSG00000197676 | RBM16 | RBM16 | • | ||||
| ENSGALG00000004555 | RBM22 | RBM22 | • | ||||
| ENSG00000119707 | RBM25 | RBM25 | • | ||||
| ENSG00000091009 | RBM27 | RBM27 | • | ||||
| ENSGALG00000011038 | CBX3 | RNPS1 | • | • | |||
| ENSG00000033030 | ZCCHC8 | ZFP8 | • | • | |||
| ENSGALG00000006113 | ZNF326 | ZFP326 | • | • | |||
| ENSG00000179950 | – | PUF60 | • | • | • | ||
| ENSG00000197381 | ADARB1 | ADAR1 | • | ||||
| ENSG00000160710 | ADAR | ADAR2 | • | ||||
| ENSGALG00000010952 | – | Requiem | • | ||||
| ENSGALG00000011570 | ILF2 | NFAT45 | • | • | • | • | |
| ENSG00000129351 | ILF3 | NFAT90 | • | • | • | • | |
| ENSG00000169564 | PCBP1 | PolyrCBP | • | ||||
| ENSGALG00000012225 | CIRBP | CIRBP | • | • | |||
| ENSG00000056097 | ZFR | ZFR | • | • | |||
| ENSGALG00000009427 | TIAL1 | TIA1 | • | ||||
| ENSGALG00000001187 | STRBP | STRBP | • | ||||
| ENSG00000136231 | IGF2BP3 | IMP3 | • | • | • | • | |
| ENSG00000060138 | CSDA | CSDA | • | • | |||
| ENSG00000121774 | KHDRBS1 | SAM68 | • | ||||
| ENSG00000126254 | - | Novel RRM | • | ||||
| ENSG00000132773 | TOE1 | TOE1 | • | ||||
| ENSG00000142864 | SERBP1 | SERBP1 | • | ||||
| Export/transcription/NMD | |||||||
| ENSGALG00000014915 | THOC1 | THO1/HPR1 | • | • | |||
| ENSGALG00000008507 | THOC2 | THO2 | • | • | • | • | |
| ENSG00000051596 | THOC3 | THO3/TEX1 | • | • | |||
| ENSGALG00000007237 | THOC4 | THO4/ALY | • | • | • | • | |
| ENSGALG00000004571 | PPP1CA | GLC7/PPP1CA | • | ||||
| ENSGALG00000002569 | RAN | Ran | • | • | |||
| ENSG00000119392 | GLE1L | GLE1L | • | ||||
| ENSGALG00000007653 | RAE1 | GLE2/RAE1 | • | • | |||
| ENSGALG00000003220 | RENT1 | UPF1/RENT | • | ||||
| ENSG00000131795 | RBM8A | Y14/RBM8A | • | • | • | ||
| ENSGALG00000002144 | THRAP3 | TRAP150 | • | • | |||
| ENSG00000172660 | TAF15 | TAF15/RBP56 | • | ||||
| ENSG00000162231 | NXF1 | TAP | • | ||||
| ENSG00000065978 | YBX1 | YBX1 | • | • | • | ||
| ENSGALG00000010689 | MAGOH | Mago nashi | • | • | • | ||
| 3′ end proc. | |||||||
| ENSG00000071894 | CPSF1 | CPSF1 | • | ||||
| ENSGALG00000010783 | CPSF2 | CPSF2 | • | ||||
| ENSGALG00000016424 | CPSF3 | CPSF3 | • | • | |||
| ENSGALG00000004714 | CPSF4 | CPSF4 | • | ||||
| ENSGALG00000003084 | CPSF5 | CPSF5 | • | • | • | ||
| ENSG00000111605 | CPSF6 | CPSF6 | • | • | |||
| ENSGALG00000011685 | CSTF3 | CSTF-77 | • | • | |||
| ENSGALG00000013943 | FIP1L1 | FIP1 | • | ||||
| ENSG00000172239 | PAIP1 | PAIP1 | • | ||||
| ENSG00000100836 | PABPN1 | PABPN1 | • | • | • | • | |
| ENSGALG00000003800 | PABPC4 | PABPC4 | • |
Table 3.
Structural, nucleoporin and novel polypeptides present in the supraspliceosomes and _in vitro_-assembled splicing complexes
| ENSEMBL accession #a | HGNCb | Polypeptidec | Gg PSd | Hs PSe | Nf | Rg | Zh |
|---|---|---|---|---|---|---|---|
| Structural | |||||||
| ENSGALG00000012533 | MYH9 | Myosin | • | • | |||
| ENSGALG00000009126 | TTN | Titin | • | ||||
| ENSGALG00000001381 | ACTG1 | Actin | • | • | |||
| ENSGALG00000002478 | MATR3 | MATRIN3 | • | • | • | • | |
| ENSGALG00000008677 | VIM | Vimentin | • | • | |||
| ENSG00000117245 | KIF17 | Kinesin KIF17 | • | ||||
| ENSGALG00000002197 | NPM1 | NUMATRIN | • | • | • | ||
| ENSGALG00000014692 | LMNB1 | Lamin B | • | • | |||
| ENSGALG00000013505 | SYNE1 | NuSpectrin | • | • | |||
| ENSG00000140259 | MFAP1 | MFAP1 | • | • | |||
| Chromatin modification | |||||||
| ENSGALG00000000360 | ARID1A | ARID1A-SWI/SNF | • | ||||
| ENSGALG00000013683 | ARID1B | ARID1B-SWI/SNF | • | ||||
| ENSGALG00000010164 | SMARCA2 | SMARCA2 | • | ||||
| ENSG00000127616 | SMARCA4 | Brahma/SMARCA4 | • | ||||
| ENSGALG00000009913 | SMARCA5 | SMARCA5 | • | ||||
| ENSGALG00000005983 | SMARCB1 | SMARCB1 | • | ||||
| ENSGALG00000005048 | SMARCC2 | BRG1-SWI/SNF | • | ||||
| ENSGALG00000005048 | SMARCC1 | SMARCC1 | • | ||||
| ENSGALP00000010010 | SMARCD1 | SMARCD1 | • | ||||
| ENSGALG00000000363 | SMARCD2 | SMARCD2 | • | ||||
| ENSGALG00000002100 | SMARCE1 | SMARCE1 | • | ||||
| Nucleoporins | |||||||
| ENSGALG00000005714 | PKD1 | PKD1 (NUP assoc) | • | ||||
| ENSGALG00000003830 | NUP214 | NUP214 | • | • | |||
| ENSGALG00000012720 | NUP153 | NUP153 | • | ||||
| ENSGALG00000005078 | NUP210 | NUP210 | • | • | |||
| ENSG00000102900 | NUP93 | NUP93 | • | ||||
| ENSG00000108559 | NUP88 | NUP88 | • | ||||
| ENSG00000111581 | NUP107 | NUP107 | • | ||||
| ENSG00000110713 | NUP98 | NUP98 | • | ||||
| ENSG00000138750 | NUP54 | NUP54 | • | ||||
| ENSG00000163002 | NUP35 | NUP35 | • | ||||
| ENSG00000069248 | NUP133 | NUP133 | • | ||||
| ENSG00000155561 | NUP205 | NUP205 | • | ||||
| Novel or unknown to splicing | |||||||
| ENSGALG00000011351 | HSP90AA1 | HSP90α | • | • | |||
| ENSGALG00000010175 | HSP90AA2 | HSP90ß | • | • | |||
| ENSGALG00000012726 | HSP90B1 | HSP108 | • | • | |||
| ENSGALG00000009967 | LRPPRC | LRP130 | • | ||||
| ENSGALG00000003693 | MACF1 | Macrophin | • | ||||
| ENSGALG00000007705 | NCL | Nucleolin | • | ||||
| ENSGALG00000015933 | C21ORF66 | GCRBF | • | ||||
| ENSGALG00000008454 | – | NOP58 | • | ||||
| ENSGALG00000009061 | ACTL6A | BAF53A | • | ||||
| ENSGALG00000007520 | SSRP1 | FACT80 | • | • | |||
| ENSGALG00000015821 | CCT8 | TCP1-theta | • | ||||
| ENSGALG00000014500 | NOL5A | Nol5A/NOP56 | • | ||||
| ENSGALG00000005624 | MED12 | TRAP230 | • | ||||
| ENSGALG00000010973 | TRA2A | TRA2α | • | • | |||
| ENSGALG00000008372 | XRN2 | RAT1 | • | • | |||
| ENSG00000197157 | – | SND1 | • | • | |||
| ENSGALG00000001948 | SAFB2 | SAFB/HSP27 | • | • | |||
| ENSGALG00000003177 | BRD8 | BRD8 | • | • | |||
| ENSGALG00000010699 | – | FOG | • | ||||
| ENSGALG00000005177 | C9ORF10 | C9ORF10 | • | • | |||
| ENSGALG00000002653 | – | ELG | • | • | |||
| ENSGALG00000016949 | WBP4 | WBP4 | • | ||||
| ENSGALG00000004133 | FUSIP1 | Fus IP | • | • | |||
| ENSGALG00000017384 | ERH | ERH | • | • | |||
| ENSG00000079246 | XRCC5 | Ku80 | • | ||||
| ENSG00000182562 | ATAD3A | ATAD3A (AAA ATPase) | • | • | |||
| ENSGALG00000001515 | ATAD3B | ATAD3B (AAA ATPase) | • | • | |||
| ENSG00000108588 | CCDC47 | CCDC47 | • |
Table 4.
Known pre-mRNA processing proteins not present in any purified splicing complex
| ENSEMBL accession #a | HGNCb | Polypeptidec |
|---|---|---|
| ENSG00000095485 | CWF19L1 | CWF19 |
| ENSG00000152404 | CWF19L2 | CWF19 |
| ENSG00000165630 | PRPF18 | PRP18 |
| ENSG00000140829 | DHX38 | PRP16 |
| ENSG00000149532 | – | CFI-59K |
| ENSG00000165494 | PCF11 | PCF11 |
| ENSG00000172409 | CLP1 | CLP1 |
| ENSG00000111880 | HCAP1 | HCE/CEG1 |
| ENSG00000146007 | ZMAT2 | SNU23 |
| ENSG00000108296 | CCDC49 | CWC25 |
| ENSG00000101138 | CSTF1 | CSTF-50 |
| ENSG00000101811 | CSTF2 | CSTF-64 |
| ENSG00000161981 | C16ORF33 | U11/U12–25K |
| ENSG00000184209 | – | U11/U12–35K |
The polypeptides identified by mass spectrometry were validated by analyzing the percent-coverage for each protein (Supplemental Table 1). Although there is a distribution of coverage for the identified proteins, we note that while many snRNP-associated factors had a large percentage of their sequence identified, some snRNP-associated proteins had <10% coverage. Differences in coverage may reflect differences in abundance, a paucity of appropriately-sized trypsin fragments or to peculiarities in the mass spectrometry detection of a particular peptide. The low-coverage of some of the novel polypeptides that may reasonably be implied to function in pre-mRNA processing (i.e. contain RNA binding motifs) may reflect their association with a smaller subset of pre-mRNAs than a general RNA binding protein such as an hnRNP. In the case of one novel factor, ZFR, the percent-coverage was low (3.4% for chicken, 8% for human) but its association with the splicing machinery was verified independently (see below).
Known snRNP-associated polypeptides
By mass spectrometry, we identified nearly all of the known pre-mRNA splicing snRNP-associated polypeptides (Table 1). We were initially surprised by the apparent absence in our preparations of a subset of snRNP-associated proteins found in most or all of the previously purified spliceosomes. However, upon closer inspection, we observed that for each polypeptide not represented in our mass spectrometry results, the inability to be detected correlated with the presence of an abundant hnRNP protein of similar molecular weight. The coverage of the major spliceosomal snRNP proteins was more complete for the chicken supraspliceosomes. Indeed, the CLEP tagging and purification procedure was sensitive enough to detect the presence of two minor AT–AC spliceosome components, the U11/U12–65K and U11/U12–48K polypeptides (34) in the chicken fractions, whereas no AT–AC specific splicing components were detected in the human complexes. The ability to detect all of the Sm proteins, but not all of the LSM proteins may reflect the difficulty in detecting all of these proteins in splicing complexes as shown previously (35,36), the 5-fold abundance differences of the two classes of proteins or perhaps due to the LSM proteins leaving the spliceosome during the process of pre-mRNA splicing (37).
By western blotting of chicken cell nuclear extracts with the Y12 monoclonal antibody, we determined that the Sm antigens in chicken cells were not reactive with the Y12 antibody (data not shown), eliminating the possibility of a direct comparison between Y12-immunopurified material from chicken and human cells.
snRNP biogenesis factors
Previous spliceosome purifications did not yield polypeptides known to be involved in snRNP biogenesis. We note in Table 1 that there are three of these present in the chicken supraspliceosomes (SIP1, SMNrp30 and Coilin). These factors, which are involved in the de novo assembly of snRNPs, are contained in Cajal bodies (CBs), nuclear organelles enriched for pre-mRNA splicing factors (38). We hypothesize that the CLEP tagging procedure may allow purification of a subset of snRNPs in the process of being re-targeted to the CBs. We did not detect this class of polypeptides in the HeLa supraspliceosomes. Other factors present in the chicken supraspliceosome but not those from HeLa cells were cyclophillins and chromatin remodeling proteins, which may be related to procedural differences in the purification methods.
Known spliceosome associated proteins (SAPs)
In the second half of Table 1, we compile a list of the 59 SAPs identified in one or more of the spliceosome purifications. The _in vivo_-assembled complexes contained pre-mRNA-interacting factors such as U2AF (39,40), PTB (41,42), and the cap binding complex proteins (43), which were present in some but not all of the _in vitro_-assembled spliceosomes. We detected the majority of PRP19-complex (NTC) related components as well, including homologues of Prp19p (44,45), Syf1p (45), Syf2p (45), Syf3/Clf1p (45,46), Isy1p (47), SKIP/Prp45p (48,49) and CDC5/Cef1p (50,51), which were also detected in vitro, and BCAS2/SPF27, which was only detected in supraspliceosomes assembled in vivo. Interestingly, our preparations included a number of polypeptides that are snRNP-associated in yeast but have not been identified in purified metazoan snRNPs or spliceosomes. Among these factors are putative orthologues of yeast Prp38p (52), Prp39p (53), Prp40p (54), Aar2p (55) and Luc7p (56).
Other polypeptides exclusively contained in the purified supraspliceosomes include, NONO/p54nrb, PNN/Pinin, CWC22, which have been previously implicated in splicing (57–60). Conversely, 13 polypeptides were exclusively associated with the in vitro purified spliceosomes, possibly reflecting specificity for the pre-mRNAs upon which these were assembled. Alternatively, the differences in composition may result from differences in the procedures. Most striking, however, is the absence of SF1/BBP from our supraspliceosome preparations. This may be due to the fact that SF1/BBP interacts very early with the pre-mRNA, remains associated for a short time and is replaced by U2 snRNP at the branchpoint sequence (61).
RNA helicase-like proteins
We identified a large number of DExH/D proteins in the preparations of supraspliceosome assembled in vivo. In addition to the RNA helicase-like polypeptides known to function in pre-mRNA splicing, such as DDX15/Prp43p (62–64), DHX8/Prp22p (65–67), DDX46/Prp5p (68–70), DDX5/p68 and DDX17/p72 (71), UAP56/Sub2p (72–74), DDX16/Prp2p (75,76) and the snRNP-associated helicases U5-200K/Brr2p (77–79) and U5-100K/Prp28p (80–82), we noted a number not previously implicated in pre-mRNA splicing and absent from the in vitro purified spliceosomes [Table 2 (‘Gg’ and ‘Hs’ to ‘N’, ‘R’ and ‘Z’)]. These include 13 additional polypeptides with sequence motifs indicative of DEAD, DEAH or Ski2p-like helicase family members, most of which were absent from spliceosomes assembled in vitro. Although we do not as yet have evidence that these proteins function in pre-mRNA splicing, the complete absence of DNA helicases in our preparations indicates a specificity (i.e. a specificity for RNA processing complexes and against chromatin components), which minimally suggests that they function in some aspect of RNA Pol II transcript processing.
Supraspliceosome-associated hnRNPs
Polypeptides termed hnRNPs are highly abundant nuclear proteins known to interact with hnRNA. We detected virtually all of the known hnRNP proteins in both human and chicken cells, as well as other hnRNP-like proteins annotated in genome databases (Table 2). We note that in the spliceosomes purified from in vitro extracts, some hnRNPs were identified; however, perhaps owing to specific binding of some hnRNPs to the bulk hnRNA and not the single transcript used in the in vitro spliceosome assembly reactions, a greater number of these polypeptides were detected in the supraspliceosomes.
SR proteins
Many splicing factors are rich in arginine and serine residues including long stretches of alternating dipeptides termed SR domains. These factors function at multiple steps in the pre-mRNA splicing pathway (83), and were constituents of the _in vitro-_purified splicing complexes. We detected 10 different SR family members in our purified supraspliceosomes from both chicken and human cells (Table 2), though not a complete set. One possible conclusion is that, due to the means by which these complexes were purified and analyzed, these SR proteins represent the major SR proteins functioning in these cells, and that those not detected in our preparations function in the splicing of a smaller subset of pre-mRNAs.
Cyclophillins
In addition to the known snRNP-associated USA–CYP (84,85), we detected five additional potential proline cis-trans isomerases co-purifying with spliceosomes from chicken, but interestingly, not from HeLa cells. Several of those from the chicken purification were also present in spliceosomes assembled in vitro (Table 3). As it is likely that these proteins also function in HeLa cells, their absence may represent operational differences in the ways in which the chicken and human cells were handled and the ways in which the complexes were purified and analyzed.
Other RNA binding proteins
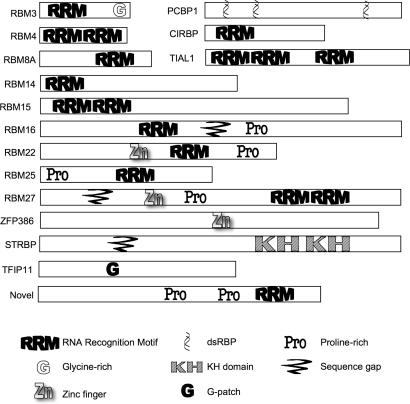
There were 32 polypeptides identified among all of the splicing complex purifications possessing sequence homology to polypeptides believed to interact with RNA by virtue of containing RNA Recognition Motifs (RRM), double-stranded RNA binding domains (dsRBD) or other motifs implicated in RNA binding. Some were identified previously, such as the ELAV/Hu protein that binds AU-rich elements in both cytoplasmic (86) and nuclear RNAs (87), the U2AF-related PUF60 protein (88), the dsRBD-motif-containing NFAT45 and NFAT90 and RNA adenosine deaminases; these were previously shown to exist in large nuclear complexes (16) and believed to function in RNA Pol II transcript metabolism. Only a single predicted RNA binding protein was found in the _in vitro_-assembled splicing complexes but not in either the chicken or HeLa supraspliceosomes, while 22 were exclusively found in supraspliceosomes, but not in the _in vitro_-formed complexes. We believe this is most likely due to the use of a single pre-mRNA in vitro, while a broader spectrum of RNA binding proteins will be associated with bulk pre-mRNA. In Figure 4 we present a graphical representation of the 16 presumptive RNA binding proteins novel to our study and highlight the sequence motifs contained in each.
Figure 4.
Novel spliceosome-associated polypeptides with predicted RNA binding motifs. Polypeptides from Table 2 with no known function in the pre-mRNA processing pathway are shown with graphical representations of the various RNA interaction or other noted motifs listed at the bottom of the Figure.
Non-spliceosomal pre-mRNA processing factors
Cap-binding proteins (CBC80 and CBC20) are present in both spliceosomes assembled in vitro and supraspliceosomes assembled in vivo (Table 1). In Table 2, we report the presence in our preparations of many 3′ end processing factors (CSPF, CSTF and poly-A binding proteins), a comprehensive set of proteins shown to be involved in mRNA export including the TREX complex (THO1/HPR1, THO2, THO3/TEX1, UAP56 and ALY) (89), export factors such as TAP (90,91), GLE1 (92), GLE2 (93) and GLC7 (94), and exon junction complex constituents including Y14 and Magoh. We also note that a single component of the nonsense-mediated decay (NMD) pathway (UPF1) (95) was identified in the chicken material. As NMD is likely to be active only in a very small subset of pre-mRNA processing complexes (96), we were surprised to observe even a single polypeptide implicated in this process.
Nuclear matrix and filament proteins
Recent data from several laboratories suggest a functional interaction between the structural proteins of the nuclear matrix and the gene expression machinery (97). Consistent with this model, we detected a number of nuclear matrix proteins in our endogenously formed pre-mRNA splicing complex preparations including actin, spectrin, matrin3, numatrin, lamin B and a matrix associated protein MAP1. Although we cannot confirm the functional relevance of these associations, we note that a few structural proteins also co-purified with _in vitro_-assembled spliceosomes. We also note that a number of hnRNPs and other known splicing factors such as Prp19p (98) were initially termed nuclear matrix-associated proteins, indicating an intimate relationship between the pre-mRNA processing machinery and the nuclear matrix. Indeed, it is an attractive hypothesis that pre-mRNA and mRNA are trafficked to the nuclear pore via the nuclear matrix.
Nuclear pore complex proteins
A substantial number of nucleoporins (NUPs) are present in the purified supraspliceosome complexes from human cells but not in spliceosomes assembled in vitro, which may perhaps be due to our use of sonication to release complexes from the purified nuclei versus salt extraction for preparation of splicing extracts. In the chicken supraspliceosomes, we detected a smaller set of NUPs, which may be due to their release by the detergent NP40 present during purification of these complexes. NP40 was absent during the purification of the human complexes, which likely maintained the integrity of hydrophobic interactions believed to stabilize the interaction of export complexes with NUPs.
Polypeptides novel to pre-mRNA splicing–SWI/SNF proteins and associated factors
A recent report from Muchardt and colleagues (99) has demonstrated that the SWI/SNF component Brahma/SMARCA4 (Brm) associates with the splicing apparatus and its presence favors the inclusion of alternatively spliced exons. The Prp4 kinase, which is present in both the human and chicken supraspliceosomes, has been reported to phosphorylate both Brm and the splicing factor U5–102K/hPrp6 (100) providing further evidence that it functions in Pol II transcript maturation. In the purified chicken supraspliceosomes, Brahma/SMARCA4, and a number of other SWI/SNF-related polypeptides were identified (Table 3), all with high degrees of confidence given the depth of the peptide identification. As our mass spectrometry data neither included structural proteins of chromatin- such as histones, nor the DNA replication machinery or other DNA binding proteins, Brahma and other polypeptides with chromatin-related functions must specifically associated with the pre-mRNA processing complexes.
Novel polypeptides present in native supraspliceosomes
Our mass spectrometry peptide data revealed several novel and intriguing polypeptides in the supraspliceosome complexes (Table 3). We found chicken homologs of the yeast splicing factors Prp38p, Prp39p and Aar2p, previously unannotated in purified pre-mRNA splicing complexes. The other polypeptides of interest in the endogenous splicing complexes include the 5′ to 3′ exonuclease XRN2/Rat1p (101–103), which has been implicated in linking transcription termination with polyadenylation. XRN2/Rat1p was found in both the human and chicken preparations, as were two uncharacterized AAA ATPases, ATAD3A and ATAD3B. The identities of 25 additional novel polypeptides are reported in Table 3.
Co-immunoprecipitation of spliceosomal components by antiserum directed against a novel polypeptide
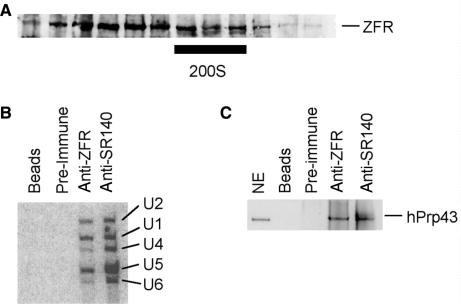
To demonstrate the authenticity and functional relevance of a novel polypeptide that co-purified with endogenous spliceosomes, we generated antiserum against ZFR (Table 2) and used it to specifically immunopurify ZFR-associated components. In Figure 5A, we show that the ZFR polypeptide is present in very high molecular weight complexes that co-migrate with supraspliceosomal material. In Figure 5B, we show that the anti-ZFR antiserum, but not the pre-immune serum or the Protein-A beads, immunoprecipitates the U1, U2, U4, U5 and U6 snRNAs. As a positive control, we showed that antiserum directed against the known spliceosomal protein SR140, prepared and analyzed under identical conditions, also immunoprecipitated all of the snRNAs. We also tested for the presence of another pre-mRNA splicing factor, hPrp43 (DHX15), in the material immunopurified with anti-ZFR; Figure 5C shows that the specific antiserum, but not the pre-immune serum or the Protein-A beads, immunoprecipitates hPrp43p/DDX15. This demonstrates that the novel spliceosome-associated factor ZFR is indeed associated with spliceosomal snRNAs and other spliceosomal proteins.
Figure 5.
The novel Zn finger protein ZFR is a bona fide spliceosomal component. (A) ZFR sediments with the 200S particle in glycerol gradients. HeLa nuclear extract was subjected to glycerol velocity gradient sedimentation analysis as in Figure 2. Proteins from the indicated fractions were electrophoresed through SDS-PAGE gels and subjected to western blot analysis using anti-ZFR antiserum. The bar below the gel denotes the 200S region. (B) ZFR is specifically associated with spliceosomal snRNAs. Equal amounts of HeLa nuclear extract were incubated with protein-A beads (beads), pre-immune serum and protein-A beads (pre-immune), anti-ZFR antiserum and protein-A beads (anti-ZFR) or anti-SR140 antiserum and protein-A beads (anti-SR140) according to the Materials and Methods. Recovered nucleic acids were subjected to northern blot analysis and probed with antisense probes to human snRNAs (identities noted to the right of the Figure). (C) ZFR is specifically associated with complexes containing spliceosomal proteins. Immunoprecipitation conditions and lanes are as described in (B). Proteins were subjected to western blot analysis using hPrp43 antiserum.
We note that there are snRNA stoichiometry differences between the two immunoprecipitations. Although we cannot be certain of exactly why this is, it may be that the ZFR polypeptide is associated with a number of different complexes, as is almost certain from its distribution in the glycerol gradient. As the immunoprecipitations were performed using nuclear extracts, and not size-selected complexes, the snRNA representation reflects the ZFR-associated material from the entire nucleus and not solely from the polyspliceosomes.
In this work, we report the composition of the endogenous pre-mRNA processing machinery from human and chicken cells and provide a comparison between native supraspliceosome complexes and spliceosomes assembled on a model single-intron substrate in vitro from salt-extracted nuclear material. These supraspliceosomes have recently been shown to be functional in add-back experiments using micrococcal nuclease-treated extracts for in vitro splicing by Sperling and colleagues (21), further enhancing the functional relevance of our findings. In addition to confirming the set of factors known to interact with Pol II transcripts during splicing, we discovered an extensive array of novel factors by purifying supraspliceosomes from two types of vertebrate cells. Many of these have been implicated in pre-mRNA maturation including a subset of the SWI/SNF chromatin remodeling complex proteins, recently shown to influence alternative splicing patterns and to co-purify with pre-mRNA splicing factors. The novel polypeptides discovered in the endogenous complexes will provide a rich source of new proteins to investigate, ultimately enhancing our understanding of this incredibly complex macromolecular machine.
Comparison with _in vitro_-assembled spliceosomes
In Tables 1–3 we present a comparison of the polypeptides present in the supraspliceosomes purified in this work with those purified in previous in vitro spliceosome preparations. The core machinery (snRNPs, SAPs, SR proteins, etc.) is well represented in the material derived from all of the purification schemes. However, a great many other factors are present in all of the preparations as well, highlighting the amazing number of proteins required to remove even the single intron used in the in vitro experiments. The methods used for the purification of all of the complexes represented in Tables 1–3 were operationally distinct and the _in vivo_-assembled spliceosomes contained a larger number of proteins than did the spliceosome preparations formed in vitro.
What is perhaps most remarkable about our results is the fact that, despite the operationally distinct purification strategies, the basal pre-mRNA processing machinery required to effect the removal of a single intron in vitro is not vastly different than that purified from complex mixtures of all of the pre-mRNAs in a vertebrate or human nucleus. The major differences in composition between the previous purifications and the one described herein involve (i) polypeptides predicted by sequence homology to interact with the pre-mRNA (ii) the depth of coverage for polypeptides involved in export and 3′ end processing and (iii) polypeptides that may require that the pre-mRNA in these complexes follow the path of RNA Pol II transcription and nuclear trafficking, such as the SWI/SNF complexes, structural proteins and NUPs.
Pre-mRNA processing factors not present in any spliceosome purification
To complete the catalog of polypeptides that participate in the nuclear pre-mRNA processing pathway, we have compiled a list of factors know to function in processing of RNA Pol II transcripts, but not present in any of the five spliceosome preparations listed in Tables 1–3. In Table 4, we outline this relatively short list of 14 factors. Table 4 does not include factors implicated in yeast splicing, but for which no identifiable human or vertebrate homologue in the genomic databases. By adding together all of the polypeptides listed in Tables 1–4, we arrive at an estimate of at least 305 for the number of proteins that co-purify with endogenous pre-mRNA splicing complexes. This is by far the largest cataloguing of factors potentially required for or participating in this process to date.
Two possible classes of polypeptides may exist that are not detected in our preparations. First are those that are underrepresented because they may interact with only a small number of pre-mRNAs, such as intron- or exon-specific binding proteins. Other classes of proteins which may participate in pre-mRNA splicing but as absent from our analyses might include tissue-type developmental stage-specific factors which would not be present in our bulk supraspliceosome preparations due to the use of only two cell types. To date, such factors have generally been elucidated via genetic or molecular strategies focused on individual pre-mRNAs. However, with the introduction of the CLEP tagging technology to more cell types (22), it may be possible to rapidly enumerate factors that function in regulated or alternative splicing.
Although we cannot completely eliminate the possibility that there may be contaminants present in our preparations, the basic strategy we adopted is validated by the presence proteins such as Brahma that were not detected in the previously characterized _in vitro_-assembled spliceosomes yet have clearly been implicated in splicing through other means. Our analyses in aggregate greatly expand our knowledge of the protein factors that function both in basal and regulated splicing in vertebrate cells.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
[Supplementary Material]
ACKNOWLEDGEMENTS
We would like to thank Jo Ann Wise for critically reviewing the manuscript, contributions of the other members of the Stevens Laboratory, Joan Steitz for the gift of Y12 antibodies, Cindy Will and Reinhard Lührmann for the hPrp43 antiserum, Phil Tucker and William Kuziel for thoughtful discussions, Hank Bose and the Bose laboratory members for advice on the DT40 system and use of their facilities and Gwen Gage for graphic art expertise. This work was supported by grants from the Welch Foundation (F-1564 to SWS), the National Science Foundation (MCB-0448556 to SWS), the American Cancer Society (RSG-05-137-01-MCB to SWS) and from the NIH (NIH P30 CA33572 to TDL).
Funding to pay the Open Access publication charges for this article was provided by NSF (MCB-0448556 to SWS).
Conflict of interest statement. None declared.
REFERENCES
- 1.Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- 2.Moore MJ, Query CC, Sharp PA. In: The RNA World. Gesteland RF, Atkins JF, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 303–357. [Google Scholar]
- 3.Shatkin AJ, Manley JL. The ends on the affair: Capping and polyadenylation. Nat. Struct. Biol. 2000;7:838–842. doi: 10.1038/79583. [DOI] [PubMed] [Google Scholar]
- 4.Reed R, Magni K. A new view of mRNA export: Separating the wheat from the chaff. Nat. Cell Biol. 2001;3:E201–E204. doi: 10.1038/ncb0901-e201. [DOI] [PubMed] [Google Scholar]
- 5.Tange TO, Nott A, Moore MJ. The ever-increasing complexities of the exon junction complex. Curr. Opin. Cell Biol. 2004;16:279–284. doi: 10.1016/j.ceb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Rappsilber J, Ryder U, Lamond AI, Mann M. Large-scale proteomic analysis of the human spliceosome. Genome Res. 2002;12:1231–1245. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z, Licklider LJ, Gygi SP, Reed R. Comprehensive analysis of the human spliceosome. Nature. 2002;419:182–185. doi: 10.1038/nature01031. [DOI] [PubMed] [Google Scholar]
- 8.Neubauer G, King A, Rappsilber J, Calvio C, Watson M, Ajuh P, Sleeman J, Lamond A, Mann M. Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat. Genet. 1998;20:46–50. doi: 10.1038/1700. [DOI] [PubMed] [Google Scholar]
- 9.Azubel M, Wolf SG, Sperling J, Sperling R. Three-dimensional structure of the native spliceosome by cryo-electron microscopy. Mol. Cell. 2004;15:833–839. doi: 10.1016/j.molcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Muller S, Wolpensinger B, Angenitzki M, Engel A, Sperling J, Sperling R. A supraspliceosome model for large nuclear ribonucleoprotein particles based on mass determinations by scanning transmission electron microscopy. J. Mol. Biol. 1998;283:383–394. doi: 10.1006/jmbi.1998.2078. [DOI] [PubMed] [Google Scholar]
- 11.Sperling R, Sperling J, Levine AD, Spann P, Stark GR, Kornberg RD. Abundant nuclear ribonucleoprotein form of CAD RNA. Mol. Cell. Biol. 1985;5:569–575. doi: 10.1128/mcb.5.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wassarman DA, Steitz JA. A base-pairing interaction between U2 and U6 small nuclear RNAs occurs in >150s complexes in HeLa-cell extracts - implications for the spliceosome assembly pathway. Proc. Natl Acad. Sci., USA. 1993;90:7139–7143. doi: 10.1073/pnas.90.15.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperling R, Koster AJ, MelamedBessudo C, Rubinstein A, Angenitzki M, BerkovitchYellin Z, Sperling J. Three-dimensional image reconstruction of large nuclear RNP (lnRNP) particles by automated electron tomography. J. Mol. Biol. 1997;267:570–583. doi: 10.1006/jmbi.1997.0898. [DOI] [PubMed] [Google Scholar]
- 14.Spann P, Feinerman M, Sperling J, Sperling R. Isolation and visualization of large compact ribonucleoprotein- particles of specific nuclear RNAs. Proc. Natl Acad. Sci. USA. 1989;86:466–470. doi: 10.1073/pnas.86.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yitzhaki S, Miriami E, Sperling R, Sperling J. Phosphorylated Ser/Arg-rich proteins: Limiting factors in the assembly of 200S large nuclear ribonucleoprotein particles. Proc. Natl Acad. Sci. USA. 1996;93:8830–8835. doi: 10.1073/pnas.93.17.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raitskin O, Cho DSC, Sperling J, Nishikura K, Sperling R. RNA editing activity is associated with splicing factors in InRNP particles: The nuclear pre-mRNA processing machinery. Proc. Natl Acad. Sci. USA. 2001;98:6571–6576. doi: 10.1073/pnas.111153798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 18.Sakharkar MK, Chow VTK, Kangueane P. Distributions of exon and introns in the human genome. In Silico Biol. 2004;4:0032. [PubMed] [Google Scholar]
- 19.Stevens SW, Ryan DE, Ge HY, Moore RE, Young MK, Lee TD, Abelson J. Composition and functional characterization of the yeast spliceosomal Penta-snRNP. Mol. Cell. 2002;9:31–44. doi: 10.1016/s1097-2765(02)00436-7. [DOI] [PubMed] [Google Scholar]
- 20.Listerman I, Sapra AK, Neugebauer KM. Cotranscriptional coupling of splicing factor recruitment and precursor messenger RNA splicing in mammalian cells. Nat. Struct. Mol. Biol. 2006;13:815–822. doi: 10.1038/nsmb1135. [DOI] [PubMed] [Google Scholar]
- 21.Buerstedde JM, Takeda S. Increased Ratio of Targeted to Random Integration after Transfection of Chicken B-Cell Lines. Cell. 1991;67:179–188. doi: 10.1016/0092-8674(91)90581-i. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y-IG, Maika SD, Stevens SW. Epitope tagging of proteins at the native chromosomal loci of genes in mice and in cultured vertebrate cells. J. Mol. Biol. 2006;361:412–419. doi: 10.1016/j.jmb.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 23.Stevens SW, Abelson J. Methods Enzymol. 2002;351:200–220. doi: 10.1016/s0076-6879(02)51849-8. [DOI] [PubMed] [Google Scholar]
- 24.Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Séraphin B. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 25.Graham DRM, Garnham CP, Fu Q, Robbins J, Van Eyk JE. Improvements in two-dimensional gel electrophoresis by utilizing a low cost “in-house” neutral pH sodium dodecyl sulfate-polyacrylamide gel electrophoresis system. Proteomics. 2005;5:2309–2314. doi: 10.1002/pmic.200401249. [DOI] [PubMed] [Google Scholar]
- 26.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 27.Davis MT, Lee TD. Rapid protein identification using a microscale electrospray LC/MS system on an ion trap mass spectrometer. J. Am. Soc. Mass Sepctrom. 1998;9:194–201. doi: 10.1016/S1044-0305(97)00282-1. [DOI] [PubMed] [Google Scholar]
- 28.Moore RE, Young MK, Lee TD. Method for screening peptide fragment ion mass spectra prior to database searching. J. Amer. Soc. Mass Spectrom. 2000;11:422–426. doi: 10.1016/S1044-0305(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 29.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass-spectral data of peptides with amino-acid sequences in a protein database. J. Amer. Soc. Mass Spectrom. 1994;5:876–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 30.Azubel M, Habib N, Sperling R, Sperling J. Native spliceosomes assemble with Pre-mRNA to form supraspliceosomes. J. Mol. Biol. 2006;356:955–966. doi: 10.1016/j.jmb.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 31.Yean SL, Lin RJ. U4 small nuclear RNA dissociates from a yeast spliceosome and does not participate in the subsequent splicing reaction. Mol. Cell. Biol. 1991;11:5571–5577. doi: 10.1128/mcb.11.11.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamond AI, Konarska MM, Grabowski PJ, Sharp PA. Spliceosome assembly involves the binding and release of U4 small nuclear ribonucleoprotein. Proc. Natl Acad. Sci. USA. 1988;85:411–415. doi: 10.1073/pnas.85.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konarska MM, Sharp PA. Electrophoretic separation of complexes involved in the splicing of precursors to mRNAs. Cell. 1986;46:845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- 34.Will CL, Schneider C, Hossbach M, Urlaub H, Rauhut R, Elbashir S, Tuschl T, Luhrmann R. The human 18S U11/U12 snRNP contains a set of novel proteins not found in the U2-dependent spliceosome. RNA. 2004;10:929–941. doi: 10.1261/rna.7320604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottschalk A, Neubauer G, Banroques J, Mann M, Lührmann R, Fabrizio P. Identification by mass spectrometry and functional analysis of novel proteins of the yeast [U4/U6•U5] tri-snRNP. EMBO J. 1999;18:4535–4548. doi: 10.1093/emboj/18.16.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens SW, Abelson J. Purification of the yeast U4/U6•U5 small nuclear ribonucleoprotein particle and identification of its proteins. Proc. Natl Acad. Sci. USA. 1999;96:7226–7231. doi: 10.1073/pnas.96.13.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan SP, Kao DI, Tsai WY, Cheng SC. The Prp19p-associated complex in spliceosome activation. Science. 2003;302:279–282. doi: 10.1126/science.1086602. [DOI] [PubMed] [Google Scholar]
- 38.Gall JG, Bellini M, Wu Z, Murphy C. Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes. Mol. Biol. Cell. 1999;10:4385–4402. doi: 10.1091/mbc.10.12.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer A, Utans U. 3 protein factors (SF1, SF3 and U2AF) function in pre-splicing complex-formation in addition to snRNPs. EMBO J. 1991;10:1503–1509. doi: 10.1002/j.1460-2075.1991.tb07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett M, Michaud S, Kingston J, Reed R. Protein components specifically associated with prespliceosome and spliceosome complexes. Genes Dev. 1992;6:1986–2000. doi: 10.1101/gad.6.10.1986. [DOI] [PubMed] [Google Scholar]
- 41.Gil A, Sharp PA, Jamison SF, Garciablanco MA. Characterization Of cDNAs Encoding The Polypyrimidine Tract-Binding Protein. Genes Dev. 1991;5:1224–1236. doi: 10.1101/gad.5.7.1224. [DOI] [PubMed] [Google Scholar]
- 42.Patton JG, Mayer SA, Tempst P, Nadalginard B. Characterization And Molecular-Cloning Of Polypyrimidine Tract-Binding Protein - A Component Of A Complex Necessary For Pre-Messenger-RNA Splicing. Genes Dev. 1991;5:1237–1251. doi: 10.1101/gad.5.7.1237. [DOI] [PubMed] [Google Scholar]
- 43.Lewis JD, Izaurralde E, Jarmolowski A, McGuigan C, Mattaj IW. A nuclear cap-binding complex facilitates association of U1 snRNP with the cap-proximal 5' splice site. Genes Dev. 1996;10:1683–1698. doi: 10.1101/gad.10.13.1683. [DOI] [PubMed] [Google Scholar]
- 44.Cheng SC, Tarn WY, Tsao TY, Abelson J. Prp19 - a Novel Spliceosomal Component. Mol. Cell. Biol. 1993;13:1876–1882. doi: 10.1128/mcb.13.3.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russell CS, Ben-Yehuda S, Dix I, Kupiec M, Beggs JD. Functional analyses of interacting factors involved in both pre-mRNA splicing and cell cycle progression in Saccharomyces cerevisiae. RNA. 2000;6:1565–1572. doi: 10.1017/s1355838200000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung SY, McLean MR, Rymond BC. Yeast ortholog of the Drosophila crooked neck protein promotes spliceosome assembly through stable U4/U6.U5 snRNP addition. RNA. 1999;5:1042–1054. doi: 10.1017/s1355838299990635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dix I, Russell C, Ben Yehuda S, Kupiec M, Beggs JD. The identification and characterization of a novel splicing protein, Isy1p, of Saccharomyces cerevisiae. RNA. 1999;5:360–368. doi: 10.1017/s1355838299981396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Figueroa JD, Hayman MJ. The human Ski-interacting protein functionally substitutes for the yeast PRP45 gene. Biochem. Biophys. Res. Comm. 2004;319:1105–1109. doi: 10.1016/j.bbrc.2004.05.096. [DOI] [PubMed] [Google Scholar]
- 49.Albers M, Diment A, Muraru M, Russell CS, Beggs JD. Identification and characterization of Prp45p and Prp46p, essential pre-mRNA splicing factors. RNA. 2003;9:138–150. doi: 10.1261/rna.2119903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai WY, Chow YT, Chen HR, Huang KT, Hong RI, Jan SP, Kuo NY, Tsao TY, Chen CH, et al. Cef1p is a component of the Prp19p-associated complex and essential for pre-mRNA splicing. J. Biol. Chem. 1999;274:9455–9462. doi: 10.1074/jbc.274.14.9455. [DOI] [PubMed] [Google Scholar]
- 51.Ben-Yehuda S, Dix I, Russell CS, McGarvey M, Beggs JD, Kupiec M. Genetic and physical interactions between factors involved in both cell cycle progression and pre-mRNA splicing in Saccharomyces cerevisiae. Genetics. 2000;156:1503–1517. doi: 10.1093/genetics/156.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanton S, Srinivasan A, Rymond BC. Prp38 encodes a yeast protein required for pre-mRNA splicing and maintenance of stable U6 snRNA levels. Mol. Cell. Biol. 1992;12:3939–3947. doi: 10.1128/mcb.12.9.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lockhart SR, Rymond BC. Commitment of yeast pre-messenger-RNA to the splicing pathway requires a novel U1 small nuclear ribonucleoprotein polypeptide, Prp39p. Mol. Cell. Biol. 1994;14:3623–3633. doi: 10.1128/mcb.14.6.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kao HY, Siliciano PG. Identification of PRP40, a novel essential yeast splicing factor associated with the U1 small nuclear ribonucleoprotein particle. Mol. Cell. Biol. 1996;16:960–967. doi: 10.1128/mcb.16.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gottschalk A, Kastner B, Luhrmann R, Fabrizio P. The yeast U5 snRNP coisolated with the U1 snRNP has an unexpected protein composition and includes the splicing factor Aar2p. RNA. 2001;7:1554–1565. [PMC free article] [PubMed] [Google Scholar]
- 56.Fortes P, Bilbao-Cortes D, Fornerod M, Rigaut G, Raymond W, Séraphin B, Mattaj IW. Luc7p, a novel yeast U1 snRNP protein with role in 5′ splice site recognition. Genes Dev. 1999;13:2425–2438. doi: 10.1101/gad.13.18.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kameoka S, Duque P, Konarska MM. P54(nrb) associates with the 5′ splice site within large transcription/splicing complexes. EMBO J. 2004;23:1782–1791. doi: 10.1038/sj.emboj.7600187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang P, Lou PJ, Leu S, Ouyang P. Modulation of alternative pre-mRNA splicing in vivo by pinin. Biochem. Biophys. Res. Comm. 2002;294:448–455. doi: 10.1016/S0006-291X(02)00495-3. [DOI] [PubMed] [Google Scholar]
- 59.Sakashita E, Tatsumi S, Werner D, Endo H, Mayeda A. Human RNPS1 and its associated factors: A versatile alternative pre-mRNA splicing regulator in vivo. Mol. Cell. Biol. 2004;24:1174–1187. doi: 10.1128/MCB.24.3.1174-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohi MD, Link AJ, Ren LP, Jennings JL, McDonald WH, Gould KL. Proteomics analysis reveals stable multiprotein complexes in both fission and budding yeasts containing Myb-related Cdc5p/Cef1p, novel pre-mRNA splicing factors, and snRNAs. Mol. Cell. Biol. 2002;22:2011–2024. doi: 10.1128/MCB.22.7.2011-2024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berglund JA, Abovich N, Rosbash M. A cooperative interaction between U2AF65 and mBBP/SF1 facilitates branchpoint region recognition. Genes Dev. 1998;12:858–867. doi: 10.1101/gad.12.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arenas JE, Abelson JN. Prp43: An RNA helicase-like factor involved in spliceosome disassembly. Proc. Natl Acad. Sci. USA. 1997;94:11798–11802. doi: 10.1073/pnas.94.22.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin A, Schneider S, Schwer B. Prp43 is an essential RNA-dependent ATPase required for release of lariat-intron from the spliceosome. J. Biol. Chem. 2002;277:17743–17750. doi: 10.1074/jbc.M200762200. [DOI] [PubMed] [Google Scholar]
- 64.Fouraux MA, Kolkman MJM, van der Heijden A, de Jong AS, van Venrooij WJ, Pruijn GJM. The human La (SS-B) autoantigen interacts with DDX15/hPrp43, a putative DEAH-box RNA helicase. RNA. 2002;8:1428–1443. doi: 10.1017/s1355838202021076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Company M, Arenas J, Abelson J. Requirement of the RNA helicase-like protein PRP22 for release of messenger RNA from spliceosomes. Nature. 1991;349:487–493. doi: 10.1038/349487a0. [DOI] [PubMed] [Google Scholar]
- 66.Wagner JDO, Jankowsky E, Company M, Pyle AM, Abelson JN. The DEAH-box protein PRP22 is an ATPase that mediates ATP- dependent mRNA release from the spliceosome and unwinds RNA duplexes. EMBO J. 1998;17:2926–2937. doi: 10.1093/emboj/17.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwer B, Gross CH. Prp22, a DExH-box RNA helicase, plays two distinct roles in yeast pre-mRNA splicing. EMBO J. 1998;17:2086–2094. doi: 10.1093/emboj/17.7.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dalbadie-McFarland G, Abelson J. PRP5: a helicase-like protein required for mRNA splicing in yeast. Proc. Natl Acad. Sci. USA. 1990;87:4236–4240. doi: 10.1073/pnas.87.11.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Day CL, Dalbadie-McFarland G, Abelson J. The Saccharomyces cerevisiae Prp5 protein has RNA-dependent ATPase activity with specificity for U2 small nuclear RNA. J. Biol. Chem. 1996;271:33261–33267. doi: 10.1074/jbc.271.52.33261. [DOI] [PubMed] [Google Scholar]
- 70.Will CL, Urlaub H, Achsel T, Gentzel M, Wilm M, Luhrmann R. Characterization of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein. EMBO J. 2002;21:4978–4988. doi: 10.1093/emboj/cdf480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin CR, Yang LQ, Yang JJ, Huang YL, Liu ZR. ATPase/helicase activities of p68 RNA helicase are required for pre-mRNA splicing but not for assembly of the spliceosome. Mol. Cell. Biol. 2005;25:7484–7493. doi: 10.1128/MCB.25.17.7484-7493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kistler AL, Guthrie C. Deletion of MUD2, the yeast homolog of U2AF65, can bypass the requirement for Sub2, an essential spliceosomal ATPase. Genes Dev. 2001;15:42–49. doi: 10.1101/gad.851301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang M, Green MR. Identification and characterization of yUAP/Sub2p, a yeast homolog of the essential human pre-mRNA splicing factor hUAP56. Genes Dev. 2001;15:30–35. doi: 10.1101/gad.851701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Libri D, Graziani N, Saguez C, Boulay J. Multiple roles for the yeast SUB2/yUAP56 gene in splicing. Genes Dev. 2001;15:36–41. doi: 10.1101/gad.852101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.King DS, Beggs JD. Interactions of PRP2 protein with pre-mRNA splicing complexes in Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:6559–6564. doi: 10.1093/nar/18.22.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teigelkamp S, McGarvey M, Plumpton M, Beggs JD. The splicing factor PRP2, a putative RNA helicase, interacts directly with pre-mRNA. EMBO J. 1994;13:888–897. doi: 10.1002/j.1460-2075.1994.tb06332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noble SM, Guthrie C. Identification of novel genes required for yeast pre-mRNA splicing by means of cold-sensitive mutations. Genetics. 1996;143:67–80. doi: 10.1093/genetics/143.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raghunathan PL, Guthrie C. RNA unwinding in U4/U6 snRNPs requires ATP hydrolysis and the DEIH-box splicing factor Brr2. Curr. Biol. 1998;8:847–855. doi: 10.1016/s0960-9822(07)00345-4. [DOI] [PubMed] [Google Scholar]
- 79.Lauber J, Fabrizio P, Teigelkamp S, Lane WS, Hartmann E, Lührmann R. The HeLa 200 kDa U5 snRNP-specific protein and its homologue in Saccharomyces cerevisiae are members of the DEXH-box protein family of putative RNA helicases. EMBO J. 1996;15:4001–4015. [PMC free article] [PubMed] [Google Scholar]
- 80.Strauss EJ, Guthrie C. A cold-sensitive mRNA splicing mutant is a member of the RNA helicase gene family. Genes Dev. 1991;5:629–641. doi: 10.1101/gad.5.4.629. [DOI] [PubMed] [Google Scholar]
- 81.Strauss EJ, Guthrie C. PRP28, a ‘DEAD-box' protein, is required for the first step of mRNA splicing in vitro. Nucleic Acids Res. 1994;22:3187–3193. doi: 10.1093/nar/22.15.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teigelkamp S, Mundt C, Achsel T, Will CL, Lührmann R. The human U5 snRNP-specific 100-kD protein is an RS domain- containing, putative RNA helicase with significant homology to the yeast splicing factor Prp28p. RNA. 1997;3:1313–1326. [PMC free article] [PubMed] [Google Scholar]
- 83.Bourgeois CF, Lejeune F, Stevenin J. Broad specificity of SR (serine/arginine) proteins in the regulation of alternative splicing of pre-messenger RNA. Prog. Nucleic Acids Res. Mol. Biol. 2004;78:37–88. doi: 10.1016/S0079-6603(04)78002-2. [DOI] [PubMed] [Google Scholar]
- 84.Horowitz DS, Kobayashi R, Krainer AR. A new cyclophilin and the human homologues of yeast Prp3 and Prp4 form a complex associated with U4/U6 snRNPs. RNA. 1997;3:1374–1387. [PMC free article] [PubMed] [Google Scholar]
- 85.Teigelkamp S, Achsel T, Mundt C, Gothel SF, Cronshagen U, Lane WS, Marahiel M, Lührmann R. The 20kD protein of human [U4/U6•U5] tri-snRNPs is a novel cyclophyllin that forms a complex with the U4/U6-specific 60kD and 90kD proteins. RNA. 1998;4:127–141. [PMC free article] [PubMed] [Google Scholar]
- 86.Myer VE, Fan XHC, Steitz JA. Identification of HuR as a protein implicated in AUUUA-mediated mRNA decay. EMBO J. 1997;16:2130–2139. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu H, Hasman RA, Barron VA, Luo GB, Lou H. A nuclear function of Hu proteins as neuron-specific alternative RNA processing regulators. Mol. Biol. Cell. 2006;17:5105–5114. doi: 10.1091/mbc.E06-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Page-McCaw PS, Amonlirdviman K, Sharp PA. PUF60: a novel U2AF65-related splicing activity. RNA. 1999;5:1548–1560. doi: 10.1017/s1355838299991938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Andres AK, Struhl K, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417:304–308. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- 90.Kang YB, Cullen BR. The human Tap protein is a nuclear mRNA export factor that contains novel RNA-binding and nucleocytoplasmic transport sequences. Genes Dev. 1999;13:1126–1139. doi: 10.1101/gad.13.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 92.Murphy R, Wente SR. An RNA-export mediator with an essential nuclear export signal. Nature. 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- 93.Blevins MB, Smith AM, Phillips EM, Powers MA. Complex formation among the RNA export proteins Nup98, Rae1/Gle2, and TAP. J. Biol. Chem. 2003;278:20979–20988. doi: 10.1074/jbc.M302061200. [DOI] [PubMed] [Google Scholar]
- 94.Gilbert W, Guthrie C. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol. Cell. 2004;13:201–212. doi: 10.1016/s1097-2765(04)00030-9. [DOI] [PubMed] [Google Scholar]
- 95.Mitchell P, Tollervey D. An NMD pathway in yeast involving accelerated deadenylation and exosome-mediated 3′-> 5′ degradation. Mol. Cell. 2003;11:1405–1413. doi: 10.1016/s1097-2765(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 96.Le Hir H, Nott A, Moore MJ. How introns influence and enhance eukaryotic gene expression. Trends In Biochem. Sci. 2003;28:215–220. doi: 10.1016/S0968-0004(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 97.de Lanerolle P, Johnson T, Hofmann WA. Actin and myosin I in the nucleus: what next? Nat. Struct. Mol. Biol. 2005;12:742–746. doi: 10.1038/nsmb983. [DOI] [PubMed] [Google Scholar]
- 98.Gotzmann J, Gerner C, Meissner M, Holzmann K, Grimm R, Mikulits W, Sauermann G. hNMP 200: A novel human common nuclear matrix protein combining structural and regulatory functions. Exp. Cell Res. 2000;261:166–179. doi: 10.1006/excr.2000.5025. [DOI] [PubMed] [Google Scholar]
- 99.Batsche E, Yaniv M, Muchardt C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat. Struct. Mol. Biol. 2006;13:22–29. doi: 10.1038/nsmb1030. [DOI] [PubMed] [Google Scholar]
- 100.Dellaire G, Makarov EM, Cowger JJM, Longman D, Sutherland HGE, Luhrmann R, Torchia J, Bickmore WA. Mammalian PRP4 kinase copurifies and interacts with components of both the U5 snRNP and the N-CoR deacetylase complexes. Mol. Cell. Biol. 2002;22:5141–5156. doi: 10.1128/MCB.22.14.5141-5156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amberg DC, Goldstein AL, Cole CN. Isolation And Characterization Of Rat1 - An Essential Gene Of Saccharomyces-Cerevisiae Required For The Efficient Nucleocytoplasmic Trafficking Of Messenger-RNA. Genes Dev. 1992;6:1173–1189. doi: 10.1101/gad.6.7.1173. [DOI] [PubMed] [Google Scholar]
- 102.Johnson AW. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol. Cell. Biol. 1997;17:6122–6130. doi: 10.1128/mcb.17.10.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim M, Krogan NJ, Vasiljeva L, Rando OJ, Nedea E, Greenblatt JF, Buratowski S. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature. 2004;432:517–522. doi: 10.1038/nature03041. [DOI] [PubMed] [Google Scholar]
- 104.Jurica MS, Moore MJ. Pre-mRNA splicing: Awash in a sea of proteins. Mol. Cell. 2003;12:5–14. doi: 10.1016/s1097-2765(03)00270-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplementary Material]