Caveolin-1-Mediated Expression and Secretion of Kallikrein 6 in Colon Cancer Cells (original) (raw)
Abstract
Kallikreins are secreted proteases that may play a functional role and/or serve as a serum biomarker for the presence or progression of certain types of cancers. Kallikrein 6 (KLK6) has been shown to be upregulated in several types of cancers, including colon. The aims of this study were to elucidate pathways that influence KLK6 gene expression and KLK6 protein secretion in the HCT116 human colon cancer cells. Our data indicate a central role for caveolin-1 (CAV-1), the main structural protein of caveolae, in both KLK6 gene expression and protein secretion. Sucrose gradient subcellular fractionation reveals that CAV-1 and KLK6 colocalize to lipid raft domains in the plasma membrane of HCT116 cells. Furthermore, we show that CAV-1, although it does not directly interact with the KLK6 molecule, enhances KLK6 secretion from the cells. Deactivation of CAV-1, through SRC-mediated phosphorylation, decreased KLK6 secretion. We also demonstrate that, in colon cancer cells, CAV-1 increased the amount of phosphorylated AKT in cells by inhibiting the activity of the AKT-negative regulators PP1 and PP2A. This study demonstrates that proteins such as CAV-1 and AKT, which are known to be altered in colon cancer, affect KLK6 expression and KLK6 secretion.
Introduction
The kallikrein protein family consists of 15 serine proteases, each of which has a unique pattern of expression and set of substrates [1]. The most widely known and used kallikrein (KLK) is KLK3, also known as prostate-specific antigen. Because of their nature as secreted proteins, kallikreins are currently under investigation as potential biomarkers. For the kallikrein family of proteins to be useful biomarkers for either the presence or the progression of cancer, it is crucial to know under which conditions kallikreins are aberrantly expressed.
Kallikrein 6 (KLK6) is being examined as a marker for certain types of ovarian and uterine cancers [2,3]. KLK6 mRNA and secreted protein were found to be significantly upregulated in uterine serous papillary cancer compared to benign tumor and endometrial carcinoma patients [3]. Patients with ovarian carcinoma also had significantly higher levels of serum KLK6, about twice the concentration of normal or benign tumor patients [2]. In both gastric and colon cancer, KLK6 mRNA was observed to be more highly expressed compared to normal mucosa [4,5]. In each previously mentioned study, above-average expression of KLK6 correlated with a poor prognosis [2–5]. This may be due to the role of KLK6 in cancer progression. KLK6 has been implicated in angiogenesis, migration, and invasion through mechanisms involving extracellular matrix (ECM) degradation [1]. Substrates for KLK6 include collagen, fibrinogen, fibronectin, and laminin [1,6]. Prezas et al. [7] demonstrated that ovarian cells that were stably transfected to express KLK4, 5, 6, and 7 were significantly more invasive in vitro and formed larger tumors in mice. Another study demonstrated that a synthetic kallikrein inhibitor attenuated tumor cell invasiveness through a matrigel substrate [8].
The protein caveolin-1 (CAV-1) is frequently expressed abnormally in colon cancer and appears to contribute to aberrant signaling and protein trafficking. CAV-1 is a structural protein required for the formation of caveolae in nonmuscle cells [9,10]. Caveolae are flask-shaped plasma membrane invaginations present in nearly all cell types which function as lipid raft and scaffolding domains within the plasma membrane [9]. There has been much controversy regarding the role of CAV-1 in cancer. It has been implicated to act as both a tumor suppressor and an oncogene, depending on the tissue of origin and stage of disease. These findings have been thoroughly summarized in several reviews [9,11–13]. In addition to varied levels of expression, the stability and activity of CAV-1 are also frequently altered in cancer. CAV-1, in its unphosphorylated form, acts to stabilize caveolae [14]. When CAV-1 is phosphorylated, most commonly at tyrosine-14 by SRC kinase, it dissociates from the caveolae, thereby destabilizing it [15–17]. SRC activity is elevated in a wide variety of other cancers, including breast, lung, pancreatic, ovarian, and gastric, and in colon cancer, SRC activity is frequently increased by an average of five- to eight-fold [18].
Another important role of CAV-1 is that of a modulator of mitogenic signaling pathways. Several studies have reported an increase in phosphatidylinositol-3-kinase (PI3K)/AKT signaling within human cell culture and patient tissues that have elevated CAV-1 expression [19–21]. One mechanism by which CAV-1 increases AKT activity is to decrease the activity of negative regulatory phosphatases PP1 and PP2A [20]. Li et al. [20] demonstrated that, in prostate cancer cells, an association between the CAV-1 scaffolding domain and the catalytic regions of PP1 and PP2A leads to reduced activity of these enzymes, thereby increasing the presence of phosphorylated AKT. They also show that this is a functional change because downstream targets of AKT are more frequently phosphorylated, all contributing to increased cell survival and proliferation. The link between PP1/PP2A and CAV-1 has thus far been limited to prostate cells and cardiomyocytes [20,22]. We wish to explore whether this effect is similar in colon cancer cells as well.
Recent findings indicate that caveolae may play an important role in protease secretion, specifically demonstrated in the proteases urokinase plasminogen activator (uPA) and cathepsin B [23,24]. Despite its controversial role in cancer, it is widely accepted that CAV-1 can modulate mitogenic signaling as well as protein trafficking, both of which are relevant to KLK6 regulation. This study investigates whether this CAV-1-dependent increase in secretion applies to another protease, KLK6, as well. To evaluate the role of CAV-1 and its kinase SRC in KLK6 regulation and KLK6 secretion, we used isogenic cells systems of which either CAV-1 or SRC expression and/or activity were stably altered [24,25]. This study will demonstrate the complex pathways involving CAV-1, SRC, AKT, and PP1/PP2A, which influence both KLK6 expression and protein secretion.
Materials and Methods
Cell Culture
All cell culture reagents were purchased from Invitrogen Corp. (Carlsbad, CA). HCT116 CAV-1 AS and CAV-1 Mock cells were stably transfected with the pAnti-caveolin-1-IRES-hrGFP-1a-puro and the pIRES-hrGFP-1a-puro vectors, respectively [24]. HCT116 SRC531 and Mock cells were stably transfected with the pcDNASRC-531 or pcDNA3.1 vectors, respectively. HCT116 CAV-1 AS and CAV-1 Mock cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin with or without selection antibiotics. Stably transfected clones were maintained in the supplemented DMEM with the addition of the following selection agents: HCT116 CAV-1 AS and Mock - 25 µg/ml puromycin. HCT116 SRC531 cells were maintained in McCoy's 5a media supplemented with 10% FBS, 1% penicillin/streptomycin, and 500 µg/ml G418.
Cell Treatment Conditions
For the AKT inhibition studies with LY294002 (Calbiochem, La Jolla, CA), cells were plated at a concentration of 5.0 x 105 cells per 60-mm plate in normal media containing 10% FBS. Twenty-four hours after plating, fresh media was added with the drug at a concentration of 50 µM or vehicle control (DMSO; Sigma-Aldrich, Life Science Research, St. Louis, MO). Twenty-four hours later, the media and drug were removed and replaced with fresh media and drug. Forty-eight hours after initial drug treatment, cell lysates, conditioned media, and RNA were collected. All drug studies were done in triplicate. The media used was DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin.
Real-Time Polymerase Chain Reaction
Reverse transcription (RT) was completed with _Taq_Man Reverse Transcription Reagents Kit (Applied Biosystems, Foster City, CA). One microgram of total RNA was transcribed into cDNA in a 50-µl reaction with random hexamers under the thermal condition recommended by the protocol. Real-time polymerase chain reaction (PCR) amplification was performed with a sequence detection system (ABI PRISM 7700 SDS; Applied Biosystems), under the universal thermal cycling conditions recommended by the Assay-on-Demand products protocol. Each 50-µl real-time PCR reaction included 25 µl of _Taq_Man Universal PCR master mix, 10 µl of the resulting cDNA from the RT step, and 15 µl of the diluted primer and probe mixes ordered from Assay-on-Demand products (Applied Biosystems). No template controls were included in each plate to monitor the potential PCR contamination. Each cell line was tested in triplicate and each reaction was run in duplicate. To determine the relative expression level of each target gene, the comparative _C_T method was used. The _C_T value of the target gene was normalized by the endogenous reference [Δ_C_T = _C_T(target) − _C_T(GAPDH)] and compared to a calibrator, in our case, control RNA [ΔΔ_C_T = Δ_C_T(target) - Δ_C_T(calibrator)]. The relative expression of each target gene was calculated using the equation: 2-ΔΔ_C_T.
Western Blot Analysis
Whole-cell lysates were collected by lysing on ice in radioimmunoprecipitation assay buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 30 µg/ml aprotinin, 100 mM sodium orthovanidate, and 10 mg/ml PMSF). Samples were kept on ice for 30 minutes, followed by centrifugation at 14,000 rpm for 10 minutes. Supernatants were collected, and protein concentration was determined using a colorimetric assay (DC Protein Assay; Bio-Rad, Hercules, CA). Sixty microgram of cell lysate was loaded per lane and run on a 12.5% SDS-PAGE gel. The proteins were transferred electrophoretically to Hybond-C nitrocellulose membrane (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) overnight. Blots were blocked in Blotto A (5% w/v nonfat dry milk, 0.1% Tween 20, and Tris-buffered saline (TBS) consisting of 10 mM Tris-HCl, pH 8.0, 150 mM NaCl) for 1 hour at room temperature. CAV-1 and KLK6 antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and flotillin-1 (Flot-1) antibody (BD Transduction Laboratories, Franklin Lakes, NJ) were diluted in Blotto A at concentrations of 1:1000, 1:200, and 1:500, respectively. AKT, p-AKT, SRC, p-SRC, p-CAV-1, PP1α, and PP2A-C subunit antibodies (Cell Signaling, Danvers, MA) were diluted in 5% BSA in TTBS at a concentration of 1:1000. The blots were washed in TBS/0.1% Tween 20. The primary antibodies were detected with an anti-rabbit (CAV-1) or anti-goat (KLK6) immunoglobulin G antibody conjugated to HRP. Blots were washed as described above, and protein was detected with an enhanced chemiluminescence detection reagent (GE Healthcare, Waukesha, WI).
ELISA for Secreted KLK6
An ELISA kit for the detection of human KLK6 was obtained from Ibex (Quebec, Canada). The assay was performed according to manufacturer's protocol. Briefly, standards were prepared at concentrations of 0, 0.2, 0.5, 2.0, 5.0, 10.0, and 20.0 ng/ml to set a standard concentration curve. Fifty microliters of either the standard or the conditioned media was added to the precoated well and incubated for 2 hours. The wells were washed six times with the provided wash buffer. One hundred microliters of KLK6 antibody-biotin was added to all the wells and incubated for 1 hour, followed by six washes. One hundred microliters of streptavidin-HRP was added and incubated for 30 minutes, followed by six washes. One hundred microliters of tetramethylbenzidine substrate was added and incubated for 30 minutes, followed by the addition of 100 µl of stop solution. All incubations were carried out on a high-speed titer plate shaker at room temperature. The plate was read at 490 nm within 10 minutes on an EL800 Universal Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT).
Sucrose Gradient Cellular Fractionation
Lipid raft fractionation was performed using a detergent-free, alkaline lysis method as described previously [26]. Briefly, HCT116 cells (7 x 106 cells per 150-mm plate) were plated for 48 hours. Each plate was then lysed with 2 ml of 500 mM sodium carbonate (pH 11.0). The lysate was sonicated for three 20-sec bursts using a Sonic Vibra Cell sonicator (Sonics & Materials Inc., Newton, CT). The lysate was then adjusted to 45% sucrose by mixing with equal volumes of 90% sucrose prepared in Mes-buffered saline (MBS - 25 mM Mes, pH 6.5, 0.15 M NaCl), and placed at the bottom of an ultracentrifuge tube. A 5% to 35% discontinuous sucrose gradient was formed above (4 ml of 5% sucrose/4 ml of 35% sucrose; both in MBS containing 250 mM sodium carbonate) and centrifuged at 39,000 rpm for 16 hours in an SW40-Ti rotor (Beckman Instruments, Palo Alto, CA). A light-scattering band at the 5% to 35% sucrose interface was observed. This fraction contains CAV-1/lipid raft proteins. Twelve 1-ml fractions were collected from top to bottom of the tube. For the detection of KLK6, CAV-1, and Flot-1 in the fractions, equal volume from each fraction were loaded on a 12.5% SDS-PAGE gel and visualized as described in the Western Blot Analysis section. The films were scanned, and densitometry was carried out using the Scion Imaging Quantification Software (Scion Corp., Frederick, MD). For each individual protein, the density values in all 12 fractions were added up. The values of CAV-1, KLK6, and Flot-1 in each single sucrose gradient fraction were calculated as a percentage of their total density value in all fractions.
Membrane and Cytosolic Fractionation
This method has been described previously [27]. Briefly, cells were suspended in 10 mM Tris, pH 7.4, 1 mM EDTA, 200 mM sucrose, and 1 mM PMSF, and then homogenized with a tight fitting douncer. The nuclei were removed from the homogenate by centrifugation at 900_g_ for 10 minutes at 4°C. The resulting supernatant was centrifuged at 110,000_g_ for 75 minutes at 4°C. The supernatant was saved as a cytosolic fraction. The remaining membrane pellet was solubilized in 10 mM Tris, pH 7.4, 1 mM EDTA, and 0.5% Triton X-100 for a minimum of 1 hour on ice with intermittent vortexing, followed by centrifugation at 13,000_g_ for 10 minutes at 4°C. The supernatant was considered as a membrane fraction.
Immunoprecipitation
The cytosolic and membrane fractions from HCT116 CAV-1 Mock and HCT116 CAV-1 AS cells were precleared with normal rabbit protein A Agarose (Santa Cruz Biotechnology, Inc.) for 1 hour at 4°C. CAV-1 was immunoprecipitated from precleared lysates with polyclonal CAV-1 antibody (Santa Cruz Biotechnology, Inc.) overnight at 4°C, in the presence of protein A Agarose. Immunoprecipitated beads were washed four times, then boiled in SDS sample buffer, separated by SDS-PAGE, and immunoblotted with KLK6 antibody (Santa Cruz Biotechnology, Inc.).
Results
KLK6 Expression and Secretion Are Decreased in the Downregulated CAV-1 Cells
HCT116 cells stably transfected with a CAV-1 antisense vector were confirmed to express a greatly reduced amount of CAV-1 than the mock-transfected control cell line (Figure 1_A_). RNA was isolated from HCT116 CAV-1 Mock and HCT116 CAV-1 AS cells after 48 hours of normal growth, in serum-containing media. As analyzed by real-time RT-PCR using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers as a control, KLK6 gene expression decreased nine-fold in HCT116 CAV-1 AS cells compared to CAV-1 Mock (Figure 1_B_). To evaluate KLK6 secretion, media was collected from cells 24, 48, and 72 hours after plating in normal growth conditions. Secretion was also significantly reduced at all time points in the HCT116 CAV-1 AS cells (Figure 1_C_).
Figure 1.
Downregulation of CAV-1 in HCT116 cells decreases the levels of KLK6 mRNA and KLK6 secretion. (A) Whole-cell lysates were collected from HCT116 CAV-1 mock and CAV-1 AS cells after 48 hours of growth under normal conditions (regular media with 10% FBS). Western blot analysis for CAV-1 was performed and β-actin was used as a loading control. (B) Real-time reverse transcription-PCR analysis was performed on RNA isolated from cells after 48 hours of growth under normal conditions (*P ≤ .05). (C) ELISA analysis for secreted KLK6 in media collected from HCT116 CAV-1 mock and AS cells 24, 48, and 72 hours after plating in normal growth conditions (*P ≤ .02).
KLK6 Localizes to CAV-1-Containing Membrane Fractions in the Presence, But Not the Absence of CAV-1
Because CAV-1 expression influenced both KLK6 gene expression and secretion in HCT116 cells, we explored the possibility that KLK6 localizes to the caveolae of these cells. Sucrose gradients were used to fractionate whole-cell lysates into density-based fractions. All fractions were analyzed for their KLK6 and CAV-1 content. Fractions 4 to 6 are most commonly enriched with CAV-1 and other lipid raft plasma membrane proteins, such as Flot-1 [28,29]. In both the HCT116 CAV-1 Mock cells and the CAV-1 AS cells, Flot-1 was observed primarily in fractions 5 and 6 (Figure 2, A and B). As expected, CAV-1 was also seen in these fractions in the mock cells, but not in the CAV-1 AS cells (Figure 2, A and B). Importantly, the levels of KLK6 in fractions 5 and 6 of the HCT116 CAV-1 Mock fractions were significantly higher than in the HCT116 CAV-1 AS fractions, indicating that in the absence of CAV-1, the level of KLK6 localizing to lipid raft membrane domains is decreased (Figure 2, A and B). The Western blots were quantified using densitometric analysis as described in Materials and Methods. The percentage of the CAV-1, KLK6, and Flot-1 contents corresponding to each individual fraction was plotted to show the distribution of these proteins in lipid rafts (Figure 2, C and D). It is notable that, overall, there was more KLK6 protein in the HCT116 CAV-1 Mock cells than in the CAV-1 AS cells, which is consistent with earlier data. The amount of KLK6 and Flot-1 localized to the caveolar fractions (fractions 4–7) was calculated as a percentage of the total amount of protein present (Table 1). In the HCT116 CAV-1 Mock cells, there was a 2.65-fold increase in KLK6 in these fractions, compared to HCT116 CAV-1 AS cells. In contrast, Flot-1 localizes to the caveolar fractions to a similar degree in the two cell lines (62.2% and 53.9%, respectively). The ratio of KLK6 to Flot-1 was then calculated to determine the relative percentage of KLK6 associated with lipid rafts. Taking values normalized to the HCT116 CAV-1 Mock cells, there was a 56% decrease in KLK6 associated with lipid rafts in the HCT116 CAV-1 AS cells.
Figure 2.
Sucrose gradient cell fractionation reveals that KLK6 associates with lipid raft domains in CAV-1 expressing cells. (A and B) Equal amounts from each of the 12 sucrose gradient fractions obtained from HCT116 CAV-1 Mock cells (A) and HCT116 CAV-1 AS cells (B) were loaded and run on a 12.5% SDS-PAGE gel, and immunoblots for KLK6, CAV-1, and Flot-1 were performed. (C) Protein localization on sucrose gradient in HCT116 CAV-1 Mock cells. (D) Protein localization on sucrose gradient in HCT116 CAV-1 AS cells. (E) KLK6 is associated with the membrane fraction but does not interact with CAV-1. CAV-1 was immunoprecipitated from the cytosolic (C) and membrane (M) fractions isolated from HCT116 CAV-1 Mock and CAV-1 AS cells as described in Materials and Methods. Western blot analysis for KLK6, CAV-1, and β-actin (used as a loading control) was performed in immunoprecipitates (IP:CAV-1) and in unprecipitated whole fractions (WF). For immunoprecipitates, 500 µg of protein was used; for whole fractions, each lane was loaded with 80 µg of protein.
Table 1.
Densitometric Quantification of the Proportion of KLK6 Associated with Lipid Rafts.
| Cell Type | Percentage of KLK6 in Caveolar Fractions (Fractions 4–7) | Percentage of Flot-1 in Caveolar Fractions (Fractions 4–7) | Ratio KLK6/Flot-1 (Normalized to HCT116 CAV-1 Mock Cells) |
|---|---|---|---|
| HCT116 CAV-1 Mock | 25.8 | 62.3 | 0.41 (1.0) |
| HCT116 CAV-1 AS | 9.7 | 53.9 | 0.18 (0.44) |
We also performed an immunoprecipitation experiment in the cytosolic and crude plasma membrane fractions of HCT116 CAV-1 Mock and HCT116 CAV-1 AS cells to assess the possible interaction of CAV-1 with KLK6. No KLK6 was detected in immunoprecipitates from the cytosolic or membrane fractions using CAV-1 antibody, showing that KLK6 was not directly associated with CAV-1 (Figure 2_E_). Western blot analysis of the level of KLK6 protein in the cytosolic and membrane fractions revealed the two-fold decrease in the KLK6 protein level in the cytosolic fraction of HCT116 CAV-1 AS cells compared to HCT116 CAV-1 Mock controls. Moreover, the levels of KLK6 in the membrane fraction of HCT116 CAV-1 AS cells was more than 4.5 times less than in the membrane fraction of HCT116 CAV-1 Mock cells (Figure 2_E_). This result is consistent with our data obtained using CAV-1-enriched membrane fractions showing a distinct effect of CAV-1 on the KLK6 intracellular level and distribution.
KLK6 Secretion Is Reduced in the Presence of Constitutively Active SRC
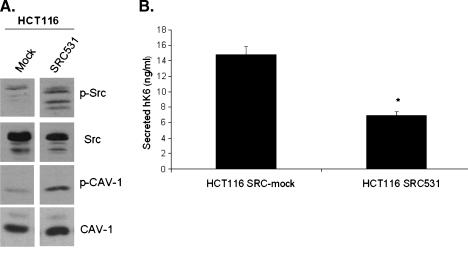
To determine whether KLK6 is sensitive to processes in the cell which alter CAV-1 activity, we used HCT116 cells stably transfected to express constitutively active SRC. These cells express SRC531, a mutant form of SRC which is insensitive to negative regulation [25]. CAV-1 is a substrate for SRC kinases. We first demonstrated that the HCT116 SRC531 cells, compared to the HCT116 SRC-mock cells, had elevated amounts of phospho-SRC as well as phospho-CAV-1 (Figure 3_A_). KLK6 gene expression was not significantly altered between the HCT116 SRC-mock and SRC531 cell lines (data not shown). KLK6 secretion in these cells was significantly decreased by nearly half in the presence of the constitutively active SRC531 (Figure 3_B_). These data suggest that the SRC-induced phosphorylation of CAV-1 ultimately leads to the decrease in secretion of KLK6.
Figure 3.
HCT116 cells with constitutively active SRC have elevated levels of phosphorylated CAV-1, leading to less KLK6 secretion. (A) Western blot analysis was performed on whole-cell lysates from HCT116 SRC-mock and HCT116 SRC531 cells collected 48 hours after plating in normal growth conditions. (B) ELISA analysis for secreted KLK6 was performed on conditioned media collected from HCT116 SRC-mock and HCT116 SRC531 cells 48 hours after plating in normal growth conditions (*P ≤ .001).
CAV-1 Downregulation Leads to Suppression of AKT Phosphorylation through an Increase in PP1 and PP2A
As demonstrated by the change in KLK6 mRNA levels in the HCT116 CAV-1 Mock versus CAV-1 AS cells, there is clearly some factor within these cells altering KLK6 gene expression. Based on published and unpublished data from our laboratory, as well as several previously published studies which correlate an increase in CAV-1 expression with an increase in AKT activity [19,20], we explored AKT-dependent signaling as a mechanism controlling increased KLK6 expression. We demonstrated that the HCT116 CAV-1 Mock cells had more phospho-AKT than do the HCT116 CAV-1 AS cells (Figure 4_A_). As indicated by previous studies, the CAV-1-dependent increase in phospho-AKT levels may involve a decrease in levels and activity of PP1 and/or PP2A [20]. The levels of PP1 and PP2A in the whole-cell lysates of HCT116 CAV-1 Mock and HCT116 CAV-1 AS cells were determined by Western blot analysis. Reduced levels of PP1 and PP2A were observed in HCT116 CAV-1 Mock cells in comparison to HCT116 CAV-1 AS cells (Figure 4_B_). These data correlate well with the relative amounts of phospho-AKT observed in these cell lines (Figure 4_A_), wherein the cells with more phospho-AKT have less PP1 and PP2A expression. The levels of PP1 and PP2A also are inversely correlated to levels of KLK6 expression because the cells that express less PP1 and PP2A express more KLK6 (Figure 1_B_).
Figure 4.
Increased KLK6 expression correlates to levels of phospho-AKT and the phosphatases PP1 and PP2A. (A) Western blot analyses of phospho-AKT and total AKT were performed on whole-cell lysates collected 48 hours after plating in normal growth conditions. (B) Western blot analyses for PP1 and PP2A were performed on whole-cell lysates collected from cells 48 hours after plating in normal growth conditions. β-Actin was used as a loading control.
Pharmacological Inhibition of AKT Leads to Reduced KLK6 Expression and KLK6 Secretion
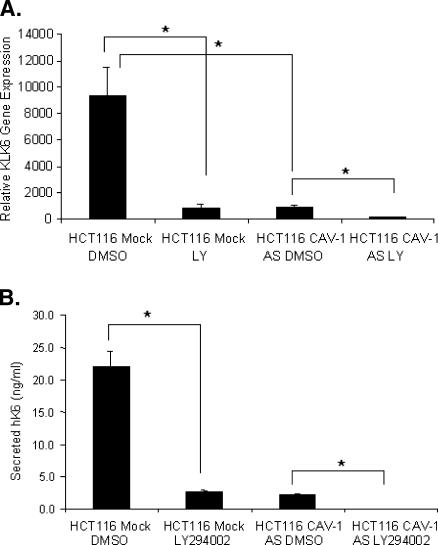
To further demonstrate that the AKT pathway positively influences KLK6, we inhibited AKT to observe changes in KLK6 expression. To disrupt the AKT signaling pathway, we used the small molecule AKT inhibitor, LY294002, at a concentration of 50 µM following the drug treatment protocol outlined in the Materials and Methods section. Real-time PCR analysis of cells treated with LY294002 reveals that KLK6 mRNA levels are significantly reduced in both the HCT116 CAV-1 Mock and CAV-1 AS cells (Figure 5_A_). Reflecting the drop in mRNA levels, the amount of secreted protein is also significantly reduced (Figure 5_B_). These data indicate that the AKT signaling pathway is a likely mechanism by which CAV-1 is influencing KLK6 gene expression.
Figure 5.
Inhibition of AKT leads to decreased KLK6 mRNA and secreted KLK6 protein. (A) Real-time PCR analysis for the detection of KLK6 mRNA was performed using RNA isolated from cells treated with either the AKT inhibitor LY294002 or the vehicle control DMSO (*P ≤ .03). The exact treatment protocol is outlined in the Materials and Methods section. (B) ELISA analysis was performed on conditioned media collected after 48 hours of treatment with LY294002 (*P ≤ .01).
Discussion
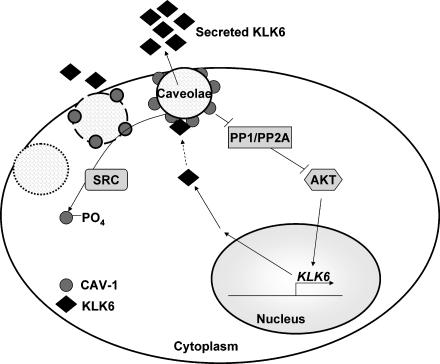
Although aberrant expression of KLK6 has been demonstrated in numerous studies, very little is known regarding the regulation of its expression or secretion. We have demonstrated here that KLK6 gene regulation is influenced by AKT signaling. AKT appears to be more active in the presence of caveolae because of the CAV-1-dependent decrease of the negative regulators PP1 and PP2A. Once synthesized, KLK6 protein is secreted, a process that is positively influenced by CAV-1. Although it has been reported in cultured endothelial, mesangial, and smooth muscle cells that different molecules might be linked to caveolae-enriched membrane domains through interactions with CAV-1 [30], we did not observe the direct association of CAV-1 and KLK6 in colon cancer cells. As has been shown previously, SRC, a CAV-1 kinase, can destabilize caveolae through the phosphorylation of CAV-1, thereby decreasing KLK6 secretion [15–17]. These findings are summarized in our proposed model (Figure 6).
Figure 6.
Proposed model of KLK6 expression and secretions: This proposed model unites the pathways regulating KLK6 gene expression and KLK6 secretion. We demonstrate that AKT plays the most pivotal role in KLK6 gene expression. AKT activity is negatively regulated by phosphatases PP1 and PP2A, which are in turn controlled at least in part by CAV-1. Colon cancer cells, which express CAV-1, express and secrete significantly more KLK6 than isogenic cells lines lacking CAV-1 expression. SRC kinase phosphorylates CAV-1, and this phosphorylation causes the decrease in KLK6 secretion.
There are key regulatory sites within the proximal KLK6 promoter region that include putative binding sites for Elk-1, AP-1, and SP-1, as well as two E-box sequences [26]. The functionality of the SP-1 binding site was confirmed using the enzyme mobility shift assay [26]. This study also showed that deletion of the portion of the promoter containing the AP-1 and E-box sequences completely abolishes promoter reporter transactivation [26]. These KLK6 promoter reporter studies were performed in MCF-7 breast cancer cells, and it will be important to perform these experiments in our colon cancer model systems to determine whether this type of regulation is conserved among cells of different tissue origins. Upregulation of AKT activity has been shown to increase downstream AP-1 signaling [31]. We have observed significantly increased AP-1 promoter reporter activity in the HCT116 CAV-1 Mock compared to the HCT116 CAV-1 AS cells (unpublished data). This indicates a potentially important avenue of study to determine whether AP-1 signaling also plays a role in KLK6 expression in colon cell model systems. In addition to the putative AP-1 regulation, the Elk-1 and SP-1 sites may also play a pivotal role in KLK6 gene regulation. Studies from our laboratory show that inhibition of the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway with the small molecule inhibitor PD98059 also reduces KLK6 expression, although to a lesser degree than AKT inhibition.
Analysis of cancerous and surrounding noncancerous human tissues reveals that the expression of CAV-1 is elevated in several types of cancers [32,33]. CAV-1 was shown to be elevated in colonic adenocarcinoma compared to both normal colonic mucosa and colon adenoma tissue [32]. This suggests an increase in CAV-1 expression with advancing tumor grade. Along those same lines, Hung et al. [33] demonstrate that through the course of oral carcinogenesis, the percentage of tissues with positive staining for CAV-1 increases along with the advancing stage of the cancer, except in the most advanced stage of metastatic oral squamous cell carcinoma, possibly indicating a biphasic expression pattern. These counter-intuitive findings underscore the fact that the state of CAV-1 expression is a controversial topic among cancer researchers. Central to this study, CAV-1 expression in several colon cancer cell lines has been analyzed. As we have demonstrated, HCT116 cells produce significant amounts of CAV-1, whereas two other colon cancer cell lines, HT29 and Caco2, produce very little or virtually none, respectively. Interestingly, these levels of CAV-1 expression correlate with the varying growth rates of these cell lines, with HCT116 cells having the fastest growth rate and Caco2 cells the slowest [34]. Increases in CAV-1 expression are also observed in azoxymethane induced rat colon adenocarcinomas, compared to surrounding normal mucosa [34].
Upregulation of CAV-1 in cancer has many implications due to its diverse roles in cells. It modulates several signaling pathway, likely due to its interaction with signaling and regulatory proteins such as PP1, PP2A and RAS [19–21,23,28,35]. When implicating protein phosphatases such as PP1 and PP2A as key regulatory proteins in a pathway, it is important to acknowledge their promiscuous nature, owing to the fact that there are far fewer protein phosphatases than there are kinases. For example, the PP2A family of phosphatases has many substrates, including Raf, MEK, ERK, and AKT, among others [22,36]. However, substrate specificity has been demonstrated through modulation of the variable B regulatory subunit of PP2A. PP2A proteins are composed of a scaffolding subunit (A), a regulatory subunit (B), and a catalytic subunit (C) [36]. It was shown that PP2A/B′ heterotrimers specifically dephosphorylated AKT, whereas PP2A/Bα and PP2A/Bδ act on the ERK pathway kinases [36]. Thus, an overall increase in PP2A may affect certain pathways more than others. A study done in cardiomyocytes, using subcellular fraction colocalization as well as immunoprecipitation, demonstrated that PP2A and CAV-1 associate in cells [22]. It has not yet been determined which specific PP2A isoforms CAV-1 affects.
In addition to its effects on mitogenic signaling, recent research, supported by the current study, demonstrates that protease secretion can be mediated by CAV-1 [23,24]. The expression and secretion of cathepsin B, a cysteine protease, uPA, a serine protease, and its receptor uPAR are decreased in HCT116 CAV-1 AS cells compared to HCT116 CAV-1 Mock cells [24]. To confirm an effect on tumorigenic phenotypes, this study also demonstrated a decrease in the degradation of the ECM protein collagen IV as well as diminished invasion through Matrigel by the HCT116 CAV-1 AS cells [24]. Increased expression of cathepsin B has been shown to be predictive of decreased overall survival rates among colon cancer patients, and upregulation of uPA/uPAR expression is known to increase tumorigenic phenotypes such as chemotaxis and evasion of apoptosis [24,37]. A recent study determined that overexpression of CAV-1 in prostate cancer correlated with more aggressive tumors (as determined by Gleason score), high preoperative prostate-specific antigen (KLK3) levels, and a high rate of cancer recurrence after a radical prostatectomy [38]. Although CAV-1 has previously been shown to enhance protease secretion, the novel findings of this study demonstrate a CAV-1-dependent upregulation of both KLK6 expression and KLK6 secretion in colon cancer.
In summary, CAV-1 influences KLK6 gene expression through its influence on PP1/PP2A and AKT. CAV-1 directly enhances protein secretion in our cell model system by facilitating KLK6 secretion through the caveolae. Determining when and why KLK6 overexpression will occur in colon cancer could have several very important implications. KLK6 has the potential to play a role as a serum marker for the presence and/or progression of several types of cancer, thus knowing exactly when and why it is upregulated is essential. KLK6 is also able to degrade ECM components, leading to more virulent tumor cell invasiveness [1]. Understanding how KLK6 is secreted could lead to therapeutic efforts to block that process, thereby conferring a less invasive phenotype to those cells.
Acknowledgments
We thank Rosalyn Irby and Timothy Yeatman for the kind gift of the HCT116 SRC531/Mock cells. Special thanks go to David Stringer for technical assistance.
Abbreviations
CAV-1
caveolin-1
ECM
extracellular matrix
ERK
extracellular signal-regulated kinase
Flot-1
flotillin-1
KLK6
kallikrein 6
MEK
mitogen-activated protein kinase kinase
PCR
polymerase chain reaction
uPA
urokinase plasminogen activator
Footnotes
1
Grant acknowledgement: CA-95060.
References
- 1.Borgono CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004;4:876–890. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- 2.Diamandis EP, Scorilas A, Fracchioli S, Van Gramberen M, De Bruijn H, Henrik A, Soosaipillai A, Grass L, Yousef GM, Stenman UH, et al. Human kallikrein 6 (KLK6): a new potential serum biomarker for diagnosis and prognosis of ovarian carcinoma. J Clin Oncol. 2003;21:1035–1043. doi: 10.1200/JCO.2003.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Santin AD, Diamandis EP, Bellone S, Soosaipillai A, Cane S, Palmieri M, Burnett A, Roman JJ, Pecorelli S. Human kallikrein 6: a new potential serum biomarker for uterine serous papillary cancer. Clin Cancer Res. 2005;11:3320–3325. doi: 10.1158/1078-0432.CCR-04-2528. [DOI] [PubMed] [Google Scholar]
- 4.Nagahara H, Mimori K, Utsunomiya T, Barnard GF, Ohira M, Hirakawa K, Mori M. Clinicopathologic and biological significance of kallikrein 6 overexpression in human gastric cancer. Clin Cancer Res. 2005;11:6800–6806. doi: 10.1158/1078-0432.CCR-05-0943. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa K, Utsunomiya T, Mimori K, Tanaka F, Inoue H, Nagahara H, Murayama S, Mori M. Clinical significance of human kallikrein gene 6 messenger RNA expression in colorectal cancer. Clin Cancer Res. 2005;11:2889–2893. doi: 10.1158/1078-0432.CCR-04-2281. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh MC, Grass L, Soosaipillai A, Sotiropoulou G, Diamandis EP. Human kallikrein 6 degrades extracellular matrix proteins and may enhance the metastatic potential of tumour cells. Tumour Biol. 2004;25:193–199. doi: 10.1159/000081102. [DOI] [PubMed] [Google Scholar]
- 7.Prezas P, Arlt MJ, Viktorov P, Soosaipillai A, Holzscheiter L, Schmitt M, Talieri M, Diamandis EP, Kruger A, Magdolen V. Overexpression of the human tissue kallikrein genes KLK4, 5, 6, and 7 increases the malignant phenotype of ovarian cancer cells. Biol Chem. 2006;387:807–811. doi: 10.1515/BC.2006.102. [DOI] [PubMed] [Google Scholar]
- 8.Wolf WC, Evans DM, Chao L, Chao J. A synthetic tissue kallikrein inhibitor suppresses cancer cell invasiveness. Am J Pathol. 2001;159:1797–1805. doi: 10.1016/S0002-9440(10)63026-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–348. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Martin S, Parton RG. Caveolin, cholesterol, and lipid bodies. Semin Cell Dev Biol. 2005;16:163–174. doi: 10.1016/j.semcdb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Le Lay S, Kurzchalia TV. Getting rid of caveolins: phenotypes caveolin-deficient animals. Biochim Biophys Acta. 2005;1746:322–333. doi: 10.1016/j.bbamcr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Schwencke C, Braun-Dullaeus RC, Wunderlich C, Strasser RH. Caveolae and caveolin in transmembrane signaling: implications for human disease. Cardiovasc Res. 2006;70:42–49. doi: 10.1016/j.cardiores.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 14.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao H, Courchesne WE, Mastick CC. A phosphotyrosinedependent protein interaction screen reveals a role for phosphorylation caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277:8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by Src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- 17.Nomura R, Fujimoto T. Tyrosine-phosphorylated caveolin-1: immunolocalization and molecular characterization. Mol Biol Cell. 1999;10:975–986. doi: 10.1091/mbc.10.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 19.Kim HA, Kim KH, Lee RA. Expression of caveolin-1 is correlated with Akt-1 in colorectal cancer tissues. Exp Mol Pathol. 2006;80:65–170. doi: 10.1016/j.yexmp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;3:389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shack S, Wang XT, Kokkonen GC, Gorospe M, Longo DL, Holbrook NJ. Caveolin-induced activation of the phosphatidylinositol 3-kinase/Akt pathway increases arsenite cytotoxicity. Mol Cell Biol. 2003;23:2407–2414. doi: 10.1128/MCB.23.7.2407-2414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuluaga S, Alvarez-Barrientos A, Gutierrez-Uzquiza A, Benito M, Nebreda AR, Porras A. Negative regulation of Akt activity by p38alpha MAP kinase in cardiomyocytes involves membrane localization of PP2A through interaction with caveolin-1. Cell Signal. 2007;19:62–74. doi: 10.1016/j.cellsig.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Cavallo-Medved D, Dosescu J, Linebaugh BE, Sameni M, Rudy D, Sloane BF. Mutant K-ras regulates cathepsin B localization on the surface human colorectal carcinoma cells. Neoplasia. 2003;5:507–519. doi: 10.1016/s1476-5586(03)80035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci. 2005;118:1493–1503. doi: 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]
- 25.Irby RB, Yeatman TJ. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer Res. 2002;62:2669–2674. [PubMed] [Google Scholar]
- 26.Pampalakis G, Sotiropoulou G. Multiple mechanisms underlie the aberrant expression of the human kallikrein 6 gene in breast cancer. Biol Chem. 2006;387:773–782. doi: 10.1515/BC.2006.097. [DOI] [PubMed] [Google Scholar]
- 27.Nagamatsu S, Kornhauser JM, Burant CF, Seino S, Mayo KE, Bell GI. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem. 1992;267:467–472. [PubMed] [Google Scholar]
- 28.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 29.Rajendran L, Le Lay S, Illges H. Raft association and lipid droplet targeting of flotillins are independent of caveolin. Biol Chem. 2007;388:307–314. doi: 10.1515/BC.2007.034. [DOI] [PubMed] [Google Scholar]
- 30.Peng F, Wu D, Ingram AJ, Zhang B, Gao B, Krepinsky JC. RhoA activation in mesangial cells by mechanical strain depends on caveolae and caveolin-1 interaction. J Am Soc Nephrol. 2007;18:189–198. doi: 10.1681/ASN.2006050498. [DOI] [PubMed] [Google Scholar]
- 31.Peloponese JM, Jr, Jeang KT. Role for Akt/protein kinase B and activator protein-1 in cellular proliferation induced by the human T-cell leukemia virus type 1 tax oncoprotein. J Biol Chem. 2006;281:8927–8938. doi: 10.1074/jbc.M510598200. [DOI] [PubMed] [Google Scholar]
- 32.Fine SW, Lisanti MP, Galbiati F, Li M. Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am J Clin Pathol. 2001;115:719–724. doi: 10.1309/YL54-CCU7-4V0P-FDUT. [DOI] [PubMed] [Google Scholar]
- 33.Hung KF, Lin SC, Lin CJ, Chang CS, Chang KW, Kao SY. The biphasic differential expression of the cellular membrane protein, caveolin-1, in oral carcinogenesis. J Oral Pathol Med. 2003;32:461–467. doi: 10.1034/j.1600-0714.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 34.Patlolla JM, Swamy MV, Raju J, Rao CV. Overexpression of caveolin-1 in experimental colon adenocarcinomas and human colon cancer cell lines. Oncol Rep. 2004;11:957–963. [PubMed] [Google Scholar]
- 35.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz DS, Lisanti MP. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 36.Van Kanegan MJ, Adams DG, Wadzinski BE, Strack S. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J Biol Chem. 2005;280:36029–36036. doi: 10.1074/jbc.M506986200. [DOI] [PubMed] [Google Scholar]
- 37.Crippa MP. Urokinase-type plasminogen activator. Int J Biochem Cell Biol. 2007;39:690–694. doi: 10.1016/j.biocel.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF. Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate. 2007;67:614–622. doi: 10.1002/pros.20557. [DOI] [PubMed] [Google Scholar]