Genetic Modifiers of dFMR1 Encode RNA Granule Components in Drosophila (original) (raw)
Abstract
Mechanisms of neuronal mRNA localization and translation are of considerable biological interest. Spatially regulated mRNA translation contributes to cell-fate decisions and axon guidance during development, as well as to long-term synaptic plasticity in adulthood. The Fragile-X Mental Retardation protein (FMRP/dFMR1) is one of the best-studied neuronal translational control molecules and here we describe the identification and early characterization of proteins likely to function in the dFMR1 pathway. Induction of the dFMR1 in _sevenless-_expressing cells of the Drosophila eye causes a disorganized (rough) eye through a mechanism that requires residues necessary for dFMR1/FMRP's translational repressor function. Several mutations in dco, orb2, pAbp, rm62, and smD3 genes dominantly suppress the sev-dfmr1 rough-eye phenotype, suggesting that they are required for dFMR1-mediated processes. The encoded proteins localize to dFMR1-containing neuronal mRNPs in neurites of cultured neurons, and/or have an effect on dendritic branching predicted for bona fide neuronal translational repressors. Genetic mosaic analyses indicate that dco, orb2, rm62, smD3, and dfmr1 are dispensable for translational repression of hid, a microRNA target gene, known to be repressed in wing discs by the bantam miRNA. Thus, the encoded proteins may function as miRNA- and/or mRNA-specific translational regulators in vivo.
THE subcellular localization and regulated translation of stored mRNAs contributes to cellular asymmetry and subcellular specialization (Lecuyer et al. 2007; Martin and Ephrussi 2009). In mature neurons, local protein synthesis at active synapses may contribute to synapse-specific plasticity that underlies persistent forms of memory (Casadio et al. 1999; Ashraf et al. 2006; Sutton and Schuman 2006; Richter and Klann 2009). During this process, synaptic activity causes local translation of mRNAs normally stored in translationally repressed synaptic mRNPs (Sutton and Schuman 2006; Richter and Klann 2009). While specific neuronal translational repressors and microRNAs have been implicated in this process, their involvement in local translation that underlies memory, as well as the underlying mechanisms, are generally not well understood (Schratt et al. 2006; Keleman et al. 2007; Kwak et al. 2008; Li et al. 2008; Richter and Klann 2009). Furthermore, it remains possible that there are neuron-specific, mRNA-specific, and stimulus-pattern specific pathways for neuronal translational control (Raab-Graham et al. 2006; Giorgi et al. 2007).
The Fragile-X Mental Retardation protein (FMRP) is among the best studied of neuronal translational repressors, in part due to its association with human neurodevelopmental disease (Pieretti et al. 1991; Mazroui et al. 2002; Gao 2008). Consistent with function in synaptic translation required for memory formation, mutations in FMRP are associated with increased synaptic translation, enhanced LTD, increased synapse growth, and also with enhanced long-term memory (Zhang et al. 2001; Huber et al. 2002; Bolduc et al. 2008; Dictenberg et al. 2008).
FMRP co-immunoprecipitates with components of the RNAi and miRNA machinery and appears to be required for aspects of miRNA function in neurons (Caudy et al. 2002; Ishizuka et al. 2002; Jin et al. 2004b; Gao 2008). In addition, FMRP associates with neuronal polyribosomes as well as with Staufen-containing ribonucleoprotein (mRNP) granules easily observed in neurites of cultured neurons (Feng et al. 1997; Krichevsky and Kosik 2001; Mazroui et al. 2002; Kanai et al. 2004; Barbee et al. 2006; Bramham and Wells 2007; Bassell and Warren 2008; Dictenberg et al. 2008). FMRP-containing neuronal mRNPs contain not only several ubiquitous translational control molecules, but also CaMKII and Arc mRNAs, whose translation is locally controlled at synapses (Rook et al. 2000; Krichevsky and Kosik 2001; Kanai et al. 2004; Barbee et al. 2006). Thus, FMRP-containing RNA particles are probably translationally repressed and transported along microtubules from the neuronal cell body to synaptic sites in dendrites where local synaptic activity can induce their translation (Kiebler and Bassell 2006; Dictenberg et al. 2008).
The functions of FMRP/dFMR1 in mRNA localization as well as miRNA-dependent and independent forms of translational control is likely to require several other regulatory proteins. To identify such proteins, we used a previously designed and validated genetic screen (Wan et al. 2000; Jin et al. 2004a; Zarnescu et al. 2005). The overexpression of dFMR1 in the fly eye causes a “rough-eye” phenotype through a mechanism that requires (a) key residues in dFMR1 that mediate translational repression in vitro; (b) Ago1, a known components of the miRNA pathway; and (c) a DEAD-box helicase called Me31B, which is a highly conserved protein from yeast (Dhh1p) to humans (Rck54/DDX6) functioning in translational repression and present on neuritic mRNPs (Wan et al. 2000; Laggerbauer et al. 2001; Jin et al. 2004a; Coller and Parker 2005; Barbee et al. 2006; Chu and Rana 2006). To identify other Me31B-like translational repressors and neuronal granule components, we screened mutations in 43 candidate proteins for their ability to modify dFMR1 induced rough-eye phenotype. We describe the results of this genetic screen and follow up experiments to address the potential cellular functions of five genes identified as suppressors of sev-dfmr1.
MATERIALS AND METHODS
Fly stocks and crosses:
Fly stocks were raised at 25° on standard cornmeal and agar media. Wild-type (Oregon-R) flies, originally from the Benzer lab, were from Ramaswami lab stocks. The UAS YFP-dFMR1 line was constructed using the Gateway vectors from DGRC for cloning and subsequent transgenesis. Mutant/P-insert lines used for screening came from Harvard, Bloomington, and Szeged stock centers or individual laboratories. Putative overexpression lines were also from various stock centers, with the exception of UAS PABP (L) which was made by P. Lasko (Sigrist et al. 2000) and UAS Orb2 from the Dickson lab (Keleman et al. 2007). UASmCD8-GFP, UAS-flip Act<CD2<Gal4 was constructed using strains from Bloomington by S. Sanyal. The sevdFMR1; Cyo/Sco stock obtained from G. Dreyfuss was used as described in Wan et al. (2000). C380; cha gal 80 was from S. Sanyal, the hs Flp; hid sensor line is previously described in Brennecke et al. (2003); Barbee et al. (2006). Recombinants for clonal analysis were made with FRT lines (42D, 80B, 82B) from the Bloomington Drosophila Stock Center.
Screening methods and scanning electron microscopy:
Rough-eye phenotypes were initially assessed under the dissection microscope and scored “blind,” wherein the observer had no knowledge of the genotype. To confirm suppression and to document phenotypes, we compared 4–12 SEM images of eyes from experimental animals with those of “average” sev-dfmr1 lines outcrossed to w1118. Procedures for SEM were as described (Barbee et al. 2006).
Cell culture, immunocytochemistry, and granule counting:
Larval ventral ganglion cells were cultured and neuritic granules visualized as described previously (Barbee et al. 2006). Primary antibodies used for neuronal granule staining were rabbit anti-PABP at 1:200 (gift from P. Lasko), described in Sigrist et al. (2000), rabbit anti-Orb2 at 1:200, a gift from K. Si (Si et al. 2003), guinea pig anti-Rm62/Dmp68 at1:100, gift from A. Spradling (Buszczak and Spradling 2006), and mouse anti-Sm protein at 1:50, Upstate Signaling (Gonsalvez et al. 2006). Secondary antibodies were Alexa 568 (Molecular Probes/Invitrogen) against corresponding animal in primary antibody.
During confocal imaging, we controlled for channel bleed-through as described (Barbee et al. 2006). Neuritic granule counting and colocalization were done using National Institutes of Health ImageJ Cell Counter Plug-in. Somatic signal was not used for colocalization analysis. The data were analyzed in Microsoft Excel.
Dendrite imaging and quantification:
Sensory neurons were visualized using a flp-out technique in Gal4477, UAS-mCD8-GFP; UAS-flp, Act<CD2<Gal4 flies, then stained, mounted, and imaged as described earlier (Barbee et al. 2006). Dendrites were quantified using Neuronmetric software (Narro et al. 2007), an ImageJ Plug-in. Settings deviating from default settings are listed as follows: rolling ball radius = 1 pixel, gap distance = 20 pixels, extend distance = 20 pixels, maximum deviation = 10 pixels, length threshold = 20 pixels. Nonparametric tests, two-way ANOVA, and Dunnett multiple mean comparison were performed using Prism statistical software.
Clone induction and imaging:
Mitotic recombination clones were induced 48 ± 2 hr after egg laying (AEL) in staged larvae by heat shock at 37° for 2 hr. Larval genotypes used were: for FRT42D, [hsFLP1; FRT42D, arm-lacZ/FRT42D, (pabp[K10109] or smD3EP(2)2176); hid reporter/+]; for FRT80B, [_hsFLP1; hid rp/+; arm-lacZ, FRT80B/orb2Δ, FRT80B_]; and for FRT82B, [hsFLP1; hid rp/+; FRT82B, arm-lacZ/FRT82B dbtP[S139602] or rm62P[01086] or dFMR150M or _dcr1Q1147X)_]. Samples were fixed with 4% formaldehyde and stained with antibodies as described earlier. Mouse anti-LacZ and FITC-goat antiGFP were from Abcam. The discs were mounted in Vectashield (Vector Labs) and analyzed by confocal microscopy (Zeiss LSM 510) with a 20× objective and digital zoom.
RESULTS
Candidate-based screen for fragile-X dominant interactors:
Expression of dFMR1 via the _sevenles_s promoter causes rough eyes in sev-FMR1 Drosophila through a mechanism that requires residues in the RNA-binding KH domain necessary for translational repression in vitro, as well as for biological function in humans and Drosophila (Siomi et al. 1994; Wan et al. 2000; Laggerbauer et al. 2001). We designed a genetic screen on the basis of the idea that the amelioration (or suppression) of the sev-dfmr1 induced rough-eye phenotype could occur when one copy of a gene required for FMR1-mediated translational control is removed, as previously shown for ago1, me31B, and tral (Jin et al. 2004b; Barbee et al. 2006). Secondary assays indicative of direct interactions with dFMR1 (described in later sections) were used to identify/rule out alternative mechanisms of phenotypic suppression (Estes et al. 2008).
We identified 43 candidate genes for which available mutant lines allowed testing for involvement in the dFMR1 pathway. Proteins encoded by these genes fall into one or more of the four following categories: (1) known RNA-binding proteins; (2) proteins present on neuronal RNA granules in mammals; (3) proteins associated with the miRNA/RNAi pathways; and finally, (4) proteins known to be present on RNPs in other systems and organisms. For each of these genes, we obtained available mutant alleles from public and private Drosophila stock collections.
In control experiments, we showed that none of 21 randomly selected P-insertion lines showed any apparent dominant interaction with the rough-eye phenotype of sev-dfmr1 flies. In contrast, from a selection of putative loss-of-function alleles for the 43 “candidate” genes, we identified nine potentially interacting genes, mutations which suppressed the rough-eye phenotype of sev-dfMR1 flies (supporting information, Table S1; materials and methods). We followed up these initial observations by analyzing an expanded numbers of alleles for each of these nine genes (Table S2) to (a) more carefully assess candidate interactions; and (b) address potential contributions from second site/genetic background mutations, which would be specific to stocks of certain alleles.
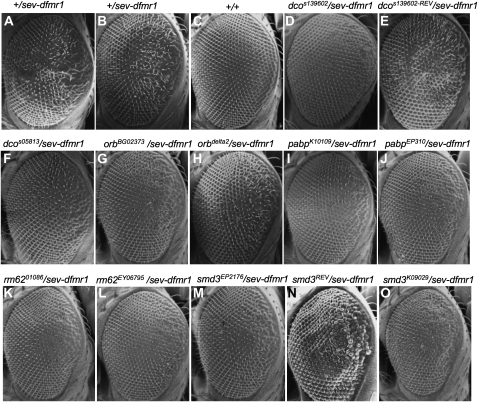
Of the initial nine genes, only five showed robust suppression with multiple, independently derived alleles (Figure 1). No mutants were identified that enhanced the rough-eye phenotype due to dFMR1 expression. The five identified genes were dco/dbt (discs overgrown/doubletime), orb2, pabp (poly-A binding protein), rm62/Dmp68, and smD3, all previously studied for their roles in Drosophila biology or RNA regulation (Figure 1; Table 1). These genetic data suggested that the five genes function in the dFMR1 pathway. Further studies addressed the underlying mechanisms.
Figure 1.—
Scanning electron micrographs of Drosophila compound eyes demonstrating suppression of sev-dfmr1 induced rough eyes by mutations in discs overgrown/doubletime (dco), orb2, poly-A binding protein (pabp), smd3, and rm62/dmp68. Two representative EMS or _P_-element associated alleles were selected with the median phenotypes and where possible, a revertant of a representative insertional allele isolated by _P_-element mobilization. A sev-dfmr1 transgene allows dFMR1 protein to be overexpressed in a subset of photoreceptors via the sevenless (sev) promoter. (A and B) Eyes of control animals. F1 progeny from a cross between sev-dfmr1 and w1118 flies showing the range of rough-eye phenotypes. (C) Wild-type (Canton S) eyes. (D) The _P_-element-associated dcoS139602 allele in trans with sev-dfmr1. (E) Eyes of phenotypic “revertant” dcoS139602-REV/sev-dfmr1 flies show reversion of suppression following _P_-element excision from dcoS139602. (F) dcoS05813/sev-dfmr1. (G and H, respectively) orb2BG02373/sev-dfmr1 and orb2delta2/sev-dfmr1. (I and J, respectively) pabpK10109/sev-dfmr1 and pabpEP310/sev-dfmr1. (K and L, respectively) rm6201086/sev-dfmr1 and rm62EY06795/sev-dfmr1. (M) smd3EP2176/sev-dfmr1 and (N) phenotypic revertant of smd3EP2176. Eyes of smd3EP2176-REV/sev-dfmr1 flies: smd3EP2176-REV was isolated by the mobilization and excision of the EP2176 P-insertion. (O) smd3K09029/sev-dfmr1.
TABLE 1.
Mutant alleles of dco, orb2, pabp, rm62, and smd3 that suppress the sev-dfmr1 induced “rough-eye” phenotype
| Gene name | Suppressing alleles |
|---|---|
| discs overgrown/doubletime | dcoj3b9, dco3, dcoS053813, dcoS139602, dcoS146009 |
| orb2 | orb2BG02373, orb2d01793, orb2c06090, orb2f01556, orb2delta2 |
| poly-A binding protein | pabpEP310, pabpK10109 |
| rm62/dmp68 | rm6210186, rm62EY10915, rm62EY06795, rm62d02918 |
| Small ribonucleoprotein D3 | smd3K09020, smd3EP2176, smd3EP2104 |
FMR1 suppressor proteins are present on neuronal granules:
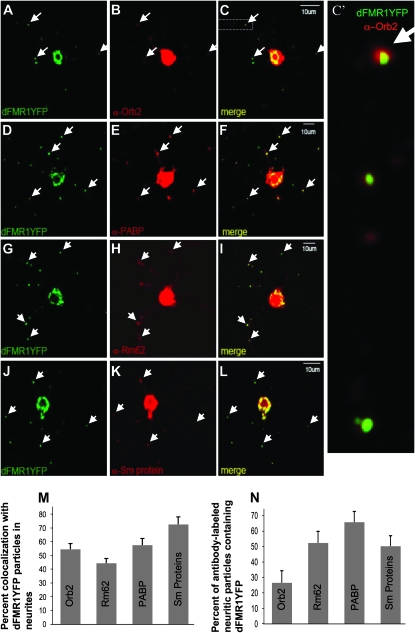
To ask whether proteins encoded by _dfmr1_-modifier genes function in physical proximity to dFMR1, we asked if these proteins were present on FMRP-positive, neuritic RNPs, previously shown to contain translationally repressed mRNAs (Mazroui et al. 2002; Kanai et al. 2004; Barbee et al. 2006; Bramham and Wells 2007; Dictenberg et al. 2008). Remarkably, four out of the five proteins colocalized, albeit at different levels, with a YFP-labeled dFMR1 granules in neurites of cultured Drosophila motor neurons (Figure 2; Barbee et al. 2006). No specific antibody was available to test the presence of Dco/Dbt in neuronal granules but studies from Saccharomyces cerevisiae have shown that a homologous protein is present on P bodies (D. Muhlrad and R. Parker, unpublished observations), which contain many of the components found in neuronal granules (Barbee et al. 2006; Cougot et al. 2008; Miller et al. 2009). Thus, we conclude that at least PABP, Orb2, Rm62, and SmD3 are components of FMRP-containing RNA granules found in neuronal processes.
Figure 2.—
Orb2, poly-A binding protein (PABP), Rm62/Dmp68, and Sm proteins are present on dFMR1-containing foci in neurites of cultured Drosophila primary neurons. Neurites of dissociated and cultured larval neurons contain distinct FMR1-containing mRNPs that also contain several molecules involved in translational control (Barbee et al. 2006; Beckham et al. 2008; Kwak et al. 2008). These particles visualized in processes of motor neuron expressing dFMR1-YFP (from C380Gal4; ChaGal80; UASdfmr1-YFP larvae) also contain Orb2 (A–C), PABP (D–F), Rm62 (G–I), and Sm (J–L) proteins. C′ shows an expanded and higher-resolution image of the boxed region in C to clearly demonstrate the observed colocalization. The percentage of colocalization between dFMR1YFP and the other proteins in neuritic granules is quantified in M (which shows the fraction of dFMR1-YFP marked neuritic particles that also contain each tested protein) and N (which indicates the fraction of neurite granules with each tested protein that also contains dFMR1-YFP). The signal in somata necessarily appears overexposed, to clearly image fainter neuritic granules that are the focus of our colocalization analysis.
Recent studies have revealed that there are different populations of neuronal RNA granules that share overlapping components (Barbee et al. 2006; Kiebler and Bassell 2006). The significance of these different granule subtypes if not yet clear but may reflect either granules with different populations of mRNAs, different kinetic stages in the function of similar granules, or differences between transport, storage, and degradative granules. Given this diversity, we desired to determine how frequently each of the suppressors colocalized with dFMR. To quantify colocalization of the specific antigen with dFMR1YFP, a number of YFP spots were first identified, then the two color channels of a confocal image were merged (green for YFP, red for antibody stain) and the number of colocalizing spots were counted. Colocalization with FMR1-positive particles was 44% for Orb2, 54% for PABP, 58% for Rm62, and 73% for Sm proteins (Figure 2M). Reverse colocalization was also done, which is defined as the percentage of antibody-labeled puncta that contain dFMR1YFP. These rates were 26% with Orb2, 52% with PABP, 65% with Rm62, and 50% with Sm Proteins. Although future work will be required to determine the significance of the granule diversity, the observed physical colocalization of proteins encoded by genes identified as dominant suppressors of sev-dfmr1, supports a model in which the gene products function in dFMR1-mediated regulation of neuronal mRNAs.
Overexpression of dFMR1 interactors inhibits dendritic branching:
Proteins colocalizing with FMR1 could be involved in translational repression or many alternative functions (see discussion). Because previous studies have suggested that overexpression of proteins repressing translation can strongly inhibit dendritic growth (Lee et al. 2003; Ye et al. 2004; Barbee et al. 2006), we tested whether the FMR1-modifier proteins had such effects on dendrite branching.
We assayed dendritic morphology in class-IV type, sensory neurons of the larval body wall, selected due to ease of identification and visualization, as well as their sensitivity to translation repression (Grueber et al. 2002). Overexpression lines for each suppressing gene were crossed to a strain that expressed a membrane-targeted GFP via a class IV-specific Gal4 driver (477) and an amplifier construct (act<CD2<Gal4; hsFLP), as described previously (Barbee et al. 2006), and dendrite morphology quantified (materials and methods; Narro et al. 2007).
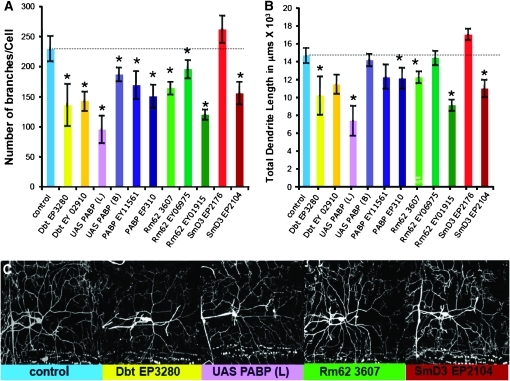
A significant observation was that following overexpression of PABP, Smd3, Rm62, and Dco, we observed statistically significant effects particularly in branch number, but also in the overall dendritic length (Figure 3). The branch number in particular is a measure of dendritic complexity. These observations are remarkably similar to those previously reported for known translational repressors including FMR1 (Lee et al. 2003; Ye et al. 2004; Barbee et al. 2006). We were unable to assess the function of Orb2 in this assay since the two available overexpression lines caused lethality (Keleman et al. 2007). The effect on dendritic complexity along with the dFMR1 colocalization data further suggest that these proteins are acting in a method similar to other known neuronal translational repressors.
Figure 3.—
Overexpression of identified proteins reduces dendritic branching and alters dendritic morphology in larval class IV sensory neurons. Each of the sev-dfmr1 interacting genes was overexpressed via UAS/Gal4 technology in sensory neurons using a flp-out technique, in which Gal4477 driver was combined with an act<CD2<Gal4, UAS Flip recombinase, to mark fine dendritic processes (Barbee et al. 2006). (A) Number of branches/cells was decreased in all lines overexpressing Discs overgrown/Doubletime (dcoEP3280, dcoEY02910), poly-A binding protein lines (UAS-pabp (L), UAS pabp (B), pabpEP310, pabpEY11561), or Rm62 (rm62EP3607, rm62EY01915, rm62 EY06975) and one of the two SmD3 (smD3EP2104) lines assayed. (B) Total dendrite length was significantly reduced in all poly-A binding protein lines (UAS-pabp (L), UAS pabp (B), pabpEP310, pabpEY11561), all Discs overgrown/Doubletime (dcoEP3280, dcoEY02910), two Rm62 (rm62EP3607, rm62EY0191), and one of the SmD3 (smD3EP2104) lines assayed. We do not have a definitive explanation why smd3EP2176 does not show a similar effect. The dashed line indicates control levels. (C) Representative images of a labeled sensory neuron from each overexpression line are presented.
None of the new dFMR1 modifier genes are required for optimal miRNA function:
Previous biochemical, genetic, and bioinformatic arguments support a role for FMRP/dFMR1 in the miRNA pathway. FMRP has been shown to have strong biochemical or genetic interactions with mammalian and Drosophila argonautes (Jin et al. 2004b), and 3′-UTRs of predicted human microRNA targets are highly enriched in potential FMRP binding elements (John et al. 2004).
Because of their genetic interaction with dFMR1/FMRP we tested whether PABP, Smd3, Rm62, Orb2, and Dco were required for the efficient function of bantam, a miRNA that represses hid mRNA translation in wing imaginal discs (Brennecke et al. 2003). To assay bantam function, we used the “hid reporter,” which carries the 3′-UTR of hid fused to the 3′ end of EGFP-coding sequence, and closely reports the bantam repression of endogenous hid mRNA (Brennecke et al. 2003). We used the hid reporter to determine how the loss of specific proteins affects miRNA-mediated repression in its natural context (Barbee et al. 2006).
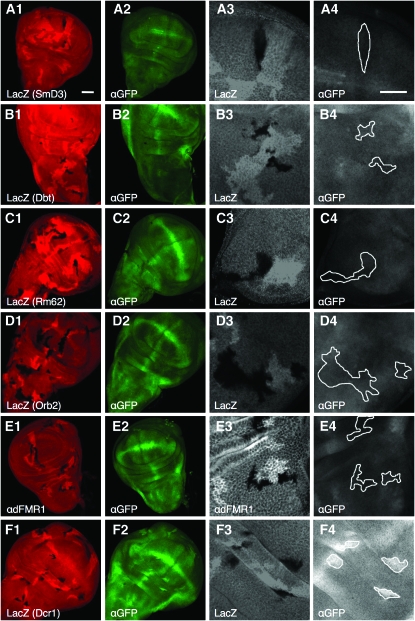
In genetically mosaic wing imaginal discs, we compared _hid-_reporter expression in marked, mutant-cell clones with that in adjacent control cells (materials and methods; Barbee et al. 2006). Mutant clones were identified either by the absence of β-galactosidase or by immunostaining for the encoded protein (Figure 4). We successfully obtained multiple loss-of-function clones for four of the five dfmr1 modifier genes; for pabp mutants, we only obtained two mutant clones from hundreds of discs examined. We found that hid reporter levels in control cells were indistinguishable from those in adjacent mutant cells that were homozygous for severe loss-of-function alleles for Rm62, orb2, smD3, and dco (Figure 4, A–D). Thus, unlike me31b (Barbee et al. 2006), rm62, dco, smd3, and orb2 are not essential for efficient bantam function in vivo.
Figure 4.—
Suppressors of sev-dfmr1 as well as the dfmr1 gene are not essential for efficient _bantam-_miRNA mediated repression of a target translational reporter (hid reporter GFP). (A–F) show analyses of _hid_-reporter expression in mutant clones of smd3 (A), dco/dbt (B), rm62 (C), orb2 (D), dfmr1(E), and dicer-1 (F). (A–F) panel 1, 20× magnification images of mutant clones in wing imaginal discs visualized by staining with a corresponding antibody or LacZ; panel 2, 20× GFP-labeled hid reporter expression, whose levels are low due to efficient _bantam_-mediated translational repression; panel 3, 60× close-up of mutant clones marked with corresponding antibody or LacZ; and panel 4, corresponding area of clones (circled) with _hid_-GFP expression. (F), 1–4, Dicer1 clones show significant upregulation of the hid reporter. See methods for larval genotypes.
In light of the above observations, we used this direct and robust assay to ask whether dFMR1 was required for _bantam_-mediated hid repression. Contrary to our expectation, loss of dfmr1 had no effect on reporter expression (Figure 4E, 1–4). In comparison, loss of Dicer-1, an essential component of the miRNA pathway, results in significant upregulation of the hid reporter levels (Figure 4F, 1–4). Thus, our data indicate that dFMR1 is not a universally required component of the miRNA pathway. However, it remains entirely possible that it is necessary for repression of a subset of miRNA targets, particularly those that contain FMRP binding motifs. Such a model would be consistent with previous observations (Ishizuka et al. 2002; Jin et al. 2004b; Siomi et al. 2004).
We note that loss of Rm62/Dmp68, a confirmed member of the Drosha complex in mammals, did not affect _hid-_reporter fluorescence. This observation may be explained by redundant processing helicases in the Drosha processing complex (see discussion) (Kahlina et al. 2004; Jalal et al. 2007).
DISCUSSION
Identification of new neuronal translational regulators:
We suggest, that as for previously identified sev-dfmr1 suppressors Ago1, Lgl, and Me31b (Jin et al. 2004b; Zarnescu et al. 2005; Barbee et al. 2006), analysis of PABP, Smd3, Rm62, Orb2, and Dco proteins, encoded by the sev-dfmr1 suppressor genes identified here, will help elucidate how dFMR1 works in translational regulation, RNA targeting and localization, and ncRNA pathway function.
FMR1 modifier proteins function in translational control:
Three lines of evidence indicate that the genes we identify encode proteins with translational repressor activity. First, with the exception of Dco, all of these proteins have been previously implicated in some aspect of RNA metabolism (discussed later in this section) and are present on dFMR1-containing neuritic granules in which RNA is repressed and transported (Bassell and Warren 2008). Second, the rough-eye phenotype observed in sev-dfmr1 has been linked to the ability of FMRP to repress mRNA translation (Wan et al. 2000; Laggerbauer et al. 2001). Thus, we would expect the phenotype to be alleviated by mutations that reduce the efficiency of translational repression. Third, overexpression of Dco, Pabp, Orb2, or Rm62 inhibits the dendritic growth of neurons, a phenotype predicted for neuronal translational repressors (Lee et al. 2003; Ye et al. 2004; Barbee et al. 2006). These observations are consistent with the idea that translation of RNAs in neurites, which promotes dendritic branching, is inhibited by overexpression of Dco, Pabp, Orb2, or Rm62 (Aakalu et al. 2001; Martin 2004). Thus, genetic interaction data, molecular localization, and one functional test in dendrites indicate that Dco/Dbt, PABP, Rm62, or SmD3 function as neuronal translational repressors.
The identification of several canonical translational-factor encoding genes as suppressors of sev-dfmr1 highlights the point that individual translational control molecules work in multicomponent complexes and therefore have several functional interactions. PABP is one example of a protein that is currently believed to perform two opposing functions of translational control. In addition to its well-studied role as a translational activator, PABP can mediate translational repression, e.g., of Vasopressin mRNA although the exact mechanism remains unclear (Mohr et al. 2001). Dual roles in activation and repression are also suggested by the observation that reduced or elevated levels of PABP have similar effects at the Drosophila neuromuscular junction (NMJ) (Sigrist et al. 2000). Additionally, PABP associates with particles containing BC1, a neuron-specific noncoding RNA with translational repressor function, as well as a CYFIP–FMRP complex that may function as a repressor in some contexts but as an activator in others (Muddashetty et al. 2002; West et al. 2002). Similarly, Orb2 homologs (CPEBs) though required for translational activation of CPE-containing mRNAs via poly-A polymerase (Wu et al. 1998; Kwak et al. 2008), also allow translational repression in combination with Maskin or Cup proteins (Barkoff et al. 2000; Nakamura et al. 2004).
It was somewhat surprising that we identify SmD3, a splicing factor, in our screen for translational repressors. However, SmD3 has additional nonsplicing functions: in Caenorhabditis elegans, the Sm proteins are required for germ cell mRNP assembly and RNA localization (Barbee et al. 2002). Such a role in translational regulation and mRNP assembly is more consistent with functions predicted by our genetic experiments.
Rm62/Dmp68 is a member of the DEAD-box helicase family that has been shown to be associated with a dFMR1-containing RNAi silencing complex (Ishizuka et al. 2002). It also has additional roles during transcription and mRNA processing as well as potentially in miRNA processing as part of the Drosha complex (Kahlina et al. 2004; Park et al. 2004; Buszczak and Spradling 2006; Camats et al. 2008). Based on the biochemical evidence for Rm62's presence in FMRP-containing complexes, it is not surprising that rm62 mutations show strong genetic interactions with dfmr1. However, the mechanism of suppression remains unknown.
Finally Dco/Dbt, is by far the most elusive protein in regard to its potential function in the translational regulatory pathway. Dco/Dbt, a casein kinase I (CKI) is best known from circadian biology where it phosphorylates Per and expedites its degradation (Preuss et al. 2004). dFMR1 protein has several phosphorylation sites, one of which in S2 cells has been demonstrated to be phosphorylated by a CKII protein (Siomi et al. 2002). While the functional requirement for CKI-dependent dFMR1 phosphorylation is as of yet not understood, there is considerable evidence that the phosphorylation state of FMRP may actually determine its role in translation. Biochemical data demonstrate that most FMRP in granules is in the phosphorylated state while FMRP in the polysome fraction is dephosphorylated (Ceman et al. 2003), suggesting a mechanism to switch state from an activator to a repressor, and an important regulatory role for kinases that phosphorylate FMRP (Narayanan et al. 2007, 2008).
Another interesting potential link between the two proteins is the behavioral observation that patients with Fragile-X Mental Retardation often display circadian disturbances (O'donnell and Warren 2002). This altered circadian rhythm is also present in the Drosophila dfmr1 mutants that usefully model fragile-X syndrome (Dockendorff et al. 2002).
The identification of these proteins as sev-dfmr1 modifiers illustrates the many possibly regulatory roles of RNA-associated proteins. In addition, the data associating Dco/Dbt with RNA regulation indicates unexplored and novel mechanisms of RNA regulation in neurons.
dFMR1/FMRP's role in the noncoding RNA pathway:
Given that dFMR1/FMRP is thought to function in miRNA-dependent translational repression, we were particularly interested in asking whether these dFMR1 interactors had any role in this pathway. To address this issue, we employed a sensitive in vivo assay that uses a fluorescent reporter to reveal the strength of translational repression via an endogenous (bantam) miRNA. When combined with genetic mosaic analysis, this assay can be used to study null mutations in candidate genes, as long as the mutations do not cause cell lethality. The assay appears more sensitive than typically used cell-based assays on the evidence of our prior analysis of Me31B, whose requirement for miRNA function, clearly seen in the in vivo assay, is only evident in double-knockdown experiments in the more commonly used cell-culture assays (Barbee et al. 2006; Chu and Rana 2006; Eulalio et al. 2007).
Our in vivo experiments revealed no requirement for the sev-dfmr1 interacting proteins Dco, Orb2, Rm62, and SmD3 in miRNA repression. For reasons explained above, it is unlikely that this reflects a weakness in the experimental assay for miRNA function. A bigger surprise was our finding that the dFMR1 itself appeared dispensable for miRNA function in vivo. Because the allele used is a well-characterized null allele, and the absence of dFMR1 in the mutant clones is confirmed by antibody staining, our conclusion that dFMR1 is not a core, essential component of the RISC/miRNA pathway is strong. This conclusion is not inconsistent with any of the existing data showing biochemical association between RISC and FMRP and genetic interactions between Ago1 and FMRP (Ishizuka et al. 2002; Jin et al. 2004b; Gao 2008; Li et al. 2008). However, it is also consistent with recent observations indicating the dispensability of FMRP for RISC function in cultured cells (Didiot et al. 2009). We suggest that the function of dFMR1 and, by extension, FMRP may be restricted to a subset of transcripts, for instance those with UTRs containing both FMRP binding motifs and miRNA target elements. Indeed similar models that account for the mRNA specificity of FMRP have been previously proposed (Siomi et al. 2004; Li et al. 2008).
Data presented here provide a foundation on which to design further experiments to understand the specific roles of FMR1 and its interacting proteins in translational control.
Acknowledgments
We thank Eimear Holohan for advice and early help with creating wing mosaics and for her comments on the manuscript; C. Boswell of the Molecular and Cellular Biology Imaging Facility for help with microscopy; D. Bentley and S. Hernandez of Arizona Research Laboratories and associated facilities (http://usif.arizona.edu/) for access to, and assistance in, scanning electron microscopy; Kyoto, Szeged, and Bloomington stock centers for Drosophila stocks; L. Restifo, R. Kraft, and M. Narro for access to computing facilities and extensive help adapting Neuronmetric software settings to our purposes; J. Price, K. Si, B. Dickson, A. Spradling, and P. Lasko for fly stocks and antibodies. This work was funded by grants from the Science Foundation of Ireland and the National Institute of Drug Abuse (to M.R.). A.-M.C. was supported by National Institute of Neurological Disorders and Stroke grant no. F31 NS055479-01.
References
- Aakalu, G., W. B. Smith, N. Nguyen, C. Jiang and E. M. Schuman, 2001. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30 489–502. [DOI] [PubMed] [Google Scholar]
- Ashraf, S. I., A. L. McLoon, S. M. Sclarsic and S. Kunes, 2006. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 124 191–205. [DOI] [PubMed] [Google Scholar]
- Barbee, S. A., P. S. Estes, A. M. Cziko, J. Hillebrand, R. A. Luedeman et al., 2006. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron 52 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee, S. A., A. L. Lublin and T. C. Evans, 2002. A novel function for the Sm proteins in germ granule localization during C. elegans embryogenesis. Curr. Biol. 12 1502–1506. [DOI] [PubMed] [Google Scholar]
- Barkoff, A. F., K. S. Dickson, N. K. Gray and M. Wickens, 2000. Translational control of cyclin B1 mRNA during meiotic maturation: coordinated repression and cytoplasmic polyadenylation. Dev. Biol. 220 97–109. [DOI] [PubMed] [Google Scholar]
- Bassell, G. J., and S. T. Warren, 2008. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham, C., A. Hilliker, A. M. Cziko, A. Noueiry, M. Ramaswami et al., 2008. The DEAD-box RNA helicase Ded1p affects and accumulates in Saccharomyces cerevisiae P-bodies. Mol. Biol. Cell 19 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc, F. V., K. Bell, H. Cox, K. S. Broadie and T. Tully, 2008. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat. Neurosci. 11 1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham, C. R., and D. G. Wells, 2007. Dendritic mRNA: transport, translation and function. Nat. Rev. Neurosci. 8 776–789. [DOI] [PubMed] [Google Scholar]
- Brennecke, J., D. R. Hipfner, A. Stark, R. B. Russell and S. M. Cohen, 2003. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113 25–36. [DOI] [PubMed] [Google Scholar]
- Buszczak, M., and A. C. Spradling, 2006. The Drosophila P68 RNA helicase regulates transcriptional deactivation by promoting RNA release from chromatin. Genes Dev. 20 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camats, M., S. Guil, M. Kokolo and M. Bach-Elias, 2008. P68 RNA helicase (DDX5) alters activity of cis- and trans-acting factors of the alternative splicing of H-Ras. PLoS ONE 3 e2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio, A., K. C. Martin, M. Giustetto, H. Zhu, M. Chen et al., 1999. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99 221–237. [DOI] [PubMed] [Google Scholar]
- Caudy, A. A., M. Myers, G. J. Hannon and S. M. Hammond, 2002. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 16 2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceman, S., W. T. O'Donnell, M. Reed, S. Patton, J. Pohl et al., 2003. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum. Mol. Genet. 12 3295–3305. [DOI] [PubMed] [Google Scholar]
- Chu, C. Y., and T. M. Rana, 2006. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 4 e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller, J., and R. Parker, 2005. General translational repression by activators of mRNA decapping. Cell 122 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougot, N., S. N. Bhattacharyya, L. Tapia-Arancibia, R. Bordonne, W. Filipowicz et al., 2008. Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation. J. Neurosci. 28 13793–13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg, J. B., S. A. Swanger, L. N. Antar, R. H. Singer and G. J. Bassell, 2008. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev. Cell 14 926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didiot, M. C., M. Subramanian, E. Flatter, J. L. Mandel and H. Moine, 2009. Cells lacking the fragile X mental retardation protein (FMRP) have normal RISC activity but exhibit altered stress granule assembly. Mol. Biol. Cell 20 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockendorff, T. C., H. S. Su, S. M. McBride, Z. Yang, C. H. Choi et al., 2002. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34 973–984. [DOI] [PubMed] [Google Scholar]
- Estes, P. S., M. O'Shea, S. Clasen and D. C. Zarnescu, 2008. Fragile X protein controls the efficacy of mRNA transport in Drosophila neurons. Mol. Cell. Neurosci. 39 170–179. [DOI] [PubMed] [Google Scholar]
- Eulalio, A., J. Rehwinkel, M. Stricker, E. Huntzinger, S. F. Yang et al., 2007. Target-specific requirements for enhancers of decapping in miRNA-mediated gene silencing. Genes Dev. 21 2558–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y., D. Absher, D. E. Eberhart, V. Brown, H. E. Malter et al., 1997. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell 1 109–118. [DOI] [PubMed] [Google Scholar]
- Gao, F. B., 2008. Post-transcriptional control of neuronal development by microRNA networks. Trends Neurosci. 31 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi, C., G. W. Yeo, M. E. Stone, D. B. Katz, C. Burge et al., 2007. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell 130 179–191. [DOI] [PubMed] [Google Scholar]
- Gonsalvez, G. B., T. K. Rajendra, L. Tian and A. G. Matera, 2006. The Sm-protein methyltransferase, dart5, is essential for germ-cell specification and maintenance. Curr. Biol. 16 1077–1089. [DOI] [PubMed] [Google Scholar]
- Grueber, W. B., L. Y. Jan and Y. N. Jan, 2002. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 129 2867–2878. [DOI] [PubMed] [Google Scholar]
- Huber, K. M., S. M. Gallagher, S. T. Warren and M. F. Bear, 2002. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA 99 7746–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka, A., M. C. Siomi and H. Siomi, 2002. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 16 2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal, C., H. Uhlmann-Schiffler and H. Stahl, 2007. Redundant role of DEAD box proteins p68 (Ddx5) and p72/p82 (Ddx17) in ribosome biogenesis and cell proliferation. Nucleic Acids Res. 35 3590–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, P., R. S. Alisch and S. T. Warren, 2004. a RNA and microRNAs in fragile X mental retardation. Nat. Cell Biol. 6 1048–1053. [DOI] [PubMed] [Google Scholar]
- Jin, P., D. C. Zarnescu, S. Ceman, M. Nakamoto, J. Mowrey et al., 2004. b Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat. Neurosci. 7 113–117. [DOI] [PubMed] [Google Scholar]
- John, B., A. J. Enright, A. Aravin, T. Tuschl, C. Sander et al., 2004. Human MicroRNA targets. PLoS Biol. 2 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlina, K., I. Goren, J. Pfeilschifter and S. Frank, 2004. p68 DEAD box RNA helicase expression in keratinocytes. Regulation, nucleolar localization, and functional connection to proliferation and vascular endothelial growth factor gene expression. J. Biol. Chem. 279 44872–44882. [DOI] [PubMed] [Google Scholar]
- Kanai, Y., N. Dohmae and N. Hirokawa, 2004. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 43 513–525. [DOI] [PubMed] [Google Scholar]
- Keleman, K., S. Kruttner, M. Alenius and B. J. Dickson, 2007. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat. Neurosci. 10 1587–1593. [DOI] [PubMed] [Google Scholar]
- Kiebler, M. A., and G. J. Bassell, 2006. Neuronal RNA granules: movers and makers. Neuron 51 685–690. [DOI] [PubMed] [Google Scholar]
- Krichevsky, A. M., and K. S. Kosik, 2001. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32 683–696. [DOI] [PubMed] [Google Scholar]
- Kwak, J. E., E. Drier, S. A. Barbee, M. Ramaswami, J. C. Yin et al., 2008. GLD2 poly(A) polymerase is required for long-term memory. Proc. Natl. Acad. Sci. USA 105 14644–14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer, B., D. Ostareck, E. M. Keidel, A. Ostareck-Lederer and U. Fischer, 2001. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum. Mol. Genet. 10 329–338. [DOI] [PubMed] [Google Scholar]
- Lecuyer, E., H. Yoshida, N. Parthasarathy, C. Alm, T. Babak et al., 2007. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell 131 174–187. [DOI] [PubMed] [Google Scholar]
- Lee, A., W. Li, K. Xu, B. A. Bogert, K. Su et al., 2003. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development 130 5543–5552. [DOI] [PubMed] [Google Scholar]
- Li, Y., L. Lin and P. Jin, 2008. The microRNA pathway and fragile X mental retardation protein. Biochim. Biophys. Acta 1779 702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, K. C., 2004. Local protein synthesis during axon guidance and synaptic plasticity. Curr. Opin. Neurobiol. 14 305–310. [DOI] [PubMed] [Google Scholar]
- Martin, K. C., and A. Ephrussi, 2009. mRNA localization: gene expression in the spatial dimension. Cell 136 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui, R., M. E. Huot, S. Tremblay, C. Filion, Y. Labelle et al., 2002. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet. 11 3007–3017. [DOI] [PubMed] [Google Scholar]
- Miller, L. C., V. Blandford, R. McAdam, M. R. Sanchez-Carbente, F. Badeaux et al., 2009. Combinations of DEAD box proteins distinguish distinct types of RNA: protein complexes in neurons. Mol. Cell. Neurosci. 40 485–495. [DOI] [PubMed] [Google Scholar]
- Mohr, E., N. Prakash, K. Vieluf, C. Fuhrmann, F. Buck et al., 2001. Vasopressin mRNA localization in nerve cells: characterization of _cis_-acting elements and _trans_-acting factors. Proc. Natl. Acad. Sci. USA 98 7072–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty, R., T. Khanam, A. Kondrashov, M. Bundman, A. Iacoangeli et al., 2002. Poly(A)-binding protein is associated with neuronal BC1 and BC200 ribonucleoprotein particles. J. Mol. Biol. 321 433–445. [DOI] [PubMed] [Google Scholar]
- Nakamura, A., K. Sato and K. Hanyu-Nakamura, 2004. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell 6 69–78. [DOI] [PubMed] [Google Scholar]
- Narayanan, U., V. Nalavadi, M. Nakamoto, D. C. Pallas, S. Ceman et al., 2007. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J. Neurosci. 27 14349–14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan, U., V. Nalavadi, M. Nakamoto, G. Thomas, S. Ceman et al., 2008. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem. 283 18478–18482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narro, M. L., F. Yang, R. Kraft, C. Wenk, A. Efrat et al., 2007. NeuronMetrics: software for semi-automated processing of cultured neuron images. Brain Res. 1138 57–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, W. T., and S. T. Warren, 2002. A decade of molecular studies of fragile X syndrome. Annu. Rev. Neurosci. 25 315–338. [DOI] [PubMed] [Google Scholar]
- Park, J. W., K. Parisky, A. M. Celotto, R. A. Reenan and B. R. Graveley, 2004. Identification of alternative splicing regulators by RNA interference in Drosophila. Proc. Natl. Acad. Sci. USA 101 15974–15979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti, M., F. P. Zhang, Y. H. Fu, S. T. Warren, B. A. Oostra et al., 1991. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66 817–822. [DOI] [PubMed] [Google Scholar]
- Preuss, F., J. Y. Fan, M. Kalive, S. Bao, E. Schuenemann et al., 2004. Drosophila doubletime mutations which either shorten or lengthen the period of circadian rhythms decrease the protein kinase activity of casein kinase I. Mol. Cell. Biol. 24 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Graham, K. F., P. C. Haddick, Y. N. Jan and L. Y. Jan, 2006. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science 314 144–148. [DOI] [PubMed] [Google Scholar]
- Richter, J. D., and E. Klann, 2009. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 23 1–11. [DOI] [PubMed] [Google Scholar]
- Rook, M. S., M. Lu and K. S. Kosik, 2000. CaMKIIalpha 3′ untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J. Neurosci. 20 6385–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt, G. M., F. Tuebing, E. A. Nigh, C. G. Kane, M. E. Sabatini et al., 2006. A brain-specific microRNA regulates dendritic spine development. Nature 439 283–289. [DOI] [PubMed] [Google Scholar]
- Si, K., S. Lindquist and E. R. Kandel, 2003. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell 115 879–891. [DOI] [PubMed] [Google Scholar]
- Sigrist, S. J., P. R. Thiel, D. F. Reiff, P. E. Lachance, P. Lasko et al., 2000. Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature 405 1062–1065. [DOI] [PubMed] [Google Scholar]
- Siomi, H., M. Choi, M. C. Siomi, R. L. Nussbaum and G. Dreyfuss, 1994. Essential role for KH domains in RNA binding: impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell 77 33–39. [DOI] [PubMed] [Google Scholar]
- Siomi, H., A. Ishizuka and M. C. Siomi, 2004. RNA interference: A new mechanism by which FMRP acts in the normal brain? What can Drosophila teach us? Ment. Retard. Dev. Disabil. Res. Rev. 10 68–74. [DOI] [PubMed] [Google Scholar]
- Siomi, M. C., K. Higashijima, A. Ishizuka and H. Siomi, 2002. Casein kinase II phosphorylates the fragile X mental retardation protein and modulates its biological properties. Mol. Cell. Biol. 22 8438–8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, M. A., and E. M. Schuman, 2006. Dendritic protein synthesis, synaptic plasticity, and memory. Cell 127 49–58. [DOI] [PubMed] [Google Scholar]
- Wan, L., T. C. Dockendorff, T. A. Jongens and G. Dreyfuss, 2000. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol. Cell. Biol. 20 8536–8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, N., A. M. Roy-Engel, H. Imataka, N. Sonenberg and P. Deininger, 2002. Shared protein components of SINE RNPs. J. Mol. Biol. 321 423–432. [DOI] [PubMed] [Google Scholar]
- Wu, L., D. Wells, J. Tay, D. Mendis, M. A. Abbott et al., 1998. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron 21 1129–1139. [DOI] [PubMed] [Google Scholar]
- Ye, B., C. Petritsch, I. E. Clark, E. R. Gavis, L. Y. Jan et al., 2004. Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr. Biol. 14 314–321. [DOI] [PubMed] [Google Scholar]
- Zarnescu, D. C., P. Jin, J. Betschinger, M. Nakamoto, Y. Wang et al., 2005. Fragile X protein functions with lgl and the par complex in flies and mice. Dev. Cell 8 43–52. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. Q., A. M. Bailey, H. J. Matthies, R. B. Renden, M. A. Smith et al., 2001. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107 591–603. [DOI] [PubMed] [Google Scholar]