Immunotherapy for Osteosarcoma: Genetic Modification of T cells Overcomes Low Levels of Tumor Antigen Expression (original) (raw)
Abstract
Human epidermal growth factor receptor 2 (HER2) is expressed by the majority of human osteosarcomas and is a risk factor for poor outcome. Unlike breast cancer, osteosarcoma cells express HER2 at too low, a level for patients to benefit from HER2 monoclonal antibodies. We reasoned that this limitation might be overcome by genetically modifying T cells with HER2-specific chimeric antigen receptors (CARs), because even a low frequency of receptor engagement could be sufficient to induce effector cell killing of the tumor. HER2-specific T cells were generated by retroviral transduction with a HER2-specific CAR containing a CD28.ζ signaling domain. HER2-specific T cells recognized HER2-positive osteosarcoma cells as judged by their ability to proliferate, produce immunostimulatory T helper 1 cytokines, and kill HER2-positive osteosarcoma cell lines in vitro. The adoptive transfer of HER2-specific T cells caused regression of established osteosarcoma xenografts in locoregional as well as metastatic mouse models. In contrast, delivery of nontransduced (NT) T cells did not change the tumor growth pattern. Genetic modification of T cells with CARs specific for target antigens, expressed at too low a level to be effectively recognized by monoclonal antibodies, may allow immunotherapy to be more broadly applicable for human cancer therapy.
Introduction
Osteosarcoma is the most common human primary bone malignancy, accounting for over 700 new diagnoses in the United States each year. Current multimodality therapy consists of radical surgery and systemic chemotherapy.1,2 Despite improvements in outcome over the past 30 years for patients with local disease, the 5-year survival rates for patients with metastatic disease at presentation or recurrent disease remain poor.3 The human epidermal growth factor receptor 2 (HER2) is expressed in up to 60% of primary osteosarcoma samples and correlates with poor outcome.4,5,6,7,8,9,10,11,12 HER2 is a validated target for breast cancer immunotherapy.13,14,15,16 However, the expression of HER2 on osteosarcoma cells is low, rendering HER2 monoclonal antibodies like trastuzumab less effective.5,17,18
Genetic modification of T cells is an attractive approach to overcome this limitation because the overall avidity of receptors arrayed on a T cell will be greater than the avidity of a bivalent antibody, and engagement of a limited number of T-cell receptor molecules may be sufficient to trigger a cytotoxic effector response.19,20,21 A HER2-specific single chain variable fragment can be prepared as part of a chimeric antigen receptor (CAR),17 and the aim of this study was to determine whether osteosarcoma expresses sufficient HER2 to induce activation and proliferation of genetically modified T cells expressing such a HER2-specific CAR that incorporated a CD28.ζ costimulatory/signaling domain. We evaluated the antitumor activity of these cells in vitro and in two xenogenic mouse models that mimic local and lung metastatic osteosarcoma.22 Our results show that genetic modification of T cells with a CAR can overcome the ineffectiveness of a monoclonal antibody (MAb) with the same specificity because HER2-specific T cells, but not HER2 MAb, killed HER2-positive osteosarcoma cells ex vivo and in both in vivo models.
Results
HER2 is expressed by osteosarcoma cells at low levels and HER2 monoclonal antibodies are ineffective
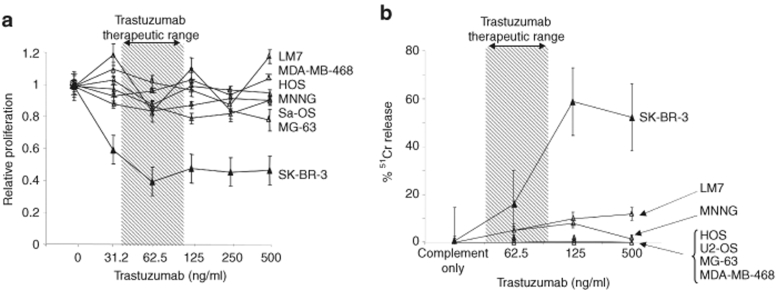
To confirm that HER2 is expressed by primary osteosarcoma cells, we analyzed HER2 expression in paraffin-embedded tissue sections as well as in osteosarcoma cell lines (Figure 1a,b). Seven of eight serial tumor samples were positive by immunohistochemistry. All 12 osteosarcoma cell lines studied expressed HER2 as determined by fluorescence-activated cell sorter (FACS) analysis. The level of HER2 expression was comparable to the low HER2 expressing breast cancer cell line MCF-7, whereas no cell line expressed HER2 at high levels equivalent to SK-BR-3. The therapeutic activity of HER2 MAb trastuzumab primarily relies on direct inhibition of the HER2 signaling cascade, reducing cell proliferation by consequent G1 phase arrest.23,24 In proliferation assays, however, we found that trastuzumab failed to inhibit the proliferation of HER2-positive osteosarcoma cell lines even at a concentration of 500 µg/ml, which is eightfold higher than the mean steady-state therapeutic serum concentration obtained in human subjects (Figure 2a). Because the antitumor effect of the antibodies in vivo may also have a component dependent on complement mediated lysis, we added complement and increasing concentrations of trastuzumab to 51Cr-labeled tumor target cells. Although the HER2 high expressing cell line SK-BR-3 was efficiently lysed, all five HER2-positive osteosarcoma cell lines tested were resistant to lysis even at concentrations of trastuzumab that are several-fold higher than the levels achievable in human subjects and even when the more potent rabbit complement was substituted for human (Figure 2b).
Figure 1.
Primary osteosarcomas and osteosarcoma cell lines express human epidermal growth factor receptor 2 (HER2) at low levels. (a) Using the HER2-specific mouse monoclonal antibody NCL-L-CB11 (Novocastra, Newcastle Upon Tyne, UK), seven of eight primary osteosarcoma samples representing different histological subtypes showed detectable HER2 expression. One of eight samples did not express HER2 (bottom right panel). (b) Fluorescence-activated cell sorter analysis: osteosarcoma cells lines (U2-OS, Sa-OS, HOS, SK-ES-1, MNNG, Hs 894, Hs 899, LM7, MG-63, SJSA-1, 143b, and 143.98.2) and breast cancer cell lines (HER2-negative: MDA-MB-468, HER2-low: MCF-7, HER2-high: SK-BR-3) were stained for HER2 expression (isotype control: open curves; HER2: solid curves. Osteosarcoma cell lines expressed HER2 at low levels comparable to MCF-7.
Figure 2.
Trastuzumab fails to inhibit the proliferation of human epidermal growth factor receptor 2 (HER2)-positive osteosarcoma cell lines. (a) Tumor cells were cultured for 4 days in the presence of increasing concentrations of trastuzumab prior to performing a proliferation assay. Trastuzumab efficiently inhibited the proliferation of the HER2-high expressing cell line SK-BR-3, where as no inhibition was observed for the HER2-low expressing cell lines Sa-OS, MG-63, HOS, MNNG, LM7 or the HER2-negative cell line MDA-MB-468. Results from two experiments; done in triplicates are shown. (b) To test for complement mediated cytotoxicity, 51Cr-labeled tumor cells incubated for 30–60 minutes with baby-rabbit complement in the presence of increasing concentrations of trastuzumab. While trastuzumab efficiently lysed the HER2-high expressing cell line SK-BR-3, no lysis was observed for the HER2-low expressing cell lines Sa-OS, U2-OS, MG-63, HOS, MNNG, LM7, or the HER2-negative cell line MDA-MB-468. Results from two experiments done in triplicates are shown.
HER2-specific T cells secrete immunostimulatory cytokines and proliferate after exposure to HER2-positive osteosarcoma cells
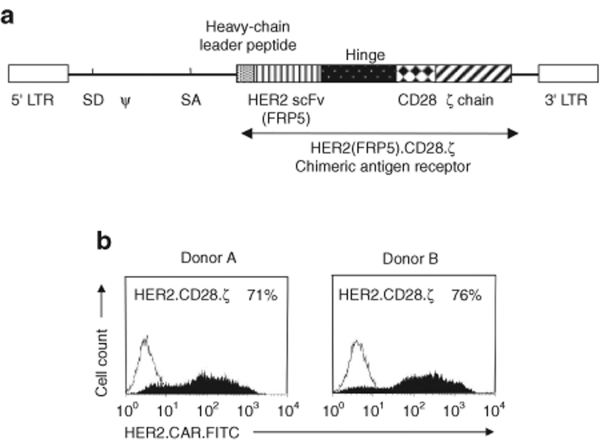
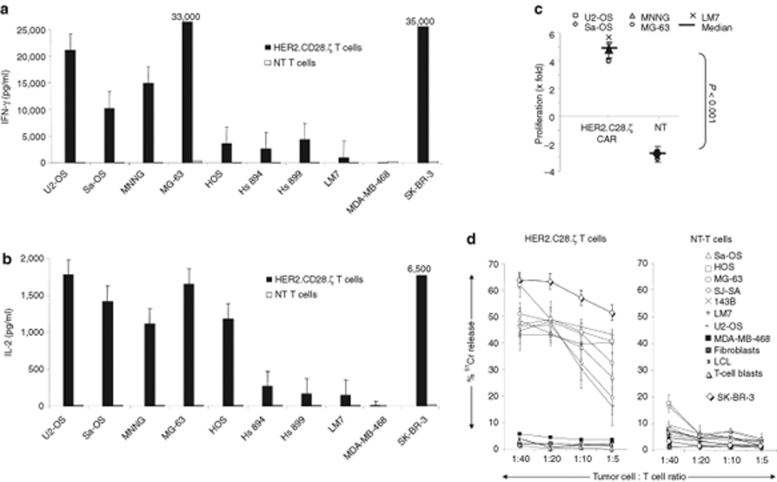
To learn whether osteosarcoma cell lines express sufficient levels of HER2 to be recognized by T cells, we generated HER2-specific T cells using a RD114-pseudotyped SFG retroviral vector encoding a CAR consisting of the HER2-specific single chain variable fragment FRP5, a CD28 transmembrane domain and a CD28.ζ signaling domain (Figure 3a).17 After transduction a median of 72% (range 45–92%) T cells expressed HER2.CD28.ζ CARs as judged by FACS analysis (Figure 3b). Nontransduced (NT) as well as HER2-specific T cells were incubated with a panel of osteosarcoma cell lines and after 24–48 hours of coculture, interferon (IFN)-γ and interleukin (IL)-2 production was determined by enzyme-linked immunosorbent assay. HER2-specific T cells only produced IFN-γ and IL-2 after exposure to HER2-positive targets, indicating that cytokine production was dependent on the presence of HER2 antigen (Figure 4a,b). HER2-specific T cells cocultured with the HER2 high expressing cell line SK-BR-3 secreted more IFN-γ and IL-2 than HER2-specific T cells cocultured with HER2 low expressing osteosarcoma cell lines, consistent with reports by others that antigen density on target cells determines T-cell activation.25 In addition, HER2-CAR triggering resulted in robust T-cell proliferation (P < 0.001; Figure 4c). These results show that osteosarcoma cells express sufficient levels of HER2 to induce cytokine secretion and T-cell proliferation.
Figure 3.
Characterization of human epidermal growth factor receptor 2 (HER2) CAR.CD28.ζ T cells. (a) Scheme of SFG retroviral vector encoding HER2.CD28.ζ CAR. (b) Depending on the donor, a median of 72% (range 45–92%) of T cells transduced with the HER2.CD28.ζ CAR retrovirus were positive for the transgene as judged by fluorescence-activated cell sorter analysis. CAR, chimeric antigen receptor.
Figure 4.
Human epidermal growth factor receptor 2 (HER2)-specific T cells secrete immunostimulatory cytokines, proliferate and kill HER2-expressing osteosarcoma cells in coculture. HER2-specific T cells were stimulated with HER2-positive (U2-OS, Sa-OS, SK-ES-1, MNNG, MG-63, HOS, Hs 894, Hs 899, and LM7) or HER2-negative (MDA-MB-468) cells. 24–48-hours poststimulation the (a) IFN-γ and (b) IL-2 concentration was determined. (c) T-cell proliferation was determined by counting viable cells (Trypan blue exclusion) 3 days poststimulation. Only HER2-specific T cells produced IFN-γ and IL-2 and proliferated after exposure to HER2-positive cells in comparison to nontransduced T cells. Results from three experiments from two donors done in duplicates are shown. (d) Only HER2-specific T cells killed HER2 positive osteosarcoma cells, in a 4 hour 51Cr-release cytotoxicity assay; nontransduced (NT) T cells did not. The HER2 negative cell line MDA-MB-468, and HER2-negative fibroblasts, lymphoblastoid B-cell lines (LCL) and T-cell blasts were not killed by HER2-specific or NT T cells. SK-BR-3 was used as a positive control. Results from three experiments done in triplicates are shown.
HER2-specific T cells kill HER2 expressing osteosarcoma cell lines in vitro
We next demonstrated that HER2-specific T cells killed HER2-positive osteosarcoma cell lines in a standard 51Cr-release assay, whereas HER2-negative control cells (MDA-MB-468) were not killed (Figure 4d). In addition, NT T cells did not kill any of the osteosarcoma cell lines, indicating that the killing of HER2-positive osteosarcoma cell lines depended on the expression of HER2-specific CARs.
Adoptive transfer of HER2-specific T cells results in regression of osteosarcoma in a SCID mouse model of locoregional disease
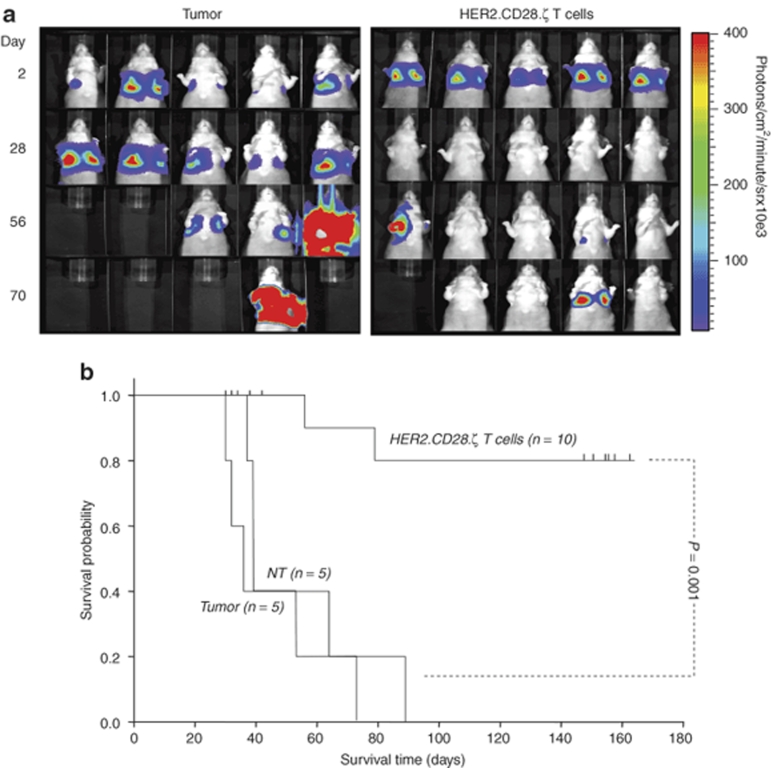
Having established that HER2 expressed on osteosarcoma is a target for HER2-specific T cells ex vivo, we determined the in vivo antitumor activity of HER2-specific T cells in a xenogeneic severe combined immunodeficient (SCID) mouse model. To allow serial imaging, osteosarcoma LM7 cells were transduced with a retroviral vector encoding an eGFP-firefly luciferase fusion gene (LM7.eGFP-FFLuc). Cells were sorted for eGFP expression, and FACS analysis confirmed that LM7.eGFP-FFLuc cells expressed HER2 at the same level as the parental cell line. Luciferase expression was confirmed ex vivo using luminometry (data not shown).
To investigate the antitumor activity of HER2-specific T cells in vivo, SCID mice were injected intraperitoneally with 2 × 106 LM7.eGFP.FFLuc cells (day 0) and treated for three consecutive days with daily intraperitoneal injections of 10 × 106 HER2.CD28.ζ T cells. We tested two groups of mice: those with small (day 2) tumors, and those with large (day 8). Bioluminescence imaging showed a 10.5-fold difference in photon emission between the day 2 and 8 malignancies (Figure 5b). Although tumors in untreated mice grew aggressively, injection of HER2-specific T cells induced tumor regression in 9/10 mice bearing small tumors and 7/9 mice bearing large tumors. To exclude nonspecific antitumor activity of T cells, a subset of tumor-bearing mice were injected with NT T cells on day 2 for three consecutive days. There was a brief, transient decrease in photon emission before the tumors started to grow at the same rate as in nontreated animals, indicating that the eradication of LM7.eGFP.FFLuc cells was dependent on HER2.CD28.ζ CAR expression.
Figure 5.
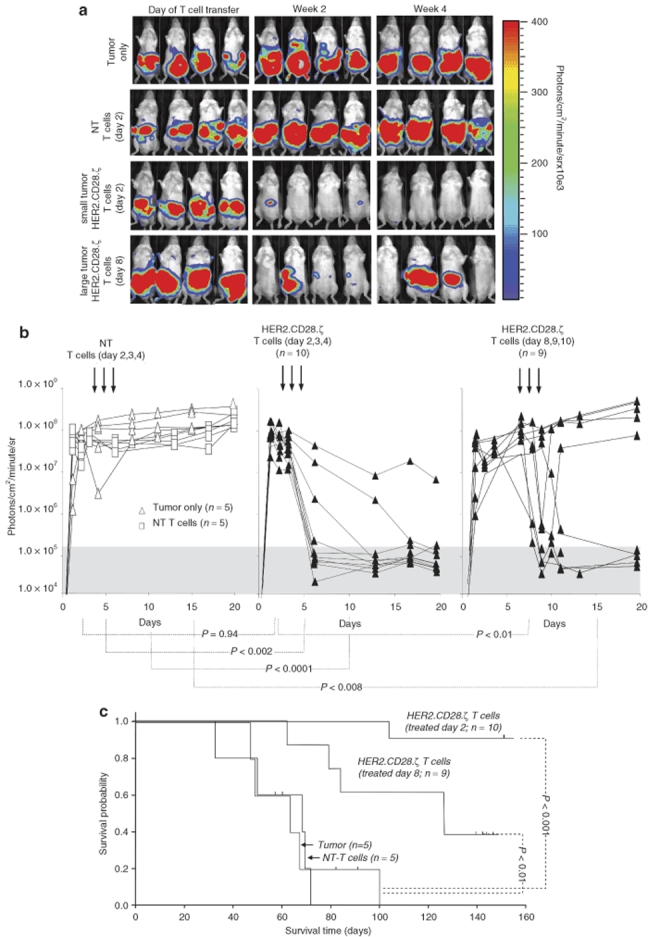
Adoptively transferred human epidermal growth factor receptor 2 (HER2)-specific T cells induce regression of intraperitoneal osteosarcoma xenograft_s_. 2 × 106 LM7.eGFP.FFLuc cells were injected intraperitoneally in 9- to 12-week-old nonobese diabetic–severe combined immunodeficient mice (day 0) followed by intraperitoneal injection of 10 × 106 HER2-specific T cells or nontransduced T cells (NT T cells) on days 2, 3 and 4 or days 8, 9 and 10 after tumor inoculation. (a) Although tumors regressed over a period of 24–72 hours in response to injection of HER2-specific T cells (two lower rows), tumors grew progressively in untreated mice as shown for four representative animals and in mice receiving nontransduced T cells (upper two rows). (b) Quantitative bioluminescence imaging: the pretreatment bioluminescence values on day 2 were comparable between animals treated with HER2-specific T cells and those treated with NT T cells (P = 0.94). As early as 24 hours after initiation of treatment, the median photon emission from animals receiving HER2-specific T cells was significantly lower than those receiving NT T cells and for untreated tumors (P < 0.002 and _P_ < 0.008 for animals treated on day 2 and those treated on day 8, respectively). Values are corrected to background bioluminescence. Two-tailed _P_ value, Mann–Whitney U test are reported. Solid arrows: time of T-cell injection. (**c**) Survival analysis was performed at day 160. Mice from the untreated and NT T-cell control groups had a median survival of 63 days (range 29–66 days) and 65 days (range 49–104 days), respectively. Injection of HER2-specific T cells into mice bearing small or large tumors resulted in a significant survival advantage; mice bearing small tumors had a median survival of greater 160 days (_P_ < 0.001) where as mice bearing large tumors had a median survival of 88 days (range 62 to >160 days; P < 0.01).
Quantification of bioluminescence signals showed no difference between groups before treatment (Figure 5b) but a statistically significant difference between the HER2-specific T-cell groups and the control groups (NT T cell and tumor only) from day 4 after T-cell injection. This difference was maintained thereafter (P < 0.002 for mice with small tumors and P < 0.008 for mice with large tumors). Hence, HER2-specific T cells have potent antitumor activity after local delivery in vivo.
All groups of mice were followed long-term with bioluminescence imaging (Figure 5c). Survival analysis at day 160 showed that mice from the untreated and NT T-cell control groups had a median survival of 63 days (range 29–66 days) and 65 days (range 49–104 days), respectively. Injection of HER2-specific T cells into mice bearing small or large tumors produced significant survival advantage; mice bearing small tumors had a median survival of >160 days (P < 0.001) whereas mice with large tumors had a median survival of 88 days (range 62 to >160 days; P < 0.01).
Regression of osteosarcoma lung metastases after adoptive transfer of HER2-specific T cells
Having shown that local injection of HER2-specific T cells results in eradication of tumor cells in vivo, we determined the antitumor activity of HER2-specific T cells in a metastatic model, because osteosarcoma primarily metastasizes to the lung in humans.26 We used the well-established LM7 metastatic model,22 and injected tumor cells into the tail vein. This route established progressive lung metastases in 100% of mice, as judged by bioluminescence imaging. In untreated mice, tumors progressively grew in all animals (n = 5; Figure 6a). In contrast, injections of HER2-specific T cells resulted in rapid regression of lung metastasis in all treated animals (n = 10). Eight of 10 animals had no evidence of tumor recurrence after >6 months of follow-up. One animal had recurrent lung metastasis and one animal was euthanized because of a metastatic deposit in the forelimb. Survival analysis revealed a significant survival advantage (P < 0.001) in animals treated with HER2-specific T cells (median 145 days; range 60–160 days) compared to untreated animals (median 65 days; range 36–89 days; Figure 6b).
Figure 6.
Adoptive transfer of human epidermal growth factor receptor 2 (HER2)-specific T cells induces regression of established osteosarcoma lung metastasis. 2 × 106 LM7.eGFP.FFLuc cells were injected systemically into the tail vein in 9- to 12-week-old Nu/Nu mice (day 0) followed by tail-vein injection of 10 × 106 HER2-specific T cells on days 2, 3, and 4 after tumor inoculation. (a) While tumors regressed in response to injection of HER2-specific T cells, tumors grew progressively in untreated mice. (b) Kaplan–Meier survival curve: mice treated with HER2-specific T cells had a significantly longer survival probability (P < 0.001) in comparison to untreated mice.
Discussion
The development of effective immunotherapies for osteosarcoma is hampered by the limited number or low expression of known target antigens and at present there are no published preclinical studies that have been able to show antitumor activity from antigen-specific vaccines or adoptively transferred T cells in xenograft models of human osteosarcoma. Here we show that the low expression of the well-known target antigen HER2 can be exploited by genetically modifying T cells by expressing CARs derived from a MAb with the same specificity. Genetically modified T cells recognized and killed HER2-positive osteosarcoma cells in vitro. In addition, adoptive transfer of HER2-specific T cells into xenogenic mouse models resulted in regression of local as well as metastatic disease.
Tumor-associated antigens expressed in osteosarcoma include HER2, MAGE and GAGE family members, NY-ESO-1, and papillomavirus binding factor.6,9,11,27 Among these HER2 is the only antigen that is expressed in unprocessed form on the cell surface, which enables the use of T cells that are genetically engineered to express HER2-specific CARs.4,7,18 Conceptually, CAR expressing T cells have several advantages over conventional antigen-specific T cells.28 In contrast to the lengthy process of generating conventional tumor antigen-specific T cells ex vivo whose frequencies are often <1 per 1,000 T cells, CAR expressing T cells can be prepared readily in large quantities by mitogen activation of all T cells.28,29 Moreover, CARs recognize antigens in a major histocompatibility complex unrestricted manner, rendering T cells immune to some of the major mechanisms by which tumors like osteosarcoma avoid major histocompatibility complex–restricted T-cell recognition.28,29
We showed in agreement with others that HER2 is expressed in primary tumors as well as osteosarcoma cell lines, however expression was low.18 Clinical studies have demonstrated that HER2 MAbs have no antitumor activity in patients with malignancies that express low levels of HER2 equivalent to those seen in human osteosarcoma.20 Our in vitro studies confirm this for osteosarcoma, because HER2 MAbs neither inhibited cell proliferation nor induced complement mediated cell lysis, even at concentrations almost 1 log higher than those used in humans. Nonetheless, HER2 expression was sufficient for T-cell recognition, and our HER2-specific T cells expressing a CAR with a CD28.ζ signaling domain were able to lyse osteosarcoma cells in vitro and in vivo. CARs with a CD28.ζ signaling domain are superior in activating T cells after antigen exposure than CARs containing a ζ signaling domain.29,30,31,32,33 CD28 signaling is critical for providing co-stimulation after initial T-cell activation and incorporation of the endodomain of CD28 into CARs results in enhanced T-cell proliferation and IL-2 secretion.29,30,31,32,33 HER2-specific T cells not only killed HER2-positive tumor cells, but also secreted IFN-γ and IL-2, and proliferated after coculture with HER2-positive tumor cells.
We tested the antitumor activity of HER2-specific T cells in two animal models. In our first model, we showed that HER2-specific T cells induced regression of locoregional osteosarcoma in 9/10 mice with small tumors and 7/9 mice with large tumors. The antitumor efficacy of HER2-specific T cells was lower in the large tumor group, likely reflecting a lower ratio of T cells to tumor cells at the time of therapy in comparison to mice with small tumors.34,35 The inclusion of additional signaling domains, such as 4-1BB (CD137) or OX40 (CD134), in antigen-specific CARs or genetically engineering T cells to secrete cytokines such as IL-2 or IL-15 may further enhance the antitumor activity of T cells.30,31,36,37
Li et al. recently demonstrated that murine sarcoma cells, genetically engineered to express human HER2, can be targeted with murine T cells expressing HER2-specific CARs.21 Our human study is consistent with these observations in mice, and shows activity against human osteosarcoma cells, in which the expression of HER2 had not been genetically manipulated. HER2-positive osteosarcoma cells obtained directly from tumors cannot be tested in these functional assays due to the limited availability of tissue and their growth characteristics in vitro. We were, however, able to characterize the expression of HER2 using immunohistochemistry of primary paraffin-embedded osteosarcoma sections and observed HER2-positivity in seven out of eight samples.
Although our locoregional model demonstrates the in vivo antitumor activity of injected HER2-specific T cells, its clinical relevance is limited because the majority of osteosarcomas occur in the extremities where aggressive surgical approaches are feasible and result in excellent local control (albeit with severe aesthetic and functional limitations). Nevertheless, achieving tumor free resection margins remains a challenge for bulky disease, and the local activity of T cells might allow limb sparing procedures for tumors which otherwise would have required amputations.
Our metastatic lung model is closer to the pattern of human disease, because lung metastases contribute significantly to the morbidity and mortality of osteosarcoma. In total, 20% of patients have bilateral lung metastases at diagnosis and >95% of patients have lung metastases on postmortem examination.26,38 In the well-established LM7 xenogenic lung metastases model,22 adoptive transfer of HER2-specific T cells resulted in regression of lung metastases and a significant survival advantage of tumor-bearing mice.
HER2 MAb may have off target effects, the most concerning of which is the poorly understood cardiac toxicity. If HER2-specific T cells are long-lived, this problem could be accentuated. Potential cardiac toxicities are difficult to model in mice because T cells that are specific for human HER2 do not recognize murine HER2. Nonetheless, we have shown that HER2-negative cells are not killed by HER2-specific T cells, and others have observed that two patients, who received HER2-specific T cells had no cardiac toxicity (see ref. 39 and S. Gottschalk, N.Ahmed, C.M. Rooney, G. Dotti, H.E. Heslop, A.P. Gee et al., unpublished results). In addition, HER2 vaccines are well tolerated, and no cardiac toxicities were observed in patients, who developed HER2-specific T-cell responses.40
In summary, genetic modification of T cells with CARs in this study has overcome the ineffectiveness of a MAb to a weakly expressed tumor-associated antigen and provides a rationale for developing a phase I clinical study for patients with recurrent HER2-positive osteosarcoma. Other tumor-associated target antigens that may be unresponsive to monoclonal antibodies may be amenable to a similar CAR T-cell approach.
Materials and Methods
Blood donors and tumor cell lines. Blood samples were obtained and studied on a protocol approved by the institutional review board of Baylor College of Medicine and informed consent was obtained from all donors. The osteosarcoma cell lines (U2-OS, Sa-OS, HOS, SK-ES-1, MNNG, Hs 894, Hs 899, MG-63, SJSA-1, 143b, and 143.98.2), the breast cancer cell lines (MDA-MB-468, MCF-7, and SK-BR-3), and 293T cells were purchased from the American Type Culture Collection (Manassas, VA). The panel of human osteosarcoma cell lines used were derived from tumors covering the clinical range of histologic appearance (U2-OS and Sa-OS, both moderately differentiated osteoblastic; HOS, fibroblastic; SK-ES-1, anaplastic; MNNG and MG-63, both fibroblastic, Hs 894 and Hs 899, both fibroblastic from lung metastatic deposits and SJSA-1, mixed histology osteosarcoma). The generation of LM7 cells, an Sa-OS derivative that has high metastatic potential to the lungs, was described previously.22 All cell lines were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) with 10% fetal calf serum (HyClone, Logan, UT), supplemented with 2 mmol/l GlutaMAX-I, 1.5 g/l sodium bicarbonate, 0.1 mmol/l nonessential amino acids, and 1.0 mmol/l sodium pyruvate (all media supplements from Invitrogen). T cells were maintained in RPMI 1,640 with 10% fetal calf serum containing 2 mmol/l GlutaMAX-I.
Generation of retroviral constructs. The HER2-specific CAR with a CD28.ζ signaling domain was constructed by subcloning the HER2-specific single chain variable fragment FRP5 into a SFG.CD28.ζ retroviral vector containing a CD28 transmembrane and a CD28.ζ signaling domain.41 A retroviral vector encoding the fusion protein eGFP-firefly luciferase (eGFP.FFLuc) was used to generate firefly luciferase–expressing LM7 cells (LM7.eGFP.FFLuc) for the in vivo studies.17,42
Retrovirus production and transduction of T cells. To produce retroviral supernatant, 293T cells were co-transfected with HER2-retroviral vector containing plasmid, Peg-Pam-e plasmid encoding the sequence for MoMLV gag-pol, and plasmid pMEVSVg containing the sequence for vesicular stomatitis virus G, using GeneJuice transfection reagent (EMD Biosciences, San Diego, CA).29,43 Supernatants containing the retrovirus were collected 48 and 72 hours later. Vesicular stomatitis virus–pseudotyped viral particles were used to transduce the FLYRD18 producer cell line for the production of RD114-pseudotyped viral particles.44
OKT3/CD28 activated T cells were transduced with retroviral vectors as described.42,43 Briefly, peripheral blood mononuclear cells were isolated by Lymphoprep (Greiner Bio-One, Monroe, NC) gradient centrifugation; 5 × 105 peripheral blood mononuclear cells per well in a 24-well plate were activated with OKT3 (OrthoBiotech, Raritan, NJ) and CD28 monoclonal antibodies (BD Biosciences, Palo Alto, CA) at a final concentration of 1 µg/ml. On day 2, recombinant human IL-2 (Chiron, Emmeryville, CA) was added at a final concentration of 100 U/ml, and on day 2 cells were harvested for retroviral transduction. For transduction, we precoated a nontissue culture treated 24-well plate with a recombinant fibronectin fragment (FN CH-296; Retronectin; Takara Bio USA, Madison, WI). Wells were washed with phosphate-buffered saline (PBS; Sigma, St Louis, MO) and incubated twice for 30 minutes with retrovirus. Subsequently, 3 × 105 T cells per well were transduced with retrovirus in the presence of 100 U IL-2/ml. After 48–72 hours cells were removed and expanded in the presence of 50–100 U IL-2/ml for 10–15 days prior to use.
Flow cytometry. For all flow-cytometric analyses, a FACScalibur instrument (Becton Dickinson, Mountain View, CA) and CellQuest software (Becton Dickinson) were used. We analyzed data on >10,000 events; in all cases negative controls included isotype antibodies. Cells were washed once with PBS containing 2% FBS and 0.1% sodium azide (Sigma; FACS buffer) prior to addition of antibodies. After 15–30 minutes of incubation at 4 °C in the dark, the cells were washed once and fixed in 0.5% paraformaldehyde/FACS buffer prior to analysis.
Tumor cell lines were analyzed with mouse anti-HER2 IgG (clone TAB250; Zymed, Carlsbad, CA) followed by a goat anti-mouse-Fc PE secondary antibody (Chemicon, Temecula, CA). T cells were analyzed with anti-CD8 FITC, -CD4 PE, and -CD3 PerCP (BD Biosciences). To determine cell-surface expression of the HER2 CAR transgene, a recombinant HER2-Fc fusion protein (R&D Systems, Minneapolis, MN) was used. Bound HER2-Fc was detected with a goat anti-Fc FITC secondary antibody (Chemicon, Temecula, CA).17
Proliferation assay. Tumor cells were cultured at 5,000 cells/well in a 96-well plate in the presence of increasing concentrations of trastuzumab. After 4 days the cells were pulsed with 1 µCi (0.037 MBq) methyl-3[H]-thymidine and cultured for an additional 18 hours. The cells were then harvested onto filters and dried; counts per minute were measured in a scintillation counter (TriCarb 2500 TR; Packard BioScience, Boston, MA). The experiments were performed in triplicate. Cells cultured without trastuzumab served as control.
Complement assay. 1 × 106 target cells were labeled with 0.1 mCi (3.7 MBq) 51Cr and different concentrations of trastuzumab. Target cells incubated in complete medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. After 30 minutes, supernatants were collected and radioactivity was measured in a gamma counter (Cobra Quantum; PerkinElmer, Wellesley, MA). The mean percentage of specific lysis of triplicate wells was calculated according to the following formula: (test release − spontaneous release)/(maximal release − spontaneous release) × 100.
Cytotoxicity assays. Cytotoxicity assays were performed as previously described.45 Briefly, target cells were labeled with 51Cr as described for the complement assay and mixed with decreasing numbers of effector cells to give effector to target ratios of 40:1, 20:1, 10:1, and 5:1. Target cells incubated in complete medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. After 4 hours, supernatants were collected and assessed as described for the complement assay.
Analysis of cytokine production and T-cell expansion. Effector T cells (HER2.CD28.ζ CAR expressing T cells and NT T cells) from healthy donors were cocultured with HER2-positive and HER2-negative cell lines at a 1:1 effector to target ratio in a 24-well plate. After 24–48-hours incubation, culture supernatants were harvested and the presence of IFN-γ and IL-2 was determined by enzyme-linked immunosorbent assay as per the manufacturer's instructions (R&D Systems). T-cell expansion was determined by counting viable cells (Trypan blue exclusion) 7 days after stimulation.
Xenogeneic mouse models. All animal experiments followed a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Animals were regularly examined for any signs of stress and euthanized according to preset criteria.
Intraperitoneal model: Recipient nonobese diabetic–SCID mice were purchased from Taconic (C.B-Igh-1 b/IcrTac-Prkdc scid; FOX CHASE CB-17 SCID ICR; Taconic, Hudson, NY). Male 9–12-week-old mice were anesthetized with rapid sequence inhalation isofluorane (Abbott Laboratories, Maidenhead, England) followed by an intraperitoneal injection of 2 × 106 LM7.eGFP.FFLuc cells (in 200 µl PBS) on day 0. Starting on day 2 or 8 animals were treated with three daily intraperitoneal injections of 10 × 106 effector cells (in 200 µl PBS).
Metastatic model: For the generation of a metastatic model of osteosarcoma, recipient Nu/Nu mice were purchased from Charles Rivers Laboratories (NU/NU-Foxn1nu; Charles River Laboratories, Wilmington, MA). Male 9–12-week-old mice were anesthetized with rapid sequence inhalation isofluorane (Abbott Laboratories) followed by tail-vein injection of 2 × 106 LM7.eGFP.FFLuc cells (in 200 µl PBS) on day 0. After evidence of tumor establishment in the lungs on day 2, animals were treated with three daily tail-vein injections of 10 × 106 effector cells (in 200 µl PBS).
Bioluminescence imaging. Isofluorane anesthetized animals were imaged using the IVIS system (Xenogen, Alameda, CA) 10 minutes after 150 mg/kg D-luciferin (Xenogen) was injected intraperitoneally.42 The photons emitted from luciferase-expressing cells within the animal body and transmitted through the tissue were quantified using “Living Image,” a software program provided by the same manufacturer. A pseudocolor image representing light intensity (blue least intense and red most intense) was generated and superimposed over the grayscale reference image. Animals were imaged after injections every other day, then twice weekly for 2 weeks then weekly thereafter. Bioluminescence imaging findings were confirmed by macroscopic examination as well as routine histology on euthanized animals. They were regularly examined for any signs of stress and euthanized according to preset criteria, in accordance the Baylor College of Medicine's Center for Comparative Medicine guidelines.
Immunohistochemistry. Serial primary osteosarcoma samples were obtained from Texas Children's Hospital Pathology Department's archives and stained for phospho-HER2 immunohistochemistry using the same protocol as previously described.46 To confirm in vivo imaging data, mice were euthanized by CO2 inhalation, dissected then fixed by immersion in 10% paraformaldehyde. Histology was performed on 10-µm serial horizontal sections. Tissue sections were stained by a standard hematoxylin and eosin technique. HER2 expression in xenografts was detected by phospho-HER2 immunohistochemistry as previously described.46
Statistical analysis. For the bioluminescence experiments, intensity signals were log-transformed and summarized using mean ± SD at baseline and multiple subsequent time points for each group of mice. Changes in intensity of signal from baseline at each time point were calculated and compared using paired _t_-tests or Wilcoxon signed-ranks test.
Acknowledgments
We thank Malcolm K. Brenner and Alberto Pappo for helpful discussion and advice, and Awateef Akrabi for assistance with FACS analysis. The authors were supported by grants from the Alex's Lemonade Stand Foundation and the Susan G. Komen for the Cure Foundation. N.A. is the recipient of the Justin Porter American Brain Tumor Association Fellowship and the American Brain Tumor Translational Grant, E.E.K. is the recipient of the NIH grant CA 42992, H.E.H. is the recipient of a Doris Duke Distinguished Clinical Scientist Award, and S.G. is the recipient of a Doris Duke Clinical Scientist Development Award.
REFERENCES
- Gurney JG, Swensen AR., and , Bulterys M. Malignant Bone Tumors. SEER Pediatric Monograph 2005100–110.Ref Type: Data File
- Link M, Gebhardt MC., and , Meyers PA.Osteosarcoma Principles and Practice of Pediatric Oncology 2006Lippincott Williams and Wilkins; 1074–1115.In: Philip, AP and David, GP (eds)5th edn [Google Scholar]
- Bacci G, Briccoli A, Longhi A, Ferrari S, Mercuri M, Faggioli F, et al. Treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol. 2005;44:748–755. doi: 10.1080/02841860500327503. [DOI] [PubMed] [Google Scholar]
- Flint AF, U'Ren L, Legare ME, Withrow SJ, Dernell W., and , Hanneman WH. Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet Pathol. 2004;41:291–296. doi: 10.1354/vp.41-3-291. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ. ERBB2 in pediatric cancer: innocent until proven guilty. Oncologist. 2005;10:508–517. doi: 10.1634/theoncologist.10-7-508. [DOI] [PubMed] [Google Scholar]
- Gorlick R, Huvos AG, Heller G, Aledo A, Beardsley GP, Healey JH, et al. Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol. 1999;17:2781–2788. doi: 10.1200/JCO.1999.17.9.2781. [DOI] [PubMed] [Google Scholar]
- Hughes DP, Thomas DG, Giordano TJ, Baker LH., and , McDonagh KT. Cell surface expression of epidermal growth factor receptor and Her-2 with nuclear expression of Her-4 in primary osteosarcoma. Cancer Res. 2004;64:2047–2053. doi: 10.1158/0008-5472.can-03-3096. [DOI] [PubMed] [Google Scholar]
- Hughes DP, Thomas DG, Giordano TJ, McDonagh KT., and , Baker LH. Essential erbB family phosphorylation in osteosarcoma as a target for CI-1033 inhibition. Pediatr Blood Cancer. 2006;46:614–623. doi: 10.1002/pbc.20454. [DOI] [PubMed] [Google Scholar]
- Morris CD, Gorlick R, Huvos G, Heller G, Meyers PA., and , Healey JH. Human epidermal growth factor receptor 2 as a prognostic indicator in osteogenic sarcoma. Clin Orthop Relat Res. 2001. pp. 59–65. [DOI] [PubMed]
- Fellenberg J, Krauthoff A, Pollandt K, Delling G., and , Parsch D. Evaluation of the predictive value of Her-2/neu gene expression on osteosarcoma therapy in laser-microdissected paraffin-embedded tissue. Lab Invest. 2004;84:113–121. doi: 10.1038/labinvest.3700006. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Bertoni F, Zanella L, Setola E, Bacchini P, Alberghini M, et al. Evaluation of P-glycoprotein, HER-2/ErbB-2, p53, and Bcl-2 in primary tumor and metachronous lung metastases in patients with high-grade osteosarcoma. Cancer. 2004;100:1936–1942. doi: 10.1002/cncr.20151. [DOI] [PubMed] [Google Scholar]
- Zhou H, Randall RL, Brothman AR, Maxwell T, Coffin CM., and , Goldsby RE. Her-2/neu expression in osteosarcoma increases risk of lung metastasis and can be associated with gene amplification. J Pediatr Hematol Oncol. 2003;25:27–32. doi: 10.1097/00043426-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Tavassoli M, Quirke P, Farzaneh F, Lock NJ, Mayne LV., and , Kirkham N. c-erbB-2/c-erbA co-amplification indicative of lymph node metastasis, and c-myc amplification of high tumour grade, in human breast carcinoma. Br J Cancer. 1989;60:505–510. doi: 10.1038/bjc.1989.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- Scotlandi K, Manara MC, Hattinger CM, Benini S, Perdichizzi S, Pasello M, et al. Prognostic and therapeutic relevance of HER2 expression in osteosarcoma and Ewing's sarcoma. Eur J Cancer. 2005;41:1349–1361. doi: 10.1016/j.ejca.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Kawaguchi S, Torigoe T, Kimura S, Murase M, Ichimiya S, et al. Prognostic impact and immunogenicity of a novel osteosarcoma antigen, papillomavirus binding factor, in patients with osteosarcoma. Cancer Sci. 2008;99:368–375. doi: 10.1111/j.1349-7006.2008.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer R, Shamon LA, Tsai MS., and , Lupu R. Herceptin: from the bench to the clinic. Cancer Invest. 2001;19:49–56. doi: 10.1081/cnv-100000074. [DOI] [PubMed] [Google Scholar]
- Li S, Yang J, Urban FA, MacGregor JN, Hughes DP, Chang AE, et al. Genetically engineered T cells expressing a HER2-specific chimeric receptor mediate antigen-specific tumor regression. Cancer Gene Ther. 2008;15:382–392. doi: 10.1038/cgt.2008.5. [DOI] [PubMed] [Google Scholar]
- Jia SF, Worth LL., and , Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17:501–506. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- Kute T, Lack CM, Willingham M, Bishwokama B, Williams H, Barrett K, et al. Development of Herceptin resistance in breast cancer cells. Cytometry A. 2004;57:86–93. doi: 10.1002/cyto.a.10095. [DOI] [PubMed] [Google Scholar]
- Weijtens ME, Hart EH., and , Bolhuis RL. Functional balance between T cell chimeric receptor density and tumor associated antigen density: CTL mediated cytolysis and lymphokine production. Gene Ther. 2000;7:35–42. doi: 10.1038/sj.gt.3301051. [DOI] [PubMed] [Google Scholar]
- Uribe-Botero G, Russell WO, Sutow WW., and , Martin RG. Primary osteosarcoma of bone. Clinicopathologic investigation of 243 cases, with necropsy studies in 54. Am J Clin Pathol. 1977;67:427–435. doi: 10.1093/ajcp/67.5.427. [DOI] [PubMed] [Google Scholar]
- Jacobs JF, Brasseur F, Hulsbergen-van de Kaa CA, van de Rakt MW, Figdor CG, Adema GJ, et al. Cancer-germline gene expression in pediatric solid tumors using quantitative real-time PCR. Int J Cancer. 2007;120:67–74. doi: 10.1002/ijc.22118. [DOI] [PubMed] [Google Scholar]
- Pule M, Finney H., and , Lawson A. Artificial T-cell receptors. Cytotherapy. 2003;5:211–226. doi: 10.1080/14653240310001488. [DOI] [PubMed] [Google Scholar]
- Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM., and , Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Finney HM, Akbar AN., and , Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Bendavid A, Waks T, Schindler D., and , Eshhar Z. Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation. Blood. 2005;105:3087–3093. doi: 10.1182/blood-2004-09-3737. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, et al. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. J Immunol. 2002;169:5780–5786. doi: 10.4049/jimmunol.169.10.5780. [DOI] [PubMed] [Google Scholar]
- Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- Duan X, Jia SF, Zhou Z, Langley RR, Bolontrade MF., and , Kleinerman ES. Association of alphavbeta3 integrin expression with the metastatic potential and migratory and chemotactic ability of human osteosarcoma cells. Clin Exp Metastasis. 2004;21:747–753. doi: 10.1007/s10585-005-0599-6. [DOI] [PubMed] [Google Scholar]
- Graat HC, Witlox MA, Schagen FH, Kaspers GJ, Helder MN, Bras J, et al. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br J Cancer. 2006;94:1837–1844. doi: 10.1038/sj.bjc.6603189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC., and , Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004. pp. 286–291. [DOI] [PubMed]
- Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nährig J, et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorf EA, Holmes JP, Ponniah S., and , Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57:1511–1521. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule M, Straathof K, Dotti G, Heslop HE, Rooney CM., and , Brenner MK. Three-module signaling endo-domain artificial T-cell receptor which transmits CD28, OX40 and CD3-zeta signals enhances IL-2 release and proliferative response in transduced primary T-cells. Blood. 2004;104:485a. [Google Scholar]
- Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosset FL, Takeuchi Y, Battini JL, Weiss RA., and , Collins MK. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101:1905–1912. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ, Pearson AD, Perry RH, Jaros E., and , Kelly PJ. Prognostic significance of the c-erbB-2 oncogene product in childhood medulloblastoma. Br J Cancer. 1995;71:473–477. doi: 10.1038/bjc.1995.96. [DOI] [PMC free article] [PubMed] [Google Scholar]