Von Hippel-Lindau Gene Product Modulates TIS11B Expression in Renal Cell Carcinoma: IMPACT ON VASCULAR ENDOTHELIAL GROWTH FACTOR EXPRESSION IN HYPOXIA (original) (raw)
Abstract
TIS11B belongs to a group of RNA-binding proteins (including TIS11/tristetraprolin and TIS11D) that share characteristic tandem CCCH-type zinc-finger domains and can be rapidly induced by multiple stimuli. TIS11B has been shown to regulate vascular endothelial growth factor (VEGF) mRNA stability in adrenocorticotropic hormone-stimulated primary adrenocortical cells. TIS11B has also been documented as a negative regulator of VEGF during development, but nothing has yet been reported in the context of human cancers. The Von Hippel-Lindau (VHL) tumor suppressor protein regulates VEGF gene expression at both the transcriptional and post-transcriptional levels in normoxia. However, whether it can do so in hypoxia is still unclear. Here, we report a unique regulatory function of VHL in VEGF expression in hypoxia that is mediated through modulation of TIS11B protein levels in renal cancer cells. In normoxia, we detected increased expression of the microRNA hsa-miR-29b in the VHL-overexpressing renal cancer cell line 786-O. We also show that this increased expression of hsa-miR-29b decreased TIS11B protein expression by post-transcriptional regulation in normoxia. In contrast, in hypoxia, increased TIS11B expression paralleled an increased TIS11B mRNA stability in VHL-overexpressing 786-O cells. This VHL-mediated TIS11B up-regulation in hypoxia may be important for TIS11B-regulated gene expression: we observed a down-regulation of VEGF mRNA in hypoxia in VHL-overexpressing cells compared with parental 786-O cells, and this effect was reversible by silencing TIS11B expression.
Introduction
Angiogenesis, the formation of new blood vessels from pre-existing vessels, is an essential process for establishing a closed circulatory system and for supplying oxygen and nutrients to tissues. Under normal circumstances, angiogenesis is a highly ordered and tightly regulated process of positive and negative regulatory pathways (1–3). Vascular endothelial growth factor (VEGF)2 is a critical signaling protein that plays a key role in developmental, physiological, and tumor angiogenesis (4–6). Of importance, up-regulation of VEGF expression by hypoxia appears to be a crucial step in the neovascularization of solid cancers (4, 6, 7). Tumor hypoxia appears to be strongly associated with tumor propagation, malignant progression, and resistance to therapy (8).
Germ line mutations of Von Hippel-Lindau (VHL), a tumor suppressor gene first described in 1993 (9), result in VHL-associated diseases such as renal cell carcinoma (RCC), hemangioblastoma, pheochromocytoma, and pancreatic cancer (10). VHL-associated tumors are highly vascularized (11) and support the existing model of VHL as a negative regulator of VEGF production (12–14). In RCC, VEGF overexpression has been shown to be regulated at both the transcriptional and post-transcriptional levels (15). In this regard, VHL has been reported to regulate VEGF transcription and mRNA stability in RCC, and these regulations are hypoxia-dependent (13, 14, 16, 17).
The immediate-early protein tristetraprolin family consists of three known members in mammals (ZFP36 or tristetraprolin, ZFP36L1 or TIS11B (tetradecanoylphorbol acetate-inducible sequence 11B), and ZFP36L2 or TIS11D) and a fourth member found only in the mouse and rat (ZFP36L3). They share characteristic tandem CCCH-type zinc-finger domains, and although they are rapidly induced by multiple stimuli, their basal mRNA level varies (18–20). During these early responses, the localization, stability, and translation of specific mRNAs are affected (21, 22), indicating the involvement of post-transcriptional regulatory mechanisms. However, their tissue-specific expression and regulation contribute to their specificity of action (23). The ACTH-regulated zinc-finger protein TIS11B was reported to interact with the 3′-untranslated region (3′-UTR) of VEGF mRNA and to decrease its stability in primary adrenocortical cells (23). The antagonistic function of TIS11B and HuR on VEGF mRNA stability in ACTH-stimulated adrenocortical cells has also been documented (24). Chorioallantoic fusion defects and embryonic lethality result from the disruption of TIS11B in mice (25). The regulatory role of TIS11B has also expanded to include the control of normal vascularization, where TIS11B has been shown to regulate VEGF expression (26). However, its role in tumor angiogenesis is largely unknown.
In this study, we investigated the role of VHL in the regulation of VEGF through TIS11B in RCC. We observed that VHL overexpression regulated TIS11B in the renal cancer cells (786-O). We also found that the microRNA (miRNA) hsa-miR-29b was overexpressed in 786-O cells expressing exogenous VHL, which could then target the TIS11B transcript to repress its expression under normoxia. However, under hypoxic stress, TIS11B mRNA became stabilized in the VHL-expressing 786-O cells and targeted the VEGF transcript for degradation.
EXPERIMENTAL PROCEDURES
Cell Culture
Human renal carcinoma cells (786-O) were purchased from American Type Culture Collection (CRL-1932) and maintained in Dulbecco's modified Eagle's medium (Invitrogen) with 10% fetal bovine serum (HyClone). 786-O cells were stably transfected with the pRC empty vector or pRC-HA-VHL expression vector to generate 786-O-neo and 786- O-VHL stable transfectants, respectively. HK-2 (CRL-2190, American Type Culture Collection), an immortalized proximal tubule epithelial cell line from normal adult human kidney, was cultured in keratinocyte serum-free medium (Invitrogen) kit catalog no. 17005-042. Cells were cultured under normoxic conditions (5% CO2, 21% O2) or hypoxic conditions (5% CO2, 3% O2).
Overexpression of VHL in 786-O Cells
For retrovirus preparation, 293T cells were seeded at a density of 6 × 106/100-mm plate 24 h before transfection. The cells were transfected with 2 μg of pBABe-puro-VHL DNA using EffecteneTM transfection reagent (Qiagen) according to the manufacturer's protocol. The medium was changed after 16 h. The retrovirus was isolated 48 h after transfection and used immediately for infection or frozen at −70 °C. The 786-O cells were seeded 24 h prior to infection. One milliliter of retrovirus solution (≅2 × 107 plaque-forming units/ml) and 5 ml of fresh medium were added to the 786-O cells with 10 μg/ml Polybrene. The medium was changed after 16 h, and 786-O cells expressing VHL (786-O+VHL) were selected with puromycin (1 μg/ml). After 24 h, 786-O cells were incubated under hypoxic conditions (5% CO2, 3% O2, and balance N2) for another 24 h.
RNA Interference
Small interfering RNA (siRNA) against TIS11B was purchased from Dharmacon (Lafayette, CO). Control siRNA from Qiagen was used. Cells were seeded at 70% confluence in Dulbecco's modified Eagle's medium 24 h prior to transfection. Before the transfection, fresh antibiotic-free Dulbecco's modified Eagle's medium was added to cells. DharmaFECT 1 (Dharmacon) was used for the transfection with 100 nm each siRNA according to the manufacturer's instructions. Cells were analyzed for loss of TIS11B mRNA expression 72 h after transfection using real-time PCR.
Western Blot Analysis
Western blot analysis was performed to detect the levels of TIS11B and VHL in RCC or HK-2 cell lysates under normoxia or hypoxia. After the treatments, cells were washed with phosphate-buffered saline and lysed with radioimmune precipitation assay buffer supplemented with a protease inhibitor mixture. Supernatant was collected by centrifugation at 13,000 rpm for 10 min. Thereafter, samples were subjected to SDS-PAGE, transferred to polyvinyl difluoride membranes, and immunoblotted. Antibody-reactive bands were detected by enzyme-linked chemiluminescence (Amersham Biosciences) and quantified by NIH Image densitometry. These experiments were repeated at least three times.
RNA Preparation
Total RNA was prepared from HK-2 and 786-O-neo cells and 786-O cells expressing VHL using the RNeasy mini kit (Qiagen). For miRNA analysis, RNA was prepared using TRIzol reagent (Invitrogen).
Quantitative Real-time PCR
cDNAs were synthesized from 1 μg of total RNA using the iScriptTM cDNA synthesis kit (Bio-Rad), and real-time PCR was performed using SYBR® Green PCR core Master Mix (Applied Biosystems) according to the manufacturer's instructions. PCRs were performed in an Applied Biosystems 7500 real-time PCR system. β-Actin (ACTB) amplification was used for cDNA normalization. TIS11B, VEGF-A, and ACTB primers were purchased from SABiosciences (Frederick, MD). Comparative real-time PCR was performed in triplicate. Relative expression was calculated using the comparative Ct method (27).
miRNA Quantification
Mature miRNA quantification was performed using the TaqMan microRNA assays (Applied Biosystems) for hsa-miR-29b according to the manufacturer's recommended protocols. Each sample was analyzed in triplicate. U6 small nuclear RNA was used for normalization. Relative expression was calculated using the comparative Ct method (27).
Target Gene Prediction of Differentially Expressed miRNAs
Analysis of miRNA predicted targets was performed using algorithms of TargetScan (Version 4.2) and miRBase (28).
Transfection of Cells
786-O-neo cells were transfected with 1 nm miRNA mimic negative control or hsa-miR-29b mimic (Dharmacon) using DharmaFECT 1. Transient transfection of 786-O-VHL or HK-2 cells with 200 nm miRNA inhibitor negative control or anti-miR-29b inhibitor oligonucleotides (Dharmacon) was also performed using DharmaFECT 1, and cells were incubated in either normoxia or hypoxia. After 72 h of transfection, cells were analyzed for TIS11B levels by Western blotting as described above using a TIS11B-specific antibody (Cell Signaling, Danvers, MA).
Luciferase Reporter Gene Assay
A luciferase reporter plasmid containing the full-length 3′-UTR of the TIS11B gene was obtained from SwitchGear Genomics (Menlo Park, CA). 293T and 786-O-neo cells were cotransfected with 5 nm scrambled miRNA or hsa-miR-29b mimic and the SwitchGear reporter plasmid using DharmaFECT Duo (Dharmacon). At 36 h post-transfection, luciferase activity was measured using Steady-Glo luciferase assay reagent (Promega, Madison, WI) in two independent experiments, each performed in triplicate.
TIS11B mRNA Stability Experiments in Normoxia and Hypoxia
The half-life of TIS11B mRNA was determined by treating 786-O and pBABe-puro-VHL-transfected 786-O cells using actinomycin D (Sigma). 786-O and 786-O+VHL cells were cultured under either normoxia or hypoxia for 24 h, and then actinomycin D (5 μg/ml) was added to the growth medium to block transcription. Immediately after the addition of actinomycin D, the cells were returned to the same culture conditions (normoxia or hypoxia). During the following 5 h, total RNA was prepared at different time points, and real-time PCR was performed as described above.
Statistical Analysis
Statistical analysis was performed with statistical SPSS software (Version 11.5). The independent samples t test was used to test the probability of significant differences between groups. Statistical significance was defined as p < 0.05; statistical high significance was defined as p < 0.01. Error bars were given on the basis of the calculated S.D.
RESULTS
VHL Controls VEGF-A Expression in Hypoxia through TIS11B
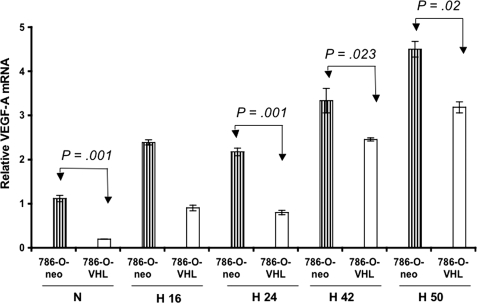
Currently, it is well documented that VHL controls VEGF expression in normoxia, but those VHL-mediated regulations are lost in hypoxia (16, 17, 29, 30). However, when we compared VEGF-A mRNA levels in 786-O-neo and 786-O-VHL cells, we observed that the VEGF-A mRNA level was significantly lower in 786-O-VHL cells than in 786-O-neo cells (p = 0.001) within 24 h of hypoxic stress (Fig. 1). This remained unaltered for up to 50 h (p = 0.02) under the same experimental conditions (Fig. 1). Thus, these findings suggest that VHL may still be functional and able to control VEGF expression under hypoxic conditions.
FIGURE 1.
VEGF-A mRNA levels in 786-O-neo and 786-O-VHL cells. RNA was collected from 786-O-neo and 786-O-VHL cells incubated under normoxic and hypoxic conditions (16–50 h). Real-time PCR for VEGF-A was done with the cDNAs from the respective samples, and β-actin was used for cDNA normalization. Subjecting both cell lines to hypoxic growth conditions resulted in significant VEGF mRNA accumulation. However, the level of VEGF-A mRNA was still considerably higher (p = 0.02) in 786-O-neo cells than in 786-O-VHL cells even after 50 h of hypoxia. Data represent the average of three independent determinations. N, normoxia; H 16, hypoxia, 16 h; H 24, hypoxia, 24 h; H 42, hypoxia, 42 h; H 50, hypoxia, 50 h.
TIS11B protein plays an important role in regulating VEGF expression at the post-transcriptional level (23, 26). Thus, we examined the expression levels of TIS11B in 786-O cells. We found a higher level of TIS11B in 786-O-neo cells (p = 0.003) than in VHL-expressing 786-O cells by Western blot analysis (Fig. 2A). However, we found an increased expression of TIS11B after 16 or 24 h (p = 0.004) of hypoxic stress in 786-O-VHL cells compared with 786-O-neo cells under similar experimental conditions (Fig. 2A). These findings suggest a possible role of VHL in regulating TIS11B expression. Normal proximal kidney epithelial cells (HK-2) also expressed an increased level TIS11B after 24 h of hypoxia compared with normoxia (Fig. 2B).
FIGURE 2.
TIS11B protein levels under normoxic and hypoxic growth conditions. A, Western blot analysis was performed with lysates from 786-O-neo and 786-O-VHL cells under normoxia or hypoxia. A significantly high level of TIS11B protein expression was detected in 786-O-neo cells (p = 0.003) compared with 786-O-VHL cells in normoxia. However, increased TIS11B expression in 786-O-VHL cells compared with 786-O-neo cells was observed after 16 and 24 h (p = 0.004) of hypoxia. α-Tubulin was used as the loading control. NIH Image quantitation data normalized with respect to α-tubulin are shown. The amount of VHL expression in 786-O-VHL cells in normoxia is also indicated. B, an up-regulation of TIS11B protein levels was also found in kidney proximal tubular epithelial cells (HK-2) after 24 h of hypoxia. Data are representative of three separate experiments, which gave similar results. N, normoxia; H 16, hypoxia, 16 h; H 24, hypoxia, 24 h.
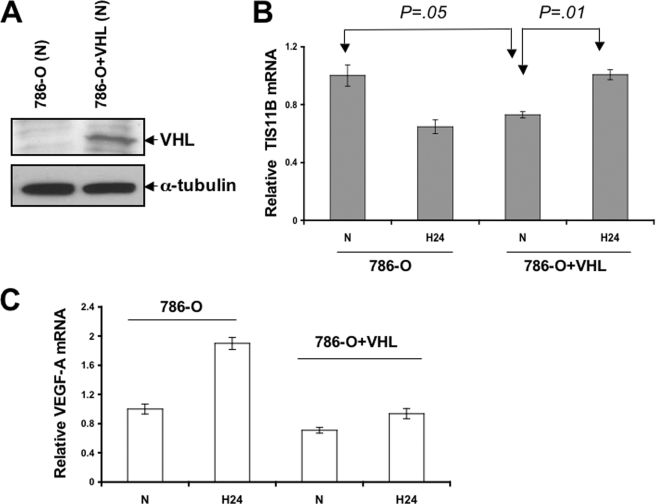
To further substantiate our observation that VHL regulates TIS11B expression, we transduced 786-O cells with a retroviral construct of VHL (see “Experimental Procedures”). VHL expression is shown in Fig. 3A. Overexpression of VHL in 786-O cells resulted in a significant down-regulation (p = 0.05) of TIS11B expression in normoxia (Fig. 3B). However in hypoxia, VHL overexpression resulted in a significant up-regulation of the TIS11B mRNA level compared with that in VHL-expressing cells in normoxia (p = 0.01) (Fig. 3B). These results suggest that VHL differentially regulates TIS11B in normoxia and hypoxia. Relative VEGF mRNA expression at these time points was also included (Fig. 3C).
FIGURE 3.
Effect of VHL overexpression in 786-O cells under normoxic and hypoxic growth conditions. 786-O cells were transfected with pBABe-puro-VHL and incubated under normoxia or hypoxia. A, Western blotting was performed to detect the level of VHL after transfection in 786-O cells in normoxia. α-Tubulin was used as the loading control. B and C, after 24 h of hypoxia, RNA was collected, and real-time PCR was performed to detect TIS11B and VEGF-A mRNA levels. Data represent the average of three independent determinations. N, normoxia; H 24, hypoxia, 24 h.
VHL Post-transcriptionally Regulates TIS11B through hsa-miR-29b in Normoxia
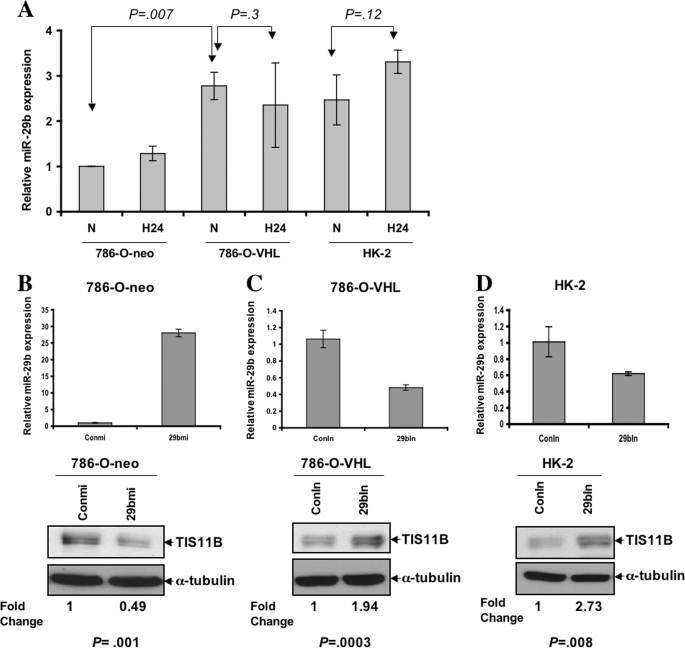
We then examined how VHL regulates TIS11B expression. With the emergence of miRNAs as potential regulators of many genes through inhibition of translations or degradation of mRNA (31), we compared the miRNA expression profiles in 786-O-neo and 786-O-VHL cells. We identified hsa-miR-29b to be up-regulated in 786-O-VHL cells (>2.5-fold; p = 0.007) compared with 786-O-neo cells in normoxia; the result did not change significantly in hypoxia (Fig. 4A). Moreover, we observed that like 786-O-VHL cells, kidney proximal epithelial cells (HK-2) also expressed a high level of miR-29b (Fig. 4A).
FIGURE 4.
A, differential expression of miR-29b. The expression levels of mature miR-29b in 786-O-neo, 786-O-VHL, and HK-2 cells were measured by real-time PCR using specific primers. Single-tube TaqMan miRNA assays were performed to quantify mature miR-29b, and they were normalized to RNU6B in triplicate. Relative expression (-fold) was calculated using the comparative Ct method. Real-time PCR analysis showed an increased expression of miR-29b in 786-O-VHL and HK-2 cells compared with 786-O-neo cells under normoxia. Under hypoxia, the expression level in all three cell lines did not change significantly. Data represent the average of three independent determinations. B, introduction of miR-29b reduces TIS11B levels in normoxia. 786-O-neo cells were transfected with 1 nm scrambled control (Conmi) or miR-29b (29bmi) mimic and analyzed for the expression of TIS11B by Western blotting. α-Tubulin was used as the loading control. Data are representative of three separate experiments, which gave similar results. The relative -fold expression of TIS11B and p values (control versus treated sample) have been included. C and D, the inhibitor of miR-29b up-regulates TIS11B expression in normoxia. 786-O-VHL and HK-2 cells transfected with the 200 nm non-targeting control (ConIn) or antisense inhibitor (29bIn) of miR-29b were analyzed for TIS11B by Western blotting. α-Tubulin was used as the loading control. Data are representative of three separate experiments, which gave similar results. The relative -fold expression of TIS11B and p values (control versus treated sample) have been indicated. N, normoxia; H 24, hypoxia, 24 h.
The results predicted using TargetScan or miRBase demonstrated that the TIS11B 3′-UTR contains a miR-29b target sequence. To examine whether miR-29b plays a role in TIS11B regulation, we transfected a miR-29b mimic in 786-O-neo cells or an antisense oligonucleotide targeting the miR-29b sequence in 786-O-VHL and HK-2 cells. Western blot analysis revealed that introduction of the miR-29b mimic in 786-O-neo cells resulted in a significant down-regulation of TIS11B expression (Fig. 4B). In contrast, up-regulation of the TIS11B level was observed upon introduction of the anti-miR-29b oligonucleotide in 786-O-VHL and HK-2 cells in normoxia (Fig. 4, C and D), but no significant change was found in 786-O-VHL cells in hypoxia (supplemental Fig. 1). Together, these observations suggest that, at least in part, hsa-miR-29b regulates TIS11B protein expression in normoxia.
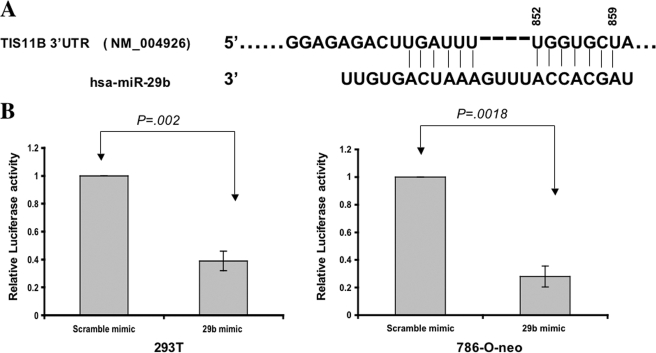
To further validate our hypothesis that miR-29b binds the TIS11B 3′-UTR, we performed an in vitro luciferase reporter gene assay. The 3′-UTR (full-length) of TIS11B was cloned immediately downstream of the luciferase gene (SwitchGear Genomics) and was cotransfected with the miR-29b mimic or non-targeting control into 293T or 786-O-neo cells. We found a direct effect of miR-29b on the TIS11B 3′-UTR: the luciferase activity was significantly repressed by miR-29b compared with the non-targeting control in both cell lines used (Fig. 5B). These results indicate that miR-29b is able to interact directly with the 3′-UTR sequence of TIS11B and subsequently represses the expression of the TIS11B gene in normoxia.
FIGURE 5.
TIS11B is a target of miR-29b in normoxia. The 3′-UTR of TIS11B enables miR-29b regulation. A, the complementarity between TIS11B cDNA and miR-29b in humans is shown. B, 293T and 786-O-neo cells were cotransfected with the miR-29b mimic or the non-targeting control and the full-length 3′-UTR of TIS11B gene downstream of the luciferase gene. A significant down-regulation of luciferase activity was recorded 36 h after transfection. Experiments were performed twice in triplicate (n = 6).
VHL Regulates TIS11B mRNA Stability in Hypoxia
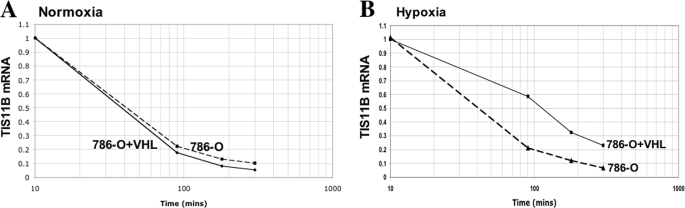
In hypoxia, we did not observe any significant modulation of the miR-29b expression levels in 786-O-neo, 786-O-VHL, or HK-2 cells (Fig. 4A) or any effect of the miR-29b inhibitor on TIS11B expression (supplemental Fig. 1). However, hypoxia induced a significant up-regulation of TIS11B in VHL-overexpressing 786-O and renal epithelial cells (Fig. 2, A and B). Therefore, we investigated whether the stability of the TIS11B transcript was altered in hypoxia. For this, 786-O and 786-O+VHL cells were treated with actinomycin D under both normoxic and hypoxic conditions. In hypoxia, an increased stability of the TIS11B mRNA was observed in 786-O cells overexpressing VHL compared with that in 786-O control cells. The TIS11B mRNA half-life was 110 min in VHL-expressing 786-O cells compared with 40 min in 786-O null cells (Fig. 6B) in hypoxia. In normoxia, there was no difference in TIS11B mRNA stability between VHL-expressing 786-O cells and 786-O null cells (Fig. 6A). In normoxia, the TIS11B mRNA half-life in both cell lines was 40 min (Fig. 6A). This result suggests that VHL could positively regulate TIS11B expression in hypoxia and thus could stabilize TIS11B mRNA in hypoxia.
FIGURE 6.
VHL regulates TIS11B mRNA stability. The half-life of TIS11B mRNA was monitored in 786-O and 786-O+VHL cells under normoxia (A) and hypoxia (B). 786-O and 786-O+VHL cells were cultured under either normoxia or hypoxia for 24 h, and then actinomycin D (5 μg/ml) was added to the growth medium. Immediately after the addition of actinomycin D, the cells were returned to the same culture conditions (normoxia or hypoxia). During the following 5 h, total RNA was prepared at different time points, and real-time PCR was performed. Data represent the average of three independent determinations.
TIS11B Regulates VEGF-A Expression in RCC
We have demonstrated increased TIS11B expression in 786-O-VHL cells under hypoxia (Fig. 2A), which seems to reduce VEGF levels significantly compared with levels in 786-O-neo cells even under prolonged hypoxia (Fig. 1). To further examine whether TIS11B indeed regulates VEGF-A expression, we used an RNA interference technique. Knocking down TIS11B in 786-O-VHL cells (Fig. 7, B and D) by siRNA resulted in an up-regulation of VEGF-A mRNA in both normoxia (Fig. 7A) and hypoxia (Fig. 7C). It appears that a 50% knockdown of TIS11B led to a >2.0-fold (p = 0.02) up-regulation of VEGF-A mRNA levels in hypoxia. Together, our results suggest the existence of a VHL-mediated unique regulation of VEGF in hypoxia due, at least in part, to modulation of TIS11B expression.
FIGURE 7.
TIS11B down-regulates VEGF-A expression. 786-O-VHL cells were transfected with the scrambled control (Consi) or TIS11B (TIS11Bsi) siRNA using DharmaFECT 1 and incubated under normoxia and hypoxia. After 72 h, total RNA was collected, and real-time PCR was performed to detect TIS11B and VEGF-A mRNA expression. After TIS11B knockdown (B and D), increased expression of VEGF-A was observed in both normoxia (A) and hypoxia (C). Data represent the average of three independent determinations. N, normoxia; H 24, hypoxia, 24 h.
DISCUSSION
RCC and other tumors that arise in patients with the VHL syndrome are characteristically well vascularized, a property that has been attributed to their consistent overexpression of the potent angiogenic factor VEGF (32–36). A number of factors have been reported to regulate VEGF in various tumors and in non-tumorigenic cells. One of the well studied regulators is hypoxic stress, and VHL has emerged as a key factor in cellular responses to hypoxia. It has been well established that VHL down-regulates VEGF gene expression at both the transcriptional and post-transcriptional levels in normoxia (12, 14, 37–39). However, the effect of VHL on VEGF regulation under hypoxic stress remains to be determined. In this study, we observed that the VEGF level in VHL-expressing 786-O cells remained significantly lower than that in the parental 786-O cells lacking endogenous VHL expression, even after prolonged hypoxia. Here, we have provided evidence as to how VHL regulates VEGF expression in hypoxia.
In normoxia, 786-O cells expressing exogenous VHL express a low level of TIS11B. However, in these same cells under hypoxia, we detected an increased level of TIS11B expression in contrast to the level in the VHL-deficient renal cancer cell line 786-O. Our results suggest that VHL regulates VEGF synthesis through TIS11B primarily by two mechanisms depending on whether the environment is normoxic or hypoxic. VHL overexpression increases the level of the miRNA miR-29b in RCC. In normoxia, miR-29b in VHL-expressing cells targets the 3′-UTR of the RNA-binding protein TIS11B to down-regulate its translation without affecting mRNA stability. Although VHL-induced miR-29b expression remains unaltered by hypoxic stress, it seems to be functionally silent, as evidenced when the introduction of an anti-miR-29b oligonucleotide did not significantly affect the level of TIS11B in hypoxia. Therefore, it appears that VHL can regulate TIS11B through an alternative mechanism in a hypoxic environment. We detected a stabilization of the TIS11B transcript for an extended period in VHL-expressing cells compared with that in the parental VHL-null 786-O cells under hypoxic conditions. Thus, enhanced mRNA stabilization contributes significantly to maintain high levels of TIS11B in 786-O-VHL cells under hypoxia. Future studies are under way to delineate the mechanism of VHL-mediated regulation of the RNA-binding protein involved in TIS11B mRNA stabilization in hypoxia. Of importance, knockdown of TIS11B in hypoxia led to a significant increase in the VEGF mRNA level. These observations present a novel inhibitory role of VHL in VEGF regulation in hypoxia through increased TIS11B expression.
Hypoxia is the major inducer of VEGF synthesis in both physiological and tumor angiogenesis. Although there are checks and balances during the physiological process of hypoxia-mediated VEGF-A synthesis, such tight control is absent in pathological angiogenesis. Hypoxia-inducible factor (HIF) and HuR are two molecules contributing mainly to the control of VEGF expression (40, 41), and VHL has been shown to down-regulate HIF-2α and HuR to modulate the VEGF level in normoxia (16, 17, 29, 30). VHL-deficient RCC and other tumor cells have been reported to express high levels of TIS11B (42). One possible reason for this high level of TIS11B expression in normoxia is to override the active positive regulators (e.g. HIF-2α and HuR) of VEGF synthesis. However, in VHL-expressing cells, even a low level of TIS11B is important for maintaining VEGF expression under a threshold value in normoxia because knockdown of TIS11B in these cells also shows an up-regulation of VEGF.
In 2006, Bell et al. (26) showed that TIS11B mutant embryos exhibited extraembryonic and intraembryonic vascular defects, cardiac abnormalities, and an elevated level of VEGF. Additionally, TIS11B has been shown to be an important regulator of myogenic differentiation (43). The lack of TIS11B expression during mid-gestation has also been linked to anomalous placentation and fetal death due to the abnormal stabilization of one or more mRNAs (25). Therefore, in VHL-expressing cells, we believe that a balanced TIS11B-regulated VEGF level in both normoxia and hypoxia is important for its physiological function. As shown here with the loss of VHL, TIS11B-regulated VEGF synthesis is also impaired and this, in turn, favors unrestricted expression of VEGF and its subsequent pathological effects.
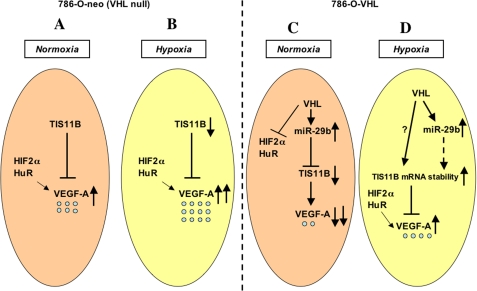
Our findings on regulation of VEGF by the VHL/TIS11B axis in RCC are summarized in Fig. 8. In normoxia, RCC 786-O cells express an increased level of TIS11B protein to override the active positive regulators (e.g. HIF-2α and HuR) of VEGF-A and synthesize a moderately high level of VEGF-A. In hypoxia, however, a decrease in TIS11B expression relieves its inhibitory effects on VEGF-A, allowing a further increase in VEGF-A synthesis under the control of HIF and HuR in 786-O cells. Interestingly, VHL overexpression in 786-O cells keeps the TIS11B level low by inhibiting TIS11B translation through miR-29b in normoxia. VEGF-A expression also remains low in VHL-overexpressing 786-O cells in normoxia because of VHL-mediated inhibition of HuR and HIF activity. Although miR-29b expression remains unaltered in VHL-overexpressing 786-O cells under hypoxia, an increased stability of TIS11B mRNA has been observed, and stabilized TIS11B maintains VEGF-A expression at ∼30% lower levels compared with the VEGF-A levels found in 786-O null cells under hypoxic stress. Here, our data uncovered a novel inhibitory pathway regulating VEGF synthesis through interplay between the VHL/miR-29b/TIS11B axis and HIF/HuR positive regulatory axis under hypoxia.
FIGURE 8.
Model for VHL-modulated TIS11B expression in regulation of VEGF-A in RCC. A, in normoxia, RCC 786-O cells express an increased level of TIS11B protein, overriding the active positive regulators (e.g. HIF-2α and HuR) of VEGF-A, and a moderately high level of VEGF-A is detected. B, in hypoxia in 786-O cells, TIS11B expression decreases, which allows for increased VEGF-A synthesis. C, in normoxia, VHL overexpression down-regulates the positive regulators of VEGF synthesis and increases the level of the miRNA miR-29b in RCC. An elevated level of miR-29b in VHL-expressing cells targets the 3′-UTR of the RNA-binding protein TIS11B and down-regulates its translation. VEGF-A expression then remains low in VHL-overexpressing 786-O cells in normoxia. D, in hypoxia, VHL-induced miR-29b expression remains unaltered. However, mRNA stabilization contributes significantly to maintaining high levels of TIS11B in 786-O-VHL cells. This stabilized TIS11B keeps VEGF-A expression low compared with the VEGF-A levels found in 786-O null cells under hypoxic stress.
Supplementary Material
Supplemental Data
Acknowledgment
We thank Julie Lau for discussions.
*
This work was supported, in whole or in part, by National Institutes of Health Grant CA78383.
2
The abbreviations used are:
VEGF
vascular endothelial growth factor
VHL
Von Hippel-Lindau
RCC
renal cell carcinoma
ACTH
adrenocorticotropic hormone
3′-UTR
3′-untranslated region
miRNA
microRNA
siRNA
small interfering RNA
HIF
hypoxia-inducible factor.
REFERENCES
- 1.Folkman J. (1996) Sci. Am. 275, 150–154 [DOI] [PubMed] [Google Scholar]
- 2.Folkman J., Klagsbrun M. (1987) Science 235, 442–447 [DOI] [PubMed] [Google Scholar]
- 3.Folkman J., Watson K., Ingber D., Hanahan D. (1989) Nature 339, 58–61 [DOI] [PubMed] [Google Scholar]
- 4.Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. (1993) Nature 362, 841–844 [DOI] [PubMed] [Google Scholar]
- 5.Millauer B., Shawver L. K., Plate K. H., Risau W., Ullrich A. (1994) Nature 367, 576–579 [DOI] [PubMed] [Google Scholar]
- 6.Shweiki D., Itin A., Soffer D., Keshet E. (1992) Nature 359, 843–845 [DOI] [PubMed] [Google Scholar]
- 7.Shweiki D., Neeman M., Itin A., Keshet E. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Höckel M., Vaupel P. (2001) J. Natl. Cancer Inst. 93, 266–276 [DOI] [PubMed] [Google Scholar]
- 9.Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L., Schmidt L., Zhou F., Li H., Wei M. H., Chen F. (1993) Science 260, 1317–1320 [DOI] [PubMed] [Google Scholar]
- 10.Maher E. R., Kaelin W. G., Jr. (1997) Medicine 76, 381–391 [DOI] [PubMed] [Google Scholar]
- 11.Kaelin W. G., Jr., Maher E. R. (1998) Trends Genet. 14, 423–426 [DOI] [PubMed] [Google Scholar]
- 12.Gnarra J. R., Zhou S., Merrill M. J., Wagner J. R., Krumm A., Papavassiliou E., Oldfield E. H., Klausner R. D., Linehan W. M. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10589–10594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliopoulos O., Levy A. P., Jiang C., Kaelin W. G., Jr., Goldberg M. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10595–10599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy A. P., Levy N. S., Iliopoulos O., Jiang C., Kaplin W. G., Jr., Goldberg M. A. (1997) Kidney Int. 51, 575–578 [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay D., Datta K. (2004) Semin. Cancer Biol. 14, 123–130 [DOI] [PubMed] [Google Scholar]
- 16.Datta K., Mondal S., Sinha S., Li J., Wang E., Knebelmann B., Karumanchi S. A., Mukhopadhyay D. (2005) Oncogene 24, 7850–7858 [DOI] [PubMed] [Google Scholar]
- 17.Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 18.Corps A. N., Brown K. D. (1995) FEBS Lett. 368, 160–164 [DOI] [PubMed] [Google Scholar]
- 19.Gomperts M., Corps A. N., Pascall J. C., Brown K. D. (1992) FEBS Lett. 306, 1–4 [DOI] [PubMed] [Google Scholar]
- 20.Varnum B. C., Ma Q. F., Chi T. H., Fletcher B., Herschman H. R. (1991) Mol. Cell. Biol. 11, 1754–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown E. J., Schreiber S. L. (1996) Cell 86, 517–520 [DOI] [PubMed] [Google Scholar]
- 22.Chen C. Y., Shyu A. B. (1995) Trends Biochem. Sci. 20, 465–470 [DOI] [PubMed] [Google Scholar]
- 23.Ciais D., Cherradi N., Bailly S., Grenier E., Berra E., Pouyssegur J., Lamarre J., Feige J. J. (2004) Oncogene 23, 8673–8680 [DOI] [PubMed] [Google Scholar]
- 24.Cherradi N., Lejczak C., Desroches-Castan A., Feige J. J. (2006) Mol. Endocrinol. 20, 916–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stumpo D. J., Byrd N. A., Phillips R. S., Ghosh S., Maronpot R. R., Castranio T., Meyers E. N., Mishina Y., Blackshear P. J. (2004) Mol. Cell. Biol. 24, 6445–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell S. E., Sanchez M. J., Spasic-Boskovic O., Santalucia T., Gambardella L., Burton G. J., Murphy J. J., Norton J. D., Clark A. R., Turner M. (2006) Dev. Dyn. 235, 3144–3155 [DOI] [PubMed] [Google Scholar]
- 27.Pichiorri F., Suh S. S., Ladetto M., Kuehl M., Palumbo T., Drandi D., Taccioli C., Zanesi N., Alder H., Hagan J. P., Munker R., Volinia S., Boccadoro M., Garzon R., Palumbo A., Aqeilan R. I., Croce C. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12885–12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths-Jones S., Grocock R. J., van Dongen S., Bateman A., Enright A. J. (2006) Nucleic Acids Res. 34, D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr. (2001) Science 292, 464–468 [DOI] [PubMed] [Google Scholar]
- 30.Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., von Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 31.Valencia-Sanchez M. A., Liu J., Hannon G. J., Parker R. (2006) Genes Dev. 20, 515–524 [DOI] [PubMed] [Google Scholar]
- 32.Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Dvorak H. F., Senger D. R. (1993) Am. J. Pathol. 143, 1255–1262 [PMC free article] [PubMed] [Google Scholar]
- 33.Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. (1989) Science 246, 1306–1309 [DOI] [PubMed] [Google Scholar]
- 34.Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. (1983) Science 219, 983–985 [DOI] [PubMed] [Google Scholar]
- 35.Takahashi A., Sasaki H., Kim S. J., Tobisu K., Kakizoe T., Tsukamoto T., Kumamoto Y., Sugimura T., Terada M. (1994) Cancer Res. 54, 4233–4237 [PubMed] [Google Scholar]
- 36.Wizigmann-Voos S., Breier G., Risau W., Plate K. H. (1995) Cancer Res. 55, 1358–1364 [PubMed] [Google Scholar]
- 37.Siemeister G., Weindel K., Mohrs K., Barleon B., Martiny-Baron G., Marmé D. (1996) Cancer Res. 56, 2299–2301 [PubMed] [Google Scholar]
- 38.Iliopoulos O., Kibel A., Gray S., Kaelin W. G., Jr. (1995) Nat. Med. 1, 822–826 [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay D., Knebelmann B., Cohen H. T., Ananth S., Sukhatme V. P. (1997) Mol. Cell. Biol. 17, 5629–5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmeliet P., Dor Y., Herbert J. M., Fukumura D., Brusselmans K., Dewerchin M., Neeman M., Bono F., Abramovitch R., Maxwell P., Koch C. J., Ratcliffe P., Moons L., Jain R. K., Collen D., Keshert E. (1998) Nature 394, 485–490 [DOI] [PubMed] [Google Scholar]
- 41.Levy N. S., Chung S., Furneaux H., Levy A. P. (1998) J. Biol. Chem. 273, 6417–6423 [DOI] [PubMed] [Google Scholar]
- 42.Carrick D. M., Blackshear P. J. (2007) Arch. Biochem. Biophys. 462, 278–285 [DOI] [PubMed] [Google Scholar]
- 43.Busse M., Schwarzburger M., Berger F., Hacker C., Munz B. (2008) Eur. J. Cell Biol. 87, 31–38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data