Mutation in the DC-SIGN cytoplasmic triacidic cluster motif markedly attenuates receptor activity for phagocytosis and endocytosis of mannose-containing ligands by human myeloid cells (original) (raw)
Abstract
The transmembrane C-type lectin, dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN), has three conserved cytoplasmic tail motifs: the tyrosine (Y)-based, dileucine (LL), and triacidic cluster (EEE), which are believed to regulate ligand binding, uptake, and trafficking. We mutated each of these motifs by alanine substitution and tested their roles in phagocytosis and receptor-mediated endocytosis of the highly mannosylated ligands, Mycobacterium tuberculosis mannose-capped lipoarabinomannan (ManLAM) and HIV-1 surface glycoprotein gp120, respectively, in transfected human myeloid K-562 cells. Compared with wild-type and other mutants, the EEE mutant of DC-SIGN showed a reduced cell-surface expression, near abolishment in the phagocytosis of ManLAM-coated beads (90.5±0.4%), and a marked reduction in the endocytosis of soluble gp120 (79.3±0.7%). Although, the Y mutant of DC-SIGN did not exhibit any effect on phagocytosis and intracellular trafficking to the phagolysosome, the LL mutant caused the majority of the receptor and/or ligands to remain bound to the cell surface, indicating a role for the LL motif as an internalization signal. The majority of the EEE mutant protein was found to be retained by the intracellular _trans_-Golgi network and not by the late endosomal/lysosomal compartment of transfected K-562 cells. Collectively, our data indicate a dual role for the EEE motif as a sorting signal in the secretory pathway and a lysosomal targeting signal in the endocytic pathway.
Keywords: C-type lectin, mannose-capped lipoarabinomannan, HIV gp120, phagosome-lysosome fusion, TGN retention
INTRODUCTION
Transmembrane C-type lectins are important host cell-surface receptors for a number of biological processes. They possess conserved motifs in their cytoplasmic tail region, which are known to regulate cell adhesion, antigen uptake, trafficking, cell signaling, and cytokine response by myeloid cells and play crucial roles in host innate immunity (reviewed in refs. [1,2,3]). Three well-characterized transmembrane C-type lectins—the macrophage mannose receptor (MR), dendritic cell (DC)-specific CD205 (DEC-205), and DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN)—have distinct extracellular carbohydrate recognition domains (CRDs) and intracellular conserved cytoplasmic motifs (see Fig. 1). The mannan-binding MR and DC-SIGN are pattern recognition receptors (PRRs), which bind to the surface structures of a variety of microbial pathogens including Mycobacterium tuberculosis and HIV-1 [4] and are felt to play important roles in tuberculosis and AIDS pathogenesis. The major M. tuberculosis surface lipoglycans, mannose-capped lipoarabinomannan (ManLAM) and higher-order phosphatidyl-_myo_-inositol mannosides (PIMs), serve as key ligands for the MR on macrophages and for DC-SIGN on DCs [5, 6]. MR-mediated phagocytosis of M. tuberculosis and beads coated with ManLAM or higher-order PIMs results in limited phagosome-lysosome (P-L) fusion [6, 7], whereas DC-SIGN-mediated phagocytosis of mycobacteria or ManLAM leads to P-L fusion [7, 8]. We hypothesize that this phenotypic difference between the MR and DC-SIGN relates to known differences in the cytoplasmic tails of these receptors.
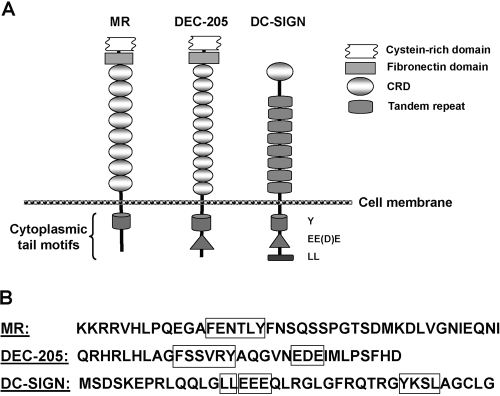
Fig. 1.
(A) Structures of the transmembrane C-type lectins: the MR, DEC-205, and DC-SIGN with their conserved cytoplasmic tail motifs. The oval-shaped, extracellular domains represent the CRDs for each lectin. The conserved motifs are shown in the cytoplasmic tail. (B) Amino acid sequence of the cytoplamic tail of each of the lectins showing their conserved motifs in boxes. The sequences shown take into account that the MR and DEC-205 are type 1 transmembrane proteins, and DC-SIGN is a type 2 transmembrane protein.
DC-SIGN has three conserved cytoplasmic motifs (see Fig. 1): tyrosine (Y)-based, dileucine (LL), and triacidic cluster (EEE), which are believed to be involved in receptor signaling for binding, phagocytosis, and intracellular trafficking of ligand molecules [1]. Specifically, the triacidic cluster (EDE) motif in DEC-205 has been reported to target endocytosed ligands to lysosomes and MHC class II-positive late endosomes [9]. The MR has only the Y-based motif in its cytoplasmic tail, which is involved in phagocytosis and endocytosis and endosomal sorting [10, 11].
In previous studies, a site-directed mutation in the LL motif of DC-SIGN caused the majority of a receptor-ligand complex [DC-SIGN and DC-SIGN antibody (Ab)] to remain on the surface of DC-SIGN-transfected cells [12, 13]. Thus, the LL motif was implicated in internalization of the soluble ligand. However, in their studies, mutagenesis of the Y and EEE motifs did not prevent internalization of the receptor-Ab complex, and the intracellular distribution of the mutant receptors was comparable with the wild-type (WT). To date, little or nothing is known about the exact roles of the DC-SIGN cytoplasmic motifs in the phagocytosis and intracellular trafficking of particulate ligands as well as in the receptor-mediated endocytosis of soluble ligands in myeloid cells.
To determine the roles of the three conserved cytoplasmic motifs of DC-SIGN in the above-mentioned cellular processes, we generated site-directed mutants of DC-SIGN for each motif, singly (LL, Y, or EEE) or in combination (LL+Y, LL+EEE, or Y+EEE). We then transfected WT and mutant receptor DNA constructs into the human myeloid cell line K-562 to study the structure-function relationships of DC-SIGN with respect to the conserved motifs in the cytoplasmic tail.
In this study, we used M. tuberculosis ManLAM-coated polystyrene beads as a phagocytic particle and HIV-1 gp120 as a soluble ligand for receptor-mediated binding and uptake studies with DC-SIGN WT and mutant-transfected K-562 cells. We examined total cell association (binding and uptake) of the ligands and P-L fusion for the intracellular trafficking of ManLAM-coated beads.
MATERIALS AND METHODS
Cell line, growth medium, ligands, and antibodies
The human erythroleukemic cell line K-562 was purchased from American Type Culture Collection (ATCC #CCL-243, Manassas, VA, USA). This cell line is known to spontaneously differentiate into recognizable progenitors of the erythrocytic, granulocytic, and monocytic series [14]. K-562 cells were routinely cultured and propagated in IMDM with glutamax (Invitrogen, Carlsbad, CA, USA) containing 10% FBS and penicillin-streptomycin (100 units or μg/ml). ManLAM was extracted and purified from plate-grown cultures of M. tuberculosis H37RV as we described previously [6]. The endotoxin level for the ManLAM preparation was calculated to be <18 pg/mg sample. HIV-1 gp120 and its corresponding mAb (17 b and ID6) were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Germantown, MD, USA). DC-SIGN rabbit polyclonal Ab (for immunofluorescence and Western blotting) was purchased from Calbiochem (San Diego, CA, USA) and GAPDH rabbit polyclonal Ab (for Western blotting) from Abcam (Cambridge, MA, USA). For immunofluorescence studies by confocal microscopy, γ-adaptin mAb was purchased from Sigma Chemical Co. (St. Louis, MO, USA); CD63, calnexin, Rab5, Rab11, and SNX1 mAb were from BD Biosciences (San Diego, CA, USA); and protein disulfide isomerase and 58K-9 mAb were from Novus Biologicals (Littleton, CO, USA). Alexa Fluor 488-conjugated goat anti-human/mouse, Alexa Fluor 546-conjugated goat anti-rabbit, and Alexa Fluor 647-conjugated goat anti-mouse IgG secondary Abs were purchased from Invitrogen. Goat anti-rabbit IgG-conjugated HRP was obtained from Bio-Rad (Hercules, CA, USA).
Site-directed mutagenesis to generate DC-SIGN cytoplasmic tail mutants
Site-directed mutagenesis was performed on plasmid DNA of a DC-SIGN expression construct [7] by using the QuikChange® site-directed mutagenesis kit (Stratagene, LaJolla, CA, USA), according to the manufacturer’s instructions. Mutations were created in the conserved amino acid sequence of DC-SIGN cytoplasmic motifs (LL, Y, and EEE) by alanine substitution and were confirmed by DNA sequencing using the ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA, USA).
Transfection and flow cytometry
K-562 cells were grown in culture medium for 2 days after expansion and transfected with empty vector (as negative control), DC-SIGN WT, or mutant DNA by nucleofection using the program T-016 of the Nucleofector Device II (Amaxa AG, Koein, Germany), according to the manufacturer’s suggestions. After an 18- to 20-h transfection period, cells were stained with PE-conjugated DC-SIGN mAb (BD PharMingen, San Diego, CA, USA) and analyzed for surface expression of DC-SIGN WT and mutant proteins by flow cytometry. The expression was measured relative to the empty vector control and expressed as mean fluorescence intensity (MFI).
Western blotting
K-562 cells transfected with DC-SIGN and its mutant derivatives were lysed in cell lysis buffer (140 mM NaCl, 20 mM Tris-HCl, 1% Triton X-100, plus protease inhibitor cocktail) for 30 min on ice. Cell lysates were centrifuged, and the supernatants were used as sources of soluble proteins. Total soluble proteins from K-562 cells were separated by 7.5% SDS-PAGE and then transferred to a nitrocellulose membrane. Blots were stained with rabbit polyclonal Abs against DC-SIGN and GAPDH (used as housekeeping protein control) followed by goat anti-rabbit IgG-conjugated HRP as secondary Ab. Blots were finally developed on X-ray film (Amersham Hyperfilm ECL) by using Amersham’s ECL Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK).
Preparation of ligand-coated polystyrene beads
Green fluorescent polystyrene beads (1 μm diameter; Polysciences Inc., Warrington, PA, USA) were coated with human serum albumin (HSA; control) or _M. tuberculosis_-derived ManLAM, according to the protocol established in our laboratory [15]. Beads were adjusted to a final concentration of 4 × 108/ml in 0.5% HSA in PBS before use in cell association and P-L fusion assays.
Quantitative cell association and P-L fusion assays using confocal microscopy
In these assays, control HSA- and ManLAM-coated beads were used as phagocytic ligands. Freshly propagated (2 days) K-562 cells were nucleofected with DC-SIGN WT and its derivative mutant DNA, grown for 10 h in a 12-well tissue-culture plate, and then transferred to polylysine-coated coverslips (2×105 cells/coverslip) to allow for adherence for 4 h. Mock-transfected K-562 cells were used as a DC-SIGN-negative control. Cell monolayers were incubated with HSA (sham control)- or ManLAM-coated beads [multiplicity of infection (MOI) 100:1] for 6 h to allow for binding and phagocytosis. Monolayers were fixed with 2% paraformaldehyde in PBS, permeabilized with 100% methanol, and blocked overnight with blocking buffer (PBS+5 mg/ml BSA+10% FBS). Permeabilization of cells was omitted in experiments intended to determine the surface expression of DC-SIGN. Cells were stained with DC-SIGN rabbit polyclonal and CD63 mouse mAb and then with Alexa Fluor 546- and 647-conjugated IgG secondary Abs, respectively. After extensive washing, coverslips were dried and mounted on glass slides, and the cell-associated fluorescent beads as well as their colocalization with the late endosomal/lysosomal marker were visualized by confocal microscopy (LSM 510, Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA). The cell surface-expressed DC-SIGN in the nonpermeabilized K-562 transfectants was measured and expressed in MFI arbitrary units by using the “ImageJ” software (NIH, http://rsb.info.nih.gov/ij/).
To demonstrate the specificity of expressed DC-SIGN for the ligand ManLAM, transfected K-562 cell monolayers were preincubated without or with mannan (4 mg/ml) at 37°C for 30 min before adding beads to the monolayers (MOI 1:50).
In other confocal experiments, transfected K-562 cells were processed for examination of the intracellular localization and/or retention of DC-SIGN within cell organelles by using Abs that recognize early endosomes, late endosomes/lysosomes, recycling and sorting endosomes, endoplasmic reticulum (ER), and Golgi or _trans_-Golgi Network (TGN).
Receptor-mediated endocytosis assay for DC-SIGN using confocal microscopy
K-562 cells were nucleofected with DC-SIGN WT or mutant DNA and grown for 14 h in a 12-well tissue-culture plate, and then cells (2×105/coverslip) were adhered to polylysine-coated coverslips for 4 h. Cell monolayers were preincubated without or with mannan (4 mg/ml) at 37°C for 30 min, followed by incubation with soluble HIV-1 gp120 (5 μg/ml) for 15 min at 37°C. Cell monolayers were fixed and permeabilized to visualize total cellular distribution of the soluble ligand and blocked overnight in blocking buffer. Permeabilization of cells was omitted in experiments intended to determine the surface expression of DC-SIGN and surface-bound ligand. Cells were then stained with gp120 mouse mAb and DC-SIGN rabbit polyclonal Ab. As secondary Abs, Alexa Fluor 546-conjugated goat anti-rabbit IgG was used to label DC-SIGN, and Alexa Fluor 488-conjugated goat anti-mouse IgG was used to label gp120 in K-562 cells. Cell surface-expressed DC-SIGN and endocytosed or surface-bound ligand gp120 were measured in MFI units by the ImageJ program.
RESULTS
Creation of site-directed mutants of the conserved cytoplasmic tail motifs of DC-SIGN
Mutagenesis in the target amino acids of the DC-SIGN sequence was performed by PCR as described in Materials and Methods. Six different site-directed mutants were generated representing mutation in a single cytoplasmic domain motif or mutation in a combination of two motifs (Fig. 1). The single mutants (LL, Y, and EEE) were derived directly from DC-SIGN WT DNA, and the double mutants YEEE, LLY, and LLEEE were derived from EE (an intermediate mutant), LL, and EEE mutant DNA, respectively. Mutants were propagated in Escherichia coli and verified by plasmid DNA analysis and nucleotide sequencing before their use in all experiments.
DC-SIGN triacidic cluster mutant shows reduced surface expression in K-562 cells
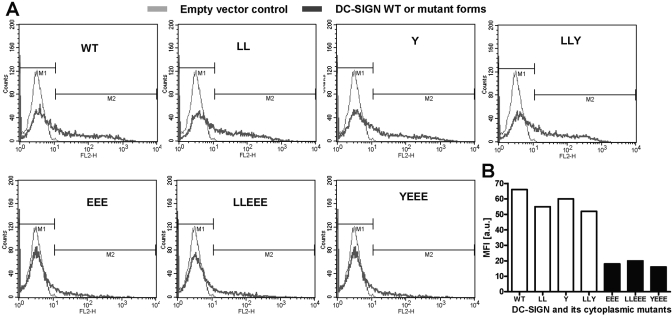
Surface expression of DC-SIGN WT and mutant proteins in transfected K-562 cells was assessed before using the cells in biological assays. Following transfection, the cells were incubated for 18–20 h and then stained with anti-DC-SIGN Ab and analyzed by flow cytometry to monitor the total surface expression of the receptor. Expression was measured as MFI and is shown in individual histograms (Fig. 2A). The levels of surface expression of DC-SIGN by EEE, LLEEE, and YEEE mutants (MFI 18, 20, and 16, respectively) were reproducibly lower than those obtained from LL, Y, and LLY mutants and WT (MFI 55, 60, 52, and 66, respectively; Fig. 2B).
Fig. 2.
Surface expression of DC-SIGN and its cytoplasmic tail mutant proteins in transfected K-562 cells. (A) K-562 cells were transfected with empty vector, DC-SIGN WT, or mutant construct DNA and after 18–20 h, stained with PE-conjugated DC-SIGN Ab. The surface expression was assessed by flow cytometry and expressed as MFI. The light gray line in the histogram represents empty vector control and the dark gray line, the expression of WT or mutant forms of DC-SIGN. Shown is a representative experiment (_n_=6). (B) A bar graph plotted from the MFI values derived from A, showing the levels of surface expression of WT and mutant DC-SIGN proteins relative to the vector control in K-562 cells. FL2-H, Fluorescence 2-height; a.u., arbitrary units.
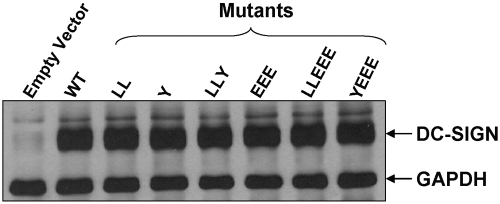
All transfectants including triacidic cluster mutants express equivalent levels of total DC-SIGN protein in K-562 cells
The reduced surface expression of DC-SIGN seen in the triacidic cluster mutants compared with the other mutants and WT (Fig. 2) prompted us to measure the total expression of receptor protein by all groups of K-562 transfectants. Following transfection with DC-SIGN empty vector, WT, or mutant DNA for 18–20 h, cells were lysed, and the total lysates were analyzed by SDS-PAGE and Western blotting for detection of DC-SIGN protein. All mutant transfectants including those involving the triacidic cluster showed equivalent levels of total DC-SIGN protein when compared with WT (Fig. 3).
Fig. 3.
Western blot analysis of K-562 WT and cytoplasmic tail mutants for total DC-SIGN protein. K-562 cells transfected with DC-SIGN empty vector (expression-negative control), WT, or mutant construct DNA were lysed, and total soluble proteins from lysates were separated by SDS-PAGE, transferred to nitrocellulose, and incubated with Abs against DC-SIGN and GAPDH (housekeeping control protein) and then with HRP-conjugated secondary Ab. Western blot shows the presence of equivalent amounts of DC-SIGN protein in WT and all mutant cell lines. Shown is a representative experiment (_n_=2).
DC-SIGN triacidic cluster mutant is markedly impaired in phagocytosis of _M. tuberculosis_-derived ManLAM-coated beads by K-562 cells
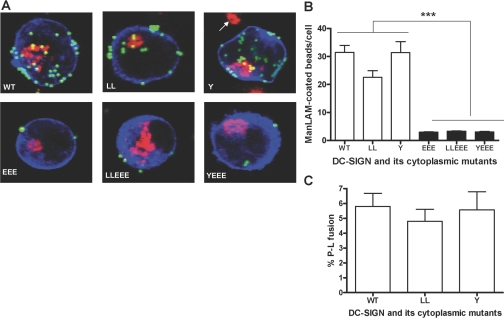
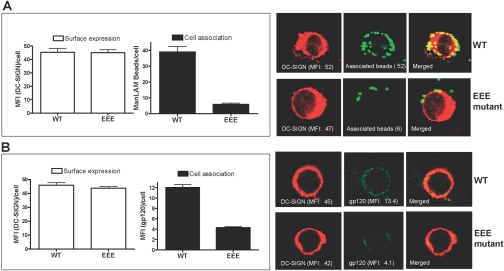
To study the intracellular localization and P-L fusion of the internalized DC-SIGN ligands in the transfected K-562 cells, we verified the presence of three late endosomal/lysosomal marker proteins (CD63, lysosome-associated membrane protein-2, and cathepsin D) in K-562 cells by Western blotting (data not shown). CD63 was chosen as the marker, and the cell association and P-L fusion (indicated by a CD63-positive compartment) assays were performed by confocal microscopy to evaluate the phagocytic ability of the K-562 transfectants expressing WT and mutant DC-SIGN. The M. tuberculosis major surface lipoglycan ManLAM, which is a key mycobacterial ligand for DC-SIGN [5, 16], was coated on the polystyrene beads and used in these assays. HSA-coated beads were used as control and were found to be poorly bound by DC-SIGN transfectants (data not shown). Representative confocal images for association of ManLAM-coated beads with each category of transfectants are shown in Figure 4A. Mean data of three independent experiments (by duplicate) for cell association and P-L fusion are shown in Figure 4, B and C, respectively.
Fig. 4.
Cell association and P-L fusion of ManLAM-coated beads in K-562 transfectants expressing DC-SIGN WT and mutant proteins. K-562 cells were transfected, adhered to polylysine-coated coverslips, and then incubated with ManLAM-coated fluorescent beads (MOI 1:100) for 6 h. Cell monolayers were fixed, permeabilized, stained with primary (DC-SIGN and CD63) Abs followed by secondary (fluorophore-conjugated IgG) Abs, and analyzed by confocal microscopy. (A) Representative confocal images of DC-SIGN WT and mutant receptor (blue)-expressing K-562 cells showing cell-associated, ManLAM-coated beads (green and yellow in the case of P-L fusion) and the intracellular lysosomal marker CD63 (red). Cell-associated beads were counted from an average of 25–30 cells/coverslip for each sample in duplicate in each experiment. The arrow (in the vicinity of the Y mutant) indicates the lysosomal staining of a neighboring, untransfected cell. Bar graphs represent the mean data ± sem of three independent experiments and display the levels of total cell association of ManLAM-coated beads (B) and P-L fusion (C) by the K-562 transfectants. Statistical analysis for significance was done by one-way ANOVA followed by Post-Tukey test (***, P<0.0001).
The LL mutant showed a small reduction (26.1±12.9%) in cell association compared with WT. We distinguished cell-bound from internalized beads by analyzing z-stacks of the confocal images. Although the WT DC-SIGN and Y mutant-transfected cells internalized 30.4 ± 2.6% and 29.5 ± 2.5%, respectively, of the total cell-associated beads, the LL mutant showed a decrease in the internalization of beads (16.6±2.7%), indicating a small defect in phagocytosis. This result was corroborated when assessing endocytosis as discussed below.
As opposed to the LL and Y mutants, all EEE mutants (single or double) showed a marked reduction (90.5±0.4%) in their cell association with ManLAM beads (***, P<0.0001, by one-way ANOVA statistical analysis). In the P-L fusion assay, the Y or LL mutant did not show a significant reduction when compared with WT. P-L fusion could not be scored in the EEE mutant-expressing cells because of the lack of bead uptake by these cells.
Phagocytosis of ManLAM-coated beads by K-562 transfectants is mediated by the surface-expressed DC-SIGN
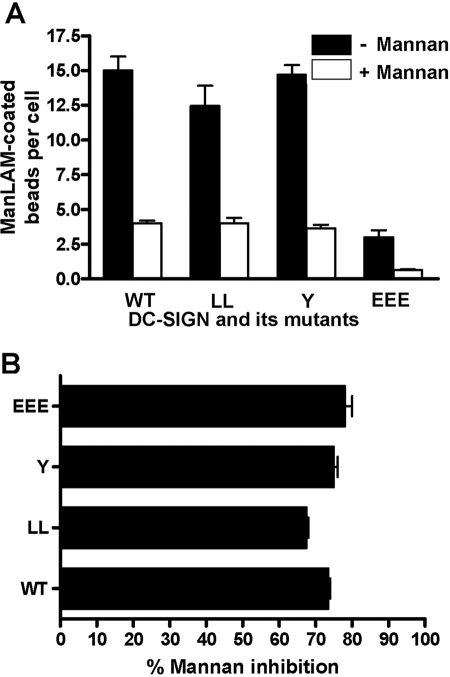
To demonstrate that the binding and subsequent phagocytosis of ManLAM-coated beads by K-562 transfectants are mediated specifically by surface-expressed DC-SIGN, we performed additional cell association assays using ManLAM beads and the single mutants LL, Y, and EEE, in addition to WT receptor-transfected cells in the presence or absence of mannan, a soluble carbohydrate-competitive inhibitor of DC-SIGN. Mannan inhibition dramatically reduced the cell association of ManLAM-coated beads with each type of transfectant (Fig. 5, A and B).
Fig. 5.
Mannan-inhibitable binding of ManLAM-coated beads to K-562 surface-expressed DC-SIGN WT and mutant receptors. K-562 cells were transfected, adhered to polylysine-coated coverslips, and then preincubated with or without mannan for 30 min before incubation with ManLAM-coated beads (MOI 1:50) for 6 h. Cell monolayers were fixed, permeabilized, stained with primary DC-SIGN Abs followed by secondary (fluorophore-conjugated IgG) Abs, and analyzed by confocal microscopy. Cell-associated beads were counted from an average of 25–30 cells/coverslip for each sample in duplicate in each experiment. Bar graphs represent the mean data ± sem of two independent experiments and show the extent of mannan inhibition of association of beads with K-562 transfectants, which are presented in bead association per cell (A) and percentage of mannan inhibition of bead association (B).
DC-SIGN triacidic cluster mutant is impaired in receptor-mediated endocytosis of the soluble ligand HIV-1 gp120 by K-562 cells
We next studied the ability of the DC-SIGN WT and EEE mutant-transfected K-562 cells to mediate receptor-mediated endocytosis using HIV-1 gp120 as the soluble ligand and mannan as its competitive inhibitor. For these studies, we also included the LL mutant because of the previous reports demonstrating the unique cell-surface distribution of this mutant receptor in response to soluble Ab ligands [12, 13, 17]. Following endocytosis of HIV-1 gp120, K-562 transfectants were stained with DC-SIGN and gp120 Abs followed by fluorophore-conjugated secondary Abs and then analyzed by confocal microscopy.
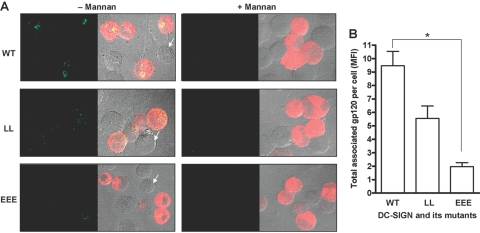
Representative confocal images are shown in Figure 6, which demonstrates that only receptor-expressing cells (red) had endocytic activity (Fig. 6A, – Mannan). The endocytosed ligand (green) showed a higher intracellular intensity in WT transfectants (top panel) than the LL mutant-expressing cells, where gp120 was visualized primarily rimming the cell, consistent with attachment and minor internalization (middle panel). On the other hand, the EEE mutant showed the least intensity for the endocytosed gp120 (bottom panel). Mannan inhibition completely abrogated the binding and uptake of gp120 in each case (Fig. 6A, + Mannan), again indicating the specificity of receptor-mediated uptake. The total gp120 cell association by each transfectant was expressed in MFI arbitrary units and is shown as a bar graph (Fig. 6B). The LL mutant showed a moderate reduction (41.8±3.4%), whereas the EEE mutant showed a marked reduction (79.3±0.7%) in endocytosis of the soluble ligand gp120 when compared with WT.
Fig. 6.
Receptor-mediated endocytosis of the soluble ligand gp120 by K-562 transfectants expressing DC-SIGN WT and LL and EEE mutant proteins. K-562 cells were transfected, adhered to polylysine-coated coverslips, and then preincubated with or without mannan for 30 min before incubation with HIV-1 gp120 for 15 min. Cell monolayers were fixed, permeabilized, stained with primary (DC-SIGN and gp120) Abs and then with secondary (fluorophore-conjugated IgG) Abs, and examined by confocal microscopy. (A, – Mannan) Representative confocal images of the endocytosed gp120 (green spots on left and yellow spots on right panels) by K-562 transfectants expressing the receptor (red on right panels) in the absence of mannan. Right panels represent the phase-contrast images. Arrows indicate representative untransfected cells without DC-SIGN expression. (A, + Mannan) Representative confocal images of the endocytosed gp120 by K-562 transfectants expressing the receptor (red on right panels) in the presence of mannan. Right panels represent the phase-contrast images. (B) Bar graph represents the mean data ± sem of two independent endocytosis experiments without mannan, where total associated gp120 was measured in MFI units from 20 to 25 cells/coverslip for each sample in duplicate in each experiment. Statistical analysis was by one-way ANOVA followed by Post-Tukey test (*, P<0.05).
DC-SIGN triacidic cluster mutant has a defect in cell association of ligands independent of its surface expression on K-562 cells
Analysis of individual cells by confocal microscopy revealed that there was a subpopulation of K-562 EEE transfectants, which displayed DC-SIGN expression equivalent to WT and other mutants. This provided us with the rationale to further compare cell association activities between cells expressing equivalent levels of cell-surface WT and EEE mutant DC-SIGN. Cell association assays were performed with K-562 transfectants using ManLAM-coated beads and gp120 as ligands, respectively, and cells were fixed without permeabilization before processing for confocal microscopy to quantify cell surface-expressed receptor protein as well as surface-bound ligands. Figure 7 shows the results using ManLAM-coated beads (Fig. 7A) and gp120 (Fig. 7B). Confocal microscopy and image analysis of 13 selected K-562 cells from each group of WT and EEE mutant transfectants with equivalent levels of DC-SIGN expression (45.4±2.7 vs. 45.0±2.3 MFI/cell) showed an approximate sevenfold reduction in cell association of ManLAM beads by the EEE mutant (5.9±0.8 beads/cell) when compared with the WT (39.1±3.4 beads/cell; Fig. 7A, left panel). Representative confocal images of DC-SIGN surface expression and cell association of ManLAM beads by the WT and EEE mutants are shown in the right panel (Fig. 7A). A similar analysis of 10 selected K-562 cells from each group of WT and EEE mutant transfectants with comparable levels of DC-SIGN expression (45.9±1.9 vs. 43.7±1.1 MFI/cell) showed an approximate threefold reduction in cell association of soluble gp120 by the EEE mutant (4.3±0.2 MFI/cell) when compared with the WT (12.1±0.6 MFI/cell; Fig. 7B, left panel). Representative confocal images of DC-SIGN surface expression and cell association of gp120 by the WT and EEE mutants are shown in the right panel (Fig. 7B). Thus, there was a marked defect in cell association by K562 cells despite good surface expression of the DC-SIGN EEE mutant protein.
Fig. 7.
Cell association of phagocytic (ManLAM beads) and endocytic (gp120) ligands by K-562 WT and EEE mutant transfectants having equivalent levels of DC-SIGN expression. K-562 cells were transfected, adhered to polylysine-coated coverslips, and then incubated with green fluorescent ManLAM beads for 5 h to allow for phagocytosis (A) or with gp120 for 15 min to allow for receptor-mediated endocytosis (B). Cell monolayers were fixed (without permeabilization), then stained with DC-SIGN (A) or with DC-SIGN and gp120 (B) Abs, followed by staining with fluorophore-conjugated secondary Abs, and examined by confocal microscopy. Expressed DC-SIGN protein and cell-associated gp120 were measured in MFI units, and cell-associated fluorescent beads were enumerated as mean (±sd) beads/cell. (A, left) Bar graphs show the mean data (MFI units or beads) ± sd of 13 selected K-562 cells from each group of WT and EEE mutant transfectants having equivalent surface expression of DC-SIGN but differential cell association of ManLAM beads. (A, right) Representative confocal images of DC-SIGN WT and EEE mutant transfectants from the phagocytosis assay. (B, left) Bar graphs show the mean data (MFI units) ± sd of 10 selected K-562 cells from each group of WT and EEE mutant transfectants having equivalent surface expression of DC-SIGN but differential cell association of gp120. (B, right) Representative confocal images of DC-SIGN WT and EEE mutant transfectants from the receptor-mediated endocytosis assay.
Newly produced DC-SIGN triacidic cluster mutant proteins have a higher intracellular retention in the TGN than do the WT and other mutant derivatives
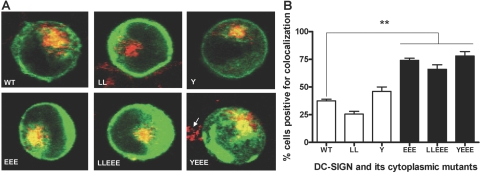
As a result of the overall reduced surface expression and limited activities of the DC-SIGN EEE mutant proteins for phagocytosis and endocytosis relative to other mutant and WT proteins, we analyzed the intracellular distribution of these proteins after their expression in transfected K-562 cells. Different intracellular organelle markers such as calnexin and protein disulfide isomerase (ER), 58K-9 (Golgi), γ-adaptin (TGN), Rab5 (early endosome), Rab11 (recycling endosome), and SNX1 (sorting endosome) were used to visualize their colocalization with the nascently produced DC-SIGN proteins in the cells by using confocal microscopy. There was no difference in colocalization of any of the markers except for the TGN marker γ-adaptin, which showed a consistent level of higher colocalization with the EEE single or double mutant proteins than with other mutant derivatives and WT (Fig. 8). The percentages of transfected K-562 cells positive for colocalization between the TGN marker and DC-SIGN protein were 37.5 ± 1.5 (WT), 25.5 ± 2.5 (LL), 46.0 ± 4.0 (Y), 74.0 ± 2.0 (EEE), 66.0 ± 4.0 (LLEEE), and 78.0 ± 4.0 (YEEE; Fig. 8B).
Fig. 8.
Comparative intracellular retention of DC-SIGN WT and mutant proteins in the TGN of the K-562-transfected cells. K-562 cells were transfected, adhered to polylysine-coated coverslips, and incubated for 18–20 h to allow for protein expression. Cell monolayers were fixed, permeabilized, stained with DC-SIGN and γ-adaptin Abs and counterstained with fluorophore-conjugated secondary Abs, and examined by confocal microscopy. (A) Representative confocal images of DC-SIGN WT and mutant receptor (green)-expressing K-562 cells showing the colocalization (yellow) between the TGN marker γ-adaptin (red) and the receptor protein. An average of 25–30 cells/coverslip in duplicate from each sample was examined for colocalization in each experiment. The arrow (in the vicinity of the YEEE mutant) indicates the TGN staining of a neighboring, untransfected cell. (B) The bar graph represents the mean data ± sem of two independent experiments and shows the percentage of each category of transfectants positive for colocalization. Statistical analysis for significance was performed by one-way ANOVA followed by Post-Tukey test (**, P<0.01).
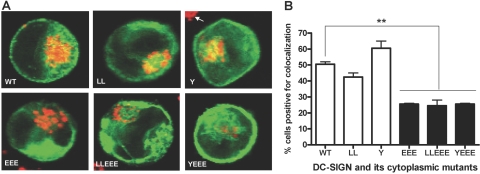
DC-SIGN triacidic cluster mutant proteins are least-targeted to the late endosomal/lysosomal compartment relative to the WT and other mutant derivatives
Based on a previous report describing the cytoplasmic tail triacidic cluster sequence of C-type lectin DEC-205 as a lysosomal targeting motif [9], we assessed the intracellular trafficking of the DC-SIGN EEE mutant protein to the late endosomal/lysosomal compartment. CD63 was used as the late endosomal/lysosomal marker to assess its colocalization with DC-SIGN WT and mutant proteins in transfected K-562 cells. The EEE single or double mutant proteins showed a reduced level of colocalization with CD63 when compared with the WT and other mutant derivatives (Fig. 9). The percentages of K-562-transfectant cells positive for colocalization between the DC-SIGN protein and CD63 were 50.5 ± 1.5 (WT), 42.5 ± 2.5 (LL), 60.5 ± 4.5 (Y), 25.5 ± 0.5 (EEE), 24.5 ± 3.5 (LLEEE), and 25.5 ± 0.5 (YEEE; Fig. 9B).
Fig. 9.
Comparative intracellular trafficking of DC-SIGN WT and mutant proteins to the late endosomal/lysosomal compartment in the K-562-transfected cells, which were transfected, adhered to polylysine-coated coverslips, and incubated for 18 h, followed by a 30-min incubation with the ligand mannan to allow for endocytic trafficking of the DC-SIGN protein. Cell monolayers were fixed, permeabilized, stained with DC-SIGN and CD63 Abs and counterstained with fluorophore-conjugated secondary Abs, and examined by confocal microscopy. (A) Representative confocal images of DC-SIGN WT and mutant receptor (green)-expressing K-562 cells showing the colocalization (yellow) of the late endosomal/lysosomal marker CD63 (red) with DC-SIGN. An average of 20–25 cells/coverslip in duplicate from each sample was examined for colocalization in each experiment. The arrow (in the vicinity of the Y mutant) indicates the lysosomal staining of a neighboring, untransfected cell. (B) The bar graph represents the mean ± sem of two independent experiments and shows the percentage of transfectants positive for colocalization. Statistical analysis for significance was performed by one-way ANOVA followed by Post-Tukey test (**, P<0.01).
DISCUSSION
Three predominant cytoplasmic tail motifs have been identified in the transmembrane C-type lectin receptors: Y-based motifs, LL motifs, and triads of acidic amino acids (composed of glutamic and/or aspartic acids), which can direct ligand uptake and the subsequent sorting of the receptor and its cargo [2, 3]. The Y-based motif acts as an internalization and/or sorting signal in the MR and DEC-205 by promoting the delivery of ligands to early endosomes, whereas the additional triacidic motif, EDE, in DEC-205 diverts this receptor to late endosomes-lysosomes [9, 11]. For Dectin-1 (a β-glucan receptor), which mediates the phagocytosis of yeast and yeast-derived zymosan particles, the Y-based and triacidic (DED) motifs are required for endocytosis [18, 19]. One of the research interest areas in our laboratory is focused on the mannose-binding C-type lectins MR and DC-SIGN [6, 7], where the latter has all three cytoplasmic motifs including the triacidic cluster, EEE [1] (see Fig. 1).
In the present study, we mutated each cytoplasmic motif of DC-SIGN, alone or in combination, and tested their specific roles in phagocytosis and receptor-mediated endocytosis by the human myeloid cell line K-562, which does not express the mannose-binding receptors such as DC-SIGN and the MR, as reported by others [8, 20] and confirmed by our current study. We used transient transfection of K-562 cells with DC-SIGN WT and mutant receptor constructs and analyzed the receptor-expressing transfectants on a single-cell basis by using confocal microscopy.
Importantly, in the current study, we assessed phagocytosis and endocytosis mediated by DC-SIGN expressed on myeloid cells using physiologically relevant ligands of DC-SIGN (M. tuberculosis ManLAM and HIV-1 gp120), which has not been done before. ManLAM and gp120 are potent, highly mannosylated ligands for this receptor [5, 21]. We demonstrate for the first time that perturbation of the triacidic cluster motif in the cytoplasmic tail of DC-SIGN causes intracellular retention of the newly produced EEE mutant protein by the TGN of the K-562 cell and results in nearly complete loss of phagocytosis and endocytosis of ligands. This intracellular retention of EEE mutant DC-SIGN protein assumes a novel pathway and leads to an aberrant biological activity of protein that does make it to the cell surface.
First, we verified surface expression of the WT and mutant DC-SIGN in the transfected K-562 cells by flow cytometry and found that all mutants exhibited comparable expression to WT, except for EEE single or double mutants, which consistently showed three- to fourfold less surface expression compared with WT (Fig. 2B). Previously, similar results were observed in transfected cells as a result of deletion of the cytoplasmic tail of DC-SIGN, which includes the EEE motif [13, 17], and mutation of the acidic residue in the cytoplasmic tail of another C-type lectin CLEC-2 [22]. However, in neither case was the resultant biological activity of the reduced expression of such cytoplasmic tail mutants investigated.
In this study, we observed that the marked defect in binding and internalization of DC-SIGN ligands by the EEE mutants (Figs. 4, A and B, and 6) correlated with reduced cell-surface expression of the receptor (Fig. 2) by the general population of EEE mutant transfectants. However, a subset of the EEE mutant population of cells expressing equivalent amounts of surface DC-SIGN when compared with WT transfectant cells also showed a similar defect in cell association (Fig. 7), indicating a dysfunctional protein. We speculate that DC-SIGN proteins lacking the EEE motif that are targeted to the cell surface by default may not be structurally competent to form tetramers, which is an essential requirement for high-affinity binding of particulate and soluble ligands by this receptor [23, 24]. Alternatively, a mutation in the EEE motif may also directly attenuate the ability of the cytoplasmic tail to signal upon the receptor-ligand interaction and hence, prevent binding and internalization.
The LL sequence was demonstrated previously to be an internalization or endocytic motif for DC-SIGN [12, 13, 17]. In our study, a mutation in the LL motif led to a small decrease in cell association (endocytosis and phagocytosis; also see below) and no significant effect on P-L fusion (Fig. 4). Our results suggest that the LL motif provides a partial signal for ligand internalization (Fig. 4B), which is in agreement with Engering et al. [12]. This study, in agreement with others [13, 17], demonstrates that the Y-based motif in DC-SIGN is not involved in internalization or intracellular trafficking (Fig. 4, B and C). This is in contrast with the role of the Y-based motif in other C-type lectins such as the MR [10, 11], DEC-205 [9], Dectin-1 [18], CLEC-2 [22], and liver and lymph node sinusoidal endothelial cell C-type lectin (LSECtin) [25]. DC-SIGN does not signal through the Y-based motif [22].
In the receptor-mediated endocytosis assay, we used HIV-1 gp120 as the soluble ligand. We opted to compare the endocytic properties between the WT and EEE mutant of DC-SIGN in this assay; however, we also included the LL mutant based on previous reports, which indicated that a mutation in LL affected the endocytosis of receptor-Ab complexes [12, 13, 17]. The overall trends in binding/uptake of the soluble ligand gp120 by the three classes of transfectants (WT, LL, and EEE) in the endocytosis assay (Fig. 6B) generally corresponded to those of the phagocytic ligand ManLAM beads in the cell association and phagocytosis assay (Fig. 4B). In the endocytosis assay, we also used mannosylated BSA, a known, soluble ligand for the MR [26,27,28,29], which was poorly recognized by DC-SIGN (data not shown).
K-562 cells transfected with the LL mutant showed retention of gp120 on the cell surface, as visualized by rimming of the ligand around the cell with a limited amount of ligand visualized intracellularly (Fig. 6A, – Mannan; middle panels). This observation confirms the cell-surface retention of the LL mutant receptor following induction with a soluble antibody ligand, as shown by previous reports using other transfected cell lines [13, 17]. Additionally, our results indicate that the lower intracellular pool of this mutant protein, as observed by its limited TGN retention (data in the text related to Fig. 8), is consistent with the higher amounts of this protein on the surface. The EEE mutant-expressing K-562 cells in our study demonstrated minimal endocytosis of gp120 (approximately fivefold less, compared with the WT-expressing cells; Fig. 6, A and B). Furthermore, we demonstrated DC-SIGN specificity by mannan inhibition in phagocytosis (Fig. 5) and endocytosis (Fig. 6A, + Mannan).
We sequenced and confirmed the entire 348-bp CRD regions (263–378 aa) [21] of DC-SIGN for the single and double EEE mutants to rule out the possibility of any additional mutation in this domain (data not shown). The reduced surface expression and poor ligand binding/internalization by the DC-SIGN EEE mutant, together with the confirmation of an intact sequence of its CRD region, led us to hypothesize a novel export pathway for this mutant protein. Our finding is, in part, supported by a recent observation of the dramatic reduction in antigen capture and uptake by K-562 cells mediated by mutation in the diglutamic (EE) and Y motifs in the cytoplasmic tail of the DC-SIGN-related lectin LSECtin [25]. However, no cellular or molecular mechanism for this observation was established.
In our current study, we found that the EEE mutant transfectants expressing less surface DC-SIGN (Fig. 2) showed no difference in total receptor protein expression when compared with WT and other mutants as demonstrated by Western blotting of total cell lysates (Fig. 3) as well as by confocal microscopy of permeabilized cells (Figs. 4A, 8A, and 9A). This observation suggested a defect in protein export and led us to hypothesize that DC-SIGN mutated in the EEE motif is retained in intracellular compartment(s) to a greater extent. Thus, we examined an array of intracellular organelle markers for colocalization with the expressed DC-SIGN proteins in K-562 cells to demonstrate their intracellular retention patterns. We found that EEE single or double mutants colocalized with the TGN marker (γ-adaptin) to a greater extent compared with WT and other derivative proteins (Fig. 8), indicating its intracellular retention within the TGN before targeting to the cell membrane. In this context, the DC-SIGN cytoplasmic EEE motif resembles the glutamic acidic (E) in the EXXXLL motif of the HIV-1 nef protein, where this acidic residue was required for efficient binding and interaction of this protein with adaptor proteins 1 and 3 (AP-1 and AP-3) in host cells [30]. AP-1 mediates vesicular transport between the TGN and endosomal compartments, whereas AP-3 mediates transport from the TGN and early endosomes to late endosomes and lysosomes. This export pathway is consistent with our findings that perturbation of the triacidic EEE motif in DC-SIGN leads to retention in the TGN rather than normal transport to other cellular compartments. We also found that the EEE mutant protein is targeted to a lesser extent to late endosomes/lysosomes in the endocytic pathway when compared with the WT and other mutant proteins in K-562 cells (Fig. 9). In this context, it is important to note that the triacidic cluster motif (EDE) in DEC-205 was described as a lysosomal targeting signal in transfected cells [9].
The expression and distribution of transmembrane proteins, such as the C-type lectins, mechanistically depend on the mode of their secretory pathways after synthesis and their endocytic pathways after induction by ligands on the cell surface. Our studies provide evidence that the DC-SIGN cytoplasmic EEE motif may have a dual function: serving as a sorting/partitioning signal in the secretory pathway and as a late endosomal/lysosomal targeting signal in the endocytic pathway. Additionally, the EEE motif may play an important role in signaling for ligand binding and/or maintaining the proper protein structure of DC-SIGN needed for oligomerization on the cell surface. Engineering inhibitors of the EEE cytoplasmic motif in DC-SIGN could potentially attenuate this PRR, which plays an important role in microbial pathogenesis.
Acknowledgments
This work was supported by the NIH/National Institute of Allergy and Infectious Diseases (NIAID) AI-052458. The following reagents were obtained through the NIH AIDS Research and Reference Program, Division of AIDS, NIAID, NIH: HIV-1 CN54 gp120 and HIV-1 gp120 mAb (17b and ID6) from Division of Acquired Immunodeficiency Syndrome (DAIDS), NIAID. We also acknowledge the support of the Campus Microscopy and Imaging Facility at The Ohio State University (Columbus, OH, USA). The authors thank Dr. Jesse Kwiek for his assistance with obtaining HIV-1 gp120 and anti gp120 mAb.
References
- Figdor C G, Van Kooyk Y, Adema G J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Cambi A, Figdor C G. Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol. 2003;15:539–546. doi: 10.1016/j.ceb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Robinson M J, Sancho D, Slack E C, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- Van Kooyk Y, Geijtenbeek T B. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- Maeda N, Nigou J, Herrmann J L, Jackson M, Amara A, Lagrange P H, Puzo G, Gicquel B, Neyrolles O. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem. 2003;278:5513–5516. doi: 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- Torrelles J B, Azad A K, Schlesinger L S. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- Kang P B, Azad A K, Torrelles J B, Kaufman T M, Beharka A A, Tibesar E, Desjardin L E, Schlesinger L S. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek T B, Van Vliet S J, Koppel E A, Sanchez-Hernandez M, Vandenbroucke-Grauls C M, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K, Guo M, Lee S, Sepulveda H, Swain S L, Nussenzweig M, Steinman R M. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–684. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal B A, Sastry K, Warner A B, Mathieu C E, Ezekowitz R A B. Phagocytic chimeric receptors require both transmembrane and cytoplasmic domains from the mannose receptor. J Exp Med. 1992;176:1673–1680. doi: 10.1084/jem.176.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Stahl P D, Rohrer J. A di-aromatic motif in the cytosolic tail of the mannose receptor mediates endosomal sorting. J Biol Chem. 2000;275:29694–29700. doi: 10.1074/jbc.M000571200. [DOI] [PubMed] [Google Scholar]
- Engering A, Geijtenbeek T B, Van Vliet S J, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor C G, Piguet V, Van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- Lozach P Y, Burleigh L, Staropoli I, Navarro-Sanchez E, Harriague J, Virelizier J L, Rey F A, Despres P, Arenzana-Seisdedos F, Amara A. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem. 2005;280:23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- Lozzio B B, Lozzio C B, Bamberger E G, Feliu A S. A multipotential leukemia cell line (K-562) of human origin. Proc Soc Exp Biol Med. 1981;166:546–550. doi: 10.3181/00379727-166-41106. [DOI] [PubMed] [Google Scholar]
- Schlesinger L S, Hull S R, Kaufman T M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- Tailleux L, Schwartz O, Herrmann J L, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod L P, Gluckman J C, Lagrange P H, Gicquel B, Neyrolles O. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh L, Lozach P Y, Schiffer C, Staropoli I, Pezo V, Porrot F, Canque B, Virelizier J L, Arenzana-Seisdedos F, Amara A. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol. 2006;80:2949–2957. doi: 10.1128/JVI.80.6.2949-2957.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herre J, Marshall A S, Caron E, Edwards A D, Williams D L, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown G D. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–4045. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- Rogers N C, Slack E C, Edwards A D, Nolte M A, Schulz O, Schweighoffer E, Williams D L, Gordon S, Tybulewicz V L, Brown G D, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Koppel E A, Ludwig I S, Hernandez M S, Lowary T L, Gadikota R R, Tuzikov A B, Vandenbroucke-Grauls C M, Van Kooyk Y, Appelmelk B J, Geijtenbeek T B. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology. 2004;209:117–127. doi: 10.1016/j.imbio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Curtis B M, Scharnowske S, Watson A J. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci USA. 1992;89:8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G L, Williams J A, Tomlinson M G, Eble J A, Hanna S L, Pohlmann S, Suzuki-Inoue K, Ozaki Y, Watson S P, Pearce A C. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg H, Mitchell D A, Drickamer K, Weis W I. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- Mitchell D A, Fadden A J, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- Dominguez-Soto A, Aragoneses-Fenoll L, Martin-Gayo E, Martinez-Prats L, Colmenares M, Naranjo-Gomez M, Borras F E, Munoz P, Zubiaur M, Toribio M L, Delgado R, Corbi A L. The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood. 2007;109:5337–5345. doi: 10.1182/blood-2006-09-048058. [DOI] [PubMed] [Google Scholar]
- Stahl P, Schlesinger P H, Sigardson E, Rodman J S, Lee Y C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980;19:207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger L S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- Beharka A A, Gaynor C D, Kang B K, Voelker D R, McCormack F X, Schlesinger L S. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- Frison N, Taylor M E, Soilleux E, Bousser M T, Mayer R, Monsigny M, Drickamer K, Roche A C. Oligolysine-based oligosaccharide clusters: selective recognition and endocytosis by the mannose receptor and DC-SIGN. J Biol Chem. 2003;278:23922–23929. doi: 10.1074/jbc.M302483200. [DOI] [PubMed] [Google Scholar]
- Coleman S H, Madrid R, Van D N, Mitchell R S, Bouchet J, Servant C, Pillai S, Benichou S, Guatelli J C. Modulation of cellular protein trafficking by human immunodeficiency virus type 1 Nef: role of the acidic residue in the ExxxLL motif. J Virol. 2006;80:1837–1849. doi: 10.1128/JVI.80.4.1837-1849.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]