The CHD3 Remodeler PICKLE Promotes Trimethylation of Histone H3 Lysine 27 (original) (raw)
Abstract
CHD3 proteins are ATP-dependent chromatin remodelers that contribute to repression of developmentally regulated genes in both animal and plant systems. In animals, this repression has been linked to a multiple subunit complex, Mi-2/NuRD, whose constituents include a CHD3 protein, a histone deacetylase, and a methyl-CpG-binding domain protein. In Arabidopsis,PICKLE (PKL) codes for a CHD3 protein that acts during germination to repress expression of seed-associated genes. Repression of seed-associated traits is promoted in pkl seedlings by the plant growth regulator gibberellin (GA). We undertook a microarray analysis to determine how PKL and GA act to promote the transition from seed to seedling. We found that PKL and GA act in separate pathways to repress expression of seed-specific genes. Comparison of genomic datasets revealed that _PKL_-dependent genes are enriched for trimethylation of histone H3 lysine 27 (H3K27me3), a repressive epigenetic mark. Chromatin immunoprecipitation studies demonstrate that PKL promotes H3K27me3 in both germinating seedlings and in adult plants but do not identify a connection between _PKL_-dependent expression and acetylation levels. Taken together, our analyses illuminate a new pathway by which CHD3 remodelers contribute to repression in eukaryotes.
The transition from inert seed to growing seedling marks the culmination of a remarkable transformation in developmental identity and transcriptional programs. During seed formation, numerous genes are expressed that contribute to establishment of the body plan, laying down of storage reserves, and desiccation tolerance (1,2). Many of these genes are widely expressed in the developing seed only to be silenced by the time the seed germinates, often for the remainder of the life cycle of the plant (3). Although several key regulators have been identified that activate various seed transcriptional programs, such as the LEAFY COTY-LEDON (LEC) genes and_ABSCISIC ACID-INSENSITIVE3_ (ABI3) (4,5), relatively little is known about the mechanisms that ensure that these programs are restricted to developing seeds. This repression is critical for subsequent stages of plant development. In particular, continued expression of LEC genes leads to continued expression of seed-specific programs and substantial alteration of seedling development (6–8).
PICKLE (PKL) has previously been shown to play a significant role in repression of seed-associated genes. In pkl plants, many seed-associated traits continue to be expressed after germination, including accumulation of seed storage reserves and the ability to undergo somatic embryogenesis (9,10). Primary roots of_pkl_ seedlings that continue to express seed-associated developmental programs stop elongating and adopt a green tuberous phenotype referred to as the “pickle root” phenotype. PKL codes for a predicted ATP-dependent chromatin remodeling factor in the CHD3 family (11,12). In animal systems, CHD3 proteins are a component of the Mi-2/NuRD complex (13–16). This complex includes several other proteins, including a methyl CpG-binding protein and a histone deacetylase, suggesting that animal CHD3 proteins play an important role in allowing DNA methylation and histone deacetylation to coordinately contribute to transcriptional repression (17). Genetic characterization of mutants that are deficient for CHD3 in several model systems is consistent with a role for CHD3 proteins in repression of transcription in animals, often of developmentally regulated genes (18,19). In Arabidopsis, loss of PKL leads to elevated expression of seed-specific genes, including the LEC genes LEC1, LEC2, and FUS3, indicating that CHD3 proteins also act to repress transcription in plants (12,20). It is unknown, however, if there is a plant equivalent of the Mi-2/NuRD complex in which PKL acts in concert with a histone deacetylase and/or is targeted by DNA methylation.
The plant growth regulator gibberellin (GA)4 has been linked to PKL and repression of seed-associated transcriptional programs. GA is well known for its ability to promote germination, shoot elongation, and flowering (21,22). Partial inhibition of GA biosynthesis in pkl seedlings substantially enhances expression of embryonic traits, in particular the expression of the pickle root phenotype (9). Interestingly,PKL and GA act during a common time to repress expression of embryonic traits in seedlings. Inhibition of GA biosynthesis during the latter portion of germination of pkl seedlings is sufficient to enhance expression of the pickle root phenotype, whereas inhibition immediately after seed wetting or after germination of the seed has no detectable effect (9). Similarly, use of a hormone-inducible PKL-GR fusion reveals that activation of PKL during germination is sufficient to repress expression of the pickle root phenotype in PKL-GR seedlings, whereas activation during seed maturation or after seed germination is ineffective (23). These analyses reveal a specific developmental window during which PKL and GA act concurrently to repress expression of seed-associated developmental programs.
A previous microarray analysis of germinating pkl seedlings identified several PKL_-dependent genes and suggested that_PKL and GA might act together to repress expression of seed-associated genes (20,24). This analysis was carried out in germinating seedlings well before emergence of the radicle (root). In light of progress in our understanding of when PKL and GA act, we have undertaken a new microarray analysis of gene expression at a later stage of germination of pkl seedlings. This analysis reveals that_PKL_ and GA act synergistically to repress expression of seed-associated genes via separate pathways. Furthermore, comparison of our expression data with data from other genomic analyses reveals a substantial overlap between PKL_-dependent genes and genes that are enriched for trimethylation of histone H3 lysine 27 (H3K27me3), an epigenetic mark that is thought to play a substantial role in silencing of genes that are expressed in a tissue-specific manner in plants (25). Analysis of H3K27me3 levels at several loci reveals that this mark is decreased in the absence of_PKL, suggesting that PKL plays a role in deposition and/or maintenance of H3K27me3 in plants.
EXPERIMENTAL PROCEDURES
Plant Material and Growth Conditions_—Wild type and_pkl-1 (9) are in the Col background. Seeds used in these studies were obtained from wild-type and_pkl_ plants grown in parallel in a CU36L5 incubator (Percival Scientific) under 24 h of illumination. Seeds were allowed to dry at least a month on the plant prior to collection. No other treatment was applied (i.e. stratification) prior to use of the seeds. For the studies described here, plants were incubated on synthetic media (9) and grown in a CU36L5 incubator under 24 h of illumination. Prior to use, seed lots were confirmed to exhibit >98% germination in 3 days and <6 h difference in time to 50% germination under the conditions described above. For all analyses during germination (microarray, qRT-PCR, and ChIP), seeds were sown at a density of 100 mg of seeds (∼3000 seeds) per 150-mm diameter Petri dish. The media were supplemented with 10-8 m uniconazole-P or solvent alone (0.01% methanol final concentration). This concentration of uniconazole-P was selected based on prior dose-response analyses that examined the effect of uniconazole-P on pickle root penetrance and germination rate (9). Samples were collected when 50% of the seeds had germinated. Length of time after seed imbibition to achieve 50% germination was 40.8 h for wild-type seeds imbibed in the absence of uniconazole-P, 43.2 h for wild-type seeds imbibed in the presence of uniconazole-P, 40.1 h for pkl seeds imbibed in the absence of uniconazole-P, and 45.5 h for pkl seeds imbibed in the presence of uniconazole-P. Alternatively, whole plants were collected 14 days post-imbibition for both ChIP and expression analysis (Fig. 7). For RNA used for microarray analysis and subsequent qRT-PCR analysis, an aliquot of seeds from each sample was saved so that the extent of germination and pickle root penetrance could be determined for each treatment. Greater than 99% of the seeds germinated for all treatments. Pickle root penetrance increased from 1.9% on MS media to 43.5% on MS media supplemented with uniconazole-P.
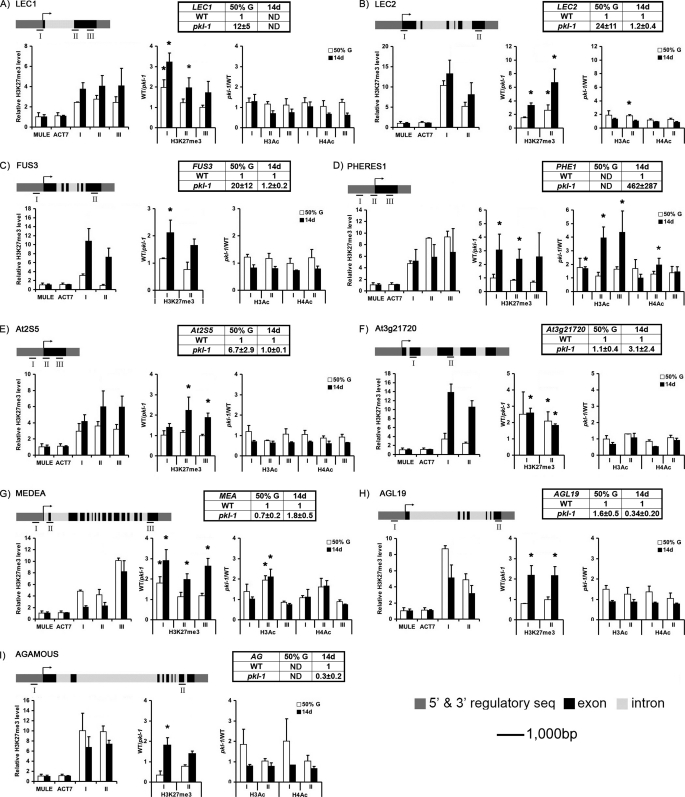
FIGURE 7.
H3K27me3 levels are decreased in pkl plants. ChIP was used to examine levels of H3K27me3, diacetylated H3 (H3Ac), and tetraacetylated H4 (H4Ac) at indicated loci in 50% germinated seedlings (white bars) or in 14-day-old plants (black bars). For each panel, a schematic of the gene of interest at the top left indicates the region that was assayed by ChIP. The graph on the_left_ indicates the level of H3K27me3 for the region of the GOI relative to MULE and ACT7 in wild-type plants. The graph in the middle indicates the level of H3K27me3 in WT relative to_pkl_ plants (thus a decrease in H3K27me3 in pkl relative to WT plants results in an increased signal). The graph on the_right_ indicates the level of H3Ac and H4Ac in pkl plants relative to WT plants (thus an increase in acetylation in pkl relative to WT plants results in an increased signal). The table in the right-hand corner of each panel indicates the expression of the gene as assayed by qRT-CR in pkl plants relative to WT plants in 50% germinated seedlings or in 14-day-old plants. ND denotes transcript not detected at indicated developmental stage. All data are the average of three biological replicates. Bars denote standard deviation.Asterisks in graphs depicting relative levels of H3K27me3, H3Ac, or H3Ac denote a fold change of at least 1.5 (95% confidence interval).
_RNA Isolation and Analysis_—Total RNA was isolated as described previously (26). All subsequent experimental manipulations for microarray analysis were carried out as per the manufacturer's instructions as described previously (20), with the modifications that Affymetrix ATH1 Gene Chips (Arabidopsis Genome Array, catalog number 900385) were used, and hybridization data were analyzed with the Affymetrix Microarray Suite version 5.0 software. Quantitative PCR was performed on an ABI Prism 7000 sequence detection system (Applied Biosystems), as described previously (23). All oligonucleotide primer sequences and primer concentrations used and the critical threshold values for the figures presented can be found in the supplemental material.
_Statistical Analysis and Archiving of Array Data_—Experiment-wise type I error rate (false-positives) was controlled using the generalized family-wise error rate methodology, GFWER(k), of Muir et al. (24) because type II errors (false-negatives) were seen as important as type I. The GFWER(k) is defined as the probability of making k or more type I errors at a given level of p. The GFWER(k) does not attempt to project a false discovery rate, rather, it only sets the maximum number of false-positives under the null hypothesis at a given level of p. We set k = 1 and p = 0.05, allowing a 5% chance of 1 or more false-positives across the experiment.
Shannon entropy was calculated based on formulas given by Schug et al. (31). The distribution and significance of the Hg and Qg statistics were based on 1,000 permutations of the data (27). Developmental samples from AtGenExpress (3) used for determination of tissue specificity are found in supplemental Table S10.
A 2 × 2 χ2 test was applied to each treatment to determine whether the effects of pkl and uniconazole were independent. The number of genes found significant were classified into four categories as up- or down-regulated by either PKL or uniconazole-P. The expected number in each cell, under the assumption of independence, was found by taking the product of the marginal totals divided by the grand total. The χ2 was then computed as the sum of the squared deviations between the observed and expected numbers divided by the expected. Significance was determined by comparison to the critical value χ2 with 1 degree of freedom. A 2 × 2 χ2 test was similarly applied to determine the independence of the effects of_pkl_ and tissue specificity (Fig. 5), uniconazole-P and tissue specificity (Fig. 5), and pkl and other remodeling pathways (Fig. 6). All values used in these χ2 analyses are found in Tables S11 and S12. The complete array data set has been deposited at the Gene Expression Omnibus (GEO) of NCBI, accession number GSE11852.
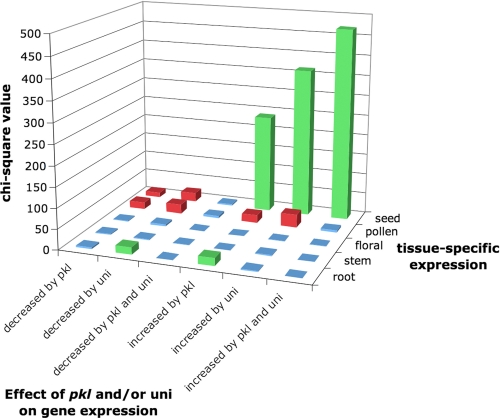
FIGURE 5.
Seed-specific genes are preferentially derepressed in the absence of_PKL_ and/or in the presence of uniconazole. χ2 analysis (see “Experimental Procedures”) was used to examine the intersection of genes that exhibit altered expression in response to_pkl_ and/or uniconazole-P (x axis) and genes preferentially expressed in a specific tissue (y axis). The χ2 value associated with each intersection is represented on the z axis. A_green bar_ denotes more genes observed in common between the two sets than expected (at p < 0.01), and a red bar denotes fewer genes observed than expected (at p < 0.01).
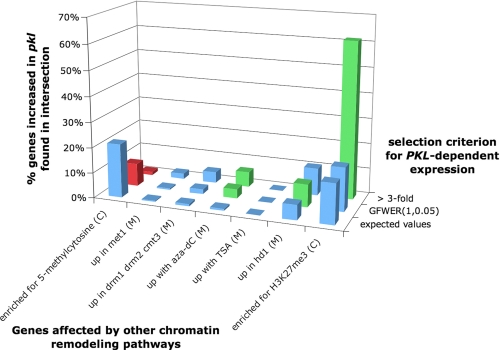
FIGURE 6.
Genes that exhibit strongly elevated expression in the absence of_PKL_ are frequently targets of H3K27me3. χ2 analysis (see “Experimental Procedures”) was used to examine the intersection of genes linked to a remodeling pathway (x axis) and genes that exhibit increased expression in response to pkl according to distinct selection criteria (y axis). The percent of genes that exhibit increased expression in pkl plants that are associated with each intersection is represented on the z axis. The expected values category on the y axis indicates the percentage of_pkl_-dependent genes expected to found in the intersection of the compared sets of genes. A green bar denotes more genes observed in common between the two sets than expected (at p < 1 × 10-4), whereas a red bar denotes fewer genes observed than expected (at p < 1 × 10-4). In x axis categories, C denotes ChIP-chip data, and M denotes microarray-derived expression data. For further discussion of the chromatin remodeling pathways investigated, please see text.
_ChIP Analysis_—50% germinated seeds or whole 14-day-old seedlings were harvested and processed based on a modified version of a previously published protocol (28). Please see supplemental material for a detailed version of the protocol.
RESULTS
Many Genes Exhibit Both PKL- and GA-dependent Repression during Late Germination_—Although inhibition of GA biosynthesis substantially increases penetrance of the pickle root phenotype (9), the mechanism by which this effect occurs has been unclear. Inhibition of GA biosynthesis in_pkl seedlings via application of the chemical inhibitor uniconazole-P was previously observed to have no detectable effect on the transcript level of the seed master regulators LEC1 and LEC2 in pkl seedlings (20). These prior analyses of LEC1 and LEC2 transcript levels were undertaken early during germination, when the testa (seed coat) had started rupturing, but well before emergence of the radicle (20). Given that PKL and GA largely act during later stages of germination to prevent expression of seed traits (9,23), we examined transcript levels of LEC1 and LEC2 in wild-type and pkl seedlings grown in the absence or presence of 10-8 m uniconazole-P developmentally staged at 50% germination (i.e. such that 50% of the seeds had radicles that protruded through the testa). We undertook this analysis at a point in development rather than a specific time after imbibition in recognition of the fact that inhibition of GA biosynthesis delays germination and the observation that the transcript levels of many genes change as a function of the stage of germination (29).
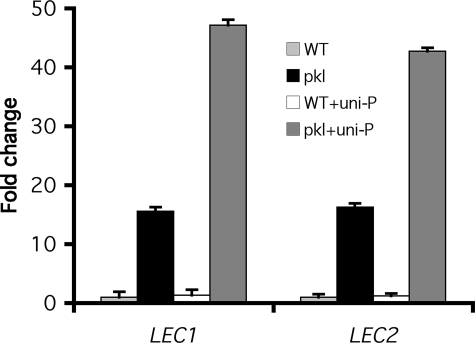
We observed that the transcript levels of LEC1 and LEC2 genes were increased in pkl seedlings by the application of uniconazole-P (Fig. 1). Treatment of wild-type seedlings with uniconazole-P had no detectable effect on expression of these genes. These data were consistent with previous observations that treatment with uniconazole-P could increase the penetrance of the pickle root phenotype in pkl plants, but could not cause expression of the pickle root phenotype in wild-type plants (9). These data thus raised the possibility that treatment of germinating pkl seedlings with uniconazole-P results in increased penetrance of the pickle root phenotype as a result of increased transcript levels of LEC1, LEC2, and similar seed-associated transcripts.
FIGURE 1.
The presence of uniconazole-P results in increased LEC1 and_LEC2_ transcript levels in germinating pkl seeds. Quantitative RT-PCR was used to determine the relative transcript levels of_LEC1_ and LEC2 in germinating WT and pkl seeds in the absence or presence of 10-8 m uniconazole-P (uni-P). Each sample was collected when 50% of the seeds had germinated under the indicated treatment. 18 S rRNA was used as a standardization control, and expression levels were normalized to wild-type seeds imbibed in the absence of uniconazole-P. Error bars represent the S.D. of the mean.
These results suggested that microarray analysis of gene expression at this point in development could clarify the mechanism by which treatment with uniconazole-P increases pickle root penetrance and identify additional genes that contribute to the expression of embryonic traits in pkl seedlings. We used Affymetrix GeneChip ATH1 arrays to query the transcriptome in pkl and wild-type seeds imbibed in the presence or absence of 10-8 m uniconazole-P. The microarray experimental design thus consisted of four treatments: untreated wild type (WT), untreated_pkl_ mutant (pkl), uniconazole-P-treated wild type, and uniconazole-P-treated pkl mutant. Each treatment was collected when 50% of the seeds in that sample had germinated. We used six biological replicates per treatment, thus allowing us to set a strict criterion by which to call a gene differentially expressed. We analyzed our data using an analysis of variance-based approach, GFWER(k) (24), and identified differentially expressed genes with the criterion that there was a 95% chance that there was one or fewer false positives included in the entire group. These selection conditions will be referred to subsequently as GFWER(1,0.05).
Using this selection criterion, 1026 genes exhibit increased transcript levels in response to the pkl mutation whereas only 695 genes exhibit decreased transcript levels (Fig. 2). We also identified 1301 genes that exhibit increased transcript levels in response to uniconazole-P treatment and 1100 genes that exhibit decreased transcript levels. qRT-PCR analysis was used to confirm that we had identified genes that exhibited increased transcript levels in response to pkl or uniconazole and that that the effect of uniconazole was reversed by application of GA (supplemental Tables S2, S3, and S4).LEC1 and LEC2 were not identified by our array analysis despite the fact that both are represented on the ATH1 array and that both exhibit robust PKL_- and uniconazole-dependent expression during this point in development (Fig. 1).LEC2 was identified, however, if array data were sorted on the basis of absence/presence calls as determined by the Affymetrix analysis software (see “Experimental Procedures”). This analysis revealed that_LEC2 was called present in all six replicates of pkl treated with uniconazole-P and called absent in all other replicates of all other treatments. This behavior was consistent with our finding that expression of_LEC2_ was greatest in uniconazole-treated pkl seedlings (Fig. 1). We queried the array data set for genes that were regulated similarly to LEC2 on the basis of absence/presence calls and identified eight other loci that were expressed similarly to LEC2 (Table 1). 7 of these 8 genes, however, were also identified using GFWER(1,0.05) indicating that this approach successfully identified the majority of genes exhibiting this type of expression. In contrast to LEC2, LEC1 was not called present under any condition. Taken together, these observations indicate that our analyses are sufficient to identify some, but not all, of the genes that exhibit _PKL_-dependent expression, including genes that are expressed at low levels such as LEC2.
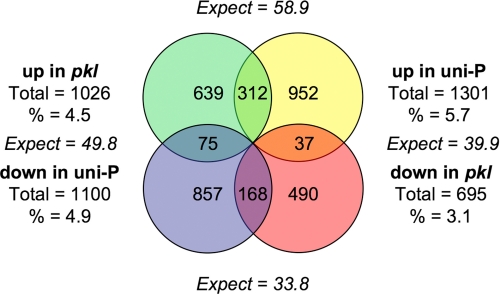
FIGURE 2.
Intersection of genes that exhibit _PKL_- and uniconazole-dependent transcript levels. The Venn diagram indicates the number of loci for which the corresponding transcript is expressed at significantly different levels in the presence of the pkl mutation and/or uniconazole-P (uni-P). Expect indicates the number of genes that would be predicted to exhibit the indicated type of behavior if there was no interaction between genotype (wild type versus pkl) and treatment (± uniconazole-P). % indicates percentage of genes represented on array that are affected by treatment. For a complete list of_PKL_- and uniconazole-dependent genes identified by our analysis, please see supplemental Table 1.
TABLE 1.
LEC2 is among 8 genes identified on the basis of absence/presence calls by Affymetrix software 8 genes (identified by AGI code and by common gene name) exhibit the following type of expression behavior: called absent on all six biological replicates of WT, uniconazole-P treated wild type, and pkl and called present on all six biological replicates of upkl. 6 of 8 of these genes are also identified by GFWER(1,0.05).
| AGI | Gene | Identified by GFWER(k)? |
|---|---|---|
| At1g05510 | Yes | |
| At1g17810 | β-TIP | Yes |
| At1g21520 | Yes | |
| At1g28300 | LEC2 | No |
| At3g26740 | CCL | Yes |
| At4g04630 | Yes | |
| At4g09610 | GASA2 | Yes |
| At5g45690 | Yes | |
| At5g63750 | No |
_PKL and GA Affect Gene Expression through Separate Pathways_—It has previously been noted that pkl plants exhibit the phenotypic hallmarks of GA-deficient plants, leading to the proposal that GA acts to promote PKL activity (9,30). If this hypothesis is true, one might expect expression of genes to be commonly affected by mutation of PKL and by application of uniconazole-P. Consistent with this hypothesis, we found that significantly more genes were commonly up-regulated (χ2 = 1,207.7, p = 1 × 10-264) or down-regulated (χ2 = 579.0, p = 6 × 10-128) by mutation of PKL or by application of uniconazole-P than would have been expected by chance (Fig. 2). In particular, >30% (312 of 1026) of the genes that exhibit increased expression in response to pkl also exhibit increased expression in response to the presence of uniconazole-P, whereas only 5.7% would have been expected by chance. Thus, these data strongly support the idea that PKL and GA act in common to affect expression of genes during germination.
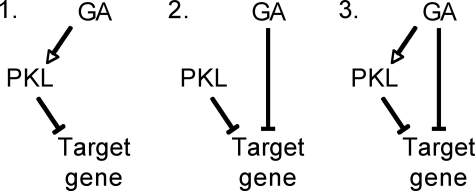
Three possible genetic models depicting the effect of PKL and GA on gene expression that are consistent with previous characterization of_pkl_ and GA signaling mutants are shown inFig. 3. In brief, the ability of GA to affect gene expression could be entirely directed through_PKL_ (model 1), PKL and GA could act through separate pathways to affect gene expression (model 2), or GA could act concurrently through distinct _PKL_-dependent and_PKL_-independent pathways to affect gene expression (model 3).
FIGURE 3.
Possible genetic models of how PKL and GA affect expression of target genes. Arrows denote activation; bars denote repression.
We examined the array data to determine how PKL and uniconazole interact to effect gene expression in an effort to distinguish among these three models. It has previously been demonstrated that although pkl-1 is not a protein null, it behaves as a phenotypic null (12,23). As a result, a strong genetic prediction of model 1 is that the expression levels of genes commonly affected by GA and PKL should be uniconazole-independent in a_pkl-1_ background. Instead, we found that for all 480 genes commonly up-regulated or down-regulated by pkl and uniconazole-P, the application of uniconazole-P to pkl seedlings resulted in enhanced differential expression relative to wild type in comparison with untreated_pkl_ seedlings (supplemental Table S5). Thus these data rule out model 1 and indicate that PKL and GA act through separate pathways to affect gene expression.
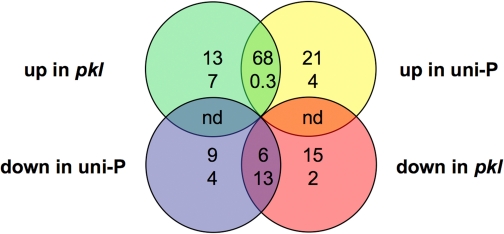
Additional support for separate pathways was provided by analysis of synergistic interactions between PKL and uniconazole-P. A t test for interaction was used to identify those genes for which the extent of the effect of pkl on gene expression was dependent on the presence of uniconazole-P and vice versa (supplemental Table S6). Synergy is defined as when pkl and uniconazole-P interact and enhance the action of each other. We examined genes that exhibit six different types of pkl_- and uniconazole-dependent expression and found that for five of six sets of genes,pkl and uniconazole preferentially act to enhance the effect of the other (Fig. 4). Most strikingly, pkl and uniconazole-P act in a synergistic fashion for 68% of genes that exhibit increased expression in response to either loss of_PKL or application of uniconazole-P. This analysis thus reveals that_PKL_ and GA preferentially act synergistically to repress gene expression and strongly supports separate pathways for each, as depicted in models 2 and 3.
FIGURE 4.
pkl and uniconazole-P act synergistically to increase gene expression. The sets of genes that exhibit PKL_- and/or uniconazole-dependent expression are depicted in a Venn diagram as inFig. 2. The top number in each set indicates the percent of genes belonging to that set for which_pkl or uniconazole-P (uni-P) enhance the effect of each other, and the bottom number in each set indicates the percent of genes for they counteract each other. nd, denotes not determined.
PKL and GA Preferentially Act to Repress Genes That Are Expressed during Seed Development_—Previous phenotypic characterization of_pkl seedlings suggested that PKL and GA act specifically to repress those genes that are preferentially expressed during seed development. An alternative hypothesis, however, is that PKL and/or GA are necessary for repression of many developmentally regulated genes during germination, but only seed-specific genes are expressed to a sufficient extent to lead to re-acquisition of a seed-like state. To distinguish between these two hypotheses, we examined whether PKL and GA repress genes that are preferentially expressed in a variety of tissues including seeds.
We analyzed developmental samples from the AtGenExpress microarray data set (3) to identify genes that exhibit significant tissue-specific expression as measured by Shannon entropy (31). By this criterion, 70 or more genes were identified that were significantly preferentially expressed in each of the following tissues: root, stem, floral, pollen, and seed (supplemental Table S7). We then examined the intersection of these developmentally regulated genes with genes that exhibited PKL_- and/or uniconazole-dependent expression during germination. Our analysis revealed that seed-specific genes were greatly over-represented in the set of genes that exhibit increased transcript levels in response to pkl or to uniconazole-P and somewhat under-represented in genes that exhibit decreased expression in response to either treatment (Fig. 5). In particular, 21% (66 of 312) of genes that exhibit increased expression in response to both_pkl and uniconazole-P are expressed in a seed-specific manner, although only 2.4% (546 of 22,651) of the genes queried exhibit seed-specific expression (p = 9.4 × 10-105). Given that addition of GA acts to suppress the effect of uniconazole on transcript levels for this class of genes (supplemental Table S4), our analysis reveals that during germination, both PKL and GA play a substantial role in the repression of seed-specific genes but not other tissue-specific genes.
Of the remaining tissue-specific genes, only root-specific genes were modestly over-represented among genes that exhibit altered expression in response to pkl or to uniconazole-P (Fig. 5). This overlap is likely to be a reflection of the profoundly altered development of primary roots in pkl seedlings. Intriguingly, we also observed a modest under-representation of pollen-specific genes among those genes that exhibit_PKL_- or uniconazole-dependent expression (either up or down), suggesting that these regulatory circuits function to some extent in a mutually exclusive fashion.
The classification of seed-specific genes also allowed us to investigate their representation among those genes for which pkl and uniconazole-P act synergistically to increase expression. Of the 213 genes that exhibit increased expression in response to pkl and uniconazole-P and for which these two treatments act synergistically to increase expression (supplemental Table S6), 30% exhibit seed-specific expression. Thus this analysis suggests a mechanism by which treatment with uniconazole-P substantially increases pickle root penetrance in pkl seedlings; uniconazole-P acts synergistically with pkl to increase the expression of seed-specific transcripts.
_Genes That Exhibit Strong PKL-dependent Repression Are Enriched for Targets of H3K27me3_—To test the hypothesis that PKL participates in the plant equivalent of a Mi-2/NuRD complex, we examined whether or not_PKL_-dependent genes are also targets of the other chromatin remodeling pathways related to Mi-2/NuRD. In particular, we determined whether genes that exhibit increased expression in pkl plants were enriched for genes identified as targets of cytosine methylation based on ChIP-chip analysis (32) as well as for genes that exhibit increased expression based on microarray analysis of plants that lack the DNA methyltransferase MET1 (32) or that lack the three DNA methyltransferases DRM1, DRM2, and CMT3 (33). We also examined genes that exhibit increased expression in plants that were treated with 5-aza-2-deoxycytosine (aza-dC), which blocks cytosine methylation (34). To examine the possible contribution of histone acetylation to PKL_-dependent expression, we examined genes that exhibit increased expression in plants that were treated with the histone deacetylase inhibitor trichostatin A (34) as well as genes that exhibit elevated expression in plants lacking the histone deacetylase_AtHD1 (35), which belongs to the same superfamily of histone deacetylases as those found in the animal Mi-2/NuRD complex (36).
We also determined if the _PKL_-dependent gene was enriched for targets of H3K27me3 (25). The motivation for this analysis was based on two sets of observations. Some notable PKL_-dependent genes such as the MADS box gene_PHERES1 and the LEC gene FUS3 are also negative regulated by the E(z) histone methyltransferases that promote the repressive epigenetic mark H3K27me3 (23,28,37). In addition, the recent determination of the distribution of H3K27me3 in the Arabidopsis genome revealed that seed-specific genes are frequently targets of H3K27me3 (25), in addition to exhibiting elevated expression in pkl plants (Fig. 5).
For this comparative analysis, we selected genes that exhibit increased expression in the absence of PKL based on two distinct criteria as follows: significantly increased expression based on the selection criterion of GFWER(1,0.05) (supplemental Table S1) and strongly increased expression based on the selection criterion of a ≥3-fold increase in transcript level in pkl plants in the absence or presence of uniconazole-P (supplemental Table S8). Given that master regulators such as the LEC genes are derepressed in pkl plants (12,20), we felt that the use of significant difference as a selection criterion would result in inclusion of many genes whose expression was indirectly affected by PKL as a result of its role in repression of the LEC genes and possibly other master regulators. Based on our previous analyses, our working model is that_LEC1_ and LEC2 represent direct targets of PKL (20,23). Given that both of these genes exhibit robust increases in transcript levels in pkl seedlings (Fig. 1), we therefore assumed that identifying genes based on a large change in transcript abundance would enrich for direct targets of PKL. The criterion of ≥3-fold was chosen in part based on the expression behavior of LEC1 and LEC2 as assayed by the ATH1 microarrays.
Our analysis led to the surprising observation that many of the genes that exhibit a strong increase in expression in pkl plants are targets of H3K27me3 (Fig. 6). 62% (56 of 91) of strongly _PKL_-dependent genes are targets of H3K27me3 (χ2 = 142, p = 1.0 × 10-32). Intriguingly, targets of H3K27me3 do not significantly overlap with those genes that GFWER(1,0.05) identifies as overexpressed in pkl plants (χ2 = 1.4, p = 0.24), suggesting that many of these genes may not be direct targets of PKL or that PKL may repress genes via multiple pathways.
Given that seed-specific genes have previously been shown to be strongly enriched for targets of H3K27me3 (25) and that seed-specific genes are over-represented among genes that exhibit increased expression in_pkl_ plants (Fig. 5), we explored the possibility that seed-specific genes were solely responsible for the observed overlap between H3K27me3 targets and _PKL_-dependent repression. Exclusion of seed-specific genes from χ2 analysis revealed that 56% (28 of 56) of genes that exhibit ≥3-fold increase in expression are targets of H3K27me3 (χ2 = 65, p = 7.6 × 10-16). Thus strong _PKL_-dependent genes that are not seed-specific are also enriched for H3K27me3 targets.
In contrast, genes that are enriched for 5-methylcytosine are significantly under-represented among genes that exhibit increased expression in_pkl_ plants (Fig. 6), suggesting that DNA methylation and PKL represent mutually exclusive repression pathways in plants. Although we observed that genes that are overexpressed in response to application of aza-dC are significantly enriched among genes that are overexpressed in pkl plants, this analysis identifies genes that exhibit altered expression in response to inhibition of DNA methylation rather than direct targets of DNA methylation. As a result, this analysis is likely to identify both direct and indirect targets. In addition, analysis of the DNA methyltransferase-defective met1 and_drm1 drm2 cmt3_ plants failed to corroborate the analysis of aza-dC-treated plants.
Analysis of plants that are impaired for histone deacetylation gave a more ambiguous result (Fig. 6). Genes that are significantly overexpressed in pkl plants by the criterion of GFWER(1,0.05) do significantly overlap with genes that are overexpressed in plants lacking the histone deacetylase HD1. This overlap was less significant that that observed for H3K27me3 (p = 5.0 × 10-5 for hd1 and p = 1.0 × 10-32 for H3K27me3) and was not observed for genes that exhibit ≥3-fold increase in expression. The number of genes altered by trichostatin A was too small to examine overlap. Given that multiple histone deacetylases exist in plants and that these deacetylases are likely to exhibit functional redundancies (36), the failure to detect a large overlap in this analysis does not exclude the possibility that CHD3 proteins in plants work with histone deacetylases as in animal systems.
_The Level of H3K27me3 Is Decreased at Target Loci in a PKL-dependent Manner_—The preceding analysis was consistent with the possibility that PKL is necessary for deposition of H3K27me3 at target loci or for a role for PKL in interpretation of H3K27me3 as a repressive mark. To distinguish between these two models, we used ChIP to examine levels of H3K27me3 at several loci in wild-type and pkl plants. Genes were selected that had previously been shown to be enriched for H3K27me3 (25) and also based in large part on previous characterization of PKL_-dependent expression.PKL_-dependent genes chosen for analysis included the three_LEC genes (LEC1, LEC2, and FUS3), the seed storage protein At2S5, and the MADS-box gene PHERES1. In addition to these genes, we chose the well characterized H3K27me3 targets MEDEA, AGL19, and AGAMOUS (25,38,39) as well as_At3g21720, which is a seed-specific gene coding for a predicted isocitrate lyase that does not exhibit _PKL_-dependent expression during germination but is enriched for H3K27me3 (25).
To confirm that selected genes were enriched for H3K27me3, we examined the amount of H3K27me3 at these loci in wild-type plants (left graph of each panel of Fig. 7). A_Mutator_-like element (MULE At2g15810) and a ubiquitously expressed actin gene (ACT7) were selected as comparative controls that were not enriched for H3K27me3. H3K27me3 ChIP data were normalized relative to ChIP using an antibody that recognized the C terminus of H3 (regardless of its modifications) so as to control for histone occupancy at the respective loci. ChIP was performed at two different developmental stages: 50% germination, representing the stage at which microarray analysis of_pkl_ seedlings was undertaken in this study, and 14-day-old plants (which have yet to make the transition to flowering under growth conditions employed for these analyses). We found that every gene we examined was enriched for H3K27me3 relative to MULE and ACT7 in 14-day-old plants, in agreement with previous characterization of the genes by ChIP-chip (25). Although most of the genes were also enriched for H3K27me3 in 50% germinated seedlings,FUS3 and At3g21720 were much less enriched for the mark (Fig. 7, C and_F_), revealing that deposition of this mark primarily occurs at some point after 50% germination for these two loci.
After confirming that the genes of interest were targets for H3K27me3, we then examined whether or not the levels of H3K27me3 were_PKL_-dependent for these genes (Fig. 7). We used ChIP to determine the level of H3K27me3 at these loci in wild-type and pkl plants at 50% germination and in 14-day-old plants. ChIP data were once again normalized relative to ChIP using an antibody that recognized the C terminus of H3 so as to control for histone occupancy at each locus in wild-type_versus pkl_ plants. Specific regions that exhibit at least a 1.5-fold increase in H3K27me3 levels in wild-type plants relative to pkl plants (95% confidence interval) are indicated by an asterisk over the relevant data point. We also determined whether or not expression of each gene of interest was _PKL_-dependent at each developmental stage to determine whether H3K27me3 levels correlated with expression status (table in right-hand corner of each panel ofFig. 7).
We found that two of three LEC genes exhibit decreased H3K27me3 levels in pkl seedlings at 50% germination. Furthermore, by 14 days of age, the level of H3K27me3 was PKL_-dependent at all nine loci tested regardless of expression status. LEC1 and LEC2 expression is elevated in pkl plants during germination, and H3K27me3 levels are reduced at both loci (Fig. 7,A and B). In adult plants, the LEC1 transcript is not detectable in either wild-type or pkl plants, whereas LEC2 is expressed in both but no longer_PKL_-dependent. Nonetheless, the level of H3K27me3 is strongly reduced at both loci (more than 6-fold for the 3′ region of LEC2). Similar to LEC2, we observed that expression of FUS3 is_PKL_-dependent at 50% germination and PKL_-independent in 14-day-old plants (Fig. 7_C_). A decrease in H3K27me3 levels is only detected in 14-day-old pkl plants however, suggesting that expression of_FUS3 is altered in germinating pkl plants via an alternative mechanism (e.g. due to increased expression of LEC1 and_LEC2 (4)).
PKL has previously been shown to act after germination to repress expression of PHERES1 (23). Consistent with prior analyses, we found that expression of PHERES1 is only_PKL_-dependent in 14-day-old plants (Fig. 7_D_). In addition, we observed that although H3K27me3 is enriched at PHERES1 at both developmental stages, it is only decreased in 14-day-old pkl plants, suggesting that PKL plays a role in repression of PHERES1 after germination because of its contribution to H3K27me3 levels after germination.
Unlike the previous four genes, At2S5 and At3g21720 are seed-specific genes that have not been previously characterized with regard to_PKL_-dependent expression. We found that At2S5 only exhibits_PKL_-dependent expression during germination (Fig. 7_E_). H3K27me3 levels are not detectably altered at 50% germination for this locus, indicating that expression of At2S5, like FUS3, is altered in pkl plants via an alternative mechanism. Yet H3K27me3 levels are still reduced for At2S5 in 14-day-old pkl plants, consistent with a general positive role for PKL in generation of this mark at this developmental stage. Analysis of At3g21720 revealed that H3K27me3 levels are reduced in pkl plants at both developmental stages (Fig. 7_F_). Expression of At3g21720, however, is _PKL_-independent during germination and only modestly increased in 14-day-old plants pkl plants. Thus reduction of H3K27me3 levels does not necessarily lead to an increase in expression of a gene during germination as well as in 14-day-old plants.
Expression of three of the previously characterized H3K27me3 targets_MEDEA, AGL19_, and AGAMOUS is only slightly altered in_pkl_ plants at either developmental stage (Fig. 7,G_–_I). None of these three exhibit a decrease in H3K27me3 levels in 50% germinated pkl seedlings, except in the 5′-transcribed region of MEDEA. Yet all three loci exhibit reduced H3K27me3 levels in 14-day-old pkl plants, again consistent with a general role for PKL in deposition of this mark at this developmental stage and further illustrating the insufficiency of a modest reduction of this mark to lead to elevation of transcript levels.
Our preceding comparative analysis of genomic data did not reveal whether or not PKL_-dependent expression was associated with an alteration of acetylation status (Fig. 6). We therefore also used ChIP to examine acetylation levels at the selected loci using an antibody that recognized diacetylated H3 (H3Ac) as well as an antibody that recognized tetraacetylated H4 (H4Ac) (Fig. 7). Both modification states are positively correlated with transcriptional activation in_Arabidopsis (40,41), and the levels of both modifications have previously been demonstrated to be decreased by mutation of a histone acetyltransferase (GCN5) or increased by mutation of a histone deacetylase (HD1) at light-responsive promoters (42).
In contrast to our analysis of H3K27me3 levels, we observed a much more modest and sporadic effect on acetylation of H3 and H4 in response to loss of_PKL_ (Fig. 7). With the exception of At3g21720 in 50% germinated seedlings, the nine loci are relatively hypoacetylated in wild-type plants (supplemental Fig. S1), as would be expected for targets of H3K27me3. Mutation of pkl results in a large increase in acetylation for only one gene; in 14-day-old pkl plants the level of H3Ac at PHERES1 is increased 4-fold relative to wild-type plants (Fig. 7_D_) and corresponds to a substantial increase in transcript levels (>400-fold). Acetylation levels in the 3′-transcribed region of LEC2 are elevated 1.8-fold in germinating pkl seedlings but are not significantly altered in 14-day-old pkl plants, despite a substantial decrease in levels of H3K27me3 (Fig. 7_B_). The 5′-transcribed region of MEDEA exhibits a roughly 2-fold increase in levels of H3Ac in both 50% germinating and 14-day-old pkl plants, but this effect is not observed in the 5′-promoter or 3′-transcribed region. Thus overall, acetylation levels are relatively unaffected at all loci examined other than PHERES1 in 14-day-old_pkl_ plants, in stark contrast to the consistent decrease in H3K27me3 levels. Our analyses thus indicate that PKL is unlikely to repress expression as a result of participating in the plant equivalent of the Mi-2/NuRD complex.
DISCUSSION
_PICKLE and GA Repress Expression of Seed-specific Genes via Separate Pathways during Germination_—Developing seeds express a highly specialized transcriptional program that is repressed during other stages of plant development. Our current analysis reveals that GA contributes to silencing of this program by acting in parallel with PKL to repress expression of seed-specific genes. GA has previously been shown to be necessary for repression of seed-specific genes. ga1 plants are profoundly defective in GA biosynthesis and strong alleles fail to germinate in the absence of exogenous GA (43,44). A previous microarray analysis of ga1 seeds revealed that the transcript level of many seed-specific genes remains elevated in ga1 seeds imbibed in the absence of GA but decreases rapidly in ga1 seeds imbibed in the presence of GA (29). In this analysis, we have perturbed GA biosynthesis in a more modest fashion: application of 10-8 m uniconazole-P delays germination in wild-type seeds by only 2.5 h relative to untreated seeds. Nonetheless, many seed-specific genes exhibit increased expression in uniconazole-treated plants relative to untreated plants at an equivalent developmental stage (50% germination), revealing that GA acts in a dose-dependent fashion to repress expression of seed-specific genes during germination.
Analysis of our microarray data provides new insight into the relationship between PKL and GA. pkl plants have previously been shown to exhibit the phenotypic hallmarks of a plant that is defective in GA signaling, including reduced sensitivity to application of GA and elevated levels of bioactive GAs (30). Based on these observations, we previously proposed that PKL is a component of a GA response pathway. Our new analysis clearly demonstrates that PKL and GA act in separate pathways during germination to repress expression of genes (supplemental Table S5). Thus if PKL is a component of a GA response pathway during germination, this pathway must be redundant with a_PKL_-independent pathway (model 3 inFig. 3).
If PKL is not a component of a GA response pathway, the question arises as to why pkl plants resemble GA response mutants. A simple answer for the germination-related phenotypes is provided by our microarray analysis of germinating seeds: PKL and GA act in a redundant fashion to repress expression of common targets. This analysis does not, however, address why adult pkl plants resemble GA response mutants. A possible explanation was provided by the characterization of plants that ectopically express FUS3 in the epidermis (8). These transgenic lines exhibit seed-associated phenotypic traits, repression of the GA biosynthetic pathway, and resemble GA-deficient plants. Based on the observation that_pkl_ plants ectopically express the FUS3 transcript (20), Gazzarrini et al. (8) proposed that the GA-deficient phenotype exhibited by pkl plants is thus a result of ectopic expression of FUS3 rather than an impairment in GA responsiveness. This model fails to account for the accumulation of bioactive GAs observed in pkl plants (30), however, which would be predicted to destabilize the FUS3 protein (8) and therefore act in opposition to expression of the FUS3 transcript. In addition, our current analysis reveals that FUS3 expression is not elevated in 14-day-old pkl plants grown on synthetic media (previous analyses used plants in pots), yet these plants continue to exhibit phenotypes associated with GA-deficient plants (30). Thus although ectopic expression of FUS3 may contribute to some of the observed GA-related phenotypes associated with pkl plants, a complete explanation is likely to require the contribution of other factors, including perhaps a positive role for PKL in GA responsiveness.
Our array data provide a simple explanation of why inhibition of GA biosynthesis during germination of pkl plants increases penetrance of the pickle root phenotype but is not sufficient to generate the pickle root phenotype in wild-type plants (9). PKL and GA not only act in separate pathways during germination, they also act in a synergistic fashion. Mutation of PKL reveals an otherwise hidden role for GA for repression of many genes (supplemental Table S6). LEC1 and_LEC2_ transcript levels, for example, are only increased in response to the addition of 10-8 m uniconazole-P if PKL is mutated (Fig. 1). Thus application of uniconazole-P to pkl seedlings results in increased expression of seed-associated transcripts that would be otherwise unaffected in wild-type seedlings. In addition, mutation of pkl enhances the effect of inhibition of GA biosynthesis on expression of many seed-specific genes (Fig. 4 and supplemental Table S6) thus further increasing the probability that seed-associated traits will be expressed in uniconazole-P-treated pkl seedlings.
Based on previous and current characterization of PKL and other CHD3 proteins, PKL is strongly predicted to act by repressing transcription. Whether GA acts to repress expression of the genes identified in this analysis by repressing transcription, decreasing transcript stability, or a combination of both remains open to question. RNA decay has previously been implicated in repression of seed-associated transcripts during germination (45). In this regard, it is intriguing to note that the examination of GA-dependent transcripts in_ga1_ seeds revealed that some genes are stably expressed at high levels in the absence of GA and exhibit a rapid reduction in expression in the presence of GA (29), suggesting either that these transcripts are relatively short lived and robustly transcribed in imbibed seeds or that the stability of these transcripts is GA-dependent.
Many of the insights regarding the respective roles of PKL and GA reflect the power and utility of using a 2 × 2 experimental design. Substantially more _PKL_- and uniconazole-dependent genes were identified by using this experimental design than would have been identified by comparing only one treatment at a time (e.g. wild-type versus pkl plants).5 Similarly, it is only by simultaneously examining the effect of both treatments (mutation of PKL and application of uniconazole-P) that we were able to definitively characterize their interaction with each other on a genome-wide basis. Given the large number of transcriptional regulators that contribute to hormone-responsive events, this approach would appear to be of general utility.
PKL Is Necessary for Wild-type Levels of H3K27me3_—Our data reveal a new pathway by which CHD3 proteins contribute to repression;PKL promotes the repressive epigenetic mark H3K27me3. Like CHD3 proteins, the histone modification H3K27me3 contributes to repression in both animal and plant systems. In animals, this mark is deposited by a SET domain histone methyltransferase (HMT), named Enhancer of zeste E(z) in_Drosophila, which is a member of a Polycomb group (PcG) complex PRC2 (46). Plants possess homologs to conserved members of the PRC2 complex, and a plant equivalent of PRC2 has been biochemically characterized (47,48). There are three SET domain HMTs that belong to the E(z) family in Arabidopsis:MEDEA (MEA), CURLY LEAF (CLF), and_SWINGER_ (SWR) (49–53). Genetic analysis reveals that these three E(z) proteins play essential roles in seed initiation, vernalization, the switch from vegetative to floral development, and flower organ development, most likely as components of distinct PRC2 complexes (47,53). Mutation of one or more of the corresponding genes leads to loss of H3K27me3 and increased expression of target loci, consistent with a role for each protein as an HMT that contributes to transcriptional repression. Homology searches suggest that there is no plant equivalent of other complexes involved in deposition of H3K27me3, however, indicating that the machinery involved in deposition of H3K27me3 is likely to differ between plants and animals. In support of such a conjecture, whole genome ChIP-chip analysis recently revealed that H3K27me3 is primarily distributed at individual loci in plants rather than in extended domains as in animal systems (25,54,55).
Previous characterization of targets of MEA, CLF, and SWN has revealed that these HMTs and PKL repress expression of common genes. In developing seeds, MEA promotes H3K27me3 at PHE1 and is required for repression of both PHE1 and FUS3 (48,56). Similarly, CLF and SWN are required for H3K27me3 and repression of PHE1 and for repression of FUS3 in adult plants (37). Furthermore, clf swn plants develop primary roots that resemble the pickle roots of_pkl_ plants and ectopically express FUS3 in addition to developing callus that produces somatic embryos revealing that both they and_PKL_ act to repress the expression of seed-associated traits in adult plants (37,51).
Our new analyses reveal that in addition to affecting expression of common targets, PKL and the E(z) genes also contribute to the abundance of H3K27me3 at those loci. H3K27me3 is reduced roughly 11-fold at PHE1 in clf swn plants (37) and 3-fold in_pkl_ plants (Fig. 7_D_). Similarly, H3K27me3 is reduced 3-fold at_AGL19_ in clf plants (39) and 2-fold in_pkl_ plants (Fig. 7_H_), whereas it is reduced 3.5–9-fold at_AG_ in clf plants (38) and 2-fold in_pkl_ plants (Fig. 7_I_). H3K27me3 is reduced 6-fold at LEC2 in_pkl_ plants, which is in the same range as that exhibited by E(z) mutants. These data suggest that PKL generally has a smaller effect on H3K27me3 levels than E(z) genes. Although quantitative comparison of ChIP data from different analyses is an exercise in optimism at best, the hypothesis that pkl has a reduced effect relative to E(z) mutants is corroborated by expression data. For example, pkl has a modest effect on H3K27me3 levels of AG and does not lead to an increase in transcript levels of AG (Fig. 7_I_). Loss of CLF, in comparison, results in a much more severe effect on levels of H3K27me3 at AG and also results in increased expression of AG (38,50). These data thus suggest that for some loci H3K27me3 levels must be reduced below some threshold to result in increased expression. The proposal that mutation of PKL has a reduced effect on levels of H3K27me3 relative to mutation of E(z) genes is also consistent with the less extreme phenotype exhibited by pkl plants relative to clf swn plants, which are only viable if the seedlings are propagated in sterile tissue culture (51).
Previous characterization of another mutant plant defective in repression of seed traits also supports a connection between PKL and H3K27me3. There are three VAL genes that code for putative transcription factors that contain a plant-specific B3 domain, a PHD-like domain, and a CW domain (57–59).val1 val2 plants exhibit pkl_-like traits, including derepression of LEC genes, GA-dependent derepression of seed traits, and somatic embryogenesis in the primary root (57,58). Microarray analysis of_val1 val2 plants (58) reveals that a broad suite of seed-specific genes is derepressed in val1 val2 plants in a similar fashion to pkl plants. Intriguingly,val1 val2 seedlings (57,58) resemble clf swn seedlings (37,51) in appearance and attributes, suggesting that VAL1 and VAL2 may also contribute to some aspect of H3K27me3-mediated repression. In fact, comparative analysis of _VAL1 VAL2_-dependent genes (58), _PKL_-dependent genes (Table S1), and H3K27me3 targets (25) reveals that VAL1 VAL2_-dependent genes strongly overlap PKL_-dependent genes and are greatly enriched for H3K27me3 targets (Table S9). It remains to be seen if_val1 val2 plants exhibit a reduction of H3K27me3 as is observed in_pkl plants or if VAL1 and VAL2 are instead involved in interpretation of H3K27me3.
Although the H3K27me3 ChIP data clearly demonstrate that this mark is_PKL_-dependent, it does not clarify whether or not PKL is directly involved in deposition of this mark. We discovered the connection between_PKL_ and H3K27me3 because many genes that exhibit strongly increased expression in the absence of PKL are also H3K27me3 targets (Fig. 6). H3K27me3 is known to be a repressive mark in plants, and genes that lose this mark can exhibit increased expression (37–39,47). A simple model to account for these observations is that PKL interacts with one or more PRC2 complexes to facilitate deposition of H3K27me3 during establishment and/or maintenance of this mark. This model is consistent with the ability of animal CHD3 proteins to participate in other complexes in addition to Mi-2/NuRD, such as the ability of the CHD3 protein Mi-2β to associate with the histone acetyltransferase p300 and promote expression of CD4 in developing T cells (60). Our data are also consistent, however, with an alternative mode of action. The closely related remodeling protein CHD1 has been shown to function as a chromatin assembly factor (61), and the CHD1 protein of Drosophila is necessary to incorporate histone variant H3.3 into male pronuclei in developing embryos (62). Perhaps PKL also plays a role in chromatin assembly, and the absence of this activity leads to a chromatin template that is a poor substrate for PRC2 complexes. Viewed in this light, it is intriguing to note that PKL is predominantly expressed in differentiating tissue (11,23), suggesting that_PKL_ plays a role in establishment of the H3K27me3 mark rather than its maintenance. Biochemical characterization of the PKL protein and its possible co-factors is likely to help distinguish between these possible models.
In light of the difference in H3K27me3-associated machinery in plants and animals, it is worth highlighting that CHD3 proteins have been linked previously to H3K27me3-associated repression in animals. In_Drosophila_, the CHD3 protein dMi-2 is recruited by Hunchback to HOX genes and is required for their repression (63). Genetic analyses support a model in which this repression reflects a role for dMi-2 as a link between Hunchback and PcG repressors. These studies led the authors to propose that dMi-2 may either interact with PcG proteins or prepare the chromatin template such that PcG proteins may interact with the target locus. Although H3K27me3 levels were not examined in the study by Kehle et al. (63), the similarity between the proposed roles of dMi-2 and PKL in PcG-mediated repression of developmental identity genes in Drosophila and Arabidopsis is readily apparent. It remains to be seen if the mechanism by which CHD3 proteins contribute to PcG-mediated repression is conserved in both systems.
Supplementary Material
[Supplemental Data]
Acknowledgments
We thank Scott Briggs, Ian Fingerman, and Hui-Chun Li for generously sharing reagents and technical assistance and Yinglin Bai for generating reagents. We thank Clint Chapple, Craig Peterson, Scott Briggs, and Ann Kirchmaier for thoughtful discussions. Microarray analyses were carried out at the Center for Medical Genomics at Indiana University School of Medicine, partly supported by the Indiana Genomics Initiative, which is partly supported by the Lilly Endowment, Inc.
The amino acid sequence of this protein can be accessed through NCBI Protein Database under NCBI accession number GSE11852.
*
This work was supported, in whole or in part, by National Institutes of Health Grants R01GM059770-01A1 and 5R01GM59770-02. This work was also supported by a grant from the Indiana 21st Century Research and Development Fund (to J. R. S.). This is journal paper number 2008-18351 of the Purdue University Agricultural Experiment Station. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
S⃞
The on-line version of this article (available athttp://www.jbc.org) contains supplemental text, additional references, Fig. S1, and Tables S1–S17.
Footnotes
4
The abbreviations used are: GA, gibberellin; aza-dC, 5-aza-2-deoxycytosine; GFWER(k), generalized family wise error rate; H3K27me3, trimethylation of histone H3 lysine 27; qRT, quantitative reverse transcription; ChIP, chromatin immunoprecipitation; HMT, histone methyltransferase; WT, wild type.
5
J. Ogas, unpublished data.
References
- 1.Girke, T., Todd, J., Ruuska, S., White, J., Benning, C., and Ohlrogge, J. (2000) Plant Physiol. 124 1570-1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruuska, S. A., Girke, T., Benning, C., and Ohlrogge, J. B. (2002) Plant Cell 14 1191-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid, M., Davison, T. S., Henz, S. R., Pape, U. J., Demar, M., Vingron, M., Scholkopf, B., Weigel, D., and Lohmann, J. U. (2005) Nat. Genet. 37 501-506 [DOI] [PubMed] [Google Scholar]
- 4.To, A., Valon, C., Savino, G., Guilleminot, J., Devic, M., Giraudat, J., and Parcy, F. (2006) Plant Cell 18 1642-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagaya, Y., Toyoshima, R., Okuda, R., Usui, H., Yamamoto, A., and Hattori, T. (2005) Plant Cell Physiol. 46 399-406 [DOI] [PubMed] [Google Scholar]
- 6.Stone, S. L., Kwong, L. W., Yee, K. M., Pelletier, J., Lepiniec, L., Fischer, R. L., Goldberg, R. B., and Harada, J. J. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 11806-11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lotan, T., Ohto, M., Yee, K. M., West, M. A., Lo, R., Kwong, R. W., Yamagishi, K., Fischer, R. L., Goldberg, R. B., and Harada, J. J. (1998) Cell 93 1195-1205 [DOI] [PubMed] [Google Scholar]
- 8.Gazzarrini, S., Tsuchiya, Y., Lumba, S., Okamoto, M., and McCourt, P. (2004) Dev. Cell 7 373-385 [DOI] [PubMed] [Google Scholar]
- 9.Ogas, J., Cheng, J. C., Sung, Z. R., and Somerville, C. (1997) Science 277 91-94 [DOI] [PubMed] [Google Scholar]
- 10.Rider, S. D., Jr., Hemm, M. R., Hostetler, H. A., Li, H. C., Chapple, C., and Ogas, J. (2004) Planta 219 489-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshed, Y., Baum, S. F., and Bowman, J. L. (1999) Cell 99 199-209 [DOI] [PubMed] [Google Scholar]
- 12.Ogas, J., Kaufmann, S., Henderson, J., and Somerville, C. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 13839-13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang, Y., LeRoy, G., Seelig, H. P., Lane, W. S., and Reinberg, D. (1998) Cell 95 279-289 [DOI] [PubMed] [Google Scholar]
- 14.Wade, P. A., Jones, P. L., Vermaak, D., and Wolffe, A. P. (1998) Curr. Biol. 8 843-846 [DOI] [PubMed] [Google Scholar]
- 15.Tong, J. K., Hassig, C. A., Schnitzler, G. R., Kingston, R. E., and Schreiber, S. L. (1998) Nature 395 917-921 [DOI] [PubMed] [Google Scholar]
- 16.Xue, Y., Wong, J., Moreno, G. T., Young, M. K., Cote, J., and Wang, W. (1998) Mol. Cell 2 851-861 [DOI] [PubMed] [Google Scholar]
- 17.Wade, P. A., Gegonne, A., Jones, P. L., Ballestar, E., Aubry, F., and Wolffe, A. P. (1999) Nat. Genet. 23 62-66 [DOI] [PubMed] [Google Scholar]
- 18.Hall, J. A., and Georgel, P. T. (2007) Biochem. Cell Biol. 85 463-476 [DOI] [PubMed] [Google Scholar]
- 19.Ahringer, J. (2000) Trends Genet. 16 351-356 [DOI] [PubMed] [Google Scholar]
- 20.Rider, S. D., Henderson, J. T., Jerome, R. E., Edenberg, H. J., Romero-Severson, J., and Ogas, J. (2003) Plant J. 35 33-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies, P. J. (ed) (1995) Plant Hormones: Physiology, Biochemistry and Molecular Biology, Kluwer Academic Publishers, Dordrecht, Netherlands
- 22.Ueguchi-Tanaka, M., Nakajima, M., Motoyuki, A., and Matsuoka, M. (2007) Annu. Rev. Plant Biol. 58 183-198 [DOI] [PubMed] [Google Scholar]
- 23.Li, H. C., Chuang, K., Henderson, J. T., Rider, S. D., Jr., Bai, Y., Zhang, H., Fountain, M., Gerber, J., and Ogas, J. (2005) Plant J. 44 1010-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muir, W. M., Romero-Severson, J., Rider, S. D., Jr., Simons, A., and Ogas, J. (2006) J. Data Sci. 4 323-341 [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, X., Clarenz, O., Cokus, S., Bernatavichute, Y. V., Pellegrini, M., Goodrich, J., and Jacobsen, S. E. (2007) Plos Biol. 5 e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verwoerd, T. C., Dekker, B. M., and Hoekema, A. (1989) Nucleic Acids Res. 17 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Churchill, G. A., and Doerge, R. W. (1994) Genetics 138 963-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohler, C., Hennig, L., Spillane, C., Pien, S., Gruissem, W., and Grossniklaus, U. (2003) Genes Dev. 17 1540-1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa, M., Hanada, A., Yamauchi, Y., Kuwahara, A., Kamiya, Y., and Yamaguchi, S. (2003) Plant Cell 15 1591-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson, J. T., Li, H. C., Rider, S. D., Mordhorst, A. P., Romero-Severson, J., Cheng, J. C., Robey, J., Sung, Z. R., De Vries, S. C., and Ogas, J. (2004) Plant Physiol. 134 995-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schug, J., Schuller, W. P., Kappen, C., Salbaum, J. M., Bucan, M., and Stoeckert, C. J., Jr. (2005) Genome Biol. 6 R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zilberman, D., Gehring, M., Tran, R. K., Ballinger, T., and Henikoff, S. (2007) Nat. Genet. 39 61-69 [DOI] [PubMed] [Google Scholar]
- 33.Zhang, X., Yazaki, J., Sundaresan, A., Cokus, S., Chan, S. W., Chen, H., Henderson, I. R., Shinn, P., Pellegrini, M., Jacobsen, S. E., and Ecker, J. R. (2006) Cell 126 1189-1201 [DOI] [PubMed] [Google Scholar]
- 34.Chang, S., and Pikaard, C. S. (2005) J. Biol. Chem. 280 796-804 [DOI] [PubMed] [Google Scholar]
- 35.Tian, L., Fong, M. P., Wang, J. J., Wei, N. E., Jiang, H., Doerge, R. W., and Chen, Z. J. (2005) Genetics 169 337-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey, R., Muller, A., Napoli, C. A., Selinger, D. A., Pikaard, C. S., Richards, E. J., Bender, J., Mount, D. W., and Jorgensen, R. A. (2002) Nucleic Acids Res. 30 5036-5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makarevich, G., Leroy, O., Akinci, U., Schubert, D., Clarenz, O., Goodrich, J., Grossniklaus, U., and Kohler, C. (2006) EMBO Rep. 7 947-952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schubert, D., Primavesi, L., Bishopp, A., Roberts, G., Doonan, J., Jenuwein, T., and Goodrich, J. (2006) EMBO J. 25 4638-4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schonrock, N., Bouveret, R., Leroy, O., Borghi, L., Kohler, C., Gruissem, W., and Hennig, L. (2006) Genes Dev. 20 1667-1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng, D. W., Chandrasekharan, M. B., and Hall, T. C. (2006) Plant Cell 18 119-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheldon, C. C., Finnegan, E. J., Dennis, E. S., and Peacock, W. J. (2006) Plant J. 45 871-883 [DOI] [PubMed] [Google Scholar]
- 42.Benhamed, M., Bertrand, C., Servet, C., and Zhou, D. X. (2006) Plant Cell 18 2893-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barendse, G. W. M., Kepczynski, J., Karssen, C. M., and Koornneef, M. (1986) Physiol. Plant. 67 315-319 [Google Scholar]
- 44.Koornneef, M., and van der Veen, J. H. (1980) Theor. Appl. Genet. 58 257-263 [DOI] [PubMed] [Google Scholar]
- 45.Goeres, D. C., Van Norman, J. M., Zhang, W., Fauver, N. A., Spencer, M. L., and Sieburth, L. E. (2007) Plant Cell 19 1549-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ringrose, L., and Paro, R. (2004) Annu. Rev. Genet. 38 413-443 [DOI] [PubMed] [Google Scholar]
- 47.Pien, S., and Grossniklaus, U. (2007) Biochim. Biophys. Acta 1769 375-382 [DOI] [PubMed] [Google Scholar]
- 48.Kohler, C., Hennig, L., Bouveret, R., Gheyselinck, J., Grossniklaus, U., and Gruissem, W. (2003) EMBO J. 22 4804-4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grossniklaus, U., Vielle-Calzada, J. P., Hoeppner, M. A., and Gagliano, W. B. (1998) Science 280 446-450 [DOI] [PubMed] [Google Scholar]
- 50.Goodrich, J., Puangsomlee, P., Martin, M., Long, D., Meyerowitz, E. M., and Coupland, G. (1997) Nature 386 44-51 [DOI] [PubMed] [Google Scholar]
- 51.Chanvivattana, Y., Bishopp, A., Schubert, D., Stock, C., Moon, Y. H., Sung, Z. R., and Goodrich, J. (2004) Development (Camb.) 131 5263-5276 [DOI] [PubMed] [Google Scholar]
- 52.Luo, M., Bilodeau, P., Dennis, E. S., Peacock, W. J., and Chaudhury, A. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 10637-10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guitton, A. E., and Berger, F. (2005) Int. J. Dev. Biol. 49 707-716 [DOI] [PubMed] [Google Scholar]
- 54.Schwartz, Y. B., Kahn, T. G., Nix, D. A., Li, X. Y., Bourgon, R., Biggin, M., and Pirrotta, V. (2006) Nat. Genet. 38 700-705 [DOI] [PubMed] [Google Scholar]
- 55.Lee, T. I., Jenner, R. G., Boyer, L. A., Guenther, M. G., Levine, S. S., Kumar, R. M., Chevalier, B., Johnstone, S. E., Cole, M. F., Isono, K., Koseki, H., Fuchikami, T., Abe, K., Murray, H. L., Zucker, J. P., Yuan, B., Bell, G. W., Herbolsheimer, E., Hannett, N. M., Sun, K., Odom, D. T., Otte, A. P., Volkert, T. L., Bartel, D. P., Melton, D. A., Gifford, D. K., Jaenisch, R., and Young, R. A. (2006) Cell 125 301-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohler, C., Page, D. R., Gagliardini, V., and Grossniklaus, U. (2005) Nat. Genet. 37 28-30 [DOI] [PubMed] [Google Scholar]
- 57.Tsukagoshi, H., Morikami, A., and Nakamura, K. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 2543-2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki, M., Wang, H. H., and McCarty, D. R. (2007) Plant Physiol. 143 902-911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsukagoshi, H., Saijo, T., Shibata, D., Morikami, A., and Nakamura, K. (2005) Plant Physiol. 138 675-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams, C. J., Naito, T., Arco, P. G., Seavitt, J. R., Cashman, S. M., De Souza, B., Qi, X., Keables, P., Von Andrian, U. H., and Georgopoulos, K. (2004) Immunity 20 719-733 [DOI] [PubMed] [Google Scholar]
- 61.Lusser, A., Urwin, D. L., and Kadonaga, J. T. (2005) Nat. Struct. Mol. Biol. 12 160-166 [DOI] [PubMed] [Google Scholar]
- 62.Konev, A. Y., Tribus, M., Park, S. Y., Podhraski, V., Lim, C. Y., Emelyanov, A. V., Vershilova, E., Pirrotta, V., Kadonaga, J. T., Lusser, A., and Fyodorov, D. V. (2007) Science 317 1087-1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kehle, J., Beuchle, D., Treuheit, S., Christen, B., Kennison, J. A., Bienz, M., and Muller, J. (1998) Science 282 1897-1900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]