Postsynaptic Regulation of Long-Term Facilitation in Aplysia (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 15.
Published in final edited form as: Curr Biol. 2008 Jun 24;18(12):920–925. doi: 10.1016/j.cub.2008.05.038
Summary
Repeated exposure to serotonin (5-HT), an endogenous neurotransmitter that mediates behavioral sensitization in Aplysia [1–3], induces long-term facilitation (LTF) of the Aplysia sensorimotor synapse [4]. LTF, a prominent form of invertebrate synaptic plasticity, is believed to play a major role in long-term learning in Aplysia [5]. Until now, LTF has been thought to be due predominantly to cellular processes activated by 5-HT within the presynaptic sensory neuron [6]. Recent work indicates that LTF depends on the increased expression and release of a sensory neuron-specific neuropeptide, sensorin [7]. Sensorin released during LTF appears to bind to autoreceptors on the sensory neuron, thereby activating critical presynaptic signals, including mitogen-activated protein kinase (MAPK) [8, 9]. Here, we show that LTF depends on elevated postsynaptic Ca2+ and postsynaptic protein synthesis. Furthermore, we find that the increased expression of presynaptic sensorin due to 5-HT stimulation requires elevation of postsynaptic intracellular Ca2+. Our results represent perhaps the strongest evidence to date that the increased expression of a specific presynaptic neuropeptide during LTF is regulated by retrograde signals.
Results
LTF of the In Vitro Sensorimotor Synapse Requires Elevated Postsynaptic Ca2+
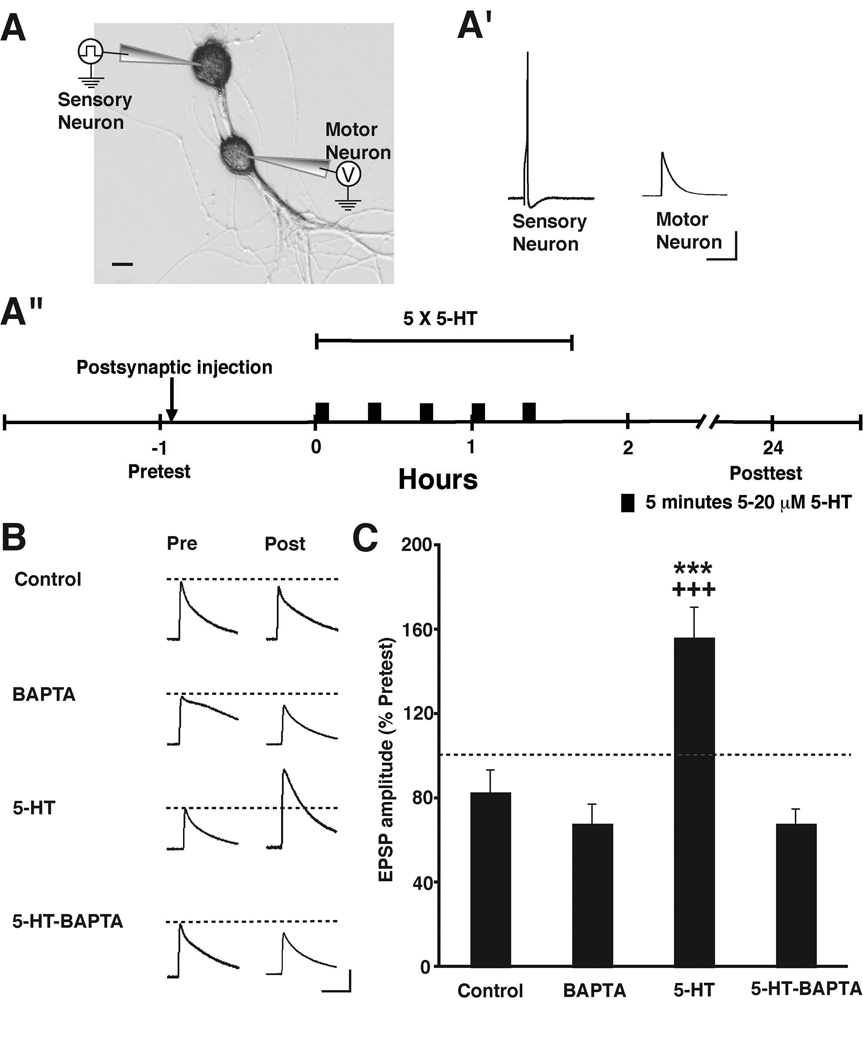
Previous investigations have shown that both associative facilitation [10–12] and intermediate-term facilitation (ITF) [13] of the Aplysia sensorimotor synapse depend on elevated postsynaptic Ca2+. In the case of ITF, the elevated intracellular Ca2+ is due to release from intracellular stores [13]. To test whether LTF due to repeated applications of 5-HT, which lasts for ≥ 24 h [4] also depends on a rise in intracellular Ca2+ in the motor neuron, we injected the rapid Ca2+ chelator 1,2-bis-(o-aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid, (BAPTA, 200 mM in the injection solution; see Experimental Procedures in the Supplemental Data available online) into the postsynaptic motor neuron 30–60 min before the start of 5-HT treatment (Figure 1A). (Note that the concentrations given for all injected compounds represent final concentrations in the injection pipette.) 1 h before the 5-HT or Control treatment, the strength of each synaptic connection was tested by evoking a single action potential in the sensory neuron and recording the excitatory postsynaptic potential (EPSP) in the motor neuron (the Pretest; Figures 1A’ and 1A“). There were no significant group differences in the mean amplitudes of the Pretest EPSPs in any of the experiments reported here (see Results in the Supplemental Data). The 5-HT treatment consisted of five 5-min pulses of 5-HT (5–20 µM) with a 20-min interpulse interval; after each pulse the drug was washed out of the cell culture dish for 15 min with normal perfusion medium (Figure 1A“ and Experimental Procedures in the Supplemental Data). The 5-HT treatment produced significant LTF of synapses in which BAPTA was not present postsynaptically compared to Control synapses that received neither 5-HT nor postsynaptic BAPTA (Figures 1B and 1C). By contrast, synapses in which BAPTA was injected into the motor neuron prior to 5-HT treatment did not exhibit LTF. Postsynaptic injection of BAPTA by itself did not cause a significant long-term change in the strength of the sensorimotor synaptic connections.
Figure 1. Postsynaptic Chelation of Intracellular Ca2+ Disrupts 5-HT-induced LTF.
(A) Experimental arrangement. Scale bar, 20 µm.
(A’) Sample electrophysiological records from a Pretest. A single action potential evoked in a sensory neuron produced a monosynaptic EPSP in the motor neuron. Note that the sensory action potentials are not included in the other pre- and posttest records presented in this and following figures. Scale bars, 20 mV and 200 ms.
(A”) Experimental protocol. Note that the sensory and motor neurons in the cocultures were given 3–4 d to form synaptic connections before the start of the experiment. (See Experimental Procedures in the Supplemental Data [online] for additional information.)
(B) Sample EPSPs for four of the experimental groups: Control, BAPTA, 5-HT, 5-HT-BAPTA. Each pair of traces shows EPSPs recorded from the same sensorimotor synapse before (Pre) and 24 h after treatment (Post). Scale bars for these and EPSPs shown in subsequent figures, 10 mV and 80 ms.
(C) Mean normalized amplitude of the EPSPs in the four experimental groups. A one-way ANOVA indicated that the differences among the groups were highly significant [F(3,49) = 11.20, p < 0.0001]. There was significant LTF of the synapses in the 5-HT-treated group (Day 2 EPSP = 155 ± 14%, n = 18), as indicated by the comparison with the Control synapses (Day 2 EPSP = 88 ± 10%, n = 14), which did not receive 5-HT (p < 0.001). By contrast, synapses in which BAPTA was injected into the motor neuron prior to 5-HT treatment (5-HT-BAPTA group) did not exhibit LTF (Day 2 EPSP = 69 ± 7%, n = 10; p < 0.001 for the comparison with the 5-HT group). Postsynaptic injection of BAPTA by itself (Day 2 EPSP = 73 ± 9%, n = 11) did not cause significant long-term changes in the strength of the sensorimotor synaptic connections (p > 0.05 for the comparison between the BAPTA alone and Control groups). * indicates significance of the difference between the 5-HT and Control groups, and + indicates significance of the difference between the 5-HT and 5-HT-BAPTA groups. Error bars represent ± SEM.
Inhibition of Postsynaptic Protein Synthesis Blocks LTF
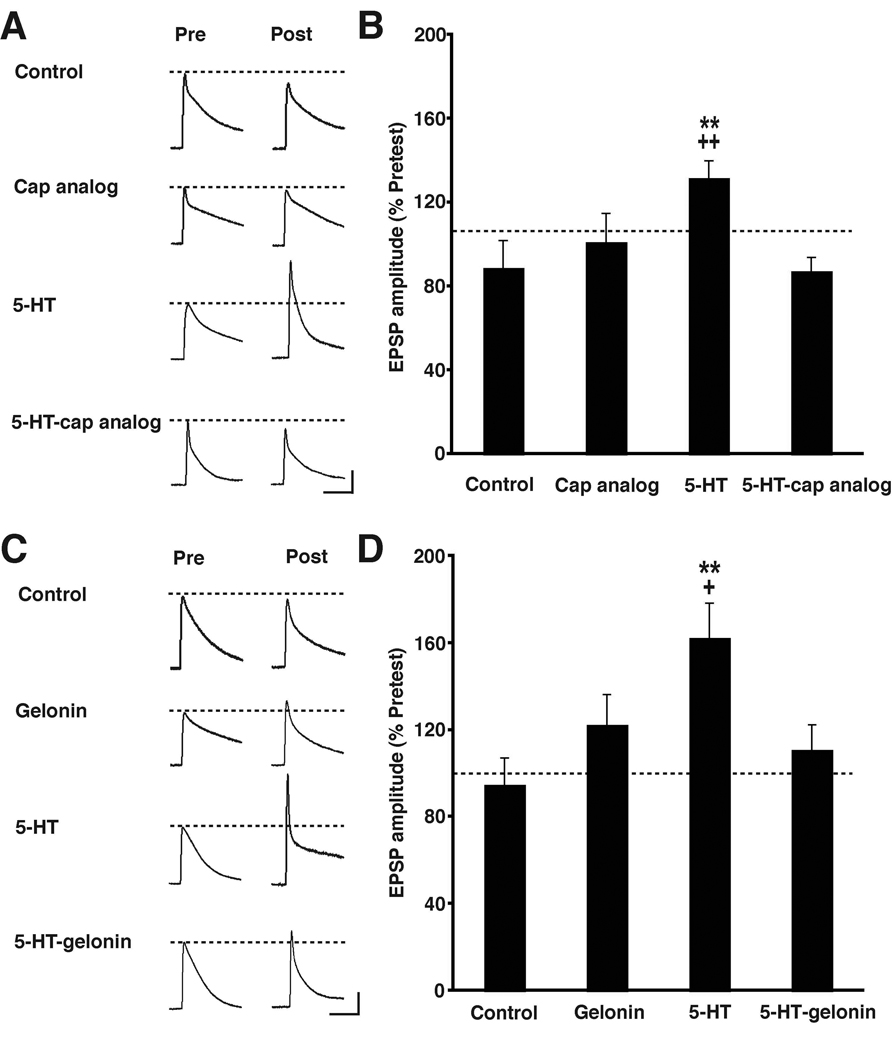
Long-term facilitation differs from short-term facilitation (STF) of the sensorimotor synapse in its requirement for both new protein synthesis and gene transcription [4, 14]. It is generally agreed that LTF requires presynaptic protein synthesis [15, 16]. By contrast, the current status of the requirement for postsynaptic protein synthesis in LTF is murky [14, 15, 17]. Recently, we have found that local postsynaptic protein synthesis is critical for ITF [18]. This finding, together with the data from our BAPTA experiments (Figure 1), led us to reexamine the question of whether postsynaptic protein synthesis plays a role in LTF. Accordingly, we injected a cell membrane-impermeant protein synthesis inhibitor, the cap analog m7GpppG (2.5 mM in the injection solution) [19], into the motor neuron of sensorimotor cocultures prior to 5-HT treatment. The cap analog competes with endogenous capped mRNA for the initiation factor eIF4E. Binding of eIF4E to mRNA facilitates initiation of translation; excess cap analog interferes with this binding, and thereby disrupts protein synthesis [20]. When the cap analog was not present in the motor neuron the 5-HT treatment produced significant LTF of the synapses compared to synapses given the Control treatment (Figure 2A and 2B). However, postsynaptic injection of the cap analog prior to 5-HT application blocked LTF. There was no long-term effect on the in vitro synaptic connections of postsynaptic injection of the cap analog by itself.
Figure 2. Postsynaptic Blockade of Protein Synthesis with the Cap Analog m7GpppG or Gelonin Disrupts LTF.
(A) Sample EPSPs recorded from cocultures in the four groups in the experiments summarized in (B).
(B) Mean normalized amplitude of the EPSPs for each group in experiments testing the effect of postsynaptic injection of the cap analog. A one-way ANOVA of the data indicated that the group difference were significant [F(3,63) = 4.89, p < 0.005]. The 5-HT treatment produced significant LTF of the synapses, as indicated by comparing the Day 2 EPSPs in the 5-HT (132 ± 8%, n = 20) and Control (89 ± 13%, n = 15) groups (p < 0.01). Synapses in which cap analog was injected into the motor neuron prior to 5-HT treatment (5-HT-cap analog group, Day 2 EPSP = 88 ± 6%, n = 18) did not exhibit LTF (p < 0.01 for the comparison with the 5-HT group). Postsynaptic injection of cap analog alone did not significantly alter the strength of the sensorimotor synaptic connections (Day 2 EPSP = 101 ± 13%, n = 14; p > 0.05 for the comparison between the Cap analog and Control groups). * indicates significance of the difference between the 5-HT and Control groups, and + indicates significance of the difference between the 5-HT and 5-HT-cap analog groups. Error bars represent ± SEM.
(C) Sample EPSPs recorded from cocultures in the four groups in the experiments summarized in (D).
(D) Mean normalized amplitude of the EPSPs for each group in experiments that examined the effect of postsynaptic injection of gelonin. The differences among the groups were highly significant [F(3,95) = 4.59, p < 0.005]. Mean normalized EPSP in the 5-HT group (163 ± 16%, n = 23) was significantly greater than that in the Control group (95 ± 12%, n = 25; p < 0.01). The mean normalized amplitude of the Day 2 EPSP in cocultures that received the postsynaptic gelonin prior to 5-HT treatment (5-HT-gelonin group, 111 ± 11%, n = 25) was significantly less than that in the 5-HT alone group (p < 0.05). Postsynaptic injection of gelonin by itself did not significantly alter the strength of the synapse (mean normalized Day 2 EPSP in the Gelonin group = 123 ± 14%, n = 26; p > 0.05 for the comparison between the with the Control group). * indicates significance of the difference between the 5-HT and Control groups, and + indicates significance of the difference between the 5-HT and 5-HT-Gelonin groups Error bars represent ± SEM.
To ensure that the disruptive effect of the cap analog on LTF was not due to some nonspecific action of the drug, we performed additional experiments using a second cell membrane-impermeant protein synthesis inhibitor, gelonin. Gelonin, a plant-derived ribosome-inactivating glycoprotein [21], has previously been used in studies of synaptic plasticity in Aplysia for cell-specific inhibition of protein synthesis [14, 15, 17]. Repeated application of 5-HT produced LTF of the in vitro synapse (Figures 2C and 2D), and this effect was blocked by the postsynaptic injection of gelonin (10–25 mM in injection buffer) 1 h before the start of 5-HT treatment.
Postsynaptic Ca2+ Regulates the Rapid Increase in Sensorin Expression Induced by Repeated Applications of 5-HT
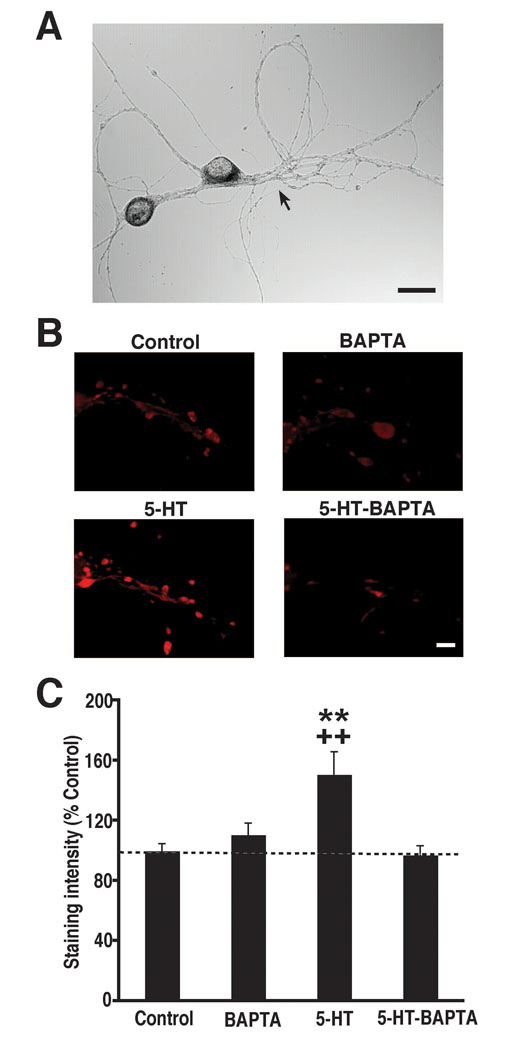
The sensory neuron-specific neuropeptide, sensorin [7], makes a critical contribution to LTF [8]. Repeated application of 5-HT to sensorimotor cocultures causes the rapid release of sensorin from presynaptic terminals [8], as well as a rapid increase in sensorin expression, particularly at presynaptic varicosities [9, 22]. Release of sensorin is necessary for LTF: application of an antibody to sensorin blocks 5-HT-dependent LTF in sensorimotor cocultures [8]. One possibility is that 5-HT acts presynaptically to alter the secretion and expression of sensorin. However, the present demonstration that LTF depends on both elevated postsynaptic Ca2+ and postsynaptic protein synthesis suggests an alternative scheme: that 5-HT’s effects on sensorin originate from postsynaptic, not presynaptic, actions of the facilitatory monoamine. According to this idea, repeated applications of 5-HT produce prolonged activation of postsynaptic G protein-coupled receptors, which leads to a significant release of Ca2+ from intracellular stores [13]; the rapid increase in postsynaptic intracellular Ca2+, in turn, activates a retrograde signal that triggers the release and increased expression of sensorin. To test whether changes in presynaptic sensorin are regulated postsynaptically during LTF, we examined whether chelating postsynaptic intracellular Ca2+ disrupts the 5-HT-induced increase in sensorin expression. Sensorin was stained in sensory neurons with a specific sensorin antibody [9]. We quantified changes in sensorin expression using fluorescence immunohistochemistry (Experimental Procedures and Figure S1 in the Supplemental Data). BAPTA (200 mM) was injected into the motor neuron of some cocultures 30–60 min before the start of 5-HT treatment. As previously reported [9, 22], five spaced applications of 5-HT produced increased expression of sensorin in sensory processes and varicosities, as indicated by enhanced sensorin immunostaining in 5-HT-treated cocultures compared to Controls (Figure 3). Prior injection of BAPTA into the motor neuron blocked the 5-HT-dependent increase in sensorin immunostaining. Postsynaptic BAPTA alone failed to alter sensorin immunostaining.
Figure 3. The Rapid Increase in Sensorin Expression Induced by 5-HT Is Blocked by Postsynaptic BAPTA.
(A) Phase contrast micrograph of a sensorimotor coculture. The arrow points to the major neurite of the motor neuron. Scale bar, 30µm.
(B) Micrographs of sensorin immunofluorescence in the experimental groups. Each micrograph depicts the region along the main axon of a sensory neuron where contacted the major neurite of the motor neuron. Previous evidence indicates that this region represents the area of maximum synaptic contact between the sensory and motor neurons [40]. In cocultures that did not receive a postsynaptic injection of BAPTA, 5-HT treatment increased sensorin staining compared to untreated Controls. In cocultures treated with 5-HT after a postsynaptic BAPTA injection the immunostaining resembled that in Controls. Scale bar, 10 µM.
(C) Intensity of sensorin immunostaining in the four experimental groups. Staining intensity was determined for each coculture by measuring the mean pixel intensity of the fluorescence in four circular regions centered on the main process of the motor neuron. The mean pixel intensity was then corrected for background fluorescence, and the result was normalized to the mean pixel intensity in Control group (Figure S1). A one-way ANOVA indicated that the group differences were highly significant [F(3,82) = 6.13, p = 0.0008]. A Post-hoc comparison showed that the normalized sensorin staining was greater in 5-HT cocultures (151 ± 15%, n = 20) than in Controls (100 ± 5%, n = 26; p < 0.01). Importantly, the mean normalized sensorin staining in 5-HT-BAPTA cocultures (97 ± 6%, n = 20) was significantly less than that in the 5-HT group (p < 0.01), and not statistically different from that in Control cocultures (p > 0.05). The postsynaptic BAPTA injection alone had little effect on sensorin staining (mean normalized staining in the BAPTA group = 111 ± 8%, n = 20; p > 0.05 for the comparison with the Controls). *, significance of the difference between 5-HT and Control groups; +, significance of the difference 5-HT and 5-HT-BAPTA groups. Error bars represent ± SEM.
Discussion
The present results demonstrate that LTF of the Aplysia sensorimotor synapse due to five spaced applications of 5-HT [4] depends crucially on postsynaptic processes, including elevated postsynaptic Ca2+ and postsynaptic protein synthesis. Although prior studies have implicated contributions from the postsynaptic target neuron to this form of LTF [23, 24], the present study unambiguously identifies some of the postsynaptic processes required. Moreover, we have shown that a specific presynaptic change required for LTF—increased expression of the sensory neuron-specific neuropeptide sensorin [9]—is regulated, at least in part, by postsynaptic Ca2+.
LTF and the Requirement for Postsynaptic Protein Synthesis: Discrepancy with Earlier Studies
Our finding that inhibiting protein synthesis in the postsynaptic motor neuron blocks LTF contrasts with those of previous studies [14, 15, 17]. What is the reason for this discrepancy? There are significant methodological differences among the studies that might account for the different findings. For example, some of the studies were performed on synapses in central ganglia [14, 17], whereas others were performed on synapses in cell culture ([15] and the present study). The studies also differed substantially in the method of 5-HT treatment: in some cases the 5-HT application was restricted to sites of sensorimotor contact [15], whereas whole ganglia were treated with the drug in others [14, 17]. (Note that our study used the original method of Montarolo et al. [4] in which 5-HT was bath-applied to sensorimotor cocultures.) One major potential source of variance is the type of postsynaptic target neuron used in the studies. We used the identified small siphon (LFS)-type motor neurons [25] as the postsynaptic neurons in our cocultures. By contrast, Trudeau and Castellucci [14], as well as Martin et al. [15], used the large gill and mantle motor neuron L7 [26] as the target in their cocultures. Why might the type of postsynaptic neuron matter? There is a striking physical difference between LFS and L7 neurons: the diameter of L7 neurons (~100–400 µM) is approximately an order of magnitude greater than that of the LFS neurons (20–50 µM in our cell cultures). This implies that a far greater quantity (> 100 times the amount) of a cell membrane-impermeant protein synthesis inhibitor, such as gelonin, must be introduced into an L7 cell than into an LFS cell in order to produce equal impairment of protein synthesis. The large size of the L7 soma becomes even more problematic if, as other evidence suggests [18, 27] (see also [28]), the site for the synthesis of critical postsynaptic proteins required for LTF induction is the neurites of the motor neuron, rather than its cell body. We therefore believe that the likely reason for the failure of Trudeau and Castellucci [14] and of Martin et al. [15] to observe a disruption of LTF due to somal injections of gelonin into L7 is that the injections failed to impair 5-HT-induced protein synthesis at critical postsynaptic sites. It is unclear, however, whether this explanation can account for the failure of postsynaptic gelonin to disrupt LTF due to repeated, spaced applications of 5-HT in Sherff and Carew’s study [17]. (See Discussion in the Supplemental Data.) Thus, although the present study unambiguously demonstrates a requirement for postsynaptic protein synthesis in the most studied form of LTF [4], it remains to be determined whether or not all forms of LTF depend on postsynaptic protein synthesis (also see [29]).
LTF of the Sensorimotor Synapse Requires Retrograde Signaling
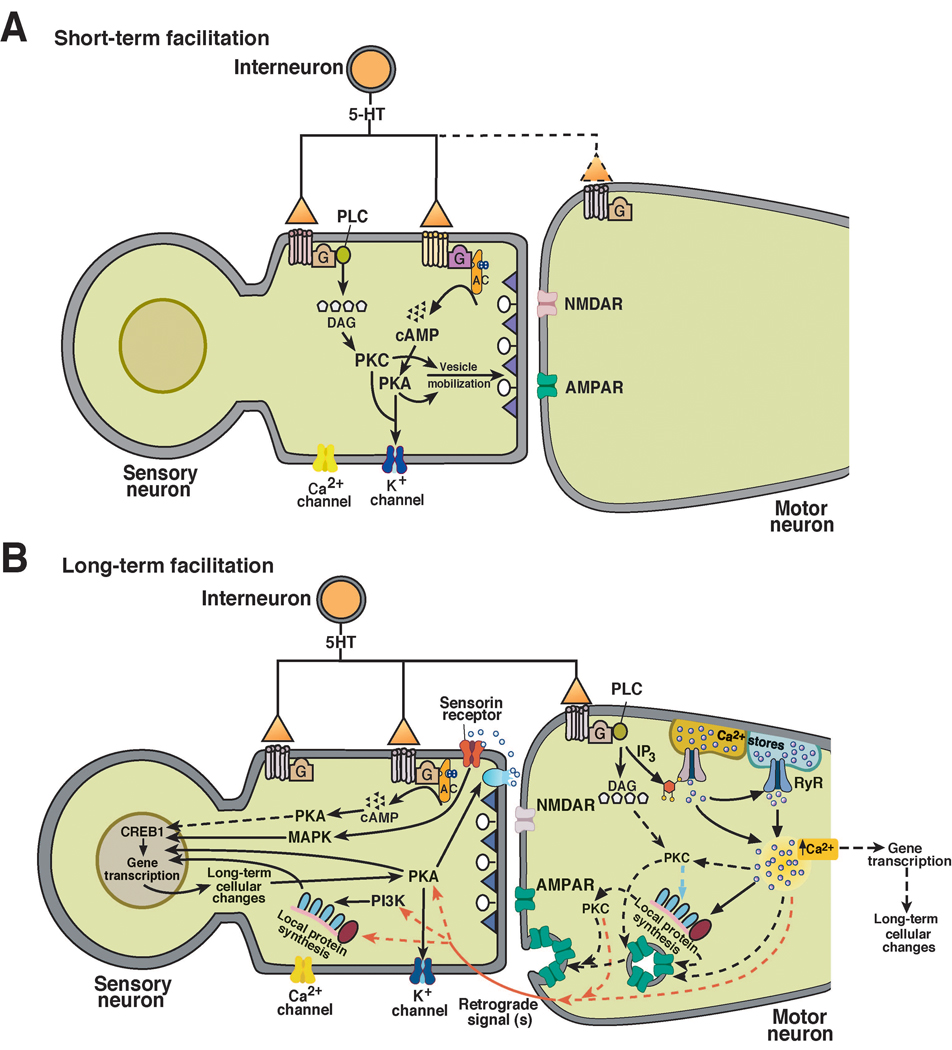
A surprising result from the present study is that presynaptic sensorin expression is regulated by postsynaptic Ca2+. This implies that the increased sensorin expression required for LTF is stimulated by one or more retrograde signals. Prior work has indicated that the enhanced expression and secretion of sensorin triggered by repeated application of 5-HT is due to activation of two signaling pathways within the sensory neuron, those involving PI3K and type II PKA [8, 9]. Likely targets for the retrograde signal(s), therefore, are presynaptic PI3K and PKA. Because we do not yet know the identity of the putative retrograde signal(s) involved in LTF, however, it is unclear how presynaptic PI3K and PKA are activated during LTF. Previously, it has been thought that the presynaptic kinases are activated by the binding of 5-HT to a G protein-coupled receptor in the presynaptic cell membrane [6, 8, 9]. Although direct actions of 5-HT on the sensory neuron may contribute to LTF, the present results show that such actions are not sufficient for LTF. An important question therefore is whether 5-HT acting on the presynaptic neuron has any mechanistic role in LTF. Recent evidence from experiments on isolated sensory neurons suggests that activation of presynaptic 5-HT receptors produces only short-term changes in the sensory neuron; elevated postsynaptic Ca2+ appears necessary for all persistent plastic changes in the sensorimotor synapse, including ITF and LTF [30]. We propose that LTF of the sensorimotor synapse is induced postsynaptically via an increase in intracellular Ca2+, and then expressed by both pre- and postsynaptic long-term changes. Among these changes are an increase in the number of presynaptic varicosities [23, 31], and a functional increase in the efficacy of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-type receptors [14, 32], possibly due to insertion of additional receptors into the postsynaptic membrane (Figure 4).
Figure 4. Cellular Models for Different Temporal Phases of Facilitation in Aplysia.
(A) Model for short-term synaptic facilitation. The serotonergic facilitatory interneuron, activated in the intact animal by noxious stimulation, releases 5-HT onto the sensory neuron. After binding to its G protein-coupled receptor, 5-HT causes rapid-onset, short-lasting (5–10 min) facilitation of the synapses via processes that involve presynaptic PKA and PKC [41, 42].
(B) Model for long-term synaptic facilitation. Binding of 5-HT to its receptors on the motor neuron causes a rise of intracellular calcium in motor neuron via activation of IP3 receptors and RyRs [13]. The rise of intracellular calcium drives local postsynaptic protein synthesis (present results) and enhancement of AMPA receptor function [14, 32]. AMPA receptor function may be enhanced through the synthesis of new AMPA receptors, exocytotic delivery of additional receptors to the postsynaptic membrane, or both [13, 18]. The postsynaptic rise in Ca2+ also activates one or more retrograde signals (present results). The retrograde signals, released from motor neuron, cause the rapid secretion and enhanced expression (via PKA and PI3K, respectively) of sensorin (Refs. [8, 9 and present results]). After binding to its autoreceptors, sensorin leads to phosphorylation of MAPK and its subsequent translocation into the nucleus of the sensory neuron. Translocated MAPK phosphorylates transcription factors that regulate the gene expression required for LTF [15, 43]. PKA, which is also translocated to nucleus, also plays a critical role in regulating long-term cellular changes accompanying LTF [44, 45]. In addition to changes in presynaptic transcription, LTF is likely to be accompanied by changes in postsynaptic transcription. Furthermore, it is possible that presynaptic effects of 5-HT also contribute to LTF [37]. The dashed lines in [A] and [B] indicate pathways whose involvement in STF/LTF is uncertain at present.
The model presented in Figure 4 has features in common with a recent scheme proposed for mossy fiber long-term potentiation (LTP) in the CA3 region of the mammalian hippocampus. This form of LTP, which is independent of _N_-methyl-d-aspartate (NMDA) receptor activation [33], appears to be induced postsynaptically by a rise in intracellular Ca2+ [although see 34], but expressed, in part, presynaptically by activation of presynaptic PKA [35, 36]. According to one model [35], elevated postsynaptic Ca2+ triggers binding of postsynaptic Eph receptors to presynaptic ephrins; the transsynaptic interaction between Eph receptors and ephrins, in turn, leads to activation of PKA in the mossy fibers. A similar transsynaptic mechanism may mediate LTF in Aplysia.
Our results do not exclude a contribution from presynaptic 5-HT receptors to LTF. Recent evidence from experiments on ITF of the sensorimotor synapse suggests that activation of presynaptic 5-HT receptors triggers an increase in spontaneous release of neurotransmitter from the sensory neuron. This increased spontaneous transmitter release, in turn, may contribute to a postsynaptic rise in intracellular Ca2+ via activation of postsynaptic metabotropic glutamate receptors (mGluRs) (see [37]. The potential involvement of mGluRs in LTF, however, remains to be determined.
Work on the mechanisms of LTF in Aplysia may provide insights into the regulation of the neurotrophin brain-derived neurotrophic factor (BDNF) during hippocampal LTP. BDNF mediates long-term presynaptic changes that accompany LTP of CA3-CA1 synapses due to a strong stimulus (200 Hz tetanus or theta burst stimulation) [38]. It has been suggested that BDNF, released from presynaptic terminals by strong synaptic stimulation, may act as an autocrine factor, binding to presynaptic tyrosine kinase B (TrkB) receptors. Activation of TrkB receptors by BDNF, in turn, would be expected to stimulate the mitogen-activated protein kinase (MAPK) pathway in CA3 neurons [39]. Interestingly, because LTP of CA3-CA1 synapses is induced postsynaptically, via activation of NMDA receptors and a postsynaptic influx of Ca2+, the enhanced presynaptic release, and possibly enhanced presynaptic expression, of BDNF may be triggered by elevated postsynaptic Ca2+. The parallels between the regulation and mechanistic role of sensorin during LTF in Aplysia and BDNF during hippocampal LTP are intriguing. Sensorin is released presynaptically and appears to activate a Trk-like receptor in sensory neurons [8], resulting in activation of MAPK. Thus, sensorin and BDNF may play similar roles in learning-related synaptic plasticity.
In summary, our study demonstrates that LTF depends critically on elevated postsynaptic Ca2+ and postsynaptic protein synthesis. Furthermore, we find that the increased expression of presynaptic sensorin during LTF requires elevation of postsynaptic intracellular Ca2+. Our results indicate that the persistent presynaptic changes that characterize LTF—which have been believed to be due to 5-HT acting on the sensory neuron—are stimulated, at least in part, by retrograde signals.
Supplementary Material
Supplemental Data
Supplemental Figure
ACKNOWLEDGMENTS
We are grateful to Dr. Samuel Schacher for providing us with the sensorin antibody. Also, we thank Drs. Daniel Fulton, Quan Li and Kelsey Martin for helpful comments on the manuscript. This research was supported by US National Institutes of Health grants (R37NS029563 and K02MH067062) to D.L.G.
References
- 1.Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 2.Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J. Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinesco S, Carew TJ. Serotonin release evoked by tail nerve stimulation in the CNS of Aplysia: characterization and relationship to heterosynaptic plasticity. J. Neurosci. 2002;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 5.Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Monosynaptic connections made by the sensory neurons of the gill-and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc. Natl. Acad. Sci. USA. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull. 2006;210:174–191. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- 7.Brunet JF, Shapiro E, Foster SA, Kandel ER, Iino Y. Identification of a peptide specific for Aplysia sensory neurons by PCR-based differential screening. Science. 1991;252:856–859. doi: 10.1126/science.1840700. [DOI] [PubMed] [Google Scholar]
- 8.Hu JY, Glickman L, Wu F, Schacher S. Serotonin regulates the secretion and autocrine action of a neuropeptide to activate MAPK required for long-term facilitation in Aplysia. Neuron. 2004;43:373–385. doi: 10.1016/j.neuron.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Hu JY, Wu F, Schacher S. Two signaling pathways regulate the expression and secretion of a neuropeptide required for long-term facilitation in Aplysia. J. Neurosci. 2006;26:1026–1035. doi: 10.1523/JNEUROSCI.4258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy GG, Glanzman DL. Enhancement of sensorimotor connections by conditioning-related stimulation in Aplysia depends upon postsynaptic Ca2+ Proc. Natl. Acad. Sci. USA. 1996;93:9931–9936. doi: 10.1073/pnas.93.18.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao JX, Kandel ER, Hawkins RD. Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture. J. Neurosci. 1998;18:458–466. doi: 10.1523/JNEUROSCI.18-01-00458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonov I, Antonova I, Kandel ER, Hawkins RD. Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron. 2003;37:135–147. doi: 10.1016/s0896-6273(02)01129-7. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: dependence upon release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis and modulation of postsynaptic AMPA receptor efficacy. J. Neurosci. 2005;25:5623–5637. doi: 10.1523/JNEUROSCI.5305-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trudeau LE, Castellucci VF. Postsynaptic modifications in long-term facilitation in Aplysia: upregulation of excitatory amino acid receptors. J. Neurosci. 1995;15:1275–1284. doi: 10.1523/JNEUROSCI.15-02-01275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin KC, Casadio A, Zhu H, E Y, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 16.Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- 17.Sherff CM, Carew TJ. Parallel somatic and synaptic processing in the induction of intermediate-term and long-term synaptic facilitation in Aplysia. Proc. Natl. Acad. Sci. USA. 2004;101:7463–7468. doi: 10.1073/pnas.0402163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villareal G, Li Q, Cai D, Glanzman DL. The role of rapid, local postsynaptic protein synthesis in learning-related synaptic facilitation in Aplysia. Curr. Biol. 2007;17:2073–2080. doi: 10.1016/j.cub.2007.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS. Phosphorylation and local presynaptic protein synthesis in calcium-and calcineurin-dependent induction of crayfish long-term facilitation. Neuron. 2001;32:489–501. doi: 10.1016/s0896-6273(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 20.Gingras A-C, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 21.Barbieri L, Battelli MG, Stirpe F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta. 1993;1154:237–282. doi: 10.1016/0304-4157(93)90002-6. [DOI] [PubMed] [Google Scholar]
- 22.Sun ZY, Wu F, Schacher S. Rapid bidirectional modulation of mRNA expression and export accompany long-term facilitation and depression of Aplysia synapses. J. Neurobiol. 2001;46:41–47. doi: 10.1002/1097-4695(200101)46:1<41::aid-neu4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Glanzman DL, Kandel ER, Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science. 1990;249:799–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- 24.Hu JY, Goldman J, Wu F, Schacher S. Target-dependent release of a presynaptic neuropeptide regulates the formation and maturation of specific synapses in Aplysia. J. Neurosci. 2004;24:9933–9943. doi: 10.1523/JNEUROSCI.3329-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost WN, Clark GA, Kandel ER. Parallel processing of short-term memory for sensitization in Aplysia. J. Neurobiol. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- 26.Koester J, Kandel ER. Further identification of neurons in the abdominal ganglion of Aplysia using behavioral criteria. Brain Res. 1977;121:1–20. doi: 10.1016/0006-8993(77)90435-8. [DOI] [PubMed] [Google Scholar]
- 27.Sherff CM, Carew TJ. Coincident induction of long-term facilitation in Aplysia: cooperativity between cell bodies and remote synapses. Science. 1999;285:1911–1914. doi: 10.1126/science.285.5435.1911. [DOI] [PubMed] [Google Scholar]
- 28.Schacher S, Wu F. Synapse formation in the absence of cell bodies requires protein synthesis. J. Neurosci. 2002;22:1831–1839. doi: 10.1523/JNEUROSCI.22-05-01831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey CH, Giustetto M, Zhu H, Chen M, Kandel ER. A novel function for serotonin-mediated short-term facilitation in Aplysia: conversion of a transient, cell-wide homosynaptic Hebbian plasticity into a persistent, protein synthesis-independent synapse-specific enhancement. Proc. Natl. Acad. Sci. USA. 2000;97:11581–11586. doi: 10.1073/pnas.97.21.11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glanzman DL. Simple minds: the neurobiology of invertebrate learning and memory. In: North G, Greenspan RJ, editors. Invertebrate Neurobiology. New York: Cold Spring Harbor Laboratory Press; 2007. pp. 347–380. [Google Scholar]
- 31.Bailey CH, Chen M. Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc. Natl. Acad. Sci. USA. 1988;85:9356–9359. doi: 10.1073/pnas.85.23.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu H, Wu F, Schacher S. Site-specific and sensory neuron-dependent increases in postsynaptic glutamate sensitivity accompany serotonin-induced long-term facilitation at Aplysia sensorimotor synapses. J. Neurosci. 1997;17:4976–4986. doi: 10.1523/JNEUROSCI.17-13-04976.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci. Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- 34.Mellor J, Nicoll RA. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nat. Neurosci. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- 35.Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- 36.Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat. Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan Q, Abrams TW. Trans-synaptic plasticity: presynaptic initiation, postsynaptic memory. Curr. Biol. 2008;18:R220–R223. doi: 10.1016/j.cub.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 38.Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 39.Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glanzman DL, Kandel ER, Schacher S. Identified target motor neuron regulates neurite outgrowth and synapse formation of Aplysia sensory neurons in vitro. Neuron. 1989;3:441–450. doi: 10.1016/0896-6273(89)90203-1. [DOI] [PubMed] [Google Scholar]
- 41.Braha O, Dale N, Hochner B, Klein M, Abrams TW, Kandel ER. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proc. Natl. Acad. Sci. USA. 1990;87:2040–2044. doi: 10.1073/pnas.87.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghirardi M, Braha O, Hochner B, Montarolo PG, Kandel ER, Dale N. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron. 1992;9:479–489. doi: 10.1016/0896-6273(92)90185-g. [DOI] [PubMed] [Google Scholar]
- 43.Purcell AL, Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Activation of a tyrosine kinase-MAPK cascade enhances the induction of long-term synaptic facilitation and long-term memory in Aplysia. Neuron. 2003;37:473–484. doi: 10.1016/s0896-6273(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 44.Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 45.Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data
Supplemental Figure