An investigation of predictors of renal insufficiency following treatment of hyperthyroidism in cats (original) (raw)
Abstract
To determine if routine pre-treatment clinical data can be used to predict the development of overt renal insufficiency following treatment of feline hyperthyroidism, we studied retrospectively all non-azotemic cats undergoing treatment for hyperthyroidism at our hospital. Medical records were reviewed for signalment, clinical signs, and serum biochemical, hematologic and urinalysis parameters before and after treatment for hyperthyroidism. Two groups – cats that developed post-treatment renal insufficiency, and those that did not – were compared. No significant differences could be detected between the groups with respect to the parameters measured. Our study suggests that the results of routine pre-treatment clinical data cannot be used to reliably predict renal function after treatment for hyperthyroidism, validating the necessity of a methimazole trial prior to definitive therapy. The widely held belief that cats with pre-treatment urine specific gravity>1.035 are at less risk for development of renal azotemia after treatment of hyperthyroidism seems unwarranted.
Renal failure is diagnosed commonly in feline medicine, with nearly 30% of cats over 15 years of age being affected (Plantinga et al 2005). Hyperthyroidism is the most common endocrine disorder diagnosed in cats, with an increasing incidence since the first documented cases in 1979 (Broussard et al 1995, Mooney and Thoday 2000, Mooney 2005). Renal disease can often be masked by the hyperthyroid state. Excess thyroid hormone can increase cardiac output while decreasing peripheral vascular resistance, causing increased glomerular filtration rate (GFR) as a result of increased renal plasma flow (Bradley et al 1974, Adams et al 1997a, Klein and Ojamaa 2001, Langston and Reine 2006). One study showed that 15% of hyperthyroid cats treated with radioiodine developed overt renal disease during a 13–18-month follow-up period (Slater et al 1994).
Previous studies have shown that mean GFR is increased in hyperthyroid cats and that treatment of hyperthyroidism leads to decreases in GFR as well as an increase in serum urea nitrogen (SUN) concentrations (Graves et al 1994, DiBartola et al 1996, Becker et al 2000). Oral medication (most commonly methimazole) is often used to control hyperthyroidism (Peterson et al 1988, Trepanier 2006). The effects of methimazole on renal function are often reversible. One study reported that GFR in two cats treated for hyperthyroidism increased after cessation of methimazole, but did not return to pre-treatment values (Becker et al 2000). Still, it is widely recommended that cats with hyperthyroidism be treated initially with methimazole because its effects can be largely reversed should renal insufficiency develop. There are disadvantages with the use of methimazole, however, including the need for twice daily dosing, and the development of side effects such as facial excoriations, gastrointestinal upset, blood dyscrasias, and hepatotoxicity (Peterson et al 1988, Mooney 2001, Trepanier 2006).
Definitive treatments for feline hyperthyroidism, such as thyroidectomy or radioiodine, are attractive alternatives to life-long anti-thyroid drug therapy because owner satisfaction is high. Radioiodine treatment, where available, is preferred to surgery because it is non-invasive, safe, and effective (Turrel et al 1984, Slater et al 1994, Peterson and Becker 1995, Mooney and Thoday 2000, Mooney 2005). Because an irreversible decline in renal function occurs with definitive treatment, it may be prudent to assess renal function once euthyroidism has been achieved. There are anecdotal reports that cats with normal SUN and serum creatinine concentrations, and with urine specific gravity (USG) measurements greater than 1.035, have a reduced risk for the development of renal insufficiency after treatment for hyperthyroidism (Mooney 2005, Garrett 2006). To our knowledge, no studies evaluating this claim have been published.
Pre-treatment GFR is also reported to be a predictor of post-treatment renal failure, with one study reporting that a pre-treatment GFR of less than 2.25 ml/kg/min was 100% sensitive and 78% specific for post-treatment renal failure (Adams et al 1997b). In that study, 15 of 22 cats were azotemic 30 days after treatment with radioiodine, although nine of these cats were also azotemic before treatment. The seven cats in that study that did not develop azotemia within 30 days of treatment all had GFR measurements above 2.25 ml/kg/min. In other studies, however, cats with pre-treatment GFR measurements considerably higher than 2.25 ml/kg/min were azotemic 30 days after treatment of hyperthyroidism (Graves et al 1994, DiBartola et al 1996), so the use of GFR measurement as a predictor of post-treatment renal function is controversial.
The ability to predict which cats will develop renal insufficiency after treatment of hyperthyroidism could help to guide treatment decisions and may result in decreased morbidity for cats. For that reason we have examined common pre-treatment clinical data from cats with hyperthyroidism, and have sought to identify predictors of post-treatment renal insufficiency.
Materials and methods
Criteria for selection of cases
Medical records of cats diagnosed with hyperthyroidism and treated at the University of Illinois between January 1998 and December 2003 were reviewed. The diagnosis of hyperthyroidism was made based on clinical signs of the disease along with findings of elevated serum concentrations of total thyroxine (TT4) or free thyroxine (measured by equilibrium dialysis) measured by widely accepted methods. Cats were excluded from the study if they were azotemic with inadequate urine concentration or if pre-treatment or follow-up blood work was not available for review. Inadequate urine concentrating ability was defined as a USG of 1.030 or less in the face of azotemia or dehydration. After review of 152 records, a total of 39 cats were eligible for inclusion into the study. Reasons for exclusion included lack of pre-treatment blood work, lack of follow-up for a minimum of 6 months following treatment, and renal disease present prior to treatment. Cats were divided into two groups; group 1 (_n_=19) was comprised of cats that did not have evidence of renal insufficiency for at least 6 months after treatment, and group 2 (_n_=20) was comprised of cats that developed renal insufficiency within 6 months after treatment as documented by azotemia and inadequate urine concentration. One cat (cat 39 in Tables 1 and 2) did not experience an increase in the serum creatinine concentration after treatment of hyperthyroidism, but was included in the group 2 because of the findings of weight loss, isosthenuria, dehydration, and increased SUN concentration following treatment of hyperthyroidism.
Table 1.
Selected pre-treatment clinical data from cats that did not (group 1, cats 1–19) or did (group 2, cats 20–39) develop renal failure after treatment of hyperthyroidism
| Sex | Breed | Age (yrs) | BW (kg) | SBP (mmHg) | TT4 (nmol/l) | Creat (μmol/l) | SUN (mmol/l) | Phos (mmol/l) | K (mmol/l) | USG | UP (g/dl) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | ||||||||||||
| 1 | FS | DSH | 14.2 | 3.7 | 160 | 165.0 | 61.9 | 9.3 | 1.6 | 4.5 | 1.052 | Trace |
| 2 | MC | DMH | 9 | 5.3 | 185 | 70.7 | 8.9 | 2.0 | 4.0 | |||
| 3 | MC | Mix | 12 | 4.9 | 122.9 | 93.7 | 7.4 | 1.1 | ||||
| 4 | MC | DSH | 11 | 6.4 | 113.0 | 88.4 | 4.6 | 1.9 | 5.4 | |||
| 5 | FS | DSH | 10 | 3.4 | 74.1 | 106.1 | 10.2 | 1.5 | 4.7 | 1.033 | Trace | |
| 6 | FS | DSH | 11 | 3.6 | 58.8 | 106.1 | 8.8 | 1.6 | 4.7 | 1.070 | Trace | |
| 7 | MC | DSH | 15 | 3.6 | 220 | 40.9 | 114.9 | 8.7 | 1.4 | 4.6 | 1.018 | Trace |
| 8 | FS | DSH | 14 | 3.1 | 123.0 | 53.0 | 7.7 | 1.4 | 3.6 | |||
| 9 | FI | DSH | 17.7 | 4.1 | 122 | 53.4 | 123.8 | 10.7 | 1.4 | 4.4 | 1.052 | 0.0 |
| 10 | MC | DSH | 13.6 | 5.3 | 168.0 | 44.2 | 7.7 | 1.4 | 3.7 | |||
| 11 | MC | Mix | 14.3 | 6.2 | 57.0 | 132.6 | 10.6 | 1.5 | 4.2 | 1.046 | Trace | |
| 12 | MC | DSH | 10 | 4.7 | 155 | 83.3 | 97.2 | 11.6 | 1.1 | 3.7 | ||
| 13 | MC | DSH | 9 | 8.7 | 46.6 | 132.6 | 7.4 | 1.4 | 4.1 | |||
| 14 | MC | DSH | 13.7 | 5.6 | 157.0 | 150.3 | 16.8 | 1.7 | 3.8 | 1.040 | 0.30 | |
| 15 | FS | DSH | 5 | 5.2 | 117.0 | 79.6 | 7.3 | 1.3 | 4.4 | 1.025 | Trace | |
| 16 | FS | Maine Coon | 9 | 5.5 | 165 | 49.1 | 97.2 | 7.1 | 1.1 | 4.9 | 1.064 | Trace |
| 17 | MC | DSH | 8 | 5.9 | 190 | 51.9 | 88.4 | 8.7 | 1.0 | 4.8 | 1.031 | 0.30 |
| 18 | FS | Mix | 12.5 | 4.8 | 60.3 | 114.9 | 12.6 | 1.3 | 4.1 | 1.033 | Trace | |
| 19 | MC | Mix | 14.5 | 4.5 | 60.0 | 114.9 | 12.7 | 1.4 | 3.8 | |||
| Group 2 | ||||||||||||
| 20 | FS | Mix | 16.6 | 3.3 | 135 | 117.0 | 106.1 | 8.0 | 1.2 | 4.4 | 1.052 | 1.00 |
| 21 | FS | DSH | 19.3 | 3.3 | 74.1 | 123.8 | 10.7 | 1.2 | 3.9 | 1.025 | 0.10 | |
| 22 | DSH | 14 | 3.5 | 52 | 123.8 | 10.7 | 1.2 | 4.2 | 1.045 | 0.30 | ||
| 23 | FS | DSH | 12 | 6.2 | 240 | 73.3 | 97.2 | 11.1 | 1.2 | 2.8 | 1.042 | 0.30 |
| 24 | FS | DSH | 13 | 8 | 64.9 | 122.0 | 9.4 | 1.5 | 4.2 | 1.045 | 1.00 | |
| 25 | MC | DLH | 12 | 3.2 | 150 | 192.0 | 114.9 | 12.9 | 1.0 | 3.6 | 1.035 | Trace |
| 26 | MC | DSH | 10 | 5.7 | 74.2 | 150.3 | 10.6 | 1.6 | 5.0 | 1.042 | Trace | |
| 27 | MC | DSH | 12 | 3.5 | 112.0 | 88.4 | 9.1 | 1.6 | 4.9 | 1.054 | Trace | |
| 28 | FS | DSH | 12 | 5.5 | 162.0 | 97.2 | 6.7 | 1.6 | 4.0 | 1.038 | 0.30 | |
| 29 | FS | DSH | 6 | 2.7 | 70.9 | 106.1 | 10.2 | 2.3 | 4.8 | 1.015 | Trace | |
| 30 | MC | Siamese | 12 | 2.7 | 140 | 107.0 | 61.9 | 10.5 | 1.3 | 3.5 | 1.016 | Trace |
| 31 | FS | Persian | 12 | 1.4 | 190 | 49.0 | 88.4 | 12.1 | 2.0 | 4.9 | 1.018 | 0.0 |
| 32 | FS | Mix | 15 | 4 | 181.5 | 79.6 | 8.2 | 1.4 | ||||
| 33 | MC | DSH | 13 | 4.2 | 195 | 146.0 | 61.9 | 9.1 | 2.0 | 3.7 | 1.058 | 1.00 |
| 34 | FS | DSH | 15 | 3.6 | 77.2 | 88.4 | 11.1 | 1.3 | 3.5 | |||
| 35 | MC | DSH | 10 | 5.5 | 89.3 | 132.6 | 13.4 | 1.9 | 4.1 | 1.042 | 1.00 | |
| 36 | FS | DSH | 17 | 3.1 | 165 | 72.5 | 123.8 | 12.0 | 1.3 | 5.0 | 1.016 | Trace |
| 37 | FS | DSH | 13 | 2.6 | 220 | 374.0 | 88.4 | 11.4 | 1.5 | 4.1 | 1.023 | Trace |
| 38 | MC | DSH | 14 | 3.7 | 68.2 | 106.1 | 7.9 | 4.6 | 1.030 | Trace | ||
| 39 | FS | DSH | 16 | 3.9 | 66.9 | 132.6 | 12.9 | 1.9 | 4.8 |
Table 2.
Time to development of post-treatment renal azotemia, and selected pre- and post-treatment clinical data in 19 cats (group 2, cats 20–39) that developed renal azotemia following treatment of hyperthyroidism
| Time to azotemia (months) | TT4 (nmol/l) | Creat (μmol/l) | SUN (mmol/l) | Phos (mmol/l) | USG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| 20 | 0.5 | 117.0 | 26.7 | 106.1 | 185.6 | 8.0 | 16.6 | 1.2 | 1.0 | 1.052 | 1.040 † |
| 21 | 5 | 74.1 | 96.9 * | 123.8 | 168.0 | 10.7 | 13.3 | 1.2 | 1.6 | 1.025 | 1.018 |
| 22 | 2 | 52 | 21 | 123.8 | 194.5 | 10.7 | 16.4 | 1.2 | 1.4 | 1.045 | 1.020 |
| 23 | 5 | 73.3 | 21.5 | 97.2 | 168.0 | 11.1 | 11.6 | 1.2 | 1.3 | 1.042 | |
| 24 | 3.5 | 64.9 | 28.3 | 122.0 | 212.2 | 9.4 | 10.5 | 1.5 | 1.1 | 1.045 | 1.025 |
| 25 | 1 | 192.0 | ND ‡ | 114.9 | 168.0 | 12.9 | 12.2 | 1.0 | 1.2 | 1.035 | |
| 26 | 3 | 74.2 | 25.8 | 150.3 | 185.6 | 10.6 | 15.0 | 1.6 | 1.2 | 1.042 | 1.031 † |
| 27 | 4 | 112.0 | 18.1 | 88.4 | 265.2 | 9.1 | 14.1 | 1.6 | 1.7 | 1.054 | 1.015 |
| 28 | 3 | 162.0 | 145 ‡ | 97.2 | 327.1 | 6.7 | 36.4 | 1.6 | 3.3 | 1.038 | 1.010 |
| 29 | 1 | 70.9 | ND * | 106.1 | 176.8 | 10.2 | 15.3 | 2.3 | 3.9 | 1.015 | |
| 30 | 2 | 107.0 | 12.8 | 61.9 | 185.6 | 10.5 | 16.5 | 1.3 | 1.6 | 1.016 | 1.014 |
| 31 | 3 | 49.0 | 25.7 | 88.4 | 221.0 | 12.1 | 31.3 | 2.0 | 3.2 | 1.018 | 1.014 |
| 32 | 6 | 181.5 | <12.87 | 79.6 | 168.0 | 8.2 | 14.9 | 1.4 | 1.0 | ND § | 1.015 |
| 33 | 6 | 146.0 | <12.87 | 61.9 | 176.8 | 9.1 | 16.0 | 2.0 | 1.6 | 1.058 | 1.020 |
| 34 | 0.75 | 77.2 | <12.87 | 88.4 | 176.8 | 11.1 | 11.7 | 1.3 | 1.2 | ND § | 1.030 |
| 35 | 2.7 | 89.3 | 33 | 132.6 | 176.8 | 13.4 | 13.1 | 1.9 | 1.9 | 1.042 | 1.015 |
| 36 | 0.75 | 72.5 | <12.87 | 123.8 | 159.1 | 12.0 | 13.9 | 1.3 | 1.1 | 1.016 | 1.015 |
| 37 | 3 | 374.0 | 18.7 | 88.4 | 176.8 | 11.4 | 17.3 | 1.5 | 1.6 | 1.023 | 1.020 |
| 38 | 1 | 68.2 | 30.89 | 106.1 | 159.1 | 7.9 | 12.2 | 1.2 | 1.030 | 1.015 | |
| 39 | 2 | 66.9 | <12.87 | 132.6 | 132.6 | 12.9 | 16.7 | 1.9 | 1.4 | ND § | 1.012 |
Procedures
Signalment, weight at diagnosis, results of initial hematological profiles, serum biochemistry analysis results, urinalysis, serum TT4 concentrations, systolic blood pressure (when available), and method of treatment for hyperthyroidism were recorded for each cat. Only 14 of 39 (36%) cats had systolic blood pressure measurements recorded at the time of diagnosis of hyperthyroidism (Table 1). Due to the low sample size, we were unable to draw any conclusions from initial blood pressure values. Eleven of 39 (28%) cats had no initial USG measurement recorded; however, these patients were not azotemic at that time and so were included in the study population. Eight of the cats without an initial USG were included in group 1, and three were from group 2. Serum TT4 concentrations were noted at the time renal failure was diagnosed in 18 of 20 cats (90% of group 2). A total of 15 cats were treated with radioactive iodine only, three from group 1 and 12 from group 2. Nineteen cats were treated with methimazole only, 13 cats in group 1 and six cats in group 2. Two cats from each group were treated with methimazole prior to radioactive iodine therapy. One cat in group 1 underwent unilateral thyroidectomy. Finally, the time in months to diagnosis of renal insufficiency after treatment of hyperthyroidism was noted, along with the follow-up time.
Statistical analysis
Groups were compared by use of the Student's _t_-test._P_-values of less than 0.05 were considered significant. All ranges are reported with mean±standard deviation, unless otherwise noted.
Results
Thirty-nine cats diagnosed with hyperthyroidism were eligible for inclusion into in the study. Group 1 was comprised of 13 domestic shorthair (DSH) cats, one domestic medium hair cat, one Maine Coon cat, and four cats listed as mixed breed. Group 2 was comprised of 15 DSH cats, one domestic longhair cat, one Persian cat, one Siamese cat, and two cats listed as mixed breed. Of those 39 cats, 20 (51%) developed renal insufficiency within 2 weeks to 6 months after treatment (2.7±1.8 months).
Group 1 was comprised of seven spayed females, one intact female, and 11 castrated males. Group 2 was comprised of 13 spayed females and seven castrated males. Age differences between groups approached significance (_P_=0.07). Cats in group 1 ranged from 5 to 17.7 years old (11.7±3.1), and group 2 cats ranged from 6.0 to 19.3 years old (13±2.9). The weight range for cats in group 1 was 3.1–8.7 kg (5.0±1.3), and for group 2 was 1.4–8.0 kg (4.0±1.5). This did represent a significant difference (_P_=0.02) with cats in group 2 having, on average, lower weights than those in group 1.
The initial serum TT4 concentration was similar for cats in both groups. The TT4 for group 1 ranged from 40.9 to 185.0 nmol/l (94.0±47.9) and 49.0–374.0 nmol/l for group 2 (111.2±74.9). The reference range for TT4 for the laboratory used was 15.0–48.0 nmol/l. The diagnosis of hyperthyroidism was confirmed by the use of free thyroxine (by equilibrium dialysis) for one of the cats in group 1 with a serum TT4 within the reference range (46.6 nmol/l). The free thyroxine level was 64 pmol/l (reference range 10–50 pmol/l). The other cat in group 1 with a TT4 within the reference range (40.9 nmol/l) was confirmed hyperthyroid with the use of nuclear scintigraphy. The right lobe of the thyroid gland showed increased uptake compared to the salivary glands at 20 and 60 min. Normal uptake was noted in the left lobe. There was no significant difference between groups with respect to serum TT4 concentration (_P_=0.20).
Serum creatinine and SUN concentrations prior to treatment were also similar for both groups. Initial serum creatinine ranged from 44.2 to 150.3 μmol/l (98.4±28.2) for group 1 and 61.9–150.3 μmol/l (104.7±23.6) for group 2. No significant difference with respect to initial serum creatinine was detected (_P_=0.23). Initial SUN concentration for group 1 cats ranged from 4.6 to 16.8 mmol/l (9.4±2.7), and from 6.7 to 13.4 mmol/l (10.4±1.8). No significant difference in initial SUN concentration was detected (_P_=0.10).
No significant difference could be detected with respect to mean pre-treatment USG (_P_=0.12) (Fig 1). Initial USG ranged from 1.018 to 1.070 (1.042±0.016) for cats in group 1 and from 1.015 to 1.058 (1.035±0.014) for cats in group 2. Ten cats in group 2 (50%) had an initial USG equal to or greater than 1.035. Eight cats in group 1 and 3 cats in group 2 did not have pre-treatment USG recorded.
Fig 1.
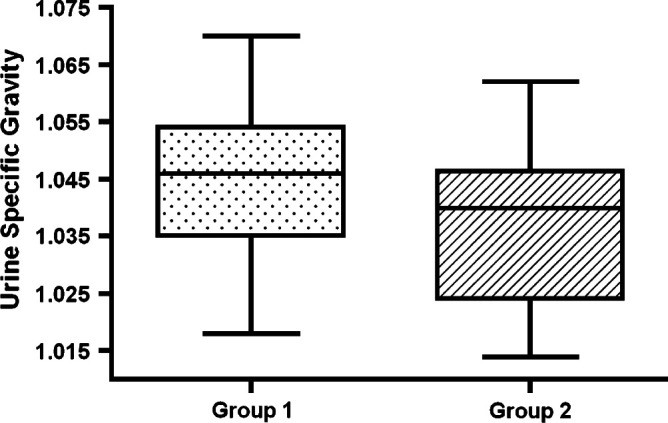
Box plots showing USG measurements in cats that did (group 2) or did not (group 1) develop overt renal insufficiency within 6 months of treatment of hyperthyroidism. The box represents the interquartile range from the 25th to the 75th percentile. The solid horizontal bar through the box represents the mean, and the 10th through 90th percentile is represented by the capped vertical bars.
There was no significant difference in the remaining hematologic, biochemical (including phosphate and electrolyte levels), or urinalysis parameters measured, however, because these would not likely be used in the evaluation of renal function, they will not be reported individually here.
Discussion
No significant differences were detected between the groups with respect to initial serum TT4, creatinine concentrations, SUN concentrations, or urine specific gravities, indicating that these values would not be useful to predict post-treatment renal insufficiency in a given hyperthyroid cat. Any assumption that cats with higher serum TT4 concentrations may have greater renal dysregulation and, therefore, are more likely to experience profound declines in GFR after treatment seems unwarranted based on our findings.
It has been reported that a methimazole trial prior to more permanent treatment for hyperthyroidism may not be necessary if the patient is not azotemic and has well-concentrated urine (Mooney 2005, Garrett 2006). The small group sizes and the fact that 11 of the 39 cats did not have an initial USG recorded may have skewed our results. It is important, however, to note that even if there was no statistical difference between the groups, the study still demonstrates that cats with well-concentrated urine before treatment can develop azotemia after establishment of euthyroidism, and this remains an important finding. Figure 1 shows that many of the cats that developed renal insufficiency subsequent to treatment with radioiodine therapy had USG measurements greater than 1.035, and even as high as 1.058, indicating that there is no suitable cut-off value for USG appropriate for clinical decision-making. Furthermore, because no difference could be found between the groups with respect to initial USG concentrations, no specific value can be used to predict post-treatment renal function.
Due to the low numbers of cats who had a systolic blood pressure measurement recorded, we were unable to assess the effects of blood pressure in this study. This may have been in part due to the fact that hyperthyroid cats are easily stressed and restraint for blood pressure measurement can be difficult. In addition, client financial concerns may have impacted the clinicians' decision to measure systolic blood pressure. Hypertension is thought to be a common complication in feline hyperthyroidism but most commonly occurs in cats with concurrent renal disease (Mooney 2005). Hypertension likely plays a role in the progression of renal disease in cats as loss of autoregulatory mechanisms may lead to the transmission of systemic blood pressures to the glomerulus. Further investigation is needed to evaluate the role of hypertension in cats with hyperthyroidism and concurrent renal disease, especially as it relates to the progression of renal disease unmasked by treatment of hyperthyroidism.
Proteinuria was measured by dipstick in some of the cats of our study population, however, no cats had urine protein to creatinine ratios performed, making accurate evaluation of proteinuria impossible. Proteinuria may be related to the severity of renal disease and may prove to be an indicator of pre-treatment renal disease, but this requires further study.
There was a significant difference in the mean weights of the cats prior to treatment, with the mean weight for cats in group 1 being higher than for cats in group 2. It is possible that cats with lower weights had decreased muscle mass, but body condition score, which may have identified more clearly cats with decreased muscle mass, was not consistently noted in the records. Because creatinine is derived from muscle, it is possible that the pre-treatment creatinine level was artificially lowered in group 2 cats.
The age difference between the two groups approached significance, with cats in group 1 appearing to have been younger, on average, than those in group 2. We feel this is likely due to an increase in the incidence of renal failure with increasing age and does not likely reflect a true difference between the groups (Polzin et al 2005).
The small study group size limited the power in this study to 0.67, with 0.8 being typical. This decrease in power makes it possible that we were unable to detect a difference between the groups when one truly exists. However, because the differences between the groups are likely to be small, it is likely that overlap between the groups will make it impossible to use routine screening tests, in particular USG, to predict post-treatment renal function. In addition, creatinine and SUN have poor sensitivity and specificity for detection of mild renal disease, typically requiring loss of greater than 75% of functioning nephrons to cause a clinically significant elevation. Furthermore, as both of these parameters can be affected by other factors including lean muscle mass, protein content of the diet, and the presence of gastrointestinal disease they are less ideal markers to use. More objective and accurate evaluations of renal function before and after treatment of hyperthyroidism may be useful to determine if accurate predictors exist. In addition, further evaluation to determine the severity and progression of renal insufficiency unmasked by treatment of hyperthyroidism would be beneficial in clinical decision-making, because it is still unknown whether development of post-treatment renal insufficiency has a significant impact on mortality and/or morbidity.
Due to the overlap in the groups, no reliable predictor of renal insufficiency after treatment for hyperthyroidism could be found. This adds weight to the recommendation that methimazole trials should be undertaken, to assess the effect of treatment on renal function, prior to radioiodine therapy or surgical thyroidectomy. The optimal duration for a methimazole trial has not been established, and there are many factors that may influence individual cases including adverse drug reactions, owner and patient compliance, and the ability of oral or topical medications to establish and maintain euthyroidism. Given these confounding factors the duration of a methimazole trial needs to be individualized for each patient. Although no reliable predictor could be identified from routine pre-treatment assessment, it is still valuable to perform these routine tests to identify concurrent medical problems. There are many limitations to our study including its retrospective nature, the small number of cases, 11 cats lacking pre-treatment urinalysis, the lack of blood pressure measurement and lack of assessment of urine protein to creatinine ratios. However, despite these limitations the important conclusion of the study is that cats with well-concentrated urine pre-treatment can still develop azotemia after establishment of euthyroidism. The practice of recommending definitive treatment in cats prior to establishment of euthyroidism with reversible medical management is strongly discouraged until reliable predictors of post-treatment renal function can be identified, and the relationship between post-renal insufficiency and mortality and morbidity is better evaluated.
References
- Adams W.H., Daniel G.B., Legendre A.M. Investigation of the effects of hyperthyroidism on renal function in the cat, Canadian Journal of Veterinary Research 61 (1), 1997a, 53–56. [PMC free article] [PubMed] [Google Scholar]
- Adams W.H., Daniel G.B., Legendre A.M., Gompf R.E., Grove C.A. Changes in renal function in cats following treatment of hyperthyroidism using 131I, Veterinary Radiology and Ultrasound 38 (3), 1997b, 231–238. [DOI] [PubMed] [Google Scholar]
- Becker T.J., Graves T.K., Kruger J.M., Braselton W.E., Nachreiner R.F. Effects of methimazole on renal function in cats with hyperthyroidism, Journal of the American Animal Hospital Association 36 (3), 2000, 215–223. [DOI] [PubMed] [Google Scholar]
- Bradley S.E., Stephan F., Coelho J.B., Reville P. The thyroid and the kidney, Kidney International 6 (5), 1974, 346–365. [DOI] [PubMed] [Google Scholar]
- Broussard J.D., Peterson M.E., Fox P.R. Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983–1993, Journal of the American Veterinary Medical Association 206 (3), 1995, 302–305. [PubMed] [Google Scholar]
- DiBartola S.P., Broome M.R., Stein B.S., Nixon M. Effect of treatment of hyperthyroidism on renal function in cats, Journal of the American Veterinary Medical Association 208 (6), 1996, 875–878. [PubMed] [Google Scholar]
- Garrett L.D. How to refer: the hyperthyroid cat, NAVC Clinician's Brief 4 (4), 2006, 79–82. [Google Scholar]
- Graves T.K., Olivier N.B., Nachreiner R.F., Kruger J.M., Walshaw R., Stickle R.L. Changes in renal function associated with treatment of hyperthyroidism in cats, American Journal of Veterinary Research 55 (12), 1994, 1745–1749. [PubMed] [Google Scholar]
- Klein I., Ojamaa K. Thyroid hormone and the cardiovascular system, New England Journal of Medicine 344 (7), 2001, 501–509. [DOI] [PubMed] [Google Scholar]
- Langston C.E., Reine N.J. Hyperthyroidism and the kidney, Clinical Techniques in Small Animal Practice 21 (1), 2006, 17–21. [DOI] [PubMed] [Google Scholar]
- Mooney C.T. Feline hyperthyroidism diagnostics and therapeutics, Veterinary Clinics of North America 31 (5), 2001, 963–983. [DOI] [PubMed] [Google Scholar]
- Mooney C.T. Hyperthyroidism. Ettinger S.J., Feldman E.D. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 6th edn, 2005, Elsevier Saunders: Saint Louis, 1544–1558. [Google Scholar]
- Mooney C.T., Thoday K.L. CVT update: medical treatment of hyperthyroidism in cats. Bonagura J.D. Kirk's Current Veterinary Therapy XIII, 2000, WB Saunders: Philadelphia, 333–337. [Google Scholar]
- Peterson M.E., Becker D.V. Radioiodine treatment of 524 cats with hyperthyroidism, Journal of the American Veterinary Medical Association 207 (11), 1995, 1422–1428. [PubMed] [Google Scholar]
- Peterson M.E., Kintzer P.P., Hurvitz A.I. Methimazole treatment of 262 cats with hyperthyroidism, Journal of Veterinary Internal Medicine 2 (3), 1988, 150–157. [DOI] [PubMed] [Google Scholar]
- Plantinga E.A., Everts H., Kastelein A.M., Beynen A.C. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets, Veterinary Record 157 (7), 2005, 185–187. [DOI] [PubMed] [Google Scholar]
- Polzin D.J., Osborne C.A., Ross S. Chronic kidney disease. Ettinger S.J., Feldman E.D. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 6th edn, 2005, Elsevier Saunders: Saint Louis, 1762. [Google Scholar]
- Slater M.R., Komkov A., Robinson L.E. Long-term follow-up of hyperthyroid cats treated with iodine-131, Veterinary Radiology and Ultrasound 35 (2), 1994, 204–209. [Google Scholar]
- Trepanier L.A. Medical management of hyperthyroidism, Clinical Techniques in Small Animal Practice 21 (1), 2006, 22–28. [DOI] [PubMed] [Google Scholar]
- Turrel J.M., Feldman E.C., Hays M., Hornof W.J. Radioactive iodine therapy in cats with hyperthyroidism, Journal of the American Veterinary Medical Association 184 (5), 1984, 554–559. [PubMed] [Google Scholar]