Deletion of IKZF1 and Prognosis in Acute Lymphoblastic Leukemia (original) (raw)
. Author manuscript; available in PMC: 2009 Jul 28.
Published in final edited form as: N Engl J Med. 2009 Jan 7;360(5):470–480. doi: 10.1056/NEJMoa0808253
Abstract
Background
Despite best current therapy, up to 20% of pediatric patients with acute lymphoblastic leukemia (ALL) have a relapse. Recent genomewide analyses have identified a high frequency of DNA copy-number abnormalities in ALL, but the prognostic implications of these abnormalities have not been defined.
Methods
We studied a cohort of 221 children with high-risk B-cell–progenitor ALL with the use of single-nucleotide–polymorphism microarrays, transcriptional profiling, and resequencing of samples obtained at diagnosis. Children with known very-high-risk ALL subtypes (i.e., _BCR-ABL1_–positive ALL, hypodiploid ALL, and ALL in infants) were excluded from this cohort. A copy-number abnormality was identified as a predictor of poor outcome, and it was then tested in an independent validation cohort of 258 patients with B-cell–progenitor ALL.
Results
More than 50 recurring copy-number abnormalities were identified, most commonly involving genes that encode regulators of B-cell development (in 66.8% of patients in the original cohort); PAX5 was involved in 31.7% and IKZF1 in 28.6% of patients. Using copy-number abnormalities, we identified a predictor of poor outcome that was validated in the independent validation cohort. This predictor was strongly associated with alteration of IKZF1, a gene that encodes the lymphoid transcription factor IKAROS. The gene-expression signature of the group of patients with a poor outcome revealed increased expression of hematopoietic stem-cell genes and reduced expression of B-cell–lineage genes, and it was similar to the signature of _BCR-ABL1_–positive ALL, another high-risk subtype of ALL with a high frequency of IKZF1 deletion.
Conclusions
Genetic alteration of IKZF1 is associated with a very poor outcome in B-cell–progenitor ALL.
CURE RATES AMONG CHILDREN WITH acute lymphoblastic leukemia (ALL) now exceed 80%,1 but current therapies have substantial toxic effects, and up to 20% of patients with ALL have a relapse after initial therapy. 2 Risk stratification in B-cell–progenitor ALL is based on a number of recurring chromosomal abnormalities, including hyperdiploidy, hypodiploidy, translocations t(12;21)(ETV6-RUNX1), t(9;22)(BCR-ABL1), and t(1;19)(TCF3-PBX1), and rearrangement of the mixed-lineage leukemia (MLL) gene. Treatment failure is common in _BCR-ABL1_–rearranged and _MLL_-rearranged ALL, but relapse occurs in all subtypes. Moreover, the biologic basis of resistance to therapy in ALL is poorly understood.
Recent genomewide analyses of DNA copy-number abnormalities have identified numerous recurring genetic alterations in ALL.3–6 Mutations of genes encoding transcriptional regulators of B lymphoid development, including PAX5, EBF1, and IKZF1, occur in more than 40% of patients with B-cell–progenitor ALL.3 Deletion of IKZF1, which encodes the lymphoid transcription factor IKAROS, is a very frequent event in _BCR-ABL1_–positive ALL and at the progression of chronic myeloid leukemia to lymphoid blast crisis.5 Other copy-number abnormalities involve tumor suppressors and cell-cycle regulators (e.g., CDKN2A/B, RB1, PTEN, and ETV6), regulators of apoptosis (BTG1), drug-receptor genes (NR3C1 and NR3C2), and lymphoid-signaling molecules (BTLA and CD200).3
We report on a study of copy-number abnormalities in 221 children with high-risk ALL. We identified a predictor of poor outcome based on copy-number abnormalities that was driven by the deletion or mutation of IKZF1, which is associated with a high risk of relapse. The correlation between this predictor and poor outcome was validated in an independent cohort of 258 patients with B-cell–progenitor ALL. The predictor was also associated with a gene-expression signature characterized by the increased expression of hematopoietic stem-cell genes and the reduced expression of B lymphoid genes.
METHODS
PATIENTS AND SAMPLES
Of the two cohorts of patients who were examined (see the Supplementary Appendix, available with the full text of this article at NEJM.org), the originalcohort comprised 221 patients with B-cell–progenitor ALL treated in the Children’s Oncology Group P9906 study; this study used an augmented reinduction–reconsolidation strategy (the Berlin–Frankfurt–Münster regimen) (Table 1 in the Supplementary Appendix).7,8 All patients were at high risk for treatment failure based on the presence of central nervous system or testicular disease, MLL gene rearrangement, or age, sex, and leukocyte count at presentation.9 Patients with _BCR-ABL1_–positive and hypodiploid ALL, infants, and patients who did not have a response to induction chemotherapy were excluded. A recurring chromosomal abnormality was not detected in 170 patients (76.9%). Patients were enrolled from May 2000 through April 2003. The median follow-up time, defined as the time from enrollment to death or the last follow-up, was 3.94 years (range, 0.16 to 6.20).
The validation cohort comprised 258 children with B-cell–progenitor ALL treated in multiple protocols3,5,10–14 at St. Jude Children’s Research Hospital. This cohort included both standard-risk and high-risk patients, patients with common aneuploidies, and patients with recurring translocations (including 21 _BCR-ABL1_–positive patients) (Table 2 in the Supplementary Appendix). Patients were enrolled from September 1986 through February 2007. The median follow-up time was 6.05 years (range, 0.27 to 21.47).
Written informed consent and institutional-review-board approval were obtained for both cohorts. In the original cohort, minimal residual disease was measured in 196 patients at day 8 (in peripheral blood) and in 204 patients at day 29 (in bone marrow) of initial induction chemotherapy, and in the validation cohort, in 161 patients at day 19 and in 160 patients at day 46. This measurement of minimal residual disease was performed with the use of immunophenotyping, as previously described.8,15,16
No commercial entity was involved in the conduct of the study, the analysis or storage of the data, or the preparation of the manuscript. The authors vouch for the completeness and accuracy of the data and the analysis.
GENOMIC ANALYSES
DNA extracted from leukemic cells obtained at diagnosis and from samples obtained during remission was genotyped with the use of 250k Sty and Nsp single-nucleotide–polymorphism (SNP) arrays (Affymetrix). Samples from patients in the validation cohort were genotyped with SNP 6.0 arrays in 36 patients, 250K Sty and Nsp arrays in 37 patients, and 250K Sty and Nsp and 50K Hind 240 and Xba 240 arrays in 185 patients. SNP array analyses, gene-expression profiling, and the use of gene set enrichment analysis17 and gene set analysis18 to compare gene-expression signatures and examine associations between gene sets and outcome are described in the Supplementary Appendix.
GENOMIC RESEQUENCING OF PAX5, EBF1, AND IKZF1
Genomic resequencing of all the coding exons of PAX5, EBF1, and IKZF1 was performed for all samples in the original cohort. The Supplementary Appendix includes a description of sequencing methods and structural modeling of PAX5 mutations.
DNA COPY-NUMBER ABNORMALITIES AND OUTCOME
Supervised principal-components analysis19,20 was used to examine associations between copy-number abnormalities and treatment outcome in a genomewide fashion (Supplementary Appendix). A modified univariate Cox score was calculated for the association between the copy-number status of each gene and the cumulative risk of any adverse events or relapse, and genes with a Cox score that exceeded a threshold that best predicted outcome were used to perform a principal-components analysis. We subsequently generated a risk score for each patient, using the first principal component. Methods used to examine associations between the supervised principal-components risk score, individual genetic lesions and relapse, adverse events, and minimal residual disease are described in the Supplementary Appendix.
RESULTS
COPY-NUMBER ALTERATIONS IN HIGH-RISK ALL
We identified a mean of 8.36 copy-number abnormalities per patient in the original cohort (Table 3 in the Supplementary Appendix), and more than 50 recurring copy-number abnormalities in which the minimal common region of change involved one or few genes (Table 4 in the Supplementary Appendix). The most common deletions involved CDKN2A/B (45.7%), the lymphoid transcription-factor genes PAX5 (31.7%) (Fig. 1 and Table 6 in the Supplementary Appendix) and IKZF1 (28.6%) (Fig. 2 and Table 8 in the Supplementary Appendix), ETV6 (also known as TEL) (12.7%), RB1 (11.3%), and BTG1 (10.4%).
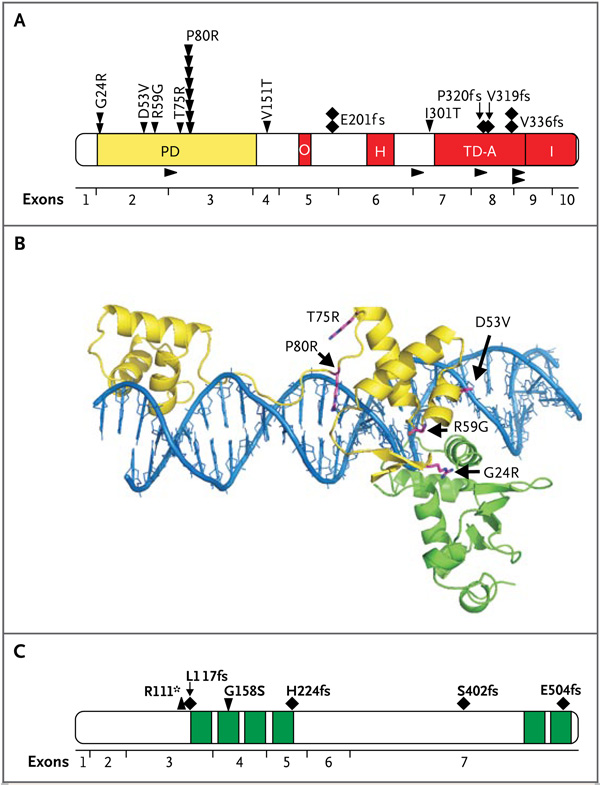
Figure 1. PAX5 and IKZF1 Sequence Mutations in High-Risk ALL in the Original Cohort.
Panel A shows the functional domains of PAX5 and the location of missense mutations (arrowheads pointing down), frameshift mutations (diamonds), and splice-site mutations (arrowheads pointing to the right) detected in this study. In the domains, A denotes activating, H homeodomain, I inhibitory, O octapeptide domain, PD paired domain, and TD transactivating domain. Panel B shows structural modeling of the location of PAX5 paired-domain mutations. The DNA double helix is blue, the bipartite PAX5 paired domain is yellow, and ETS1, which interacts with and increases the affinity of DNA binding of PAX5, is green. Each mutation is predicted to disrupt the normal interaction of PAX5 with DNA, ETS1, or both. G24R is predicted to alter the flexibility of the DNA binding loop and interfere with the interaction of PAX5 with ETS1. D53V aligns R56, which in turn directly contacts DNA. R59G occurs at the junction with ETS1 and DNA and is likely to increase flexibility and destabilize both interactions. T75R clashes and causes electrostatic repulsion at R71, which is adjacent to the DNA binding site, and P80R has a direct effect on DNA binding, as previously described. 3 Panel C shows the primary structure of IKAROS and the location of the six zinc fingers (green) and missense (arrowhead pointing down), frameshift (diamonds), and nonsense (arrowhead pointing up) mutations.
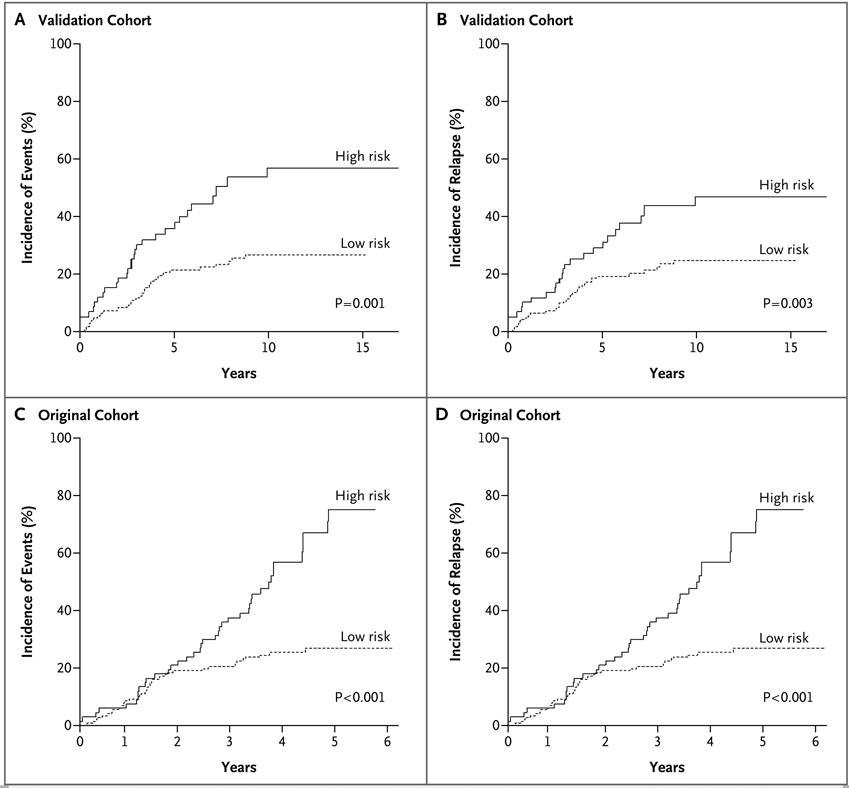
Figure 2. Associations between DNA Copy-Number Abnormality Predictors and Outcome in the Original and Validation Cohorts.
Panel A shows the cumulative incidence of any event (relapse, death, or second malignant condition), and Panel B shows the cumulative incidence of any relapse among patients in the validation cohort after risk stratification with the use of the copy-number abnormality predictor based on data from the original cohort. Panel C shows the cumulative incidence of any event, and Panel D shows the cumulative incidence of relapse in the original cohort after risk stratification with the use of the copy-number abnormality predictor based on data from the validation cohort. High risk refers to patients who are predicted to be at high risk for events or relapse, and low risk refers to patients who are predicted to be at low risk for events or relapse.
Twenty-two patients had 27 PAX5 sequence mutations (Table 7 in the Supplementary Appendix). The most frequent mutation was the previously identified P80R mutation in the paired domain of PAX5 that attenuates the DNA-binding and transactivating activity of PAX53 (Fig. 1A). Several novel paired-domain missense (R59G, T75R) and transactivating domain splice-site and frameshift mutations were identified. Each of the paired-domain mutations is predicted to result in impaired binding of PAX5 to DNA, or disruption of the interaction of PAX5 with ETS1, which is required for high-affinity binding of PAX5 to target DNA sequences21 (Fig. 1B).
Among the 221 patients, the entire IKZF1 locus was deleted in 16 (Tables 4 and 8 and Fig. 2 in the Supplementary Appendix); in 47 additional patients, a subgroup of exons or the genomic region immediately upstream of IKZF1 was deleted. In 20 of these 47 patients, there was a deletion of coding exons 3 through 6, which results in expression of a dominant-negative form of IKAROS, Ik6, which lacks all N-terminal, DNA-binding zinc fingers.5 We also identified six novel missense, frameshift, and nonsense IKZF1 mutations (Fig. 1C), each of which is predicted to impair IKAROS function. A mutation of G158 is known to attenuate the DNA-binding activity of IKAROS,22 and thus the G158S mutation we identified would probably act as a dominant-negative IKAROS allele. Overall, 66.5% of the patients with high-risk ALL had at least one mutation of genes regulating B lymphoid development (Tables 4 and 9 in the Supplementary Appendix), with significant variation in the frequency of lesions among ALL subtypes (Table 10 in the Supplementary Appendix).
ASSOCIATIONS WITH OUTCOME
Using supervised principal-components analysis of the original cohort, we identified associations between the copy-number status of 20 genes and treatment outcome (Table 11 in the Supplementary Appendix). The risk score based on the supervised principal-components analysis was significantly associated with poor outcome in the validation cohort. The 10-year incidence of events among patients who were predicted to be at high risk according to the supervised principal-components analysis was 56.9% (95% confidence interval [CI], 41.5 to 72.3), as compared with 26.8% (95% CI, 19.5 to 34.1) among patients who were predicted to be at low risk (P = 0.001) (Fig. 2A). The 10-year incidence of relapse was 47.0% (95%CI, 31.9 to 62.0) among high-risk patients, as compared with 24.6% (95% CI, 17.5 to 31.7) among low-risk patients (P = 0.003) (Fig. 2B). Conversely, with the use of the validation cohort as the training set, a supervised principal-components predictor was identified that was associated with poor outcome in the original cohort. The 5-year incidence of adverse events among high-risk patients was 75.0% (95% CI, 59.7 to 90.3), as compared with 27.0% (95% CI, 19.4 to 34.6) among low-risk patients (P<0.001) (Fig. 2C). The 5-year incidence of relapse was 73.8% (95% CI, 58.4 to 89.3) among high-risk patients, as compared with 25.0% (95% CI, 17.6 to 32.4) among low-risk patients (P<0.001) (Fig. 2D).
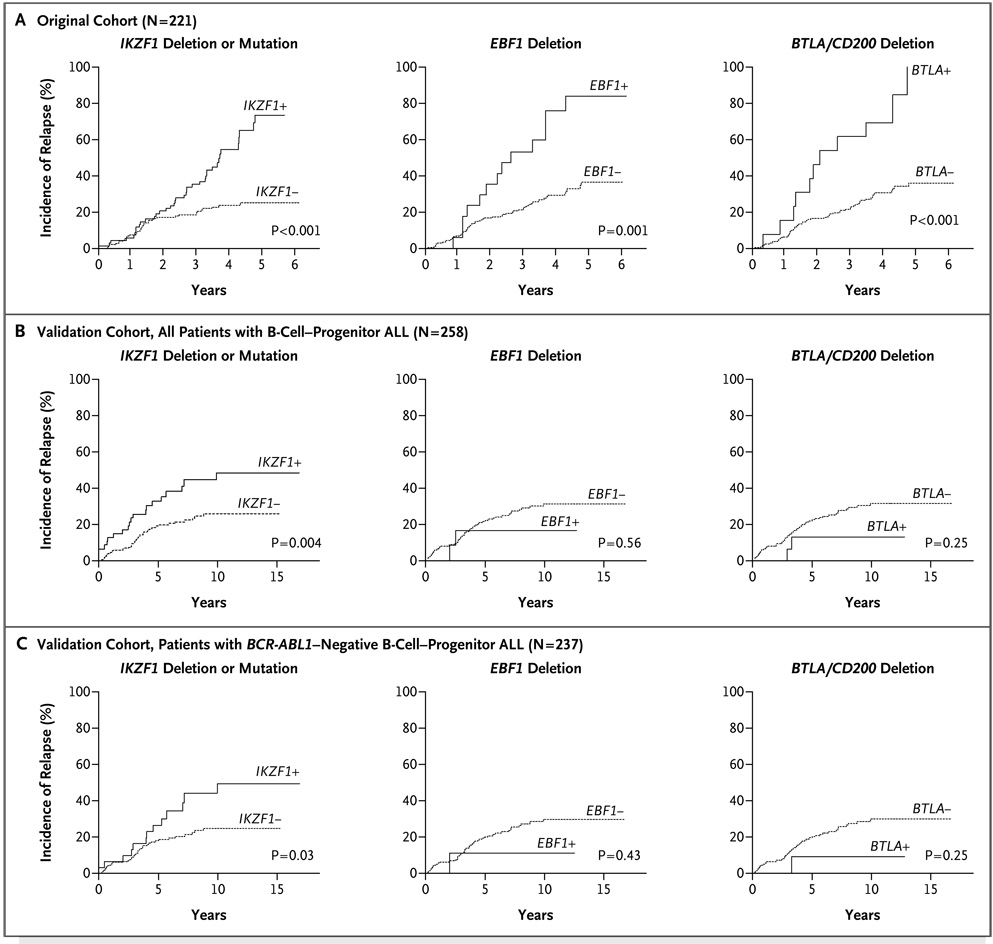
Of the genetic alterations that were significantly associated with the supervised principal-components predictor in the original cohort (Table 11 in the Supplementary Appendix), only IKZF1 was also significantly associated with the predictor defined in the validation cohort (Table 12 in the Supplementary Appendix). Deletion or mutation of IKZF1 was significantly associated with an increased risk of relapse and adverse events in both cohorts (Table 1 and Fig. 3A and 3B, and Tables 13 through 15 in the Supplementary Appendix). IKZF1 deletions were also associated with a poor outcome in patients with _BCR-ABL1_–negative ALL in the validation cohort (Fig. 3C). Furthermore, alteration of IKZF1 had an independent association with outcome after adjusting for age, leukocyte count at presentation, and cytogenetic subtype (Table 15 in the Supplementary Appendix). Deletions of EBF1 and BTLA/CD200 were associated with a poor outcome only in the original cohort. Although a high cumulative number of genetic alterations targeting B-cell development per patient was also associated with a poor outcome (Tables 13 through 15 in the Supplementary Appendix), no independent association between PAX5 lesions and outcome was observed in either cohort.
Table 1.
Alterations in IKZF1, EBF1, and BTLA/CD200 and Outcome in the Original and Validation Cohorts.*
| Outcome and Alteration | Original Cohort (N=221) | Validation Cohort (N=258) | ||||||
|---|---|---|---|---|---|---|---|---|
| All Patients | Patients with Event | Incidence at 4 or 5 Yr† | P Value | All Patients | Patients with Event | Incidence at 10 Yr | P Value | |
| no. | % | no. | % | |||||
| Hematologic relapse | ||||||||
| IKZF1 alteration | ||||||||
| No | 154 | 19 | 14.0±3.1 | 210 | 38 | 22.5±3.5 | ||
| Yes | 67 | 27 | 55.2±8.6 | <0.001 | 48 | 19 | 46.3±8.4 | 0.002 |
| EBF1 alteration | ||||||||
| No | 204 | 34 | 22.5±4.1 | 246 | 56 | 28.7±3.6 | ||
| Yes | 17 | 12 | 78.0±12.6 | <0.001 | 12 | 1 | 8.3±8.4 | 0.26 |
| BTLA/CD200 alteration | ||||||||
| No | 208 | 39 | 18.3±2.9 | 242 | 55 | 28.7±3.6 | ||
| Yes | 13 | 7 | 38.5±14.4 | 0.003 | 16 | 2 | 13.0±9.0 | 0.35 |
| Any relapse | ||||||||
| IKZF1 alteration | ||||||||
| No | 154 | 36 | 25.2±3.8 | 210 | 44 | 25.6±3.6 | ||
| Yes | 67 | 39 | 73.4±8.0 | <0.001 | 48 | 20 | 48.4±8.4 | 0.004 |
| EBF1 alteration | ||||||||
| No | 204 | 62 | 36.7±4.4 | 246 | 62 | 31.3±3.6 | ||
| Yes | 17 | 13 | 83.9±11.7 | <0.001 | 12 | 2 | 16.7±11.3 | 0.56 |
| BTLA/CD200 alteration | ||||||||
| No | 208 | 64 | 30.7±3.4 | 242 | 62 | 31.5±3.7 | ||
| Yes | 13 | 11 | 69.2±13.9 | <0.001 | 16 | 2 | 13.0±9.0 | 0.25 |
| Any event | ||||||||
| IK_Z_F1 alteration | ||||||||
| No | 154 | 39 | 27.1±3.9 | 210 | 48 | 27.6±3.7 | ||
| Yes | 67 | 40 | 74.6±7.9 | <0.001 | 48 | 25 | 60.5±8.4 | <0.001 |
| EBF1 alteration | ||||||||
| No | 204 | 66 | 38.7±4.5 | 246 | 71 | 35.5±3.8 | ||
| Yes | 17 | 13 | 83.9±11.7 | <0.001 | 12 | 2 | 16.7±11.3 | 0.40 |
| BTLA/CD200 alteration | ||||||||
| No | 208 | 68 | 32.6±3.3 | 242 | 69 | 35.2±3.8 | ||
| Yes | 13 | 11 | 69.2±13.9 | <0.001 | 16 | 4 | 25.5±11.5 | 0.83 |
Figure 3. Genetic Alterations of IKZF1, EBF1, and BTLA/CD200 and the Cumulative Incidence of Any Relapse in the Original Cohort.
Panel A shows the cumulative incidence of relapse in the entire original cohort of patients with B-cell–progenitor ALL according to IKZF1, EBF1, and BTLA/CD200 alteration status. Panel B shows the cumulative incidence of relapse in the entire validation cohort, including patients with _BCR-ABL1_–positive B-cell–progenitor ALL. Panel C shows the cumulative incidence of relapse in the validation cohort after exclusion of patients with _BCR-ABL1_–positive B-cell–progenitor ALL. Only IKZF1 alterations were associated with poor outcome in both the original and validation cohorts. Five-year estimates of relapse are shown in the original cohort, and 10-year estimates are shown in the validation cohort. P values are calculated with the use of Gray’s test.
ASSOCIATIONS WITH MINIMAL RESIDUAL DISEASE
Consistent with previous data,8,15,16 elevated levels of minimal residual disease were strongly associated with an increased risk of relapse in both cohorts (at day 8 and day 29 in the original cohort and at day 19 and day 46 in the validation cohort) (P<0.001 for both comparisons). _IKZF1_ and _EBF1_ alterations were strongly associated with elevated levels of minimal residual disease at day 29 in the original cohort. Of 67 patients with deleted or mutated _IKZF1_, 16 (23.9%) had high-level (>1%) minimal residual disease at day 29, as compared with 6.6% of patients without this abnormality (P = 0.001) (Table 2, and Table 17 in the Supplementary Appendix). These associations remained significant in multivariable analyses adjusted for age, leukocyte count at presentation, and genetic subtype (odds ratio for the association of EBF1 alterations with elevated levels of minimal residual disease, 9.0; P<0.001; odds ratio for IKZF1 alterations, 3.71; P<0.001) (Table 18 in the Supplementary Appendix). The associations of IKZF1 abnormalities with relapse and adverse events remained significant after adjusting for age, leukocyte count, subtype, and minimal residual disease in this cohort (Table 19 in the Supplementary Appendix).
Table 2.
Associations between IKZF1 Alterations and Levels of Minimal Residual Disease.
| Cohort | IKZF1 Deletion or Mutation | Patients | Level of Minimal Residual Disease* | P Value | |
|---|---|---|---|---|---|
| Low | Intermediate | High | |||
| no. | no. of patients (%) | ||||
| Original | |||||
| Day 8 | No | 133 | 26 (19.5) | 50 (37.6) | 57 (42.9) |
| Yes | 63 | 7 (11.1) | 17 (27.0) | 39 (61.9) | 0.04 |
| Day 29 | No | 137 | 100 (73.0) | 28 (20.4) | 9 (6.6) |
| Yes | 67 | 31 (46.3) | 20 (29.9) | 16 (23.9) | 0.001 |
| Validation | |||||
| Day 19 | No | 140 | 69 (49.3) | 58 (41.4) | 13 (9.3) |
| Yes | 21 | 2 (9.5) | 6 (28.6) | 13 (61.9) | <0.001 |
| Day 46 | No | 139 | 119 (85.6) | 19 (13.7) | 1 (0.7) |
| Yes | 21 | 7 (33.3) | 7 (33.3) | 7 (33.3) | <0.001 |
IKZF1 alterations were also associated with outcome in the subgroup of 160 patients for whom we had data on minimal residual disease in the validation cohort (Tables 20 and 21 in the Supplementary Appendix). A deletion or mutation of IKZF1 was strongly associated with elevated levels of minimal residual disease in this subgroup of patients. High levels of residual disease (≥1.0%) at day 19 were detected in 13 patients with a IKZF1 deletion or mutation (61.9%), as compared with 9.3% of patients without deletion or mutation of IKZF1 (P<0.001) (Table 2, and Table 22 in the Supplementary Appendix). This association was also observed for minimal residual disease at day 46 (33.3% vs. 0.7%, P<0.001) (Table 2, and Table 23 in the Supplementary Appendix). IKZF1 status was also associated with minimal residual disease at both day 19 (P = 0.001) and day 46 (P = 0.001) in patients in the _BCR-ABL1_–negative validation cohort (Tables 24 and 25 in the Supplementary Appendix).
GENE-EXPRESSION PROFILING OF HIGH-RISK ALL
The association between IKZF1 alterations and outcome in both cohorts, as well as previous data showing that deletion of IKZF1 is frequent in BCR-ABL1 ALL,5 suggests that IKAROS abnormalities are important in the pathogenesis of both _BCR-ABL1_–positive B-cell–progenitor ALL and _BCR-ABL1_–negative ALL that is associated with a poor outcome. To explore this possibility, we used gene-set enrichment analysis to compare the gene-expression signatures of patients with ALL who had a poor outcome in the original and validation cohorts. We also used this form of analysis to compare the gene-expression signatures of _BCR-ABL1_–positive ALL and _BCR-ABL1_–negative ALL associated with a poor outcome in the original cohort. This analysis revealed a significant similarity of signatures in the patients with ALL who had a poor outcome in the original and validation cohorts (Fig. 3A and 3B in the Supplementary Appendix).
Using gene-set enrichment analysis, we also observed a highly significant similarity between the signature of high-risk, _BCR-ABL_–negative ALL (derived from the original cohort) and the signature of _BCR-ABL1_–positive ALL in the validation cohort (Fig. 3C and 3D in the Supplementary Appendix). Moreover, 61 of the 100 most differentially expressed genes in patients with ALL with a poor outcome in the original cohort were present in patients with the BCR-ABL1 signature in the validation cohort (at a false discovery rate of 5%), indicating substantial similarity between the two signatures. These findings suggest that genetic alterations of IKZF1 influence the transcriptome of both _BCR-ABL1_–positive ALL and _BCR-ABL1_–negative ALL with a poor outcome. We also observed the enrichment of genes up-regulated in hematopoietic stem cells and progenitor cells23 in patients with ALL who had a poor outcome in both the original and validation cohorts (Table 26 and Fig. 3E in the Supplementary Appendix) and a relative lack of expression of genes mediating B-lymphocyte– receptor signaling and development24 in patients with a poor outcome in the original cohort (Table 27 and Fig. 3F in the Supplementary Appendix), suggesting that IKZF1 alterations result in developmental arrest and impaired B-cell development. Finally, gene-set analysis18 with the use of time to first event as the phenotype showed that the BCR-ABL1 signature was the gene set most strongly predictive of a poor outcome in the original (_BCR-ABL1_–negative) cohort (P<0.001).
DISCUSSION
Accurate risk stratification is critical for ensuring that patients with high-risk ALL receive treatment of appropriate intensity and that low-risk patients are spared unnecessary toxic effects. Current risk stratification is based primarily on clinical variables, immunophenotype, detection of sentinel cytogenetic or molecular lesions, and early response to therapy.1 However, a substantial proportion of patients who have a relapse have no known poor-risk factors at the time of diagnosis.
We used high-resolution, genomewide copy-number analysis to identify genetic lesions associated with clinical outcome. Most striking was the strong association between deletions or mutations of IKZF1 and a poor outcome in two independent cohorts notable for different sample composition and treatment schedules. In multivariate analysis, the association between IKZF1 status and outcome was independent of age, leukocyte count at presentation, cytogenetic subtype, and levels of minimal residual disease; this indicates that detection of IKZF1 alterations at diagnosis might be useful in identifying patients with a high risk of treatment failure. Moreover, the gene-expression signatures of patients with poor-outcome (_IKZF1_-deleted) ALL in the original and validation cohorts were very similar to each other and to the signature of _BCR-ABL1_–positive ALL, a subtype of ALL in which IKZF1 deletion is very common. Since BCR-ABL1 ALL has a poor prognosis, these findings suggest that the mutation of IKZF1 is a key determinant of a poor outcome both in patients with _BCR-ABL1_–positive and patients with _BCR-ABL1_–negative disease. The similarity of the gene-expression signatures of _BCR-ABL1_–negative ALL with a mutation of IKZF1 and _BCR-ABL1_–positive ALL raises the possibility that patients with _BCR-ABL1_–negative ALL, deletion of IKZF1, and a poor outcome may have hitherto unidentified activating mutations in tyrosine kinases.
IKAROS is a transcription factor with well-established roles in lymphopoiesis and cancer.25 Normal IKAROS contains four N-terminal zinc fingers, which are required for DNA binding, and two C-terminal zinc fingers that mediate dimerization of IKAROS with itself and with other IKAROS family members. The development of all lymphoid lineages requires IKAROS,26 and in mice that are heterozygous for a dominant-negative Ikzf1 mutation, aggressive T-lineage hematopoietic disease develops.27 Ikzf1 is also a common target of integration in retroviral mutagenesis studies in mice.28
Alternative IKAROS transcripts have been detected in normal hematopoietic cells and leukemic blasts.25 Isoforms lacking most or all of the N-terminal zinc fingers have attenuated DNA-binding capacity but retain their ability to undergo homodimerization and heterodimerization, and they thus act as dominant-negative inhibitors of IKAROS.29 Previous studies have shown expression of these aberrant IKAROS isoforms in ALL.25 Recently, we reported a very frequent deletion of IKZF1 in _BCR-ABL1_–positive ALL and lymphoid blast crisis of chronic myeloid leukemia, suggesting that perturbation of IKAROS is a key event in the pathogenesis and progression of BCR-ABL1 leukemia.5 Moreover, there was complete correlation between the extent of genomic deletion and the expression of aberrant IKAROS isoforms.5 For example, all patients expressing the dominant-negative Ik6 isoform, which lacks coding exons 3 through 6 and all N-terminal zinc fingers, had genomic deletions of exons 3 through 6.5
The present study shows that IKZF1 alterations occur in a substantial proportion of patients with _BCR-ABL1_–negative B-cell–progenitor ALL, predominantly in patients without other common recurrent cytogenetic abnormalities (38.8% of patients in the original cohort and 22.8% of the patients in the validation cohort with normal or miscellaneous karyotypic abnormalities had alterations of IKZF1). As in _BCR-ABL1_–positive ALL, IKZF1 deletions involved either the entire locus or sets of exons, and they are predicted to result in either haploinsufficiency or the expression of dominant-negative IKAROS isoforms. Moreover, we have identified sequence mutations of IKZF1 in ALL that are predicted to result in the loss of normal IKAROS function or expression of a novel dominant-negative isoform, G158S.
Using gene-set enrichment analysis, we found enrichment of hematopoietic stem-cell and progenitor genes and underexpression of B lymphoid genes in patients with ALL who had a poor outcome. This finding is consistent with the requirement for IKAROS in lymphoid development26 and the demonstration that expression of dominant-negative IKAROS isoforms impairs B lymphoid differentiation.30 Together, these data suggest that attenuation of normal IKAROS activity and the resulting block in lymphoid maturation render leukemic cells relatively resistant to eradication by chemotherapy. The clinical consequences of enrichment for genes that are characteristic of leukemia-initiating cells or stem cells, including their inherent drug-resistant mechanisms, remain to be determined.31
We did not find an association between clinical outcome and extensively studied loci such as CDKN2A/B32,33 or PAX5 status, despite the finding that PAX5 alterations were the most common lesions in the B-cell–differentiation pathway in both cohorts. PAX5 alterations may be important in establishing the leukemic clone, whereas alterations of IKZF1 may also contribute to resistance to chemotherapy. This finding is supported by recent data showing that IKZF1 alterations also emerge as new genetic alterations at the time of relapse in ALL.34 In summary, we identified an association between alterations of IKZF1 and the clinical outcome in B-cell–progenitor ALL in childhood. Integrated genomic analysis suggests that IKZF1 contributes directly to treatment resistance in ALL. These results provide a rationale for the integration of IKZF1 status in the evaluation of patients with ALL.
Supplementary Material
appndx
Acknowledgments
Supported by funds provided as a supplement to the Children’s Oncology Group Chair’s Award (CA098543, to Dr. Hunger); a Strategic Partnering to Evaluate Cancer Signatures Program award (CA114762, to Drs. Carroll, Chen, Harvey, and Willman) from the National Cancer Institute (NCI); grants from the National Institute of General Medical Sciences Pharmacogenetics Research Network and Database (U01 GM61393 and U01GM61374, to Dr. Relling), a Cancer Center core grant (21765, to Drs. Downing, Mullighan, and Pui), and a grant R01 CA86011, to Dr. Borowitz) from the National Institutes of Health (NIH); a Leukemia and Lymphoma Society Specialized Center of Research grant (7388-06, to Dr. Willman); a grant from CureSearch National Childhood Cancer Foundation; a National Health and Medical Research Council (Australia) C.J. Martin Traveling Fellowship (to Dr. Mullighan); and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital. The sequencing was supported by funds from the NCI and NIH to Agencourt Bioscience under contract N01-C0-12400.
Dr. Cheng reports serving on the advisory board for Enzon Pharmaceuticals; Dr. Borowitz, receiving consulting fees and grant support from Becton Dickinson Biosciences; Dr. Reaman, serving on the advisory board for Enzon Pharmaceuticals; Dr. Willman, receiving grant support from Xanthus Pharmaceuticals; and Drs. Downing and Mullighan, being co-inventors on a pending patent application concerning tests for IKZF1 mutations that was filed by St. Jude Children’s Research Hospital, which has not entered into any licenses related to this application. No other potential conflict of interest relevant to this article was reported.
We thank Zhongling Cai and Claire Boltz for technical assistance and Stephen Smale for helpful discussions.
REFERENCES
- 1.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 2.Rivera GK, Zhou Y, Hancock ML, et al. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 2005;103:368–376. doi: 10.1002/cncr.20743. [DOI] [PubMed] [Google Scholar]
- 3.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper RP, Schoenmakers EF, van Reijmersdal SV, et al. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21:1258–1266. doi: 10.1038/sj.leu.2404691. [DOI] [PubMed] [Google Scholar]
- 5.Mullighan CG, Miller CB, Phillips LA, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 6.Kawamata N, Ogawa S, Zimmermann M, et al. Molecular allelokaryotyping of pediatric acute lymphoblastic leukemias by high-resolution single nucleotide polymorphism oligonucleotide genomic microarray. Blood. 2008;111:776–784. doi: 10.1182/blood-2007-05-088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 8.Borowitz MJ, Devidas M, Hunger SP. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuster JJ, Camitta BM, Pullen J, et al. Identification of newly diagnosed children with acute lymphocytic leukemia at high risk for relapse. Cancer Res Ther Control. 1999;9:101–107. [Google Scholar]
- 10.Pui CH, Boyett JM, Rivera GK, et al. Long-term results of Total Therapy studies 11, 12 and 13A for childhood acute lymphoblastic leukemia at St Jude Children’s Research Hospital. Leukemia. 2000;14:2286–2294. doi: 10.1038/sj.leu.2401938. [DOI] [PubMed] [Google Scholar]
- 11.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 12.Kishi S, Griener J, Cheng C, et al. Homocysteine, pharmacogenetics, and neurotoxicity in children with leukemia. J Clin Oncol. 2003;21:3084–3091. doi: 10.1200/JCO.2003.07.056. [DOI] [PubMed] [Google Scholar]
- 13.Pui CH, Relling MV, Sandlund JT, Downing JR, Campana D, Evans WE. Total Therapy study XV for newly diagnosed childhood acute lymphoblastic leukemia: study design and preliminary results. Ann Hematol. 2006;85 Suppl 1:88–91. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 14.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370:240–250. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 15.Coustan-Smith E, Behm FG, Sanchez J, et al. Immunological detection of minimal residual disease in children with acute lymphoblastic leukaemia. Lancet. 1998;351:550–554. doi: 10.1016/S0140-6736(97)10295-1. [DOI] [PubMed] [Google Scholar]
- 16.Coustan-Smith E, Sancho J, Hancock ML, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96:2691–2696. [PubMed] [Google Scholar]
- 17.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efron B, Tibshirani R. On testing the significance of sets of genes. Ann Appl Stat. 2007;1:107–129. [Google Scholar]
- 19.Bair E, Hasle H, Debashis P, Tibshirani R. Prediction by supervised principal components. J Am Stat Assoc. 2006;101:119–137. [Google Scholar]
- 20.Bair E, Tibshirani R. Semi-supervised methods to predict patient survival from gene expression data. PLoS Biol. 2004;2(4):E108. doi: 10.1371/journal.pbio.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier H, Ostraat R, Parenti S, et al. Requirements for selective recruitment of Ets proteins and activation of mb-1/Ig-alpha gene transcription by Pax-5 (BSAP) Nucleic Acids Res. 2003;31:5483–5489. doi: 10.1093/nar/gkg785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 2000;14:2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgantas RW, III, Tanadve V, Malehorn M, et al. Microarray and serial analysis of gene expression analyses identify known and novel transcripts overexpressed in hematopoietic stem cells. Cancer Res. 2004;64:4434–4441. doi: 10.1158/0008-5472.CAN-03-3247. [DOI] [PubMed] [Google Scholar]
- 24.Buhl AM, Nemazee D, Cambier JC, Rickert R, Hertz M. B-cell antigen receptor competence regulates B-lymphocyte selection and survival. Immunol Rev. 2000;176:141–153. doi: 10.1034/j.1600-065x.2000.00613.x. [DOI] [PubMed] [Google Scholar]
- 25.Rebollo A, Schmitt C. Ikaros, Aiolos and Helios: transcription regulators and lymphoid malignancies. Immunol Cell Biol. 2003;81:171–175. doi: 10.1046/j.1440-1711.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- 26.Georgopoulos K, Bigby M, Wang JH, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 27.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83:289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 28.Uren AG, Kool J, Matentzoglu K, et al. Large-scale mutagenesis in p19(ARF)-and p53-deficient mice identifies cancer genes and their collaborative networks. Cell. 2008;133:727–741. doi: 10.1016/j.cell.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Liu A, Georgopoulos K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 1996;15:5358–5369. [PMC free article] [PubMed] [Google Scholar]
- 30.Tonnelle C, Bardin F, Maroc C, et al. Forced expression of the Ikaros 6 isoform in human placental blood CD34(+) cells impairs their ability to differentiate toward the B-lymphoid lineage. Blood. 2001;98:2673–2680. doi: 10.1182/blood.v98.9.2673. [DOI] [PubMed] [Google Scholar]
- 31.le Viseur C, Hotfilder M, Bomken S, et al. In childhood acute lymphoblastic leukemiablasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14:47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calero Moreno TM, Gustafsson G, Garwicz S, et al. Deletion of the Ink4-locus (the p16ink4a, p14ARF and p15ink4b genes) predicts relapse in children with ALL treated according to the Nordic protocols NOPHO-86 and NOPHO-92. Leukemia. 2002;16:2037–2045. doi: 10.1038/sj.leu.2402697. [DOI] [PubMed] [Google Scholar]
- 33.Mirebeaux D, Acquaviva C, Suciu S, et al. The prognostic significance of CDKN2A, CDKN2B and MTAP inactivation in B-lineage acute lymphoblastic leukemia of childhood: results of the EORTC studies 58881 and 58951. Haematologica. 2006;91:881–885. [PubMed] [Google Scholar]
- 34.Mullighan CG, Phillips LA, Su X, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322:1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
appndx