Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially (original) (raw)
Abstract
Eosinophils are innate immune leukocytes implicated in the initiation and maintenance of type 2 immune responses, including asthma and allergy. The ability to store and rapidly secrete preformed cytokines distinguishes eosinophils from most lymphocytes, which must synthesize cytokine proteins prior to secretion and may be a factor in the apparent Th2 bias of eosinophils. Multiple studies confirm that human eosinophils from atopic or hypereosinophilic donors can secrete over 30 cytokines with a varying and often opposing immune-polarizing potential. However, it remains unclear whether all of these cytokines are constitutively preformed and available for rapid secretion from eosinophils in the circulation of healthy individuals or are restricted to eosinophils from atopic donors. Likewise, the relative concentrations of cytokines stored within eosinophils have not been studied. Here, we demonstrate that human blood eosinophils are not singularly outfitted with Th2-associated cytokines but rather, constitutively store a cache of cytokines with nominal Th1, Th2, and regulatory capacities, including IL-4, IL-13, IL-6, IL-10, IL-12, IFN-γ, and TNF-α. We demonstrate further rapid and differential release of each cytokine in response to specific stimuli. As agonists, strong Th1 and inflammatory cytokines elicited release of Th2-promoting IL-4 but not Th1-inducing IL-12. Moreover, a large quantity of IFN-γ was secreted in response to Th1, Th2, and inflammatory stimuli. Delineations of the multifarious nature of preformed eosinophil cytokines and the varied stimulus-dependent profiles of rapid cytokine secretion provide insights into the functions of human eosinophils in mediating inflammation and initiation of specific immunity.
Keywords: innate cells, immunomodulation, intracellular granule
INTRODUCTION
Eosinophils are innate immune leukocytes classically associated with type 2 immune responses, evoked by parasitic helminths and atopic diseases (e.g., asthma and allergy). Eosinophils participate in allergic diseases and helminthic infections as early initiators of polarized type 2 immunity and as effector cells in ensuing tissue responses. IL-4-producing eosinophils and basophils are rapidly recruited to tissues and contribute to type 2 responses following infection of wild-type and immunodeficient mice with the helminth Nippostrongylus brasiliensis [1,2,3]. Th2 cell IL-13 production is inhibited in the absence of eosinophils in OVA-sensitized mice [4], and murine eosinophils trafficking to draining lymph nodes function as APCs to preferentially promote Th2 differentiation [5,6,7,8]. In established allergic and anthelminthic responses, eosinophil-derived protein and lipid mediators act on tissue cells to exacerbate type 2 effector processes. These functions may at least partially reflect a Th2-favored cytokine potential of eosinophils, as suggested by observations of constitutive IL-4 and IL-13 mRNA expression early in eosinophil ontogeny and early IL-4 and IL-13 protein production by mature eosinophils [2].
Eosinophils are distinguished from most lymphocytes in their capacity for rapid cytokine secretion. The capability for immediate release of specific cytokines from eosinophils derives from the existence of intracellular stores of preformed cytokines [9], secreted through a regulated, vesicular-based process, termed piecemeal degranulation (PMD) [10, 11]. In addition to IL-4 and IL-13, human eosinophils can produce, store, and secrete over 30 cytokines, including Th1-associated IL-12 [12] and IFN-γ [13]. Whether all of these cytokines are constitutively stored in preformed caches within circulating human eosinophils from normal individuals and whether immune-polarizing cytokine stimulation might elicit rapid differential eosinophil cytokine secretion have not been studied. Here, we asked whether the purported Th2 bias of eosinophils might be associated with a predilection toward storage of preformed Th2 over Th1 cytokines within human circulating eosinophils or with a tendency favoring Th2-associated cytokine secretion. Toward this end, we investigated the relative abundance of seven cytokines [IL-4, IL-13, IL-6, IL-10, IFN-γ, IL-12(p70), TNF-α], all produced by human eosinophils and associated with Th1, Th2 (allergic and inflammatory subsets), and T regulatory responses. Our findings demonstrate that human blood eosinophils constitutively store all seven cytokines intracellularly, with IL-13, TNF-α, and IFN-γ detected in greatest abundance. Thus, eosinophils in the circulation are not armed solely with Th2-related mediators but rather, maintain an arsenal of cytokines with nominal Th1, Th2, and regulatory capacities. As agonists, strong Th1 and inflammatory cytokines elicited differential patterns of cytokine release from eosinophils, which consistently included IL-4. In contrast, the hallmark inducer of Th1 differentiation, IL-12, was not detected in response to Th1, Th2, or inflammatory stimuli, suggesting that under these conditions, secretion of Th2-promoting IL-4 is favored in immune-polarized microenvironments over Th1-inducing IL-12. However, all seven cytokines could be secreted rapidly from eosinophils in response to at least one of the stimuli analyzed, including IL-12, which was released in response to stimulation with the regulatory cytokine IL-10. These findings demonstrate preferential Th2 cytokine secretion by eosinophils in response to immune-polarizing cytokine signals and provide insights into the immunomodulatory potential of circulating human eosinophils. Moreover, we document a large overlay of IFN-γ secretion, paralleling IL-4 release from eosinophils in response to Th1, Th2, and inflammatory signals, highlighting an ill-appreciated identification of IFN-γ as a major product of human eosinophils.
MATERIALS AND METHODS
Eosinophil isolation
The Committee on Clinical Investigation approved the experiments, and informed consent was obtained from all subjects, which included normal donors (nine) and those with histories of mild atopy (nine). Total eosinophil recoveries and atopic status of individual donors are detailed online in Supplemental Table 1. The current study was not designed to evaluate the relationship of atopy status to cytokine content or release. Differences in eosinophil cytokine content or secretion could not be correlated with the atopic history of the donors (as a result of insufficient sample sizes of normal and atopic populations, making appropriate power analysis impossible). Granulocytes were isolated from blood of donors as described [14], with the exception that RBC lysis was omitted. Eosinophils were purified by negative selection, and contaminating RBCs were eliminated by antiglycophorin antibodies included in the negative selection eosinophil enrichment antibody cocktail (StemSep, StemCell Technologies, Vancouver, Canada). Eosinophil purity was >99%.
Subcellular fractionation
Freshly isolated eosinophils were lysed using nitrogen cavitation, and postnuclear supernatants were centrifuged over a density gradient (Optiprep™, Axis-Shield PoC, Norton, MA, USA), as described previously [15]. Fractions were collected from the bottom (heaviest) and frozen at –80°C until analysis. Thus, cytosolic components excluded from the gradient medium were found within the lightest fraction(s). We have previously localized cellular constituents within these subcellular fractions through quantification of eosinophil granule major basic protein (MBP) and β-hexosaminidase to identify granule-enriched fractions and analyses of arylsulfatase B and lactate dehydrogenase to localize small vesicles and cytosol, respectively (data not shown). Localizations of granule- and vesicle-enriched fractions were validated further through quantification of eosinophil peroxidase (EPO; see Fig. 1) and immunoblotting for detection of lysosome-assocaited membrane protein 2 (LAMP-2) and vesicle-associated membrane protein 2 (VAMP-2; Supplemental Fig. 1). Quantification of EPO was achieved by oxidation of O-phenylenediamine (OPD) in the presence of H2O2. Briefly, subcellular fractions were mixed 1:2 with a 2-mM OPD solution containing 0.1% Triton X-100, 1 mM H2O2, and 50 mM Tris and incubated in the dark. Reactions were stopped after 30 min by addition of 4 M sulfuric acid and OD measured at 492 nm. Antibody clone H-207 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-VAMP-2 rabbit antiserum (Stressgen, Canada) were used in immunoblotting experiments to detect LAMP-2 and VAMP-2, respectively.
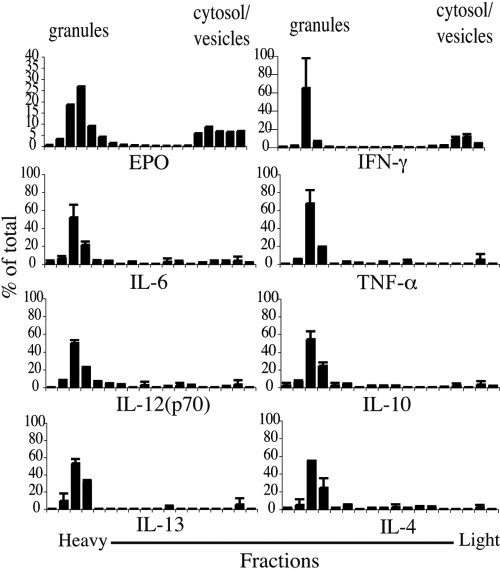
Fig. 1.
Preformed cytokines are stored within intracellular granules. Eosinophil subcellular fractions, prepared and evaluated per Materials and Methods, were analyzed by multiplex analysis for cytokine content. Fractions containing granules and small, less-dense vesicles and cytosol are indicated. Data are from eosinophils from one donor and are representative of studies of eosinophils from three donors.
Lysate preparation
Eosinophil lysates were prepared by resuspending freshly isolated eosinophils in lysis buffer containing 1% Triton X-100 to a final concentration of 5 × 107 cells/mL and rocking for 20 min at 4°C. Cleared supernatants were collected after centrifuging at 15,500 g for 20 min at 4°C and stored at –80°C until analysis.
Cell stimulation
Eosinophils were resuspended in 200 μL RPMI without phenol red (BioWhittaker, Walkersville, MD, USA), supplemented with 0.1% OVA (Sigma Chemical Co., St. Louis, MO, USA) at a final concentration of 3 × 106 cells/mL and incubated at 37°C ± recombinant (r)IL-12, rTNF-α, rIL-27, rIL-4, and rIL-13 (R&D Systems, Minneapolis, MN, USA) or rIFN-γ (BioSource, Camarillo, CA, USA) for the indicated times. Following stimulation, cell viability was confirmed by Trypan blue exclusion, and cell-free supernatants were collected and stored at –80°C until analysis.
ELISA and multiplex analyses
IFN-γ concentrations were determined from serial dilutions of eosinophil lysates using a standard sandwich ELISA protocol with anti-IFN-γ clone NIB42 (BD PharMingen, San Diego, CA, USA) to capture and biotinylated anti-IFN-γ clone 4S.B3 (BD PharMingen) to detect soluble IFN-γ. Multiplex analyses were performed on serial dilutions of whole eosinophil lysates using custom-designed BioPlex assays (BioRad, Hercules, CA, USA) for the simultaneous quantification of IFN-γ, IL-4, IL-6, IL-10, TNF-α, IL-13, and IL-12(p70). IL-5 was also included in initial multiplex analyses. However, only low quantities (∼3 pg/mL), close to the lower limits of assay sensitivity, were detected (_n_=4, data not shown). IL-12 (p40) concentrations were quantified from whole eosinophil lysates using a Beadlyte assay kit (Upstate, Charlottesville, VA, USA). Cell supernatants and subcellular fractions were assayed as undiluted samples using BioPlex assay kits. All multiplex data were acquired using the BioPlex System (BioRad). All samples were assayed in duplicate or triplicate wells.
Statistical analysis
Data are presented as the mean (±sd). The significances of differences between groups were analyzed using Student’s _t_-test. P values <0.05 were considered statistically significant.
RESULTS
Freshly isolated human eosinophils constitutively contain preformed Th1, Th2, and immunoregulatory cytokines
Human eosinophils are known to produce and secrete over 30 cytokines, chemokines, and growth factors with varying immunomodulatory and inflammatory potentials [9]. No studies have yet investigated the relative quantities of these various preformed cytokines within eosinophils or confirmed their availability in nonstimulated, circulating eosinophils. We investigated by multiplex analyses the abundance of seven preformed cytokine proteins [IL-4, IL-6, IL-10, IL-12(p70), IFN-γ, IL-13, and TNF-α], resident within whole cell lysates of freshly isolated eosinophils from normal and moderately atopic donors. As depicted in Table 1, IL-13 was the most abundant cytokine measured in eosinophils from every donor tested. Surprisingly, the cytokines second in abundance were Th1-associated IFN-γ and proinflammatory TNF-α. IFN-γ-specific ELISA assays of lysates from several donors confirmed the high levels of preformed IFN-γ content detected by multiplex analyses (data not shown). Levels of preformed TNF-α demonstrated the greatest range of variability between donors (nearly 100-fold), and concentrations of IL-4, IFN-γ, and IL-12(p70) remained the most consistent (less than or equal to threefold variance). Notably, antibodies used for the detection of the Th1 initiator IL-12 recognize only the p70 heterodimer, formed through the covalent interaction between p35 and p40 subunits. Hence, IL-12 is stored in its biologically active form within blood eosinophils. Intracellular IL-4 levels were consistently lower than all other cytokines detected, although this may reflect inefficiencies in IL-4 detection, as IL-4 has proven notoriously difficult to quantify [16].
TABLE 1.
Abundance of Preformed Cytokines within Human Eosinophil Lysates
| Donor # | IFN-γ | IL-4 | IL-6 | TNF-α | IL-10 | IL-12 (p70) | IL-13 |
|---|---|---|---|---|---|---|---|
| 1 | 565 (49) | 88 (25) | 135 (44) | 1135 (290) | 172 (6) | 251 (11) | 3970 (368) |
| 2 | 810 (0) | 85 (7) | 65 (7) | 40 (0) | 120 (14) | 160 (0) | 1670 (439) |
| 3 | 810 (0) | 70 (28) | 45 (7) | 20 (0) | 160 (21) | 130 (14) | 1330 (99) |
| 4 | 1455 (106) | 70 (42) | 165 (64) | 1815 (870) | 80 (42) | 160 (71) | 5557 (2515) |
| 5 | 1200 (170) | 115 (21) | 495 (21) | 525 (403) | 105 (35) | 140 (14) | 997 (361) |
| 6 | 1140 (552) | 160 (99) | 1230 (594) | 1920 (467) | 2095 (841) | 275 (21) | 14,050 (2461) |
Intracellular localization of preformed cytokines
Under physiological conditions, the most relevant mechanism of storage and secretion of preformed mediators from human eosinophils is a vesicular-based process, PMD, whereby a granule-stored cytokine is mobilized into budding vesicles, which travel to the plasma membrane for extracellular release [17,18,19]. Preformed stores of several cytokines, including IL-4 [20], IL-6 [21], IL-13 [22], and TNF-α [23], have been localized to intracellular granules within human eosinophils. However, specific intracellular localizations of IFN-γ, IL-12(p70), and IL-10 have not been confirmed. To verify that each of the intracellular cytokines that we detected in blood eosinophil lysates were stored within intragranular pools, subcellular fractionation was performed by layering eosinophil cavitates over an isotonic gradient to optimally preserve vesicular compartments. Cytokine concentrations were determined by multiplex analysis from the isolated fractions. As seen in Figure 1, the bulk of all seven cytokines colocalized to granule-enriched fractions [as identified by colocalization of EPO, MBP, and LAMP-2 and by electron microscopy analysis of isolated fractions (Fig. 1 and Supplemental Fig. 1, and data not shown)]. In addition to intragranular stores, EPO [24] and several cytokines, including IL-4 [18] and TGF-α [25], have been reported to reside within vesicles, suggestive of a putative “secretory-ready” mediator pool. Intriguingly, although the majority of IFN-γ signals colocalized to granule-enriched fractions (∼70%), a second peak of IFN-γ (∼20%) appeared within lighter fractions, corresponding to small vesicles and cytosolic proteins (Supplemental Fig. 1), presumably representing a pool of vesicle-contained cytokines (Fig. 1). Thus, IFN-γ resides in granular and vesicular compartments within circulating eosinophils.
Th1, Th2, and inflammatory and regulatory cytokines are secreted rapidly and differentially from eosinophils following specific cytokine stimulation
Upon identifying preformed, intracellular pools of Th1- and Th2-related cytokines within circulating eosinophils, we investigated the relative potential for rapid secretion (within 30–60 min) of these cytokines in response to immune-polarizing cytokine stimuli and found that all seven cytokines analyzed could be rapidly secreted from eosinophils in response to specific stimulation (Fig. 2). The following stimuli were used to represent Th1 (IL-12, IL-27, IFN-γ), Th2 (IL-4, IL-13), and inflammatory (TNF-α) and regulatory (IL-10) microenvironments. Supernatants collected after 30, 60, or 90 min of stimulation yielded secreted cytokine values within comparable ranges, as demonstrated in Supplemental Figure 2 for the most robust stimuli, IL-12 and TNF-α. Neither IL-27, an IL-12 family member implicated in the differentiation of Th1 [26] and IL-10-producing T regulatory type 1 cells [27] and in the inhibition of Th17 development [28, 29], nor IL-13, a major factor in Th2 immunity, elicited detectable release within 60 min of any cytokine measured (data not shown). As exemplified by meager secretion of TNF-α, despite its intracellular abundance, rapid secretion of cytokines from eosinophils did not merely reflect relative intracellular concentrations. Notably, as demonstrated in Supplemental Figure 3, profiles of cytokines secreted in response to specific cytokine stimulation were remarkably similar between donors.
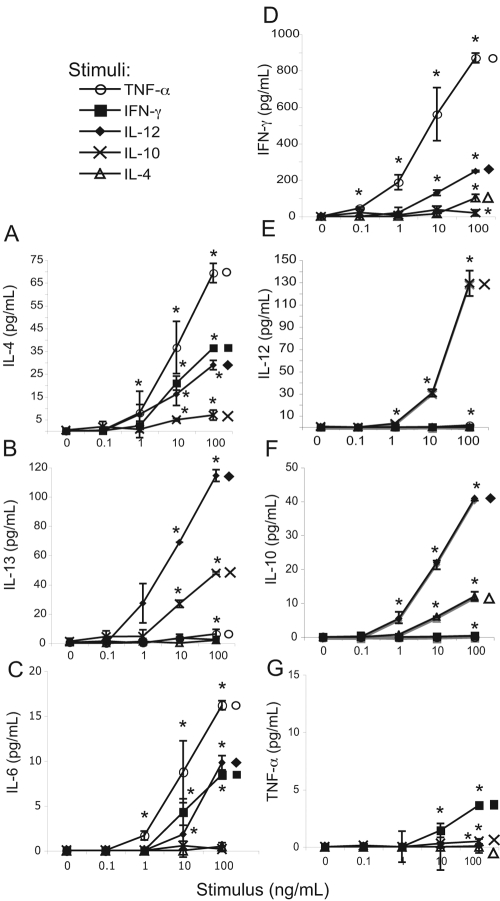
Fig. 2.
Dose-dependent release of cytokines from eosinophils in response to cytokine stimuli. Eosinophils (3×106/mL) were incubated for 30 min at 37°C with increasing doses of indicated cytokine stimuli. Following stimulation, cell-free supernatants were analyzed by multiplex analysis for the presence of IL-4 (A), IL-13 (B), IL-6 (C), IFN-γ (D), IL-12(p70) (E), IL-10 (F), and TNF-α (G). Data are presented as means (±sd) of duplicate or triplicate wells of one experiment representative of at least three independent experiments. *, P ≤ 0.05, versus nonstimulated controls.
In support of an inherent disposition toward Th2 immunity, IL-4 was readily secreted in response to Th1 and inflammatory stimuli at stimulus concentrations of 10–100 ng/mL (Fig. 2A and Supplemental Fig. 2). Despite some overlapping functions, release of IL-4 was not coupled to IL-13 secretion (compare Fig. 2, A and B). Unexpectedly, the Th1 product IFN-γ was also detected in eosinophil supernatants in response to Th1, inflammatory, and Th2 stimuli (Fig. 2D). Stimulations of IL-4, IL-6, and IFN-γ secretions were similar in a hierarchical pattern (TNF-α>IL-12>IFN/IL-4>IL-10) and in minimum stimulus concentration required, with the exception that IFN-γ secretion could be detected in response to a tenfold lower concentration of TNF-α (compare Fig. 2, A, C, and D).
Although IFN-γ may augment IL-12-induced Th1 differentiation, this cytokine is not a direct inducer of Th1 T cell polarization [30]. Of note, secretion of the Th1 initiatory cytokine IL-12 was not observed in response to Th1, Th2, or inflammatory stimuli (Fig. 2E), although preformed IL-12(p70) was detected within eosinophil lysates (Table 1). IL-12 secretion was detected in response to stimulation with anti-inflammatory IL-10 (Fig. 2E). Notably, IL-12 was the only cytokine detected in eosinophil supernatants following stimulation with IL-10 at 1 ng/mL. Reciprocally, IL-12 stimulation elicited the most robust IL-10 secretion (Fig. 2F).
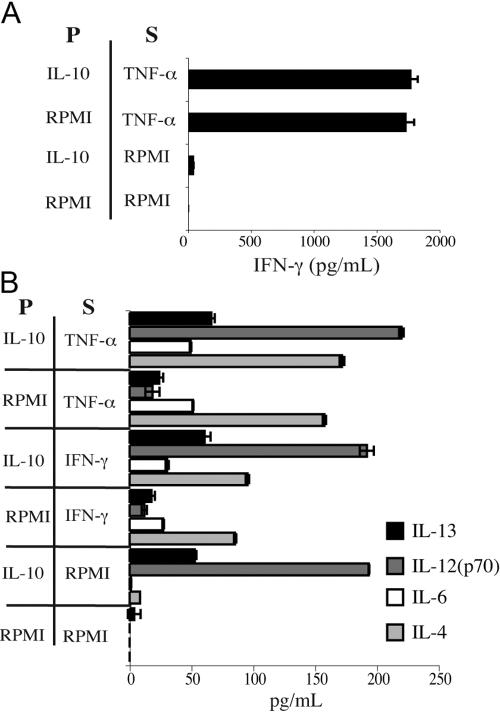
IL-10 is recognized for its inhibition of Th1 immunity, including targeted impairment of T cell IFN-γ [31] and monocyte TNF-α [32] production and inhibition of Staphylococcus aureus- or LPS-induced IL-12 production from human PBMC [33]. Thus, we investigated whether IL-10 pretreatment might dampen subsequent secretion of IFN-γ from human eosinophils. As shown in Figure 3A, IL-10 pretreatment did not affect TNF-α-induced secretion of IFN-γ from eosinophils. Moreover, in light of our unexpected finding of IL-10-induced IL-12(p70) secretion, we analyzed this response further to determine if IL-10 pretreatment might alter the type 2 bias of eosinophils upon subsequent cytokine stimulation. Notably, secretions of IL-4, IL-6, and IL-13 from eosinophils, elicited by IFN-γ or TNF-α, remained intact following pretreatment with IL-10 (Fig. 3B).
Fig. 3.
Pretreatment with IL-10 does not affect eosinophil cytokine secretion. Eosinophils (3×106/mL) were preincubated for 20 min at 37°C ± 100 ng/mL rIL-10 (P) prior to addition of 100 ng/mL rTNF-α or rIFN-γ for a subsequent 20 min stimulation (S). Cell-free supernatants were analyzed as above. Data are the means (±sd) of duplicate wells from one experiment, representative of three independent experiments.
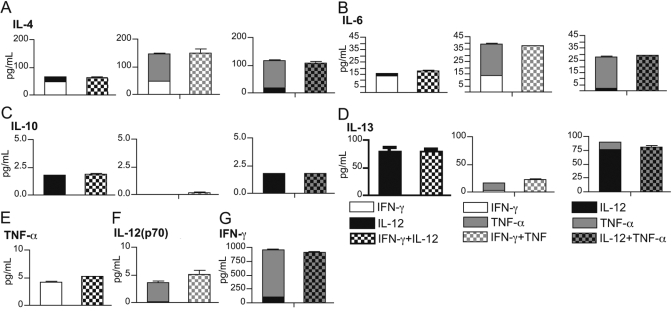
Eosinophils recruited to tissue sites in vivo may concurrently encounter myriad signals. Thus, to determine whether patterns of cytokine secretion observed in response to single stimuli are relevant to more complex in vivo responses, supernatants were recovered from eosinophils following incubation with IL-12, IFN-γ, and TNF-α, alone or in combination, and assayed for the secretion of IFN-γ, IL-4, TNF-α, IL-13, IL-6, IL-10, and IL-12(p70). Secreted cytokine concentrations in each case fell within the anticipated range for additive stimulus effects (Fig. 4), suggesting that synergy between cytokine stimuli does not influence rapid eosinophil cytokine release and demonstrating that eosinophils may respond simultaneously to multiple cytokine stimuli.
Fig. 4.
Simultaneous stimulation with multiple cytokines elicits additive effects on eosinophil cytokine secretion. Eosinophils (3×106 per mL) were stimulated with 100 ng/mL IFN-γ, IL-12, and TNF-α, alone or in various combinations, for 30 min at 37°C. Cell-free supernatants were analyzed as above for the presence of IL-4 (A), IL-6 (B), IL-10 (C), IL-13 (D), TNF-α (E), IL-12 (F), and IFN-γ (G). Means (±sd) of stimulation with individual cytokines are stacked and plotted beside results from the appropriate dual stimulation.
DISCUSSION
Eosinophils can participate in the initiation and maintenance of type 2 immune responses. One attribute of their immunobiology preparing eosinophils for these tasks is the ability to store preformed cytokines, available for immediate secretion. Although eosinophils are known to produce and secrete over 30 cytokines, the majority of these cytokines was identified within eosinophils isolated from tissues or from atopic or hypereosinophilic patients. In the present study, we queried whether human eosinophils, isolated directly from blood of normal or mildly atopic donors, might be predisposed toward the storage of Th2-associated cytokines, thus favoring early secretion of type 2 immune-polarizing signals upon entry into tissue sites. Moreover, we questioned whether rapid secretion of eosinophil-derived cytokines in response to immune-polarizing cytokine agonists might favor Th2-associated proteins.
Despite the association of eosinophils with Th2 immunity, we demonstrate that preformed cytokine stores within circulating human eosinophils are not restricted to Th2-related cytokines but rather, include cytokines with nominal Th1, Th2, and inflammatory and regulatory capacities, including the Th1 initiator, IL-12; the prime product of a Th1 response, IFN-γ; and proinflammatory TNF-α. In the case of the first, stored IL-12 was detectable in the biologically active p70 form. Moreover, we demonstrate that appropriate stimulation of human blood eosinophils can lead to the rapid, differential release of all seven cytokines examined. Importantly, eosinophil cytokine secretion was stimulus- and dose-dependent and revealed consistent patterns of cytokine release remarkably comparable among donors. Concentrations of cytokines secreted by eosinophils within 30 min of stimulation were relatively low (compared, for example, with T lymphocyte cytokine production), consistent with local functions of innate immune leukocytes within sequestered microenvironments, as opposed to systemic effects mediated by T cell cytokine secretion.
Notably, secretion of cytokines was stimulus-specific, demonstrating the differential nature of early cytokine release from human eosinophils. Mechanisms governing transduction of exogenous stimulatory signals and the differential sorting, packaging, and secretion of granule-stored cytokines from within eosinophils remain to be fully elucidated. However, toward this end, our lab has previously demonstrated participation of intracellular lipid signals in specific cytokine secretion from eosinophils [34] and implicated cognate receptor-mediated vesicular transport as a means of sorting and mobilizing specific granule-stored cytokine targets [15]. A series of studies by Moqbel and Coughlin [11] reveals that appropriate trafficking of cargo-loaded secretory vesicles is likely mediated through specific Rab-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-mediated vesicle movement, specifically involving the eosinophil secretory vesicle-expressed SNARE VAMP-2.
Despite preformed, intragranular stores of IL-12(p70) and IL-4, human blood eosinophils responded to Th1 and proinflammatory cytokine stimuli dose-dependently with secretion of IL-4 but not IL-12. In the same hierarchical order, these cytokine stimuli also elicited IL-6 secretion from eosinophils. In addition to proinflammatory effects, IL-6 has been shown to inhibit Th1 and encourage Th2 differentiation through induction of naïve T cell IL-4 production [35]. Thus, eosinophils trafficking to cytokine-rich T cell zones of lymph nodes (as observed for endobronchial eosinophils [5]) may inherently encourage Th2 differentiation, even in the presence of IL-12, by converting type 1-inducing IL-12 signals into Th2-promoting IL-4 and IL-6. Intracellular, preformed levels of IL-12, available for rapid secretion, are likely not the limiting factor in our analyses of rapid release, as in addition to stored IL-12(p70), intracellular concentrations of IL-12(p40) subunits within eosinophils from four donors ranged from 70 to 1000 pg/mL, and rIL-10 stimulation induced rapid release of IL-12(p70) heterodimers (Fig. 2E). Indeed, we previously demonstrated IL-12 secretion within 1 h of cross-linking membrane CD9 or leukocyte Ig-like receptor 7 receptors [36]. Grewe et al. [12] demonstrated release of biologically active IL-12 from human eosinophils, preceded by IL-12(p40) and p35 mRNA induction, following 24 h of stimulation with IL-4, suggesting newly formed IL-12 may be released at later time-points and highlighting the likelihood for time-dependent alteration of eosinophil cytokine secretion profiles that may reflect compound functions of eosinophil-derived cytokines early in initiating an immune response as opposed to during established tissue responses.
Notably, TNF-α proved a potent stimulus, eliciting secretion of IL-4, IL-6, and IFN-γ from eosinophils. TNF-α was shown recently to be essential for IFN-γ-induced secretion of Th1-type chemokines and to enhance IL-4-induced secretion of Th2-type chemokines by human eosinophils [37].
Unexpectedly, in parallel to IL-4, a large quantity of IFN-γ, the classic product of Th1 cells, was released from eosinophils in response to cytokine stimulation. Early secretion of IFN-γ may at least partially reflect its presence within an extragranular, vesicular compartment (Fig. 1), as was established previously for IL-4. Rapid secretion of IFN-γ from eosinophils may be especially intriguing in light of the emerging, paradoxical view of IFN-γ as a master regulator of inflammation, implicated as a pro- and anti-inflammatory mediator (reviewed in ref. [38]), and suggests eosinophil secretion of IFN-γ may be more prominent than anticipated previously.
Quite unanticipated was the finding of IL-10-induced secretion of IL-12 from human eosinophils, as IL-10 is typically antagonistic to Th1 responses [31,32,33, 39]. It is plausible that eosinophil-derived IL-12 may function primarily as an autocrine factor, inducing the release of IL-4 and IL-13 from eosinophils through a feedback loop (see Fig. 2, A and B). The extent and significance of autocrine recapture of eosinophil-derived cytokines remain important subjects yet to be delineated.
These results establish the multifarious-preformed cytokine potential of circulating human eosinophils and document rapid, differential cytokine release from human blood eosinophils elicited by exogenous cytokine stimuli. The strong elicitation of eosinophil-derived cytokines in response to inflammatory TNF-α, IL-12, and IFN-γ alone or in combination suggests that eosinophils infiltrating inflamed tissues contribute rapidly to the cytokine milieu. Moreover, preferential secretion of IL-4 and IL-6 over IL-12 in response to Th1 and inflammatory stimuli suggests eosinophils recruited into tissues and lymph nodes may help to balance early type 1 immune-inducing signals to promote Th2 immunity. These findings provide insights into the functions of human eosinophils pertinent to an evolving understanding of the critical contributions of eosinophilic leukocytes to inflammatory processes and to initiation of type 2 immunity.
Supplementary Material
[Supplemental figures and table]
Acknowledgments
This work was supported in part by National Institutes of Health grants AI20241, HL70270, and AI051645 to P. F. W. and an Interest Section and Faculty Development Awards to L. A. S. from the American Academy of Allergy, Asthma and Immunology.
References
- Shinkai K, Mohrs M, Locksley R M. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Shinkai K, Locksley R M. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan E S, Urban J F, Jr, Dvorak A M, Finkelman F D, LeGros G, Paul W E. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes J, Yang M, Mahalingam S, Kuehr J, Webb D C, Simson L, Hogan S P, Koskinen A, McKenzie A N, Dent L A, Rothenberg M E, Matthaei K I, Young I G, Foster P S. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H Z, Humbles A, Gerard C, Jin Z, Weller P F. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie J R, Mattes J, Dent L A, Foster P S. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167:3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- Padigel U M, Lee J J, Nolan T J, Schad G A, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun. 2006;74:3232–3238. doi: 10.1128/IAI.02067-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H B, Ghiran I, Matthaei K, Weller P F. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- Lacy P, Moqbel R. Eokines: synthesis, storage and release from human eosinophils. Mem Inst Oswaldo Cruz. 1997;92:125–133. doi: 10.1590/s0074-02761997000800017. [DOI] [PubMed] [Google Scholar]
- Moqbel R, Coughlin J J. Differential secretion of cytokines. Sci STKE. 2006;2006:pe26. doi: 10.1126/stke.3382006pe26. [DOI] [PubMed] [Google Scholar]
- Grewe M, Czech W, Morita A, Werfel T, Klammer M, Kapp A, Ruzicka T, Schopf E, Krutmann J. Human eosinophils produce biologically active IL-12: implications for control of T cell responses. J Immunol. 1998;161:415–420. [PubMed] [Google Scholar]
- Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon γ): inhibition by immunoglobulin A complexes. J Exp Med. 1999;190:487–495. doi: 10.1084/jem.190.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira-Melo C, Gillard G, Ghiran I, Weller P F. EliCell: a gel-phase dual antibody capture and detection assay to measure cytokine release from eosinophils. J Immunol Methods. 2000;244:105–115. doi: 10.1016/s0022-1759(00)00264-7. [DOI] [PubMed] [Google Scholar]
- Spencer L A, Melo R C, Perez S A, Bafford S P, Dvorak A M, Weller P F. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci USA. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech J T, Bainbridge T, Thompson S J. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997;205:163–168. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- Dvorak A M, Furitsu T, Letourneau L, Ishizaka T, Ackerman S J. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. Part I. Piecemeal degranulation of specific granules and distribution of Charcot-Leyden crystal protein. Am J Pathol. 1991;138:69–82. [PMC free article] [PubMed] [Google Scholar]
- Melo R C, Spencer L A, Perez S A, Ghiran I, Dvorak A M, Weller P F. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo R C, Perez S A, Spencer L A, Dvorak A M, Weller P F. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005;6:866–879. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqbel R, Ying S, Barkans J, Newman T M, Kimmitt P, Wakelin M, Taborda-Barata L, Meng Q, Corrigan C J, Durham S R, Kay A B. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995;155:4939–4947. [PubMed] [Google Scholar]
- Lacy P, Levi-Schaffer F, Mahmudi-Azer S, Bablitz B, Hagen S C, Velazquez J, Kay A B, Moqbel R. Intracellular localization of interleukin-6 in eosinophils from atopic asthmatics and effects of interferon γ. Blood. 1998;91:2508–2516. [PubMed] [Google Scholar]
- Woerly G, Lacy P, Younes A B, Roger N, Loiseau S, Moqbel R, Capron M. Human eosinophils express and release IL-13 following CD28-dependent activation. J Leukoc Biol. 2002;72:769–779. [PubMed] [Google Scholar]
- Beil W J, Weller P F, Tzizik D M, Galli S J, Dvorak A M. Ultrastructural immunogold localization of tumor necrosis factor-α to the matrix compartment of eosinophil secondary granules in patients with idiopathic hypereosinophilic syndrome. J Histochem Cytochem. 1993;41:1611–1615. doi: 10.1177/41.11.8409368. [DOI] [PubMed] [Google Scholar]
- Dvorak A M, Ackerman S J, Furitsu T, Estrella P, Letourneau L, Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vesicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am J Pathol. 1992;140:795–807. [PMC free article] [PubMed] [Google Scholar]
- Egesten A, Calafat J, Knol E F, Janssen H, Walz T M. Subcellular localization of transforming growth factor-α in human eosinophil granulocytes. Blood. 1996;87:3910–3918. [PubMed] [Google Scholar]
- Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak T W, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Carrier Y, Peron J P, Bettelli E, Kamanaka M, Flavell R A, Kuchroo V K, Oukka M, Weiner H L. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Batten M, Li J, Yi S, Kljavin N M, Danilenko D M, Lucas S, Lee J, de Sauvage F J, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- Stumhofer J S, Laurence A, Wilson E H, Huang E, Tato C M, Johnson L M, Villarino A V, Huang Q, Yoshimura A, Sehy D, Saris C J, O'Shea J J, Hennighausen L, Ernst M, Hunter C A. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Wenner C A, Guler M L, Macatonia S E, O'Garra A, Murphy K M. Roles of IFN-γ and IFN-α in IL-12-induced T helper cell-1 development. J Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]
- Moore K W, de Waal Malefyt R, Coffman R L, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- De Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira-Melo C, Woods L J, Phoofolo M, Weller P F. Intracrine cysteinyl leukotriene receptor-mediated signaling of eosinophil vesicular transport-mediated interleukin-4 secretion. J Exp Med. 2002;196:841–850. doi: 10.1084/jem.20020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedla N, Bandeira-Melo C, Tassinari P, Sloane D E, Samplaski M, Cosman D, Borges L, Weller P F, Arm J P. Activation of human eosinophils through leukocyte immunoglobulin-like receptor 7. Proc Natl Acad Sci USA. 2003;100:1174–1179. doi: 10.1073/pnas.0337567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L Y, Bates M E, Jarjour N N, Busse W W, Bertics P J, Kelly E A. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-α. J Immunol. 2007;179:4840–4848. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]
- Zhang J. Yin and yang interplay of IFN-γ in inflammation and autoimmune disease. J Clin Invest. 2007;117:871–873. doi: 10.1172/JCI31860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissert S, Hosoi J, Grabbe S, Asahina A, Granstein R D. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J Immunol. 1995;154:1280–1286. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental figures and table]