PK11195 labels activated microglia in Alzheimer’s disease and in vivo in a mouse model using PET (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 1.
Abstract
Activated microglia may promote neurodegeneration in Alzheimer’s disease (AD) and may also help in amyloid clearance in immunization therapies. In vivo imaging of activated microglia using positron emission tomography (PET) could assist in defining the role of activated microglia during AD progression and therapeutics. We hypothesized that PK11195, a ligand that binds activated microglia, could label these cells in postmortem AD tissues and in vivo in an animal model of AD using PET. [3H](R)-PK11195 binding was significantly higher in AD frontal cortex compared to controls and correlated mainly with the abundance of immunohistochemically labeled activated microglia. With age, the brains of APP/PS1 transgenic mice showed progressive increase in [3H](R)-PK11195 binding and [11C](R)-PK11195 retention in vivo assessed using microPET, which correlated with the histopathological abundance of activated microglia. These results suggest that PK11195 binding in AD postmortem tissue and transgenic mice in vivo correlates with the extent of microglial activation and may help define the role of activated microglia in the pathogenesis and treatment of AD.
Keywords: Alzheimer’s disease, microglia, PET, PK11195, Peripheral benzodiazepine Receptor, Astrocyte
Introduction
AD, the leading cause of dementia, is characterized histopathologicaly by the presence of neurofibrillary tangles and plaques consisting of extracellular amyloid surrounded by activated microglia (McGeer et al., 1988). Activated microglia are thought to secrete neurotoxins that trigger various cellular processes including cell death cascades in neurons (Bianca et al., 1999, Tan et al., 1999). On the other hand, microglial phagocytosis is one of the proposed mechanisms of amyloid clearance from the brain in both animal models and human subjects immunized against amyloid (Bard et al., 2000, Nicoll et al., 2003, Ferrer et al., 2004, Wilcock et al., 2004, Masliah et al., 2005). These studies suggest multiple roles for activated microglia in the pathogenesis and treatment of AD (Morgan et al., 2005). Were it possible to image microglia during life, the dynamic role played by activated microglia in AD and therapies targeted at Aβ clearance might be better defined.
PK11195, a ligand that binds to the peripheral benzodiazepine receptor (PBR) present in low levels in astrocytes and microglia in the normal brain, shows increased uptake in subjects with AD in vivo using PET imaging (Cagnin et al., 2001). Since it is not possible to determine the cellular sources of PK11195 binding in human PET studies in vivo, the contributions of astrocytes versus microglia to PK11195 binding in AD are not known. Further, it is not known if PK11195 labels activated microglia in transgenic mouse models of AD in vivo. To address these questions we hypothesized that PK11195 binding in human brain tissue from AD cases and APP/PS1 Tg mice assessed in vivo using PET correlates with the extent and distribution of microglial activation.
Methods
Animals
The University of Pittsburgh Institutional Animal Care and Use Committee approved all experiments and mice were housed according to standards of the Association for Assessment and Accreditation of Laboratory Animal Care. APPSwe/PSEN1DeltaE9 (APP/PS1) Tg heterozygote (n=9) and control wild type (n=6) mice on a B6C3 background (retired breeders obtained from the Jackson Laboratory, Maine, USA) between the ages of 13–19 months were used in this study.
AD brain tissues
The University of Pittsburgh Institutional Review Board for procurement of human tissues approved all procedures for harvesting post mortem brain tissues and experiments conducted on post mortem brain tissues. Frozen brain tissue from the frontal cortex and the cerebellum of 5 AD cases and 6 control cases were obtained from the University of Pittsburgh Alzheimer’s Disease Research Center neuropathology core. AD cases were grade 3 Tau pathology as proposed by the International Classification of Diseases of the Nervous System (ICDNS, www.icdns.org) (Graeber et al., 2004). 3 of these cases were Braak stage 6 and 2 cases with Braak stage 5, mean age 80 ± 6, 1 male and 4 females. All 6 controls were grade 1 Tau pathology as proposed by ICDNS (2 cases with Braak stage 1–2, 3 cases with Braak stage 1 and 1 case with Braak stage 0, mean age 65 ± 7, 5 male and 1 female. The post mortem interval was less than 12 hrs in all cases.
Filtration and radioligand binding assays
Filtration binding assays were performed as described previously (Venneti et al., 2004). In brief, brain tissues were homogenized (protein concentration 150 to 200 μg) and incubated with 0.5–100 nM [3H](R)-PK11195 (sp. Act., 89.9 Ci/mmol; NEN Life Sciences Products, Boston, MA) at 4°C for 2 hr in a final volume of 250 μl of HEPES (50 mM, pH 7.4). Nonspecific binding was determined by the inclusion of 10 μM PK11195. All samples were run in duplicate. Bmax in fmols/mg protein measuring the maximal number of binding sites and reflective of the total number of receptors and KD, the dissociation constant reported in nM and inversely proportional to the binding affinity of the ligand were determined using PRISM software (Graphpad, San Diego, CA).
PET imaging
High specific activity [11C](R)-PK11195 ([N-methyl-11C]-PK11195) was produced at the University of Pittsburgh PET Facility as previously described (Venneti et al., 2004). Chemical and radiochemical purities were ≥95% with specific activities ≥2.0 Ci/μmol at the end of 40 min synthesis with yields of high purity [11C](R)-PK11195 in the range of 40–100 mCi.
Mice were initially anesthetized by isoflurane induction (5.0%) in a small induction chamber. After anesthetic induction, mice were removed and placed on an open circuit anesthesia system which delivered isoflurane (1–2% as needed) via a nose cone. The exact amount of anesthesia delivered to each animal was not determined, however, the depth of anesthesia was monitored by periodic observations of the respiratory rate of the animals with modulation of the anesthetic concentration to maintain a constant respiratory rate of ~40 breaths/min. APP/PS1 (Tg) (n=9) and control wild type (n=6) mice were injected with [11C](R)-PK11195 (750 –1500 μCi) i.v. and imaged using microPET (Concorde Microsystems, Knoxville TN). Emission data collected over 60 minutes were reconstructed using 2D filtered backprojection and corrected for photon attenuation and scatter. Regional brain radioactivity concentration were summed over the scan frames and normalized to both the injected dose of [11C](R)-PK11195 and the body mass of the animal (%ID/Kg*g) to represent a semi-quantitative measure of [11C](R)-PK11195 retention. Regions-of-interest (ROI) were defined on summed PET images and applied to the dynamic PET images to generate time-activity curves. Due to spatial resolution limitations of microPET and the small size of the mouse brain, it is not practical to define ROIs on small brain structures. Hence ROI parameters were defined in regions approximating the whole mouse brain (indicated by the boxes in figure 3) and applied to all animals. After PET scans, animals were sacrificed and brain tissue was fixed and paraffin embedded for immunohistochemistry.
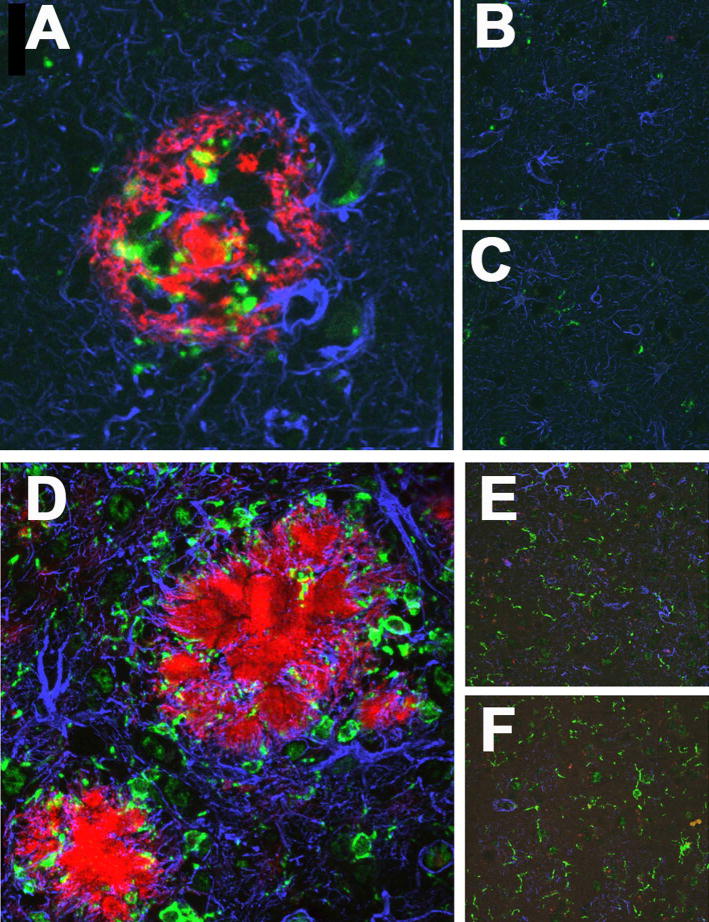
Figure 3. Immunohistochemical evaluation of activated microglia and reactive astrocytosis in AD and Tg mice.
(A–C) Frontal tissue from AD in regions containing plaques (A) showed increased staining for microglia (CD68, green), astrocytes (GFAP, blue) and Aβ (red) compared to plaque free areas in the same cases (B) and controls (C).
(D–F) Frontal tissue from APP/PS1 mice showed increased staining for microglia (Iba-1, green), astrocytes (GFAP, blue) and Aβ (red) in regions containing plaques (D) compared to plaque free areas in the same mice (E) and control, wild type mice (F).
Immunohistochemical labeling and confocal microscopic quantification
Immunostaining and laser confocal microscopic quantification were performed as described previously (Venneti et al., 2004). Paraffin-embedded brain sections from the frontal cortex of transgenic mice, AD cases and controls were stained with antibodies to GFAP (rabbit polyclonal, DAKO, Carpinteria, CA) or Aβ (mouse monoclonal, DAKO) used at concentrations 1:1000 and 1:100 respectively. Activated microglia were stained with antibodies to Iba1 (rabbit polyclonal, cat # 01–1974, WAKO chemicals, Richmond, VA) or CD68 (mouse monoclonal, DAKO) at concentrations of 1:1000 and 1:100 in mouse and human brain tissues respectively. Immunostained sections were scanned and quantified on a laser confocal microscope equipped with an argon laser with 458 nm, 477 nm, 488 nm and 514 nm primary emission lines (LSM 150, Zeiss, Heidelberg, Germany). Each section was scanned along the _z_-axis to define the middle optical plane used in quantification (262,144 pixels/plane; 1 pixel= 0.25 μm2). Scanning parameters such as laser power aperture, gain, and photomultiplier tube settings were kept constant for each wavelength.
Activated microglial and reactive astrocytes were quantified in Tg and AD brain frontal cortical tissues in regions containing plaques (including an area surrounding and inside the plaques) and compared to regions without plaques and with controls. In each case, an individual blinded to the experimental design imaged 10 such areas (40X) encompassing 106,100 μm2. For each cell phenotype scanned, contribution to signal intensity from autofluorescence was minimized using a threshold that was kept constant. In each area the average pixel fluorescence and the pixel counts for a given cell phenotype marker that exceeded the threshold were enumerated. The average pixel fluorescence was multiplied by the total number of pixels to represent the total florescence for that cell phenotype marker in that area. The total fluorescence values determined from the 3 scanned areas in one brain region were averaged to represent a measure of the cell phenotype in that brain region.
Statistical analysis
Data were analyzed using PRISM software (Graphpad, San Diego, CA). One-way ANOVA tests with 95% confidence intervals were used to analyze data. Two-way ANOVA using the weights of the animals as a co-variable was used to analyze [11C](R)-PK11195 PET retention data. Nonparametric correlations using 95% confidence intervals were performed to quantify the relationship between (R)-PK11195 binding in AD and Tg mice with various parameters described in Table 1. Results from correlation analyses are represented by r, the Spearman’s coefficient.
Table 1.
(R)-PK11195 binding correlates best with activated microglia
| Parameter | Marker | Statistical value | [3H](R)-PK11195 binding in AD post mortem tissues | [3H](R)-PK11195 binding in APP/PS1 brain tissues | [11C](R)-PK11195 retention in APP/PS1 mice in vivo |
|---|---|---|---|---|---|
| Microglia | CD68/Iba1 | r value | 0.9393 | 0.9657 | 0.7690 |
| p value | 0.0002 | 0.0007 | 0.0433 | ||
| Astrocytes | GFAP | r value | 0.6729 | −0.3203 | 0.4100 |
| p value | 0.0390 | 0.5360 | 0.3610 | ||
| Age | Months | r value | NA | 0.9823 | 0.7656 |
| p value | NA | 0.0005 | 0.0162 |
Results
[3H](R)-PK11195 binding is higher in AD frontal cortex
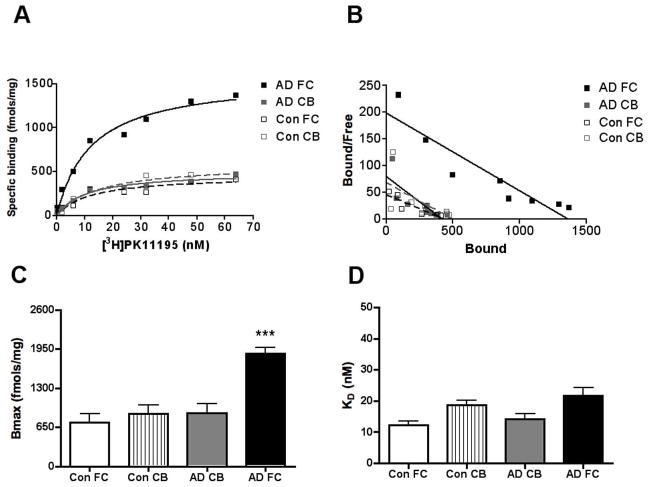
We used saturation filtration binding to compare [3H](R)-PK11195 specific binding in AD with controls. Bmax, reflective of the number of binding sites was significantly higher in the frontal cortex of AD brain tissue but did not differ in the cerebellum (Figure 1A–C, _p_=0.0002). The binding affinity of [3H](R)-PK11195 reflected by the KD did not differ between AD and control brain tissue in either brain region (Figure 1D, _p_=0.3187).
Figure 1. [3H](R)-PK11195 is higher in the AD frontal cortex.
Filtration binding with [3H](R)-PK11195 was assessed in the frontal cortex (FC) and cerebellum (CB) of AD cases (n=5) and controls (Con) (n=6).
(A & B) Representative curves (A) and scatchard plots (B) from the FC (black squares) of AD showed significantly higher specific binding (per mg protein) than brain tissue obtained from the CB of AD cases (gray squares), FC (black open squares) and CB (gray open squares) brain tissue obtained from controls.
(C) Bmax (reflective of the total number of binding sites) with [3H](R)-PK11195 was significantly higher in AD FC (black bars) compared with AD CB (gray bars), FC (clear bars) and CB (hatched bars) tissue obtained from controls (_p_=0.0002).
(D) The KD (reflective of the binding affinity) was not significantly different in all the conditions (_p_=0.3187). Data was analyzed using one-way ANOVA with 95% confidence intervals.
[3H](R)-PK11195 binding increases in transgenic mice with age
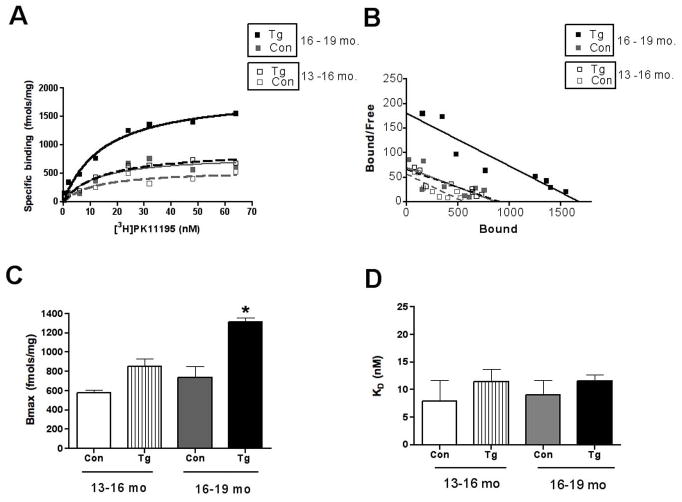
Tg animals, but not wild type control animals showed an age dependent increase [3H](R)-PK11195 binding (Figure 2A–C, Table 1). Bmax values were significantly higher in animals between the ages of 16–19 months compared to controls and Tg and controls between the ages of 13–16 months (Figure 2A–C, _p_=0.0023). The binding affinity of [3H](R)-PK11195 reflected by the KD did not differ between Tg and control brain tissue in both age groups (Figure 2D, _p_=0.7249).
Figure 2. [3H](R)-PK11195 increases in Tg mice with age.
Filtration binding with [3H](R)-PK11195 was assessed in the frontal cortex (FC) of Tg mice and wild type controls (Con) in the age groups 13–16 and 16–19 months (n=3, each).
(A & B) Representative curves (A) and scatchard plots (B) of Tg mice in the age groups 16–19 months (black squares) showed significantly higher specific binding (per mg protein) than Tg mice in the age group 13–16 months (gray squares) and both control (black and gray squares) groups.
(C) Bmax (reflective of the total number of binding sites) with [3H](R)-PK11195 was significantly higher in Tg mice in the age groups 16–19 months (black squares) than Tg mice in the age group 13–16 months (gray bars), and both control groups (clear and hatched bars) (_p_=0.0023).
(D) The KD (reflective of the binding affinity) was not significantly different in all the conditions (_p_=0.7249). Data was analyzed using one-way ANOVA with 95% confidence intervals.
Increased [3H](R)-PK11195 binding correlates best with activated microglia in AD postmortem tissue
The abundance of activated microglia and reactive astrocytes, assessed by CD68 and GFAP staining respectively was determined in the frontal cortex of AD postmortem tissue and controls in the same cases used for filtration binding analyses. Microglia were significantly increased in AD in areas containing plaques compared to areas with no plaques and controls (Figure 3A–C and Figure 4A, p < 0.0001). GFAP staining was also significantly higher in regions with plaques compared to plaque free areas and control brain tissue (Figure 3A–C and Figure 4B, p = 0.0003).
Figure 4. Quantification of activated microglia and reactive astrocytosis in AD and Tg mice.
(A & B) Microglia stained with CD68 (A) and astrocytes with GFAP (B) were quantified in AD frontal cortical tissue (n=5, each). Regions containing plaques had significantly higher microglia (A, black bars, p < 0.0001) and astrocytes (B, black bars, p = 0.0003) than regions without plaques (A & B, grey bars) and controls (A & B, clear bars).
(C & D) Microglia stained with Iba-1 (C) and astrocytes stained with GFAP (D) were quantified in APP/PS1 (Tg) and wild type control (Con) mice in two age groups: 13–16, and 16–19 months. Regions containing plaques had significantly higher microglia (C, black bars) in Tg (n=5) compared to controls (n=3) (D, hatched bars) mice in the 16–19 months age group (p = 0.048), but not in Tg (n=4) and control (n=3) mice in the 13–16 month age groups (C, clear and grey bars, p = 0.6383). GFAP labeled astrocytes were significantly higher in Tg mice compared to controls in both 13–16 (D, clear and grey bars, p = 0.0416) and 16–19 (D, hatched and black bars, p = 0.0224). Data was analyzed using ANOVA with 95% confidence intervals, ***p<0.001, **p<0.01, *p<0.05.
To determine the cell type responsible for PK11195 binding we determined the relationship between [3H](R)-PK11195 specific binding (Bmax) with the abundance of both immunolabeled activated microglia and astrocytes. [3H](R)-PK11195 binding correlated significantly with the abundance of CD68 stained microglia (Figure 5A, r = 0.9393, p = 0.0002) and to a lesser extent with the distribution of astrocytes (Figure 5B, r = 0.6729, p = 0.0390) (Table 1).
Figure 5. [3H](R)-PK11195 binding corrleates with activated microglia in AD and Tg brain tissues.
(A & B) [3H](R)-PK11195 Bmax (Y-axis, fmols/mg) values derived from AD and control cases correlated with the abundance of CD68 labeled activated microglia (A, X-axis, CD68 units) assessed in the same area in these same cases (r = 0.9393, p = 0.0002). [3H](R)-PK11195 Bmax values (Y-axis, fmols/mg) correlated weakly with the extent of reactive astrocytosis (B, X-axis, GFAP units, r = 0.6729, p = 0.0390).
(C & D) [3H](R)-PK11195 Bmax (Y-axis, fmols/mg) values derived from Tg and controls correlated with the abundance of Iba-1 labeled activated microglia (C, X-axis, Iba-1 units) assessed in the same area in these same animals (r = 0.9657, p = 0.0007). [3H](R)-PK11195 Bmax values (Y-axis, fmols/mg) did not correlated with the extent of reactive astrocytosis (D, X-axis, GFAP units, r = -0.3203, p = 0.5360).
Increased [3H](R)-PK11195 binding in older Tg animals correlates with activated microglia
The abundance of activated microglia and reactive astrocytes, assessed by Iba1 and GFAP staining respectively was determined in the frontal cortex of Tg animals and wild type controls in the same cases used for filtration binding analyses. Iba-1 staining did not differ in mice in the age groups of 13–16 months from controls (4 C, p = 0.6383), but was significantly higher in mice between the ages of 16–19 month compared to the rest of the animals (Figure 3 and Figure 4C, p = 0.048). GFAP staining was significantly higher in Tg mice in both age groups compared to controls in regions containing plaques (Figure 4D, p = 0.0224). Both Iba-1 and GFAP staining in Tg mice in regions devoid of plaques did not differ from controls (Figure 3E and F). [3H](R)-PK11195 binding in these same animals correlated significantly with the abundance of Iba1stained microglia (Figure 5C, r = 0.9657, p = 0.0017) but not with the distribution of GFAP labeled astrocytes (Figure 5D, r = −0.3203, p = 0.5360) (Table 1).
In vivo retention of [11C](R)-PK11195 in the brain of older Tg mice correlates with the abundance of microglia
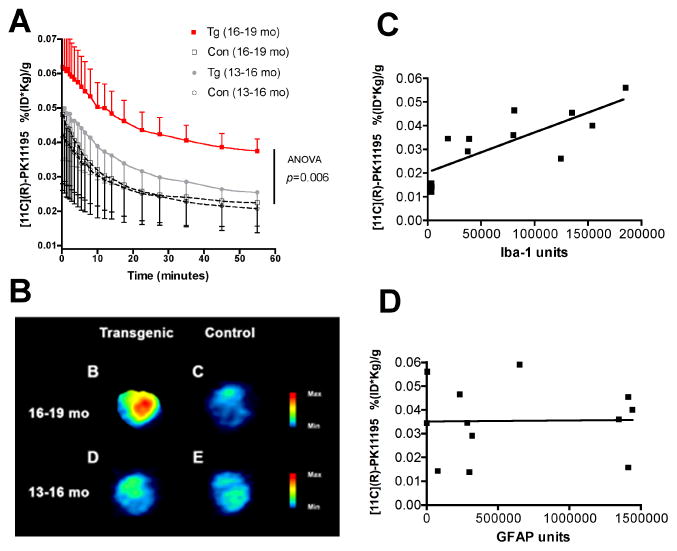
PET imaging using [11C](R)-PK11195 was assessed in 9 APP/PS1 Tg and 6 control mice between the ages of 13 to 19 months based on animal availability. Tg animals showed an age dependent increase in brain retention of [11C](R)-PK11195 in animals between the ages of 16–19 months compared to controls and Tg and controls between the ages of 13–16 months (Figure 6A-B, Two way ANOVA, _p_=0.006 and Table 1). As [11C](R)-PK11195 retention in the brains of Tg and control mice were normalized to the weights of the animals, we determined if the weights of the animals in both Tg and control groups in the two age groups were significantly different. The mean weights (grams) along with standard deviations of the animals are follows: (1) Tg 13–16 months: 37±10, (2) control 13–16 months: 31±2, (3) Tg 16–19 months: 33±8 and (4) control 16–19 months: 26±4. The weights on comparison were not significantly different (p = 0.2242, ANOVA). Further, correlational analyses between the weights of the animals and [11C](R)-PK11195 retention did not show any significant relationship ((r = 0.2981, p = 0.3466). Finally, in vivo [11C](R)-PK11195 retention correlated significantly with Iba-1 stained microglia (Figure 6C, r = 0.7690, p = 0.0433), but not with GFAP stained astrocytes (Figure 6D, r = 0.4100, p = 0.3610) (Table 1).
Figure 6. PET imaging with [11C](R)-PK11195 shows an age-dependent increase in APP/PS1 (Tg) mice, but not controls.
(A) APP/PS1 (Tg) and wild type control (Con) mice in the age groups 13–16 and 16–19 months were imaged with microPET using [11C](R)-PK11195. Retention of the radioligand in the brain is represented as radioactivity concentrations summed over the scan frames and normalized to both the injected dose of [11C](R)-PK11195 and the body mass of the animal (%ID/g*kg, Y-axis) and is plotted against time in minutes (X-axis). [11C](R)-PK11195 retention was not different between Tg (n=4, grey squares) and Con (n=3, clear squares) in the 13–16 month age group, but was higher in Tg mice 16–19 month age group (n=5, red squares) compared to all the other animal groups (_p_=0.001). Data was analyzed using one-way ANOVA with 95% confidence intervals.
(B) Representative coronal PET images from 16–19 month animals (Tg, and control) and 13–16 month animals (Tg, and control) showing increased [11C](R)-PK11195 retention in the 16–19 month Tg compared to all the other animal groups.
(C and D) [11C](R)-PK11195 binding values (%ID/g*kg, Y-axis) derived from Tg and controls correlated with the abundance of Iba-1 labeled activated microglia (C, X-axis, Iba-1 units) assessed in the same area in these same animals (r = 0.7690, p = 0.0433). [11C](R)-PK11195 binding values (%ID/g*kg, Y-axis) did not correlated with the extent of reactive astrocytosis (D, X-axis, GFAP units, r = 0.4100, p = 0.3610)
Discussion
Activated microglia as sources of neurotoxins have been hypothesized to promote the degenerative process in AD (Smith et al., 1997, Bianca et al., 1999, Tan et al., 1999). However, recent evidence suggests that the ability of activated microglia to phagocytose Aβ promotes the clearance of amyloid deposits. The roles played by activated microglia in both the pathogenesis and treatments of AD may be better delineated by in vivo longitudinal assessments of activated microglia in AD.
We sought to determine if PK11195 could label microglial activation in AD postmortem tissue and in vivo in APP/PS1 transgenic mice. In AD tissues, [3H](R)-PK11195 binding was increased in the frontal cortex but not the cerebellum, suggesting that the increase in [3H](R)-PK11195 binding was specific to regions of AD pathology, as the cerebellum is usually not affected until end-stage disease (Figure 1). [3H](R)-PK11195 binding increased in an age dependent manner in frontal cortical tissues of APPS/PS1 mice, but not in control wild type mice, consistent with progression in pathology (Borchelt et al., 1997) (Figure 2 and Table 1). This was paralleled by an age dependent increase in both [11C](R)-PK11195 retention and the abundance of activated microglia in Tg mice between the ages of 16–19 months compared to Tg mice between the ages 13–16 months and control wild type mice in both age groups (Figure 3–6). In both AD tissues and Tg mice, PK11195 binding correlated with the abundance of activated microglia. In AD postmortem tissues, but not Tg mice, [3H](R)-PK11195 binding correlated weakly with reactive astrocytosis (Figure 5 and Table 1). However, both in vivo [11C](R)-PK11195 retention and [3H](R)-PK11195 binding in brain tissues in Tg mice did not show significant correlations with GFAP labeled astrocytes. While PK11195 binding in brain tissues as well as in vivo correlated with the abundance of Iba-1 labeled activated microglia, this correlation was more robust in brain tissues compared to in vivo PET data. This discrepancy may be due to differences in resolution and sampling size between PET, postmortem tissue-filtration binding analyses, and histopathological assessment. Neuropathological studies were performed on sections from the frontal cortex and postmortem binding analysis was restricted to a mass of frontal cortical brain tissue (~100 mg) in contrast to whole brain estimates in PET analyses due to limited spatial resolution. However, despite these limitations, in both postmortem tissue and in vivo PET analyses PK11195 binding correlated with the histopathological abundance of activated microglia, but not reactive astrocytes. Ubiquitous distribution of pathology in the APP1/PS1 Tg brains, microPET resolution limitations and the small size of the mouse brain result in a uniform PET signals without discernment of specific anatomical brain areas. Moreover, the rate of clearance of [11C](R)-PK11195 is considerably faster than many other widely used PET radioligands (e.g. [11C]McN5652 and [11C]α-CFT) leading to a very low ratio of specific to non-specific binding. The small brain and blood volume of the laboratory mouse, as well as the finite spatial resolution of the microPET instrumentation, further limit the degree of quantitative information that can be gleaned from in vivo microPET studies of mice. Despite these limitations, useful observations are still possible using this technology. In this case, the microPET studies indicate that it is conceivable to demonstrate the co-localization of microglial activation and Alzheimer’s pathology non-invasively in human subjects, which could ultimately provide a missing piece of the puzzle of the pathogenesis of AD. These results support the overall hypothesis that PK11195 binds to activated microglia in AD post mortem tissues and can be used to image activated microglia in vivo in transgenic mouse models of AD.
Initial studies with [11C]PK11195 in subjects with AD failed to show differences from controls (Groom et al., 1995). A subsequent imaging study with the higher affinity R-enatimomer, [11C](R)-PK11195 showed increased PET ligand retention in various brain regions such as the temporal gyri, cingulate cortex and entorhinal cortex in 8 subjects with AD compared to controls (Cagnin et al., 2001). However, the cellular source of [11C](R)-PK11195 binding cannot be determined in human subjects in vivo. Further, it is not known if [11C](R)-PK11195 can be used to image activated microglia in Tg mouse models of AD. We addressed these questions by comparing [3H](R)-PK11195 binding in AD postmortem tissues with histopathological assessments of both activated microglia and astrocytes from the same brain regions derived from the same cases. Furthermore, animal models provide the advantage of direct comparisons of in vivo PET data with histopathologic findings. Our data suggest that the majority of PK11195 binding in both human AD tissue and from Tg mice in vivo correspond to the histological abundance of activated microglia.
The relative contributions of astrocytes versus microglia to PK11195 binding in neurological disorders is a subject of continuing debate. Several reports suggest that [3H](R)-PK11195 corresponds to activated microglia in rat models of stroke (Myers et al., 1991) and ischemia (Stephenson et al., 1995), experimental autoimmune encephalitis (Vowinckel et al., 1997), multiple sclerosis (Vowinckel et al., 1997, Banati et al., 2000), facial nerve axotomy in rats (Banati et al., 1997), brain trauma in rats (Raghavendra Rao et al., 2000), SIV encephalitis in macaques (Venneti et al., 2004) and hippocampal lesions in rodents (Pedersen et al., 2006). [3H](R)-PK11195 biniding to astrocytes following an initial increase in microglia is observed in brain tissues obtained from rodents treated with the neurotoxin trimethyltin (Kuhlmann et al., 2000) and cuprizone (Chen et al., 2004). We addressed this question in AD by comparing immunohistochemical distribution of activated microglia and astrocytes with [3H](R)-PK11195 binding. In human AD tissues, reactive astrocytosis certainly seem to contribute to [3H](R)-PK11195 binding. However, statistical parameters suggest that [3H](R)-PK11195 binding is better correlated the abundance of activated microglia. In Tg mice, we observed significant correlations with both [3H](R)-PK11195 and [11C](R)-PK11195 to activated microglia only. This may possibly reflect one of the many differences between AD and mouse models of the disease. For example, the PET ligand PIB binding in mice is different from that seen in AD suggesting that the amyloid deposits in humans and transgenic mice may not be the same (Klunk et al., 2005). While our data suggest that PK11195 binding in Tg mice correlates with the abundance of activated microglia and not reactive astrocytes, it is possible that this may not entirely reflect the in vivo nature of PK11195 binding in human subjects with AD. Another distinction between human AD and Tg mice brain tissue was the difference in the KD values with values in AD tissues being approximately two fold higher compared to Tg tissues. This might likely reflect a species variation as this difference in KD values were observed in controls as well. Alternatively, it is likely that postmortem time intervals as well as archival periods of human brain tissue might influence the qualtity of tissues in contrast to animal studies where these variables are more easily controlled.
While the deposition of Aβ has been well documented in mouse models, the extent of microglial activation reported varies with both the histological markers used and the type and extent of transgene(s) expressed in mice (Dickson, 1999, Morgan et al., 2005). We observed significant increases in Iba-1 staining in Tg mice from controls only after 16 months of age, which corresponded closely with PET retention of [11C](R)-PK11195 in these same animals. These data are similar to age dependent increases in activation of microglia reported in APP/PS1 and other transgenic models (Stalder et al., 1999, Kitazawa et al., 2005, Simard et al., 2006). The animals obtained form Jackson laboratories (see methods) were between 13 – 19 months of age and defined the starting and cut off age points of this study. We were unable to assess both Iba-1 staining, [3H](R)-PK11195 binding and [11C](R)-PK11195 PET imaging in animals younger than 13 months or older than 19 months of age due to limitations of animal availability.
The role of activated brain microglia in AD is not clearly understood (reviewed in (Bamberger et al., 2001, Schenk et al., 2002)). The neurotoxic role of brain macrophages draws support from several in vitro studies (reviewed in (Gonzalez-Scarano et al., 1999, Benveniste et al., 2001)), while other studies suggest a beneficial role of activated microglia in promoting phagocytosis of Aβ (Malm et al., 2005, Simard et al., 2006). Moreover, clinical trials aimed at reducing the extent of inflammation in the brain have suggested either reduction in the occurrence of the disease prior to the onset of AD (Stewart et al., 1997, in t’ Veld et al., 2001) or no effect in patients with mild-moderate AD (Aisen et al., 2003). Activated microglia may also play a role in immunization therapies by promoting phagocytosis of Aβ (Schenk et al., 1999, Bard et al., 2000, Nicoll et al., 2003, Ferrer et al., 2004). Detecting activated microglia in vivo longitudinally in AD as well as Tg models of AD may be of vital importance in defining the role of these cells in progression of disease as well as the effectiveness of therapeutic strategies targeting modulation of the immune system. Our studies suggest that PK11195 may be one such tool, which may help delineate the role of activated microglia in the pathogenesis and treatment of AD.
Acknowledgments
We thank Aaron Schmookler, William Paljug, Jonette Werley, James Kasenchak, Jason Nugyen, and Susan Slagel for technical help and James Ruskiewicz for help with the PET scans. This work was supported by the National Institutes of Health RO1 MH64921 (CAW), K24 MH01717 (CAW), R21 AG025829 (CAW), RO1 AG020226 (WEK) and P50 AG05133-21-Alzheimer Disease Research Center (RLH).
Footnotes
Disclosure statement: All authors declare no proprietary interest or any conflict of interest related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Landreth GE. Microglial interaction with beta-amyloid: implications for the pathogenesis of Alzheimer’s disease. Microsc Res Tech. 2001;54:59–70. doi: 10.1002/jemt.1121. [DOI] [PubMed] [Google Scholar]
- Banati RB, Myers R, Kreutzberg GW. PK (‘peripheral benzodiazepine’)--binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol. 1997;26:77–82. doi: 10.1023/a:1018567510105. [DOI] [PubMed] [Google Scholar]
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123(Pt 11):2321–2337. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Benveniste EN, Nguyen VT, O’Keefe GM. Immunological aspects of microglia: relevance to Alzheimer’s disease. Neurochem Int. 2001;39:381–391. doi: 10.1016/s0197-0186(01)00045-6. [DOI] [PubMed] [Google Scholar]
- Bianca VD, Dusi S, Bianchini E, Dal Pra I, Rossi F. beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J Biol Chem. 1999;274:15493–15499. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Chen MK, Baidoo K, Verina T, Guilarte TR. Peripheral benzodiazepine receptor imaging in CNS demyelination: functional implications of anatomical and cellular localization. Brain. 2004;127:1379–1392. doi: 10.1093/brain/awh161. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Microglia in Alzheimer’s disease and transgenic models. How close the fit? Am J Pathol. 1999;154:1627–1631. doi: 10.1016/S0002-9440(10)65416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Lowe J, Radotra B. A free community approach to classifying disease. PLoS Med. 2004;1:e16. doi: 10.1371/journal.pmed.0010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom GN, Junck L, Foster NL, Frey KA, Kuhl DE. PET of peripheral benzodiazepine binding sites in the microgliosis of Alzheimer’s disease. J Nucl Med. 1995;36:2207–2210. [PubMed] [Google Scholar]
- in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, Abrahamson EE, Debnath ML, Holt DP, Huang GF, Shao L, DeKosky ST, Price JC, Mathis CA. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer’s disease brain but not in transgenic mouse brain. J Neurosci. 2005;25:10598–10606. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem. 2000;74:1694–1704. doi: 10.1046/j.1471-4159.2000.0741694.x. [DOI] [PubMed] [Google Scholar]
- Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’;s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE. Macrophage and astrocyte populations in relation to [3H]PK11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab. 1991;11:314–322. doi: 10.1038/jcbfm.1991.64. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Pedersen MD, Minuzzi L, Wirenfeldt M, Meldgaard M, Slidsborg C, Cumming P, Finsen B. Up-regulation of PK11195 binding in areas of axonal degeneration coincides with early microglial activation in mouse brain. Eur J Neurosci. 2006;24:991–1000. doi: 10.1111/j.1460-9568.2006.04975.x. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol. 2000;161:102–114. doi: 10.1006/exnr.1999.7269. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schenk DB, Yednock T. The role of microglia in Alzheimer’s disease: friend or foe? Neurobiol Aging. 2002;23:677–679. doi: 10.1016/s0197-4580(02)00034-9. discussion 683–674. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci. 1995;15:5263–5274. doi: 10.1523/JNEUROSCI.15-07-05263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286:2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wang G, Bissel SJ, Mathis CA, Meltzer CC, Boada F, Capuano S, 3rd, Kress GJ, Davis DK, Ruszkiewicz J, Reynolds IJ, Murphey-Corb M, Trichel AM, Wisniewski SR, Wiley CA. PET imaging of brain macrophages using the peripheral benzodiazepine receptor in a macaque model of neuroAIDS. J Clin Invest. 2004;113:981–989. doi: 10.1172/JCI20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowinckel E, Reutens D, Becher B, Verge G, Evans A, Owens T, Antel JP. PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci Res. 1997;50:345–353. doi: 10.1002/(SICI)1097-4547(19971015)50:2<345::AID-JNR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, Wilson D, Wilson N, Freeman MJ, Gordon MN, Morgan D. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]