DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status (original) (raw)
Abstract
A complex combination of adult health-related disorders can originate from developmental events that occur in utero. The periconceptional period may also be programmable. We report on the effects of restricting the supply of specific B vitamins (i.e., B12 and folate) and methionine, within normal physiological ranges, from the periconceptional diet of mature female sheep. We hypothesized this would lead to epigenetic modifications to DNA methylation in the preovulatory oocyte and/or preimplantation embryo, with long-term health implications for offspring. DNA methylation is a key epigenetic contributor to maintenance of gene silencing that relies on a dietary supply of methyl groups. We observed no effects on pregnancy establishment or birth weight, but this modest early dietary intervention led to adult offspring that were both heavier and fatter, elicited altered immune responses to antigenic challenge, were insulin-resistant, and had elevated blood pressure–effects that were most obvious in males. The altered methylation status of 4% of 1,400 CpG islands examined by restriction landmark genome scanning in the fetal liver revealed compelling evidence of a widespread epigenetic mechanism associated with this nutritionally programmed effect. Intriguingly, more than half of the affected loci were specific to males. The data provide the first evidence that clinically relevant reductions in specific dietary inputs to the methionine/folate cycles during the periconceptional period can lead to widespread epigenetic alterations to DNA methylation in offspring, and modify adult health-related phenotypes.
Evidence from both epidemiological studies in humans and direct intervention studies in animals indicates that altering key developmental processes in utero can predispose offspring to many late-onset diseases such as dyslipidemia, type II diabetes, and heart disease (1, 2). In this regard, the effects of gross nutrient or protein deficiencies in maternal diet during pregnancy are well documented (3), although little is known about the effects of specific nutrients or the timing and mechanistic basis of nutrient programming (4). Here we investigated the effects of restricting the supply of specific B group vitamins (i.e., vitamin B12 and folate) and sulfur amino acids (in particular, methionine) from the diet of adult female sheep from 8 weeks preceding until 6 days after conception, within physiological ranges encountered in both sheep (5) and humans (i.e., within the 5th and 95th percentiles) (6, 7). These micronutrients are important intermediates and/or have specific regulatory functions in the linked methionine–folate cycles (5, 7). In rodents, maternal supraphysiological methyl group supply and a low-protein diet (50% control) offered throughout pregnancy altered DNA methylation of candidate genes (agouti, glucocorticoid receptor, and peroxisomal proliferator-activated receptor-α) (8, 9), but the extent of methylation change in these or more clinically relevant diets is not known. Gametes and preimplantation embryos undergo significant DNA methylation reprogramming (10), leading us to test the extent to which periconceptional availability of methyl groups altered DNA methylation of gene-associated CpG islands and affected adult health status. We elected to test this hypothesis in sheep because it is a large outbred species, whose pre- and postnatal development and physiology approximates that of humans (2). Sheep also lend themselves to the establishment of singleton pregnancies, obviating complications of data interpretation associated with large litters (11). Dietary manipulation of methionine–folate metabolism has been extensively studied in this species (12), assisting diet formulation that, in all other respects, matched their nutrient requirements (13).
Results
Embryo-Donor Metabolism.
Embryo-donor ewes (n = 50) were randomly allocated to either a control or “methyl-deficient” (MD) diet. The MD diet reduced peripheral concentrations of vitamin B12 (P < 0.001), folate (P < 0.01), and methionine (P < 0.05) in the weeks leading up to and around the time of conception (see information for plasma in Table 1). Homocysteine concentrations in ovarian follicular fluid precisely matched those of plasma, and both were greater (P < 0.001) in ewes offered the MD than those offered the control diet (see information for plasma in Table 1). Similarly, the concentration of homocysteine was greater (P < 0.05) in granulosa cell lysates from animals offered the MD than those offered the control diet (see information for granulosa cells in Table 1). The incorporation of [35S]methionine into _S_-adenosyl methionine (SAM) pools and the ratio of SAM to _S_-adenosyl homocysteine (SAH) were both reduced (P < 0.05) in granulosa cells from MD relative to control animals (see information for granulosa cells in Table 1). Animals exhibited no signs of clinical deficiency such as loss of appetite and/or weight loss [supporting information (SI) Table 3].
Table 1.
Effects of dietary treatments on key methyl cycle metabolite concentrations (mean ± SEM) in the peripheral circulation of embryo-donating ewes and ovarian granulosa cells
| Metabolite | Control | MD | Significance |
|---|---|---|---|
| Plasma | |||
| Vitamin B12, pM | 1,000.5 ± 72.7 | 198.2 ± 68.2 | <0.001 |
| Folate, nM | 6.90 ± 0.72 | 4.42 ± 0.43 | <0.01 |
| Methionine, μM | 39.1 ± 3.0 | 30.8 ± 1.7 | <0.05 |
| Homocysteine, μM | 9.6 ± 0.8 | 19.3 ± 1.7 | <0.001 |
| Granulosa cells | |||
| Homocysteine, μM | 0.54 ± 0.09 | 1.10 ± 0.20 | <0.05 |
| SAM, pmol per 106 cells per 2 h | 99.4 ± 16.3 | 53.0 ± 5.0 | <0.05 |
| SAM:SAH ratio | 14.6 ± 1.4 | 10.7 ± 0.7 | <0.05 |
Pregnancy Outcome.
Day-6 blastocysts recovered after artificial insemination were transferred singly to 203 normally fed surrogate ewes. Pregnancy rates averaged 55.7%, were within the range encountered for single embryo transfers for this genotype (14), and did not differ between treatments. Of a cohort of 41 term singleton pregnancies (gestation = 146 d), 37 survived to adulthood. The four animals that died were MD males. One animal was euthanized humanely at ≈1 year of age after a self-inflicted injury. The other three died at birth, two as a result of obstetrical complications, and the remaining animal was delivered naturally but died shortly thereafter; the cause of death is unknown. Birth weights were typical for the genotype in question (14). They averaged 6.57 and 5.54 kg for male and female offspring respectively [standard error of mean difference (SED) = 0.36; P < 0.001], but did not differ between treatments. Growth rates to weaning (at 3 months) were greater for MD than control offspring (338 vs. 295 g/d; SED = 17.1; P < 0.05). This treatment-specific postnatal difference in growth persisted in animals at 22 months of age, resulting in heavier MD offspring (85.9 vs. 80.4 kg; SED = 2.29, P < 0.01).
Immune Function in Offspring.
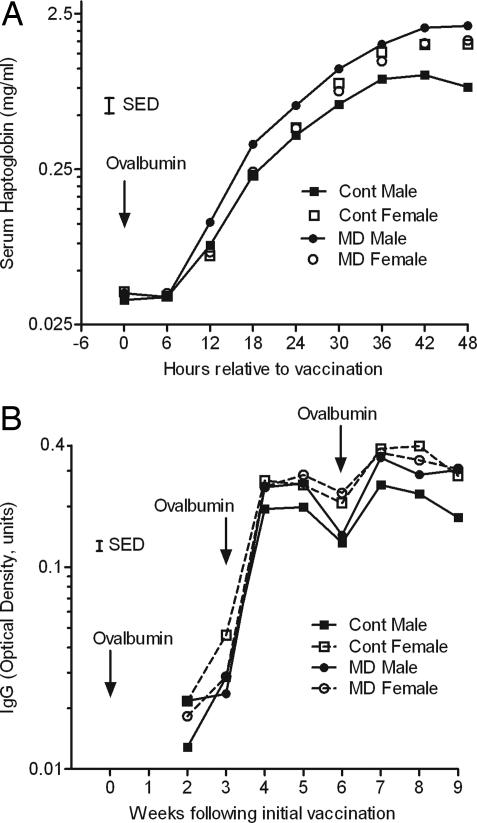
Both the innate and acquired immune responses to purified ovalbumin were assessed at 12 months. The acute-phase (serum haptoglobin) response to a single i.m. bolus of ovalbumin in Quil A adjuvant, determined at 1 year of age, was greater (P < 0.05) in female than in male offspring and led to a significant (P < 0.001) gender x treatment interaction (Fig. 1A). Although female offspring showed no effect of maternal (i.e., embryo donor) diet, the serum haptoglobin response to ovalbumin during the initial 48 h increased to a greater extent in MD than in control males. A similar gender effect (P < 0.05) was observed for serum IgG measured at weekly intervals for 9 weeks. However, serum IgG did not differ significantly between treatments (P = 0.064; Fig. 1B).
Fig. 1.
Innate and adaptive immune responses to ovalbumin in Quil A adjuvant determined in offspring, derived from the embryos of ewes offered either a control or MD diet, at 1 year of age. (A) Acute-phase (serum haptoglobin) response measured over a 48-h period after the initial vaccination. (B) Serum IgG measured at weekly intervals over 9 weeks, during which time additional boluses of ovalbumin were administered at weeks 3 and 6. Error bars are SED.
Body Composition and Glucose Metabolism.
By 12 months of age, female offspring were proportionately fatter (determined by computed tomography) than males (0.23 vs. 0.19; SED = 0.010; P < 0.001), but there were no differences in body fatness between treatment groups. By 22 months, all animals were fatter, and females were once again proportionately fatter than males (0.38 vs. 0.27; SED = 0.90; P < 0.001). Female body composition at 22 months was unaffected by experimental treatment. In contrast, MD males were proportionately fatter than control males (0.290 vs. 0.261; SED = 0.011; P < 0.05) and had proportionately less muscle mass (0.539 vs. 0.570; SED = 0.010; P < 0.05). For full details, see SI Table 4. To detect early signs of dysregulation of glucose–insulin homeostasis, offspring were challenged with an i.v. glucose infusion. Female offspring were less tolerant to infused glucose than males (see information for plasma glucose in Table 2), an effect negated when data were adjusted for body fatness. However, there was a significant treatment effect on insulin response to infused glucose (see information for plasma insulin in Table 2) that persisted when the data were adjusted for body fat. Once again, MD male offspring elicited the greatest response, exhibiting clear signs of insulin resistance that were independent of differences in adiposity.
Table 2.
Glucose–insulin homeostasis in offspring, derived from the embryos of ewes offered either a control or MD diet at 22 months of age
| Parameter | Male | Female | Significance | ||||
|---|---|---|---|---|---|---|---|
| Control | MD | Control | MD | G | D | G × D | |
| Plasma glucose | |||||||
| Peak, mM | 19.2 ± 0.6 | 19.4 ± 0.5 | 20.8 ± 0.4 | 22.2 ± 0.4 | <0.001 | <0.1 | |
| k, percent per min | 1.74 ± 0.01 | 1.83 ± 0.12 | 1.57 ± 0.04 | 1.66 ± 0.09 | <0.001 | ||
| AUC, units | 509 ± 46 | 531 ± 42 | 631 ± 35 | 692 ± 36 | <0.001 | ||
| Plasma insulin | |||||||
| Peak, microunits/ml | 77.6 ± 6.6 | 117.1 ± 7.5 | 66.6 ± 5.9 | 72.1 ± 6.2 | <0.001 | 0.01 | |
| ΔMax, microunits/ml | 44.3 ± 4.3 | 82.9 ± 6.9 | 41.2 ± 5.2 | 44.8 ± 5.4 | <0.01 | <0.001 | <0.01 |
| AUC, units | 3,362 ± 497 | 6,057 ± 588 | 3,446 ± 459 | 4,316 ± 484 | <0.001 | <0.1 |
Cardiovascular Function.
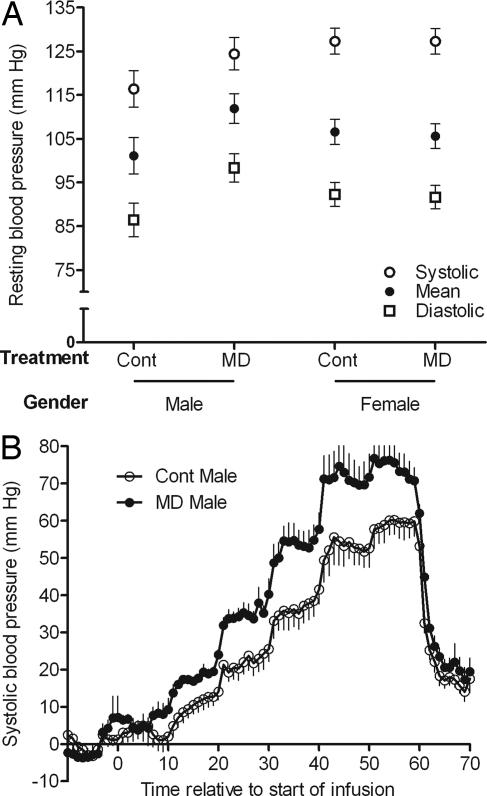
Cardiovascular function was assessed at 23 months of age. Resting heart rate was greater (P < 0.05) in females than in males (98.4 ± 3.8 vs. 85.1 ± 4.9 beats per min), but did not differ between treatments and were within the normal range for nonstressed sheep at this age. Body fat-adjusted resting blood pressure did not differ between the sexes, but there were significant treatment x gender interactions for diastolic (P < 0.01) and mean (P < 0.02) arterial blood pressure (Fig. 2A), indicating that, relative to their respective controls, blood pressure was increased by 11 mm of Hg in MD male, but not MD female, offspring. We next determined the effects of incremental angiotensin II infusion on cardiovascular function. Once again, relative to their respective controls, systolic (P = 0.02), diastolic (P < 0.01), and mean arterial (P = 0.02) pressor responses were greater in MD male (Fig. 2B), but not in MD female, offspring (values for the latter were similar to those for control males and are not shown).
Fig. 2.
Cardiovascular function in offspring, derived from the embryos of ewes offered either a control or MD diet, at 23 months of age. (A) Resting, body-fat-adjusted systolic, diastolic, and mean arterial blood pressure. (B) Change in systolic blood pressure during angiotensin II infusion. Values are minute means for a baseline period of 10 min (adjusted to zero), followed by 1 h of angiotensin II infusion (increments from 2.5 to 60 ng·kg−1·min−1 every 10 min) and 10 min of recovery. Error bars are SEM.
Epigenetic Modifications to DNA.
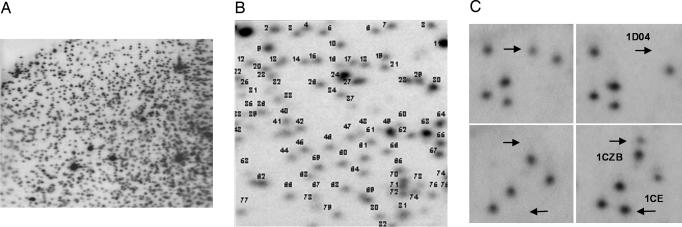
To investigate whether these altered clinical parameters were associated with dietary-induced epigenetic modifications to DNA, we looked for changes in the methylation status of 1,400 CpG sites (mostly gene promoter-associated CpG islands that are dispersed throughout the genome) by using restriction landmark genome scanning (RLGS). Unmethylated CpG sites were digested with the methylation-sensitive restriction enzyme, NotI, resulting in spots on an autoradiograph, whereas methylated (undigested) sites were absent from the same positions (Fig. 3). RLGS was chosen as a nonselective screen in the sheep (for which only limited genomic sequence information is available) to define the proportion of genes that could be affected by periconceptional maternal diet. However, the >1 μg of DNA required to perform RLGS precluded analysis of oocytes or preimplantation embryos, thus we examined fetal liver at gestational day 90.
Fig. 3.
RLGS profile of sheep liver at fetal day 90. (A) Spots present in the representative autoradiograph represent unmethylated CpG sites in the genome that are digested with the methylation-sensitive enzyme NotI. The first-dimension separation of RLGS fragments (NotI/EcoRV) extends horizontally, whereas the second dimension (NotI/HinfI) extends vertically in the profile. (B) Enlarged representation of quadrant 2B in the master profile, for which letters/numbers allocated to each RLGS fragment have been assigned. (C) Example methylation differences observed between control and MD individuals. RLGS fragment 1D04 represents a methylation event, with spots disappearing from the profile; fragments 1CZB and 1CE represent demethylation events, with spots appearing in the profile.
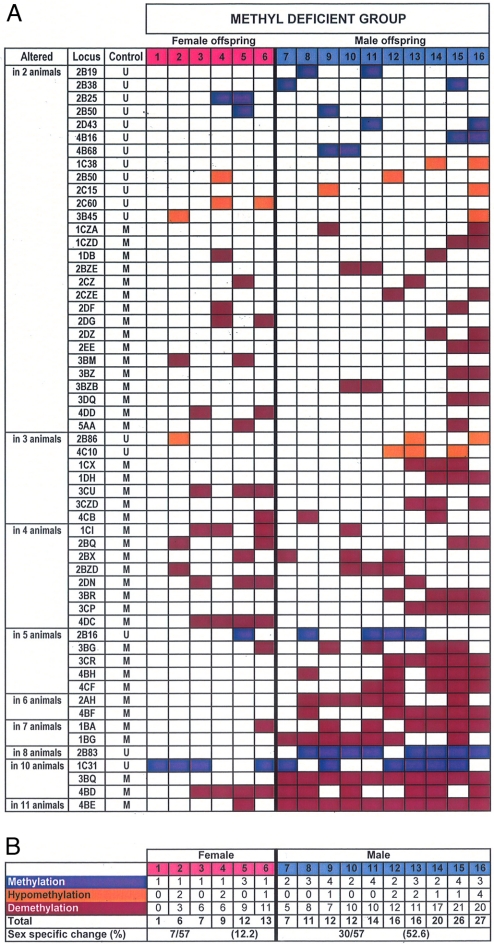
To address the possibility that some of the RLGS fragments were due to NotI genetic polymorphisms between the outbred individuals examined, and not to methylation differences, we excluded loci that varied among control autoradiographs from the analysis. Overall, 71 loci were identified that varied from the entire control group in only a single MD individual, likely representing stochastic changes (SI Fig. 5). However, a further 57 loci (representing 4% of the 1,400 CpG loci examined) were altered in two or more MD animals (Fig. 4), which is more (P < 0.001) than would be expected to occur if such events had been distributed randomly between control and MD animals. Of these 57 loci, 28 were affected in 2 MD animals, 7 in 3 animals, 8 in 4 animals, 5 in 5 animals, 2 in 6 animals, 2 in 7 animals, 1 in 8 animals, 3 in 10 animals, and 1 in 11 animals (Fig. 4). Furthermore, 88% of these loci were unmethylated or hypomethylated relative to controls. The remaining loci were hypermethylated. Intriguingly, 53% of altered loci were specific to males, whereas only 12% were specific to females (P < 0.01).
Fig. 4.
RLGS of DNA methylation differences between control and MD liver at fetal day 90. (A) The unique identifier codes (locus) of fragments that varied between each MD individual and all of the controls are shown. RLGS fragments that varied among control-group individuals were not scored to minimize scoring of genetic polymorphisms as DNA methylation differences. “Control” indicates whether each locus was methylated (M) or unmethylated (U) in the control group. Data representing the loci that differed from the control group in 2–11 MD individuals are presented in ascending order. Blue boxes indicate loci methylated in the indicated MD individuals and unmethylated in all controls. Purple boxes indicate MD loci unmethylated in MD individuals and methylated in controls. Orange boxes indicate MD loci hypomethylated in MD individuals. (B) Summary of methylation differences between the control group and the MD individuals.
Discussion
The findings from the current study are significant for the following reasons. They demonstrate in an animal model that clinically relevant reductions in the availability of vitamin B12, folate and the amino acid methionine from maternal diet around the time of conception lead to widespread epigenetic modifications to the genome associated with increased adiposity, insulin resistance, altered immune function, and high blood pressure in adult offspring. Importantly, these clinically relevant phenotypic effects were most evident in males. Moreover, over half of the affected loci were specific to males. Male-specific demethylation at these loci could provide a tantalizing explanation for the phenotypic sex differences observed in this study.
It is possible that our method of pregnancy establishment somehow “sensitized” the egg or preimplantation embryo to the nutritional treatments, although at present this remains pure conjecture. To test this hypothesis would require generating a group of naturally mated controls. This is less satisfactory than it might seem, for with naturally mated controls there would be a significant carryover effect of maternal nutrition from the periods of oocyte growth and preimplantation development to the later stages of gestation (4). Furthermore, with naturally mated controls, one would have little control on litter size and litter-size-related effects (2). Collectively, these issues have complicated the interpretation of data from many previous studies with rodents and large animal species (4). In contrast, our experimental approach (i.e., day-6 embryo recovery and transfer to surrogates) ensured that the timing of dietary treatments was limited specifically to the periods of oocyte growth and early postfertilization development when the mammalian genome is known to undergo significant DNA methylation reprogramming (10). Consequently, the findings are particularly relevant for nutritional advice offered to intending mothers, because the message to date has focused on the protective effects of folic acid around the time of conception against the development of neural tube defects (15). The current data indicate that there are subtle, long-term programming effects associated with modest reductions in B vitamin and methionine status around the time of conception. In this respect it is worth emphasizing that nutrient restriction was within physiological ranges encountered in both sheep and humans (5–7) and that animals exhibited no signs of clinical deficiency. Importantly, key components of the methionine cycle within the ovarian follicle were altered, including the ratio of SAM to SAH, which is associated with the extent of DNA methylation (16).
The timing of nutrient restriction may have contributed to the system-wide alterations to physiology and metabolism observed among offspring. However, it may be that there is a causal link between these clinically related phenotypes. For example, the haptoglobin gene is expressed in white adipose tissue and circulating haptoglobin is positively correlated to fat mass in humans (17). Serum haptoglobin responses to ovalbumin were greater in the fatter female than in male offspring. Furthermore, although these responses were established at a time (i.e., 12 months of age) when there were no measurable difference in body fat content between control and MD males, the heavier MD males went on to become fatter at 22 months of age. The quantity (kilograms) of body fat was estimated to be 25% greater in MD than in control males by 22 months. Obesity is a low-grade systemic inflammatory condition in which several inflammation-sensitive serum proteins, including haptoglobin, are elevated. In humans, these inflammation-related proteins are associated with the development of both type II diabetes and cardiovascular disease (18, 19).
The methylation status of 4% of 1,400 CpG islands examined by RLGS in the fetal liver was altered by maternal B vitamin and methionine status around the time of conception. Because ≈5% of RLGS fragments differ between profiles of different mouse tissues (20), 1% of CpG islands vary within human tissues as they age (21) and <3.4% of loci vary between primary tumors and their normal tissue counterparts (22), this represents a degree of difference expected to have marked biological significance. The vast majority (88%) of loci were unmethylated or hypomethylated relative to controls; the remaining loci were hypermethylated, and this is reminiscent of the unexplained scenario in tumors, in which some loci gain methylation amidst a background of mostly hypomethylated regions (23). A small number of loci were altered in a large proportion of MD offspring, suggesting that some changes occur at highly susceptible regions of the genome. The nonrandom susceptibility of some loci to environmental perturbation is similar to our recent observations that, in blastocyst-derived human embryonic stem cells, notably >80% of the loci altered in undifferentiated cells persisted after differentiation (24). Because we wish to sustain the experimental animals in the current study to determine their later-life disease phenotypes, we have not yet been able to determine whether the methylation changes observed in fetal liver persist into adulthood. However, because subtle early developmental changes in pluripotent or early embryonic progenitor cells could explain the diverse programming phenotypes observed, changes that persist into adulthood may not necessarily be the most functionally important.
Individual MD animals exhibited between 1–27 and 57 “susceptible” loci (Fig. 4) with a methylation status that varied from controls. This individual variation in the number of affected loci (and in clinical parameters; Table 2 and Figs. 1 and 2) may, in part, have arisen as a consequence of differing metabolic responses to diet between embryo donor ewes (Table 1). This in turn may have been due to variable maternal metabolite stores and/or nutrient–gene interactions resulting from polymorphic variances in key metabolic enzymes, such as 5,10-methylenetetrahydrofolate reductase (25). Although such variations confound strict interpretation of the experimental effects of maternal diet, they recapitulate the normal scenario in human populations.
In conclusion, this study demonstrates that clinically relevant reductions in specific dietary inputs to the methionine/folate cycles during the periconceptional period can affect DNA methylation of a significant proportion of the genome in offspring with long-term implications for adult health. These observations provide an important impetus for the identification of specific loci that contribute to adult disease, ultimately leading to the provision of nutritional advice to intending mothers for the improved long-term health of their children.
Materials and Methods
Animals and Experimental Diets.
All procedures were reviewed by the Animal Experiments Committee of the University of Nottingham and conducted in accordance with the requirements of the U.K. Home Office Animals (Scientific Procedures) Act of 1986. Mature (n = 310) Scottish Blackface ewes (5–6 years old) were purchased from a single source (University of Nottingham Farm) and randomly allocated as embryo donors (n = 50), embryo-transfer recipients (n = 220), or were reserved for metabolic analyses (n = 30). Peripheral metabolites were measured in all animals, but metabolite concentrations within the ovarian follicle were limited to this latter group of animals. Animals were offered a complete diet calculated to fully meet their energy and protein requirements (13). The two experimental diets were chemically identical, except for their content of elemental cobalt and sulfur, which were reduced in the MD diet relative to the control diet to diminish the capacity of rumen microorganisms to synthesize sulfur amino acids and vitamin B12, respectively (13) (SI Table 5). Embryo-donor ewes were randomly allocated to one of these two experimental diets from 8 weeks before until 6 days after conception.
Pregnancy Establishment.
Estrous synchronization, ovarian stimulation, and embryo recovery and transfer were fully described in ref. 14. Semen from a single Suffolk sire was used throughout so that all progeny were either full or half siblings. Morphologically similar embryos (day-6 blastocysts) were transferred singly to synchronized recipients, and pregnancies were confirmed at day 35 and again at day 70 by transabdominal ultrasonography. A cohort of pregnancies was terminated at day 90 for tissue-specific DNA methylation analysis (18 control and 16 MD). A further cohort of 41 pregnancies went to term, and 37 offspring (consisting 8 control males, 11 control females, 8 MD males, and 10 MD females) were subsequently reared to adulthood.
Metabolic Analyses.
Plasma vitamin B12 and folate were measured using a commercially available kit (Simultrac Radioassay Vitamin B12 [57Co]/Folate [125I] Kit; MP Biomedicals). Ovaries were recovered post mortem from donor ewes, and follicular fluid and granulosa cells were aspirated from all visible antral follicles. Aspirants were centrifuged at 500 × g for 10 min, and follicular fluid was stored at −80°C before analysis. Granulosa cell pellets were washed in PBS (Sigma–Aldrich) and either stored at −80°C, awaiting analysis, or transferred to 500-μl vials for immediate culture and subsequent SAM/SAH analysis. Thiols in plasma, follicular fluid, and granulosa cell lysates were analyzed using an Agilent 1100 HPLC system (Agilent Technologies), and amino acids in plasma were analyzed using an amino acid analyzer (Amersham Pharmacia) with ninhydrin detection, according to published in-house protocols (26). SAM and SAH in freshly recovered granulosa cells were labeled by incubation for 2 h with [35S]methionine (74 kBq/nmol). After washing, the cells were extracted in 0.4 M perchloric acid, and SAM and SAH were analyzed by HPLC. The radioactive peaks were identified by cold SAM and SAH standards, and the specific fractions were transferred to vials containing scintillation fluid and were counted.
Phenotypic Measurements in Offspring.
All offspring were offered a standard diet from weaning, which was balanced for all major and micronutrients (Clover Pellets; Harbro), with supplemental hay.
Body composition.
Body composition was determined at 11 and 22 months of age by computed tomography using a Siemens Esprit scanner with spiral scanning and 3D reconstruction according to our standard protocols (27). Briefly, a longitudinal topogram was taken to identify locations for the three sites along the body (caudal ischium, the fifth lumbar, and the eighth thoracic vertebrae) where cross-sectional tomograms were taken. Images obtained were analyzed using the Sheep Tomogram Analysis Routines software (STAR, version 0.6; SAC-BioSS). This software determined the total area of fat, muscle, and bone, with a measurement resolution of 2 mm from which body proportions were predicted (_r_2 > 0.96).
Immune function.
At 12 months, acute-phase and adaptive immune responses to purified ovalbumin, administered i.m. (100 μg per dose) on three occasions at 3-week intervals in 2 ml containing 5 mg of Quil A adjuvant plus PBS, were assessed. Serum haptoglobin was measured in samples, which were collected at 6-h intervals for 48 h after the initial vaccination, by colorimetric analysis (28) using a commercially available kit (Tridelta Development) on a Cobas Miras Plus autoanalyzer (ABX Diagnostics). Quantification of serum IgG antibodies to ovalbumin, from samples collected at weekly intervals, were performed by an ELISA in a similar manner to that described previously for monitoring IgG antibody responses in sheep (29).
Glucose metabolism.
Glucose tolerance and insulin resistance were determined using standard in-house protocols (30). Briefly, basal plasma insulin and glucose concentrations were established 4 h after the animals' morning meal. i.v. infusion of sterile glucose (0.4 g·kg−1 body weight offered as a single bolus) was followed by blood sample collection from indwelling jugular catheters at 5, 10, 20, 30 40, 60, 90, and 120 min. Plasma glucose concentrations were determined on a Cobas Miras Plus autoanalyzer (ABX Diagnostics); the kit for glucose was supplied by Randox Laboratories (catalog no. GL2623). Plasma insulin concentrations were determined by radioimunnoassay (30).
Cardiovascular function.
Throughout the entire experimental period, animals were kept in adjacent individual pens and were accustomed to regular handling and restraint in stalls. Carotid and jugular catheters were inserted into each animal under general anesthesia, and animals were allowed 2 days of postoperative recovery before the commencement of procedures. Resting and stimulated cardiophysiology were determined on the third day (31). After a period of stabilization (typically lasting 30 min), resting systolic, diastolic, and mean arterial blood pressure and heart rate were measured (at 1-sec intervals) during the course of a further 30 min by precalibrated pressure transducers (SensorNor 840, S 4925; Horten) linked to a data-acquisition system (Po-Ne-Mahphysiology platform, Model P3P; Linton Instruments). This procedure was followed by stepwise incremental i.v. infusion of angiotensin II (0, 2.5, 5, 10, 20, 40, and 60 ng·kg−1·min−1) administered every 10 min, followed by a 10-min recovery period.
Restriction Landmark Genome Scanning.
DNA was extracted from snap-frozen liver tissue (18 control and 16 MD fetuses) as described in refs. 21 and 23. RLGS was performed as described in ref. 23. Briefly, randomly sheared genomic DNA ends (1.5 μg) were blocked by incubation with DNA polymerase I (2 units; Roche) and a mixture of nucleotide analogues (1.6 mM αS-dGTP, 0.8 mM αS-dCTP, 1.6 μM ddATP, and 1.6 μMddTTP; Amersham Biosciences) for 20 min at 37°C. DNA digested with NotI (20 units; Promega) for 2 h at 37°C was end-labeled with 1.3 units Sequenase (USB), 1 μl of [α-32P]-dGTP (6,000 Ci/mmol), and 1 μl of [α-32P]-dCTP (3,000 Ci/mmol; Amersham Biosciences) for 30 min at 37°C. Labeled DNA was then digested with EcoRV (20 units; Promega) for 2 h at 37°C and then was electrophoresed through a 0.8% agarose tube gel at 230 V for 19 h. Agarose tube gels were then digested with HinfI (700 units; NEB) for 2 h at 37°C and were electrophoresed in a 5% nondenaturing polyacrylamide gel in the second dimension at 180 V overnight. Gels were then dried and exposed to x-ray films (Kodak X-Omat-AR) in the presence of intensifying screens (Quanta III; Dupont) for up to 14 days. A region of the autoradiograms that covered 1,400 NotI/EcoRV/HinfI DNA fragments was consistently analyzable on all profiles. An RLGS “master” profile (32) was produced from control animal 1672 as a reference autoradiogram against which all others were compared. The locations of fragments that were present in the profiles of other individuals but that was absent from the profile of animal 1672 were added to the master profile manually. All fragments were then given a unique identifier code based on a grid system, allocating a first-dimension gel (NotI/EcoRV) coordinate (from A to E) and a second dimension gel (NotI/HinfI) coordinate (from 1 to 5) and a fragment number to each spot (e.g., 1D04). Coordinates for RLGS fragments that were not present in the master profile from sample 1672 were indicated with a four-character code, representing the same first and second letter as the master profile and a new letter identifying the fragment (e.g., 1CZB). Changes in fragment intensity were designated as gain or loss of methylation when at least 30% intensity difference was observed. Duplicate RLGS gels were prepared for each liver sample with 100% concordance between replicate profiles.
Statistical Analysis.
Metabolite concentrations in granulosa cells and body fluids of embryo donors were analyzed by repeated measures ANOVA (33); vitamin B12 data were log transformed before analysis. Growth, body composition, and metabolic and physiological parameters in offspring were analyzed using a mixed linear restricted maximum likelihood (REML) algorithm in which experimental treatment by gender formed the fixed effects and embryo donor formed the random effect; that is, this model recognized the embryo donor as the experimental replicate. Where appropriate, data were adjusted for body fatness by introducing this term into the fixed model.
A χ2 test was used to compare the number of loci showing altered methylation in two or more MD-deficient fetuses (Fig. 4) to the value expected for randomly occurring events with the same mean proportion (total number altered loci per total possible number of altered loci). The number of abnormally methylated loci, for each fetus, as a proportion of the 57 loci showing altered methylation in two or more MD-deficient fetuses (Fig. 4) was further analyzed for gender differences by using a generalized linear mixed REML model (33), assuming binomial errors with a logit-link function. This model used the fixed and random effects described for the REML analysis above.
Supplementary Material
Supporting Information
Acknowledgments
We thank J. Milne (Britbreed, Midlothian, U.K.) for assistance in conducting embryo transfers; staff at the computer tomography unit (Scottish Agricultural College); animal care staff at the University of Nottingham; and Christoph Plass (Ohio State University, Columbus, OH) for providing training in RLGS. This work was supported by National Institute of Child Health and Human Development Cooperative Program on Female Health and Egg Quality Grant U01-HD044638. S.A.C. receives funding from the Scottish Executive Rural Directorate.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Barker DJ. Eur J Clin Invest. 1995;25:457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 2.McMillen IC, Robinson JS. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 3.Langley-Evans SC. Proc Nutr Soc. 2006;65:97–105. doi: 10.1079/pns2005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinclair KD, Singh R. Theriogenology. 2007;67:43–53. doi: 10.1016/j.theriogenology.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Underwood EJ, Suttle NF. The Mineral Nutrition of Livestock. 3rd Ed. Oxon, UK: CAB International; 1999. [Google Scholar]
- 6.Wright JD, Bialostosky K, Gunter EW, Carroll MD, Najiar MF, Bowman BA, Johnson CL. Vital Health Stat 11. 1998;243:1–78. [PubMed] [Google Scholar]
- 7.Midttum Ø, Hustad S, Schneede J, Vollset SE, Ueland PM. Am J Clin Nutr. 2007;86:131–138. doi: 10.1093/ajcn/86.1.131. [DOI] [PubMed] [Google Scholar]
- 8.Waterland RA, Jirtle RL. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lillycrop KA, Philips ES, Jackson AA, Hanson MA, Burdge GC. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 10.Morgan HD, Santos F, Green K, Dean W, Reik W. Hum Mol Gen. 2005;14:R47–R58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 11.Wainwright PE. J Nutr. 1998;128:661–663. doi: 10.1093/jn/128.3.661. [DOI] [PubMed] [Google Scholar]
- 12.Snoswell AM, Xue GP. Comp Biochem Physiol B. 1987;88:383–394. doi: 10.1016/0305-0491(87)90317-8. [DOI] [PubMed] [Google Scholar]
- 13.Agricultural Research Council. The Nutrient Requirements of Ruminant Livestock. Slough, UK: Commonwealth Agricultural Bureaux; 1980. [Google Scholar]
- 14.Sinclair KD, Dunne LD, Maxfield MK, Maltin CA, Young LE, Wilmut I, Robinson JJ, Broadbent PJ. Reprod Fertil Dev. 1998;10:263–269. doi: 10.1071/r98021. [DOI] [PubMed] [Google Scholar]
- 15.Pitkin RM. Am J Clin Nutr. 2007;85(Suppl):285–288. [Google Scholar]
- 16.Pogribny IP, Ross SA, Wise C, Pogribna M, Jones EA, Tryndyak VP, James SJ, Dragan YP, Poirier LA. Mut Res. 2006;593:80–87. doi: 10.1016/j.mrfmmm.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Chiellini C, Santini F, Marsili A, Berti P, Bertacca A, Pelosini C, Scartabelli G, Pardini E, López-Soriano J, Centoni R, et al. J Clin Endocrinol Metab. 2004;89:678–2683. doi: 10.1210/jc.2003-031965. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuji MI, Tracy RP, Heiss G. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 19.Engstrom G, Hedblad B, Stavenow L, Jonsson S, Lind P, Janzon L, Lindgarde F. Arterioscler Tromb Vasc Biol. 2004;24:1498–1502. doi: 10.1161/01.ATV.0000134293.31512.be. [DOI] [PubMed] [Google Scholar]
- 20.Song F, Smith JF, Kimura MT, Morrow AD, Matsuyama T, Nagase H, Held WA. Proc Natl Acad Sci USA. 2005;102:3336–3341. doi: 10.1073/pnas.0408436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tra J, Kondo T, Lu Q, Kuick R, Hanash S, Richardson B. Mech Age Dev. 2002;123:1487–1503. doi: 10.1016/s0047-6374(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 22.Smiraglia DJ, Plass C. Oncogene. 2002;21:5414–5426. doi: 10.1038/sj.onc.1205608. [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich M. Curr Top Microbiol Immunol. 2006;310:251–274. doi: 10.1007/3-540-31181-5_12. [DOI] [PubMed] [Google Scholar]
- 24.Allegrucci C, Wu Y-Z, Thurston A, Denning CN, Priddle H, Mummery CL, Ward-van Oostwaard D, Andrews PW, Stojkovic M, Smith N, et al. Hum Mol Genet. 2007;16:1253–1268. doi: 10.1093/hmg/ddm074. [DOI] [PubMed] [Google Scholar]
- 25.Fredriksen A, Meyer K, Ueland PM, Vollset SE, Grotmol T, Schneede J. Hum Mut. 2007;28:856–865. doi: 10.1002/humu.20522. [DOI] [PubMed] [Google Scholar]
- 26.Steele W, Allegrucci C, Singh R, Lucas E, Priddle H, Denning C, Sinclair K, Young L. Reprod Biomed Online. 2005;10:755–766. doi: 10.1016/s1472-6483(10)61120-0. [DOI] [PubMed] [Google Scholar]
- 27.Glasby CA, Young MJ. Appl Stat. 2002;51:209–221. [Google Scholar]
- 28.Skinner JG, Roberts L. Vet Rec. 1994;134:33–36. doi: 10.1136/vr.134.2.33. [DOI] [PubMed] [Google Scholar]
- 29.Stanley AC, Buxton D, Innes EA, Huntley JF. Vaccine. 2004;22:3929–3941. doi: 10.1016/j.vaccine.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Adamiak SJ, Mackie K, Watt RG, Webb R, Sinclair KD. Biol Reprod. 2005;73:918–926. doi: 10.1095/biolreprod.105.041483. [DOI] [PubMed] [Google Scholar]
- 31.Gardner DS, Pearce S, Dandrea J, Walker R, Ramsay MM, Stephenson T, Symonds ME. Hypertension. 2004;43:1290–1296. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costello JF, Smiraglia DJ, Plass C. Methods. 2002;2:144–149. doi: 10.1016/s1046-2023(02)00067-1. [DOI] [PubMed] [Google Scholar]
- 33.Genstat. Release 6 Reference Manual. Oxford: Claredon; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information