Lymphocyte/Macrophage Interactions: Biomaterial Surface-Dependent Cytokine, Chemokine, and Matrix Protein Production (original) (raw)
. Author manuscript; available in PMC: 2013 Dec 18.
Published in final edited form as: J Biomed Mater Res A. 2008 Dec 1;87(3):10.1002/jbm.a.31630. doi: 10.1002/jbm.a.31630
Abstract
The role of lymphocytes in the biological response to synthetic polymers is poorly understood despite the transient appearance of lymphocytes at the biomaterial implant site. To investigate cytokines, chemokines, and extracellular matrix (ECM) proteins produced by lymphocytes and macrophages in response to biomaterial surfaces, human peripheral blood monocytes and lymphocytes were co-cultured on polyethylene terephthalate (PET)-based material surfaces displaying distinct hydrophobic, hydrophilic/neutral, hydrophilic/anionic, and hydrophilic/cationic chemistries. Antibody array screening showed the majority of detected proteins are inflammatory mediators that guide the early inflammatory phases of wound healing. Proteomic ELISA quantification and adherent cell analysis were performed after 3, 7, and 10 days of culture. IL-2 and IFN-γ were not detected in any co-cultures suggesting lack of lymphocyte activation. The hydrophilic/neutral surfaces increased IL-8 relative to the hydrophobic PET surface (p<0.05). The hydrophilic/anionic surfaces promoted increased TNF-α over hydrophobic and cationic surfaces and increased MIP-1β compared to hydrophobic surfaces (p<0.05). Since enhanced macrophage fusion was observed on hydrophilic/anionic surfaces, the production of these cytokines likely plays an important role in the fusion process. The hydrophilic/cationic surface promoted IL-10 production and increased matrix metalloproteinase (MMP)-9/tissue inhibitor of MMP (TIMP) relative to hydrophilic/neutral and anionic surfaces (p<0.05). These results suggest hydrophilic/neutral and anionic surfaces promote pro-inflammatory responses and reduced degradation of the ECM, whereas the hydrophilic/cationic surfaces induce an anti-inflammatory response and greater MMP-9/TIMP with an enhanced potential for ECM breakdown. The study also underscores the usefulness of protein arrays in assessing the role of soluble mediators in the inflammatory response to biomaterials.
Keywords: PET biomaterials, lymphocytes, macrophages, cytokines, matrix metalloproteinases
Introduction
The development of novel biomaterials, biomedical devices, or tissue-engineered constructs necessitates a thorough understanding of the biological responses to implanted materials. Lymphocytes and macrophages both exist at the implant site, but lymphocytes appear transiently at the implant site and predominately during the chronic inflammation phase.1,2 To date, the majority of studies center around investigating the role of macrophages in inflammation, wound healing, and the foreign body reaction subsequent to biomaterial implantation. Meanwhile, lymphocyte activities and interactions with macrophages in response to synthetic polymers are unclear.
Although the lymphocyte role in the biological response to biomaterials is poorly understood, there is evidence that lymphocytes can participate in this response. Lymphocytes have been shown to adhere to biomaterial surfaces in vitro.3–6 In lymphocyte/macrophage co-cultures, adherent lymphocytes are predominately associated with macrophages rather than the biomaterial surface indicating molecular interactions between the two cells (i.e. juxtacrine interaction).7 IL-4 and IL-13, known lymphokines, have been demonstrated to participate in macrophage fusion to form foreign body giant cells (FBGCs).8–10 In vitro lymphocyte/macrophage interactions at the material surface have been shown to enhance the adhesion and fusion of macrophages as well as stimulate lymphocyte proliferation primarily through paracrine-mediated mechanisms.7 Therefore, both direct (juxtacrine) and indirect (paracrine) mechanisms of lymphocyte/macrophage interactions may play an integral part in the inflammatory and wound healing events that occur at the implant site.
The lymphocyte population, consisting of T lymphocytes (T cells), B lymphocytes (B cells), and natural killer (NK) cells, responds to stimuli utilizing various mechanisms of action. B cells are involved in the recognition of foreign substances and producing antibodies for the elimination of the antigens. NK cells are known for mediating the killing of cells by inducing apoptosis. T lymphocytes, which comprise the largest percentage, are divided into cytotoxic (CD8+) and T helper (CD4+) subpopulations. The CD8+ cells destroy cells in a similar manner as NK cells. CD4+ T cells are further separated into type 1 (Th1) and type 2 (Th2) T helper subsets. These T cells can communicate and direct other cells types either directly or indirectly through soluble factors (i.e. cytokines) to induce a variety of responses.
T lymphocytes are capable of engaging in juxtacrine cell-cell interactions with macrophages in immune activation. Macrophages can act as antigen presenting cells (APC) to initiate an immune response by phagocytosing, processing, and presenting foreign materials to lymphocytes. As a result, stimulated T lymphocytes can secrete interleukin-2 (IL-2), which mediates activation and proliferation of lymphocytes. Cellular activation can also result in secretion of varying effector molecules. For instance, Th1 type T cells produce IFN-γ, IL-2, and TNF-β while Th2 type T cells produce IL-4, IL-5, IL-10, and IL-13.11 Contact-mediated activation of macrophages by activated lymphocytes triggers production of reactive oxygen species (ROS), nitric oxide (NO), IL-1β, and TNF-α.11,12
T lymphocytes and macrophages are not only capable of activating each other but they are also able to induce immune suppressive effects. For instance, T cell receptor (TCR) activation by macrophage major histocompatibility complex (MHC) without a secondary co-stimulatory signal can render the T lymphocyte unresponsive (anergy); as a result, the T cells fail to proliferate.13 Additionally, macrophages can drive the differentiation of regulatory T cells capable of actively suppressing immune responses via the expression of inhibitory cell surface molecules and/or generation of IL-10 and TGF-β.14 These are mechanisms for active suppression of the immune response to self and foreign antigen and establishing peripheral tolerance.
Material surfaces have been shown to be capable of dictating lymphocyte and macrophage behavior. Marques et al. demonstrated that in mixed populations of monocytes/macrophages and lymphocytes, starch-based polymers and poly-L-lactide induced different levels of macrophage activation as measured by cytokine secretion.15 Trinidade et al. did not find a synergistic macrophage and lymphocyte cytokine response to orthopedic biomaterials; however, their investigation focused primarily on IL-6 and TNF-α production. Previous reports from our laboratory have shown that material surface chemistries are capable of modulating surface-adherent macrophage activation as measured by cytokine, chemokine, and matrix protein production.16,17 Using our lymphocyte/macrophage co-cultures system, our laboratory has demonstrated that material surface chemistries can dictate macrophage adhesion, fusion, and lymphocyte proliferation. In addition, paracrine interactions between lymphocytes and macrophages via soluble factors were shown to stimulate macrophage fusion.18 We hypothesized that biomaterial surface chemistries could modulate the production of inflammatory mediators from lymphocytes and macrophages in response to interactions with biomaterials. This study investigated the cytokines, chemokines, and extracellular matrix proteins produced by lymphocyte/macrophage interactions using a protein array as a general screening approach in response to biomaterial surfaces displaying distinct hydrophobic, hydrophilic/neutral, and hydrophilic/charged surfaces. Biomaterial-dependent differences in soluble mediator production were identified and correlated with macrophage fusion. This study illustrates the feasibility of using protein arrays to dissect the complex interaction of lymphocytes and monocytes with biomaterial surfaces.
Materials and Methods
Biomaterial Surfaces
The polyethylene terephthalate (PET)-based photograft copolymerized surfaces displaying distinct hydrophobic, hydrophilic/neutral, hydrophilic/anionic, and hydrophilic/cationic characteristic surface chemistries were synthesized as described previously.19 Sheets of Mylar® PET were modified with poly(benzyl N,N-diethyldithiocarbamate-co-styrene) (BDEDTC) coating for the hydrophobic surface. Polyacrylamide (PAAm), polyacrylic acid (PAANa), and N-methiodide of dimethylamino propylacrylamide (DMAPAAmMeI) provided hydrophilic/neutral, hydrophilic/anionic and hydrophilic/cationic surfaces, respectively. These surfaces were characterized, confirmed, and prepared for cell culture as described previously.16
Monocyte and Lymphocyte Isolation and Culture
Peripheral blood monocytes and lymphocytes were isolated from healthy adult blood donors by a non-adherent centrifugation method utilizing a Percoll gradient as previously described.20 Separate monocyte and lymphocyte populations were washed twice with phosphate-buffered saline (PBS) (Invitrogen, Grand Island, NY) containing magnesium chloride and calcium chloride, resuspended in serum free medium (SFM) (Invitrogen, Grand Island, NY) supplemented with L-glutamine, antibiotics, and antimycotics, and kept at 4°C prior to plating. The isolated lymphocyte population was composed of, on average, 51.3% ± 4.1% CD4+ T lymphocytes, 30.8% ± 5.2% CD8+ T lymphocytes, 10.7% ± 1.1% NK cells, 5.6% ± 0.5% B lymphocytes, and 1.6% ± 0.1% contaminating monocytes while the isolated monocyte population was composed of, on average, 60.5% ± 9.1% monocytes and 39.5 ± 9.1% contaminating lymphocytes, as determined by flow cytometry. The lymphocyte and monocyte populations were suspended in supplemented SFM containing 20% autologous serum (AS) for plating. Lymphocyte and monocytes were co-cultured in 1 mL of SFM containing 20% AS on the biomaterial surfaces using either 1.5 × 106 cells of the isolated lymphocyte population alone (low monocyte culture) for a lymphocyte:monocyte ratio of approximately 1.0:0.015, or together with 1.0 × 106 cells of the isolated monocyte population for a lymphocyte:monocyte ratio of approximately 1.0:0.3 (high monocyte culture). The cultures were then incubated at 37°C and 5% CO2 for periods of 3, 7, and 10 days. Non-adherent cells and supernatant were collected on days 3, 7, and 10 and separated by centrifugation at 10,000 rpm for 5 minutes. Supernatants were stored at −80°C until cytokine analysis.
Determination of Adherent Cell Densities and Fusion
After culture periods of 3, 7, and 10 days, adherent cells were fixed, stained, and analyzed as previously published.18 The density of adherent monocytes, macrophages, and foreign body giant cells were determined by counting nuclei from 5–20x objective fields and expressed as cells/mm2. Fusion of macrophages were expressed as percent fusion and calculated by dividing the number of nuclei contained within the multinucleated giant cells by the total number of nuclei located within the field of view.
Protein Array Screening of Cytokines/Chemokines
Supernatant collected on days 3 and 10 from lymphocyte/monocyte co-cultures on the different biomaterial surface were screened with the RayBio® Human Cytokine Antibody Array V (RayBiotech, Inc., Norcross, GA) which detects 79 cytokines, chemokines, and growth factors simultaneously. The screening was performed according to the manufacturer’s protocol. Detection was performed by a BioRad Versadoc Chemiluminescence Imaging System with corresponding Versadoc Chemiluminescence Software (BioRad Laboratories, Inc., Hercules, CA). Membranes were stored at −20°C or −80°C for future reference. Analysis of results involved the intensity rating of positive signals from 0 to 4 representing no signal to strong signal, respectively. Media control signal ratings were subtracted from supernatant signal ratings. Since detection sensitivities for each cytokine vary as illustrated in Table 1, comparisons between cytokines were not done. The variation in the level of each individual cytokine across material surface chemistries was determined from 3 different donors. The level of variation (variation index) was calculated for each cytokine by taking the average of the absolute intensity rating differences of that particular cytokine produced in response to the various biomaterial surfaces (BDEDTC, PAAm, PAANa, and DMAPAAmMeI) using the following formula (averaged over 3 different experiments):
VariationIndex=(∣BDEDTC-PAAm∣+∣BDEDTC-PAANa∣+∣BDEDTC-DMAPAAmMeI∣+∣PAAm-PAANa∣+∣PAAm-DMAPAAmMeI∣+∣PAANa-DMAPAAmMeI∣)/6
Table 1.
Minimum detection limit for the utilized protein detection techniques
| Cytokines | Array (pg/mL)a | ELISA (pg/mL)b |
|---|---|---|
| IL-1β | 100 | 1 |
| IL-2 | 25 | 7 |
| IL-6 | 1 | 0.7 |
| IL-8 | 1 | 3.5 |
| TNF-α | 10 | 15.6 |
| IL-10 | 10 | 3.9 |
| TGF-β2 | 1000 | 7 |
| MIP-1β | 10 | 4 |
| TIMP-1 | 100 | 80 |
| TIMP-2 | 1 | 11 |
| MMP-9 | 1000 | 156 |
Select cytokines based on strength of signal, importance, and material variability were then chosen for further quantification by ELISA.
In addition, to determine whether individual cytokines may be preferentially over- or under-produced on PAANa compared to the other biomaterial surfaces, a PAANa comparative index was generated. This was calculated by subtracting the signal rating of each soluble factor as determined by the cytokine array on the individual biomaterial surfaces from that produced in response to PAANa and averaging the result using the following formula (for 3 separate experiments):
ComparativeIndex=[(PAANa-BDEDTC)+(PAANa-PAAm)+(PAANa-DMAPAAmMeI)]/3
A negative number indicates the factor is under-produced in supernatants from cells exposed to PAANa compared to the other biomaterial surfaces and a positive number indicates the factor is over-produced in supernatants from cells exposed to PAANa compared to the other biomaterial surfaces.
ELISA Quantification of Cytokine Production
After screening by cytokine antibody array, targeted cytokines were quantified by ELISA (R&D systems, Minneapolis, MN) and the assay was performed according to manufacturer’s instructions. Microplate color intensity was measured by an EL808 ultra microplate reader with KC Junior software (Bio-Tek Instruments, Inc., Winooski, Vermont). For MMP-9, TIMP-1, and TIMP-2, the data provided was used to determine molar concentrations and calculate molar ratios of MMP-9 to TIMP-1 (MMP-9/TIMP-1) and MMP-9 to TIMP-2 (MMP-9/TIMP-2).21–25 These measured quantities were compared across the experimental conditions: material surface chemistry, co-culture ratio, and time. Table 1 lists the cytokines being quantified by ELISA with their respective sensitivities.
Statistical Analysis
All data are expressed as an average ± the standard error of the mean (SEM) of at least 3 replicate experiments with 3 different donors to account for donor variability. All data were analyzed by ANOVA with Tukey’s test for pair-wise comparisons at a 95% confidence level utilizing Minitab (Minitab Inc., State College, PA).
Results
Adhesion and Fusion Analysis
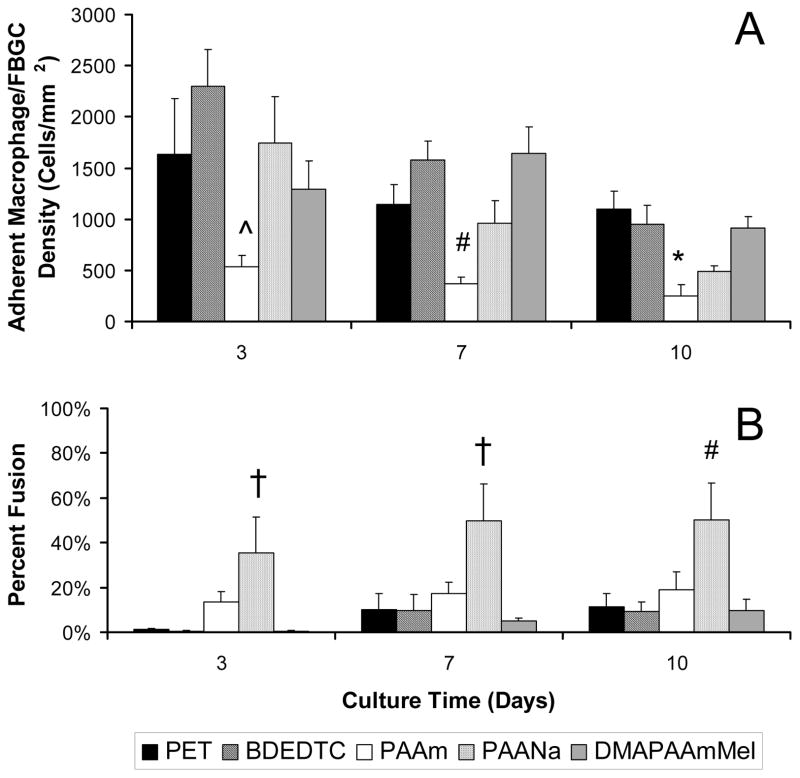
The lymphocyte/monocyte co-cultures on the biomaterial surfaces displayed material-dependent monocyte/macrophage/FBGC adhesion as well as macrophage/FBGC fusion. The adhesion and fusion analysis of high monocyte co-cultures are shown in Figure 1. Examination of surface adherent cells showed that PAAm, the hydrophilic/neutral surface, minimized adhesion relative to all surfaces. Adhesion on the hydrophilic/neutral surface (PAAm) was significantly less than on the hydrophobic surface, BDEDTC on day 3 (p < 0.05), significantly less than the hydrophobic (BDEDTC) and the hydrophilic/cationic (DMAPAAmMeI) surfaces on day 7 (p < 0.01), and less than the hydrophobic surface (PET) at day 10 (p < 0.05).
Figure 1.
(A) Macrophage/FBGC adhesion and (B) percent fusion from high monocyte co-cultures over 10 days. Data represents the mean ± standard error of the mean (SEM), n = 4. *Significance relative to PET (p < 0.05). ^Significance relative to BDEDTC (p < 0.05). #Significance relative to BDEDTC and DMAPAAmMeI (p < 0.05). †Significance relative to PET, BDEDTC, and DMAPAAmMeI (p <0.05).
The hydrophilic/anionic (PAANa) surfaces increased macrophage fusion compared to the other surfaces. The percentage of fusion on PAANa was significantly greater than on PET, BDEDTC, and DMAPAAmMeI until day 7 (p < 0.05) and greater than on BDEDTC and DMAPAAmMeI on day 10 (p < 0.05).
Protein Array Analysis
Since different biomaterial surface chemistries are capable of eliciting different biological responses, a general screen of 79 inflammatory cytokines, chemokines, growth factors, and matrix proteins produced by the cell populations on the various biomaterial surfaces was performed using a cytokine array. This approach was undertaken as an attempt to identify cytokines that could potentially account for differences in the biomaterial-dependent cellular responses. Analysis revealed 43 proteins that were produced and/or demonstrated material variability. Table 2 shows a summary of the analysis for the high monocyte co-culture of 43 proteins which includes the average signal ratings for the media control, the average rating of the co-culture supernatants from all materials, and a measure of the biomaterial dependence of the cytokine, the variability index (see Materials and Methods). The presented ratings take into account the level in the autologous serum (i.e. the level of protein in the control media was subtracted out). Signal ratings ranged from −1.5 to 4 and variability indices ranged from 0 to 1.3. A negative signal rating indicates the factor was present at lower concentrations in the co-cultures on biomaterials than that detected in autologous serum. The low monocyte co-culture revealed similar findings (data not shown). Based on the analysis shown in Table 2, targets that were produced at high levels (signal rating > 2) and those believed to be important cytokines with material variability were chosen for further quantification. These included TNF-α, IL-1β, IL-6, IL-10, IL-8, MIP-1β and TIMP-1, TIMP-2. Although several other proteins satisfied the criteria, not all were able to be further analyzed in this study. As shown in Table 2, 24 of the proteins were present at some level in autologous control serum. Soluble factors not detected in either the high or low monocyte co-cultures include: GCSF, IL-1α, IL-2, IL-3, IL-4, IL-5, IL-7, IL-12p40p70, IL-13, IL-15, IFN-γ, MIG, SCF, SDF-1, TNF-β, IGF-1, Oncostatin M, Thrombopoietin, VEGF, BDNF, BLC, ck β 8–1, Eotaxin, Eotaxin-3, FGF-4, FGF-6, FGF-7, FGF-9, Fit-3 Ligand, Fractalkine, GCP-2, IGFBP-4, IL-16, MIF, NT-3, NT-4, and Osteoprotegerin.
Table 2.
Signal intensity ratings and material variability indices*
| Protein£ | Media Control | Day 3† | Day 10† | PAANa Comparative Index# | ||
|---|---|---|---|---|---|---|
| Signal Rating | Variability Index | Signal Rating | Variability Index | |||
| ENA-78 | 0.00 | 1.08 | 0.39 | 1.58 | 0.72 | 0.11 |
| GM-CSF | 0.00 | 0.08 | 0.17 | 0.00 | 0.00 | 0.00 |
| GRO | 1.00 | 0.92 | 0.39 | 0.42 | 0.72 | −0.11 |
| GRO-α | 0.00 | 1.33 | 0.33 | 0.92 | 0.83 | −0.78 |
| I-309 | 0.00 | 0.83 | 0.78 | 1.08 | 1.17 | −0.11 |
| IL-1β | 0.00 | 2.42 | 0.94 | 2.58 | 0.72 | −0.33 |
| IL-6 | 0.50 | 3.92 | 0.17 | 2.83 | 0.22 | 0.22 |
| IL-8 | 1.33 | 2.92 | 0.39 | 1.92 | 0.17 | 0.11 |
| IL-10 | 0.33 | 0.42 | 0.61 | 0.33 | 0.33 | 0.00 |
| MCP-1 | 2.17 | 0.08 | 0.39 | 0.00 | 0.33 | 0.00 |
| MCP-2 | 0.33 | 0.08 | 0.17 | −0.50 | 0.33 | −0.22 |
| MCP-3 | 0.00 | 0.92 | 0.72 | 0.17 | 0.22 | 0.22 |
| MCSF | 0.00 | 0.00 | 0.00 | 0.08 | 0.17 | 0.33 |
| MDC | 0.00 | 1.00 | 0.33 | 0.42 | 0.50 | −0.11 |
| MIP-1β | 0.33 | 0.00 | 0.44 | 0.33 | 0.33 | 0.44 |
| MIP-1δ | 1.00 | 0.33 | 0.56 | −0.83 | 0.33 | −0.22 |
| RANTES | 2.33 | −0.67 | 0.00 | −1.50 | 1.00 | 0.67 |
| TARC | 0.00 | 0.92 | 0.39 | 0.25 | 0.39 | −0.33 |
| TGF-β1 | 0.00 | 0.00 | 0.00 | 0.08 | 0.17 | −0.11 |
| TNF-α | 0.17 | 0.67 | 0.89 | 0.25 | 0.50 | 0.56 |
| TNF-β | 0.33 | −0.67 | 0.00 | 0.08 | 0.17 | −0.11 |
| EGF | 2.50 | −0.25 | 0.17 | 0.08 | 0.72 | 0.33 |
| Angiogenin | 2.50 | −1.00 | 0.00 | 0.17 | 0.67 | 0.22 |
| PDGF-BB | 3.17 | −0.83 | 0.67 | 0.17 | 0.78 | −0.22 |
| Leptin | 2.67 | −1.17 | 0.78 | −0.25 | 0.72 | −0.56 |
| Eotaxin-2 | 0.33 | 1.50 | 0.22 | 1.50 | 0.67 | 0.22 |
| GDNF | 0.33 | −0.33 | 0.44 | 0.17 | 0.33 | 0.67 |
| HGF | 1.17 | −0.33 | 0.33 | 1.00 | 0.78 | 0.00 |
| IGFBP-1 | 0.00 | 0.08 | 0.17 | 0.33 | 0.56 | 0.44 |
| IGFBP-2 | 1.50 | −0.33 | 0.00 | 0.08 | 0.50 | −0.11 |
| IGFBP-3 | 0.00 | 0.00 | 0.00 | 0.17 | 0.33 | −0.22 |
| IP-10 | 0.00 | 0.08 | 0.17 | 0.50 | 0.78 | −0.67 |
| LIF | 0.00 | 0.08 | 0.17 | 0.00 | 0.00 | 0.00 |
| LIGHT | 0.00 | 0.08 | 0.17 | 0.00 | 0.00 | 0.00 |
| MCP-4 | 0.00 | 0.08 | 0.17 | 0.00 | 0.00 | 0.00 |
| MIP-3α | 0.00 | 1.00 | 1.22 | 1.00 | 0.89 | −0.44 |
| NAP-2 | 2.33 | −0.33 | 0.00 | −0.25 | 0.39 | 0.33 |
| PARC | 2.83 | −1.00 | 0.33 | −0.17 | 1.11 | 0.22 |
| PIGF | 0.00 | 0.08 | 0.17 | 0.00 | 0.00 | 0.00 |
| TFG-β2 | 1.67 | −0.92 | 0.17 | 0.25 | 0.17 | 0.11 |
| TGF-β3 | 0.00 | 0.08 | 0.17 | 0.00 | 0.00 | 0.00 |
| TIMP-1 | 2.00 | −0.67 | 0.00 | −0.25 | 0.61 | −0.11 |
| TIMP-2 | 2.67 | −0.08 | 0.17 | 0.17 | 0.33 | 0.22 |
ELISA Analysis
After cytokine array screening, production of TNF-α, IL-1β, IL-6, IL-10, IL-8, MIP-1β and TIMP-1 by the lymphocyte/macrophage co-cultures were quantified by ELISA. Additionally, IL-2, TGF-β2, and MMP-9 were selected for quantification because they were proteins of importance in lymphocyte proliferation (IL-2), wound healing (TGF-β), or are targets of proteins detected in array analysis (MMP-9). Two co-culture ratios (high and low monocyte) were utilized in order to examine the effect of depleted monocytes/macrophages on lymphocyte/macrophage interactions and the production of these various factors. IL-1β, IL-10, TGF-β2, MIP-1β, MMP-9, TIMP-1, and TIMP-2 were present in autologous serum containing media controls at concentrations of 24 ± 7 pg/mL, 4 ± 1 pg/mL, 57 ± 2 pg/mL, 24 ± 1 pg/mL, 44 ± 14 ng/mL, 32 ± 4 ng/mL, and 20 ± 2 ng/mL, respectively. These amounts were subtracted from the quantities in the media exposed to the co-cultures to determine the produced quantities. No IL-2 was detected while TGF-β2 was found to be minimally produced (data not shown).
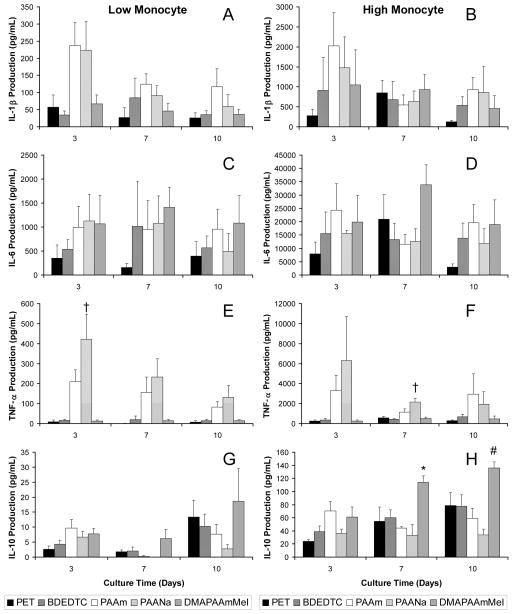
Cytokines
Figure 2 shows the quantity of inflammatory cytokines, IL-1β, IL-6, TNF-α, and IL-10, produced over time. In general, the low monocyte and high monocyte co-cultures showed similar material trends although there was variability in the amount of cytokines produced by high and low monocyte co-cultures on the different material surfaces. The hydrophilic PAAm surface tended to induce an increased production of IL-1β relative to other surfaces, hydrophobic PET and BDEDTC in particular, in both co-culture ratios (Figure 2A–2D). IL-1β production was initially higher on PAAm and PAANa surfaces and decreased by day 7 while all other surfaces remained relatively constant. IL-6 on all surfaces remained relatively constant over time.
Figure 2.
IL-1β production from (A) low and (B) high monocyte co-cultures over 10 days. IL-6 production from (C) low and (D) high monocyte co-cultures over 10 days. TNF-α production from (E) low and (F) high monocyte co-cultures over 10 days. IL-10 production from (E) low and (F) high monocyte co-cultures over 10 days. Data represents the mean ± standard error of the mean (SEM), n = 4. *Significance relative to PAANa (p < 0.05). #Significance relative to PAAm and PAANa (p < 0.05). †Significance relative to PET, BDEDTC, and DMAPAAmMeI (p < 0.05).
TNF-α levels were highest on the hydrophilic/anionic (PAANa) surfaces across all time points and in both co-culture ratios as shown in Figure 2E and 2F. The levels on PAANa were significantly greater than hydrophobic (PET and BDEDTC) and hydrophilic/cationic (DMAPAAmMeI) surfaces in low monocyte co-cultures at initial day 3 and high monocyte co-cultures at day 7 (p < 0.005). Levels were increased on the hydrophilic/neutral (PAAm) surfaces compared to PET, BDEDTC and DMAPAAmMeI although the difference was not statistically significant. Over time, however, TNF-α levels on these two surfaces (PAAm and PAANa) decreased while the other surfaces produced lower levels that remained relatively constant or slightly increased.
Whereas IL-1β, TNF-α, and IL-6 either decreased over time or remained the same, IL-10 production generally increased with time as shown in Figure 2G and 2H. Significant differences were most evident in the high monocyte co-cultures. At day 7, the IL-10 level was significantly higher on the hydrophilic/cationic surface, DMAPAAmMeI, relative to the hydrophilic/neutral surface (p < 0.01). By day 10, DMAPAAmMeI showed greater production of IL-10 than both hydrophilic/neutral (p < 0.05) and hydrophilic/anionic surfaces (p < 0.005).
Chemokines
IL-8 was produced at the highest concentration amongst all proteins quantified. IL-8 production on the various materials across time is shown in Figure 3A and 3B. IL-8 levels were produced at a level approximately 10 fold greater in high monocyte co-cultures relative to low monocyte co-cultures. The hydrophilic/neutral surface, PAAm, in high monocyte co-cultures, in particular, showed increased IL-8 levels relative to the hydrophobic surfaces over the entire culture period with significance compared to PET after 10 days of culture (p < 0.05).
Figure 3.
IL-8 production from (A) low and (B) high monocyte co-cultures over 10 days. MIP-1β production from (C) low and (D) high monocyte co-cultures over 10 days. Data represents the mean ± standard error of the mean (SEM), n = 4. *Significance relative to PET (p < 0.05). †Significance relative to PET and BDEDTC (p < 0.05).
MIP-1β, on the other hand, began decreasing after the initial day 3 time point over the time course regardless of culture ratio (Figure 3C and 3D). The quantity in high monocyte co-cultures declined at a much faster rate than in the low monocyte co-cultures. MIP-1β levels in the high monocyte co-cultures were initially greater at day 3 than those in low monocyte co-cultures by a factor of two to eight depending on the surface. By 10 days, MIP-1β levels on all materials were lower in high monocyte co-cultures than in low monocyte co-cultures. The highest quantity of MIP-1β was seen on the hydrophilic/anionic surface, PAANa, over time and material. Levels on PAAm were slightly increased compared to hydrophobic and cationic surfaces although not statistically significant. The trend was the same for both co-culture ratios. However, in low monocyte co-cultures, MIP-1β production after 3 days was significantly higher on PAANa relative to PET and significantly higher than PET and BDEDTC after 7 days (p < 0.05).
Extracellular Matrix Proteins
MMP-9 production increased after day 3 over the 10 day culture period and diverged according to material surface (Figure 4A and 4B). Both TIMP-1 and TIMP-2 mirrored MMP-9 in regards to increased production after day 3 as demonstrated in Figure 4, C – D and E – F, respectively. TIMP-1 also had a small amount produced at day 3 (10 ng/mL for the low monocyte co-culture and 100 ng/mL for the high monocyte co-culture) while TIMP-2 was minimally produced in both co-cultures. DMAPAAmMeI diminished TIMP-1 and TIMP-2 production at later time points showing significantly in high monocyte co-cultures after 7 days (Figure 4D and 4F).
Figure 4.
MMP-9 production from (A) low and (B) high monocyte co-cultures over 10 days. TIMP-1 production from (C) low and (D) high monocyte co-cultures over 10 days. TIMP-2 production from (E) low and (F) high monocyte co-cultures over 10 days. Data represents the mean ± standard error of the mean (SEM), n = 4. *Significance relative to PAAm (p < 0.05). †Significance relative to PAAm and PAANa (p < 0.05).
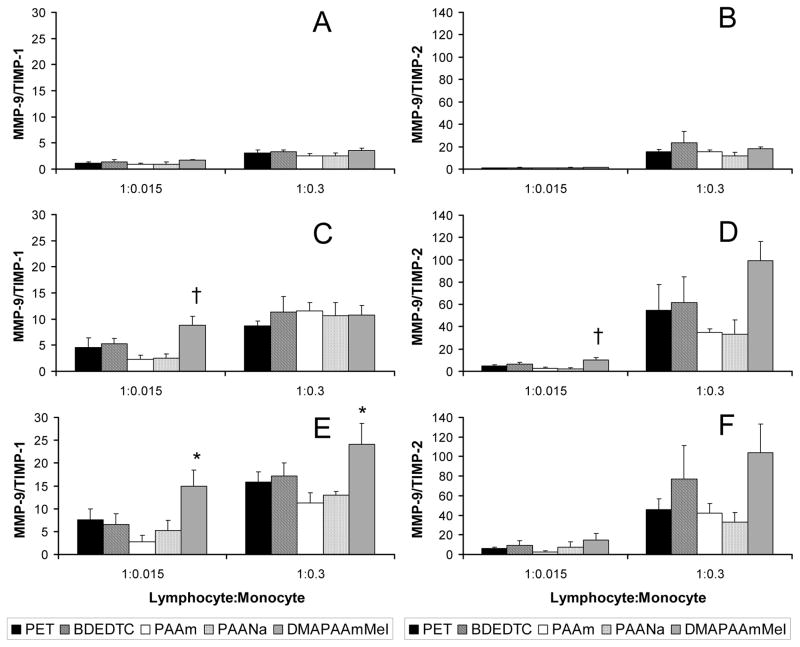
TIMPs bind and inhibit MMPs in a 1:1 stoichiometric ratio.46 Molar concentrations of MMP-9, TIMP-1, and TIMP-2 were calculated and subsequently used to determine MMP-9/TIMP-1 and MMP-9/TIMP-2 in order to quantitatively show the balance between matrix metalloproteinase and its inhibitor (TIMPs). These ratios are presented at 3, 7, and 10 day time points in Figure 5. In both low and high monocyte co-cultures, MMP-9/TIMP-1 (Figure 5A, 5C, 5E) and MMP-9/TIMP-2 (Figure 5B, 5D, 5F) both increased with time on PET, BDEDTC, the most on DMAPAAmMeI, and least if at all on PAAm and PAANa surfaces. Initially, at day 3, MMP-9/TIMP-1 was close to 1 (< 5) on all surfaces and in general, increased on DMAPAAmMeI over time at a rate higher than on all other surfaces. By day 7 in low monocyte co-cultures, MMP-9/TIMP-1 on PAAm and PAANa were at the lowest levels while the ratio on DMAPAAmMeI was significantly higher (p < 0.05). By day 10, both co-cultures demonstrated lower ratios on PAAm and PAANa with those on PAAm significantly lower compared to DMAPAAmMeI (p < 0.05). Like MMP-9/TIMP-1, MMP-9/TIMP-2 also increased with time and showed an identical material-dependent trend. That is, after day 3, the ratio on hydrophilic/neutral (PAAm) and hydrophilic/anionic (PAANa) surfaces tended to be lower while the hydrophilic/cationic (DMAPAAmMeI) surface showed the highest levels. The ratio on DMAPAAmMeI in low monocyte co-cultures was significantly higher than both PAAm and PAANa at day 7 (p < 0.05).
Figure 5.
Ratio of MMP-9/TIMP-1 in co-cultures over (A) 3 days, (C) 7 days, and (E) 10 days. Ratio of MMP-9/TIMP-2 in co-cultures over (B) 3 days, (D) 7 days, and (F) 10 days. Data represents the mean ± standard error of the mean (SEM), n = 4. *Significance relative to PAAm (p < 0.05). †Significance relative to PAAm and PAANa (p < 0.05).
Finally, since PAANa induced significantly greater macrophage fusion than on other surfaces, the protein arrays were utilized to identify soluble factors that could account for the enhanced fusion. A comparison between the levels of these factors in supernatants from lymphocyte and monocyte co-cultures exposed to the fusion–promoting hydrophilic/anionic surface, PAANa, and those of the other biomaterials was determined using a PAANa comparative index (see Materials and Methods and Table 2). Cytokines and chemokines MIP-1β, RANTES, TNF-α, GDNF, and IGFBP-1 appeared to be over-produced in supernatants from co-cultures exposed to PAANa compared to other substrates and positively correlated with macrophage fusion while the soluble factors GRO-α, Leptin, IP-10, and MIP-3α appeared negatively correlated with fusion. These differences were verified in the subsequent ELISA analysis for two factors, TNF-α and MIP-1β (Figures 2 and 3). Thus, the use of cytokine arrays is a feasible approach to identify soluble factors that may be important for biomaterial-dependent processes such as macrophage fusion.
Discussion
The objectives of this study were to examine the response of lymphocyte/macrophage interactions to biomaterials and the effect of material surface chemistry on these interactions. Our findings show that in response to biomaterial surfaces, cytokines, chemokines, as well as matrix proteins are produced, that are capable of guiding cellular behavior and the development of the extracellular matrix surrounding the implantation site, and biomaterial surface chemistry influences the particular cytokine profiles produced by the inflammatory cells. Additionally, the concentration of monocytes in the lymphocyte/macrophage co-cultures can alter the level of inflammatory mediators produced in this experimental system.
Cell derived proteins from lymphocyte/macrophage co-cultures include cytokines, chemokines, growth factors, and matrix proteins with pleiotropic effects on multiple inflammatory and wound healing cell types. These include inflammatory cytokines such as IL-1β, IL-6, IL-10, and TNF-α as well as chemokines (e.g. ENA-78, NAP-2, IL-8, MCP, GRO, MIP-1β, IP-10, PARC) that target inflammatory effector cells such as neutrophils, monocyte/macrophages, and lymphocytes. Many of these chemokines are capable of attracting more than one cell type. IL-8 chemotactically attracts neutrophils and T lymphocytes and also activates neutrophils. MIP-1β is known as a chemotactic protein for monocytes/macrophages, but it is also capable of chemotactically targeting CD4+ T lymphocytes.26 MCP-1 has also been shown to play a role in the biological response to biomaterials in promoting macrophage fusion to form foreign body giant cells.27 In addition, many of the cytokines and chemokines are capable of influencing lymphocyte behaviors. I-309, IL-8, MCPs, MDC, MIP-1β, MIP-3α, RANTES, PARC, IP-10, and TARC can all chemoattract specific lymphocyte subpopulations such as T lymphocytes.28 Cytokines such as IL-1β and IL-6 are capable of enhancing T lymphocyte activation and proliferation with IL-1β augmenting IL-2 production and IL-6 increasing responsiveness to IL-2.29,30 Chemokines such as MIP-1β, MCP-1, and RANTES have been shown to induce activation and proliferation of NK cells.31 Moreover, RANTES can activate and induce proliferation of T cells independent of other stimuli.32 Nonetheless, T lymphocyte activation was not observed in our studies as neither IL-2 nor IFN-γ was detected in the co-cultures.
The majority of the soluble factors produced over the 10 days of culture act primarily to direct the early part of the normal wound healing response. As expected, TNF-α and IL-1β were initially high and decreased over the 10 day period. However, IL-10, a cytokine suppressing the inflammatory response, generally increased over the 10 day cultures suggest dampening of the immune response after 10 days. These results mirror time-dependent resolution of the inflammatory response in normal wound healing.33 Additionally, MMP-9, TIMP-1, and TIMP-2 all increased in production after 3 days of culture. MMP-9 readily digests extracellular matrix molecules such as gelatin, elastin, and collagens and is inhibited by the TIMPs.34 During the inflammatory phase, proteases and MMPs are elaborated in order to degrade the damaged extracellular matrix and facilitate cellular migration through the matrix. In general, the balance between protease and inhibitor tilted towards protease action and matrix breakdown. IL-1β and TNF-α, which were produced at high levels at day 3, induce MMP transcription.33 Thus, the increase in MMP-9 after day 3 is consistent with the natural wound healing progression. The continued production of MMP-9 over its inhibitor was also likely due to the lack of TGF-β in the co-cultures. TGF-β, a major growth factor in the later stages of the wound healing response, upregulates TIMPs while decreasing MMP production but was not detected in our system.33 Therefore, lymphocytes and macrophages in culture demonstrated the capability of guiding the inflammatory phase of the wound healing response.
IL-2 and IFN-γ were undetected while considerable levels of IL-10 were found. The lack of IL-2 suggests the absence of classic T lymphocyte activation and proliferation in response to synthetic polymers. Macrophages are capable of rendering T cells unresponsive, or anergic, as a result of TCR/MHC interaction in the absence of costimulation.13 IL-10 inhibits the activation of macrophages and downregulates macrophage co-stimulatory activity.35,36 Furthermore, IL-10 can directly inhibit T cell activation and induce T cell anergy.13,37 Therefore, the results from this in vitro study suggest that the lack of T cell activation as determined by cytokine release may be mediated by IL-10 inhibition either directly or indirectly. Our findings are similar to those described by Hoves, et al. They found substantial levels of IL-10, low levels of IL-2 and IFN-γ, and minimal T lymphocyte proliferation in in vitro co-cultures, and showed that human monocyte-derived macrophages produced anergic T cells.14 The absence of IL-3 and IL-5, which are produced by CD4+ T lymphocytes, further suggest lymphokine suppression and lack of T lymphocyte activation.
As shown above, material surface chemistries are capable of dictating cellular behaviors such as adhesion, fusion, and protein production. Hydrophilic/neutral PAAm surfaces diminished adhesion while all other surfaces, hydrophobic and hydrophilic/charged, promoted adhesion. Overall cellular (macrophage/FBGC) adhesion on PAANa also diminished over time as macrophages fused into FBGCs. The adhesion results confirm previous material surface chemistry dependency shown in our laboratory.40 Although adhesion on PAAm was inhibited, fusion was still capable of occurring. The amount of fusion on PAAm was higher in these co-cultures compared to fusion in adherent monocyte/macrophage cultures.16 This is likely due to the presence of lymphocytes in culture since lymphocytes have been shown to facilitate fusion.7 Additionally, hydrophilic/anionic PAANa increased macrophage fusion while hydrophobic and hydrophilic/cationic surfaces inhibited fusion. The PAANa fusion promotion confirms previous findings in our laboratory.18
The protein array analysis showed many cytokines exhibited potential material-dependent production based on a calculated variability index. Furthermore, the array analysis identified soluble factors that could account for the biomaterial-dependent differences in fusion. MIP-1β and TNF-α were found to positively correlate with fusion and could be proteins that are produced and also possibly utilized during macrophage fusion to form FBGCs. On the other hand, GRO-α, a chemokine that can attract monocytes, negatively correlated with macrophage fusion suggesting that this chemokine may be preferentially utilized during the fusion process. Quantification by ELISA confirmed the correlation of MIP-1β and TNF-α with fusion on the biomaterial surfaces. These results indicate that the use of protein arrays is a feasible approach to identify soluble factors that may be important for biomaterial-dependent processes such as macrophage fusion. However, the variability in the detection sensitivities for the protein targets on the array (from 1 pg/mL to 2000 pg/mL) somewhat limits its usefulness since biomaterial-dependent changes in signal intensity could be due to either trivial or rather large changes in the concentration of the molecules. Therefore, more precise quantification of molecules of interest by ELISA helps substantiate the observed differences.
The hydrophilic/neutral (PAAm) and hydrophilic/anionic (PAANa) surfaces were both shown to promote a pro-inflammatory response albeit through different cytokine secretion profiles while the hydrophilic/cationic surfaces (DMAPAAmMeI) promoted a more anti-inflammatory response. Despite the inhibition of cellular adhesion on PAAm, higher levels of IL-8, IL-1β, and TNF-α, and lower levels of IL-10 were induced on PAAm compared to other surfaces. TNF-α and MIP-1β were over-produced in response to PAANa with significantly less IL-10 secreted. DMAPAAmMeI induced greater IL-10 production and relatively low levels of pro-inflammatory mediators such as IL-1β, TNF-α, and MIP-1β compared to the other surfaces. IL-10 suppresses inflammatory cytokines specifically inhibiting cytokines such as IL-1β, IL-6, IL-8, TNF-α, MIP-1β in monocytes/macrophages in addition to its effects on co-stimulation mentioned above.41,42
Aside from its effects on metalloproteinases, TNF-α also induces cellular apoptosis. Brodbeck et al. demonstrated that TNF-α was responsible for biomaterial-mediated spontaneous apoptosis.43 Furthermore, hydrophilic/neutral and hydrophilic/anionic biomaterial surfaces were shown to induce a higher level of adherent macrophage apoptosis compared to hydrophobic and cationic surfaces both in vitro and in vivo.44,45 The results from this study are consistent with these findings in that TNF-α was produced at the highest levels on both hydrophilic/neutral (PAAm) and hydrophilic/anionic (PAANa) surfaces.
MMPs degrade the damaged extracellular matrix and also facilitate cellular migration through the matrix. These actions ultimately facilitate the progression of the immune response to injury from inflammatory to the proliferative and ultimately the remodeling phases. The actions of the MMPs are inhibited and regulated by the TIMPs in a 1:1 stoichiometric ratio,46 and the balance between protease and inhibitor determines the degradative state of the ECM. The hydrophilic/neutral (PAAm) and hydrophilic/anionic (PAANa) surfaces were both shown to minimize MMP-9/TIMP suggesting a reduction in degradation of extracellular matrix while the hydrophilic/cationic surfaces (DMAPAAmMeI) promoted greater MMP-9/TIMP suggesting the potential for greater extracellular matrix breakdown. Based upon the cumulative actions of the inflammatory mediators, hydrophilic/neutral and hydrophilic/anionic surfaces induce a pro-inflammatory state with a potentially inhibited progression from the inflammatory phase. Conversely, hydrophilic/cationic surfaces induce an anti-inflammatory state while possibly promoting progression through the wound healing phases.
In comparing the high monocyte to low monocyte co-cultures, the ratios of MIP-1β and TIMP-2 production were less than the ratio of monocytes (high:low) on all materials. TNF-α showed both higher and lower ratios depending on the surface. These ratio differences suggest that cell numbers and/or lymphocyte/macrophage interactions can affect the amounts of soluble factors produced. Levels of MIP-1β were markedly reduced in high monocyte co-cultures relative to low monocyte co-cultures as time progressed. This could be due to enhanced lymphocyte-mediated activation of macrophages resulting in increased utilization or breakdown of the chemokine or a reflection of lymphocyte-mediated inhibition of chemokine production. MIP-1β, a known chemokine, may also be involved in the fusion process since macrophages are required to come together in close proximity. Increased macrophage activation in higher macrophage co-cultures would then lead to increased utilization of this factor. TIMP-2 production also demonstrated a similar decreased response. Unlike MIP-1β, which is a chemokine that targets monocytes/macrophages, TIMP-2 is a matrix protein that acts on the extracellular matrix. In this case, inhibited production is more likely than increased utilization. TNF-α levels were also reduced in high monocyte co-cultures compared to low monocyte co-cultures on PAANa and earlier time points on PAAm where the concentrations were relatively high. On the other hand, enhanced production of TNF-α in high monocyte-co-cultures relative to low monocyte co-cultures was evident on PET, BDEDTC, and DMAPAAmMeI where TNF-α levels were comparatively low. This variable response suggests that there may be a feedback mechanism leading to inhibition of the specific cytokine production. Therefore, the cellular interactions at the tissue/material interface and the subsequent response are potentially influenced by material surface chemistries as well as lymphocyte or macrophage numbers. In contrast, Trindade et al. suggested that there was a lack of synergism between the lymphocytes and macrophages with respect to IL-6 and TNF-α production in response to particles composed of orthopedic materials.47 Our findings prompt further investigation into possible lymphocyte-mediated monocyte/macrophage activation, inflammatory mediator production, and material dependency of these interactions and responses.
This study contributes to understanding lymphocyte/macrophage interactions that occur at the implant site of a biomaterial. The inability of T lymphocytes to produce IL-2 or IFN-γ when interacting with macrophages indicates the lack of classic T lymphocyte activation. However, the results suggest lymphocytes may modulate the production of soluble factors by macrophages. Our findings provide quantitative evidence to support our hypothesis that material surface chemistry is capable of modulating cytokine, chemokine, and matrix protein production from lymphocyte/macrophage interactions. More specifically, material surface characteristics or chemistries can dictate the production profile of different inflammatory mediators. Further studies are necessary to better understand the importance of juxtacrine and paracrine-mediated lymphocyte/macrophage interactions and the material surface-dependent effects on those interactions.
Acknowledgments
Contract Grant Sponsor: National Institutes of Health (NIH)
National Institute of Biomedical Imaging and Bioengineering (NIBIB)
Contract Grant Number: EB-000275
EB-000282
T32 GM07250
References
- 1.Anderson JM. Inflammation and the foreign body response. Problems in General Surgery. 1994;11(2):147–60. [Google Scholar]
- 2.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 3.Maeda M, Kimura M, Inoue S, Kataoka K, Okano T, Sakurai Y. Adhesion behavior of rat lymphocyte subpopulations (B cell and T cell) on the surface of polystyrene/polypeptide graft copolymer. J Biomed Mater Res. 1986;20(1):25–35. doi: 10.1002/jbm.820200104. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama M, Nakahashi T, Nishimura T, Maeda M, Inoue S, Kataoka K, Sakurai Y. Adhesion behavior of rat lymphocytes to poly(ether)-poly(amino acid) block and graft copolymers. J Biomed Mater Res. 1986;20(7):867–78. doi: 10.1002/jbm.820200702. [DOI] [PubMed] [Google Scholar]
- 5.Groth T, Altankov G, Klosz K. Adhesion of human peripheral blood lymphocytes is dependent on surface wettability and protein preadsorption. Biomaterials. 1994;15(6):423–8. doi: 10.1016/0142-9612(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 6.Bergman AJ, Zygourakis K. Migration of lymphocytes on fibronectin-coated surfaces: temporal evolution of migratory parameters. Biomaterials. 1999;20(23–24):2235–44. doi: 10.1016/s0142-9612(99)00154-4. [DOI] [PubMed] [Google Scholar]
- 7.Brodbeck WG, Macewan M, Colton E, Meyerson H, Anderson JM. Lymphocytes and the foreign body response: lymphocyte enhancement of macrophage adhesion and fusion. J Biomed Mater Res A. 2005;74(2):222–9. doi: 10.1002/jbm.a.30313. [DOI] [PubMed] [Google Scholar]
- 8.McNally AK. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147(5):1487–99. [PMC free article] [PubMed] [Google Scholar]
- 9.DeFife KM, Jenney CR, McNally AK, Colton E, Anderson JM. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J Immunol. 1997;158(7):3385–90. [PubMed] [Google Scholar]
- 10.Kao WJ, McNally AK, Hiltner A, Anderson JM. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res. 1995;29(10):1267–75. doi: 10.1002/jbm.820291014. [DOI] [PubMed] [Google Scholar]
- 11.Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999;5(4):285–94. doi: 10.1097/00054725-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Burger D, Dayer JM. The role of human T-lymphocyte-monocyte contact in inflammation and tissue destruction. Arthritis Res. 2002;4 (Suppl 3):S169–76. doi: 10.1186/ar558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoves S, Krause SW, Schutz C, Halbritter D, Scholmerich J, Herfarth H, Fleck M. Monocyte-derived human macrophages mediate anergy in allogeneic T cells and induce regulatory T cells. J Immunol. 2006;177(4):2691–8. doi: 10.4049/jimmunol.177.4.2691. [DOI] [PubMed] [Google Scholar]
- 15.Marques AP, Reis RL, Hunt JA. Cytokine secretion from mononuclear cells cultured in vitro with starch-based polymers and poly-L-lactide. J Biomed Mater Res A. 2004;71(3):419–29. doi: 10.1002/jbm.a.30155. [DOI] [PubMed] [Google Scholar]
- 16.Jones JA, Chang DT, Colton E, Kwon IK, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31221. In Press. [DOI] [PubMed] [Google Scholar]
- 17.Jones JA, McNally AK, Chang DT, Qin LA, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Matrix Metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res. 2007 doi: 10.1002/jbm.a.31220. In Press. [DOI] [PubMed] [Google Scholar]
- 18.MacEwan MR, Brodbeck WG, Matsuda T, Anderson JM. Monocyte/lymphocyte interactions and the foreign body response: in vitro effects of biomaterial surface chemistry. J Biomed Mater Res A; Student Research Award in the Undergraduate Degree Candidate category, 30th Annual Meeting of the Society for Biomaterials; Memphis, Tennessee,. April 27–30, 2005; 2005. pp. 285–93. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama Y, Anderson JM, Matsuda T. Laboratory-scale mass production of a multi-micropatterned grafted surface with different polymer regions. J Biomed Mater Res. 2000;53(5):584–91. doi: 10.1002/1097-4636(200009)53:5<584::aid-jbm19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.McNally AK, Anderson JM. Complement C3 participation in monocyte adhesion to different surfaces. Proc Natl Acad Sci USA. 1994;91:10119–23. doi: 10.1073/pnas.91.21.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J Biol Chem. 1997;272(47):29975–83. doi: 10.1074/jbc.272.47.29975. [DOI] [PubMed] [Google Scholar]
- 22.Xue M, Thompson PJ, Clifton-Bligh R, Fulcher G, Gallery ED, Jackson C. Leukocyte matrix metalloproteinase-9 is elevated and contributes to lymphocyte activation in type I diabetes. Int J Biochem Cell Biol. 2005;37(11):2406–16. doi: 10.1016/j.biocel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Mott JD, Thomas CL, Rosenbach MT, Takahara K, Greenspan DS, Banda MJ. Post-translational proteolytic processing of procollagen C-terminal proteinase enhancer releases a metalloproteinase inhibitor. J Biol Chem. 2000;275(2):1384–90. doi: 10.1074/jbc.275.2.1384. [DOI] [PubMed] [Google Scholar]
- 24.Osthues A, Knauper V, Oberhoff R, Reinke H, Tschesche H. Isolation and characterization of tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) from human rheumatoid synovial fluid. FEBS Lett. 1992;296(1):16–20. doi: 10.1016/0014-5793(92)80393-u. [DOI] [PubMed] [Google Scholar]
- 25.Stetler-Stevenson WG, Liotta LA, Kleiner DE., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. Faseb J. 1993;7(15):1434–41. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 26.Schall TJ, editor. The Cytokine Handbook. New York: Academic Press; 1994. pp. 419–460. [Google Scholar]
- 27.Kyriakides TR, Foster MJ, Keeney GE, Tsai A, Giachelli CM, Clark-Lewis I, Rollins BJ, Bornstein P. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165(6):2157–66. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward SG, Westwick J. Chemokines: understanding their role in T-lymphocyte biology. Biochem J. 1998;333 (Pt 3):457–70. doi: 10.1042/bj3330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houssiau FA, Coulie PG, Van Snick J. Distinct roles of IL-1 and IL-6 in human T cell activation. J Immunol. 1989;143(8):2520–4. [PubMed] [Google Scholar]
- 30.Le JM, Fredrickson G, Reis LF, Diamantstein T, Hirano T, Kishimoto T, Vilcek J. Interleukin 2-dependent and interleukin 2-independent pathways of regulation of thymocyte function by interleukin 6. Proc Natl Acad Sci U S A. 1988;85(22):8643–7. doi: 10.1073/pnas.85.22.8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maghazachi AA, Al-Aoukaty A, Schall TJ. CC chemokines induce the generation of killer cells from CD56+ cells. Eur J Immunol. 1996;26(2):315–9. doi: 10.1002/eji.1830260207. [DOI] [PubMed] [Google Scholar]
- 32.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269(5231):1727–30. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 33.Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 34.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184(1):1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151(3):1224–34. [PubMed] [Google Scholar]
- 37.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184(1):19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7–H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–61. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 39.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 40.Brodbeck WG, Nakayama Y, Matsuda T, Colton E, Ziats NP, Anderson JM. Biomaterial surface chemistry dictates adherent monocyte/macophage cytokine expression in vitro. Cytokine. 2002;18(6):311–19. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 41.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groux H, Cottrez F. The complex role of interleukin-10 in autoimmunity. J Autoimmun. 2003;20(4):281–5. doi: 10.1016/s0896-8411(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 43.Brodbeck WG, Shive MS, Colton E, Ziats NP, Anderson JM. Interleukin-4 inhibits tumor necrosis factor-alpha-induced and spontaneous apoptosis of biomaterial-adherent macrophages. J Lab Clin Med. 2002;139(2):90–100. doi: 10.1067/mlc.2002.121260. [DOI] [PubMed] [Google Scholar]
- 44.Brodbeck WG, Patel J, Voskerician G, Christenson E, Shive MS, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc Natl Acad Sci U S A. 2002;99(16):10287–92. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brodbeck WG, Shive MS, Colton E, Nakayama Y, Matsuda T, Anderson JM. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. J Biomed Mater Res. 2001;55(4):661–8. doi: 10.1002/1097-4636(20010615)55:4<661::aid-jbm1061>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 46.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 47.Trindade MC, Lind M, Sun D, Schurman DJ, Goodman SB, Smith RL. In vitro reaction to orthopaedic biomaterials by macrophages and lymphocytes isolated from patients undergoing revision surgery. Biomaterials. 2001;22(3):253–9. doi: 10.1016/s0142-9612(00)00181-2. [DOI] [PubMed] [Google Scholar]