Generation of Novel, Secreted Epidermal Growth Factor Receptor (EGFR/ErbB1) Isoforms Via Metalloprotease-Dependent Ectodomain Shedding and Exosome Secretion (original) (raw)
. Author manuscript; available in PMC: 2016 Jun 27.
Published in final edited form as: J Cell Biochem. 2008 Apr 15;103(6):1783–1797. doi: 10.1002/jcb.21569
Abstract
Exosomes are small membrane vesicles derived from intracellular multivescicular bodies (MVBs) that can undergo constitutive and regulated secretion from cells. Exosomes can also secrete soluble proteins through metalloprotease-dependent ectodomain shedding. In this study, we sought to determine whether ErbB1 receptors are present within exosomes isolated from the human keratinocyte cell line, HaCaT, and whether exosome-associated ErbB1 receptors can undergo further proteolytic processing. We show that full-length transmembrane ErbB1 is secreted in HaCaT exosomes. EGF treatment and calcium flux stimulated the release of phosphorylated ErbB1 in exosomes but only ligand-stimulated release was blocked by the ErbB1 kinase inhibitor, AG1478, indicating that ligand-dependent ErbB1 receptor activation can initiate ErbB1 secretion into exosomes. In addition, other immunoreactive but truncated ErbB1 isoforms were detected in exosomes suggestive of additional proteolytic processing. We demonstrate that cellular and exosomal ErbB1 receptors can undergo ectodomain shedding to generate soluble N-terminal ectodomains and membrane-associated C-terminal remnant fragments (CTFs). ErbB1 shedding was activated by calcium flux and the metalloprotease activator APMA (4-aminophenylmercuric acetate) and was blocked by a metalloprotease inhibitor (GM6001). Soluble ErbB1 ectodomains shed into conditioned medium retained the ability to bind exogenous ligand. Our results provide new insights into the proteolysis, trafficking and fate of ErbB1 receptors and suggest that the novel ErbB1 isoforms may have functions distinct from the plasma membrane receptor.
Keywords: ErbB1, EGF, exosome, metalloprotease, signaling, ectodomain shedding
ErbB1 is a member of the ErbB receptor family and plays an important role in development, proliferation, migration, differentiation, apoptosis and in tumorigenesis [Olayioye et al., 2000; Yarden and Sliwkowski, 2001]. Whilst the classical signaling role of ErbB receptors as cell surface receptor tyrosine kinases (RTKs) is well established, more recent findings suggest that ErbBs can undergo a variety of pre- and post-translational modifications to generate novel proteins with differing functions [Rio et al., 2000; Lin et al., 2001; Ni et al., 2001; Anido et al., 2006]. Upon ligand binding, the ErbB4 receptor can undergo ectodomain cleavage by the disintegrin metalloprotease TACE/ADAM17 to shed a soluble extracellular domain [Rio et al., 2000]. Following this cleavage event, the residual transmembrane ErbB4 remnant becomes susceptible to regulated intramembrane processing and generates a cytosolic C-terminal fragment (CTF) that is kinase active and can regulate transcription after nuclear translocation [Ni et al., 2001; Williams et al., 2004; Clark et al., 2005]. In other situations, alternate transcripts of ErbB1 and ErbB3 encode soluble N-terminal ectodomain receptor isoforms that are constitutively secreted from cells [Ullrich et al., 1984; Lee and Maihle, 1998; Baron et al., 2003]. Interestingly, secreted ErbB1 levels are significantly decreased in patients with epithelial ovarian cancer, raising the possibility that soluble ErbB1 could play an as yet unrealized role as a suppressor of ErbB1-dependent tumor growth [Baron et al., 2003]. In addition, ErbB2 CTFs can be generated via alternate initiation of translation from downstream initiation codons that retain kinase activity, have nuclear localization and are highly tumorigenic [Anido et al., 2006]. Furthermore, in some cell types, full-length ErbB receptor proteins can be trafficked to the nucleus and can also regulate transcription [Lin et al., 2001; Offterdinger et al., 2002; Wang et al., 2004; Giri et al., 2005; Lo and Hung, 2006].
Exosomes are small (30–100 nm diameter) membrane vesicles that are formed by intraluminal budding within the late endosomal compartment termed multivesicular bodies (MVBs). Exosomes can be secreted from cells upon fusion of MVBs with the plasma membrane [Thery et al., 2002; Andre et al., 2002a; Raiborg et al., 2003]. Exosome secretion was originally associated with the removal of unwanted membrane components but recent data suggests a functional role of exosomes in vivo, particularly within the immune system [Keller et al., 2006; Li et al., 2006; Johnstone, 2006]. The soluble forms of several proteins including CD46, tumor necrosis factor receptor 1 (TNFR1) and fibroblast growth factor-2 (FGF-2) can be released from cells within exosomes [Taverna et al., 2003; Hakulinen et al., 2004; Hawari et al., 2004]. Moreover, recent studies suggest ADAM-mediated ectodomain shedding of a variety of transmembrane proteins including CD46, CD44 and L1 can also occur in exosomes, thus providing another mechanism by which soluble forms of these molecules can be generated and released into the extracellular environment [Gutwein et al., 2003, 2005; Nagano et al., 2004; Stoeck et al., 2006].
In mammalian cells, EGF stimulation and calcium flux can promote MVB formation and intraluminal budding. Upon ligand binding, ErbB1/ligand complexes are trafficked from the cell surface into MVBs and both proteins are subsequently degraded or the receptor is rerouted to the cell surface [de Gassart et al., 2004; White et al., 2006]. Although the ErbB1 receptor is an established marker of MVBs [White et al., 2006], the expression and fate of ErbB1 in exosomes has not been defined. In this study, we demonstrate that novel ErbB1 isoforms are present in exosomes derived from HaCaT cells. Both calcium flux and EGF treatment increased trafficking of ErbB1 into exosomes but only EGF stimulated trafficking was dependent on ErbB1 receptor kinase activity. Other novel truncated ErbB1 isoforms and metalloprotease-dependent ErbB1 ectodomain shedding stimulated by calcium flux and APMA could be detected in exosomal and cellular compartments of HaCaT cells. We suggest that the generation and secretion of soluble ErbB1 isoforms from cellular and exosomal compartments may provide alternate paradigms for signal transduction pathways associated with the ErbB1 receptor.
Experimental Procedures
Cells
The spontaneously immortalized human keratinocyte HaCaT cell line has been described previously [Crusius et al., 1998]. HaCaT cells were cultivated in DMEM supplemented with 10% fetal bovine serum (FBS), Penicillin/Streptomycin and 10 mM Glutamine at 37°C, 5%CO2 and 100% humidity.
Chemicals and Antibodies
The polyclonal rabbit anti-ErbB1 C-terminus, monoclonal mouse anti-ErbB1 N-terminal ectodomain (clone 528) and anti-HSP70 antibodies were obtained from Santa Cruz Biotechnology (CA). The goat anti-ErbB1 ectodomain antibody was purchased from R&D Systems (MN) and the mouse anti-phospho Tyrosine1068 ErbB1 antibody was from BIOMOL International (PA). The antibody to the C-terminus of ADAM10 (serum 71) has been previously described [Gutwein et al., 2003] and the antibody to the ectodomain of ADAM17 (mAb34/4D) was a kind gift from Dr. Martin Humphries (University of Manchester, UK). The mouse monoclonal antibodies to annexin-1, LAMP-1 and BIP/GRP78 were purchased from BD Biosciences (NJ). The mouse anti-moesin (38/87) antibody was a kind gift from Dr. Reinhardt Schwartz-Albiez (DKFZ, Heidelberg, Germany). APMA(4-aminophenylmercuric acetate) and PMA (phorbol 12-myristate 13-acetate) were obtained from Sigma (Taufkirchen, Germany). Ionomycincalcium salt, the metalloprotease inhibitor GM 6001 and the ErbB1 kinase inhibitor AG1478 were from Calbiochem (Bad Soden, Germany). Recombinant EGF was purchased from Roche Applied Sciences (Mannheim, Germany) and recombinant BTC and the rabbit anti-BTC polyclonal antibody were from GroPep Limited (Adelaide, Australia).
Isolation of Membrane Vesicles From Conditioned Media
Cells were cultivated for 24 h in serum-free medium for collection of constitutive vesicles or treated with or without ionomycin (1 μM) or PMA (50 ng/ml) for 2 h for collection of vesicles following cell activation. Tissue culture supernatants were collected and centrifuged for 20 min at 10,000_g_ to remove cellular debris. Membrane vesicles were collected by centrifugation at 100,000_g_ for 2.5 h at 4°C using a Beckman SW 40 rotor. Vesicles were directly dissolved in SDS sample buffer or processed further for gradient centrifugation as described below.
FACS Analysis
FACS analysis of isolated vesicles was done after adsorbing of isolated vesicles to 4 mm (Surfactant-free) aldehyde-sulfate latex beads (Interfacial Dynamics Corp., Portland, OR) as described [Stoeck et al., 2006]. The staining of beads with mAbs and PE-conjugated secondary antibodies has been described [Stoeck et al., 2006]. The indication of apoptosis was measured using the FITC-Annexin-V kit from BD (BD Bioscience, Heidelberg, Germany). Stained beads or cells were analyzed with a FACScan using Cellquest software (Becton & Dickinson, Heidelberg, Germany).
Homogenization of Cells for Membrane Vesicle Preparation
HaCaT cells were scraped from the tissue culture plate surface and taken up in 10 ml PBS. Cells were then centrifuged at 300_g_, resuspended in a buffer containing 0.25 M sucrose, Tris-buffered saline (TBS), 1 mM magnesium acetate and complete protease inhibitor cocktail (Roche) and homogenized with a stainless steel ball-bearing homogenizer as described previously [Gutwein et al., 2003]. In order to separate cellular vesicle compartments, the homogenate was loaded onto a sucrose gradient as described below.
Sucrose Density Gradient Fractionation of Vesicles From CM or Cell Homogenates
Vesicles from CM or cell homogenates were loaded onto the top of a step gradient comprising layers of 2, 1.3, 1.16, 0.8, 0.5, and 0.25 M sucrose as described [Gutwein et al., 2003, 2005]. The gradients were centrifuged for 2.5 h at 100,000_g_ using a Beckman SW40 rotor. Twelve 1 ml fractions were collected from the top of the gradient and proteins precipitated with chloroform/methanol. Samples were analyzed by SDS–PAGE and Western blotting.
Cell Surface Biotinylation
Cell surface proteins were biotinylated using EZ-Link Sulfo-NHS-Biotin-Reagent (Pierce). After washing cells for 2 times with icecold PBS, cell surface proteins were labeled by incubation with 0.5 mg/ml biotin reagent for 30 min at 4°C. The reaction was stopped by washing the cells with 100 mM glycine/PBS.
Isolation of Shed ErbB1 Ectodomains From CM
For constitutive shedding of ErbB1, HaCaT cells were cultured for 24 h in serum-free medium. For activated shedding, cells were treated for 2 h using ionomycin (1 μM), APMA (50 μM) or PMA (50 ng/ml). CM was collected and cellular debris and membrane vesicles removed by centrifugation at 10,000_g_ for 20 min and 100,000_g_ for 2.5 h, respectively. The cleared supernatant was then incubated overnight at 4°C with 2 μg of the mouse anti-ErbB1 ectodomain antibody in the presence of complete protease inhibitor cocktail (Roche). Protein A-Sepharose (GE Healthcare, Freiburg, Germany) was then added and the mixture further incubated for 4 h at 4°C. Immunoprecipitates were isolated by centrifugation at 10,000_g_ and washed twice with ice-cold PBS. Proteins were solubilized by direct addition of SDS–PAGE sample buffer in preparation for Western blot.
Biochemical Analysis
SDS–PAGE under reducing conditions and transfer of proteins to Immobilon membranes (Millipore) using semi-dry blotting has been described previously [Gutwein et al., 2000, 2003]. After blocking with 5% non-fat milk in TBS, blots were developed with the respective primary antibody followed by peroxidase conjugated secondary antibody and ECL detection.
Confocal Immunohistochemistry and Electron Microscopy
The staining of cells grown on glass cover slips with primary antibodies and Cy3-conjugated secondary antibodies has been described previously [Mechtersheimer et al., 2001]. Vesicles from HaCaT cells cultured under constitutive conditions for 24 h in serum-free DMEM were collected and analyzed using a Zeiss 10 Å electron microscope as previously described [Gutwein et al., 2003].
Binding of ErbB1 Ectodomains to Betacellulin
To analyze the ability of shed ErbB1 ectodomains to bind EGF-like factors, the ErbB1 ectodomains were first concentrated. One hundred fifty milliliters of CM from ten 150 mm plates of confluent HaCaT cells treated with ionomycin was first cleared of exosomes and other membranes by ultracentrifugation as described above. The cleared CM was then concentrated to 2 ml using centrifugal filter devices (Millipore, 100 kDa cut-off). Recombinant betacellulin (BTC) was then mixed overnight at 4°C with 500 μl of concentrated CM in the presence of complete protease inhibitor cocktail and 0.01% sodium azide. Immunoprecipitation was then performed with the mouse monoclonal anti-ErbB1 ectodomain antibody as described above. Following washing four times with ice-cold PBS, immunoprecipitates were solubilized in SDS–PAGE sample buffer and analyzed by Western blot using the rabbit anti-BTC polyclonal and goat anti-ErbB1 ectodomain antibodies.
Results
The ErbB1 Receptor is Secreted From HaCaT Cells in Exosomes
Previous studies have demonstrated that internalized ErbB1 receptors accumulate within MVBs and associated intra luminal vesicles (ILVs)prior to degradation in lysosomes [Futter et al., 1993, 1996; de Gassart et al., 2004; White et al., 2006]. To investigate whether ErbB1 receptors could also be released within exosomes from cells, we examined constitutive and regulated exosomal release from human keratinocyte HaCaT cells, which express endogenous ErbB1 receptors. Treatment of HaCaT cells with ionomycin, but not phorbol esters, led to a decrease in total cellular levels of ErbB1 (Fig. 1A). Two ErbB1 isoforms were identified of approximately 185 and 120 kDa that correspond to the major full-length glycosylated and minor full-length non-glycosylated proteins, respectively [Gamou and Shimizu, 1988].
Fig. 1.
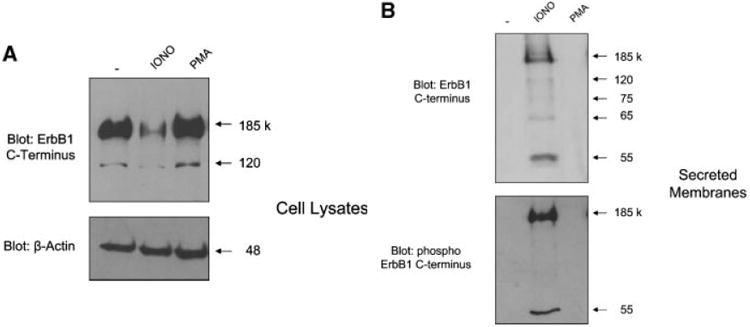
Identification of soluble ErbB1 associated with secreted membrane vesicles. A: Equal numbers of HaCaT cells were treated for 2 h in serum-free medium with or without ionomycin (1 μM) or PMA (50 ng/ml). Cell lysates were collected by direct lysis in SDSPAGE sample buffer and then analyzed by Western blot using antibodies against the ErbB1 C-terminus and β-Actin. B: Conditioned media (CM) from HaCaT cells treated with ionomycin or PMA, as above, were collected and membrane vesicles isolated by ultracentrifugation. Vesicles were directly taken up in SDS–PAGE sample buffer and analyzed by Western blot using antibodies against the ErbB1 C-terminus and phospho ErbB1 C-terminus (residue Tyr1068). C: Vesicles from HaCaT CM were isolated as in (B). The vesicle pellet is a mixture of exosome and membrane bleb components [Stoeck et al., 2006]. To separate these components, vesicles were centrifuged on a sucrose step gradient as described in the experimental procedures section. Protein fractions from the gradient were analyzed by Western blotting using the anti-ErbB1 C-terminus antibody and a range of other exosomal marker proteins and disintegrin metalloproteases as indicated. The density of each fraction of the gradient is shown. D: Vesicles from HaCaT cells were collected by ultracentrifugation and then resuspended in PBS in preparation for electron microscopy at 48,000× magnification. Exosomes displayed a disk like structure consistent with the findings of other groups [Andre et al., 2002b; Gill et al., 2005]. E: Isolated vesicles were adsorbed to latex beads and subjected to FACS analysis using an antibody to the ectodomain of ErbB1.
To determine whether the loss of cellular ErbB1 upon ionomycin treatment was due to release of membrane- and/or vesicle-associated ErbB1 from cells, conditioned media (CM) from HaCaT cells was ultracentrifuged and pelleted membranes were examined for ErbB1 expression. An antibody to the C-terminus of ErbB1 readily detected the full-length 185 kDa ErbB1 receptor in the membrane pellet of ionomycin treated cells (Fig. 1B). Moreover, smaller ErbB1 fragments were detected implying that ErbB1 may undergo proteolytic processing. In addition, the full-length ErbB1 and an additional 55 kDa ErbB1 C-terminal fragment were phosphorylated at the autophosphorylation site tyrosine1068 [Hunter and Cooper, 1985].
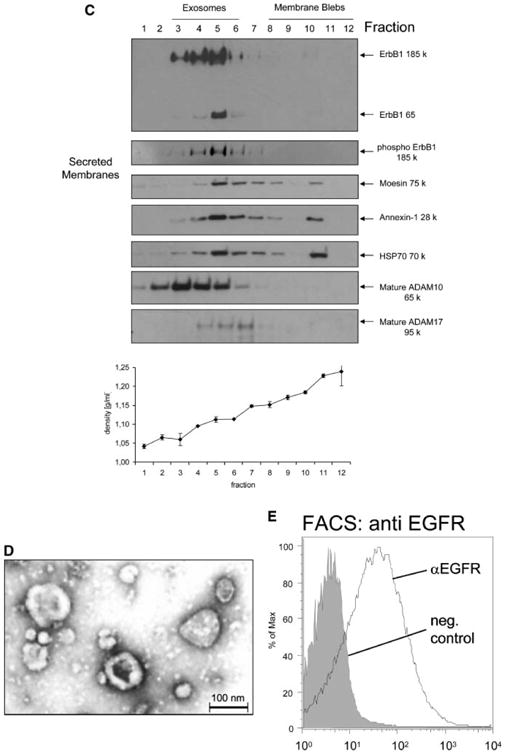
Exosomes are released from cells by fusion of MVBs with the plasma membrane and express specific marker proteins including moesin, annexin-1, heat shock protein 70 (HSP70) and the metalloproteases ADAM10 and ADAM17 [Stoeck et al., 2006]. To determine whether the ErbB1 proteins were present in exosomes, the vesicle pellet from ionomycin-treated HaCaT cells was resuspended and submitted to sucrose density fractionation followed by analysis of expression of exosomal markers. Western blot analysis of fractionated sucrose density gradients showed that the majority of immunoreactive ErbB1 and phospho-ErbB1 was detected within the exosomal fractions 3–6 of the gradient confirming that ErbB1 was indeed released from cells within exosomes (Fig. 1C). ErbB1-containing exosomes could also be isolated from CM of cells cultured for 24 h in serum-free DMEM with a density of 1.06– 1.11 g/ml implying that constitutive secretion of ErbB1 in exosomes can occur under physiological conditions in vivo. Vesicles from HaCaT cells were also analyzed by electron microscopy (EM). Consistent with previous reports [Andre et al., 2002b; Gill et al., 2005], vesicles from HaCaT cells showed a typical disk-like exosomal structure with a diameter of between approximately 30 and 100 nm (Fig. 1D).
Exosomal transmembrane proteins have an orientation identical to that of cell surface proteins. To determine the orientation of ErbB1 on within exosomes, we immobilized HaCaT derived exosomes onto latex beads and carried out FACS analysis. Vesicles were readily stained with an antibody against the ectodomain of ErbB1, supporting the notion that HaCaT exosomes contain ErbB1 and that the receptor has the same orientation as its cell surface counterpart (Fig. 1E).
ErbB1 Within Exosomes Is Derived From an Intracellular Vesicle Pool
Exosomes are formed by invagination and budding from the limiting membrane of late endosomes and accumulate within MVBs from where they are released by fusion with the plasma membrane [Raiborg et al., 2003]. To investigate the cellular origin of exosomes detected in CM, a sucrose gradient fractionation procedure was used to separate distinct subcellular membrane compartments within untreated HaCaT cell homogenates [Stoeck et al., 2006]. ErbB1 localized at the plasma membrane and within the intracellular vesicle/Golgi/Trans Golgi Network (TGN) fractions of the gradient (Fig. 2A). These ErbB1-positive intracellular vesicles had the same buoyant density as ErbB1-positive secreted exosomes (see Figs. 1C and 2A) and had a similar profile of ErbB1 immunoreactive fragments as secreted exosomes (Fig. 2B and refer also to Fig. 1B), whereas the plasma membrane/endoplasmic reticulum fractions only contained the intact, full-length glycosylated (185 kDa) and non-glycosylated (120 kDa) ErbB1 receptor isoforms (Fig. 2B). This data is consistent with the ErbB1-immunoreactive intracellular vesicles being functionally and biochemically related to the ErbB-immunoreactive secreted exosomes.
Fig. 2.
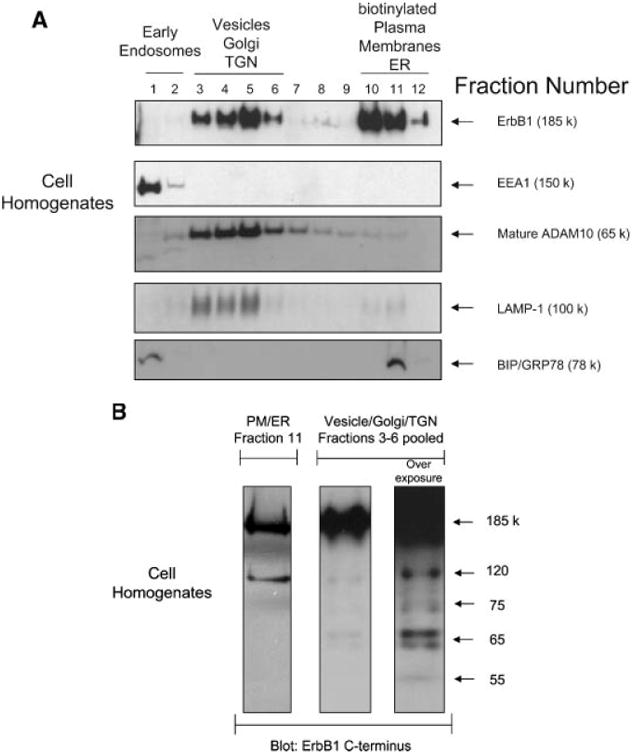
Intracellular vesicles containing ErbB1 are released from the cell as exosomes. A: Cell homogenates were layered on a step sucrose gradient to separate intracellular vesicles and plasma membranes. Gradient fractions were analyzed by Western blot using an antiErbB1 C-terminus antibody. In addition, antibodies to marker proteins were used in order to confirm the separation of organelles. EEA1, early endosome antigen 1; BIP/GRP78, binding protein/glucose-regulated protein 78 (endoplasmic reticulum marker); LAMP-1, lysosome associated membrane protein-1; TGN, trans Golgi network; ER, endoplasmic reticulum. Plasma membrane fractions were identified by cell surface biotinylation followed by homogenization and gradient fractionation. Biotinylated proteins were detected with Streptavidin-HRP. Note that ADAM10 has previously been shown to predominantly localize to the Golgi, TGN and intracellular vesicles by cell fractionation and immunohistochemistry [Gutwein et al., 2003]. B: Fractions 3–6 from the cell homogenate gradient in A were pooled and ErbB1 protein fragments analyzed by Western blotting with the anti-ErbB1 C-terminus antibody. In order to visualize less represented smaller fragments, the blot was overexposed (right panel). Fraction 11 of the gradient, representing the plasma membrane (PM) and ER components (left panel) was also analyzed alongside the vesicle fractions.
Because exosome formation in MVBs can be stimulated by calcium ionophores [Savina et al., 2003], we next investigated the effect of ionomycin treatment on the distribution of ErbB1 within intracellular vesicles. To exclude any toxic effects, we first tested the effect of ionomycin on cell viability using annexin-V staining as an early marker of apopotosis. As shown in Figure 3A, treatment of HaCaT cells with ionomycin at 1 μM for up to 60 min did not induce significant Annexin-V staining. The stimulation of HaCaT cells for 10 min with ionomycin led to a decrease of ErbB1 at the cell surface, whereas ErbB1 levels within the vesicle/Golgi/TGN fractions increased (Fig. 3B). This redistribution of ErbB1 into the vesicle/ Golgi/TGN fractions was particularly evident with the 120 kDa ErbB1 form. Following 30 to 60 min of treatment, ErbB1 levels at the cell surface and in the vesicle/Golgi/TGN fractions decreased. Concurrently, levels of ErbB1-containing exosomes secreted into CM increased over 10, 30, and 60 min of ionomycin treatment (Fig. 3C).
Fig. 3.
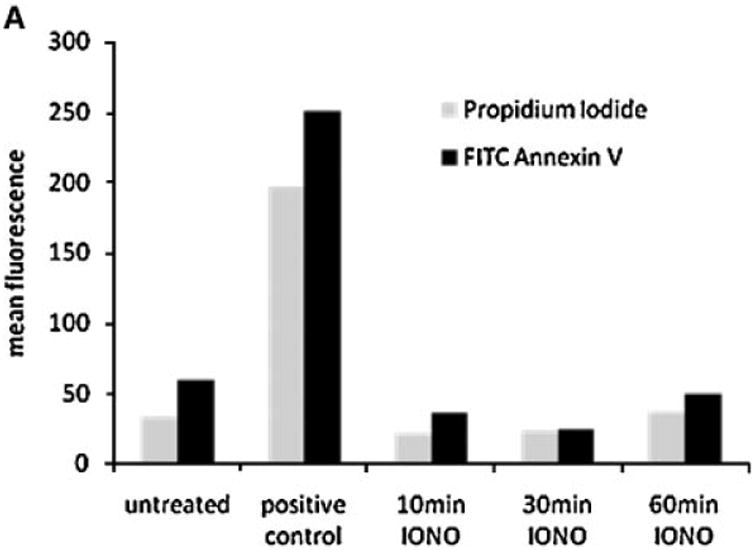
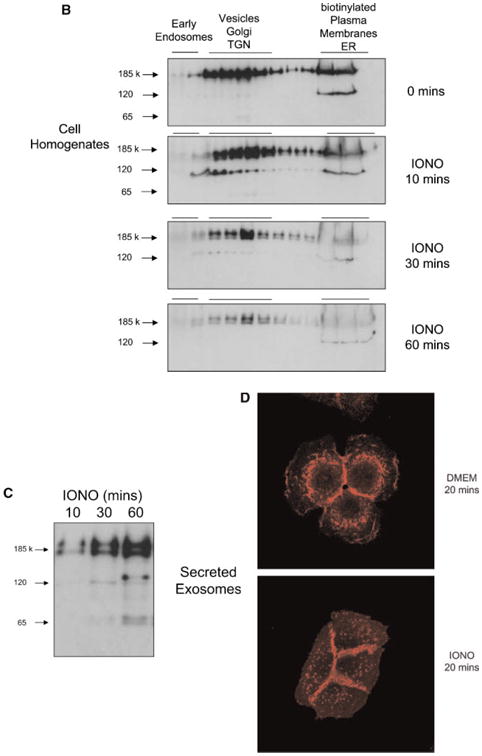
Ionomycin leads to ErbB1 trafficking to MVB compartments. A: HaCaT were treated with 1 mM ionomycin for the indicated length of time and cells were analyzed for apoptosis using Annexin-V and PI staining and FACS analysis. Treatment with staurosporine (1 μM) for 16 h served as positive control. B, C: HaCaT cells were treated with ionomycin (1 μM) for 10, 30, and 60 min and cell homogenates layered on a step sucrose gradient as described above. Homogenate gradient fractions and exosomes isolated from CM were analyzed by Western blot using the anti-ErbB1 C-terminus antibody. D: HaCaT cells were grown on glass cover slips and treated with or without ionomycin (1 μM) for 20 min. Cells were fixed and permeabilized in 3% paraformaldehyde/Triton X100 and stained with a mouse anti-ErbB1 ectodomain antibody followed by a Cy3-conjugated antimouse IgG secondary antibody. Confocal images were taken in the DKFZ microscopy division. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The effects of ionomycin on ErbB1 trafficking to exosomes were visualized by immunoflorescence analysis using an antibody to the extracellular ectodomain of ErbB1. In untreated HaCaT cells, ErbB1 was localized within the cell and at the cell surface between cell–cell contacts (Fig. 3D). Treatment with ionomycin for 20 min resulted in the formation of multiple punctate vesicle structures which were ErbB1-positive. Taken together, these results indicate that ionomycin redirects the ErbB1 receptor from the plasma membrane and possibly from the secretory pathway into an intracellular vesicle pool within MVBs whereupon these vesicles are released from the cell as exosomes.
Ligand Engagement Leads to Release of ErbB1 in Exosomes
It is well recognized that ligand stimulation causes the internalization, endosomal sorting and accumulation of ErbB1 into MVBs [Futter et al., 1993, 1996; de Gassart et al., 2004; White et al., 2006]. We next investigated whether ligand binding could induce the release of ErbB1-containing exosomes. EGF treatment alone or in combination with ionomycin resulted in higher levels of phosphorylated ErbB1 being secreted in exosomes (Fig. 4A). Based on the expression of the exosomal marker moesin, the overall level of exosome release was not dramatically increased by EGF treatment suggesting that ErbB1 and its phosphorylated form were being selectively recruited into released exosomes.
Fig. 4.
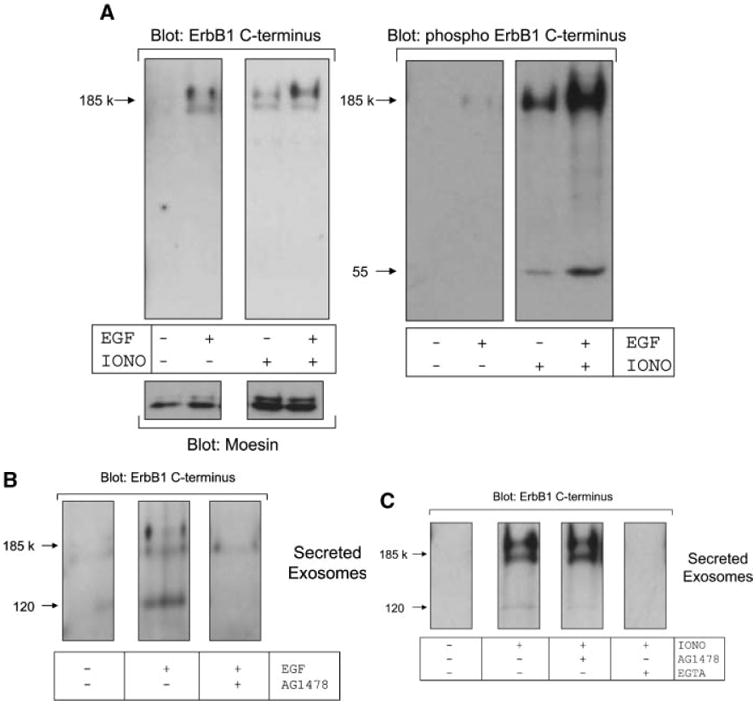
EGF activates release of ErbB1 in exosomes. A: HaCaT cells were treated in the presence or absence of EGF (20 ng/ml) and ionomycin-calcium salt (1 μM) for 2 h. Exosomes were isolated from conditioned media by ultracentrifugation and then analyzed by Western blot using antibodies against the ErbB1 C-terminus, phospho-ErbB1 C-terminus and the exosomal marker protein moesin. B: HaCaT cells were pre-treated with or without AG1478 (0.25 μM) for 30 min prior to stimulation with EGF (20 ng/ml). Exosomes were analyzed by Western blot for ErbB1 as in (A). C: HaCaT cells were pre-incubated for 30 min in AG1478 (0.25 μM) or EGTA (10 mM) and then treated with ionomycin (1 μM). Exosomes were then analyzed by Western blot for ErbB1 as in A.
To determine whether the kinase activity of ErbB1 was necessary for its secretion in exosomes, we pre-incubated HaCaT cells with the ErbB1 kinase inhibitor AG1478 prior to stimulation. EGF-induced release of ErbB1 containing vesicles was blocked by AG1478 (Fig. 4B), whereas ionomycin-induced secretion of ErbB1-containing exosomes was not affected by AG1478 (Fig. 4C). However, depletion of extracellular calcium with the chelator EGTA blocked ionomycin-induced exosome release confirming a role for cellular calcium flux in this process. These observations are not only in agreement with previous findings on the accumulation of ErbB1 in MVBs upon ligand stimulation [White et al., 2006], but suggest that physiological stimuli such as ligand-dependent ErbB1 activation is also involved in exosome release from cells.
Soluble Isoforms of ErbB1 Are Generated by Metalloprotease-Mediated Ectodomain Shedding
We have previously shown that ectodomain shedding of the transmembrane proteins CD44 and L1 can occur at the plasma membrane and in intracellular vesicles and secreted exosomes [Gutwein et al., 2005; Stoeck et al., 2006]. The possibility for such processing of ErbB1 in HaCaT cells was suggested by the detection of smaller immunoreactive C-terminal ErbB1 fragments in secreted exosomes (Figs. 1 and 2).
Further analysis of the total exosome lysate with an antibody against the C-terminus of ErbB1 identified the full-length 185 kDa receptor and a ladder of smaller fragments (Fig. 5A). An anti-ErbB1 ectodomain antibody also detected the full-length receptor and an additional 150 kDa isoform. This 150 kDa isoform and a less represented 100 kDa form were also found to be released into CM and lacked the ErbB1 C-terminus as confirmed by their inability to be detected by the anti-C-terminus antibody. These soluble ErbB1 isoforms in CM are consistent with proteolytically shed ErbB1 ectodomains. The association of the soluble 150 and 100 kDa isoforms with exosomes could possibly occur via homodimerization with another full-length transmembraneErbB1 molecule on the exosome surface. Several smaller exosomal ErbB1 bands recognized by the anti-C-terminus antibodies (65 and phosphorylated 55 kDa) were not detected by the anti-ectodomain antibody. This suggests that they could be C-terminal remnant forms generated following proteolytic ectodomain shedding.
Fig. 5.
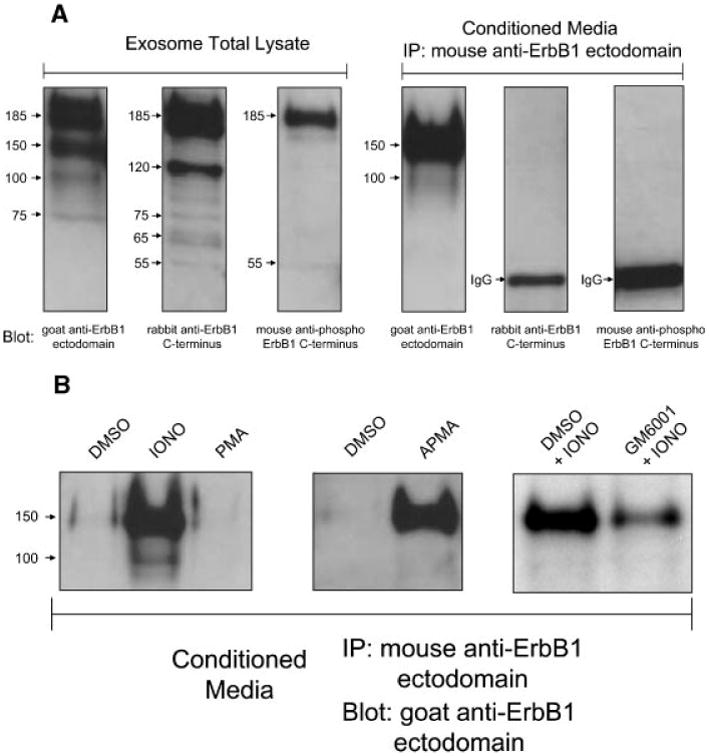
Characterization of ErbB1 isoforms in exosomes and soluble forms generated by metalloprotease cleavage. A: Exosomes from HaCaT cell CM were pelleted by ultracentrifugation as described above. Following this, soluble non-membrane associated ErbB1 ectodomains in the supernatant were immunoprecipitated using a mouse anti-ErbB1 ectodomain antibody. Exosome and IP samples were analyzed by SDSPAGE and Western blotting for the ErbB1 C-terminus, phospho-ErbB1 C-terminus and N-terminal ErbB1 ectodomain. B: HaCaT cells were treated for 2 h with; serum-free media with DMSO (vehicle), ionomycin (1 μM) or PMA (50 ng/ml; left panel), DMSO or the metalloprotease activator APMA (middle panel), or ionomycin in the presence of DMSO or the metalloprotease inhibitor GM6001 (right panel). Exosomes were removed from the CM by ultracentrifugation and IP performed as described in A. Samples were then analyzed for soluble ErbB1 levels by Western blot analysis with a goat anti-ErbB1 ectodomain antibody.
Levels of the soluble 150 kDa ErbB1 isoform in CM could be increased following ionomycin treatment (Fig. 5B). Ionomycin and other calcium ionophores are strong activators of ADAM10-dependent ectodomain shedding at the cell surface as well as in intracellular vesicles and exosomes [Sanderson et al., 2005; Stoeck et al., 2006]. PMA is a well-characterized activator of ADAM17-mediated shedding events which occur at the plasma membrane [Nagano et al., 2004; Stoeck et al., 2006]. Interestingly, PMA did not activate ErbB1 release into CM suggesting that ErbB1 is not subject to ADAM17mediated cleavage.
The role of a metalloprotease in ErbB1 shedding was confirmed by the stimulatory effect of the metalloprotease activator APMA (Fig. 5B). In addition, pre-incubation of HaCaT cells with the metalloprotease inhibitor GM6001 reduced levels of soluble 150 kDa ErbB1 in CM following ionomycin treatment. Interestingly, in the case of constitutive shedding over 24 h of culture in serum-free media, metalloprotease inhibitors and inhibitors of other protease classes (serine, cysteine and aspartic proteases and aminopeptidases) were ineffective in blockingErbB1 shedding (data not shown). This could reflect that constitutive proteolytic shedding of the ErbB1 ectodomain occurs predominantly in an intracellular compartment which is not efficiently accessible to such chemical inhibitors.
Shed ErbB1 Binds the Ligand Betacellulin
A soluble form of the ErbB3 ectodomain, generated by alternate mRNA splicing, binds the EGF-like factor Neuregulin-1 (NRG1) with similar affinity to the transmembrane form of ErbB3 [Lee et al., 2001]. We therefore investigated whether shed ErbB1 could bind exogenous ligand betacellulin (BTC) [Dunbar et al., 1999]. BTC was incubated with concentrated HaCaT cell CM overnight at 4°C. For isolation of complexes between shed ErbB1 and BTC, immunoprecipitation was performed using an anti-ErbB1 ectodomain antibody. Immunoprecipitates were then analyzed by Western blot using anti-BTC and antiErbB1 ectodomain antibodies. BTC was immunoprecipitated with shed ErbB1 indicating an interaction between these proteins (Fig. 6). This suggests that soluble forms of ErbB1 could modulate the function of soluble EGF-like factors.
Fig. 6.
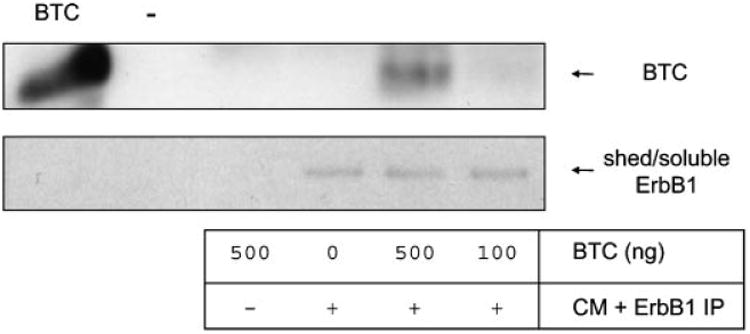
ErbB1 ectodomains bind the ligand betacellulin (BTC). Concentrated HaCaT CM free of exosomes was incubated with recombinant BTC and then the ErbB1 ectodomains immunoprecipitated using the mouse anti-ErbB1 ectodomain antibody and protein-A sepharose. As controls, BTC or CM alone were used. Immunoprecipitates were analyzed by Western blot using rabbit anti-BTC and goat anti-ErbB1 ectodomain antibodies.
Discussion
ErbB1 is a member of the ErbB receptor family which plays an important role in the normal development and homeostasis of multiple tissues and also in tumorigenesis [Olayioye et al., 2000; Yarden and Sliwkowski, 2001]. In addition to acting as cell surface receptors, ErbBs and other transmembrane proteins can undergo a variety of post-translational modification events to generate novel proteins with differing functions. In the case of ErbB2 and ErbB4, proteolytic processing by metalloproteases sheds soluble ectodomain forms of each molecule [Rio et al., 2000; Molina et al., 2001; Liu et al., 2006a, b]. Whilst sequential processing of ErbB4 generates a C-terminal fragment (CTF) which migrates to the nucleus and affects transcription events [Ni et al., 2001]. Full-length ErbB receptor proteins can be trafficked to the nucleus in certain cell types [Lo and Hung, 2006], whereas other full-length transmembrane proteins can be secreted in exosomes [Taverna et al., 2003; Hakulinen et al., 2004; Hawari et al., 2004; Stoeck et al., 2006]. In this study, we demonstrate that the full-length and smaller processed forms of ErbB1 are secreted in exosomes and that the soluble immunoreactive ErbB1 fragments are generated by proteolytic processing.
Secretion of full-length and proteolysed ErbB1 isoforms in exosomes was upregulated by treatment with EGF and the calcium ionophore ionomycin. The generation of exosomes in response to calcium flux is a well-characterized process [Savina et al., 2003], whereas upon ligand binding, ErbB1/ligand complexes are trafficked from the cell surface into MVBs and both proteins are subsequently degraded or the receptor is re-routed to the cell surface [Futter et al., 1993, 1996; de Gassart et al., 2004; White et al., 2006]. Our findings indicate that ligand (EGF) binding also results in a portion of ErbB1 being secreted from MVBs in exosomes. Consistent with earlier findings that the kinase domain of ErbB1 is required for intracellular trafficking to MVBs [Felder et al., 1990], we demonstrated that the ErbB1 kinase inhibitor AG1478 blocked secretion of ErbB1-containing exosomes following EGF treatment. Trafficking of ErbB1 to exosomes following EGF or ionomycin treatment likely represents a common pathway. Formation of exosomes following calcium flux and also the recruitment of ErbB1/ligand complexes to MVBs are dependent on the calcium-binding protein annexin-1 [Radke et al., 2004; Gerke et al., 2005]. Calcium flux leads to the redistribution of annexin-1 to the plasma membrane and the inward formation of intracellular endosomal vesicles [Rescher and Gerke, 2004]. In this report, and our earlier work in ovarian carcinoma cell lines [Stoeck et al., 2006], we demonstrated that ionophore-induced exosomes contain annexin-1. Following ligand binding, ErbB1 binds and phosphorylates annexin-1 and this process drives trafficking of the receptor/ligand complex to MVBs [Futter et al., 1993; Radke et al., 2004]. In addition, other calcium binding proteins including calmodulin have been demonstrated to interact with the ErbB1 receptor and modulate its endosomal/lysosomal trafficking and recycling to the plasma membrane [Li and Villalobo, 2002; Tebar et al., 2002]. Further work is warranted to elucidate the role of annexin-1 and other calcium-regulated proteins in ErbB1 trafficking to exosomes.
In addition to the trafficking of ErbB1 into exosomes, calcium flux and the metalloprotease activator APMA stimulated proteolytic shedding of the ErbB1 ectodomain. We have previously found that these two stimuli are strong activators of the metalloprotease ADAM10 [Sanderson et al., 2005; Stoeck et al., 2006]. Ectodomain shedding of ErbB1 generated a major 150 kDa soluble form and smaller membrane-associated C-terminal fragments (CTFs) lacking the ectodomain. These remnant forms were only present in the Golgi/TGN/ vesicle fractions of cell homogenate gradients suggesting that, like the L1 adhesion molecule [Stoeck et al., 2006], shedding of ErbB1 occurs predominantly inside the cell. Consistent with this, ADAM10 was present predominantly in the same intracellular fractions as full-length and cleaved ErbB1 isoforms. Numerous studies have identified that ligands for ErbB receptors (EGF-like factors) such as HB-EGF and TGFα undergo ADAM-mediated shedding in response to pharmacological agents including phorbol esters and ionophores [Pandiella and Massague, 1991a, b] as well as physiological activators such as G-protein coupled receptor (GPCR) agonists [Prenzel et al., 1999]. Shed EGF-like factors then ‘transactivate’ the ErbB1 receptor and this process appears to be particularly important for driving the growth of certain cancer cell lines [Fischer et al., 2003]. We have now identified that ErbB1 is also proteolysed to a soluble form following cell stimulation and that shed ErbB1 retained the ability to bind the EGF-like factor BTC. These findings raise the possibility that soluble ErbB1 forms could regulate the activity of shed EGF-like factors and cell growth and this may highlight an as yet uncharacterized component of the transactivation process.
In summary, the work presented here identifies novel protein forms of ErbB1 which are generated by proteolytic processing and secretion in exosomes. It is possible that each ErbB1 isoform could play a novel and as yet unrecognized function in the normal and tumorigenic activity of ErbB1 and such investigations are warranted in the future.
Acknowledgments
We thank Verena Gschwend and Natalie Erbe for their excellent technical assistance. This work was supported by a grant from Deutsche Krebshilfe (Schwerpunktprogramm Migration and Invasion) and the European Community (EC-Strep Signaling & Traffic) to Peter Altevogt and by grants from the NIH (DK59778 and DK63363) and from the Crohn's and Colitis Foundation of America to P. Dempsey. We also thank Prof. Dr. Hanswalter Zentgraf and Dr. HerbertSpring (both at DKFZ, Heidelberg) for their help with electron and confocal microscopy, respectively.
Grant sponsor: Deutsche Krebshilfe (Bonn, Germany); Grant sponsor: European Community (EC-Strep Signaling & Traffic); Grant sponsor: NIH; Grant numbers: DK59778, DK63363; Grant sponsor: Crohn's and Colitis Foundation of America.
Abbreviations used
ADAM
a disintegrin and metalloprotease
APMA
4-aminophenylmercuric acetate
BTC
beta-cellulin
CM
conditioned media
DMEM
Dulbecco's modified Eagle's medium
DMSO
dimethyl sulfoxide
EGF
epidermal growth factor
EGFR
EGF receptor
ER
endoplasmic reticulum
FBS
fetal bovine serum
HB-EGF
heparin-binding EGF-like growth factor
LAMP-1
lysosome-associated membrane protein
MVB
multivesicular body
NRG
neuregulin
PM
plasma membrane
TACE
tumor necrosis factor-α-converting enzyme
TBS-T
Tris-buffered saline-tween
TGFα
transforming growth factor α
TGN
Trans Golgi network
References
- Andre F, Schartz NE, Chaput N, Flament C, Raposo G, Amigorena S, Angevin E, Zitvogel L. Tumor-derived exosomes: A new source of tumor rejection antigens. Vaccine. 2002;20(Suppl. 4):A28–A31. doi: 10.1016/s0264-410x(02)00384-5. [DOI] [PubMed] [Google Scholar]
- Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- Anido J, Scaltriti M, Bech Serra JJ, Santiago Josefat B, Todo FR, Baselga J, Arribas J. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J. 2006;25:3234–3244. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron AT, Cora EM, Lafky JM, Boardman CH, Buenafe MC, Rademaker A, Liu D, Fishman DA, Podratz KC, Maihle NJ. Soluble epidermal growth factor receptor (sEGFR/sErbB1) as a potential risk, screening, and diagnostic serum biomarker of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:103–113. [PubMed] [Google Scholar]
- Clark DE, Williams CC, Duplessis TT, Moring KL, Notwick AR, Long W, Lane WS, Beuvink I, Hynes NE, Jones FE. ERBB4/HER4 potentiates STAT5A transcriptional activity by regulating novel STAT5A serine phosphorylation events. J Biol Chem. 2005;280:24175–24180. doi: 10.1074/jbc.M414044200. [DOI] [PubMed] [Google Scholar]
- Crusius K, Auvinen E, Steuer B, Gaissert H, Alonso A. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp Cell Res. 1998;241:76–83. doi: 10.1006/excr.1998.4024. [DOI] [PubMed] [Google Scholar]
- de Gassart A, Geminard C, Hoekstra D, Vidal M. Exosome secretion: The art of reutilizing nonrecycled proteins? Traffic. 2004;5:896–903. doi: 10.1111/j.1600-0854.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- Dunbar AJ, Priebe IK, Belford DA, Goddard C. Identification of betacellulin as a major peptide growth factor in milk: Purification, characterization and molecular cloning of bovine betacellulin. Biochem J. 1999;344Pt 3:713–721. [PMC free article] [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31:1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- Futter CE, Felder S, Schlessinger J, Ullrich A, Hopkins CR. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993;120:77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamou S, Shimizu N. Glycosylation of the epidermal growth factor receptor and its relationship to membrane transport and ligand binding. J Biochem (Tokyo) 1988;104:388–396. doi: 10.1093/oxfordjournals.jbchem.a122478. [DOI] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Gill DB, Spitzer D, Koomey M, Heuser JE, Atkinson JP. Release of host-derived membrane vesicles following pilus-mediated adhesion of Neisseria gonorrhoeae. Cell Microbiol. 2005;7:1672–1683. doi: 10.1111/j.1462-5822.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G, Wang SC, Hung MC. Endosomal transport of ErbB-2: Mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25:11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutwein P, Oleszewski M, Mechtersheimer S, Agmon-Levin N, Krauss K, Altevogt P. Role of Src kinases in the ADAM-mediated release of L1 adhesion molecule from human tumor cells. J Biol Chem. 2000;275:15490–15497. doi: 10.1074/jbc.275.20.15490. [DOI] [PubMed] [Google Scholar]
- Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Gast D, Joumaa S, Zentgraf H, Fogel M, Altevogt DP. ADAM10-mediated cleavage of L1 adhesion molecule at the cell surface and in released membrane vesicles. FASEB J. 2003;17:292–294. doi: 10.1096/fj.02-0430fje. [DOI] [PubMed] [Google Scholar]
- Gutwein P, Stoeck A, Riedle S, Gast D, Runz S, Condon TP, Marme A, Phong MC, Linderkamp O, Skorokhod A, Altevogt P. Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin Cancer Res. 2005;11:2492–2501. doi: 10.1158/1078-0432.CCR-04-1688. [DOI] [PubMed] [Google Scholar]
- Hakulinen J, Junnikkala S, Sorsa T, Meri S. Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur J Immunol. 2004;34:2620–2629. doi: 10.1002/eji.200424969. [DOI] [PubMed] [Google Scholar]
- Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: A mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA. 2004;101:1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Cooper JA. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Lee H, Maihle NJ. Isolation and characterization of four alternate c-erbB3 transcripts expressed in ovarian carcinoma-derived cell lines and normal human tissues. Oncogene. 1998;16:3243–3252. doi: 10.1038/sj.onc.1201866. [DOI] [PubMed] [Google Scholar]
- Lee H, Akita RW, Sliwkowski MX, Maihle NJ. A naturally occurring secreted human ErbB3 receptor isoform inhibits heregulin-stimulated activation of ErbB2, ErbB3, and Erb B4. Cancer Res. 2001;61:4467–4473. [PubMed] [Google Scholar]
- Li H, Villalobo A. Evidence for the direct interaction between calmodulin and the human epidermal growth factor receptor. Biochem J. 2002;362:499–505. doi: 10.1042/bj3620499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XB, Zhang ZR, Schluesener HJ, Xu SQ. Role of exosomes in immune regulation. J Cell Mol Med. 2006;10:364–375. doi: 10.1111/j.1582-4934.2006.tb00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- Liu PC, Liu X, Li Y, Covington M, Wynn R, Huber R, Hillman M, Yang G, Ellis D, Marando C, Katiyar K, Bradley J, Abremski K, Stow M, Rupar M, Zhuo J, Li YL, Lin Q, Burns D, Xu M, Zhang C, Qian DQ, He C, Sharief V, Weng L, Agrios C, Shi E, Metcalf B, Newton R, Friedman S, Yao W, Scherle P, Hollis G, Burn TC. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol Ther. 2006;5:657–664. doi: 10.4161/cbt.5.6.2708. [DOI] [PubMed] [Google Scholar]
- Liu X, Fridman JS, Wang Q, Caulder E, Yang G, Covington M, Liu C, Marando C, Zhuo J, Li Y, Yao W, Vaddi K, Newton RC, Scherle PA, Friedman SM. Selective inhibition of ADAM metalloproteases blocks HER-2 extracellular domain (ECD) cleavage and potentiates the anti-tumor effects of trastuzumab. Cancer Biol Ther. 2006;5:648–656. doi: 10.4161/cbt.5.6.2707. [DOI] [PubMed] [Google Scholar]
- Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: Linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, Postina R, Fahrenholz F, Fogel M, Lemmon V, Altevogt P. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol. 2001;155:661–673. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, Nakajima M, Shinohara M, Saya H. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol. 2004;165:893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni CY, Murphy MP, Golde TE, Carpenter G. Gammasecretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Offterdinger M, Schofer C, Weipoltshammer K, Grunt TW. c-erbB-3: A nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157:929–939. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiella A, Massague J. Cleavage of the membrane precursor for transforming growth factor alpha is a regulated process. Proc Natl Acad Sci USA. 1991a;88:1726–1730. doi: 10.1073/pnas.88.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiella A, Massague J. Multiple signals activate cleavage of the membrane transforming growth factor-alpha precursor. J Biol Chem. 1991b;266:5769–5773. [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Radke S, Austermann J, Russo-Marie F, Gerke V, Rescher U. Specific association of annexin 1 with plasma membrane-resident and internalized EGF receptors mediated through the protein core domain. FEBS Lett. 2004;578:95–98. doi: 10.1016/j.febslet.2004.10.078. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- Rescher U, Gerke V. Annexins—Unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/H ER4. J Biol Chem. 2000;275:10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- Sanderson MP, Erickson SN, Gough PJ, Garton KJ, Wille PT, Raines EW, Dunbar AJ, Dempsey PJ. ADAM10 mediates ectodomain shedding of the betacellulin precursor activated by p-aminophenylmercuric acetate and extracellular calcium influx. J Biol Chem. 2005;280:1826–1837. doi: 10.1074/jbc.M408804200. [DOI] [PubMed] [Google Scholar]
- Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F, Gutwein P, Ludwig A, Rubinstein E, Altevogt P. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD4. Biochem J. 2006;393:609–618. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Ghersi G, Ginestra A, Rigogliuso S, Pecorella S, Alaimo G, Saladino F, Dolo V, Dell'Era P, Pavan A, Pizzolanti G, Mignatti P, Presta M, Vittorelli ML. Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. J Biol Chem. 2003;278:51911–51919. doi: 10.1074/jbc.M304192200. [DOI] [PubMed] [Google Scholar]
- Tebar F, Villalonga P, Sorkina T, Agell N, Sorkin A, Enrich C. Calmodulin regulates intracellular trafficking of epidermal growth factor receptor and the MAPK signaling pathway. Mol Biol Cell. 2002;13:2057–2068. doi: 10.1091/mbc.01-12-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, Downward J, Mayes ELV, Whittle N, Waterfield MD, Seeburg PH. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK, Hung MC. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–261. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167:469–478. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]