In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations (original) (raw)
Abstract
In a recent study of older participants (age ≥60 years) in the 1999–2002 National Health and Nutrition Examination Survey (NHANES), we showed that a combination of high serum folate and low vitamin B12 status was associated with higher prevalence of cognitive impairment and anemia than other combinations of vitamin B12 and folate status. In the present study, we sought to determine the joint influence of serum folate and vitamin B12 concentrations on two functional indicators of vitamin B12 status, total homocysteine (tHcy) and methylmalonic acid (MMA), among adult participants in phase 2 of the NHANES III (1991–1994) and the NHANES 1999–2002. Exclusion of subjects who were <20 years old, were pregnant, had evidence of kidney or liver dysfunction, or reported a history of alcohol abuse or recent anemia therapy left 4,940 NHANES III participants and 5,473 NHANES 1999–2002 participants for the study. Multivariate analyses controlled for demographic factors, smoking, alcohol use, body mass index, self-reported diabetes diagnosis, and serum concentrations of creatinine and alanine aminotransferase revealed significant interactions between serum folate and serum vitamin B12 in relation to circulating concentrations of both metabolites. In subjects with serum vitamin B12 >148 pmol/liter (L), concentrations of both metabolites decreased significantly as serum folate increased. In subjects with lower serum vitamin B12, however, metabolite concentrations increased as serum folate increased starting at ≈20 nmol/L. These results suggest a worsening of vitamin B12's enzymatic functions as folate status increases in people who are vitamin B12-deficient.
Keywords: NHANES, cobalamin, methionine synthase, l-methylmalonyl-CoA mutase
Vitamin B12 participates as a coenzyme in two reactions, the remethylation of homocysteine (Hcy) to methionine and the isomerization of l-methylmalonyl-CoA to succinyl-CoA (1–3). The former reaction is catalyzed by methionine synthase in the cytosol, and the latter is catalyzed by l-methylmalonyl-CoA mutase in the mitochondria. Vitamin B12 deficiency is associated with anemia, megaloblastosis, neuropathy, and neuropsychiatric disorders (4), all of which could theoretically be linked to the block in Hcy remethylation or the resulting hyperhomocysteinemia (5). However, the block in the isomerization reaction and resulting buildup of methylmalonic acid (MMA) could also play a role (6).
Early case reports of vitamin B12 deficiency due to pernicious anemia described the alleviation of anemia but the precipitation or exacerbation of neurologic or neuropsychiatric sequelae after folic acid administration, an early form of treatment based on the mistaken idea that folate deficiency was the problem (7). However, a lack of confirmatory data from animal models or human studies has generated controversy about the true consequences of folic acid administration in vitamin B12 deficiency (8), which is now known to encompass more than pernicious anemia (9, 10).
We recently examined the interaction between serum folate and vitamin B12 status in relation to anemia, macrocytosis, and cognitive impairment in older participants (age ≥60 years) in the 1999–2002 United States National Health and Nutrition Examination Survey (NHANES) (11). As expected, low vitamin B12 status was associated with all three abnormalities. Furthermore, when vitamin B12 status was normal, high serum folate was related to protection from cognitive impairment. Among those with low vitamin B12 status, however, high serum folate was directly associated with both anemia and cognitive impairment. Consistent with our findings for subjects with normal vitamin B12 status, several cross-sectional studies (12–15), as well as some prospective investigations (16–19), have reported direct relations between folate status and cognition. On the other hand, Morris et al. (20) found that higher folate intake was associated with more rapid cognitive decline in a cohort of ≈4,000 community-dwelling elderly people whose vitamin B12 status was unknown.
In India, where vitamin B12 deficiency is common, authors of the Pune Maternal Nutrition Study found that the combination of high blood folate and low vitamin B12 levels during pregnancy was associated with elevated risk of insulin resistance in the children at age six (21). Insulin resistance is not an established consequence of vitamin B12 deficiency, but the wide-ranging known effects of the vitamin suggest that it also may have unknown benefits, and its deficiency may have unknown risks (22).
Our goal in the present study was to determine whether higher folate status in vitamin B12 deficiency was associated with higher circulating levels of the functional indicators of low vitamin B12 status, total (t) Hcy, and MMA.
Results
Subject Characteristics and Serum Vitamin B12.
The prevalence of serum vitamin B12 <148 pmol/liter (L) was 1.6% in the NHANES III and 2.2% in the NHANES 1999–2002. The demographic distribution of the subjects reflected the national representation of the survey and our focus on adults of all ages (Table 1). Supplement use was much more common during the more recent survey, when serum folate was also much higher, and tHcy was lower. In both surveys, low serum vitamin B12 was significantly less common among non-Hispanic blacks than it was in non-Hispanic whites. Also consistent across surveys were strong associations between low serum vitamin B12 status and nonuse of dietary supplements, low folate status, and high tHcy and MMA concentrations as well as at least marginally significant associations with higher age, cigarette smoking, and lower alanine aminotransferase concentration. A notable difference between the results of the two surveys pertained to body mass index, which was significantly inversely associated with serum vitamin B12 in the more recent NHANES only.
Table 1.
Characteristics of eligible adult participants in the NHANES III and the NHANES 1999–2002 by serum vitamin B12 concentration*
| Characteristic | NHANES III | NHANES 1999–2002 | ||||||
|---|---|---|---|---|---|---|---|---|
| Serum B12, pmol/L | OR (95% C.I.) | P value | Serum B12, pmol/L | OR (95% C.I.) | P value | |||
| ≥148 | <148 | ≥148 | <148 | |||||
| n = 4,823 | n = 117 | n = 5,343 | n = 130 | |||||
| Age, years | 45 ± 1.0 | 53 ± 4.0 | 1.1 (1.0–1.2)† | 0.057 | 47 ± 0.4 | 51 ± 1.6 | 1.1 (1.02–1.13)† | 0.016 |
| Female, % | 57 | 64 | 1.4 (0.7–2.6) | 0.640 | 74 | 70 | 1.7 (1.04–2.9) | 0.018 |
| Nonhispanic white, % | 82 | 79 | 1.0 | Referent | 57 | 83 | 1.0 | Referent |
| Nonhispanic black, % | 8.4 | 2.7 | 0.3 (0.2–0.8) | 0.011 | 9.6 | 3.7 | 0.4 (0.2–0.8) | 0.015 |
| Mexican-American, % | 4.3 | 4.7 | 1.1 (0.5–2.4) | 0.745 | 6.2 | 4.6 | 0.7 (0.4–1.3) | 0.236 |
| Cigarette smoker, %‡ | 25 | 32 | 1.9 (0.9–4.0) | 0.096 | 25 | 31 | 1.7 (1.03–2.7) | 0.037 |
| Supplement user, %‡ | 38 | 16 | 0.3 (0.1–0.8) | <0.015 | 55 | 49 | 0.6 (0.3–0.95) | 0.044 |
| Alcohol per day, g‡ | 10.0 | 9.2 | 1.0 (1.0–1.01) | 0.711 | 8.7 | 14.8 | 1.0 (1.0–1.01) | 0.147 |
| Creatinine, μmol/L‡§ | 71 ± 0.3 | 74 ± 1.6 | 1.0 (1.0–1.03) | 1.0 | 72 ± 0.8 | 69 ± 1.9 | 1.0 (1.0–1.01) | 0.363 |
| Alanine aminotransferase, units/L‡§ | 15.2 ± 0.2 | 12.7 ± 0.8 | 0.9 (0.9–1.0) | 0.057 | 20.9 ± 0.02 | 17.8 ± 0.8 | 0.4 (0.2–0.8) | 0.001 |
| Body mass index¶ | 26.4 | 26.6 | 1.0 (1.0–1.0) | 0.927 | 27.7 | 29.1 | 1.04 (1.02–1.2) | 0.002 |
| Serum folate, nmol/L†§‖ | 15.3 ± 1.0 | 10.6 ± 0.8 | 0.94 (0.92–0.96) | <0.001 | 30.8 ± 0.5 | 25.0 ± 1.2 | 0.84 (0.8–0.9) | <0.001 |
| Hcy, μmol/L§‖ | 8.5 ± 0.1 | 13.2 ± 2.2 | 1.1 (1.1–1.2) | <0.001 | 7.7 ± 0.1 | 10.7 ± 0.8 | 1.2 (1.1–1.2) | <0.001 |
| MMA, nmol/L§‖** | 189 ± 7.5 | 317 ± 134 | 1.2 (0.99–1.3) | <0.078 | 132 ± 1.6 | 245 ± 47 | 1.4 (1.3–1.5) | <0.001 |
Serum Folate and tHcy and MMA.
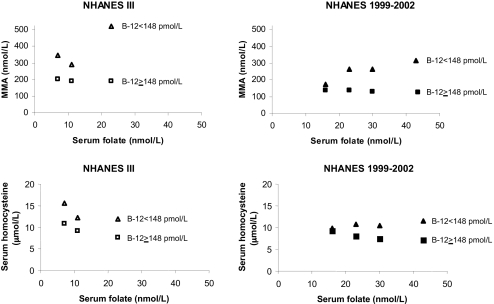
Among participants in both surveys, serum folate interacted significantly with serum vitamin B12 in relation to tHcy (NHANES III, P < 0.002; NHANES 1999–2002, _P_ = 0.005). Specifically, although geometric mean Hcy decreased across increasing serum folate categories among survey participants in both surveys with serum vitamin B12 >148 pmol/L (P < 0.001), a decreasing trend across all serum folate categories was not observed for subjects with serum vitamin B12 <148 pmol/L (Fig. 1). The highest geometric mean tHcy that we estimated was for the NHANES III participants with serum vitamin B12 <148 pmol/L and serum folate in the low-to-deficient range. Furthermore, geometric mean tHcy for NHANES 1999–2002 subjects with low serum vitamin B12 and serum folate <19.3 nmol/L was in the normal range and close to that of subjects in the same serum folate category with higher serum vitamin B12 (Table 2). However, the mean for the group with low serum vitamin B12 and serum folate >32.6 nmol/L was nearly 12 μmol/L, a commonly used cut-off point for hyperhomocysteinemia. In fact, in subjects with serum B12 <148pmol/L, tHcy increased significantly across the four serum folate categories represented in the NHANES 1999–2002 (P = 0.003).
Fig. 1.
Trends in serum MMA and Hcy concentrations with increasing serum folate in the United States population over the age of 20.
Table 2.
Least-square geometric mean (95% C.I.) Hcy and MMA concentration by serum vitamin B12 and serum folate category,* NHANES
| Serum folate,nmol/L | Hcy | MMA† | ||||
|---|---|---|---|---|---|---|
| Serum B12, pmol/L | Serum B12, pmol/L | |||||
| <148 | ≥148 | P value | <148 | ≥148 | P value | |
| 1991–1994 | ||||||
| 7 | 15.5 (13.2–18.1) | 10.9 (10.5–11.3) | <0.001 | 347 (178–676) | 202 (180–228) | 0.194 |
| 11 | 12.2 (10.4–14.2) | 9.2 (8.8–9.2) | <0.001 | 290 (155–543) | 191 (176–206) | 0.140 |
| 23 | 11.9 (8.7–16.3) | 7.6 (7.5–7.8) | 0.009 | 523 (333–821) | 191 (180–202) | 0.002 |
| 1999–2002 | ||||||
| 16 | 9.9 (8.8–11.1) | 9.2 (9.0–9.4) | 0.222 | 175 (149–204) | 138 (133–145) | 0.005 |
| 23 | 10.8 (9.4–12.4) | 8.0 (7.8–8.2) | <0.001 | 265 (218–322) | 137 (132–142) | <0.001 |
| 30 | 10.5 (9.1–12.0) | 7.5 (7.4–7.7) | 0.001 | 265 (194–363) | 132 (127–137) | <0.001 |
| 44 | 11.8 (10.1–13.8) | 7.1 (7.0–7.2) | <0.001 | 314 (234–421) | 128 (125–130) | <0.001 |
Serum folate interacted significantly with serum vitamin B12 in relation to serum MMA concentrations only for participants in the NHANES 1999–2002 (P = 0.003). Among participants in that survey with serum vitamin B12 <148 pmol/L, geometric mean serum MMA increased significantly across categories of increasing serum folate (_P_ = 0.008), while a modest but significant decrease in MMA with increasing serum folate was observed among subjects with serum vitamin B12 >148 pmol/L (P < 0.001). Although no significant trends emerged for participants in the NHANES III, the data for subjects in the two surveys with serum vitamin B12 <148 pmol/L suggested a decrease in serum MMA as serum folate increased up to a point, but highly elevated MMA concentrations at serum folate concentrations >19.3 nmol/L.
Discussion
Associations between low vitamin B12 status and both hyperhomocysteinemia and elevated MMA concentrations are well established, as is an inverse relation between folate status and circulating Hcy under normal circumstances. A possible link between folate status and MMA has not been considered, because folate is not known to participate in pathways affecting MMA accumulation. In this study of the general United States population spanning pre- and postfolate fortification eras, we found that tHcy does not generally decrease with increasing folate status among people with low serum vitamin B12 concentrations. On the contrary, in people with low serum B12 concentrations, high tHcy levels were found at both very low and very high serum folate levels, with lower tHcy levels in between. Furthermore, whereas MMA data for people with low levels of both vitamin B12 and folate were limited, the MMA results were consistent with the tHcy results in revealing a narrow range of folate values associated with near-normal metabolite levels and elevated metabolite levels among those with low and high folate concentrations.
In vitamin B12 deficiency, high tHcy reflects impaired methionine synthase activity, whereas high MMA indicates impaired methylmalonyl-CoA mutase activity. Consequently, our findings suggest that both pathways of vitamin B12 metabolism are adversely affected by high serum folate despite the direct involvement of folate only in methionine synthase activity.
Simultaneous impairment of both pathways is normally caused by vitamin B12 deficiency or by the disruption of those early steps in vitamin B12 processing that are common in both pathways (1, 23): (i) the intrinsic factor mediated B12 absorption by the intestine; (ii) formation of the B12-transcobalamin II complex in plasma; (iii) transport of the complex into peripheral tissue by a receptor-mediated endocytosis and incorporation of the complex into the lysosomes; (iv) dissociation of the complex and exit of vitamin B12, in the form of acquocob(III)alamin, into the cytosol; and (v) reduction to cob(II)alamin for subsequent migration either for association with the methionine synthase (MS) in the cytosol or to enter the mitochondria.
It is difficult to envision how folate could interfere with any of these steps. The alternate possibility is that this interference occurs after the diversion of the vitamin to the respective compartments in the course of rendering the vitamin available for interaction with the respective enzyme proteins or during catalysis. In the cytosol, the cob(II)alamin associates with the MS to form a cob(II)alamin–MS complex. This complex is then reduced by a closely associated enzyme, methionine synthase reductase (MTR) to form a cob(I)alamin–MS that is catalytically active. The MS enzyme contains a binding site for 5-methyltetrahydrofolate, which is more specific for polyglutamyl derivatives (2). In the enzyme catalysis, the cob(I)alamin acquires a methyl group from bound 5-methyltetrahydrofolate by means of an oxidation addition mechanism forming a methyl–cob(III)alamin prosthetic group (24). This bond between the methyl group and the cobalt ion is then cleaved heterolytically, leaving both bonding electrons on the cob(I)alamin and transferring the methyl group to homocysteine in the form of a carbocation, CH3+ (24). Cob(I)alamin is remethylated by 5-methyltetrahydrofolate. However, the enzyme-bound cob(I)alamin occasionally becomes oxidized to cob(II)alamin, and this enzyme form is catalytically inactive. The enzyme is reactivated by a reductive methylation in which the methyl group is derived from adenosylmethionine (25).
In the mitochondria, the cob(II)alamin undergoes a reduction to cob(I)alamin by an electron donor followed by adenosylation in a reaction that involves ATP, to form the 5′-deoxyadenosyl-cob(III)alamin (Adenosylcobalamin) (1, 2, 23). This is then associated with the mutase by the action of adenosyltransferase. A mitochondrial protein, methylmalonic aciduria cblA type protein (MMAA), associates with the mutase to protect the mutase from inactivation (26).
In the MMA–CoA mutase reaction, the bond between the methylene group of 5′-deoxyadenosine and the cobalt ion undergoes homolytic cleavage, thus resulting in free radicals both on the methylene group and the cobalt ion. The free radical on the methylene group is transferred to the MMA–CoA molecule to allow isomerization to succinyl-CoA, which is accompanied by the transfer of the free radical back onto the methylene group for subsequent rebonding with the cobalt ion (1, 2, 23).
Although the reduction of cob(II)alamin to cob(I)alamin occurs in both compartments, the process is substantially different for the two. In the cytosol, this reduction takes place while cob(II)alamin is bound to the enzyme. In the mitochondrion, on the other hand, this reduction precedes the association of the mutase. This difference and the fact that these reductions occur in separate compartments render it difficult to envision interference by folate.
Another possibility is that folate interferes with the catalytic action of MS, and the impairment of the mutase is secondary to the interaction with the MS. This possibility would be analogous to the observed effects of nitric oxide on B12 metabolism. Nitric oxide is a strong oxidizing agent that irreversibly inactivates methionine synthase by oxidizing enzyme-bound vitamin B12 to form a free radical (27). There is no known direct effect of nitrous oxide on the mutase. Nevertheless, both humans who inhaled the gas and laboratory animals subjected to prolonged exposure had elevated plasma and spinal fluid levels of not only tHcy but also MMA (28–32).
Using a methionine-independent human glioma cell line (P60H), Reidel et al. (33) showed that exposure to nitrous oxide resulted in cellular depletion of methylcobalamin and, after a lag period, decreased adenosylcobalamin and conversion of the mutase holoenzyme to its apoenzyme. These data suggest a coordinate distribution of cobalamin cofactors between the mitochondria and the cytosol compartments.
High serum folate as encountered in this study was attributable to high intake of folic acid from supplements and fortified food, which undoubtedly resulted in the appearance of unmetabolized folic acid in plasma (34, 35). In the Framingham Study, the percent of folate consumed as folic acid increased sharply as plasma folate increased, from 50% among those with plasma folate ≤30 nmol/L to 80% among those with plasma folate >50 nmol/L (S. Choumenkovitch, P.F.J., and J.S., unpublished work).
A high intake of folic acid in vitamin B12 deficiency would change the concentration and character of cellular folate, particularly tetrahydrofolate (36). A central hypothesis to account for the observed effect of folic acid intake in the remission of the hematological complications in pernicious anemia is that the flux of unmethylated folate into the bone marrow cells circumvents the inhibition of DNA synthesis caused by the trapping of folate coenzymes as methyltetrahydrofolate (37). Folate coenzymes participating in DNA synthesis include formylated tetrahydrofolate, which is used for purine synthesis, and 5,10- methylenetetrahydrofolate, which is used for thymidylate synthesis. Unsubstituted tetrahydrofolate and dihydrofolate are the respective products of these syntheses.
A common denominator for some of these folates is that they can serve as electron acceptors or oxidation agents. Formylated tetrahydrofolate can be reduced to 5,10- methylenetetrahydrofolate; 5,10- methylenetetrahydrofolate can be reduced to 5-methyltetrahydrofolate, whereas folic acid and dihydrofolate acquire electrons for conversion to tetrahydrofolate. Based on these properties, it is tempting to suggest that folic acid or its partially reduced form, dihydrofolate, as well as formylated and methylene tetrahydrofolate could act as electron acceptors and thereby accelerate the rate of oxidation of MS cob(I)alamin to MS cob(II)alamin. The enzyme activity could be even more compromised because of the lower _S_-adenosylmethionine (SAM) availability and lower enzyme synthesis due to B12 deficiency (38, 39).
This proposed interaction between folate derivatives (folic acid, dihydrofolate, etc.) and a heterologous protein (i.e., MS) is not unprecedented: 5-methyltetrahdrofolate is an activator of cystathionine γ-synthase in Neurospora crassa (40), an inhibitor of glycine methyltransferase in mammalian systems (41), and binds to cytosolic serine hydroxymethylase (42). Binding to serine hydroxymethylase is associated with diminished synthesis of methionine and diversion of the flow of one carbon units toward thymidylate synthesis. Dihydrofolate is a competitive inhibitor of pig liver methylenetetrahydrofolate reductase (MTHFR) (43). In all cases, these associations are enhanced when the respective folates are polyglutamated, which is consistent with the idea that the great majority of folate enzymes have a common binding site for the glutamic acid side chain. As pointed out above, MS also contains a binding site for methyltetrahydrofolate and its product, tetrahydrofolate (2).
Based on this paradigm, we propose that the high serum MMA associated with the combination of low B12 status and high plasma folate status stems from the disruption of B12 hemostasis in the mitochondria, analogous to the effect of nitric oxide.
In conclusion, we have extended the findings of our recently published study of the interaction between vitamin B12 status and folate status in relation to anemia, macrocytosis, and cognitive impairment (11) by demonstrating that, among people with low serum vitamin B12 concentrations, high plasma folate is associated with higher concentrations of the two functional indicators of impaired B12 status, Hcy and MMA. These observations provide a possible biochemical explanation for high folic acid intake's exacerbation of the clinical manifestations of vitamin B12 deficiency.
Methods
Study Population.
The NHANES, which is currently implemented as a continuous annual survey, monitors the nation's health and nutritional status by using a complex multistage probability design to select a representative sample of the noninstitutionalized United States civilian population. To increase the precision of estimates derived from the survey, adolescents, the elderly, Mexican Americans, and blacks are oversampled. The protocols for conducting the NHANES were approved by the institutional review board of the National Center for Health Statistics, Centers for Disease Control and Prevention, and informed consent was obtained from all participants (44). For this investigation, we used data from phase 2 (1991–1994) of the third NHANES (NHANES III) and the NHANES conducted in 1999–2000 and 2000–2002. Consistent with NHANES analytic guidelines, we combined data from these latter two surveys into a single dataset (45).
Trained interviewers used a computer-assisted personal interview system to interview participants in their homes. The participants were also asked to report to a mobile examination center (MEC) to provide further interview data and undergo a physical examination that included phlebotomy. A detailed description of blood collection and processing can be found in the NHANES Phlebotomy Manual (46). Although the survey included people of all ages, we focused our attention on adults (i.e., those aged >19 years).
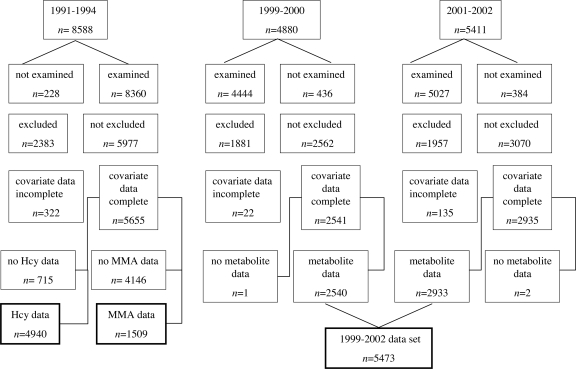
Besides participants <20 years old, we excluded pregnant women and men and women who reported a history of heavy alcohol use or recent anemia therapy as well as those with serum creatinine concentration (based on Jaffe reaction) indicative of renal dysfunction (i.e., men, >131 μmol/L; women, >115 μmol/L) or alanine aminotransferase >40 units/L, which suggests liver disease (47). Of the 8,588 adult participants in phase 2 of the NHANES III, 4,940 were included in this study (Fig. 2). Of the 10,291 adult participants in the NHANES 1999–2002, 5,473 were included in this study.
Fig. 2.
Creation of study datasets from NHANES datasets.
Biochemical Measurements.
Priority analyses were carried out at the Inorganic Toxicology and Nutrition Branch or the Division of Laboratory Sciences, National Center for Environmental Health, or at central laboratories that varied with survey years. Serum concentrations of folate and vitamin B12 were measured by using the Quantaphase II Radioassay Kit (Bio-Rad). Serum alanine aminotransferase was measured by using an autoanalyzer (NHANES III, Hitachi; NHANES 1999–2001, Hitachi; NHANES 2002, Beckman; Synchron LX20). In all survey years, serum creatinine concentration was based on the Jaffe reaction, but values varied greatly among the NHANES III, NHANES 1999–2000, and NHANES 2001 and 2002. Also, only the values reported for the last 2-year period were consistent with results using a gold-standard method (46). After Coresh et al. (48), we subtracted 20 μmol/L (0.23 mg/dl) from the NHANES III values. We also added 11.5 μmol/L (0.13 mg/dl) to the NHANES 1999–2000 values as recommended by NHANES documentation (46).
Concentrations of MMA and tHcy were measured in NHANES III as phase 2 (1991–1994) surplus serum projects carried out at the United States Department of Agriculture Human Nutrition Research Center on Aging after approval by the New England Medical Center Human Investigations Review Committee and the Surplus Sera Bank Steering Committee. Surplus serum samples were stored at −70°C and underwent one to four freeze–thaw cycles before tHcy and MMA analyses. Total Hcy and MMA concentrations have been shown to remain stable in long-frozen samples (49, 50). In 1994, Hcy concentrations were measured in serum samples from participants ≥12 years old by the HPLC method of Araki and Sako (51). In 1998, MMA concentrations were measured in surplus serum samples from 65-year-old participants (n = 1,145) and, for comparison purposes, in a random sample of available serum samples from 30- to 39-year-old participants (n = 1,026). Serum MMA concentrations were measured by gas chromatography/mass spectrometry using the solid extraction method and derivatization with cyclohexanol as described by Rasmussen (52).
In the NHANES 1999–2002, measurement of plasma tHcy and MMA were priority analyses. tHcy was measured by using a fully automated fluorescence polarization immunoassay (Abbott Laboratories). Plasma levels were calculated by using a machine- stored calibration curve (Abbott IMx analyzer, 1999–2001; Abbott AxSYManalyzer, 2002). Plasma MMA was measured by gas chromatography/mass spectrometry with cyclohexanol derivatization (53). It is important to note that MMA measurements for the NHANES 1999–2002 were much lower than the measurements recorded in the NHANES III. Pfeiffer et al. (53) attributed the different MMA values obtained in the two surveys to differences in the laboratories conducting the analyses and the matrices and methods used.
Statistical Analyses.
Data analyses were performed by using SUDAAN release 9.0 (Research Triangle Institute) with appropriate sampling weights and masked variance pseudostratum and pseudoPSU variables available from the NHANES website to account for the survey's complex sampling design (44). P < 0.05 was considered statistically significant for all tests.
We first used SUDAAN PROC REGRESS, SUDAAN PROC CROSSTAB, and SUDAAN PROC RLOGIST to describe the qualifying participants via least-squares means and proportions and to perform comparisons between subjects with serum vitamin B12 below the conventional cut-off point for deficiency of 148 pmol/L and the remaining subjects.
For our main data analyses, we used SUDAAN PROC REGRESS to estimate least-square geometric mean (95% C.I.) circulating tHcy and MMA for serum folate categories after multivariate adjustment for age, gender, race/ethnicity, body mass index, smoking status, alcohol intake, self-reported diabetes diagnosis, and serum concentrations of creatinine and alanine aminotransferase. These analyses were performed stratified by vitamin B12 status after interactions between vitamin B12 status and folate status were tested. Because of the much smaller number of subjects in the group with lower serum vitamin B12 as compared with the number with higher serum vitamin B12 values, we used the distribution of the former group to determine the serum folate category cut-off points.
The availability of tHcy and MMA values for NHANES III participants allowed us to examine interrelations among folate, vitamin B12, and the metabolites across a broader range of folate levels than the NHANES 1999–2002 dataset afforded given the institution of government- mandated food folic acid fortification between the two surveys. We made no attempt to combine data from the NHANES III with data from the NHANES 1999–2002. We divided participants in the 3-year second phase of the NHANES III into three folate categories, and we divided participants in the 4-year NHANES 1999–2002 into four folate categories (Table 3).
Table 3.
Category definitions and sample sizes*
| Serum folate, nmol/L | NHANES III† | NHANES 1999–2002 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hcy | MMA | Hcy/MMA | ||||||||
| <9.1 | 9.1–13.5 | ≥13.6 | ≤9.7 | 9.7–13.5 | ≥13.6 | <19.3 | 19.3–25.9 | 26–32.5 | ≥32.6 | |
| Serum B12 ≥ 148 pmol/L, n | 1,581 | 1,271 | 1,970 | 501 | 309 | 655 | 1,032 | 1,152 | 892 | 2,267 |
| Serum B12 < 148 pmol/L, n | 39 | 41 | 37 | 15 | 13 | 16 | 32 | 33 | 32 | 33 |
Acknowledgments
This material is supported by the United States Department of Agriculture under agreement no.1950-51520-008-00D and United States Department of Agriculture Grant 2006-35200-17198. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the United States Department of Agriculture.
Footnotes
The authors declare no conflict of interest.
References
- 1.Banerjee R. ACS Chem Biol. 2006;1:149–159. doi: 10.1021/cb6001174. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig ML, Matthews RG. Annu Rev Biochem. 1997;66:269–313. doi: 10.1146/annurev.biochem.66.1.269. [DOI] [PubMed] [Google Scholar]
- 3.Savage DG, Lindenbaum J. In: Folate in Health and Disease. Bayley LB, editor. NY: Marcel Decker; 1995. pp. 237–285. [Google Scholar]
- 4.Baik HW, Russell RM. Annu Rev Nutr. 1999;19:357–377. doi: 10.1146/annurev.nutr.19.1.357. [DOI] [PubMed] [Google Scholar]
- 5.Scott JM. Proc Nutr Soc. 1999;58:441–448. doi: 10.1017/s0029665199000580. [DOI] [PubMed] [Google Scholar]
- 6.Allen RH, Stabler SP, Lindenbaum J. Eur J Pediatr. 1998;157(Suppl 2):S122–S126. doi: 10.1007/pl00014295. [DOI] [PubMed] [Google Scholar]
- 7.Chodos RB, Ross JF. Blood. 1951;6:1213–1233. [PubMed] [Google Scholar]
- 8.Reynolds E. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 9.Carmel R. Am J Hematol. 1990;34:108–114. doi: 10.1002/ajh.2830340206. [DOI] [PubMed] [Google Scholar]
- 10.Miller JW. Nutr Rev. 2002;60:142–144. doi: 10.1301/00296640260093805. [DOI] [PubMed] [Google Scholar]
- 11.Morris MS, Jacques PF, Rosenberg I, Selhub J. Am J Clin Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ. Am J Clin Nutr. 2002;75:908–913. doi: 10.1093/ajcn/75.5.908. [DOI] [PubMed] [Google Scholar]
- 13.Lindeman RD, Romero LJ, Koehler KM, Liang HC, LaRue A, Baumgartner RN, Garry PJ. J Am Coll Nutr. 2000;19:68–76. doi: 10.1080/07315724.2000.10718916. [DOI] [PubMed] [Google Scholar]
- 14.Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, Miller JW. Am J Clin Nutr. 2005;82:1346–1352. doi: 10.1093/ajcn/82.6.1346. [DOI] [PubMed] [Google Scholar]
- 15.Wahlin A, Hill RD, Winblad B, Backman L. Psychol Aging. 1996;11:487–496. doi: 10.1037//0882-7974.11.3.487. [DOI] [PubMed] [Google Scholar]
- 16.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., III Am J Clin Nutr. 2005;82:627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 17.Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Lancet. 2007;369:208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 18.Nurk E, Refsum H, Tell GS, Engedal K, Vollset SE, Ueland PM, Nygaard HA, Smith AD. Ann Neurol. 2005;58:847–857. doi: 10.1002/ana.20645. [DOI] [PubMed] [Google Scholar]
- 19.Kado DM, Karlamangla AS, Huang MH, Troen A, Rowe JW, Selhub J, Seeman TE. Am J Med. 2005;118:161–167. doi: 10.1016/j.amjmed.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, Schneider JA. Arch Neurol. 2005;62:641–645. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 21.Yajnik C. Nutr Rev. 2006;64:S50–S51. doi: 10.1301/nr.2006.may.s50-s51. [DOI] [PubMed] [Google Scholar]
- 22.Volkov I, Press Y, Rudoy I. J Nippon Med Sch. 2006;73:65–69. doi: 10.1272/jnms.73.65. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblatt DS, Cooper BA. Blood Rev. 1987;1:177–182. doi: 10.1016/0268-960x(87)90033-6. [DOI] [PubMed] [Google Scholar]
- 24.Smith AE, Matthews RG. Biochemistry. 2000;39:13880–13890. doi: 10.1021/bi001431x. [DOI] [PubMed] [Google Scholar]
- 25.Jarrett JT, Hoover DM, Ludwig ML, Matthews RG. Biochemistry. 1998;37:12649–12658. doi: 10.1021/bi9808565. [DOI] [PubMed] [Google Scholar]
- 26.Lerner-Ellis JP, Dobson CM, Wai T, Watkins D, Tirone JC, Leclerc D, Dore C, Lepage P, Gravel RA, Rosenblatt DS. Hum Mutat. 2004;24:509–516. doi: 10.1002/humu.20104. [DOI] [PubMed] [Google Scholar]
- 27.Ng J, Frith R. Lancet. 2002;360:384. doi: 10.1016/S0140-6736(02)09611-3. [DOI] [PubMed] [Google Scholar]
- 28.Waclawik AJ, Luzzio CC, Juhasz-Pocsine K, Hamilton V. Wis Med J. 2003;102:43–45. [PubMed] [Google Scholar]
- 29.Drummond JT, Matthews RG. Biochemistry. 1994;33:3732–3741. doi: 10.1021/bi00178a033. [DOI] [PubMed] [Google Scholar]
- 30.Kondo H, Osborne ML, Kolhouse JF, Binder MJ, Podell ER, Utley CS, Abrams RS, Allen RH. J Clin Invest. 1981;67:1270–1283. doi: 10.1172/JCI110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rask H, Olesen AS, Mortensen JZ, Freund LG. Scand J Haematol. 1983;31:45–48. doi: 10.1111/j.1600-0609.1983.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 32.Vieira-Makings E, Chetty N, Reavis SC, Metz J. Biochem J. 1991;275(Pt 3):585–590. doi: 10.1042/bj2750585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riedel B, Fiskerstrand T, Refsum H, Ueland PM. Biochem J. 1999;341(Pt 1):133–138. [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney MR, McPartlin J, Weir DG, Daly L, Scott JM. Br J Nutr. 2006;95:145–151. doi: 10.1079/bjn20051618. [DOI] [PubMed] [Google Scholar]
- 35.Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, et al. J Nutr. 2006;136:189–194. doi: 10.1093/jn/136.1.189. [DOI] [PubMed] [Google Scholar]
- 36.Stokstad EL, Reisenauer A, Kusano G, Keating JN. Arch Biochem Biophys. 1988;265:407–414. doi: 10.1016/0003-9861(88)90143-9. [DOI] [PubMed] [Google Scholar]
- 37.Scott JM, Weir DG. Lancet. 1981;2:337–340. doi: 10.1016/s0140-6736(81)90650-4. [DOI] [PubMed] [Google Scholar]
- 38.Oltean S, Banerjee R. J Biol Chem. 2003;278:20778–20784. doi: 10.1074/jbc.M300845200. [DOI] [PubMed] [Google Scholar]
- 39.Oltean S, Banerjee R. J Biol Chem. 2005;280:32662–32668. doi: 10.1074/jbc.M501964200. [DOI] [PubMed] [Google Scholar]
- 40.Selhub J, Savin MA, Sakami W, Flavin M. Proc Natl Acad Sci USA. 1971;68:312–314. doi: 10.1073/pnas.68.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook RJ, Wagner C. Proc Natl Acad Sci USA. 1984;81:3631–3634. doi: 10.1073/pnas.81.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbig K, Chiang EP, Lee LR, Hills J, Shane B, Stover PJ. J Biol Chem. 2002;277:38381–38389. doi: 10.1074/jbc.M205000200. [DOI] [PubMed] [Google Scholar]
- 43.Matthews RG, Baugh CM. Biochemistry. 1980;19:2040–2045. doi: 10.1021/bi00551a005. [DOI] [PubMed] [Google Scholar]
- 44.National Center for Health Statistics. [Accessed September 1, 2005];National Health and Nutrition Examination Survey. 2005 Available at www.cdc.gov/nchs/data/nhanes/gendoc.pdf.
- 45.National Center for Health Statistics. [Accessed December 31, 2005];National Health and Nutrition Examination Survey: Analytic Reporting Guidelines. 2005 Available at www.cdc.gov/nchs/data/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf.
- 46.National Center for Health Statistics. [Accessed September 1, 2005];National Health and Nutrition Examination Survey: Lab 18, Biochemistry Profile. 2007 Available at www.cdc.gov/nchs/data/nhanes/frequency/phdoc.pdf.
- 47.Ioannou GN, Boyko EJ, Lee SP. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 48.Coresh J, Eknoyan G, Levey AS. J Am Soc Nephrol. 2002;13:2811–2812. doi: 10.1097/01.asn.0000037420.89149.c9. [DOI] [PubMed] [Google Scholar]
- 49.Israelsson B, Brattstrom L, Refsum H. Scand J Clin Lab Invest. 1993;53:465–469. doi: 10.3109/00365519309092541. [DOI] [PubMed] [Google Scholar]
- 50.Stabler SP, Marcell PD, Podell ER, Allen RH, Savage DG, Lindenbaum J. J Clin Invest. 1988;81:466–474. doi: 10.1172/JCI113343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Araki A, Sako Y. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 52.Rasmussen K. Clin Chem. 1989;35:260–264. [PubMed] [Google Scholar]
- 53.Pfeiffer CM, Gunter EW. Clin Chem. 1999;45:A166. abstr 593. [PubMed] [Google Scholar]