One-Year Treatment With Exenatide Improves β-Cell Function, Compared With Insulin Glargine, in Metformin-Treated Type 2 Diabetic Patients: A randomized, controlled trial (original) (raw)
Abstract
OBJECTIVE
Traditional blood glucose–lowering agents do not sustain adequate glycemic control in most type 2 diabetic patients. Preclinical studies with exenatide have suggested sustained improvements in β-cell function. We investigated the effects of 52 weeks of treatment with exenatide or insulin glargine followed by an off-drug period on hyperglycemic clamp–derived measures of β-cell function, glycemic control, and body weight.
RESEARCH DESIGN AND METHODS
Sixty-nine metformin-treated patients with type 2 diabetes were randomly assigned to exenatide (n = 36) or insulin glargine (n = 33). β-Cell function was measured during an arginine-stimulated hyperglycemic clamp at week 0, at week 52, and after a 4-week off-drug period. Additional end points included effects on glycemic control, body weight, and safety.
RESULTS
Treatment-induced change in combined glucose- and arginine-stimulated C-peptide secretion was 2.46-fold (95% CI 2.09–2.90, P < 0.0001) greater after a 52-week exenatide treatment compared with insulin glargine treatment. Both exenatide and insulin glargine reduced A1C similarly: −0.8 ± 0.1 and −0.7 ± 0.2%, respectively (P = 0.55). Exenatide reduced body weight compared with insulin glargine (difference −4.6 kg, P < 0.0001). β-Cell function measures returned to pretreatment values in both groups after a 4-week off-drug period. A1C and body weight rose to pretreatment values 12 weeks after discontinuation of either exenatide or insulin glargine therapy.
CONCLUSIONS
Exenatide significantly improves β-cell function during 1 year of treatment compared with titrated insulin glargine. After cessation of both exenatide and insulin glargine therapy, β-cell function and glycemic control returned to pretreatment values, suggesting that ongoing treatment is necessary to maintain the beneficial effects of either therapy.
Type 2 diabetes is characterized by β-cell dysfunction against a background of obesity-related insulin resistance (1). When lifestyle measures and oral blood glucose–lowering medications fail to sustain glycemic control, current guidelines advise the use of basal insulin (2). Data from the U.K. Prospective Diabetes Study suggest that glycemic control progressively worsens over time, and this deterioration has been attributed to a progressive loss of β-cell function that occurs irrespective of whether metformin, sulfonylureas, or insulin are used (3). Therefore, therapeutic approaches, which may prevent or delay the decline of β-cell function in type 2 diabetes, are eagerly awaited.
Exenatide is synthetic exendin-4, first identified and isolated from the salivary secretions of the Gila monster (Heloderma suspectum). Exendin-4 shares 53% amino acid sequence identity with human glucagon-like peptide (GLP)-1 and binds directly to GLP-1 receptors. Placebo-controlled (4–7) and comparator-controlled (8–10) clinical studies have demonstrated that exenatide improves glycemic control and reduces body weight in patients with type 2 diabetes. These studies also showed amelioration of surrogate measures of β-cell function (4–6,8). Accordingly, improvements have been demonstrated in first- and second-phase glucose-stimulated insulin secretion and in meal-derived indexes of β-function compared with placebo (11,12). In animals, exenatide has been shown to sustain improvements in β-cell function or even increase β-cell mass (13).
Previously, we showed that 26-week exenatide therapy lowered A1C similarly to insulin glargine in patients with type 2 diabetes who were treated with metformin and a sulfonylurea (8). However, at present, no data exist regarding the relative effects of these treatments on β-cell function, nor is it known whether the effects of either therapy are sustained after discontinuation. The aim of the current study was to assess the effects of treatment with exenatide or insulin glargine on β-cell function, glycemic control, body weight, and safety, after 52 weeks of treatment and during a 12-week off-drug period.
RESEARCH DESIGN AND METHODS
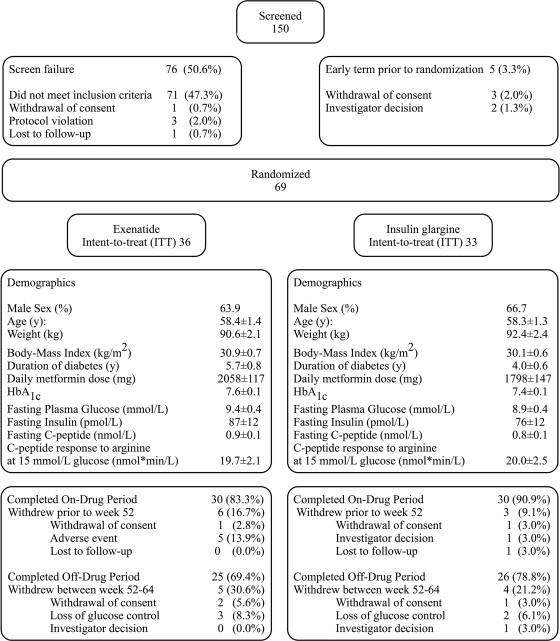
The study was performed between 27 September 2004 and 13 September 2007 at three study sites, in Sweden, Finland, and the Netherlands. In total, 69 patients were randomly assigned using a permutated block randomization scheme stratified by site and screening A1C to receive exenatide or insulin glargine, in addition to ongoing metformin treatment (Fig. 1). Inclusion criteria were age 30–75 years, A1C 6.5–9.5%, BMI 25–40 kg/m2, and metformin treatment at a stable dose for at least 2 months. No other blood glucose–lowering agents were allowed within 3 months before screening. No changes in other agents known to affect β-cell function (such as ACE inhibitors and angiotensin receptor blockers) were allowed during the study. The study protocol was approved by the ethics review committee at each site and was in accordance with the principles described in the Declaration of Helsinki. All participating patients gave their written informed consent before screening.
Figure 1.
Protocol flow chart and baseline characteristics of the study population. Data represent means ± SEM.
Patients randomly assigned to exenatide (n = 36) initiated treatment at a dose of 5 μg b.i.d., injected 15 min before breakfast and dinner, for a period of 4 weeks, followed by a dose increase to 10 μg b.i.d. Exenatide was titrated to a maximum dose of 20 μg t.i.d., or the maximum tolerated dose, when A1C ranged from 7.1 to 7.5% at two consecutive visits or when A1C was ≥7.6% at any given visit. Patients randomly assigned to insulin glargine (n = 33) started at an initial dose of 10 IU q.d., injected at bedtime. Patients were instructed to increase the daily dose based on their fasting self-monitored blood glucose (SMBG) levels, according to a prespecified algorithm (14). When fasting SMBG was ≥5.6 mmol/l on 3 consecutive days, the insulin dose was increased by 2 units until finally fasting SMBG would range between 4.5 and 5.5 mmol/l. If a hypoglycemic event (<3.3 mmol/l) occurred, patients were instructed to refrain from increasing the insulin glargine dose for 7 days and to contact the study physician. When necessary, the importance of proper titration of insulin was emphasized.
Study end points
Insulin secretion and sensitivity were measured during a combined euglycemic-hyperinsulinemic and hyperglycemic clamp procedure (supplementary Fig. 1A, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc08-1797/DC1) (15,16). First- and second-phase C-peptide secretion was calculated as area under the curve (AUC)180–190 min and AUC190–260 min. Arginine-stimulated C-peptide secretion (AIRarg) was calculated as the incremental AUC260–270 min above the fasting C-peptide concentration. Arginine was administered during a hyperglycemic clamp to measure maximum insulin secretory capacity at a steady-state glucose concentration of 15 mmol/l (17). Clamps were performed before randomization, after 52 weeks of treatment, and after a 4-week off-drug period. After an overnight fast, an indwelling cannula was inserted into an antecubital vein for infusion of glucose and insulin. To obtain arterialized venous blood samples, an cannula was inserted in a retrograde fashion into a dorsal hand or wrist vein and maintained in a heated box at 50°C. During the clamp at week 52, patients randomly assigned to exenatide, were given the study drug 15 min before the onset of the hyperglycemic clamp, and patients randomly assigned to insulin glargine received their last insulin dose the night before at bedtime.
A1C (normal range: 4.3–6.1%, Diabetes Control and Complications Trial standardized Bio-Rad assay) was measured using the fasting plasma glucose, and safety parameters were measured before randomization and during each follow-up visit until the end of the 12-week off-drug period by a central laboratory (Quintiles, Livingston, U.K.). Patients were instructed to record seven-point (fasting, 2 h after breakfast, before lunch, 2 h after lunch, before dinner, 2 h after dinner, and at bedtime) SMBG profiles using an OneTouch Ultra blood glucose meter (LifeScan, Milpitas, CA) before each visit. Plasma glucose concentrations during the clamp were measured using an YSI 2300 STAT Plus analyzer (YSI, Yellow Springs, OH) in Sweden and the Netherlands and using a Beckman Coulter Glucose Analyzer II (Beckman Coulter, Fullerton, CA) in Finland. C-peptide samples were analyzed at the VU University Medical Center using an immunoradiometric assay (Centaur; Bayer Diagnostics, Mijdrecht, Netherlands).
Statistical analysis
The primary efficacy end point of this study is the treatment effect on β-cell function as measured by the ratio of week 52 combined glucose- and arginine-stimulated insulin secretion during a hyperglycemic clamp. A sample size of 26 patients per group was required to provide 90% power to detect a between-group significant difference in arginine-stimulated insulin secretion between the two treatment groups, assuming that the mean incremental AUC value at baseline is 200 pmol · min−1 · l−1 for both groups and values at week 52 are 1,100 and 300 pmol · min−1 · l−1 for the exenatide and insulin glargine groups, respectively (11,18).
All outcome measures were compared between the two treatment groups using an ANCOVA model. The dependent variable used in the model is the loge-transformed ratio to pretreatment for the β-cell function parameters (AIRarg, first phase, and second phase). For all other end points the dependent value used is the mean at the corresponding visit. The model includes factors for treatment group (exenatide/glargine), site (Netherlands/Sweden/Finland), and baseline A1C stratum (≤8.5%/>8.5%), and the pretreatment variable of the corresponding dependent variable as a covariate.
Statistical analysis was done using SAS software (SAS Institute, Cary, NC). All inferential statistical tests were conducted at a significance level of 0.05 (two-sided). Unless otherwise stated, data are presented as means ± SEM.
RESULTS
Patient disposition and baseline clinical characteristics
Patient disposition and baseline clinical characteristics are shown in Fig. 1. Sixty patients completed the 52-week treatment period. Of the patients randomly assigned to exenatide, 62.1% (n = 18) were treated with exenatide 10 μg b.i.d. at 52 weeks of treatment. Five (17.2%) patients were using 20 μg t.i.d., two (6.9%) were using 10 μg t.i.d., one (3.4%) was using 15 μg b.i.d., and one (3.4%) was using 15 μg of t.i.d. The daily exenatide dose was reduced to 5 μg b.i.d. in two patients (6.9%). Despite this increase in daily exenatide dose, none of these patients reached the A1C target of <7.1%. At 52 weeks, the mean ± SEM insulin glargine dose used was 33.6 ± 3.5 units/day. The corresponding fasting SMBG in the insulin glargine–treated group was 5.6 ± 0.2 mmol/l.
A1C and fasting plasma glucose
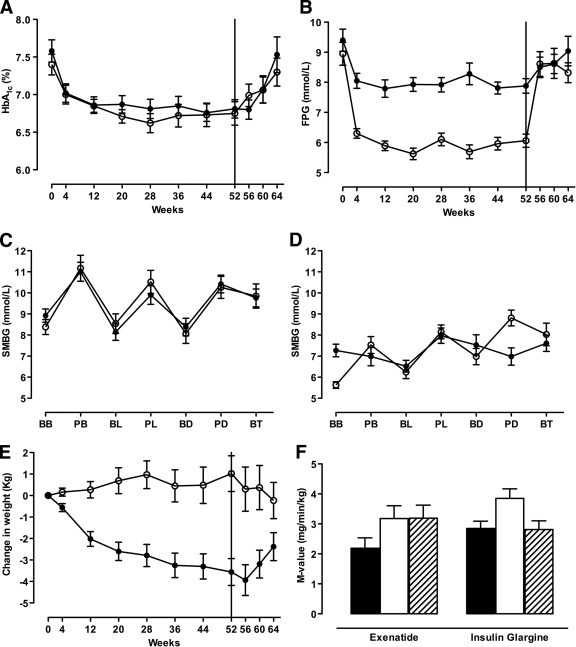
Exenatide and insulin glargine treatment resulted in similar reductions in A1C (0.8 ± 0.1 and 0.7 ± 0.2%, respectively; P = 0.55), with both groups achieving a mean A1C of 6.8% at 52 weeks. The insulin glargine group showed a significantly greater reduction in fasting plasma glucose compared with the exenatide group (−2.9 ± 0.4 vs. −1.6 ± 0.3 mmol/l, respectively; P < 0.0001), whereas SMBG profiles demonstrated significantly greater reductions in postprandial glucose excursions in the exenatide-treated patients (Fig. 2C and D). During the off-drug period, both A1C and fasting plasma glucose increased in both groups and were not significantly different compared with pretreatment values after 12 weeks off-drug (Fig. 2A and B).
Figure 2.
Time course for A1C (A) and fasting plasma glucose (B). SMBG concentrations before (C) and after (D) 52 weeks of treatment. Changes in body weight (E) and insulin sensitivity were measured as the M value (F). Data are means ± SEM. ●, exenatide; ○, insulin glargine; ■, pretreatment; □, 52 weeks on-drug; ▨, 4 weeks off-drug. Vertical black line at 52 weeks represents cessation of study medication. BB, before breakfast; AB, after breakfast; BL, before lunch; AL, after lunch; BD, before dinner; AD, after dinner; BT, bedtime.
Body weight and insulin sensitivity
Fifty-two weeks of exenatide treatment resulted in a lowering of body weight of −3.6 ± 0.6 kg, whereas treatment with insulin glargine resulted in a body weight increase of +1.0 ± 0.8 kg (between-group difference, −4.6 ± 1.1 kg; P < 0.0001) (Fig. 2E). During the 12-week off-drug period, body weight trended toward baseline values with both therapies (between-group difference −2.4 ± 1.1 kg; P = 0.03).
At baseline, insulin-mediated glucose uptake did not differ between the two treatment groups (Fig. 2F). Treatment with exenatide and insulin glargine improved insulin sensitivity to the same extent by 0.9 ± 0.3 and 1.1 ± 0.3 mg · min−1 · kg−1, respectively (P = 0.49). After a 4-week discontinuation of study medication, the M value was not significantly different from pretreatment values in the insulin glargine–treated group, whereas it remained significantly higher in the exenatide-treated group (between-group difference 0.8 ± 0.4 mg/min/kg; P = 0.03).
Hyperglycemic clamp–derived measures of β-cell function
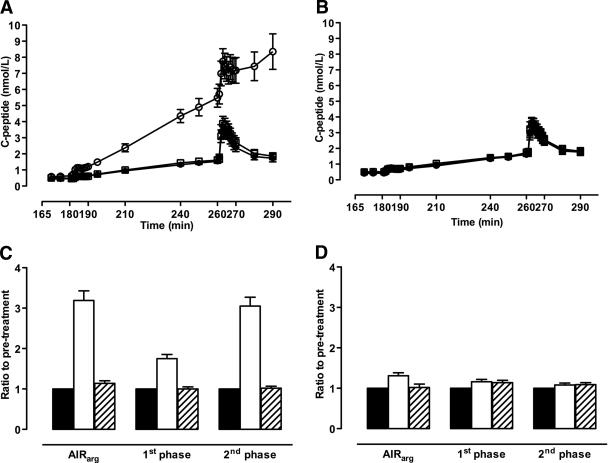
At baseline, both glucose- and arginine-stimulated C-peptide secretion did not differ between the two treatment groups (Table 1; Fig. 3A–D). After 52 weeks of treatment, the exenatide group demonstrated a significant increase in all measures of β-cell function. Accordingly, exenatide treatment significantly increased first- and second-phase glucose-stimulated C-peptide secretion by 1.53 ± 0.11- and 2.85 ± 0.22-fold, respectively (P < 0.0001), compared with insulin glargine. The C-peptide response to arginine during hyperglycemia increased 3.19 ± 0.24-fold from pretreatment in the exenatide group compared with a 1.31 ± 0.07-fold increase in the insulin glargine group (between-group difference 2.46 ± 0.20-fold; P < 0.0001). After 4 weeks discontinuation of the study medication, measures of β-cell function returned to pretreatment values in both groups.
Table 1.
Measures of β-cell secretory function during hyperglycemic clamp and ratio to pretreatment in the exenatide- and insulin glargine–treated groups
| Pretreatment (week −2) | On-drug (week 52) | Off-drug (week 56) | On-drug ratio to pretreatment (week 52) | Off-drug ratio to pretreatment (week 56) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric mean | Between-group difference | P | Geometric mean | Between-group difference | P | ||||
| First phase | |||||||||
| Insulin glargine | 5.4 ± 0.6 | 6.1 ± 0.5 | 6.1 ± 0.6 | 1.17 ± 0.06 | 1.13 ± 0.05 | ||||
| Exenatide | 5.4 ± 0.6 | 9.4 ± 1.0 | 5.0 ± 0.6 | 1.78 ± 0.11 | 1.53 ± 0.11 | <0.0001 | 1.00 ± 0.05 | 0.90 ± 0.06 | 0.1188 |
| Second phase | |||||||||
| Insulin glargine | 77.4 ± 8.8 | 80.7 ± 6.9 | 86.2 ± 9.1 | 1.08 ± 0.05 | 1.10 ± 0.05 | ||||
| Exenatide | 78.5 ± 8.3 | 235.6 ± 23.0 | 79.5 ± 9.1 | 3.05 ± 0.22 | 2.85 ± 0.22 | <0.0001 | 1.01 ± 0.04 | 0.92 ± 0.06 | 0.1996 |
| AIRarg | |||||||||
| Insulin glargine | 20.0 ± 2.5 | 24.8 ± 2.2 | 21.4 ± 2.5 | 1.31 ± 0.07 | 1.03 ± 0.08 | ||||
| Exenatide | 19.7 ± 2.1 | 62.2 ± 7.0 | 22.0 ± 2.6 | 3.19 ± 0.24 | 2.46 ± 0.20 | <0.0001 | 1.12 ± 0.06 | 1.08 ± 0.10 | 0.4052 |
Figure 3.
C-peptide concentrations during hyperglycemic clamp and ratio to pretreatment in the exenatide (A and C)- and insulin glargine (B and D)-treated group. Data represent mean ± SEM in A and B and geometric mean ± SEM in C and D. AIRarg, C-peptide response to arginine at 15 mmol/l glucose concentration; 1st phase, first-phase C-peptide response to glucose; 2nd phase, second-phase C-peptide response to glucose. See research design and methods for calculations of β-cell function measures. ●, ■, pretreatment; ○, □, 52-weeks on-drug; ■, ▨, 4 weeks off-drug.
Adverse effects and tolerability
The most frequently observed adverse event in exenatide-treated patients was mild-to-moderate nausea (50%). Other gastrointestinal adverse events were reported more commonly in exenatide-treated patients, including vomiting, diarrhea, and abdominal distension. Biochemically confirmed hypoglycemia (<3.3 mmol/l) was observed more frequently in the insulin glargine group (24.2%) than in the exenatide-treated patients (8.3%). There was no severe hypoglycemia with either treatment. Other adverse events observed more frequently in the insulin glargine group included influenza and gastroenteritis. One patient randomly assigned to exenatide developed pancreatitis, which resolved after withdrawal of the study medication.
CONCLUSIONS
This study demonstrates that 52 weeks of treatment with exenatide significantly improves β-cell function compared with insulin glargine in metformin-treated type 2 diabetic patients. In addition, exenatide treatment achieved similar improvements in glycemic control, reduced body weight, and resulted in fewer hypoglycemic events. Both exenatide and insulin glargine resulted in similar improvements in whole-body insulin sensitivity. After cessation of both treatments, end point measures returned to pretreatment values.
In type 2 diabetes, defects in β-cell function include an absent first-phase insulin response and a gradually diminishing second-phase response to glucose (1). This progressive loss of β-cell function is considered to be the main factor responsible for the gradual increase of glycemia over time, regardless of the therapy used (3). Acute and chronic exposure to exenatide has been shown to improve β-cell function (4–6,8). However, there have been no studies comparing β-cell function after long-term exposure to exenatide or other glucose-lowering therapies. In the current study, exenatide therapy for 1 year significantly improved β-cell function compared with titrated insulin glargine therapy, in the presence of comparable improvements in glycemic control.
A number of prior studies have demonstrated that exenatide is not inferior to insulin regimens as a glucose-lowering treatment option in type 2 diabetic patients with inadequate glucose control (8–10). These previous insulin comparator trials have been criticized for not achieving optimal insulin doses in the comparator arm of the study (19) despite mean reductions in A1C levels that were within the range of reductions observed in comparable insulin trials. In the current study, insulin titration resulted in a mean daily insulin dose of 34 ± 19 units. Although this insulin glargine dose is lower than that used in other studies of type 2 diabetes (20,21), SMBG targets were achieved in the current study, suggesting that insulin doses were appropriately titrated in the majority of patients. In addition, the intensive treatment with both therapies reduced mean A1C values after 52 weeks to 6.8%. In the LANMET study (22), in which, comparable to our study, insulin glargine was added to metformin monotherapy in patients with type 2 diabetes, a higher dose of insulin glargine (68 units/day) was used. These apparent differences in insulin glargine dose may be attributed to the relatively good baseline A1C in our population compared with that of the LANMET participants (A1C 9.5%).
One limitation of the current study should be highlighted: exenatide was given 15 min before the start of the hyperglycemic clamp study. We therefore cannot discriminate between acute and long-term effects of exenatide on β-cell function. The current study does, however, support the observation that longer-term treatment with exenatide does not attenuate the known acute effects of this therapy on β-cell function, whereas active insulin therapy did not improve β-cell function to the same degree. Because the primary study objective was to determine the effects of both active exenatide and insulin therapy on β-cell function in type 2 diabetic patients, we decided to perform the tests on therapy, in accordance with previous studies (18,23,24). These findings support the idea that longer-term exenatide treatment could have an enduring effect on β-cell function.
In the current study, treatment with both exenatide and insulin glargine resulted in a similar reduction in A1C. Of interest, the two therapies achieved this result through different ways: exenatide primarily affected postprandial glucose excursions, with a modest effect on fasting glucose, whereas insulin glargine predominantly reduced fasting plasma glucose, without influencing postprandial glucose elevations. The resultant average glucose concentrations are similar in both treatment groups, as reflected by the A1C concentration. Albeit modest, the improved glycemic control in either group may have lowered the hyperglycemia-associated oxidative stress burden, which in part may explain the modest improvement in β-cell function in the insulin glargine group (25). Obviously, patients treated with exenatide showed a greater improvement in acute β-cell function, which is considered to be the result of binding to the β-cell GLP-1 receptor (13). These findings are consistent with work in a number of animal models, in which exenatide or GLP-1 has been shown to increase β-cell mass and to reduce apoptosis (13). Collectively, these observations lead to the question whether long-term exenatide administration to patients with type 2 diabetes may restore some of the β-cell functionality that is lost as part of the natural history of the disease.
In our study the improved β-cell function at 52 weeks was lost 4 weeks after cessation of either study treatment, and this was accompanied by an increase in plasma glucose and A1C to pretreatment values in both study arms. Interestingly, insulin sensitivity remained significantly improved after 4 weeks cessation of treatment in the exenatide-treated group only. This finding may suggest additional effects of exenatide, possibly mediated by weight loss, which may be of longer duration. Whether longer-term exposure to exenatide can alter functional β-cell mass in the absence of active exenatide treatment will require further study. These effects may be dependent on other factors including diabetes duration, the amount of functional β-cells present at the initiation of therapy and overall metabolic control achieved. To study a possible preserving effect on β-cell function, an additional 2-year extension trial is currently underway.
Both titrated exenatide and insulin glargine were generally well tolerated with >80% of patients in both groups completing 1 year of therapy. These therapeutic approaches differed in that the most common side effects with exenatide were of gastrointestinal origin, occurring in a proportion of patients similar to that reported in previous studies (4–10). Mild to moderate nausea (47%) was the most commonly reported adverse event with exenatide. In contrast, hypoglycemia was the most common adverse event reported in 25% of the insulin glargine–treated patients.
In summary, this study uniquely demonstrates that 1 year of treatment with exenatide significantly improved β-cell function and reduced body weight in the presence of similar improvements in glycemic control compared with insulin glargine treatment. After cessation of therapy, the beneficial effects on β-cell function, glycemic control, and body weight were not sustained, suggesting that active treatment is necessary to maintain these beneficial effects of exenatide in patients in whom oral blood glucose–lowering therapy has failed.
Supplementary Material
Online-Only Appendix
Acknowledgments
The study was sponsored by Amylin Pharmaceuticals and Eli Lilly and Company. M.C.B. has received travel expenses and fees for speaking at meetings from Eli Lilly and Amylin (which developed and markets BYETTA [exenatide]). M.D. has received travel expenses, fees for speaking at meetings, and grant support from Eli Lilly. B.E. has received travel expenses and fees for speaking at meetings and has served on the advisory board for Eli Lilly. D.M.K. was an employee and stockholder of Amylin. M.-R.T. received travel expenses, fees for speaking at meetings, and grant support from Eli Lilly. U.S. has received fees for speaking at meetings and served on the advisory board for Amylin H.Y.J. has received consultation fees from Amylin and grant support from Eli Lilly. No other potential conflicts of interest relevant to this article were reported.
The study was collectively initiated and designed by the investigators from the three study sites.
Parts of this study were presented in abstract form at the 68th Scientific Sessionsof the American Diabetes Association, San Francisco, California, -10 June 2008.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Kahn SE: The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003; 46: 3– 19 [DOI] [PubMed] [Google Scholar]
- 2.Riddle MC: Timely initiation of basal insulin. Am J Med 2004; 116: 3S– 9S [DOI] [PubMed] [Google Scholar]
- 3.Turner RC, Cull CA, Frighi V, Holman RR: the UKPDSG. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999; 281: 2005– 2012 [DOI] [PubMed] [Google Scholar]
- 4.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD: the Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27: 2628– 2635 [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28: 1092– 1100 [DOI] [PubMed] [Google Scholar]
- 6.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28: 1083– 1091 [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Hoogwerf BJ, Duran Garcia S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG: The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146: 477– 485 [DOI] [PubMed] [Google Scholar]
- 8.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG: Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143: 559– 569 [DOI] [PubMed] [Google Scholar]
- 9.Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodows R, Trautmann M: A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a noninferiority study. Diabetologia 2007; 50: 259– 267 [DOI] [PubMed] [Google Scholar]
- 10.Barnett AH, Burger J, Johns D, Brodows R, Kendall DM, Roberts A, Trautmann ME: Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29: 2333– 2348 [DOI] [PubMed] [Google Scholar]
- 11.Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, Fineman MS, Kim DD, Nauck MA: Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocr Metab 2005; 90: 5991– 5997 [DOI] [PubMed] [Google Scholar]
- 12.Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A: Mathematical modeling shows exenatide improved β-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res 2006; 38: 838– 844 [DOI] [PubMed] [Google Scholar]
- 13.Drucker DJ: The biology of incretin hormones. Cell Metab 2006; 3: 153– 165 [DOI] [PubMed] [Google Scholar]
- 14.Yki-Jarvinen H, Juurinen L, Alvarsson M, Bystedt T, Caldwell I, Davies M, Lahdenpera S, Nijpels G, Vahatalo M: Initiate insulin by aggressive titration and education (INITIATE): a randomized study to compare initiation of insulin combination therapy in type 2 diabetic patients individually and in groups. Diabetes Care 2007; 30: 1364– 1369 [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 1979; 237: E214– E223 [DOI] [PubMed] [Google Scholar]
- 16.Van Cauter E, Mestrez F, Sturis J, Polonsky KS: Estimation of insulin secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992; 41: 368– 377 [DOI] [PubMed] [Google Scholar]
- 17.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D, Jr: Diminished β-cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984; 74: 1318– 1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zander M, Madsbad S, Madsen JL, Holst JJ: Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet 2002; 359: 824– 830 [DOI] [PubMed] [Google Scholar]
- 19.Home PD, Nauck MA, Duran S, Kim D, et al. : Comment on: A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50: 1561– 1564 [DOI] [PubMed] [Google Scholar]
- 20.Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, Bode B, Garber A, Group IS: Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 2005; 28: 260– 265 [DOI] [PubMed] [Google Scholar]
- 21.Rosenstock J, Dailey G, Massi-Benedetti M, Fritsche A, Lin Z, Salzman A: Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 2005; 28: 950– 955 [DOI] [PubMed] [Google Scholar]
- 22.Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, Tulokas T, Hulme S, Hardy K, McNulty S, Hanninen J, Levanen H, Lahdenpera S, Lehtonen R, Ryysy L: Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 2006; 49: 442– 451 [DOI] [PubMed] [Google Scholar]
- 23.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O: One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and α- and β-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 2004; 53: 1187– 1194 [DOI] [PubMed] [Google Scholar]
- 24.Vilsboll T, Brock B, Perrild H, Levin K, Lervang H, Kolendorf K, Krarup T, Schmitz O, Zdravkovic M, Le-Thi T, Madsbad S: Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med 2008; 25: 152– 156 [DOI] [PubMed] [Google Scholar]
- 25.Yki-Jarvinen H: Glucose toxicity. Endocr Rev 1992; 13: 415– 431 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online-Only Appendix