Cardiac Outcomes After Screening for Asymptomatic Coronary Artery Disease in Patients With Type 2 Diabetes: The DIAD Study: A Randomized Controlled Trial (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 1.
Published in final edited form as: JAMA. 2009 Apr 15;301(15):1547–1555. doi: 10.1001/jama.2009.476
Abstract
Context
Coronary artery disease (CAD) is the major cause of mortality and morbidity in patients with type 2 diabetes. But the utility of screening patients with type 2 diabetes for asymptomatic CAD is controversial.
Objective
To assess whether routine screening for CAD identifies patients with type 2 diabetes as being at high cardiac risk and whether it affects their cardiac outcomes.
Design, Setting, and Patients
The Detection of Ischemia in Asymptomatic Diabetics (DIAD) study is a randomized controlled trial in which 1123 participants with type 2 diabetes and no symptoms of CAD were randomly assigned to be screened with adenosine-stress radionuclide myocardial perfusion imaging (MPI) or not to be screened. Participants were recruited from diabetes clinics and practices and prospectively followed up from August 2000 to September 2007.
Main Outcome Measure
Cardiac death or nonfatal myocardial infarction (MI).
Results
The cumulative cardiac event rate was 2.9% over a mean (SD) follow-up of 4.8 (0.9) years for an average of 0.6% per year. Seven nonfatal MIs and 8 cardiac deaths (2.7%) occurred among the screened group and 10 nonfatal MIs and 7 cardiac deaths (3.0%) among the not-screened group (hazard ratio [HR], 0.88; 95% confidence interval [CI], 0.44–1.88; _P_=.73). Of those in the screened group, 409 participants with normal results and 50 with small MPI defects had lower event rates than the 33 with moderate or large MPI defects; 0.4% per year vs 2.4% per year (HR, 6.3; 95% CI, 1.9–20.1; _P_=.001). Nevertheless, the positive predictive value of having moderate or large MPI defects was only 12%. The overall rate of coronary revascularization was low in both groups: 31 (5.5%) in the screened group and 44 (7.8%) in the unscreened group (HR, 0.71; 95% CI, 0.45–1.1; _P_=.14). During the course of study there was a significant and equivalent increase in primary medical prevention in both groups.
Conclusion
In this contemporary study population of patients with diabetes, the cardiac event rates were low and were not significantly reduced by MPI screening for myocardial ischemia over 4.8 years.
Almost 200 million people worldwide have type 2 diabetes.1 Coronary artery disease (CAD) is a major health concern and the leading cause of death in individuals with type 2 diabetes.2 CAD is often asymptomatic in these patients until the onset of myocardial infarction or sudden cardiac death.3 Type 2 diabetes is also widely recognized as a CAD risk equivalent.4
The current standard of care for type 2 diabetes emphasizes the reduction of cardiovascular risk factors.2,5 However, there has also been substantial interest in the early detection of asymptomatic CAD by screening of patients with type 2 diabetes.6 Recent studies have shown that CAD can be detected noninvasively in a significant number of these individuals.7–9 Inducible ischemia7,10,11 and coronary artery calcium9,11 each have been shown to be associated with worse cardiac outcomes. However, the potential of routine screening to alter treatment and to prevent cardiac events in persons without clinically apparent CAD is largely unknown.6,12,13 Thus, although endorsed by some professional organizations,6,14 screening of patients with type 2 diabetes and no symptoms of CAD remains highly controversial in the absence of prospective outcome studies supporting its utility.6,12,13,15,16
The Detection of Ischemia in Asymptomatic Diabetics (DIAD) study is a randomized controlled trial in which participants were randomly assigned either to be systematically screened with stress myocardial perfusion imaging (MPI) or not to be screened.8 The aim of DIAD was to test the hypothesis that systematic screening would identify higher-risk individuals and beneficially affect their risk of myocardial infarction or cardiac death.
METHODS
The DIAD study prospectively assessed the prevalence, predictors, and outcomes of inducible ischemia in patients with type 2 diabetes without symptomatic or previously recognized CAD.8 Inclusion criteria were that onset of type 2 diabetes occurred at age 30 years or older with no history of ketoacidosis and that patients were between the ages of 50 and 75 years at enrollment. Exclusion criteria included (1) angina pectoris or chest discomfort evaluated with a positive Rose questionnaire8; (2) stress test or coronary angiography within the prior 3 years; (3) history of myocardial infarction, heart failure, or coronary revascularization; (4) abnormal rest electrocardiographic results, ie, pathological Q waves, ischemic (≥1 mm depression) ST segments, deep negative T waves, or complete left bundle-branch block; (5) any clinical indication for stress testing; (6) active bronchospasm precluding the use of adenosine; and (7) limited life expectancy due to cancer or end-stage renal or liver disease.8
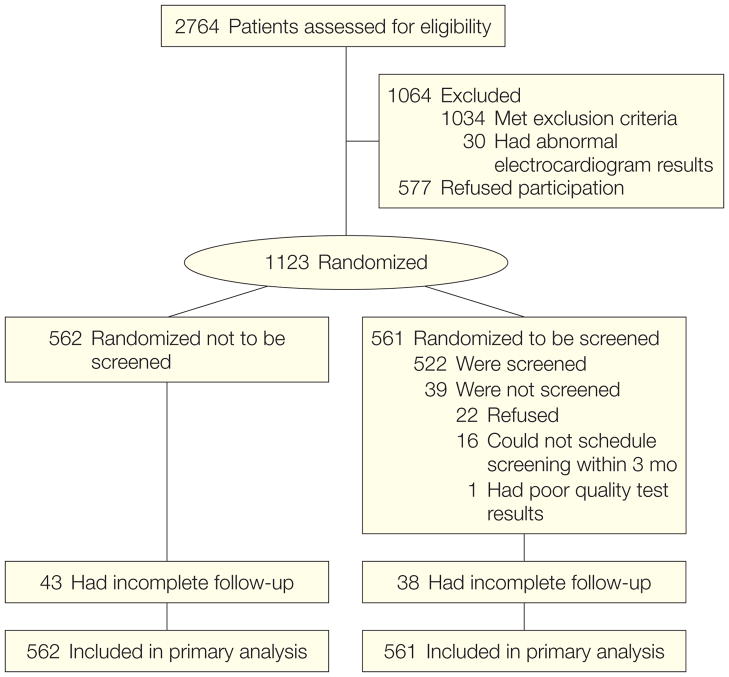
Of 2764 patients screened, 1034 were excluded because they failed to satisfy the clinical inclusion criteria and 30 were excluded because of significant Q-waves on electrocardiogram (Figure 1). Of the 1700 eligible participants, 1123 (66%) consented to participate and were enrolled at 14 centers in the United States and Canada between July 2000 and August 2002.8 The DIAD protocol was approved by the institutional review boards at each participating center. The study design and procedures were explained by a member of the local research team to potential participants. Those who agreed to participate subsequently provided written informed consent.
Figure 1.
Flow of Study Participants
Medical history and demographics were obtained. Ethnic/racial origin was recorded as stated by the participants to confirm equal distribution in the randomized groups and to provide insight into the generalizability of the results. All participants had a physical examination, including assessment of diabetic neuropathy and cardiac autonomic dysfunction,8,17 and underwent blood and urine laboratory testing.
After baseline testing, the participants were assigned a sequential identification number at each site and a corresponding sealed envelope was opened. Random permuted blocks (block size 6) were used to assign the randomization sequence 1:1 at each site.8
In total, 561 participants were randomized to screening with adenosine Tc-99m sestamibi MPI and 562 to no screening.8
Evaluation and Follow-up
Nuclear cardiologists at each site interpreted the stress MPI studies and communicated the results to the participants and their personal physicians.8 Participants also received copies of their laboratory results, including hemoglobin A1c and lipid levels. The screening MPI tests were also interpreted by an independent expert panel, with computer quantification of perfusion abnormalities as percent of the left ventricle at the core laboratory.8 Nonperfusion abnormalities, including ischemic electrocardiographic changes, transient left ventricle dilation, or baseline left ventricle dysfunction, were also assessed.8 The quality of images was deemed excellent or good in 509 (97%), poor in 13 (2%), and not interpretable in 1 (<1%). The panel’s interpretations were used in analyzing risk for cardiac events but were not sent to the participants or their providers.
The DIAD study was not designed as a treatment trial and did not mandate coronary angiography or any specific treatment plan for patients with abnormal screening tests. The protocol design therefore reflects routine clinical practice, in which management decisions are made according to the best judgment of patients’ medical providers. Thus, the DIAD study also assessed the value and effect of CAD screening on the overall medical management of participants.
Participants were asked about their health status, medications, intervening cardiac events, additional stress testing, coronary angiography, and revascularization at 6-month intervals.8 Cardiac events were independently adjudicated by a committee, which was blinded to the randomization status.
Cardiac Events
The primary end point consisted of the composite of nonfatal myocardial infarction and cardiac death. Cardiac death included fatal myocardial infarction (within 30 days); death due to heart failure or arrhythmia; or sudden cardiac death. Secondary end points included unstable angina, heart failure, stroke, and coronary revascularization.
Statistical Analysis
All analyses were conducted with SAS statistical software version 9.1 (SAS Institute Inc, Cary, North Carolina). Bivariate associations, according to losses to follow-up, randomization status, and factors associated with cardiac events, were first tested using t tests, Wilcoxon rank sum, χ2, and Fisher exact analyses. All analyses were 2-sided. Changes in medications were assessed using the McNemar test and logistic regression. Actuarial survival analysis was used to assess cardiac events according to randomization status and then in the screening group with stratification by screening results. Cox proportional hazards regression was used to determine hazard ratios (HRs) comparing (1) events in screened vs non-screened participants; (2) events in participants with normal MPI vs non-perfusion, small or moderate or large perfusion defects, as well as those who were randomized to screening but who did not undergo MPI or who had an uninterpretable result (n=39); and (3) participants with and without a primary event in unadjusted and age- and sex-adjusted models.
The study was designed with an anticipated 5% to 10% cardiac event rate over a projected 5-year follow-up period. We estimated that 500 participants would be required in each group to have a power of 80% to detect a 20% difference between the 2 groups with a 2-sided α of .05.
RESULTS
Of the 561 participants randomized to screening, 522 underwent stress MPI and 39 did not. Of the latter, 22 refused, 16 were unable to schedule their MPI within the required 3-month time window, and the screening MPI of 1 participant was poor quality and not interpretable (Figure 1). These 39 participants without MPI were included in the analysis on an intention-to-screen basis. The total cohort of 1123 individuals was used in the analysis, with participants censored at the time of last follow-up (Figure 1). Table 1 shows the baseline characteristics of the participants in the screening and no-screening groups. The mean (SD) follow-up was 4.8 (0.9) years and median was 5 years. Follow-up was 97% complete at 3.5 years. Eighty-one participants, evenly distributed between groups, did not have complete follow-up visits, Notably, the prevalence of ischemia did not differ in screened participants with complete or incomplete follow-up. The last follow-up data were collected in September 2007.
Table 1.
Baseline Characteristics According to Randomization
| No Screening (n = 562) | Screening (n = 561) | |
|---|---|---|
| Mean (SD) | ||
| Age, y | 60.8 (6.4) | 60.7 (6.7) |
| Diabetes duration, y | 8.9 (6.9) | 8.2 (7.1) |
| Body mass indexa | 31.0 (6.1) | 31.1 (6.5) |
| Hemoglobin A1C, % | 7.0 (1.5) | 7.2 (1.6) |
| Serum creatinine, mg/dL | 0.99 (0.33) | 0.95 (0.29) |
| Clinical risk factors | ||
| Cholesterol, mg/dL | ||
| LDL | 114 (33) | 114 (32) |
| HDL | 49 (15) | 50 (15) |
| Triglycerides, mg/dL | 168 (101) | 172 (118) |
| Blood pressure, mm Hg | ||
| Systolic | 132 (16) | 133 (17) |
| Diastolic | 79 (8) | 80 (9) |
| No. (%) of Patients | ||
| Male sex | 311 (55) | 290 (52) |
| Nonwhite race | 129 (23) | 122 (22) |
| Diabetes treatment | ||
| Oral agents | 356 (64) | 346 (63) |
| Insulin | 50 (9) | 59 (11) |
| Insulin and oral agent | 76 (13) | 75 (13) |
| Diet only | 80 (14) | 81 (14) |
| Retinopathy | 92 (16) | 81 (14) |
| Microalbumin:creatinine ratio, μg/mgc | ||
| <30 | 411 (75) | 425 (78) |
| 30–299 | 113 (20) | 104 (19) |
| >300 | 27 (5) | 19 (3) |
| Peripheral neuropathy | ||
| Numbness | 214 (38) | 178 (32) |
| Pain | 73 (13) | 56 (10) |
| Tingling | 158 (28) | 156 (28) |
| Erectile dysfunction | 149 (48) | 140 (48) |
| Cardiac autonomic dysfunction, lowest quartile | 125 (22) | 120 (21) |
| Peripheral vascular disease | 51 (9) | 52 (9) |
| Current smoking | 52 (9) | 57 (10) |
| Family history of premature CADb | 98 (17) | 117 (21) |
Primary Outcomes
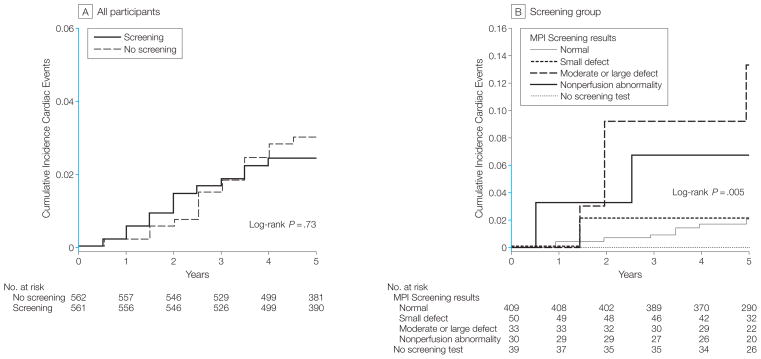
Table 2 and Figure 2A show cardiac events according to randomization status. Overall during the follow-up period, there were 32 cardiac events: 17 participants developed nonfatal MIs and 15 had cardiac deaths, of which 13 were sudden and unexpected. There were also 18 noncardiac deaths. The overall cumulative 5-year cardiac event rate was 2.9% and averaged 0.6% per year. When analyzed according to randomization, there were 15 events (7 nonfatal MIs; 8 cardiac deaths) in the screening group vs 17 events (10 nonfatal MIs; 7 cardiac deaths) in the no-screening group (HR, 0.88; 95% confidence interval [CI], 0.44–1.8; log-rank 0.12; _P_=.73).
Table 2.
Events in No-Screening vs Screening Group
| No. (%) of Patients | HR (95% CI)a | Log-Rank P Valueb | ||
|---|---|---|---|---|
| No Screening (n = 562) | Screening (n = 561) | |||
| Primary events | 17 (3.0) | 15 (2.7) | 0.88 (0.44–1.8) | .73 |
| Myocardial infarction | 10 (1.7) | 7 (1.3) | 0.82 (0.34–2.0) | .66 |
| Cardiac death | 7 (1.2) | 8 (1.4) | 1.1 (0.41–3.1) | .80 |
| Secondary events | 14 (2.5)c | 21 (3.7) | 1.5 (0.77–3.0) | .23 |
| Unstable angina | 3 (0.5) | 4 (0.7) | 1.3 (0.30–6.0) | .70 |
| Heart failure | 7 (1.2) | 7 (1.2) | 1.0 (0.35–2.9) | .99 |
| Stroke | 5 (0.9) | 10 (1.8) | 2.0 (0.69–5.9) | .20 |
| Revascularizations | 44 (7.8)d | 31 (5.5) | 0.71 (0.45–1.1) | .14 |
| PTCA | 27 (4.8) | 15 (2.7) | 0.90 (0.48–1.7) | .74 |
| CABG surgery | 20 (3.6) | 16 (2.9) | 0.81 (0.42–1.6) | .76 |
| Death All cause | 15 (2.7) | 18 (3.2) | 1.2 (0.69–2.4) | .60 |
| Noncardiac | 8 (1.4) | 10 (1.8) | 1.3 (0.49–3.2) | .63 |
Figure 2. Cumulative Incidence of Cardiac Events in Participants With Type 2 Diabetes Without Symptomatic or Previously Diagnosed Coronary Artery Disease.
A, Cumulative incidence of cardiac events in 561 participants randomized to systematic baseline screening with stress myocardial perfusion imaging (MPI) and 562 participants randomized to receive no screening. B, Cumulative incidence of cardiac events according to results of systematic screening with stress MPI: normal, small defect, moderate or large defect, and nonperfusion abnormality. No cardiac events occurred in participants who were randomized to but did not complete screening MPI. The y_-_axis scale in blue indicates range from 0 to 0.06.
Table 3 and Figure 2B show cardiac events in the screening group, which varied significantly according to the results of stress MPI (log-rank, 14.93; _P_=.005). Four hundred nine participants (78%) had normal test results and 50 (10%) had small MPI defects. Only 8 (2%) of 409 participants with normal MPI results and 1 (2%) of 50 participants with small MPI defects had hard cardiac events, in contrast to 4 (12.1%) of 33 with moderate or large MPI defects (HR, 6.3; 95 CI, 1.9–20.1; _P_=.001). In addition, 2 (6.7%) of 30 participants with nonperfusion abnormalities had cardiac events (HR, 3.5; 95% CI, 0.8–16.7; _P_=.08). The mean (SD) MPI defect size was 4.1% (6.6%) of left ventricle in participants with cardiac events and 1.4% (2.2%) of left ventricle in participants without events (_P_=.12). The negative predictive value of having a normal MPI was 98% (401 of 409). The positive predictive value was only 6% (7 of 113) of patients for any MPI abnormality and 12% (4 of 33) of patients for moderate or large MPI defects.
Table 3.
Events According to Findings of Screening Myocardial Perfusion Imaging (n = 522)
| No. (%) of Patients With Normal Imaging (n = 409) | Small Perfusion Defect (n = 50) | Moderate or Large Perfusion Defect (n = 33) | Nonperfusion Abnormality (n = 30) | P Value Log-Rankc | ||||
|---|---|---|---|---|---|---|---|---|
| No. (%)of Patients | HR (95% CI)a | No. (%)of Patients | HR (95% CI)a | No. (%)of Patients | HR (95% CI)a | |||
| Percentage of patients | 78 | 10 | 6 | 6 | ||||
| Primary events | 8 (2.0) | 1 (2.0) | 1.1 (0.1–8.5) | 4 (12.1) | 6.3 (1.9–20.1) | 2 (6.7) | 3.5 (0.8–16.7) | .005 |
| Myocardial infarction | 7 (1.7) | 0 | NA | 0 | NA | 2 (6.7) | 4.1 (0.8–19.6) | .14 |
| Cardiac death | 2 (0.5) | 1 (2.0) | 4.3 (0.4–47.4) | 4 (12.1) | 25.7 (4.7–140) | 1 (3.3) | 7.0 (0.6–77.8) | <.001 |
| Secondary events | 13 (3.2) | 1 (2.0) | NA | 3 (9.1) | 3.0 (0.8–10.4) | 4 (13.3) | 4.6 (1.5–14.0) | .01 |
| Unstable angina | 1 (0.2) | 0 | NA | 1 (3.0) | 13.1 (0.8–210) | 2 (6.7) | 28.4 (2.6–313) | <.001 |
| Heart failure | 5 (1.2) | 0 | NA | 0 | NA | 2 (6.7) | 5.4 (1.1–27.9) | .08 |
| Stroke | 7 (1.7 ) | 1 (2.0) | 1.3 (0.2–10.4) | 2 (6.1) | 3.7 (0.8–17.7) | 0 | NA | .31 |
| Revascularization | 16 (3.9) | 2 (4.0) | 1.1 (0.2–4.6) | 7 (21.2) | 6.4 (2.6–15.6) | 6 (20) | 5.6 (2.2–14.4) | <.001 |
| PTCA | 9 (2.2) | 1 (2.0) | 0.2 (0.1–1.9) | 3 (9.1) | 0.7 (0.2–2.8) | 2 (6.7) | 2.1 (0.4–10.1) | .43 |
| CABG surgery | 7 (1.7) | 1 (2.0) | 1.2 (0.2–10) | 4 (12.1) | 7.9 (2.3–27) | 4 (13.3) | 8.3 (2.4–28.2) | <.001 |
| Death All cause | 9 (2.2) | 2 (4.0) | 1.9 (0.4–8.9) | 5 (15.2) | 7.2 (2.4–21.4) | 1 (3.3) | 1.6 (0.2–12.3) | .002 |
| Noncardiacb | 7 (1.7) | 1 (2.0) | 1.3 (0.2–10.2) | 1 (3.0) | 1.9 (0.2–15) | 0 | NA | .90 |
Secondary Outcomes
Seven participants developed unstable angina, and 14 developed heart failure with a similar incidence in the 2 groups (Table 2). There were 15 strokes, with a trend toward more in the screened participants, but none of the strokes resulted from coronary angiography or revascularization.
Coronary Angiography and Revascularization
Coronary angiography was performed within 120 days after screening in 25 (4.4%)of561participants(Table 4), including in 5 (15%) of 33 with moderate or large defects. In comparison, only 3 (0.5%) of 562 participants in the no-screening group underwent angiography within 120 days after randomization (P<.001; Table 4). Nine of the 25 screened participants had significant CAD that was treated with revascularization (6 coronary artery bypass surgery and 3 percutaneous transluminal coronary angioplasty) vs 2 of 3 participants in the no-screening group. There were no procedural complications, but 1 of those screened experienced sudden cardiac death a year after undergoing CABG. Among the 28 participants with moderate or large defects on screening who did not undergo early angiography, only 3 had cardiac events over the ensuing 5 years, although an additional 6 ultimately underwent angiography. One of these latter participants had 3-vessel CAD, which was not amenable to surgery, and died a year later; 3 others had percutaneous transluminal coronary angioplasty.
Table 4.
Follow-up and Medication Use
| No. (%) of Patients | P Value | ||||
|---|---|---|---|---|---|
| No Screening (n = 562) | Screening (n = 561) | ||||
| Additional cardiac testing | |||||
| Nonprotocol stress test | 170 (30) | 118 (21) | <.001 | ||
| Abnormal nonprotocol stress test | 45 (26) | 28 (24) | .60 | ||
| Coronary angiogram <120 d | 3 (0.5) | 25 (4.4) | <.001 | ||
| Revascularization <120 d | 2 (0.36) | 9 (1.6) | .03 | ||
| Total coronary angiograms | 66 (12)a | 80 (14) | .20 | ||
| No. of vessels >70% stenosis | |||||
| 0 | 22 (33) | 40 (50) |  |
.05 | |
| 1 | 21 (32) | 11 (14) | |||
| 2 | 13 (20) | 19 (23) | |||
| 3 | 10 (15) | 10 (12) | |||
| Baseline | 5 Years | Baseline | 5 Years | ||
| Pharmacological treatment | |||||
| Insulin treatment | 126 (22) | 141 (29)b | 134 (24) | 171 (35)b | .54 |
| Oral anti-hyperglycemic agents | 482 (86) | 444 (91)b | 480 (86) | 447 (92)b | .81 |
| Lipid-lowering drugs | 272 (48) | 377 (78) | 255 (45) | 365 (76)b | .32 |
| Statins | 228 (41) | 327 (67)b | 209 (37) | 324 (67)b | .25 |
| Antihypertensive drugs | 320 (57) | 362 (75) | 315 (56) | 355 (74)b | .79 |
| ACE or angiotensin receptor blockers | 229 (41) | 218 (45)b | 206 (37) | 210 (43)b | .17 |
| Aspirin | 261 (46) | 356 (73)b | 241 (43) | 364 (74)b | .24 |
Additional participants underwent coronary angiography and revascularization during the 5-year follow-up period because of unstable angina, chest pain, or perceived high risk. An additional 55 screened participants (10%) and 63 non-screened participants (11%) underwent angiography more than 120 days after randomization (Table 4). Also, 22 screened participants (4%) and 42 nonscreened participants(7%)underwent coronary revascularization more than120 days after randomization (Table 4).
Additional Testing
Participants in both groups were referred clinically for nonprotocol stress tests during follow-up, but this occurred more frequently in the unscreened group (P<.001; Table 4). Approximately one-fourth of tests were abnormal in both groups. By study design, all screened participants who had not experienced cardiac events or revascularization were also invited to return for stress MPI 3 years after entry; repeat tests were performed for 358 participants and overall showed significant improvement in MPI defects as previously reported.18
Medical Treatment
The use of statins, angiotensin-converting enzyme inhibitors, antihypertensive and antihyperglycemic medications, and aspirin for primary medical prevention increased significantly from baseline to 5 years later in the study (Table 4). However, the increased use of these medications was not different in screened and not-screened patients.
Predictors of Cardiac Events
Clinical factors associated with primary events are shown in Table 5. In unadjusted bivariate comparisons, as well as with adjustment for age and sex, we found that male sex, diabetes duration, microalbuminuria/proteinuria, serum creatinine, symptoms of peripheral neuropathy (numbness, pain), diminished peripheral sensation, cardiacautonomic dysfunction, peripheral vascular disease, elevated low-density lipoprotein levels, and family history of premature CAD were associated with development of a primary event. The low number of events precluded multivariate assessment; however, further exploratory analyses with combinations of these factors, suggested an independent role of male sex, serum creatinine, cardiac autonomic dysfunction, peripheral vascular disease, and low-density lipoprotein levels.
Table 5.
Factors Associated With Primary Events
| Event (n = 32) | No Event (n = 1091) | Unadjusted, HR (95% CI), % | Age- and Sex-Adjusted, HR (95% CI), %a | P Value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | ||||||
| Age, y | 62.7 (5.9) | 60.7 (6.6) | 1.04 (0.99–1.10) | 1.04 (0.99–1.10) | .09 | |
| Diabetes duration, y | 12.0 (8.3) | 8.4 (6.9) | 1.05 (1.02–1.10) | 1.05 (1.01–1.10) | .004 | |
| Body mass indexb | 28.7 (4.8) | 31.2 (6.3) | 0.93 (0.86–0.99) | 0.94 (0.87–1.01) | .03 | |
| Hemoglobin A1C, % | 7.5 (1.5) | 7.1 (1.5) | 1.17 (0.98–1.40) | 1.18 (0.99–1.42) | .11 | |
| Waist circumference, cm | 41 (4.9) | 41 (5.9) | 0.99 (0.93–1.05) | 0.99 (0.93–1.05) | .75 | |
| Hip circumference, cm | 107 (10.4) | 112 (14.5) | 0.93 (0.87–1.00) | 0.99 (0.93–1.05) | .02 | |
| Serum creatinine, mg/dL | 1.24 (0.69) | 0.96 (0.29) | 1.14 (1.09–1.18) | 1.13 (1.08–1.18) | .03 | |
| Cholesterol, mg/dL | ||||||
| LDL | 129 (41) | 114 (32) | 1.14 (1.04–1.24) | 1.14 (1.04–1.24) | .01 | |
| HDL | 47 (12) | 50 (15) | 1.00 (0.86–1.08) | 1.00 (0.86–1.14) | .31 | |
| Triglycerides, mg/dL | 203 (149) | 169 (108) | 1.01 (1.00–1.02) | 1.00 (0.99–1.00) | .20 | |
| Antihypertensive drugs | 19 (59) | 616 (57) | 1.12 (0.55–2.27) | 1.15 (0.56–2.33) | .74 | |
| Blood pressure, mm Hg | ||||||
| Systolic | 134 (19) | 131 (16) | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) | .36 | |
| Diastolic | 78 (7.7) | 79 (8.4) | 0.99 (0.95–1.03) | 0.99 (0.95–1.03) | .59 | |
| No. (%) of Patients | ||||||
| Male sex | 23 (72) | 578 (53) | 2.18 (1.01–4.72) | 2.15 (1.00–4.66) | .03 | |
| Insulin treatment | 11 (34) | 249 (23) | 1.82 (0.88–3.78) | 1.77 (0.85–3.68) | .13 | |
| Retinopathy | 6 (19) | 167 (15) | 1.32 (0.54–3.21) | 1.22 (0.50–2.98) | .59 | |
| Microalbumin:creatinine ratio, μg/mg | ||||||
| <30 | 17 (59) | 819 (77) |  |
4.78 (1.84–12.40) | 4.59 (1.76–11.92) | .001 |
| 30–299 | 7 (24) | 210 (19) | ||||
| ≥300 | 5 (17) | 41 (4) | ||||
| Peripheral neuropathy | ||||||
| Numbness | 17 (53) | 375 (34) | 2.17 (1.09–4.35) | 2.23 (1.11–4.48) | .03 | |
| Pain | 7 (22) | 122 (11) | 2.22 (0.96–5.14) | 2.36 (1.01–5.45) | .06 | |
| Tingling | 11 (34) | 303 (28) | 1.36 (0.66–2.82) | 1.44 (0.69–2.83) | .41 | |
| Absent vibration | 9 (28) | 317 (29) | 0.98 (0.45–2.12) | 0.85 (0.39–1.87) | .91 | |
| Absent sensation | 9 (28) | 127 (12) | 2.99 (1.39–6.47) | 2.83 (1.31–6.13) | .005 | |
| Absent reflex | 10 (31) | 348 (32) | 0.96 (0.45–2.02) | 0.87 (0.41–1.86) | .93 | |
| Autonomic neuropathy | ||||||
| Bloating | 6 (19) | 160 (15) | 1.35 (0.56–3.28) | 1.55 (0.69–4.69) | .52 | |
| Dizziness | 4 (13) | 161 (15) | 0.84 (0.29–2.39) | 0.86 (0.30–2.48) | .72 | |
| Erectile dysfunction | 14 (61) | 275 (48) | 1.69 (0.73–3.90) | 1.45 (0.61–3.43) | .21 | |
| Cardiac autonomic dysfunction, lowest quartilec | 17 (53) | 228 (21) | 4.28 (2.14–8.58) | 4.33 (2.14–8.75) | <.001 | |
| Peripheral vascular disease | 9 (28) | 94 (9) | 4.06 (1.88–8.77) | 4.65 (2.14–10.1) | <.001 | |
| Current smoking | 5 (16) | 104 (10) | 1.80 (0.69–4.69) | 1.95 (0.74–5.12) | .25 | |
| Lipid-lowering drugs | 15 (47) | 512 (47) | 0.98 (0.49–1.95) | 0.95 (0.47–1.91) | .98 | |
| Aspirin use | 16 (50) | 486 (45) | 1.25 (0.62–2.49) | 1.18 (0.59–2.36) | .54 | |
| Family history of premature CAD | 11 (34) | 204 (19) | 2.27 (1.09–4.70) | 2.52 (1.21–5.26) | .03 |
COMMENT
The DIAD study is the first large-scale prospective study to randomize type 2 diabetes patients with no symptoms of CAD to screening for inducible ischemia or no-screening and follow them up for clinical outcomes. Overall, cardiac event rates in this population were much lower than anticipated. Within this population of patients with asymptomatic type 2 diabetes, use of MPI screening had no discernable effect on subsequent cardiac events. The results also show that significant MPI abnormalities on screening were associated with a greater incidence of cardiac events, although the positive predictive value of such abnormalities was low and events also occurred in participants with normal screening tests.
The strategy of routine screening for CAD in patients with type 2 diabetes is based on the premise that testing could accurately identify a significant number of individuals at particularly high risk and lead to various interventions that prevent cardiac events. However, the results of the DIAD study would appear to refute this notion. Although type 2 diabetes is considered to be a CAD equivalent,4 participants had a low cardiac event rate (average, 0.6% per year) and the identification of participants with abnormal screening results did not serve to eliminate their risk over 5 years of follow-up.
The overall cardiac event rate is 3- to 4-fold lower than those previously reported in retrospective analyses of patients with diabetes who had no symptoms of CAD but were referred to nuclear cardiologylaboratories.7,19 However, such referred patients had a much higher incidence of peripheral arterial disease, renal insufficiency, and many were referred for preoperative evaluation.7,19 Referred patients also have a 2- to 3-fold higher incidence of myocardial perfusion abnormalities than those in the DIAD study. These studies highlight the observation that patients referred for specific cardiac testing have a much higher risk than the overall population of patients with type 2 diabetes whose risk is better reflected by the DIAD study group. The event rate in DIAD is similar to that in prior research studies that screened for asymptomatic ischemia in type 2 diabetes.20–22 For instance in the Milan study,22 the overall event rates are quite comparable, probably reflecting the offsetting factors of healthier patients capable of exercise testing and the more intensive medical therapy in the DIAD study.
Comparison of the cardiac event rates (0.6% per year) with those reported recently in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial for the subgroup of patients with type 2 diabetes without previous cardiac events (1.4% per year) is also informative.23 When one accounts for the inclusion of noncardiac vascular events in the ACCORD primary outcome definition and the selection of older patients with specific additional risk factors for cardiovascular disease, the event rates in ACCORD would appear favorable and compatible with those in DIAD.
The favorable cardiac outcomes among participants in the DIAD study likely reflect, in part, the impact of aggressive, guideline-driven management of cardiac risk factors, which is known to improve outcomes in patients with type 2 diabetes.24 As such, the study reflects contemporary medical practice in which most participants had hemoglobin A1c and lipid levels that were at or near the targeted range. Admittedly, a healthier cohort might have also been selected because prior stress testing was an exclusion criterion. Prior to study start, screening for CAD in patients with type 2 diabetes with 2 or more cardiac risk factors had been endorsed by an expert panel of the American Diabetes Association.14 Although these recommendations were not evidence-based,5,25 higher-risk individuals may have already been screened in clinical practice, excluding them from participation. We have also recently reported that DIAD patients frequently had resolution of inducible ischemia when rescreened 3 years after randomization,18 which may partially explain their low overall cardiac event rate. This prior analysis did not include patients with intervening cardiac events or revascularization suggesting that the resolution of inducible ischemia was associated with more aggressive therapy of cardiovascular risk factors.18
DIAD study participants also had other characteristics that would predict at least an intermediate cardiac risk, including long-standing diabetes, older age, and obesity with more than 60% of participants having 2 or more additional cardiac risk factors at baseline. Moreover, half of the participants were unable to walk even at a slow rate on a flat treadmill during adenosine infusion.8 The inability to exercise has been associated with higher cardiac risk in individuals with type 2 diabetes.20
Screening did identify participants, albeit a small number, who were at relatively greater risk than others, consistent with prior studies using stress MPI7,9,19 and stress echocardiography in patients with diabetes.10,26 DIAD study participants with moderate or large MPI defects had a 6-fold greater cardiac risk than those with normal studies or small defects. Nonperfusion abnormalities tended to convey an increased risk, as previously reported in symptomatic patients with suspected CAD.27 However, even the group of 33 participants with moderate or large defects had an annual event rate of only 2.4% per year, which would fall only into an intermediate cardiac risk group (between 1% and 3% per year28). Thus, even moderate or large MPI defects had a positive predictive value of only 12%. Seventy-eight percent of participants had normal screening tests, which had a negative predictive value of 98%. However, even the low incidence of events after a normal MPI was somewhat problematic in this low-risk population, in that more than half of the cardiac events occurred in participants with a normal screening test.
Current clinical recommendations advise intensive cardiac risk factor modification in all patients with type 2 diabetes.5,29 Thus, the validity of selective risk-factor intervention based on the results of screening, has been questioned.29 In the DIAD study, the use of statins, angiotensin-converting enzyme inhibitors, aspirin, and other evidence-based medications was better than in the general population of patients with type 2 diabetes.30 Medication use increased significantly during the course of study but did so comparably in the 2 groups (Table 4). These results suggest that ischemia detected by screening did not necessarily trigger more aggressive risk factor intervention in contemporary practice.
Screening for critical CAD to identify patients who might benefit from prophylactic revascularization also remains controversial.6,14,29 In the DIAD study, management decisions were made according to the clinical judgment of treating physicians,8 who referred only 4.4% of participants for coronary angiography within the first 120 days after screening, even though 22% had abnormal test results. Only 9 of these participants (1.6% of the screened group overall) underwent early revascularization, with 6 having CABG, indicating the severity of disease in this small group. Despite the small number of early revascularization in the screened participants, the overall rate of revascularization was similar in the 2 groups. However, DIAD was not designed to address whether asymptomatic ischemiais best treated with revascularization, because treatment was not randomized and relatively few participants underwent revascularization. Some of these individuals also had CAD that was not amenable to intervention and revascularization did not fully prevent cardiac events, consistent with prior findings in the diabetic population.31–34 Thus, it remains uncertain whether asymptomatic patients with type 2 diabetes benefit from revascularization after the identification of inducible ischemia, as was suggested in a prior retrospective database analysis34 and a small randomized pilot study.35 The on-going Bypass Angioplasty Revascularization Investigation(BARI)2DiabetesTrial may shed additional light on this issue.36
Limitations
The cardiac event rates were significantly lower than originally anticipated at the time of the design of the study and therefore the DIAD study does not have the power to exclude a small difference between the screened and unscreened participants. Based on the observed cardiac event rate, we would estimate that the study only had 14% power to detect a 20% difference between the 2 groups. A 3- to 4-fold larger study would be required to exclude such a difference,13 and it is not clear that a reduction in cardiac events from 0.6% to 0.5% per year even if proved would justify cardiac screening.
Another potential limitation to consider is that nonprotocol stress tests were done during follow-up when clinically indicated in both groups. In addition, screening led to only a modest reduction in subsequent diagnostic testing. Testing was typically performed to evaluate potential cardiac symptoms but may have also been undertaken for risk stratification in some participants. In the no-screening group, such testing represents a crossover to a physician-directed screening strategy and theoretically might have counter-balanced a benefit of protocol-mandated systematic screening. However, because the DIAD study did not prohibit physician-directed cardiac evaluation, the results are more applicable to current day medicine in which patients are often evaluated for symptoms or preoperative risk stratification or when considered particularly high risk by their physicians.
Clinical Implications
In the light of our findings, routine screening for inducible ischemia in asymptomatic patients with type 2 diabetes cannot be advocated for 4 reasons. First, the yield of detecting significant inducible ischemia is relatively low.8 Second, the overall cardiac event rate is low. Indeed, even our participants with moderate or large defects and the highest event rate would be conventionally assigned to an intermediate-risk category. Third, routine screening does not appear to affect overall outcome. Finally, routine screening of millions of asymptomatic diabetic patients would be prohibitively expensive.
However, rather than viewing this study, as a negative screening study, clinicians might consider the results as a positive message: patients with type 2 diabetes without symptoms to suggest CAD, receiving contemporary medical care, close follow-up, and appropriate diagnostic evaluation for symptoms of ischemia have relatively favorable outcomes in the current era.
Acknowledgments
Funding/Support: This work was performed with the support of National Institute of Health grants M01-RR-00125 through the General Clinical Research Centers at Yale University, 5M01-RR-00847 through the University of Rochester, and 6M01-RR-05096 through Tulane University. In addition, The DIAD study was supported by grants from Bristol Myers-Squibb Medical Imaging, North Billerica, Massachusetts, and Astellas Pharma, Deerfield, Michigan, which also provided technetium-99m sestamibi and adenosine for study patients.
Role of the Sponsor: The industrial sponsors had no role in the design or conduct of the study, in the collection, analysis, or interpretation of data, or in the preparation of the manuscript.
Additional Contributions: We thank Karen Furie, MD, MPH, Department of Neurology, Massachusetts General Hospital, Boston, who did not recieve any compensation for her contribution.
DIAD Study Investigators
Principal Investigator and Chair: Frans J. Th. Wackers, MD (Yale University School of Medicine, New Haven, Connecticut).
Coprincipal Investigators: Lawrence H. Young, MD, Silvio E. Inzucchi, MD (Yale University School of Medicine, New Haven, Connecticut) and, Deborah A. Chyun, RN, MSN, PhD (New York University College of Nursing at the College of Dentistry, New York).
Study Coordinator: Janice A. Davey, MSN, APRN (Yale University School of Medicine, New Haven, Connecticut).
Clinical Centers: University of Montreal, Montreal, Quebec, Canada: Raymond Taillefer, MD, Sylvain Prévost, MD, Carole Benjamin, and Andre Gagnon; Med-Star Research Institute, Washington, DC: Robert Ratner, MD, Maureen Passaro, MD, Evelyn Robinson, RN, and Amy Smith; Hartford Hospital, Hartford, Connecticut: Gary V. Heller, MD, PhD, Deborah Katten, RN, MPH; Tulane University, New Orleans, Louisiana: Vivian Fonseca, MD, Richard Campeau, MD, Rhonda Fontenot, RN, and Sunil Asnani, MD; University of Alabama, Birmingham: Ami E. Iskandrian, MD, Fernando Ovalle, MD, Mary Beth Hall, RN, and Misty Collins, RN; and University of Virginia, Charlottesville: Eugene Barrett, MD, George Beller, MD, Wendie Price, RN, and Denny Watson, PhD. Sound-view Research Associates, Norwalk, Connecticut: Samuel Engel, MD, Mindy Sotsky, MD, Stella Carolan, RN, and Angela Martin, APRN; University of Rochester, Rochester, New York: Steven Wittlin, MD, Ronald Schwartz, MD, and Mary Kelly, RN; University of North Carolina, Chapel Hill: Elisabeth Fasy, MD, Jean Dostou, MD, Leigh Gosnell, RD, Joe Largay, Michelle Duclos, MPH, John Buse, MD, and Steven Falen, MD; MidOhio Cardiology and Vascular Consultants Columbus, Ohio: Dennis Calnon, MD, and Connie Zimmerman; Maine Cardiology Associates, Portland, Maine: Mylan Cohen, MD, and Jennifer Powers, RN; Cardiology Consultants, Calgary, Alberta, Canada: Neil Filipchuk, MD, Marie Small, RN, and Melina Krulc, RN.
Kansas City Cardiology, Kansas City, Missouri: Timothy Blackburn, MD, Eric Hockstad, MD, and Terry Plesser, RN; Yale University Radionuclide Core Laboratory and Data Coordinating Center: Donna Natale, Roberta O’Brien, and Laurie Finta.
Advisory Board and Data Safety and Monitoring Board: Barry L. Zaret, MD, Section of Cardiovascular Medicine, and Robert, S. Sherwin, MD, Section of Endocrinology, Department of Internal Medicine, Yale University; and Ralph I. Horwitz, MD, Department of Internal Medicine, Stanford University, Palo Alto, California.
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT00769275
Financial Disclosures: None reported.
Author Contributions: Dr Wackers had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Young, Wackers, Chyun, Inzucchi.
Acquisition of data: Young, Wackers, Chyun, Davey, Barrett, Taillefer, Heller, Iskandrian, Wittlin, Filipchuk, Ratner, Inzucchi.
Analysis and interpretation of data: Young, Wackers, Chyun, Inzucchi.
Drafting of the manuscript: Young, Wackers, Chyun, Inzucchi.
Critical revision of the manuscript for important intellectual content: Young, Wackers, Chyun, Davey, Barrett, Taillefer, Heller, Iskandrian, Wittlin, Filipchuk, Ratner, Inzucchi.
Statistical analysis: Chyun.
Obtained funding: Wackers.
Administrative, technical, or material support: Young, Wackers, Chyun.
Study supervision: Wackers, Davey.
Ajudication of cardiac events: Young, Wackers, Chyun.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for health-care professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 3.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26(20):2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Howard B, Smith S, Jr, Eckel R, Redberg R, Bonow RO. Prevention Conference VI: Diabetes and Cardiovascular Disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2002;105(18):2231–2239. doi: 10.1161/01.cir.0000013952.86046.dd. [DOI] [PubMed] [Google Scholar]
- 6.Bax JJ, Young LH, Frye RL, Bonow RO, Steinberg HO, Barrett EJ American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30(10):2729–2736. doi: 10.2337/dc07-9927. [DOI] [PubMed] [Google Scholar]
- 7.Rajagopalan N, Miller TD, Hodge DO, Frye RL, Gibbons RJ. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol. 2005;45(1):43–49. doi: 10.1016/j.jacc.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 8.Wackers FJ, Young LH, Inzucchi SE, et al. Detection of Ischemia in Asymptomatic Diabetics Investigators. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27(8):1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 9.Anand DV, Lim E, Hopkins D, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27(6):713–721. doi: 10.1093/eurheartj/ehi808. [DOI] [PubMed] [Google Scholar]
- 10.Marwick TH, Case C, Sawada S, Vasey C, Short L, Lauer M. Use of stress echocardiography to predict mortality in patients with diabetes and known or suspected coronary artery disease. Diabetes Care. 2002;25(6):1042–1048. doi: 10.2337/diacare.25.6.1042. [DOI] [PubMed] [Google Scholar]
- 11.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43(9):1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 12.Beller GA. Noninvasive screening for coronary atherosclerosis and silent ischemia in asymptomatic type 2 diabetic patients: is it appropriate and cost-effective? J Am Coll Cardiol. 2007;49(19):1918–1923. doi: 10.1016/j.jacc.2007.01.079. [DOI] [PubMed] [Google Scholar]
- 13.Diamond GA, Kaul S, Shah PK. Screen testing cardiovascular prevention in asymptomatic diabetic patients. J Am Coll Cardiol. 2007;49(19):1915–1917. doi: 10.1016/j.jacc.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 14.Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10–11 February 1998, Miami, Florida. American Diabetes Association. Diabetes Care. 1998;21(9):1551–1559. doi: 10.2337/diacare.21.9.1551. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons RJ. Asymptomatic patients with diabetes mellitus should not be screened for coronary artery disease. J Nucl Cardiol. 2006;13(5):616–620. doi: 10.1016/j.nuclcard.2006.06.130. [DOI] [PubMed] [Google Scholar]
- 16.Miller TD, Redberg RF, Wackers FJ. Screening asymptomatic diabetic patients for coronary artery disease: why not? J Am Coll Cardiol. 2006;48(4):761–764. doi: 10.1016/j.jacc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 17.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 18.Wackers FJ, Chyun DA, Young LH, et al. Detection of Ischemia in Asymptomatic Diabetics (DIAD) Investigators. Resolution of asymptomatic myocardial ischemia in patients with type 2 diabetes in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study. Diabetes Care. 2007;30(11):2892–2898. doi: 10.2337/dc07-1250. [DOI] [PubMed] [Google Scholar]
- 19.Zellweger MJ, Hachamovitch R, Kang X, et al. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25(7):543–550. doi: 10.1016/j.ehj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Vanzetto G, Halimi S, Hammoud T, et al. Prediction of cardiovascular events in clinically selected high-risk NIDDM patients: prognostic value of exercise stress test and thallium-201 single-photon emission computed tomography. Diabetes Care. 1999;22(1):19–26. doi: 10.2337/diacare.22.1.19. [DOI] [PubMed] [Google Scholar]
- 21.De Lorenzo A, Lima RS, Siqueira-Filho AG, Pantoja MR. Prevalence and prognostic value of perfusion defects detected by stress technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography in asymptomatic patients with diabetes mellitus and no known coronary artery disease. Am J Cardiol. 2002;90(8):827–832. doi: 10.1016/s0002-9149(02)02702-9. [DOI] [PubMed] [Google Scholar]
- 22.Faglia E, Favales F, Calia P, et al. Milan Study on Atherosclerosis and Diabetes (Mi SAD) Cardiac events in 735 type 2 diabetic patients who underwent screening for unknown asymptomatic coronary heart disease: 5-year follow-up report from the Milan Study on Atherosclerosis and Diabetes (MiSAD) Diabetes Care. 2002;25(11):2032–2036. doi: 10.2337/diacare.25.11.2032. [DOI] [PubMed] [Google Scholar]
- 23.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons RJ, Balady GJ, Bricker JT, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2002;106(14):1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 26.Bigi R, Desideri A, Cortigiani L, Bax JJ, Celegon L, Fiorentini C. Stress echocardiography for risk stratification of diabetic patients with known or suspected coronary artery disease. Diabetes Care. 2001;24 (9):1596–1601. doi: 10.2337/diacare.24.9.1596. [DOI] [PubMed] [Google Scholar]
- 27.Abbott BG, Afshar M, Berger AK, Wackers FJ. Prognostic significance of ischemic electrocardiographic changes during adenosine infusion in patients with normal myocardial perfusion imaging. J Nucl Cardiol. 2003;10(1):9–16. doi: 10.1067/mnc.2002.127625. [DOI] [PubMed] [Google Scholar]
- 28.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines:37. [Accessed March 19, 2009]; http://www.acc.org/qualityandscience/clinical/guidelines/stable/stable_clean.pdf.
- 29.Rutter MK, Nesto RW. The changing costs and benefits of screening for asymptomatic coronary heart disease in patients with diabetes. Nat Clin Pract Endocrinol Metab. 2007;3(1):26–35. doi: 10.1038/ncpendmet0352. [DOI] [PubMed] [Google Scholar]
- 30.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among US adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29(3):531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 31.Penfornis A, Zimmermann C, Boumal D, et al. Use of dobutamine stress echocardiography in detecting silent myocardial ischaemia in asymptomatic diabetic patients: a comparison with thallium scintigraphy and exercise testing. Diabet Med. 2001;18(11):900–905. doi: 10.1046/j.1464-5491.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- 32.Gazzaruso C, Garzaniti A, Giordanetti S, et al. Assessment of asymptomatic coronary artery disease in apparently uncomplicated type 2 diabetic patients: a role for lipoprotein(a) and apolipoprotein(a) polymorphism. Diabetes Care. 2002;25(8):1418–1424. doi: 10.2337/diacare.25.8.1418. [DOI] [PubMed] [Google Scholar]
- 33.Giri S, Shaw LJ, Murthy DR, et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002;105(1):32–40. doi: 10.1161/hc5001.100528. [DOI] [PubMed] [Google Scholar]
- 34.Sorajja P, Chareonthaitawee P, Rajagopalan N, et al. Improved survival in asymptomatic diabetic patients with high-risk SPECT imaging treated with coronary artery bypass grafting. Circulation. 2005;112(9 suppl):I311–I316. doi: 10.1161/CIRCULATIONAHA.104.525022. [DOI] [PubMed] [Google Scholar]
- 35.Faglia E, Manuela M, Antonella Q, et al. Risk reduction of cardiac events by screening of unknown asymptomatic coronary artery disease in subjects with type 2 diabetes mellitus at high cardiovascular risk: an open-label randomized pilot study. Am Heart J. 2005;149(2):e1–e6. doi: 10.1016/j.ahj.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group. Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am Heart J. 2008;156(3):528–536. doi: 10.1016/j.ahj.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]