Fibrinogen–like–protein 1, a hepatocyte derived protein is an acute phase reactant (original) (raw)
. Author manuscript; available in PMC: 2009 Jan 25.
Published in final edited form as: Biochem Biophys Res Commun. 2007 Nov 26;365(4):729–734. doi: 10.1016/j.bbrc.2007.11.069
Abstract
Fibrinogen-like-protein 1 (FGL1) is a hepatocyte derived protein that is upregulated in regenerating rodent livers following partial hepatectomy. It has been implicated as a mitogen for liver cell proliferation. In this study, we show that recombinant human IL-6 induces FGL1 expression in Hep G2 cells in a pattern similar to those of acute phase reactants. Following induction of acute inflammation in rats by subcutaneous injection of turpentine oil, serum FGL1 levels are also enhanced. Although, a recent report suggests that FGL1 associates almost exclusively with the fibrin matrix, we report here that approximately 20% of the total plasma FGL1 remains free. The enhancement of FGL1 levels in vitro by IL-6 and its induction after turpentine oil injection suggest that it is an acute phase reactant. Its presence in bound and free forms in the blood also implies biological roles that extend beyond the proposed autocrine effect it has on hepatocytes during regeneration.
Keywords: fibrinogen-like-protein 1, fibrinogen, serum amyloid, liver regeneration, acute phase, interleukin 6, turpentine, inflammation
Introduction
Acute phase proteins (APPs) are plasma proteins secreted mainly by liver in response to altered homeostasis. This can be as a result of injury, infection or neoplastic growth [1]. It is believed that synthesis of APPs represent the most acute line of defense following injury, occurring prior to the synthesis of specific antibodies. Although, APP profiles vary among different species, an accepted definition is a greater than 25% rise in plasma concentration following stimuli [2]. The elaboration of APPs result in the decreased activity of tissue proteases through increased synthesis of proteinase inhibitors, reduction in hemorrhagic insult by increased activity of coagulation factors, enhanced removal of foreign materials by increased level of binding proteins, and suppression of inflammation by modulation of immunological response[3]. Although APP biological activities are mostly protective, they can also play roles in pathologic conditions. For example, C-reactive protein (CRP), serum amyloid protein A (SAA) and hepcidin, are implicated in disease states[4; 5].
IL-6 mediates a significant fraction of the early phase response during liver regeneration, accounting for more than 30% of transcriptional induction [6]. The induction of acute phase proteins by IL-6 is primarily through an exocrine mechanism [7; 8; 9] suggesting that systemic disturbances at sites distant from the liver can stimulate responses similar to those that occur locally after liver injury.
Fibrinogen-like-protein 1 (FGL1, also called FREP1 or hepassocin) is a hepatocyte secreted protein which was initially cloned from and found to be over-expressed in human hepatocellular carcinoma [10]. It contains a fibrinogen related domain in its C-terminal portion [11; 12] similar to tenascins, fibroleukin, angiopoietins and fibrinogen β and γ chains. It however lacks fibrinogen’s three functional domains: the platelet binding-site, the cross-linking region and the thrombin-sensitive site. Because FGL1 is up-regulated in the regenerating liver and stimulates 3H-thymidine uptake in primary hepatocytes it has been suggested that it promotes hepatocyte proliferation [12] and as a result it has been deemed a liver regeneration factor. Paradoxically, a second study has shown that FGL1 has growth suppressive effects on hepatocellular carcinoma cells suggesting an anti-proliferative role in liver proliferation. Thus the true action of FGL1 on liver cell proliferation remains unclear.
Here, we show that FGL1 is induced by recombinant human IL-6 (rhIL-6) in Hep G2 cells in a dose dependent manner. We show that the pattern and magnitude of FGL1 induction in Hep G2 cells is similar to that of the alpha chain of fibrinogen, an acute phase reactant. Induction of acute inflammation in rats through turpentine oil injection also enhances FGL1 expression to levels that are typical for acute phase response proteins. Therefore, induction of FGL1 synthesis occurs not exclusively as a result of liver injury but also in states of enhanced IL-6 expression. Like many other acute phase reactants we believe that it may serve as a diagnostic or prognostic biological marker in certain inflammatory conditions.
Materials and Methods
Material
Human hepatocellular carcinoma cell line, Hep G2 and MEM medium were purchased from ATCC, Manassas, VA. rhIL-6 was purchased from Biomyx technology (San Diego, CA). Dexamethasone and turpentine were from Sigma-Aldrich (St. Louis, MO). Mouse anti human FGL1 monoclonal antibody, goat anti human FGL1 polyclonal antibody, purified rat FGL1 and FGL1 ELISA/assay kit were from R&D systems (Minneapolis, MN). All remaining reagents unless specified were from Invitrogen (Carlsbad, CA).
Cell Culture, immunoblots and polymerase chain reactions
Hep G2 cells were grown under standard conditions [13]. 5 × 104 cells per well of Hep G2 cells were plated on 24-well plates and grown to near confluency. The growth medium was replaced by serum free media and varying concentrations of hrIL-6 was added with or without 1 μM dexamethasone. Cells were cultured for 1–3 days in 1.5 ml medium per well. At the end of incubation, 20 μl from each sample group was electrophoresed on SDS PAGE, and immunoblotted as previously described [14]. cDNA was synthesized from total cellular RNA using the SuperScript II reverse transcriptase according to the manufacturer’s guidelines. Q-PCR analysis was performed using LightCycler (Roche Applied Science) according to manufacture’s manual. Primers used for Q-PCR are shown in Table S1.
In vivo studies
Animal experiments were performed according to institutional guidelines of Harvard Medical School. Male Sprague Dawley rats (200 – 400 gm body weight) were purchased from Charles River Laboratories (Wilmington MA). Rats were fed a normal diet with free access to food and water. For experimental induction of localized acute inflammation, rats were injected subcutaneously with 100 μl of steam distilled turpentine or with sterile phosphate buffered saline (PBS), at two different sites in the lumbar area. Blood samples were taken from the tail vein prior to and at time points after injection (24 hours, 48 hours, 72 hours and 96 hours). For plasma collection, 5 μl of 0.34 M of K3EDTA was added to 25 μl of whole blood. For serum collection the same amount of K3EDTA was added 1 hour after the blood was collected. Plasma and serum FGL1 levels were determined by ELISA kit according to standard ELISA protocol with purified rat FGL1 as a standard.
Results
IL-6 induces expression of FGL1 in Hep G2 cells
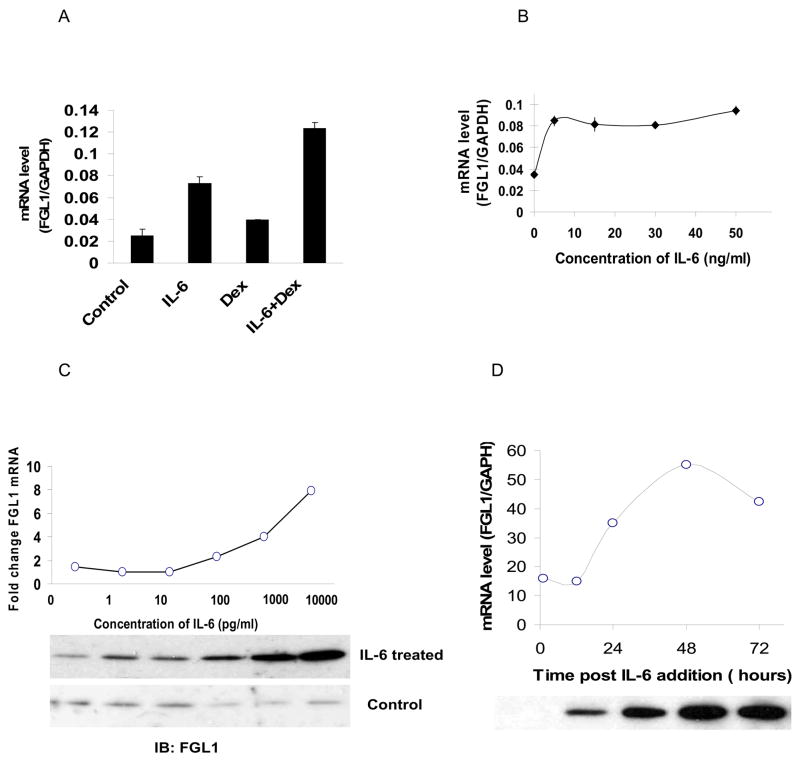
Because FGL1 is induced during liver regeneration [15], we evaluated whether IL-6 enhances its expression. When Hep G2 cells were treated with 50 ng/ml of rhIL-6, we noted a greater than two fold induction of FGL1 mRNA within 24 hours compared to cells treated with bovine serum albumin (BSA) (Figure 1A compare bar 2 to 1). Treatment with 1μM of dexamethasone resulted in a smaller but reproducible increase (Figure 1A, compare bar 3 to 1) while the addition of both rhIL-6 and dexamethasone led to a greater than four fold increase in FGL1 mRNA (Figure 1, compare bar 4 to 1). The augmentation of the effect of IL-6 by dexamethasone is expected in these in vitro assays because dexamethasone stimulates the surface delivery of the hepatocyte IL-6 receptor [16; 17]. These results show that IL-6 enhances FGL1 expression in Hep G2 cells. We next determined the effective concentration range of IL-6 for induction of FGL1 expression. Figure 1B shows that in the presence of 1 μM dexamethasone, maximal induction of FGL1 mRNA expression is achieved at 5 ng/ml which is the lowest concentration tested. We then examined the effect of IL-6 induction of FGL1 mRNA at a dose range that has been reported in various mammalian conditions (0 pg/ml to 10, 000 pg/ml). For comparison we determined the effect of similar concentrations of BSA on cells in parallel experiments. Figure 1C (graph), shows a representative experiment of the fold induction of FGL1 mRNA in IL-6 treated cells in comparison to BSA treated cells. FGL1 mRNA begins to increase around 0.1 ng/ml but is maximal at 10 ng/ml of IL-6. Not surprisingly, the enhancement of FGL1 transcription leads to increased protein secretion into the culture media as shown in Western blots of the culture media (Figure 1C, upper panel of autoradiograph). Cells treated with equivalent concentration of BSA show a basal level of FGL1 (Figure 1C, bottom panel of autoradiograph). We then used rhIL-6 at 10 ng/ml to determine the time dependent induction of FGL1 in Hep G2 cells. Figure 1D, shows a representative experiment with both mRNA and protein expression levels as a function of time. mRNA level of FGL1 peaked at 48 hours before beginning to decline (graph in figure 1D). Corresponding Western blots also show a time dependent increase in FGL1 protein accumulation in the supernatant (Figure 1D, autoradiograph). The induction of FGL1 expression by IL-6 and the effect of dexamathasone are characteristic of APPs and suggested to us that FGL1 may be an acute phase reactant.
Figure 1.
IL-6 induces expression of FGL1 in Hep G2 cells: A) Relative mRNA level of FGL1 in Hep G2 cells treated with 50 ng/ml BSA (control), 50 ng/ml of rhIL-6 (IL-6), 1 μM dexamethasone (Dex) and rhIL-6 in the presence of 1 μM dexamethasone for 48 hours. IL-6 treatment results in a 3 fold increase in FGL1 message (compare bar 2 to 1). Dexamethasone alone results in small but reproducible increase in FGL1 levels (bar 3) but augments the effects of IL-6 (compare bar 4 to 2). mRNA levels were normalized to GAPDH. B) Concentration dependence of IL-6 effect on Hep G2 cells. Dose response of Hep G2 cells treated with 0 ng/ml, 5 ng/ml, 15 ng/ml, 30 ng/ml and 50 ng/ml of IL-6 shows that concentration of 5ng/ml result in maximal induction of FGL1 mRNA. Samples were normalized to GAPDH. C) Representative dose response of Hep G2 cells treated with IL-6 at concentrations of 1 to 104 pg/ml. Cells treated with equivalent amounts of BSA were used as control. Fold change in mRNA was determined by dividing the relative level of FGL1 mRNA in IL-6 treated cells by that of FGL1 mRNA in BSA treated cells. FGL1 levels begin to rise 100 pg/ml (graph) and are more profound as expected at 10 ng/ml. β-actin mRNA level was used for normalization. (Bottom, panel) Western blots of cell culture supernatant shows the presence of accumulation of FGL1 protein with increasing concentrations of IL-6 (top). There is no effect of BSA addition on FGl1 protein level (bottom). D) Time dependent effect of IL-6 on FGL1 levels. Representative experiment shows the quantitation of FGL1 mRNA and protein levels following treatment with 10 ng/ml of IL-6. mRNA induction begins at 12 hours and peaks 48 hours after treatment (graph), FGl1 protein accumulates in the culture media as a function of time (bottom, gel). mRNA levels were normalized to GAPDH.
FGL1 is an acute phase reactant
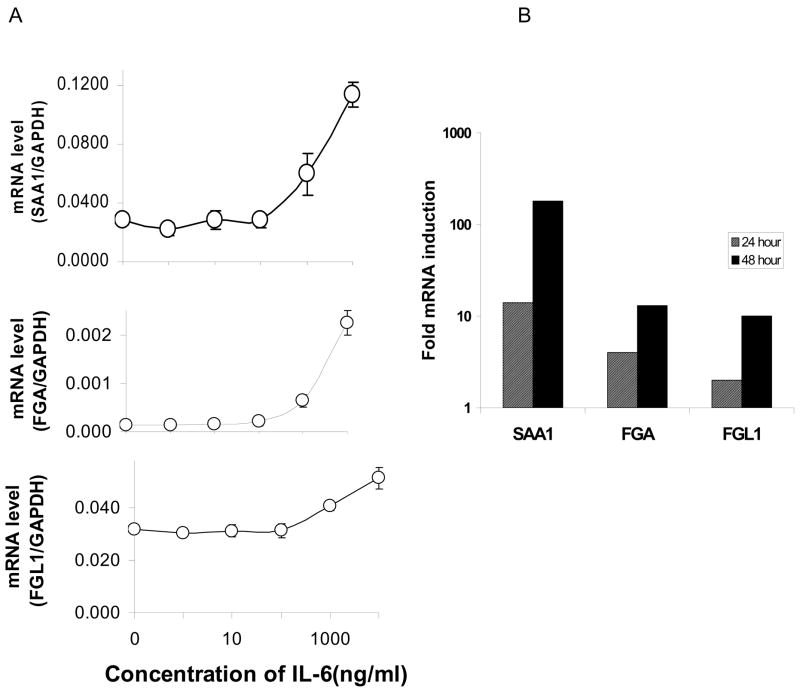
Like FGL1, many acute phase reactants are liver derived secretory proteins whose expressions are regulated by IL-6. We therefore wondered whether FGL1 is an acute phase protein. We evaluated the expression pattern of FGL1 in comparison with those of two well characterized acute phase response proteins, serum amyloid protein A1 (SAA1) and fibrinogen alpha (FGA) in IL-6 treated Hep G2 cells. Figure 2A shows the effect of varying concentrations of IL-6 on SAA1 (top), FGA (middle) and FGL1 (bottom) on cells harvested 24 hours after addition of the cytokine. Enhancement of expression in all cases begins at concentrations greater than 0.1 ng/ml for all three factors. Although the temporal pattern of expression is similar, the magnitude of enhancement of SAA1 is more profound than those of FGL1 and FGA. Figure 2B shows mRNA induction at 10 ng/ml of IL-6 at 24 and 48 hours for SAA1, FGA and FGL1. FGA and FGL1 are elevated approximately 10 fold versus that of SAA1 of over 100 fold at 48 hours. These results suggest that FGL1 is an acute phase reactant given a similar pattern of induction to known APPs (SAA1 and FGA) and the similarity in the magnitude of induction (FGA).
Figure 2.
FGL1 is an acute phase reactant. A) mRNA levels of SAA1 (top), FGA (middle) and FGL1 (bottom), 24 hours after treatment with varying doses of IL-6. The pattern of induction is similar for all three genes, with increase in mRNA notable beginning at 100 ng/ml. B) Fold inductions of all three genes at 24 and 48 hours shows that SAA1 levels are at least an order of magnitude higher than those of FGA and FGL1. At 48 hours SAA1 is induced 180 fold compared with 14 fold for FGA and 10 fold for FGL1. mRNA levels were normalized to GAPDH.
FGL1 levels are enhanced in an experimental model of acute inflammation
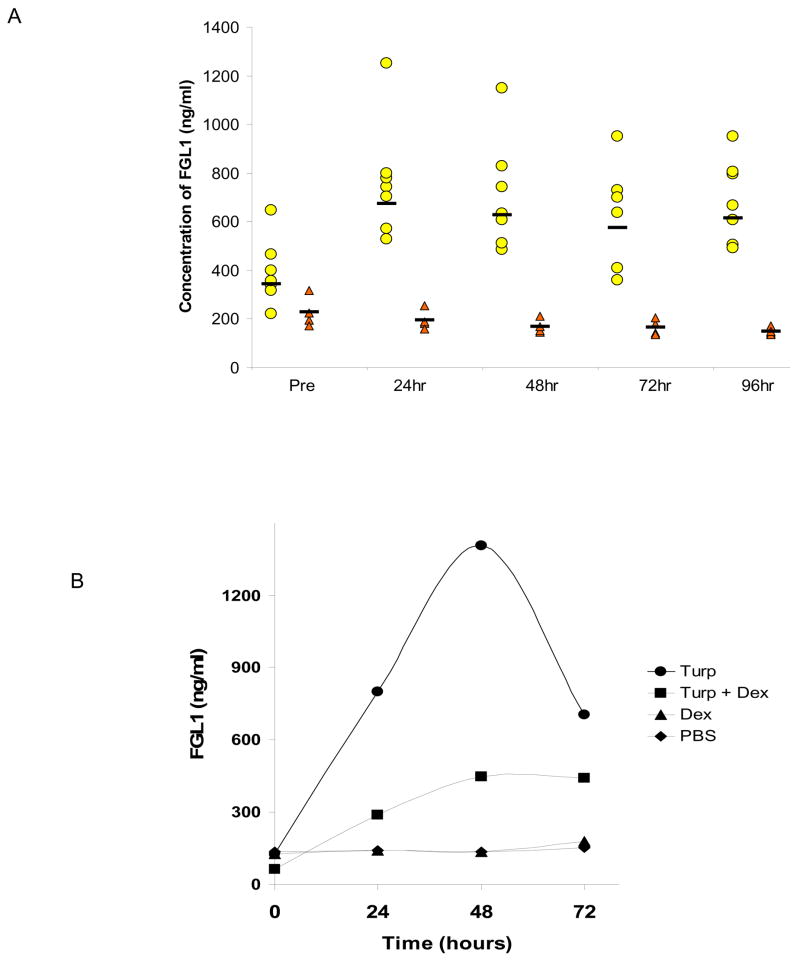
The experiments in figures 1 and 2 clearly suggest that IL-6 induces FGL1 expression in a manner similar to that of known acute phase reactants. If liver injury was the only stimulant for the synthesis of FGL1 in vivo, then extrahepatic induction of inflammation would have no effect on FGL1 levels. We experimentally induced localized acute inflammation through the subcutaneous injection of turpentine oil in rats [7; 8]. We assayed serum levels of FGL1 at various time points using a sandwich ELISA assay. We used purified rat FGL1 protein as a positive control to determine serum concentrations. Figure 3A shows that subcutaneous injection of turpentine oil, a known enhancer of IL-6 levels results in elevated serum levels of FGL1 when compared to serum from control injected rats. Although there were variations among the animals in their response to turpentine, dexamethasone or PBS injections, the pattern of response is illustrated in the representative experiment in Figure 3B. Injection of PBS and dexamethasone did not enhance serum FGL1 levels. In contrast to the situation in vitro where the permissive effect of dexamethasone results in an enhanced stimulation of IL-6 transcription, in vivo, dexamethasone actually dampened the effect of turpentine in enhancing FGL1 levels (Figure 3B). This inhibitory effect of dexamethasone on FGL1 release was expected because dexamethasone suppresses of IL-6 biosynthesis [18; 19]. The injection of turpentine leads to a time dependent increase in FGL1 protein concentration (Figure 3B). These experiments demonstrate that in vivo extra-hepatic induction of inflammation induces FGL1 synthesis and release from hepatocytes.
Figure 3.
Subcutaneous Turpentine oil injection results in enhancement of serum levels of FGL1 in rats.
ELISA assays of FGL1 serum levels at various time points following the subcutaneous injection of turpentine oil (circles) or PBS (triangles). Stimulation of inflammation at an extrahepatic site results in enhanced levels of FGL1. Horizontal bars represent the mean B) Representative experiment on the effect of dexamethasone on turpentine induced FGL1 expression. Injection of dexamethasone dampens effect of turpentine. Compare squares to circles.
A fraction of FGL1 remains free in the serum after clot formation
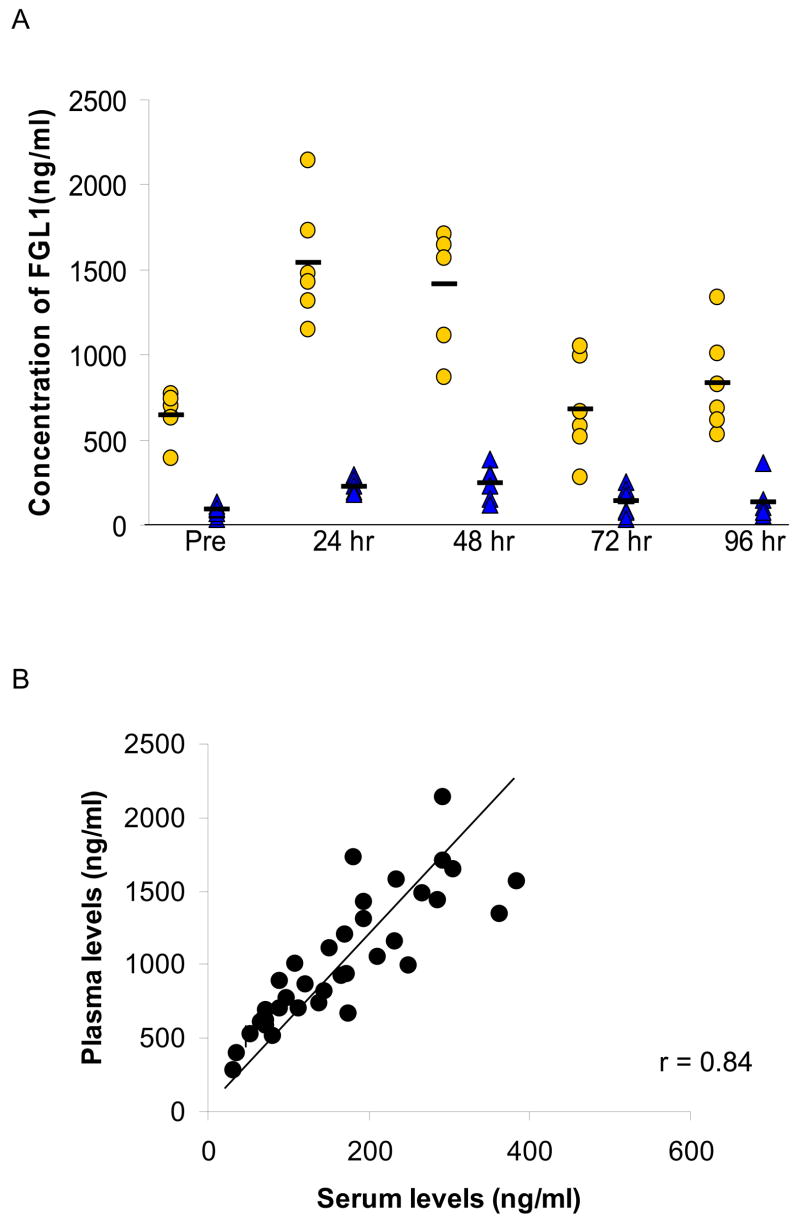
It was recently reported that FGL1 is present in the fibrin matrix of a plasma clot and under this condition, most of the FGL1 was contained in this fraction not in the serum [20]. To assess what proportion of the total FGL1 remained in the serum after coagulation, we generated concurrent plasma and serum samples at time points prior to and after the injection of turpentine oil in six rats. Figure 4A shows the levels of FGL1 in plasma and serum. Approximately 20% of the FGL1 remained unassociated with the fibrin clot at all time points (Figure 4A). Plasma levels were linearly correlated (R=0.84) with serum levels of FGL1 (Figure 4B). At the mid point of the line, the ratio of serum to plasma FGL1 is 0.2 indicating that around 20% of the FGL1 was free. Although the function of FGL1 either within the organized clot or in its free state remains unclear, these data suggest that in addition to its role in liver regeneration, FGL1 may play a role in modulating coagulation and it is available for regulating cellular functions at extrahepatic sites.
Figure 4.
FGL1 is present in the plasma and serum. A) Detection of FGL1 levels in the plasma (circles) and serum (triangles) show that approximately 20% of FGL1 remains in the serum after blood coagulation. The horizontal bars represent the mean. A plot of plasma FGL1 against serum FGL1 shows a near linear correlation with a co-efficient of 0.84. Approximately 20% of FGL1 is in the serum at all times.
Discussion
FGL1 is a hepatocyte derived protein that contains the fibrinogen related domain in its C-terminal portion. This domain is present in fibrinogen β and γ and also in a number of other proteins including angiopoietins, fibroleukin and tenascins. Despite extensive knowledge about the function of many of the members of this family of proteins, the literature concerning FGL1 remains sparse.
The exact role of FGL1 in liver cell physiology is controversial. The cDNA was first identified as a transcript over expressed in hepatocellular cancer [10]. However, a follow-up study provided supporting evidence for its role as a tumor suppressor down regulated in HCC [21]. Secondly, FGL1 has been implicated as a mitogenic factor in liver regeneration for two reasons: 1) It is induced during the early response phase of liver regeneration following partial hepatectomy and 2) Addition to primary hepatocytes results in the increased incorporation of 3H-thymidine into liver cells [12].
Whereas a liver specific role for FGL1 might well exist, we have demonstrated that the protein is induced by conditions that are unlikely to have effect on hepatoyte cell proliferation. We show that in cultured cells, IL-6 alone or in combination with dexamethasone enhances FGL1 expression in a manner that suggests that it is an acute phase response protein. This observation is not surprising given the pivotal role of IL-6 in the transcription of genes during the early phase of liver regeneration and its role in regulating the acute phase response. NF-IL6 and STAT-3 have been identified as downstream factors in IL-6 signal transduction pathways including those that mediate APP elaboration [22; 23]. An analysis of the 5′ flanking region of FGL1 in humans and rats shows conserved potential DNA binding sites for STAT3 and NF-IL6 (Liu and Ukomadu, unpublished). Thus it appears that the presence of IL-6 itself even in the absence of liver specific injury may be enough to induce expression of FGL1. This is strongly supported by the observation that the intra peritoneal injection of rhIL-6 (Liu and Ukomadu, not shown) result in the enhancement of serum levels of FGL1.
It has recently been reported that FGL1 is abundantly associated with the fibrin matrix after clot formation. These studies suggest that FGL1 may play a role at extra hepatic sites including the regulation fibrin polymerization and that FGL1 may interact with fibrinogen in the fibrin matrix [20]. We have confirmed that FGL1 is present in plasma of rats but we also note that a stable fraction of it remains free in the serum at all times. In fact the near linear correlation (Figure 4B) suggests that the free fraction is maintained at all times even during acute phase induction when the level of its presumed interactor fibrinogen is high. These observations suggest that unbound FGL1 may have other biologic roles distinct from that in liver regeneration and clot formation.
This works represents a step towards deciphering the biologic effects of FGL1. It is noteworthy that structurally related proteins (angiopoietins, tenascins, fibrinogen) have been implicated in multiple cellular processes including angiogenesis, proliferation, apoptosis and extracellular matrix modulation [24; 25; 26; 27] suggesting a potential role for FGL1 in these processes. Additionally, the presence of FGL1 in rat serum following cytokine stimulation suggests that it may serve as a biological marker for systemic inflammation.
Supplementary Material
01
Acknowledgments
This work was supported by R21DK073236 to CU. We thank Drs David Cohen and Jerry Trier for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushner I. The acute phase response: an overview. Methods Enzymol. 1988;163:373–83. doi: 10.1016/0076-6879(88)63037-0. [DOI] [PubMed] [Google Scholar]
- 3.Koj A. Biological functions of acute-phase proteins. In: Gordon AH, Koj A, editors. The acute phase response to injury and infection. Elsheiver Science; New York: 1985. pp. 145–160. [Google Scholar]
- 4.Gertz MA, Kyle RA. Secondary systemic amyloidosis: response and survival in 64 patients. Medicine (Baltimore) 1991;70:246–56. [PubMed] [Google Scholar]
- 5.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–3. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 6.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 7.Geiger T, Andus T, Klapproth J, Hirano T, Kishimoto T, Heinrich PC. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur J Immunol. 1988;18:717–21. doi: 10.1002/eji.1830180510. [DOI] [PubMed] [Google Scholar]
- 8.Marinkovic S, Jahreis GP, Wong GG, Baumann H. IL-6 modulates the synthesis of a specific set of acute phase plasma proteins in vivo. J Immunol. 1989;142:808–12. [PubMed] [Google Scholar]
- 9.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987;84:7251–5. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T, Gotoh M, Sasaki H, Terada M, Kitajima M, Hirohashi S. Molecular cloning and initial characterization of a novel fibrinogen-related gene, HFREP-1. Biochem Biophys Res Commun. 1993;193:681–7. doi: 10.1006/bbrc.1993.1678. [DOI] [PubMed] [Google Scholar]
- 11.Hara H, Uchida S, Yoshimura H, Aoki M, Toyoda Y, Sakai Y, Morimoto S, Fukamachi H, Shiokawa K, Hanada K. Isolation and characterization of a novel liver-specific gene, hepassocin, upregulated during liver regeneration. Biochim Biophys Acta. 2000;1492:31–44. doi: 10.1016/s0167-4781(00)00056-7. [DOI] [PubMed] [Google Scholar]
- 12.Hara H, Yoshimura H, Uchida S, Toyoda Y, Aoki M, Sakai Y, Morimoto S, Shiokawa K. Molecular cloning and functional expression analysis of a cDNA for human hepassocin, a liver-specific protein with hepatocyte mitogenic activity. Biochim Biophys Acta. 2001;1520:45–53. doi: 10.1016/s0167-4781(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 13.Hill DB, Schmidt J, Shedlofsky SI, Cohen DA, McClain CJ. In vitro tumor necrosis factor cytotoxicity in Hep G2 liver cells. Hepatology. 1995;21:1114–9. [PubMed] [Google Scholar]
- 14.Ukomadu C, Dutta A. p21-dependent inhibition of colon cancer cell growth by mevastatin is independent of inhibition of G1 cyclin-dependent kinases. J Biol Chem. 2003;278:43586–94. doi: 10.1074/jbc.M307194200. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Ying H, Gu F, He J, Li YL, Liu HM, Xu YH. Cloning and characterization of a mouse liver-specific gene mfrep-1, up-regulated in liver regeneration. Cell Res. 2002;12:353–61. doi: 10.1038/sj.cr.7290137. [DOI] [PubMed] [Google Scholar]
- 16.Snyers L, Fontaine V, Content J. Modulation of interleukin-6 receptors in human cells. Ann N Y Acad Sci. 1989;557:388–93. 394–5. doi: 10.1111/j.1749-6632.1989.tb24031.x. [DOI] [PubMed] [Google Scholar]
- 17.Fischer CP, Bode BP, Takahashi K, Tanabe KK, Souba WW. Glucocorticoid-dependent induction of interleukin-6 receptor expression in human hepatocytes facilitates interleukin-6 stimulation of amino acid transport. Ann Surg. 1996;223:610–8. 618–9. doi: 10.1097/00000658-199605000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helfgott DC, May LT, Sthoeger Z, Tamm I, Sehgal PB. Bacterial lipopolysaccharide (endotoxin) enhances expression and secretion of beta 2 interferon by human fibroblasts. J Exp Med. 1987;166:1300–9. doi: 10.1084/jem.166.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohase M, Henriksen-Destefano D, Sehgal PB, Vilcek J. Dexamethasone inhibits feedback regulation of the mitogenic activity of tumor necrosis factor, interleukin-1, and epidermal growth factor in human fibroblasts. J Cell Physiol. 1987;132:271–8. doi: 10.1002/jcp.1041320211. [DOI] [PubMed] [Google Scholar]
- 20.Rijken DC, Dirkx SP, Luider TM, Leebeek FW. Hepatocyte-derived fibrinogen-related protein-1 is associated with the fibrin matrix of a plasma clot. Biochem Biophys Res Commun. 2006;350:191–4. doi: 10.1016/j.bbrc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Yan J, Yu Y, Wang N, Chang Y, Ying H, Liu W, He J, Li S, Jiang W, Li Y, Liu H, Wang H, Xu Y. LFIRE-1/HFREP-1, a liver-specific gene, is frequently downregulated and has growth suppressor activity in hepatocellular carcinoma. Oncogene. 2004;23:1939–49. doi: 10.1038/sj.onc.1207306. [DOI] [PubMed] [Google Scholar]
- 22.Wegenka UM, Buschmann J, Lutticken C, Heinrich PC, Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993;13:276–88. doi: 10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 24.Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346 Pt 3:603–10. [PMC free article] [PubMed] [Google Scholar]
- 25.Procopio WN, Pelavin PI, Lee WM, Yeilding NM. Angiopoietin-1 and -2 coiled coil domains mediate distinct homo-oligomerization patterns, but fibrinogen-like domains mediate ligand activity. J Biol Chem. 1999;274:30196–201. doi: 10.1074/jbc.274.42.30196. [DOI] [PubMed] [Google Scholar]
- 26.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–8. [PubMed] [Google Scholar]
- 27.El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol. 2007;211:86–94. doi: 10.1002/path.2099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01