Oligoclonal Myelin-reactive T-cell Infiltrates Derived from Multiple Sclerosis Lesions are Enriched in Th17 Cells (original) (raw)
. Author manuscript; available in PMC: 2020 Jan 15.
Published in final edited form as: Clin Immunol. 2008 Nov 5;130(2):133–144. doi: 10.1016/j.clim.2008.08.030
Abstract
In this study, acute and chronic brain and spinal cord lesions, and normal appearing white matter (NAWM), were resected post-mortem from a patient with aggressive relapsing-remitting multiple sclerosis (MS). T-cell infiltrates from the central nervous system (CNS) lesions and NAWM were separated and characterized in-vitro. All infiltrates showed a proliferative response against multiple myelin peptides. Studies of the T-cell receptor (TCR)Vβ and Jβ usage revealed a very skewed repertoire with shared complementarity-determining region (CDR)3 lengths detected in all CNS lesions and NAWM. In the acute lesion, genomic profiling of the infiltrating T-cells revealed up-regulated expression of TCRα and β chain, retinoic acid-related orphan nuclear hormone receptor C (RORC) transcription factor, and multiple cytokine genes that mediate Th17 cell expansion. The differentially expressed genes involved in regulation of Th17 cells represent promising targets for new therapies of relapsing-remitting MS.
Keywords: human, T-cells, demyelinating CNS inflammatory lesions, autoimmunity
Introduction
Inflammatory cells play a central role in MS lesions development [1, 2]. Therefore, their detailed characterization in the CNS lesions at various stages of progression provides a framework for better understanding the pathogenesis of the disease [3]. Studies of human CNS lesions, and particularly of the infiltrating inflammatory cells, have been hampered by the limited availability of tissue from active MS lesions, the small number of infiltrating inflammatory cells, and technical challenges involved in their separation and in-vitro expansion [4].
Longitudinal studies of auto-reactive cells in the peripheral circulation have determined that the chronic inflammatory response in MS is mediated by oligoclonal T-cell populations [5]. Studies of the TCRVβ repertoire of peripheral blood mononuclear cells (PBMC)s and cerebrospinal fluid (CSF)-derived T-cells from MS patients have identified a “skewed” repertoire, suggesting that a limited number of T-cell clones drive the chronic immune response in MS [6]. These auto-reactive cells persist in the circulation for years and expand during clinical relapses [7]. As the blood brain barrier becomes permeable, those selected T-cells migrate to the multiple CNS lesions during disease exacerbations.
The present study has identified, for the first time, the presence of myelin-reactive T-cells in CNS demyelinating lesions and NAWM, a skewed TCRVβ repertoire dominated by a limited number of T-cell clones in all CNS regions, and gene and protein expression profiles of T-cells infiltrating CNS MS lesions at various stages of progression.
Results
Inflammatory Cells are Prominent in the Acute CNS Lesion, Persist in the Chronic Lesions, and Diffusely Infiltrate the NAWM
A twelve-year-old African-American female presented initially with a subacute paraparesis. Magnetic resonance imaging (MRI) scan of the brain revealed multiple T2 and contrast-enhancing lesions, while subsequent spinal cord imaging revealed T2 signal abnormalities at C6 and Th4-Th8 levels, with patchy contrast enhancement, all consistent with disseminated demyalinating disease. CSF analysis detected elevated IgG synthesis and oligoclonal bands. Five months after the initial presentation, the patient developed acute optic neuritis. Due to the involvement of the spinal cord and optic nerves, a differential diagnosis of neuromyelitis optica (NMO) was considered [8; 9]. Unfortunately, since serum sample was not available for the NMO Ab testing, it was not possible to completely rule out this variant of disseminated demyelinating disease. Since the initial studies demonstrated multiple inflammatory contrast-enhancing brain lesions and positive CSF studies, and subsequent gene expression studies documented aquaporin-4 expression in the NAWM and thoracic lesion tissue, we established a diagnosis of aggressive CNS inflammatory demyelinating disease, consistent with MS. Aggressive MS with opticospinal involvement is reported with higher frequency in African American patients in than in Caucasian Americans [10], while NMO is more prevalent in oriental population. One month prior to the last hospitalization, the patient experienced difficulties with swallowing and dysarthria, suggestive of brainstem disease activity. Emergent admission was arranged following respiratory failure. A brain MRI scan obtained three days prior to death revealed new right pontine hyperintense T2 and fast fluid-attenuated inversion-recovery signal abnormalities suggestive of an inflammatory demyelinating lesion. According to the patient’s living will and the family’s decision, respiratory support was withdrawn, and an autopsy was performed 6 h after death.
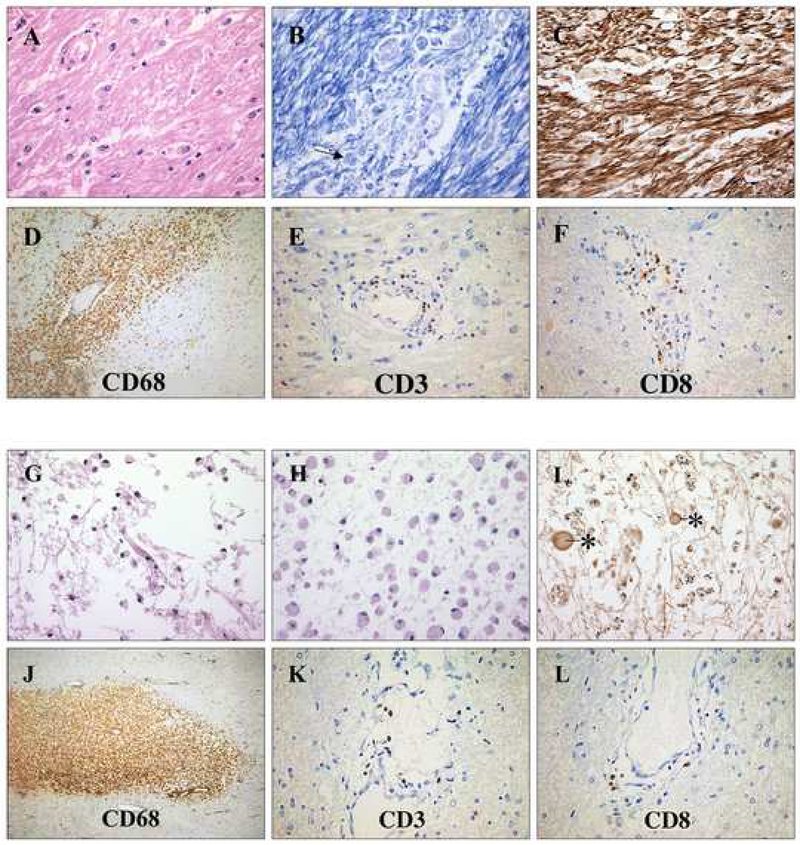
Lesions were harvested at the autopsy based on the MRI findings and macroscopic pathological localization. The acute pontine (AP) lesion, identified only on the final MRI scan, was hypercellular (Figure 1A) with an abundance of CD68+ macrophages (Figure 1D) and CD3+, predominantly CD8+ lymphocytes (Figure 1E and F). In these regions, acute myelin degradation was signified by macrophages containing Luxol-fast blue (LFB) -positive intracellular material (Figure 1B), while the axons were relatively preserved (Figure 1C). A chronic periventricular (CP) lesion, identified four years earlier by MRI scan, was well-demarcated, hyperintense on T2 and hypointense on T1 scans. It was histologically characterized by chronic inflammatory infiltrate (Figure 1G), a completely demyelinated cystic lesion center (Figure 1H), and severe axonal loss (Figure 1I). Inflammatory infiltrate was less prominent when compared to the AP lesion (Figure 1J, K, L). A chronic thoracic (CTh) lesion was identified in spinal cord segments Th4–8. Microscopic evaluation revealed increased vascularity, minimal inflammatory infiltrate, and extensive demyelination and axonal loss. NAWM tissue obtained from right frontal lobe contained diffuse macrophage and predominantly CD8 lymphocitic infiltrate. Threfore, the histopathological studies confirmed the CNS inflammatory demyelinating disease consistent with MS.
Figure 1.
Histopathological characteristics of the AP and CP MS lesions. A) Hematoxylin and Eosin staining of the AP lesion detected perivascular inflammatory infiltrates, activated microglia and a decreased number of oligodendrocytes. B) LFB staining revealed active demyelination with phagocytosed LFB-positive myelin within macrophages (arrow). C) Axonal staining showed a relatively preserved axonal network. D), E) and F) The perivascular inflammatory infiltrate was composed of CD68+ macrophages and predominantly CD8+ lymphocytes. G) The CP lesion showed cystic changes with scattered mononuclear cells. H) Myelin staining revealed complete demyelination. I) Axonal staining showed profound axonal loss with axonal swelling and spheroids (asterisk). J), K) and L) The residual inflammatory infiltrate, which was less prominent when compared to the AP lesion, was composed of CD68+ macrophages and CD3+ lymphocytes.
Inflammatory Demyelinating CNS Lesions and NAWM Infiltrates are Enriched in Myelin-reactive T-cells
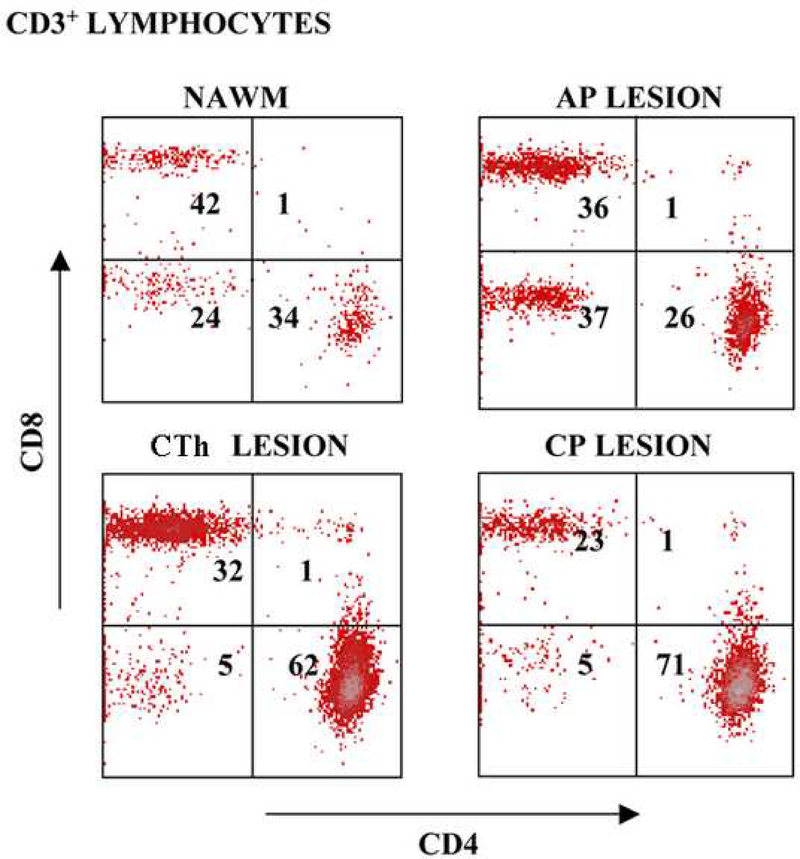
Post-mortem flow cytometry studies on non-manipulated T-cell infiltrates contained predominantly CD45RO+ memory cells: 93% of CD3+ cells in the NAWM, 97% in the AP lesion, and 98% of cells in the CP lesion. Ten days following the initial phytohaemaglutinin (PHA) stimulation, the analysis of CD3+ gated cells derived from the NAWM and the AP lesion contained higher number of CD8+ lymphocytes (CD4+ to CD8+ ratios of 0.8 and 0.6, respectively), while infiltrates from the CTh and CP lesions contained higher percentage of CD4+ cells (CD4+ to CD8+ ratios of 1.9 and 3.1, respectively), as presented in Figure 2. In order to ensure a sufficient number of cells for further studies, the T-cells were expanded with PHA over three rounds of re-stimulation.
Figure 2.
Phenotypic characterization of lesion infiltrates. Flow cytometry plots represent CD3+ gated viable cells derived from acute and chronic lesions upon PHA expansion (10 days). CD4+ and CD8+ cell percentages of gated cells are indicated in the plot.
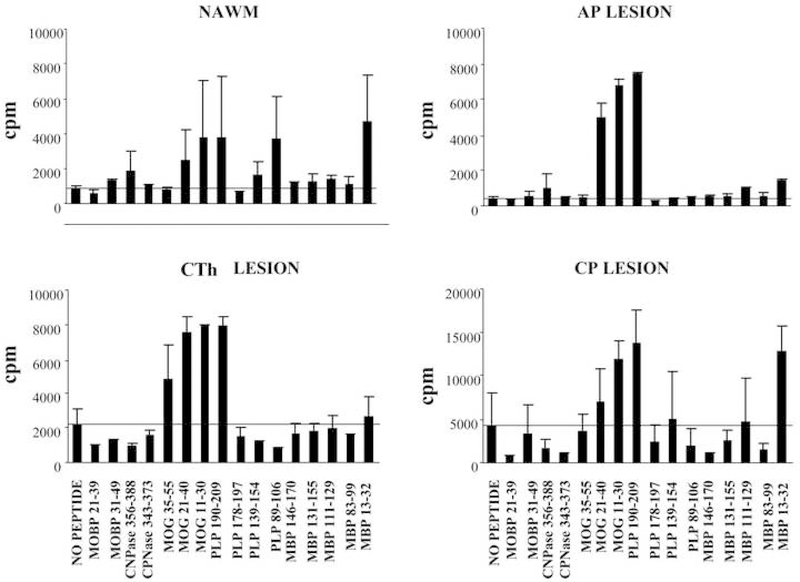
The antigen specificity of NAWM and inflammatory demyelinating lesion-derived T-cells was tested against a panel of 13 immunodominant myelin-derived peptides, identified in studies of MS patients’ T-cell reactivity and in studies of experimental autoimmune encephalomyelitis (EAE) [2], Figure 3. The highest proliferative response was detected against proteolipid protein (PLP) 190–209 in the AP lesion, which also induced a proliferative response (stimulation index, SI>2) in the CTh, CP lesions, and NAWM. Cells derived from NAWM exhibited a proliferative response against six myelin peptides: cyclic nucleotide phosphodiesterase (CNPase) 356–388, myelin oligodendrocyte glycoprotein (MOG) 21–40, MOG 11–30, PLP 190–209, PLP 89–106, and myelin basic protein (MBP) 13–32. The AP lesion infiltrate shared the proliferative response against 5 peptides, with an additional response against MBP 111–129. MOG 35–55, MOG 21–40, MOG 11–30 and PLP 190–209 peptides induced a proliferative response by the T-cells derived from the CTh lesion, and MOG 11–30, PLP 190–209 and MBP 13–32 peptides by the T-cells derived from the CP lesion. The frequency of antigen-specific cells (~10–4) was estimated by dividing a number of positive wells (SI>2) with a number of plated cells per individual antigen.
Figure 3.
Proliferation of lesion-infiltrating T-cells against myelin peptides. The antigen specificity of NAWM and MS-lesion-derived T-cells was tested against a panel of 13 immunodominant myelin-derived peptides. The results are presented as average cpm from three wells±SD.
CNS T-cell Infiltrates Have a Highly Restricted TCRVβ Repertoire
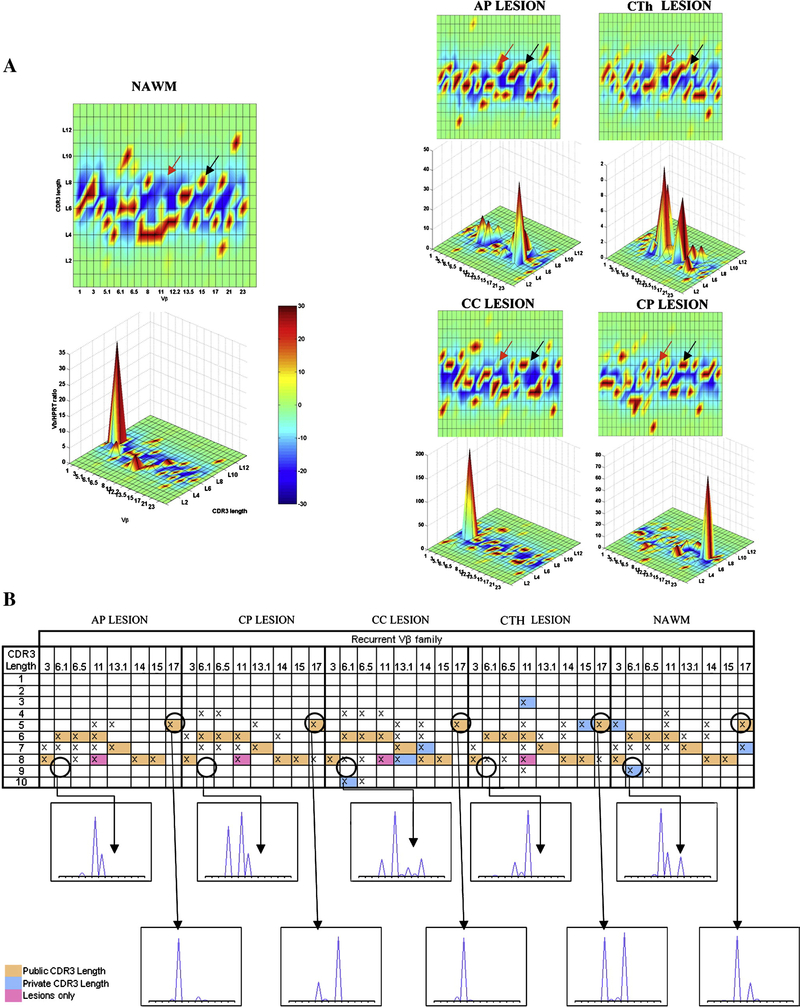
In order to characterize the TCR usage of infiltrating cells derived from CNS demyelinating lesions and NAWM, we used Immunoscope® and Tclandscape® technologies. Cell infiltrates derived from NAWM, AP, CTh, chronic cervical (CC), and CP lesions displayed highly altered TCRVβ repertoires (red spots on Figure 4A). The global percentages of CDR3-LD alterations reached 60.54% for the AP lesion, 51.75% for the CTh, 64.66% for the CC, and 58.25% for the CP lesion. Interestingly, the NAWM sample also yielded high selection of TCRVβ (67.33% CD3-LD alteration). The cell infiltrates from the five different CNS regions showed CDR3-LD alterations associated with high Vβ mRNA accumulation. In the AP lesion, Vβ17 with a CDR3 length of five amino-acids showed a large amount of mRNA, as indicated by a Vβ/HPRT ratio of 45. This Vβ family corresponded to a skewed Vβ family infiltrating all the lesions and NAWM (“shared” alteration). Vβ mRNA accumulations were also seen in NAWM (Vβ2), in the CP lesion (Vβ21) and in the spinal cord lesions (Vβ6.1, Vβ6.5, Vβ8 and Vβ13.1 for the CTh; Vβ 5.1 for the CC lesion). The results are presented as the TcLandscapes in Figure 4A. Such profiles represent highly restricted repertoires, indicating an extremely high T-cell clonal selection.
Figure 4.
TCRVβ repertoire of infiltrating T cells derived from MS lesions and NAWM. A) Alterations of CDR3-LD are represented in a global bi-dimensional and a three-dimensional representation. The X axis displays the 26 human Vβ families, the 13 possible CDR3 lengths per Vβ family are on the Y axis, and the Vβ/HPRT transcript ratios are on the Z axis. The percentages of alterations are represented by a color code ranging from deep blue (≤ −30%) to dark red (≥ +30%) in the integrated landscapes. Black arrows indicate an example of “shared” alteration (shared CDR3 length of eight amino-acids for Vβ15 in all the lesions and NAWM). Red arrows indicate an example of lesion alteration (Vβ11 with a CDR3 length of eight amino-acids present in all lesions, but not in NAWM). B) Recurrent Vβ and CDR3 length distributions were detected in multiple infiltrates from MS lesions and NAWM. The figure presents 8 Vβ families containing shared CDR3 lengths of expanded infiltrating cells in five tested CNS regions. Shared alterations (CDR3 lengths shared by all CNS regions) are highlighted by orange boxes; shared alterations present in MS lesions, but not in NAWM are presented as pink boxes; while private alterations, corresponding to a CDR3 length that is present only in one studied lesion are presented as blue boxes.
To ensure that these selective profiles were not related to the short-term PHA expansion, control PBMCs derived from 5 RR MS patients were expanded with PHA over 1.5 months and tested for TCRVβ repertoire by TcLandscapes. These control experiments identified polyclonal profiles on the TCR landscape after PHA expansion (data not shown). In contrast, the T-cells derived from CNS inflammatory demyelinating lesions and NAWM exhibited very restricted oligoclonal repertoires, indicating that CNS infiltrating cells are selected prior to PHA expansion. Furthermore, a TcLandscape performed on the RNA samples derived from non-manipulated NAWM tissue also revealed high degree of T-cell clonal selection (data not shown) consistent with the report by Muraro et al. [11].
In order to analyse the presence of individual TCRVβ clones in NAWM and in different CNS lesions, the CDR3-LD profiles were compared within each family. Figure 4B shows eight Vβ families (Vβ3, 6.1, 6.5, 11, 13.1, 14, 15 and Vβ17) containing transcripts with CDR3 lengths shared by all lesions and the NAWM cell infiltrates (referred to as “shared” alterations; Figure 4B, orange boxes). These shared alterations represented 3 % of the total CDR3 lengths present in all CNS regions (eight out of 240 CDR3 lengths), while 16.25 % (39 out of 240 CDR3 lengths) corresponded to “private” CDR3 lengths (i.e. present only in one studied region; Figure 4B, blue boxes). The alterations shared by all lesions but not NAWM (1.25 %, three out of 240 CDR3 lengths), corresponded to Vβ11, Vβ 21 and Vβ 22 (Figure 4B, pink boxes). Therefore, most of the CDR3 lengths (79.5%, 190 out 240 CDR3 lengths) were present in two or three different CNS regions. However, each CDR3 length may contain several CDR3 sequences and does not necessarily correspond to a single clonotype. In order to address this possibility, we performed a Jβ analyses of the two Vβ families (Vβ15 and Vβ15, shared by all CNS regions. We identified the presence of identical CD3 lengths in five CNS regions (Jβ 1.4 and Jβ 2.5 for Vβ15; Jβ 2.2 for Vβ17), confirming the presence of shared T-cell clones between all CNS regions.
Genomic and Proteomic Profiling of the T-cell Infiltrates Reveal Accumulation of Th17 Cells in the Acute MS Lesion
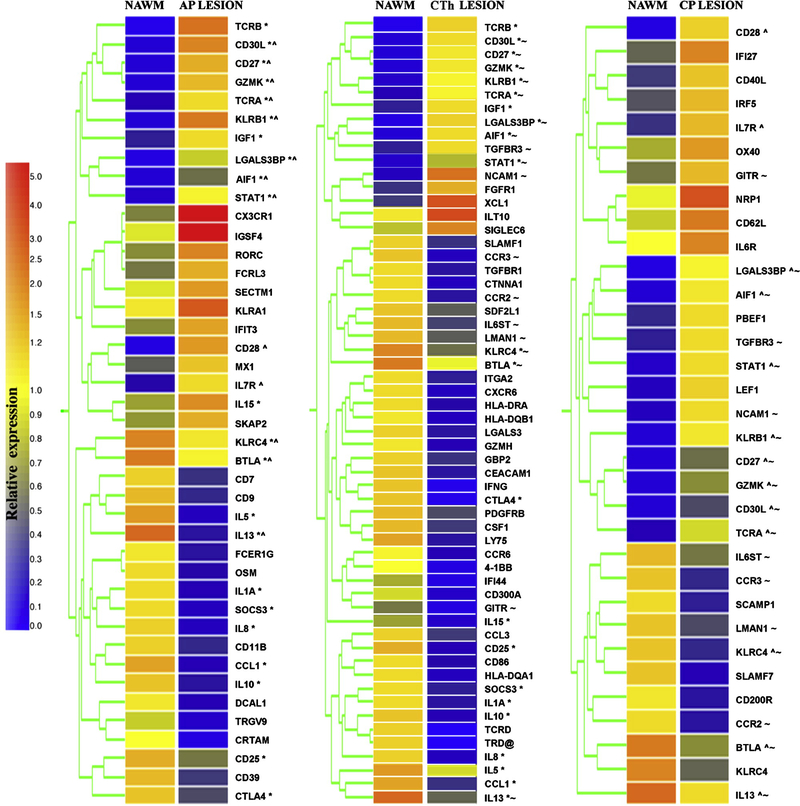
Studies of EAE have established that activated T-cells entering CNS play a critical role in the disease development, warranting their detailed gene expression profiling. However, gene array (GA) studies of lesion tissue have identified immune response (IR) genes at a significantly lower proportion (20%) of differentially expressed genes (DEGs) than studies of PBMCs (40–50%) [12], reflecting the relatively low numbers of inflammatory cells within the CNS. In order to study the gene expression profiles of T-cells infiltrating inflammatory CNS lesions, we focused on differentially expressed IR genes in T-cells infiltrating AP, CTh and CP lesions in comparison to NAWM. NAWM was used as a reference, since it also contained diffuse cellular infiltrate that allowed comparison with the gene expression profiles from the lesion cell infiltrates. While NAWM does not represent a normal brain parenchyma, it is the best reference for the gene expression profiles from inflammatory demyelinating lesions’ cellular infiltrates in an individual patient. Figure 5 summarizes the results for IR genes with >5-fold change in expression, while the remaining DEGs are listed in the Supplementary Table 1.
Figure 5.
Gene expression profiling of T-cell infiltrates derived from MS lesions in comparison to NAWM. Tree graphs summarize the gene expression results for IR genes with >5-fold change in MS lesions in comparison to NAWM. They represent normalized expression levels for all IR DEGs in colored bars and the clustering of genes according to their expression patterns. Superscript symbols denote DEGs in multiple lesions: *denotes DEGs shared between AP and CTh lesion, ^denotes DEGs shared between AP and CP lesion, and ~ denotes DEGs shared between CTh and CP lesion. Complete gene names are listed in the Supplementary Table 1.
All three lesions shared 11 differentially expressed IR genes, as indicated in Figure 5. While the shared DEGs reflect an inflammatory process common for MS lesions at various stages of progression, differentially expressed genes in the acute lesion characterize the immune response that initiates inflammatory demyelinating lesion formation. We focused on IR DEGs in the AP lesion and extended analysis to the genes with >1.5-fold changes in comparison to NAWM, as presented in Table 1. Gene expression profiling of the AP lesion infiltrate revealed up-regulation of TCRVβ and TCRα, but a decreased expression of TCR gamma chain TCRGV9. Signaling molecule gene expression changes included the up-regulation of RORC and STAT1, while regulatory T-cell markers forkhead box protein P3 (FOXP3) and IL2RA were down-regulated. Cytokines/cytokine receptors showed the strongest up-regulation of IL-15, followed by IL-7R, both implicated in the long-term survival of memory cells. IL-17D and IL-26, which are secreted by Th-17 cells, were up-regulated, while IFN-γ, IL-4, and IL-10, which inhibit Th17-cell differentiation, were down-regulated in the AP lesion. The Th2 cytokines IL-4, IL-5 and IL-13 were inhibited in all three lesions, with the strongest down-regulation in the AP lesion. Gene expression for chemokines and their receptors in the AP lesion revealed up-regulation of the Th1 chemokine receptors CX3CR1 and CCR5, and down-regulation of Th2 chemokines CCL3, CCL1, and the chemokine receptor CCR8. Among the co-stimulatory molecules, we detected upregulation of CD30L, CD28, CD27, CD69, CD40L, and the glucocorticoid-induced TNF receptor, and down-regulation of the inhibitory co-stimulatory molecules B and T lymphocyte attenuator (BTLA) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).
Table 1.
Gene expression profiling of the T-cell infiltrate derived from the AP lesion. The table lists cytokines/receptors, chemokines/receptors, co-stimulatory and signalling molecules whose gene expression was changed in T-cell infiltrates derived from the AP lesion. In order to include all genes of interest, we extended the analysis to >1.5-fold changed genes in each MS lesion in comparison to NAWM.
| Name | Gene Bank | Description | Fold Changes in Gene Expression in the Lesion vs. NAWM | ||
|---|---|---|---|---|---|
| AP | CTh | CP | |||
| Cytokines/Receptors | |||||
| IL-15 | NM_000585 | Interleukin-15 | 8.3 | −5.6 | 1.7 |
| IL7R | NM_002185 | Interleukin-7 receptor | 7.4 | −2.4 | 7.9 |
| TGFBR1 | AV00621 | TGFB receptor 1 | 4.6 | 8.0 | 3.1 |
| IL18BP | AI521549 | Interleukin-18 binding protein | 4.5 | 1.3 | 2.0 |
| IFNGR1 | AI458949 | Interferon gamma receptor 1 | 3.9 | 2.9 | 1.6 |
| IL17D | AI669535 | Interleukin-17D | 3.6 | 1.1 | −2.2 |
| IL26 | NM_018402 | Interleukin- | 3.0 | 1.0 | 1.3 |
| IL11RA | NM_004512 | Interleukin-11 receptor, alpha | 2.8 | 4.2 | 1.8 |
| IL16 | M90391 | Interleukin-16 | 2.7 | 1.5 | −1.7 |
| IFNAR2 | AI653318 | Interferon alpha receptor 2 | 2.5 | 4.9 | 2.2 |
| IL12RB2 | R01220 | Interleukin-12 receptor B2 | 2.2 | 3.2 | 2.4 |
| IFNAR1 | NM_000629 | Interferon alpha receptor 1 | 2.0 | 1.4 | 1.0 |
| IL21R | NM_021798 | Interleukin-21 receptor | −2.1 | −1.5 | 2.8 |
| ILST | AB015706 | Interleukin-6 signal transducer, gp130 | −2.2 | −7.4 | −5.2 |
| IL4 | NM_000589 | Interleukin-4 | −2.2 | 1.2 | −1.5 |
| IFNG | M29383 | Interferon gamma | −4.0 | −99.5 | −1.9 |
| IL2RA | K03122 | Interleukin-2 receptor, alpha | −7.4 | −18.3 | −2.3 |
| IL1A | M15329 | Interleukin-1. alpha | −9.3 | −6.7 | 1.6 |
| IL10 | NM_000572 | Interleukin-10 | −9.4 | −11.6 | −1.4 |
| IL8 | NM_000584 | Interleukin-8 | −11.4 | −12.3 | 4.3 |
| IL5 | NM_000879 | Interleukin-5 | −37.9 | −6.3 | −5.0 |
| IL13 | NM_002188 | Interleukin-13 | −42.2 | −21.2 | −7.8 |
| Chemokines/Receptors | |||||
| CX3CR1 | U20350 | Chemokine (C-X3-C motif) receptor 1 | 92.0 | 1.2 | 1.1 |
| CCR5 | NM_000579 | Chemokine (C-C motif) receptor 5 | 2.1 | 1.0 | 1.1 |
| CCL4 | NM_002984 | Chemokine (C-C motif) ligand 4 | −2.3 | −2.5 | −1.2 |
| CCL28 | AW083576 | Chemokine (C-C motif) ligand 28 | −2.9 | −1.9 | −3.2 |
| CCR8 | NM_005201 | Chemokine (C-C motif) receptor 8 | −3.1 | 4.0 | 1.0 |
| XCL1 | NM_003175 | Chemokine (C motif) ligand 2 | −3.5 | 7.0 | 1.0 |
| CCL3 | NM_002983 | Chemokine (C-C motif) ligand 3 | −3.8 | −5.3 | −1.4 |
| CCL1 | NM_002981 | Chemokine (C-C motif) ligand 1 | −24.3 | −13.9 | −2.8 |
| Costimulatory Molecules | |||||
| TNFSF8/CD30L | AI936516 | Tumor necrosis factor (ligand) superfamily, member 8 | 81.4 | 17.7 | 5.2 |
| CD28 | NM_006139 | CD28 molecule | 78.8 | 1.0 | 29.0 |
| TNFRSF7/CD27 | NM_001242 | Tumor necrosis factor receptor superfamily, member 7 | 37.7 | 15.1 | 6.2 |
| TNFSF13B/CD257 | AW151360 | Tumor necrosis factor receptor superfamily, member 13b | 4.5 | −1.7 | 1.7 |
| CD69 | BF439675 | CD69 molecule | 4.0 | 6.0 | 3.1 |
| TNFSF5/CD40L | NM_000074 | Tumor necrosis factor (ligand) superfamily, member 5 | 3.9 | 1.3 | 6.5 |
| CD5 | AI797836 | CD5 molecule | 3.8 | −1.7 | −1.1 |
| TNFSF13 | BF448647 | Tumor necrosis factor (ligand) superfamily, member 13 | 3.5 | 1.8 | 1.1 |
| CD97 | NM_001784 | CD97 molecule | 3.1 | 1.1 | −1.1 |
| CD53 | AI022073 | CD53 molecule | 2.9 | 1.0 | 1.0 |
| TNFRSF11A/CD265 | AW026379 | Tumor necrosis factor receptor superfamily, member 11a | 2.8 | 1.7 | −1.2 |
| CD200R | AF497548 | Cell surface glycoprotein receptor CD200 | 2.5 | −1.7 | −2.4 |
| CD37 | NM_001774 | CD37 molecule | 2.4 | 1.3 | 1.2 |
| TNFRSF18/GITR | AF117297 | Tumor necrosis factor receptor superfamily, member 18 | 2.3 | −8.0 | 5.6 |
| TNFRSF14, HVEM | BC002794 | Tumor necrosis factor receptor superfamily, member 14 | 2.2 | 1.0 | 1.2 |
| TNFRSF10B/CD262 | AF016266 | Tumor necrosis factor receptor superfamily, member 10b | 2.2 | 2.5 | 1.4 |
| CD48 | AI378026 | CD48 molecule | 2.1 | 2.5 | 1.7 |
| CD14 | AA993683 | CD14 molecule | 2.0 | −1.6 | −2.0 |
| CD32 | J04132 | CD32 molecule | 2.0 | −1.7 | −1.4 |
| CD58 | D28586 | CD58 molecule, lymphocyte function-associated antigen 3 | −2.3 | −2.8 | −1.6 |
| CD80 | AY081815 | CD80, B7–1 molecule | −2.3 | 1.3 | 1.3 |
| CD226 | NM_006566 | CD226 molecule | −2.3 | −2.2 | −1.9 |
| CD109 | AL110152 | CD109 molecule | −2.7 | 1.6 | −1.3 |
| CD86 | L25259 | CD86, B7–2 molecule | −2.7 | −6.6 | 1.3 |
| TNFRSF8/CD30 | NM_001243 | Tumor necrosis factor receptor superfamily, member 8 | −3.0 | −1.5 | −1.2 |
| TNFSF11/CD254 | AF053712 | Tumor necrosis factor receptor superfamily, member 11 | −3.4 | −1.6 | 1.1 |
| CD74 | M28590 | CD74 molecule | −3.8 | −8.4 | 1.6 |
| CD7 | NM_006137 | CD7 molecule | −5.3 | −2.1 | −1.7 |
| CTLA4 | U90273 | Cytotoxic T-lymphocyte-associated protein 4 | −7.9 | −15.8 | −3.1 |
| BTLA | AW294080 | B and T lymphocyte associated | −8.3 | −9.3 | −12.9 |
| CD9 | NM_001769 | CD9 molecule | −10.3 | −3.0 | −3.5 |
| Signaling Molecules | |||||
| RORC | AI218580 | Retinoic acid-related orphan nuclear hormone receptor C | 11.3 | 1.0 | 1.0 |
| STAT1 | AI539443 | Signal transducer and activator of transcription 1 | 9.6 | 6.5 | 12.8 |
| SOCS2 | NM_003877 | Suppressor of cytokine signaling 2 | 2.1 | −1.1 | −1.4 |
| STAT6 | BC004973 | Signal transducer and activator of transcription 6 | 2.1 | 2.2 | 1.3 |
| FOXP3 | AF277993 | Forkhead box P3 | −3.7 | −3.5 | −1.7 |
| SOCS3 | AI244908 | Suppressor of cytokine signaling 3 | −10.5 | −6.4 | 1.2 |
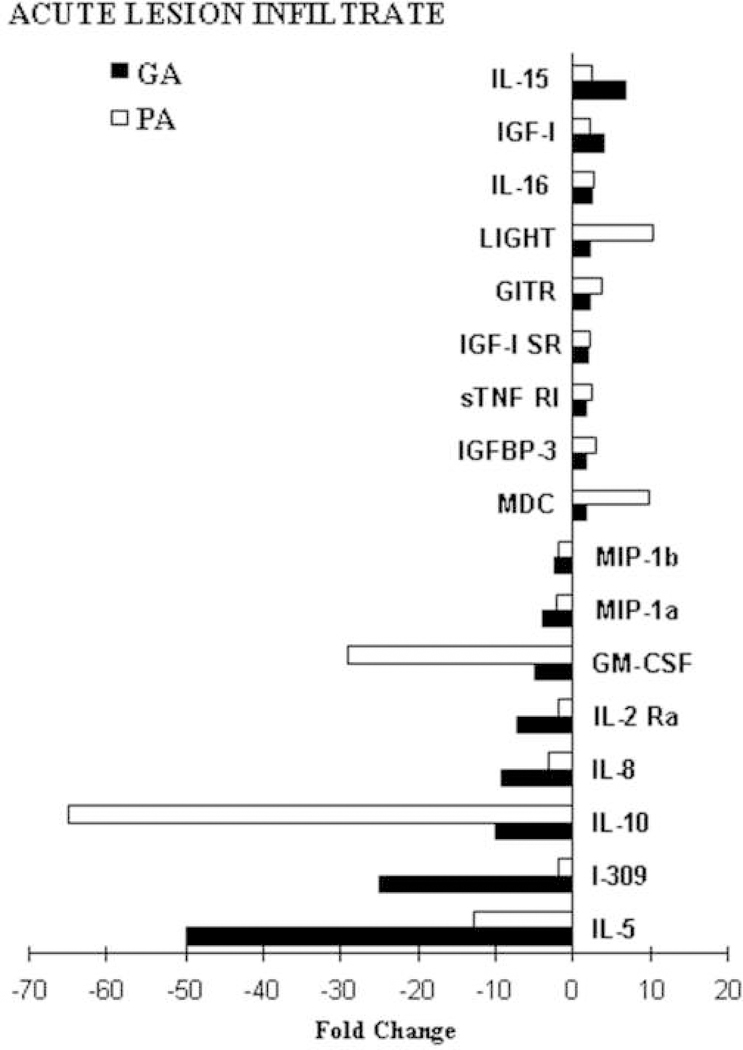
Protein array (PA) analysis of secreted cytokines and their receptors by the T-cells derived from the AP lesion revealed increased levels of TNF-β, IL-1α, TGF-β3, IL-1Ra, IL-1β, IL-12 p70, IL-16, and IL-12p40. IL-5 and IL-10 were decreased in the supernatants of T-cells derived from the AP lesion in comparison to the NAWM. PA results on 17 proteins from the supernatant of T-cells infiltrating the AP lesion confirm the gene array data from the same cultures (>1.5-fold change in both gene and protein expression), including the IL-15 and IL-16 increase and IL-10 and IL-5 decrease in comparison to the NAWM, as presented in Figure 6.
Figure 6.
Protein expression profiling of the T-cell infiltrate derived from the AP MS lesion. Gene array results from T-cell infiltrate derived from the acute MS lesion were compared to protein array data obtained from the supernatants of cells used for GA analysis. GA results for 17 genes were confirmed at the protein level for the genes and proteins with fold changes >1.5-fold.
Discussion
We have characterized, for the first time, the inflammatory T-cells obtained post-mortem from MS lesions at various stages of progression, and primarily focused on the T-cell infiltrate derived from an acute lesion whose onset correlated with the neurological symptoms of the fatal clinical exacerbation. Immunohistologically, the acute lesion showed prominent perivascular and parenchimal inflammatory infiltrates. When analyzed by flow cytometry, the infiltrate contained predominantly CD45RO+CD8+ cells, consistent with previous studies of MS lesion tissue [13; 14]. Despite its brief duration, the acute lesion was characterized by active demyelination with myelin proteins-laden macrophages, decrease in oligodendrocyte numbers, and axonal transection, confirming that demyelination and axonal transection are early events in MS lesion formation [15; 16]. The analysis of the CP lesion, whose presence was documented by MRI over four years, indicates that inflammatory cells persist in MS lesions as a chronic cell depository. CD45RO+ memory cells constituted nearly all of the T-cells derived from NAWM and MS lesions, suggesting that their selective migration into the CNS is determined by their previous exposure to antigens. In support of that, we detected a high frequency of T-cells reactive to multiple myelin peptides in the infiltrates from all CNS regions. Consistent with reports on myelin antigens recognition in the peripheral circulation [17], PLP 190–209 elicited the strongest proliferative response by T-cells derived from the acute lesion, and induced a (SI>2) proliferative response by the cells derived from other lesions and NAWM.
TCRVβ repertoire studies of T-cells infiltrating MS lesions and NAWM revealed restrictive clonal selection. We can only speculate whether clonal selection occurs as T-cells cross the blood brain barrier or only selected clones remain in the lesion due to recurrent antigen recognition. Our studies of the TCRVβ repertoire in multiple MS lesions and NAWM revealed a skewed repertoire dominated by a limited number of clones. Comparison of the CDR3-LD profiles of MS lesions and NAWM revealed 8 Vβ families containing transcripts with CDR3 lengths shared among all tested CNS regions. Those shared CDR3 alterations represent 3% of the total CDR3 lengths, while most of the CDR3 lengths are present in two or more different CNS lesions. Our results establish the presence of shared CDR3 lengths from the same Vβ and Jβ families in different CNS regions, suggesting closely related clonotypes and their role in MS lesion development.
The low numbers of infiltrating T-cells harvested from the MS lesions required PHA polyclonal expansion in order to study transcriptional profiles of the CNS-infiltrating inflammatory cells. While studies of the TCRVβ repertoire using in-vitro PHA expansion provide CDR3 spectra that are similar to the results from the amplified RNA derived from non-manipulated cells [11], the short-term PHA culture might have introduced some bias in T-cell selection from the original lymphocyte culture, and thus may have affected the level of lymphocyte activation of the expanded cultures. In this respect, the extremely high level of clonal selection must be interpreted with caution, even though non-manipulated NAWM tissue also exhibited a very skewed TCRVβ repertoire. The very high level of selected CDR3 lengths in the expanded culture suggests a high proportion of T-cells selected by common antigen. The estimated frequency of ~1×10–4 myelin-reactive T-cells in the CNS infiltrates is significantly higher than the reported 1×10–6 precursor frequency of MBP and PLP-reactive cells in the peripheral blood of MS patients [18].
In order to detect patterns of gene expression changes at various stages of MS lesion progression, we performed Affymetrix gene array analysis of the T-cells infiltrating the AP, CTh, CP lesion, and NAWM. Differentially expressed IR genes in the acute lesion reflect characteristics of the inflammatory response initiating an autoimmune response within the CNS. TCRβ and TCRα genes displayed increased expression in the AP lesion, while TCR gamma chain 9 showed higher expression in the NAWM, consistent with decreased gamma-delta T-cell-mediated apoptosis of encephalitogenic T-cells in an acute lesion [19]. Markers of Treg cells (CD25 (IL2RA) and FOXP3) are also up-regulated in the NAWM in comparison to the acute lesion. Up-regulation of RORC, a transcription factor that orchestrates Th17 differentiation and induces IL17 production [20], as well as cytokines secreted by Th17 cells (IL-17D and IL-26) [21] reflect the accumulation of this cell subset in the acute lesion. IL-15, a cytokine that induces IL-17 production [22; 23], is up-regulated in the acute lesion infiltrate, consistent with a report of increased expression of IL-15 in PBMCs and CSF-derived mononuclear cells in MS patients [24]. Suppressor of cytokine signaling 3, a major inhibitor of IL-23-mediated STAT3 phosphorylation and Th17 generation [25], has increased expression in the NAWM, consistent with a negative regulation of the JAK/STAT signaling pathway. The T-cells derived from the AP lesion exhibit increased STAT-1 expression, reflecting the role of Th1 cells in the inflammatory CNS response and/or a significant cell subset that produces both Th1 and Th17 cytokines in humans [26]. In contrast, we identified a relatively higher expression of cytokines that inhibit Th17 expansion, including IFN-γ, IL-4, and IL-10 in the NAWM infiltrate, as well as the Th2 cytokines IL-5 and IL-13. The expression of the IL-7R gene, recently associated with susceptibility to MS [27], is up-regulated in the acute lesion infiltrate. Among the co-stimulatory molecules, the elevated gene expression of CD28, CD27, and CD30L and a decreased gene expression of the inhibitory molecules BTLA and CTLA-4 indicate a T-cell activated state.
Our results indicate that immune dysregulation governs the inflammatory response and lesion formation in MS, as illustrated by the accumulation of Th17 cells in the acute lesion and higher expression of genes for anti-inflammatory cytokines, inhibitory co-stimulatory molecules, regulatory gamma-delta TCRD9 chain-positive cells and Treg genes (FOXP3) in NAWM, reflecting a breakdown of the immune tolerance in MS lesions. The study identified several molecules involved in Th17 cell differentiation, which may serve as selective targets for new therapies in RR MS.
Materials and Methods
MS Lesion Resection and Phenotypical Characterization of T-cell Infiltrates
Brain and spinal cord tissue was obtained six hours post mortem from a 16-year-old African American female who suffered from an aggressive RR MS. The study was approved by an institutional review committee and was performed upon obtaining a signed consent by family members. Five lesions at various stages of progression and areas of NAWM were identified by MRI over a four-year clinical course. The final MRI study, obtained three days prior to death, was correlated with the macroscopic tissue exam at the time of post-mortem lesion resection. Hematoxylin-Eosin, LFB and silver impregnation staining were used to demonstrate cell morphology, myelin and axons. Inflammatory infiltrates were analyzed using immunohistochemical stains: CD68 for macrophages and CD3, CD4 and CD8 for T-lymphocytes, as reported previously [28].
Upon resection, the tissue was immediately homogenized and incubated with 0.25% Trypsin for 1 h at 37°C. Inflammatory cells were separated using Percoll gradient centrifugation and stained with the following antibodies: CD3-Cy, CD4-FITC, CD8-PE, CD45RO-FITC, CCR7-FITC, CD27-FITC and CD28-FITC (all from BD Bioscience, San Diego, CA), as described previously [29]. Ten days after an initial PHA expansion, the cells were stained again for more detailed quantitative analysis of T-cells upon acquisition of 10 000 events.
In order to obtain sufficient cell numbers for further studies, we expanded the CNS infiltrating lymphocytes by PHA polyclonal activation, as this method allows a 1000-fold increase in cell number over three cycles of re-stimulation. Cells were plated at 104 cells/well (Costar, Cambridge, MA) with 105/well irradiated allogenic feeders (PBMCs) and PHA (Remel, Lenexa, KS) at 1 μg/ml in media consisting of: RPMI 1640; 10% human serum (Gemini Bioproducts, Woodland, CA); 1% penicillin/streptomycin; 2% glutamine; 1% NEAA; 1% HEPES buffer and sodium piruvate. Medium was exchanged and 100 IU/ml of human rIL-2 (NCI, Bethesda, MD) was added every 3–4 days. T-cell cultures were expanded every 14 days in-vitro with PHA and allogenic feeders, and after three rounds of re-stimulation, sufficient cell numbers were obtained for detailed characterization.
CNS-infiltrating T-cells’ Proliferative Response Against Myelin-derived Peptides
T-cells derived from MS lesions and NAWM were plated in triplicates at 104 cells/well in 96-well plates in serum free medium (Opti MEM, Gibco, Grand Island, NY). Peptide recognition was measured in a split-well assays at 10 μg/ml, as previously described [17]. Irradiated PBMCs from a donor matched for the patient’s major histocomaptiblity complex class II allele DRB1*0701/09 were used as antigen presenting cells at105 cells/well. A proliferative response was considered antigen-specific when stimulation index (SI) = cpm response to peptide/cpm background was >2.
TCRVβ Repertoire Analysis
The concentration and quality of RNA derived from cells infiltrating the MS lesions and NAWM were determined using nanoRNA Chips (Agilent®, Edinburgh, UK). cDNA was obtained from 2 μg of RNA reverse-transcribed using an Invitrogen cDNA synthesis kit (Boehringer Mannheim, Indianapolis, IN). CDR3 was amplified by quantitative polymerase chain reaction (PCR) using 26 different Vβ primers and a Cβ primer in a 9700 thermocycler (Applied Biosystems, Foster City, CA). The PCR fragments’ migration in acrylamide gel gave the distribution of the different CDR3 lengths, and analyses were performed using Immunoscope® software [30]. The percentage of CDR3 length distribution (CDR3-LD) alterations for each Vβ family and the global percentage of CDR3-LD alteration for each sample were calculated as described previously [31]. Briefly, the percentage of alteration was defined as the difference between the frequency of each CDR3 length in the distribution profile of the studied Vβ family and the average control distribution calculated from 14 healthy individuals [6]. The magnitude of the CDR3-LD alteration compared to controls is displayed as a bi-dimensional TopView and the global three-dimensional TcLandscape® [32]. MatLab® 5.3 software (The MathWorks Inc., Natick, MA) was used to compute and display the data.
Gene Expression Profiling of T-cells Derived from MS Lesions and NAWM
In order to simultaneously capture the complex changes of gene expression in T-cells derived from the CNS inflammatory demyelinating lesions, we used Affymetrix Human Genome U133 (HG-U133) arrays. The arrays contain ~45,000 probe sets representing more than 39,000 transcripts derived from approximately 33,000 human genes. Briefly, 5×106 resting cells were cultured in serum-free medium over 24 h, and the total RNA was isolated by RNAeasy Kit (Quiagen, Valencia, CA). Seven μg of total RNA was used for cDNA synthesis using a custom cDNA kit (Life Technologies, Gaithersburg, MD). The arrays were hybridized for 16 hours at 45° C in a GeneChip Hybridization Oven 640, washed, stained with R-phycoerythrin streptavidin in a GeneChip Fluidics Station 400, and scanned with a Hewlett Packard GeneArray Scanner. The results were expressed as the relative expression for the individual gene, normalized per chip. Genes were considered differentially expressed when >5-fold change in the gene expression was detected in T-cells derived from an MS lesion in comparison to the NAWM. Gene tree clustering was carried out using Gene Spring software (Agilent Technologies, Palo Alto, CA). The gene expression results were confirmed by real-time PCR.
Profiling of Proteins Secreted by T-cells Derived from MS Lesions And NAWM
5×106/ml T-cells derived from CNS inflammatory demyelinating lesions and NAWM were cultured in serum-free medium for 24 h, as described above. Supernatants were collected and quantitative analysis of 120 secreted proteins was performed using human cytokine protein arrays (Ray Biotech, Norcross, GA) according to the manufacturer’s instructions. Briefly, after blocking, membranes were incubated with 1 ml of supernatant sample in 1:250 diluted biotin-conjugated primary antibodies, followed by the incubation with 1:1000-diluted HRP-conjugated streptavidin and the detection buffer. Membranes were exposed to Premium Autoradiography Film (Denville Scientific Inc., Metuchen, NJ). The films were scanned with an EPSON Expression 1680 Scanner (Epson America Inc., Long Beach, CA), and the signal intensities quantified using Meta Imaging Series 5.0 software (Molecular Devices, Downingtown, PA). The lowest measurement on the membrane was subtracted from all measured proteins as the background, and the results were normalized by the sum of optical density values for all proteins on the membrane. Results were expressed as a fold change of the normalized protein level in the supernatant of T-cells derived from the MS lesion in comparison to NAWM. Fold-change of >1.5 in both gene expression and the level of secreted protein was considered confirmatory.
Supplementary Material
01
Supplementary Table 1. DEGs in AP, CTh, and CP lesion-derived T-cells in comparison to the NAWM. The table presents fold changes and the frequencies of identical genes with >5-fold change detected by replicates of the same probe, and genes coding for the same protein detected with different probes on the array.
Acknowledgements
The study was supported by NIH grant NS045871-04 (S.M.P.) and National Multiple Sclerosis Society Center Award (S.M.P. and X.Z.). We thank Dr W. Brueck (Institute for Neuropathology, Göttingen, Germany) for performing the immunohistochemistry staining. Dr. T. W. Bouldin (Department of Pathology) and J. M. Smrtka (Department of Neurology, University of North Carolina at Chapel Hill) provided an invaluable help during the autopsy. We thank Drs. W. Powers, R. Meeker and M. Sospedra for critical reading of the manuscript.
Abbreviations
NAWM
normal appearing white matter
MS
multiple sclerosis
CNS
central nervous system
TCR
T-cell receptor
CDR
complementarity-determining region
RORC
retinoic acid-related orphan nuclear hormone receptor C
PBMC
peripheral blood mononuclear cells
CSF
cerebrospinal fluid
MRI
magnetic resonance imaging
NMO
neuromyelitis optica
AP
acute pontine
LFB
luxol fast blue
CP
chronic periventricular
CTh
chronic thoracic
PHA
phytohemagglutinin
EAE
experimental autoimmune encephalomyelitis
PLP
proteolipid protein
SI
stimulation index
CNPase
cyclic nucleotide phosphodiesterase
MOG
myelin oligodendrocyte glycoprotein
MBP
myelin basic protein
CC
chronic cervical
GA
gene array
IR
immune response
DEGs
differentially expressed genes
FOXP3
forkhead box protein P3
BTLA
B and T lymphocyte attenuator
CTLA-4
cytotoxic T-lymphocyte-associated protein 4
PA
protein array
PCR
polymerase chain reaction
Footnotes
Conflict of interest: The authors do not have conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sospedra M, and Martin R, Immunology of multiple sclerosis. Annu Rev Immunol 23 (2005) 683–747. [DOI] [PubMed] [Google Scholar]
- [2].Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, McFarland HF, and Martin R, Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 6 (2000) 1167–75. [DOI] [PubMed] [Google Scholar]
- [3].Markovic-Plese S, and McFarland HF, Immunopathogenesis of the multiple sclerosis lesion. Curr Neurol Neurosci Rep 1 (2001) 257–62. [DOI] [PubMed] [Google Scholar]
- [4].Arenz M, Herzog-Hauff S, Meyer zum Buschenfelde KH, and Lohr HF, Antigen-independent in vitro expansion of T cells does not affect the T cell receptor V beta repertoire. J Mol Med 75 (1997) 678–86. [DOI] [PubMed] [Google Scholar]
- [5].Muraro PA, Wandinger KP, Bielekova B, Gran B, Marques A, Utz U, McFarland HF, Jacobson S, and Martin R, Molecular tracking of antigen-specific T cell clones in neurological immune-mediated disorders. Brain 126 (2003) 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laplaud DA, Ruiz C, Wiertlewski S, Brouard S, Berthelot L, Guillet M, Melchior B, Degauque N, Edan G, Brachet P, Damier P, and Soulillou JP, Blood T-cell receptor beta chain transcriptome in multiple sclerosis. Characterization of the T cells with altered CDR3 length distribution. Brain 127 (2004) 981–95. [DOI] [PubMed] [Google Scholar]
- [7].Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, Wekerle H, Hohlfeld R, and Goebels N, Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A 101 (2004) 2428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wingerchuk DM, Hogancamp WF, O’Brien PC, and Weinshenker BG, The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 53 (1999) 1107–14. [DOI] [PubMed] [Google Scholar]
- [9].Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, and Weinshenker BG, Revised diagnostic criteria for neuromyelitis optica. Neurology 66 (2006) 1485–9. [DOI] [PubMed] [Google Scholar]
- [10].Cree BA, Khan O, Burdette D, Goodin DS, Cohen JA, Marrie RA, Glidden D, Weinstock-Guttman B, Reich D, Patterson N, Haines JL, Pericak-Vance M, DeLoa C, Oksenberg JR, and Hauser SL, Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 63 (2004) 2039–2045. [DOI] [PubMed] [Google Scholar]
- [11].Muraro PA, Cassiani-Ingoni R, Chung K, Packer AN, Sospedra M, and Martin R, Clonotypic analysis of cerebrospinal fluid T cells during disease exacerbation and remission in a patient with multiple sclerosis. J Neuroimmunol 171 (2006) 177–83. [DOI] [PubMed] [Google Scholar]
- [12].Comabella M, and Martin R, Genomics in multiple sclerosis--current state and future directions. J Neuroimmunol 187 (2007) 1–8. [DOI] [PubMed] [Google Scholar]
- [13].Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, and Rajewsky K, Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med 192 (2000) 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Junker A, Ivanidze J, Malotka J, Eiglmeier I, Lassmann H, Wekerle H, Meinl E, Hohlfeld R, and Dornmair K, Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain 130 (2007) 2789–99. [DOI] [PubMed] [Google Scholar]
- [15].Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, and Bo L, Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338 (1998) 278–85. [DOI] [PubMed] [Google Scholar]
- [16].Barnett MH, and Prineas JW, Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55 (2004) 458–68. [DOI] [PubMed] [Google Scholar]
- [17].Markovic-Plese S, Fukaura H, Zhang J, al-Sabbagh A, Southwood S, Sette A, Kuchroo VK, and Hafler DA, T cell recognition of immunodominant and cryptic proteolipid protein epitopes in humans. J Immunol 155 (1995) 982–92. [PubMed] [Google Scholar]
- [18].Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, and Hafler DA, Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med 179 (1994) 973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ponomarev ED, and Dittel BN, Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol 174 (2005) 4678–87. [DOI] [PubMed] [Google Scholar]
- [20].Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, and Littman DR, The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126 (2006) 1121–33. [DOI] [PubMed] [Google Scholar]
- [21].Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, and de Waal Malefyt R, Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8 (2007) 950–7. [DOI] [PubMed] [Google Scholar]
- [22].Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, and Maslinski W, High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol 164 (2000) 2832–8. [DOI] [PubMed] [Google Scholar]
- [23].Cho ML, Ju JH, Kim KW, Moon YM, Lee SY, Min SY, Cho YG, Kim HS, Park KS, Yoon CH, Lee SH, Park SH, and Kim HY, Cyclosporine A inhibits IL-15-induced IL-17 production in CD4+ T cells via down-regulation of PI3K/Akt and NF-kappaB. Immunol Lett 108 (2007) 88–96. [DOI] [PubMed] [Google Scholar]
- [24].Pashenkov M, Mustafa M, Kivisakk P, and Link H, Levels of interleukin-15expressing blood mononuclear cells are elevated in multiple sclerosis. Scand J Immunol 50 (1999) 302–8. [DOI] [PubMed] [Google Scholar]
- [25].Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, and O’Shea JJ, Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A 103 (2006) 8137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen Z, and O’Shea JJ, Regulation of IL-17 production in human lymphocytes. Cytokine 41 (2008) 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, and Hauser SL, Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357 (2007) 851–62. [DOI] [PubMed] [Google Scholar]
- [28].Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, and Lassmann H, Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 38 (1995) 788–96. [DOI] [PubMed] [Google Scholar]
- [29].Peng X, Jin J, Giri S, Montes M, Sujkowski D, Tang Y, Smrtka J, Vollmer T, Singh I, and Markovic-Plese S, Immunomodulatory effects of 3-hydroxy-3methylglutaryl coenzyme-A reductase inhibitors, potential therapy for relapsing remitting multiple sclerosis. J Neuroimmunol 178 (2006) 130–9. [DOI] [PubMed] [Google Scholar]
- [30].Pannetier C, Even J, and Kourilsky P, T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today 16 (1995) 176–81. [DOI] [PubMed] [Google Scholar]
- [31].Gorochov G, Neumann AU, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, and Debre P, Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med 4 (1998) 215–21. [DOI] [PubMed] [Google Scholar]
- [32].Sebille F, Gagne K, Guillet M, Degauque N, Pallier A, Brouard S, Vanhove B, Delsuc MA, and Soulillou JP, Direct recognition of foreign MHC determinants by naive T cells mobilizes specific Vbeta families without skewing of the complementarity-determining region 3 length distribution. J Immunol 167 (2001) 3082–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
Supplementary Table 1. DEGs in AP, CTh, and CP lesion-derived T-cells in comparison to the NAWM. The table presents fold changes and the frequencies of identical genes with >5-fold change detected by replicates of the same probe, and genes coding for the same protein detected with different probes on the array.