Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance (original) (raw)
Abstract
The balance between germ-line stem cell (GSC) self-renewal and differentiation in Drosophila ovaries is mediated by the antagonistic relationship between the Nanos (Nos)-Pumilio translational repressor complex, which promotes GSC self-renewal, and expression of Bam, a key differentiation factor. Here, we find that Bam and Nos proteins are expressed in reciprocal patterns in young germ cells. Repression of Nos in Bam-expressing cells depends on sequences in the nos 3′-UTR, suggesting that Nos is regulated by translational repression. Ectopic Bam causes differentiation of GSCs, and this activity depends on the endogenous nos 3′-UTR sequence. Previous evidence showed that Bgcn is an obligate factor for the ability of Bam to drive differentiation, and we now report that Bam forms a complex with Bgcn, a protein related to the RNA-interacting DExH-box polypeptides. Together, these observations suggest that Bam-Bgcn act together to antagonize Nos expression; thus, derepressing cystoblast-promoting factors. These findings emphasize the importance of translational repression in balancing stem cell self-renewal and differentiation.
Keywords: Drosophila, germ cell, oogenesis
Stem cells sustain many metazoan tissues by providing new stem cells, as well as terminally differentiating cells (1). Understanding the molecular mechanisms that mediate the balance between self-renewal and differentiation in stem cell systems has important implications for many areas of biology, including new cell-based therapies for a host of diseases. Drosophila germ-line stem cells (GSCs) have been a fruitful system for studying stem cell biology and dissecting the interactions between stem cells and associated stromal cells.
In the ovary of the adult fruit fly, somatically-derived cap cells form a niche that supports 2–3 germ-line cells by providing signals that prevent germ cell differentiation (2–4). The BMP-like proteins Dpp and Gbb are the most important signaling ligands in the niche (2, 5), and act through a classical Smad pathway in germ cells. Once activated, the Smad complex binds to a silencer element in the promoter of the key differentiation factor bam, blocking transcription (3, 5). Adherens junctions that attach the GSCs to cap cells are critical, because they keep GSCs within the peak region of niche signaling (6). After GSC division, the distal daughter cell (the cystoblast; CB) moves away from the niche, Dpp signaling declines, and bam is transcribed to initiate germ cell differentiation (2–4). Repression of Bam transcription is critical, because ectopic expression of bam in GSCs can induce differentiation and cause stem cell depletion (7).
Direct observation of GSC loss is possible in Drosophila ovaries, and this method has proven to be powerful for identifying genes that are essential for GSC maintenance (2, 7–9). Loss of any one of several genes can cause GSCs to exit the niche, and undergo differentiation, forming a differentiating germ cell, and thus, depleting the supply of stem cells. In addition to transcriptional control of stem cell maintenance, genetic studies unexpectedly implicated a role for translational regulation in GSC maintenance. This role was initially uncovered by the observation that loss of function mutations in nanos and pumilio caused GSC loss (8–10). These genes encode proteins that function as components of a translational repressor complex (11), and similar translational repressor proteins (e.g., PUF family) are present throughout metazoans (12–15). The Nanos-Pumilio (Nos-Pum) complex silences translation of mRNAs that carry Nos response element (NRE) in 3′-UTRs (11, 16, 17). Nos-Pum complexes are hypothesized to serve a similar role in GSCs to suppress translation of mRNAs that promote germ cell differentiation, but no targets have been identified to date.
Other intrinsic factors necessary for GSC maintenance include components of the microRNA (miRNA) silencing machinery, implicating another translational repressor in GSC maintenance. The core components of the miRNA processing machinery, including the endonuclease Dicer (Dcr)-1 and the Dcr-1 accessory protein Loquacious, are required in GSCs for their maintenance (18–21). Similarly, Argonaute (Ago)1, the catalytic member of the RNA-induced silencing complex (RISC), is required for GSC maintenance. Strikingly, Ago1 overexpression in germ cells can expand GSC number, indicating a central role for miRNA-dependent gene silencing in GSC identity (22).
The observation that Nos-Pum and RISC translational repression complexes promote stem cell maintenance predicts that mechanisms to antagonize these translational repression pathways may be needed to promote germ cell differentiation. This idea is supported by the recent report describing the Trim-NHL-domain containing protein Mei-P26 as an Ago1 binding partner and antagonist (23). Mei-P26 is expressed in early germ cells, and is required for proper differentiation, because mei-p26 mutant germ cells fail to form functional cysts (24).
Germ cells lacking both bam and pum differentiate into CBs, suggesting that bam might antagonize translational repression to promote CB differentiation (25, 26). However, the molecular mechanism for Bam activity is unknown. Biochemical strategies to identify its function have been impeded by the very low abundance and limited expression of protein. Comparing the sequence of Bam to other proteins has been uninformative, except to emphasize that bam is a rapidly evolving gene (27). Here, we report that Bam represses Nos accumulation. Bam-positive cells do not express detectable Nos, whereas Nos accumulation is widespread in the absence of bam. We demonstrate that Nos repression in Bam-expressing cells depends on sequences in the 3′-UTR of nos mRNA, and that _bam_-dependent differentiation also acts through the nos 3′-UTR. Bam forms a complex with its obligate cofactor, benign gonial cell neoplasm (Bgcn). Bgcn is distantly related to the DExH-box family of RNA-interacting proteins that are key components of translation and splicing complexes (28). Together, these observations suggest that Nos translational repression occurs downstream of Bam expression. These findings highlight the role of translational repression in regulating the balance between GSC self-renewal and differentiation, and may reflect a more general role for translational regulation in stem cell biology.
Results
Nos and Bam Protein Show Reciprocal Expression.
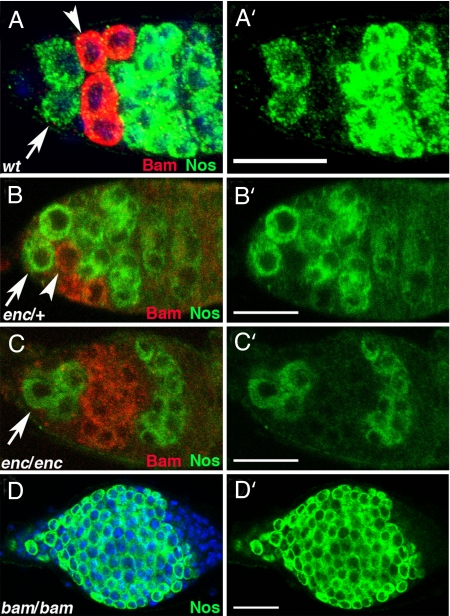
Previous genetic studies suggest bam promotes differentiation by antagonizing the action of the Nos-Pum complex (25, 26). To characterize this antagonistic relationship further, we examined the relationship between Bam expression and the accumulation of the Nos-Pum complex. We found Nos accumulation was reciprocal to Bam expression (Fig. 1A). Additional experiments that examined Nos expression when levels of Bam were manipulated supported the idea that Bam antagonized Nos-Pum accumulation. First, in encore mutants (29), Bam expression was extended into 16-cell cysts (Fig. 1C). This expanded Bam expression correlated with delayed Nos expression in maturing cysts (Fig. 1C). Second, Nos was expressed in all _bam_−/− germ cells (Fig. 1D). Together, these data suggest that Bam expression antagonizes Nos accumulation.
Fig. 1.
Bam and Nos are expressed in reciprocal patterns. (A) Bam protein (red, detected by anti-GFP against a Bam:GFP rescuing transgene) and Nos protein (green, detected by anti-myc against a Nos:Myc rescuing transgene) are expressed in nonoverlapping domains in early germ cells. Stem stems (arrows), CBs (arrowheads). (B and C) Germ cells from encore mutant flies (C) display an expansion of Bam expression compared with enc/+ and (B) a corresponding delay in late Nos accumulation (red, anti-Bam; green, Nos:GFP, detected by anti-GFP against a rescuing transgene). Stem cells (arrows), CBs (arrowheads). Cystoblasts are out of plane of focus in C. (D) Nos (green) remains high in germ cells lacking Bam. (A′–D′) Nos channel alone. (Magnification bars, 25 μm.)
Genetic Interaction Links Bam Expression to Reduced Nos Activity.
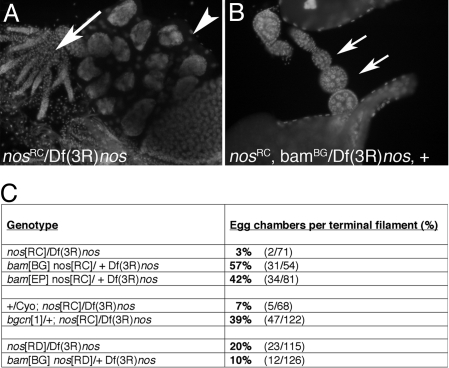
If bam expression caused the decline of Nos protein, nos+ activity would be predicted to respond to bam gene dosage. We compared the ovarian phenotypes of females that were homozygous for either of 2 strong nos alleles in bam+/+ or _bam_null/+ backgrounds. Females carrying _nos_RC alleles had ovaries that were largely agametic. However, the ovaries of _nos_RC females that were also heterozygous for bam produced many egg chambers (Fig. 2B). Removing 1 copy of bam increased egg chamber formation at least 10-fold in the _nos_RC/Df(3R)nos background (Fig. 2C). This suppression required some Nos protein, because it was not observed with _nos_RD (Fig. 2C), a null allele (30, 31). The suppression of the nos phenotypes by bam was, therefore, not the result of a bypass effect, but rather due to raising the effective level of nos+ activity. Similarly, removing a single copy of bgcn suppressed the _nos_RC phenotype (Fig. 2C).
Fig. 2.
Genetic interactions reveal an antagonistic relationship between bam and nos. (A) Ovaries from _nos_[RC] mutants are rudimentary (arrow) with few maturing egg chambers (arrowhead). (B) Introduction of a single loss of function bam allele suppressed the _nos_[RC] phenotype and ovaries contained many more maturing egg chambers (arrows). (C) Suppression was quantified by counting the number of egg chambers per terminal filament in ovaries from _nos_[RC] and _nos_[RD] ± bam or bgcn. The difference between _nos_[RC]/Df and _nos_[RD]/Df may reflect suppressing background mutation(s) on the _nos_[RD] chromosome, because the _bam_[BG] _nos_[RD] recombinant chromosome produced a more severe phenotype over Df than the original _nos_[RD] chromosome.
Posttranscriptional Control of Nos Accumulation.
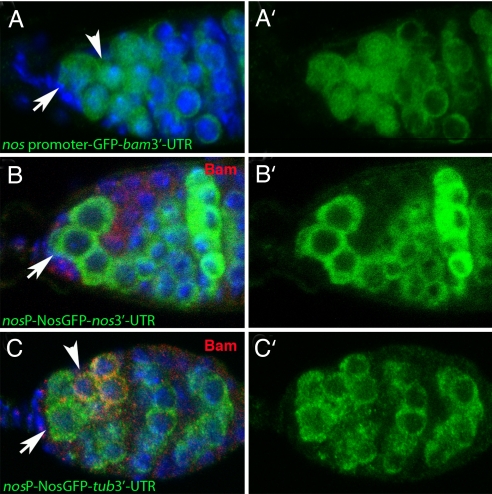
To explore the mechanism by which Bam represses Nos expression, we considered several possibilities. Transcriptional repression of nos was unlikely because transcriptional reporters, which recapitulated RNA in situ hybridization (32), produced a pattern of expression that was different from the Nos protein pattern (Fig. 3) (32). The nos promoter was uniformly active in GSCs, CBs, and cystocytes of 2-, 4-, and 8-cell cysts (Fig. 3A), whereas Nos protein distribution was more dynamic (Fig. 3B). Nos accumulated to high levels in GSCs, declined slightly in the CB, was nearly undetectable in mitotically active cysts and then accumulated again to high levels in 16-cell cysts and beyond (Fig. 3B) (31, 32).
Fig. 3.
Nos accumulation depends on the 3′-UTR. (A) Nos promoter driving GFP expression accumulates GFP throughout region 1 of the germarium. Stem cells (arrows), CBs (arrowheads). (B) Nos protein (green, anti-GFP against a rescuing _nos_-GFP transgene) accumulates in stem cells (arrow) and precystoblasts, is lost in Bam+ cells (red, anti-Bam), and reaccumulates in 16-cell cysts where Bam expression is no longer detected. (C) Nos expressed from the nos promoter but with the heterologous tubulin 3′-UTR accumulates uniformly in region 1 of the germarium, including in Bam+ cells (arrowhead). (A′–C′) Nos channel alone.
Several posttranscriptional mechanisms could explain the reciprocal pattern of Bam and Nos. We hypothesized Bam might destabilize or otherwise block translation of nos mRNA or cause Nos protein turnover by posttranslational modification. Previous studies in maturing egg chambers and embryos had already validated that nos mRNA was the target of translational control via sequences embedded in its 3′-UTR (34, 35). We characterized the ovarian expression of a transgene in which the native nos 3′-UTR had been replaced with the tubulin 3′-UTR. Protein produced from the transgene bearing the tubulin 3′-UTR was widely expressed in the germarium, including in bam positive cells (Fig. 3C). Therefore, we conclude the regulation of Nos protein expression in wild-type germaria depends on sequences in the 3′-UTR of the transcript.
To determine the regions of nos 3′-UTR that were critical for its regulation in CBs, we generated several transgenes that contained deletions in the nos 3′-UTR. We found that sequences within the first 100 bp of the nos UTR were essential for wild-type Nos accumulation pattern (Fig. S1). This region contains regulatory elements that are also known to be important for nos regulation later in oogenesis and in embryogenesis (34–37).
Bam-Induced Differentiation Acts via the nos 3′-UTR.
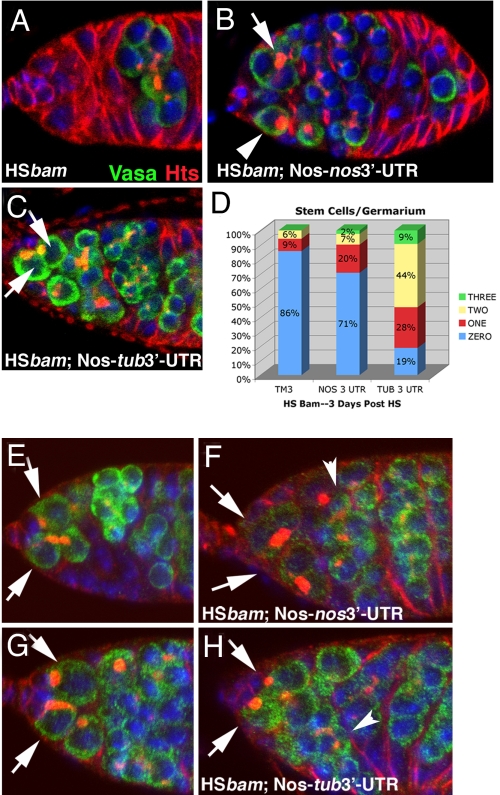
To test the hypothesis that Bam induced differentiation by reducing Nos expression, we exploited the fact that ectopic Bam expression causes GSC-to-CB differentiation and subsequent rapid loss of stem cells (7). Heat-shock induced Bam expression eliminated GSCs (Fig. 4A) and GSCs were ablated even when females carried an extra copy of nos (Fig. 4B). Strikingly, however, HS-Bam did not eliminate GSCs when females expressed nos with the tubulin 3′-UTR (Fig. 4C). Three days after heat shock, 70–80% of germaria were devoid of stem cells when HS-Bam was expressed in wild-type, or in animals bearing a nos transgene with the native 3′-UTR (Fig. 4D). In contrast, when HS-Bam was expressed together with a nos transgene containing the tub 3′-UTR, stem cells were retained in 80% of the germaria (Fig. 4D).
Fig. 4.
Bam-dependent GSC loss acts via the nos 3′-UTR. (A–C) Green, Vasa; Red, Hts; and Blue, DNA. (A) The p[HS-_bam_] ovaries maintained at 37 °C for 1 h were examined 3 days post heat shock; 86% of these germaria were devoid of stem cells. (B) Germaria from animals carrying a wild-type nos transgene were similarly depleted of stem cells by HS-bam treatment (71% of germaria had no stem cells). Arrow points to a 4-cell cyst and arrowhead indicates a CB or 2-cell cyst in the GSC position. (C) Germaria from animals carrying a nos transgene with a tubulin 3′-UTR were protected from HS-bam induced stem cell depletion. Arrows indicate 2 GSCs (81% of germaria contained 1 or more stem cells). (D) Quantification of GSC retention or loss after ectopic Bam expression. (E–H) Green, Nos:GFP; Red, Hts; and Blue, DNA. Arrows point to GSCs. Detectible Nos declines in stem cells after HS-bam, except when Nos is expressed with the tubulin 3′-UTR. (E) Nos:GFP expression levels in germarium of p[_nos_P-Nos:GFP-_nos_3′-UTR] animal without heat shock. (F) Nos:GFP levels in p[_nos_P-Nos:GFP-_nos_3′-UTR] animal 9.5 h after heat shock. Arrows indicate GSCs; arrowheads indicate 16-cell cysts. (G) Nos:GFP expression levels in germarium of p[_nos_P-Nos:GFP-_tub_3′-UTR] animal without heat shock. (H) Nos:GFP expression levels in germarium of p[_nos_P-Nos:GFP-_tub_3′-UTR] animal 9.5 h after heat shock. Arrows indicate GSCs; arrowheads indicate 16-cell cysts.
We showed directly that ectopic Bam expression in GSCs reduced the levels of Nos protein by comparing the abundance of Nos:GFP from the p[_nos_P-Nos:GFP-_nos_3′-UTR] transgene before and after heat shock induction of Bam (compare Fig. 4 E and F). In contrast, heat shock-induced Bam expression had no effect on the levels of Nos produced from the p[_nos_P-Nos:GFP-tubulin 3′-UTR] transgene (compare Fig. 4 G and H). Together, these data demonstrated that Bam repressed Nos expression via sequences in the nos 3′-UTR. Nos accumulation in 16-cell cysts is not affected by expression of Bam (compare Fig. 4 F and H), which may indicate a limit to the expression of cofactors required for this response.
Protein Interaction Partners of Bam.
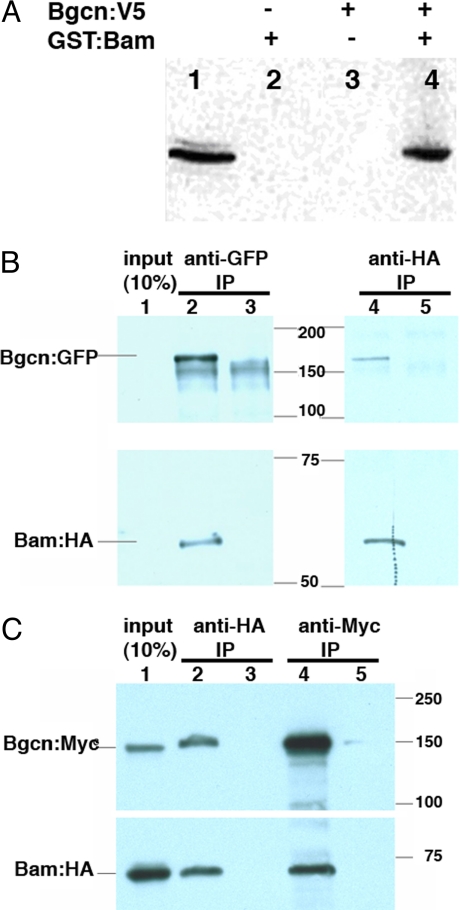
Our genetic analysis and protein expression data strongly implied that Bam negatively affected Nos accumulation, either directly or indirectly, by influencing nos translation or mRNA stability. However, the Bam protein lacks recognizable biochemical motifs so the mechanism of this regulation was unclear. We had previously found that Bgcn, a member of the RNA helicase family, was required genetically for Bam to promote CB differentiation (28, 33); therefore, we tested whether Bam and Bgcn formed a complex. A 2-hybrid screen using a Bgcn “bait” protein (Materials and Methods) recovered the C-terminal 97 aa of Bam. Subsequent in vitro “GST-pulldowns” using full-length recombinant proteins verified that the Bam-Bgcn interaction could be recapitulated with Escherichia coli and yeast protein extracts (Fig. 5A).
Fig. 5.
Bam and Bgcn form a complex. (A) Immunoblot probed with anti-V5 antibody after “pulldown” experiments with epitope-tagged Bam:GST and Bgcn:V5 proteins. (lane 1) Yeast extract expressing Bgcn:V5. (lanes 2–4) Samples from pellets recovered by incubation with glutathione-coupled agarose beads (GST pulldowns). Bgcn:V5 was recovered in the pellet only when the extracts from Bgcn:V5 yeast were incubated with Bam:GST bacterial extracts. (B) Bam and Bgcn coimmunoprecipitate from ovaries. Ovarian extracts from flies expressing Bgcn:GFP and Bam:HA (lane 2) or _w_1118 flies (lane 3) were incubated with anti-GFP or anti- HA antibodies for immunoprecipitation and the immunoblots were probed with anti-GFP and anti-HA antibodies separately. Lane 1, 10% of extract; lane 2, flies _w_−;[w+,_bam_P-Bam:HA];[w+,_bgcn_P-Bgcn:GFP] anti-GFP IP; lane 3, flies _w_1118 anti-GFP IP; lane 4, flies _w_−;[w+,_bam_P-Bam:HA];[w+,_bgcn_P-Bgcn:GFP] anti-HA IP; lane 5, flies _w_1118 anti-HA IP. (C) Bam and Bgcn coimmunoprecipitate from S2 cells that expressed tagged proteins. Tagged proteins (Bam:HA; Bgcn:Myc) were over expressed in S2 cells and the cell lysate were used for anti-HA or anti-Myc immunoprecipitation. Lane 1, 10% of S2 cell lysate; lane 2, S2 cell lysate expressing Bam:HA and Bgcn:Myc immunoprecipitated with anti-HA antibody; lane 3, S2 cell lysate expressing Bgcn:Myc immunoprecipitated with anti-HA antibody; lane 4, S2 cell lysate expressing Bam:HA and Bgcn:Myc immunoprecipitated with anti-Myc antibody; lane 5, S2 cell lysate expressing Bam:HA immunoprecipitated with anti-Myc antibody.
Bam and Bgcn complex formation was verified in vivo using the ovaries of flies expressing epitope-tagged Bam and Bgcn proteins expressed from their native promoters. Transgenes carrying Bam tagged with hemagglutinin antigen (Bam:HA) fully rescued bam mutant flies, whereas Bgcn tagged with GFP (Bgcn:GFP) also rescued oogenesis and spermatogenesis in bgcn mutants (Materials and Methods). Immunoprecipitation performed in both directions succeeded in coprecipitating the corresponding partner protein from cotransfected S2 cells (Fig. 5B; Fig. S2), and from ovarian lysates from Bam:HA; Bgcn:GFP flies (Fig. 5C). These results confirmed our earlier prediction from genetic experiments that Bam and Bgcn work together to regulate germ cell differentiation (28, 33).
Discussion
Previous studies show GSC maintenance dually depends on Nos expression to suppress CB differentiation (10) and on transcriptional silencing of bam (3, 5). Expression of Bam acts as a developmental switch, and is both necessary and sufficient to drive germ cell differentiation. Elucidating the biochemical activity of Bam has been impeded, however, because both the low abundance and lack of recognizable functional domains of the protein. The goals of the experiments presented in this article were to provide new insights into the function of Bam by finding protein partners and the downstream targets of Bam action.
Nos:Pum Regulation Downstream of Bam:Bgcn.
The translational repressor proteins Pum and Nos are critical GSC maintenance factors and suppress CB differentiation (8–10), perhaps by repressing translation of a pool of CB promoting mRNAs stored in GSCs (25, 38). The dynamic pattern of Nos accumulation in the germarium suggested the protein disappears as CB differentiation begins. Decreasing levels of Nos expression during CB differentiation are unlikely to reflect changes in the transcription, because a GFP reporter fused to the nos promoter remains active throughout the germarium. Instead, Nos elimination within early cysts is mediated by sequences in the 3′-UTR of the transcripts. Substituting a tubulin 3′-UTR for the endogenous nos 3′-UTR resulted in uniform Nos protein expression throughout the germarium. Further work has narrowed down the region responsible for translational repression of nos in the germarium to the first 100 bases of the 3′-UTR of the transcript.
Genetic experiments with bam and pum alleles had suggested that the 2 genes exerted opposite actions on CB differentiation (25, 26). Nos accumulation declined when Bam was expressed ectopically in several genetic backgrounds (this article), suggesting that Nos accumulation can be linked directly to Bam protein levels and not to signals from somatic cells in the germarium. Data showing that diminished bam or bgcn gene dosage could suppress the germ cell loss phenotype of nos alleles provided additional evidence for the inverse relationship of bam and nos expression. A reduction in bam or bgcn dose may have decreased the likelihood that a _nos_− GSC differentiated precociously, because stem cells were more likely to be maintained in these ovaries. The relevant antagonism could take place within the transient cell identified as the “precystoblast” (39, 40). It is also possible that _nos_− primordial germ cells were more likely to be captured as stem cells during gonadogenesis when bam or bgcn levels were reduced.
The nos 3′-UTR was essential for proper regulation, because the CB differentiation induced by ectopic Bam expression failed when [hs-Bam] flies carried a Nos-_tub_3′-UTR transgene. Surprisingly, cyst formation proceeded normally in ovaries carrying the Nos-_tub_3′-UTR transgene even though ectopic expression would be expected to promote GSC self-renewal. One possible explanation is provided by observations of Forbes and Lehmann (9), who noted that Pum levels also fall as CBs differentiate. Pum levels become limiting even as Nos continues to accumulate from the p[_nos_P-Nos-tub 3′-UTR] transgene (Fig. S3). Likewise, redundant pathways may exist to derepress translation of CB-promoting mRNAs, just as multiple pathways appear to exist to silence those mRNAs. If, like Nos-Pum, the miRNA pathway is down-regulated to initiate differentiation, derepresion of CB-promoting mRNAs might occur even if Nos expression was maintained during CB and cyst stages. Because ectopic Ago1 expression, but not Nos, produced extra GSCs (22), miRNAs might be separate and prominent repressors of CB differentiation to maintain GSCs.
The Bam/Bgcn Complex.
Together, our genetic and biochemical experiments suggest that Bam and Bgcn form a complex that represses nos translation, either directly or indirectly. Mechanistically, we considered it possible that Bam-Bgcn and perhaps other proteins directly repress nos mRNA by binding sequences in the nos 3′-UTR. However, we have been unable to demonstrate a direct physical interaction between Bam-Bgcn and nos mRNA, either from ovary extracts or in vitro. Likewise, our attempts to reconstitute Bam-Bgcn-dependent translational repression of the nos 3′-UTR in S2 cells failed. These experiments may have failed because S2 cells lack important, but as yet unidentified, cofactors found specifically in germ cells, or because Bam-Bgcn regulate nos translation via an indirect mechanism. For example, it is plausible that Bam-Bgcn promote the expression of the early response target mRNAs, and 1 or more of these factors could repress nos translation. Alternative mechanisms of action for Bam-Bgcn are unclear as Bam lacks any defined sequence motifs (41) and Bgcn, whereas related throughout the length of the protein to RNA/DNA helicases, lacks the motifs to be a functional helicase (28). Outside the Bam-interacting domains, Bgcn contains a pair of ankyrin repeats that could mediate other protein–protein interactions (28).
One potential component of the Bam-Bgcn complex, Mei-P26, was suggested by previous genetic experiments. Page et al. (24) identified mei-P26 as a gene required for early germ cell differentiation and meiosis, and showed that mei-P26 activity depended on the proper dosage of bam. Recently, Knoblich and coworkers (23) reported that bam required mei-P26 to deplete stem cells, and similarly, mei-P26 required bam to function properly. These observations could imply a close working relationship between bam and mei-P26. However, the interactions and interrelated functions of Bam, Bgcn, and Mei-P26 are likely to be complex. For example, although the phenotypes of bam and bgcn mutations are indistinguishable (33), the mei-P26 mutant phenotype is distinct. Germ cells lacking mei-P26 apparently form CBs, because they produce Bam-positive cysts with branched fusomes (23). Given the results presented here and elsewhere (23), exploring the functional significance of Bam, Bgcn, and Mei-P26 interactions will be important.
Implications for Stem Cell Derivation.
The view of stem cells that emerges from these studies has several striking elements: (i) that repression mechanisms control many stem cell differentiation circuits, and (ii) that translational regulation has an integral role in these decisions. The GSC model highlights an intrinsic capacity to differentiate and the need to apply brakes (Nos-Pum) to retard differentiation. Perhaps this mechanism was advantageous to prevent all gametes from maturing at once in animals that developed with a finite number of germ cells. Of course, differentiation would require a mechanism (Bam-Bgcn) to override the brakes. Within this framework, a stem cell population could arise when a group of stromal cells captured germ cells and produced signals that could repress expression of the factor(s) that would antagonize the brakes. Natural selection would rapidly fix this event, because it would greatly expand the number of gametes produced from individuals by establishing a stem cell as a renewable source of germ cells. This mode of niche evolution might also explain the appearance of stem cell populations in most organs, because it would be expected to enhance fitness by permitting larger body size, lengthening the fecund lifespan and increasing survivability of trauma by providing a mechanism for tissue regeneration. If the mechanisms at work in Drosophila GSCs apply to many stem cells, we should expect to find stem cells enriched for many more antidifferentiation genes than true stemness genes.
Materials and Methods
Transgenes.
The p[w+; _bam_P-Bam:HA] encodes a Bam protein tagged with the HA-epitope at the C terminus, expressed from a bam promoter. The Bam:HA protein rescued bam null mutants.
The p[_bgcn_P-Bgcn:GFP] uses 2,000 nt of a putative bgcn promoter to drive bgcn cDNA joined to GFP; bgcn mutant females carrying this transgene were fertile and had morphologically normal ovaries. The sibling transgenic males were also fertile.
The Nos Promoter-GFP transgene was constructed from 800 bp of genomic sequence upstream of nos ATG fused to GFP. HS-Bam-HA was constructed from the HS-Bam construct described previously (7), with a single, C-terminal HA epitope tag. Heat-shock expression rescues oogenesis in bam females.
Drosophila Strains.
The _bam_[Δ86] and _bam_[z3–2884] have been described elsewhere (7, 33, 42). Other stocks included p[_nos_P-Nos-GFP-nos 3′-UTR] and p[_nos_P-Nos-GFP-tub 3′-UTR] (43), _nos_[RC], _nos_[RD], nos Df, p[_nos_P-Nos-Myc] (31), _encore_[R1] (29), _bam_[BG] (25), and _bam_[EP667] (44). All other stocks were obtained from the Drosophila Stock Center (http://flystocks.bio.indiana.edu/).
Immumohistochemistry.
Ovaries were fixed as described (39), and imaged on a Zeiss LSM 510 confocal microscope. Primary antibodies included rabbit anti-GFP (1:5,000; Invitrogen), mouse anti-Hts (1:500) (45), rat anti-Pum (1:2,000) (46), mouse anti-Bam (1:75) (39), mouse anti-myc (1:1,000; Developmental Studies Hybridoma Bank), and rabbit anti-Vasa (1:250) (47). The secondary antibodies used were conjugated with Alexa 568, and 488 (1:500; Molecular Probes).
Yeast 2-Hybrid Screen.
Full-length Bgcn cDNA was subcloned into pEG202 and transformed into EGY48 to construct a Bgcn bait strain (48). Appropriate controls verified that the Bgcn:LexA fusion protein was stable in yeast cells and did not induce autoactivation (48).
Yeast carrying the Bgcn bait plasmid were transformed with a Drosophila ovarian cDNA library (gift of C. Nüsslein-Volhard) constructed in the vector pJG45 (48) and screened for leucine prototrophy and LacZ expression. Of 1 × 107 colonies screened, 293 positive clones were recovered. Most (267/293) encoded α-spectrin, a component of the fusome in germ cells (49). The Bgcn/α-spectrin interaction did not appear to be specific, however, because many fragments of Bgcn could bind to the α-spectrin clones when tested in GST-pulldown assays.
Fusion Proteins and in Vitro Pulldown Assays.
Full-length Bgcn cDNA was subcloned into pYESTRYP2 vector (Invitrogen) and were expressed in yeast. Soluble yeast extracts were prepared by vortexing with glass beads and yeast lysis buffer (YLB) (0.1 M KCl, 50 mM EGTA). Western blotting confirmed recovery of bgcn-V5 fusion protein in the soluble fraction.
BamGST fusion protein was purified from E. coli extracts. Equal volumes of crude yeast protein extracts or purified GST fusion proteins were incubated overnight at 4 °C. Crude yeast extracts were mixed with PBS, and GST fusions were mixed with YLB as negative controls. The extracts were then incubated with glutathione-Sepharose (Pierce) for 2 h at room temperature. The glutathione-Sepharose beads were pelleted and washed 4 times with PBST. Proteins extracted from the beads were immunoblotted with mouse anti-V5 antibodies (Invitrogen) at 1:2,500.
Mapping Interaction Domain Between Bam and Bgcn.
Bam or truncated Bam (with Flag and HA tag) associated with Bgcn or truncated Bgcn (with Flag and Myc tag) were cotransfected into S2 cells. The cells were lysed in lysis buffer (50 mM Tris·HCl, pH 7.8/150 mM NaCl/0.1% Nonidet P-40). The expression of the protein (input) was detected with anti-Flag antibody. The cell lysates were immunoprecipitated with anti-HA antibodies and immunoblotted for Bam with anti-HA antibody and Bgcn with anti-Myc antibody.
Immunoprecipitation from Ovaries.
Antibodies used for immunoprecipitations were Rat monoclonal anti-HA 3F-10 (Roche) at 1:50, and rabbit anti-GFP (Invitrogen) at 1:100. Primary antibodies used for Western blotting were rat anti-HA 3F-10 (Roche) at 1:5,000, goat anti-GFP (Abcam) at 1:1,000. Secondary antibodies used were goat anti-rat-HRP and rabbit anti-goat-HRP (Bio-RAD) at 1:2,000 dilution. Protein G-Sepharose 4 Fast Flow (AP Biotech) was used to immunoprecipitate protein complexes.
Lysates were prepared by dissecting 100 ovaries from females carrying the p[_bam_P-Bam:HA] and p[_bgcn_P-Bgcn:GFP] trangenes into ice-cold EBR or PBS and grinding for 30 s in 500 μL of lysis buffer (50 mM Hepes, pH 7.5/250 mM NaCl/0.1% Nonidet P-40/0.2% Triton X-100) plus protease inhibitor mixture (Roche). The lysate was spun in a microcentrifuge at maximum speed for 15 min at 4 °C, and the supernatant was added to 40 μL of Protein G beads. After 1-h incubation at 4 °C, the beads were pelleted at 8,000 × g and the supernatant was collected.
GFP antibody (rabbit; Invitrogen) was added to the ovary extracts at 1:100 and incubated at 4 °C for 2 h. Protein G beads (Roche) were added and incubated with the lysate and immunoprecipitating antibody overnight at 4 °C. After 14 h, the beads were washed 4 times in lysis buffer for 20 min, resuspended in an equal volume of protein loading buffer, and immunoblotted with rat anti-HA at 1:5,000.
For more details, see SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments.
We thank contributions from past members of the McKearin lab, particularly D. Chen and C. Lavoie, for transgenic constructs used in this study, E. Gavis (Princeton University, Princeton, NJ) and R. Wharton (The Ohio State University, Columbus, OH) for contributing nos transgenic flies, M. Kuhn for technical assistance, Y. Sun for advice on biochemical experiments, M. Buszczak for engaging scientific discussions and for critical evaluation of the manuscript, and anonymous reviewers whose comments improved the manuscript. Critical evaluations of the manuscript were also provided by H. Kramer, B. Ohlstein, and R. Galindo. This work was supported by the National Institutes of Health Grant GM45820.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Casanueva MO, Ferguson EL. Germline stem cell number in the Drosophila ovary is regulated by redundant mechanisms that control Dpp signaling. Development. 2004;131:1881–1890. doi: 10.1242/dev.01076. [DOI] [PubMed] [Google Scholar]
- 5.Song X, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 6.Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- 7.Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- 8.Lin H, Spradling AC. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development. 1997;124:2463–2476. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- 9.Forbes A, Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development. 1998;125:679–690. doi: 10.1242/dev.125.4.679. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Lin H. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science. 2004;303:2016–2019. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- 11.Sonoda J, Wharton RP. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev. 1999;13:2704–2712. doi: 10.1101/gad.13.20.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamore PD, Williamson JR, Lehmann R. The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. RNA. 1997;3:1421–1433. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B, et al. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature. 1997;390:477–484. doi: 10.1038/37297. [DOI] [PubMed] [Google Scholar]
- 14.Wickens M, Bernstein DS, Kimble J, Parker R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 2002;18:150–157. doi: 10.1016/s0168-9525(01)02616-6. [DOI] [PubMed] [Google Scholar]
- 15.Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- 16.Murata Y, Wharton RP. Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell. 1995;80:747–756. doi: 10.1016/0092-8674(95)90353-4. [DOI] [PubMed] [Google Scholar]
- 17.Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, Kobayashi S. Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat Cell Biol. 1999;1:431–437. doi: 10.1038/15666. [DOI] [PubMed] [Google Scholar]
- 18.Jin Z, Xie T. Dcr-1 maintains Drosophila ovarian stem cells. Curr Biol. 2007;17:539–544. doi: 10.1016/j.cub.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 19.Jiang F, et al. Dicer-1 and R3D1-L catalyze microRNA maturation in Drosophila. Genes Dev. 2005;19:1674–1679. doi: 10.1101/gad.1334005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forstemann K, et al. Normal microRNA maturation and germ-line stem cell maintenance requires Loquacious, a double-stranded RNA-binding domain protein. PLoS Biol. 2005;3:e236. doi: 10.1371/journal.pbio.0030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JK, Liu X, Strauss TJ, McKearin DM, Liu Q. The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells. Curr Biol. 2007;17:533–538. doi: 10.1016/j.cub.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, et al. Argonaute 1 regulates the fate of germline stem cells in Drosophila. Development. 2007;134:4265–4272. doi: 10.1242/dev.009159. [DOI] [PubMed] [Google Scholar]
- 23.Neumuller RA, et al. Mei-P26 regulates microRNAs and cell growth in the Drosophila ovarian stem cell lineage. Nature. 2008;454:241–245. doi: 10.1038/nature07014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page SL, McKim KS, Deneen B, Van Hook TL, Hawley RS. Genetic studies of mei-P26 reveal a link between the processes that control germ cell proliferation in both sexes and those that control meiotic exchange in Drosophila. Genetics. 2000;155:1757–1772. doi: 10.1093/genetics/155.4.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D, McKearin D. Gene circuitry controlling a stem cell niche. Curr Biol. 2005;15:179–184. doi: 10.1016/j.cub.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Szakmary A, Cox DN, Wang Z, Lin H. Regulatory relationship among piwi, pumilio, and bag-of-marbles in Drosophila germline stem cell self-renewal and differentiation. Curr Biol. 2005;15:171–178. doi: 10.1016/j.cub.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Civetta A, Rajakumar SA, Brouwers B, Bacik JP. Rapid evolution and gene-specific patterns of selection for three genes of spermatogenesis in Drosophila. Mol Biol Evol. 2006;23:655–662. doi: 10.1093/molbev/msj074. [DOI] [PubMed] [Google Scholar]
- 28.Ohlstein B, Lavoie CA, Vef O, Gateff E, McKearin DM. The Drosophila cystoblast differentiation factor, benign gonial cell neoplasm, is related to DExH-box proteins and interacts genetically with bag-of-marbles. Genetics. 2000;155:1809–1819. doi: 10.1093/genetics/155.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins NC, Thorpe J, Schupbach T. Encore, a gene required for the regulation of germ line mitosis and oocyte differentiation during Drosophila oogenesis. Development. 1996;122:281–290. doi: 10.1242/dev.122.1.281. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann R, Nusslein-Volhard C. The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development. 1991;112:679–691. doi: 10.1242/dev.112.3.679. [DOI] [PubMed] [Google Scholar]
- 31.Verrotti AC, Wharton RP. Nanos interacts with cup in the female germline of Drosophila. Development. 2000;127:5225–5232. doi: 10.1242/dev.127.23.5225. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Dickinson LK, Lehmann R. Genetics of nanos localization in Drosophila. Dev Dyn. 1994;199:103–115. doi: 10.1002/aja.1001990204. [DOI] [PubMed] [Google Scholar]
- 33.Lavoie CA, Ohlstein B, McKearin DM. Localization and function of Bam protein require the benign gonial cell neoplasm gene product. Dev Biol. 1999;212:405–413. doi: 10.1006/dbio.1999.9346. [DOI] [PubMed] [Google Scholar]
- 34.Dahanukar A, Wharton RP. The Nanos gradient in Drosophila embryos is generated by translational regulation. Genes Dev. 1996;10:2610–2620. doi: 10.1101/gad.10.20.2610. [DOI] [PubMed] [Google Scholar]
- 35.Gavis ER, Lunsford L, Bergsten SE, Lehmann R. A conserved 90 nucleotide element mediates translational repression of nanos RNA. Development. 1996;122:2791–2800. doi: 10.1242/dev.122.9.2791. [DOI] [PubMed] [Google Scholar]
- 36.Smibert CA, Wilson JE, Kerr K, Macdonald PM. smaug protein represses translation of unlocalized nanos mRNA in the Drosophila embryo. Genes Dev. 1996;10:2600–2609. doi: 10.1101/gad.10.20.2600. [DOI] [PubMed] [Google Scholar]
- 37.Kalifa Y, Huang T, Rosen LN, Chatterjee S, Gavis ER. Glorund, a Drosophila hnRNP F/H homolog, is an ovarian repressor of nanos translation. Dev Cell. 2006;10:291–301. doi: 10.1016/j.devcel.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Maines JZ, Park JK, Williams M, McKearin DM. Stonewalling Drosophila stem cell differentiation by epigenetic controls. Development. 2007;134:1471–1479. doi: 10.1242/dev.02810. [DOI] [PubMed] [Google Scholar]
- 39.McKearin D, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- 40.Gilboa L, Lehmann R. Repression of primordial germ cell differentiation parallels germ line stem cell maintenance. Curr Biol. 2004;14:981–986. doi: 10.1016/j.cub.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 41.McKearin DM, Spradling AC. bag-of-marbles: A Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- 42.Clark KA, McKearin DM. The Drosophila stonewall gene encodes a putative transcription factor essential for germ cell development. Development. 1996;122:937–950. doi: 10.1242/dev.122.3.937. [DOI] [PubMed] [Google Scholar]
- 43.Forrest KM, Clark IE, Jain RA, Gavis ER. Temporal complexity within a translational control element in the nanos mRNA. Development. 2004;131:5849–5857. doi: 10.1242/dev.01460. [DOI] [PubMed] [Google Scholar]
- 44.Schulz C, et al. A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics. 2004;167:707–723. doi: 10.1534/genetics.103.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaccai M, Lipshitz HD. Role of Adducin-like (hu-li tai shao) mRNA and protein localization in regulating cytoskeletal structure and function during Drosophila Oogenesis and early embryogenesis. Dev Genet. 1996;19:249–257. doi: 10.1002/(SICI)1520-6408(1996)19:3<249::AID-DVG8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Macdonald PM. The Drosophila pumilio gene: An unusually long transcription unit and an unusual protein. Development. 1992;114:221–232. doi: 10.1242/dev.114.1.221. [DOI] [PubMed] [Google Scholar]
- 47.Lasko PF, Ashburner M. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 1990;4:905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- 48.Brent R, Finley RL., Jr Understanding gene and allele function with two-hybrid methods. Annu Rev Genet. 1997;31:663–704. doi: 10.1146/annurev.genet.31.1.663. [DOI] [PubMed] [Google Scholar]
- 49.de Cuevas M, Lee JK, Spradling AC. alpha-spectrin is required for germline cell division and differentiation in the Drosophila ovary. Development. 1996;122:3959–3968. doi: 10.1242/dev.122.12.3959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information