Factors associated with uptake of treatment for recent hepatitis C virus infection in a predominantly injecting drug user cohort: the ATAHC Study (original) (raw)
. Author manuscript; available in PMC: 2011 Mar 1.
Abstract
Despite that the majority of hepatitis C virus (HCV) infection occurs among injection drug users (IDUs), little is known about HCV treatment uptake in this group, particularly during recent infection. We evaluated uptake of treatment for recent HCV infection, including associated factors, within a population predominantly made up of IDUs. The Australian Trial in Acute Hepatitis C was a study of the natural history and treatment of recent HCV infection. All participants with detectable HCV RNA at screening were offered HCV treatment, assessed for eligibility and those initiating treatment were identified. Logistic regression analyses were used to identify predictors of HCV treatment uptake. Between June 2004 and February 2008, 163 were enrolled, with 146 positive for HCV RNA at enrolment. The mean age was 35 years, 77% (n=113) participants had ever injected illicit drugs and 23% (n=34) reported having ever received methadone or buprenorphine treatment. The uptake of HCV treatment was 76% (111 of 146) among those who were eligible on the basis of positive HCV RNA. Estimated duration of HCV infection (OR=1.03 per week, 95% CI=1.00–1.06, _P_=0.035) and log10 HCV RNA (OR=1.92 per log10 increase, 95% CI=1.36–2.73, P<0.001) were independently associated with treatment uptake whereas injection drug use was not. This study demonstrates that a high uptake of HCV treatment can be achieved among participants with recently acquired HCV infection. Decisions about whether to initiate treatment for recently acquired HCV were mainly driven by clinical factors, rather than factors related to sociodemographics or injecting behaviors.
Keywords: hepatitis C virus, acute, treatment, injection drug users, pegylated interferon
1.0 Introduction
Among injection drug users (IDUs), hepatitis C virus (HCV) infection remains a significant medical co-morbidity. There is a growing awareness of the need for improved access to HCV treatment for IDUs, but treatment uptake in this population has been low (Grebely et al., 2009; NCHECR., 2008). Among health care providers/practitioners, there are still concerns about suitability of treatment due to patient motivation and adherence, psychosocial issues, medical and psychiatric co-morbidities, risk of re-infection and the lack of infrastructure to ensure access to care during treatment (Edlin et al., 2005). However, little is known about factors influencing HCV treatment uptake, particularly in the setting of recent infection.
The early treatment of HCV, with the shorter duration of treatment and a simpler regimen using pegylated interferon (PEG-IFN) for 12–24 weeks (De Rosa et al., 2006; Jaeckel et al., 2001; Kamal et al., 2006; Wiegand et al., 2006) is an attractive option to IDUs, many of whom experience significant social and clinical issues apart from HCV. In a group with high rates of co-morbid psychiatric illness, a shorter duration of a treatment regimen known to have neuropsychiatric adverse effects would be an advantage.
We investigated the uptake of treatment for recent HCV infection, including identifying factors associated treatment uptake in the Australian Trial in Acute Hepatitis C (ATAHC), a prospective trial of the natural history and treatment of recently acquired HCV infection, consisting mainly of IDUs.
2.0 Methods
2.1 Study design
ATAHC was a multicenter, prospective cohort study of the natural history and treatment of recent HCV infection commencing in June 2004 and is described in detail elsewhere (Dore et al., 2009). The definition of recent infection included participants with recent HCV infection according to the following eligibility criteria:
First positive anti-HCV antibody within 6 months of enrolment; and either
- Acute clinical hepatitis C infection, defined as symptomatic seroconversion illness or alanine aminotransferase (ALT) level greater than 10 times the upper limit of normal (>400 IU/mL) with exclusion of other causes of acute hepatitis, at most 12 months before the initial positive anti-HCV antibody; or
- Asymptomatic hepatitis C infection with seroconversion, defined by a negative anti-HCV antibody in the two years prior to the initial positive anti-HCV antibody. This broader seroconversion window was used to identify participants across a wide range of estimated dates of infection, given that the period defining acute HCV infection and response to therapy in this setting is still unclear.
Other eligibility criteria included being age 16 years or above, having a negative pregnancy test, and ability to provide written, informed consent. Heavy alcohol intake and active illicit drug use were not exclusion criteria. Enrolment was encouraged in all people who met the entry criteria whether or not antiviral treatment was required and participants with detectable HCV RNA at screening were assessed for HCV treatment. Antiviral treatment comprised of PEG-IFN alfa-2a for 24 weeks (plus ribavirin for HIV/HCV coinfected participants) (Dore et al., 2009). The study protocol was approved by the Human Research Ethics Committee at St Vincent’s Hospital and was conducted according to the Declaration of Helsinki and ICH/GCP guidelines.
2.2 HCV virological assessment
HCV RNA was assessed at screening with a qualitative HCV-RNA assay (TMA assay, Versant, Bayer, Australia, lower limit of detection 10 IU/ml) and if positive a quantitative HCV RNA assay (Versant HCV RNA 3.0 Bayer, Australia lower limit of detection 615 IU/ml). HCV genotype (Versant LiPa2, Bayer, Australia) was performed on HCV RNA positive participants at screening. A questionnaire was administered at screening to obtain information on injecting behaviours, social functioning (Opiate Treatment Index Social Functioning Scale) (Darke et al., 1992) and depression (Mini-International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998)].
2.3 Statistical analyses
HCV treatment uptake was evaluated based on the identification of all participants receiving at least one injection with PEG-IFN alfa-2a. Logistic regression analyses were used to identify predictors of HCV treatment uptake. It was hypothesized that at the time of screening, given the high potential for spontaneous HCV clearance, treatment uptake would be associated with clinical factors. Some individuals went on to clear their infection between screening and proposed treatment initiation (baseline) which was up to 12 weeks (Dore et al., 2009); therefore an additional analysis was performed to evaluate HCV treatment uptake among those who did not spontaneously clear infection by the date of anticipated treatment initiation (HCV RNA positive at treatment baseline). It was hypothesized that treatment uptake among those still positive for HCV RNA at treatment baseline would be associated with social and behavioural factors.
Potential predictors were determined a priori and included sex, age, tertiary education (post high-school), employment, accommodation, social functioning, methadone or buprenorphine treatment, depression (based on the MINI), ethnicity, injecting drug use characteristics, alcohol consumption, estimated duration of HCV infection, presentation (acute symptomatic, acute biochemical, asymptomatic), peak and screening ALT level, screening HCV RNA levels and HCV genotype. These factors were hypothesized to be associated with treatment uptake either based on the literature (Grebely et al., 2008; Grebely et al., 2009; Mehta et al., 2008; Strathdee et al., 2005) or clinical anecdotal evidence. Presentation of HCV at the time of diagnosis was classified as either acute clinical or asymptomatic infection. Acute clinical infection included those with either a documented clinical history of symptomatic seroconversion illness and those without clinical symptoms but with a documented peak ALT above 400 IU/ml at or prior to the time of diagnosis. Participants with asymptomatic infection included participants with anti-HCV antibody seroconversion but no acute clinical symptoms or documented peak ALT above 400 IU/ml. The estimated date of infection for acute clinical infection was calculated as six weeks prior to onset of seroconversion illness if present or six weeks prior to the first ALT reading above 400 IU/ml. The estimated date of infection for asymptomatic infection was calculated as the mid-point between the last negative anti-HCV antibody and the first positive anti-HCV antibody test result. For participants who were anti-HCV antibody negative and HCV RNA positive at screening, the estimated date of infection was designated to be six weeks prior to screening. Social functioning was calculated using a validated scale from the Opiate Treatment Index (Darke et al., 1992) that addresses employment, residential stability, and inter-personal conflict (Darke et al., 1992). Current depression and suicide risk were evaluated using the Mini-International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998).
The multivariate models for predictors of treatment uptake were determined using a backwards stepwise approach, considering factors that were significant at the 0.10 level in univariate analysis. The final multivariate models included only factors that remained significant at the 0.05 level. All analyses were performed using the statistical package Stata.
3.0 Results
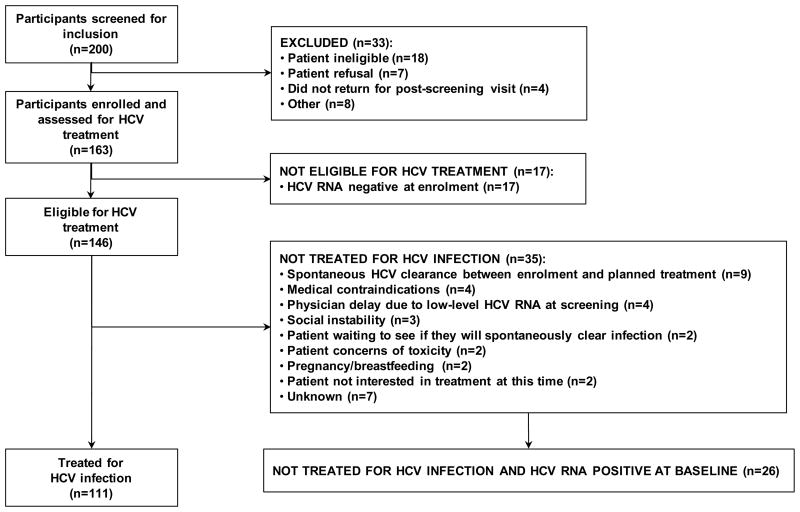
Over the period June 2004 through February 2008, 163 participants were enrolled in this study (Figure 1). At enrolment, 17 participants were negative for HCV RNA. The remaining 146 participants were positive for HCV RNA at enrolment and were thus eligible to receive treatment for HCV infection.
Figure 1.
Participant disposition in the ATAHC study.
The enrolment characteristics of those eligible to receive to receive treatment (n=146) are shown in Table 1, stratified by treated (n=111) and untreated (n=35) participants. The majority were male (73%) and did not have full-time employment (58%). Injecting drug use was the most likely mode of HCV acquisition (71%).
Table 1.
Factors associated with uptake of treatment for recent HCV infection (n=146)
| Overall | No HCV treatment | HCV treatment | Odds ratio | 95% CI | P | P overall | odds ratio | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total participants, (n) | 146 | 35 | 111 | - | - | - | - | - | - | - |
| Sex, n (%) | ||||||||||
| Male | 107 (73%) | 23 (66%) | 84 (76%) | 1.00 | - | - | - | - | - | - |
| Female | 39 (27%) | 12 (34%) | 27 (24%) | 0.62 | 0.27–1.40 | 0.25 | - | - | - | - |
| Age (yrs), mean/SD | 34.6 (10.0) | 34.9 (8.9) | 34.5 (10.0) | 1.00 | 0.96–1.04 | 0.85 | - | - | - | - |
| Ethnicity, n (%) | ||||||||||
| Caucasian | 133 (91%) | 34 (97%) | 99 (89%) | 1.00 | - | - | - | - | - | - |
| Other | 13 (9%) | 1 (3%) | 12 (11%) | 4.12 | 0.52–32.88 | 0.11 | - | - | - | - |
| Tertiary education or greater, n (%) | ||||||||||
| Yes | 60 (41%) | 9 (26%) | 51 (46%) | 1.00 | - | - | - | 1.00 | - | - |
| No | 86 (59%) | 26 (74%) | 60 (54%) | 0.41 | 0.17–0.96 | 0.037 | - | 0.43* | 0.17–1.08 | 0.071 |
| Accommodation | ||||||||||
| Rent | 90 (62%) | 25 (71%) | 65 (59%) | 1.00 | - | - | - | - | - | - |
| Privately owned | 37 (25%) | 5 (14%) | 32 (29%) | 2.46 | 0.86–7.03 | 0.092 | 0.24 | - | - | - |
| Other | 19 (13%) | 5 (14%) | 14 (13%) | 1.08 | 0.35–3.30 | 0.90 | - | - | - | - |
| Full-time or part-time employment, n (%) | ||||||||||
| Yes | 61 (42%) | 9 (26%) | 52 (47%) | 1.00 | - | - | - | 1.00 | - | - |
| No | 85 (58%) | 26 (74%) | 59 (53%) | 0.39 | 0.17–0.91 | 0.030 | - | 0.44* | 0.18–1.10 | 0.080 |
| Prison ever, n (%) | ||||||||||
| Yes | 22 (15%) | 6 (18%) | 16 (15%) | 1.00 | - | - | - | - | - | - |
| No | 121 (85%) | 28 (82%) | 93 (85%) | 1.25 | 0.45–3.49 | 0.68 | - | - | - | - |
| Methadone or buprenorphine treatment | ||||||||||
| Ever (not current) | 16 (11%) | 4 (11%) | 12 (11%) | 1.00 | - | - | - | - | - | - |
| Current | 18 (12%) | 6 (17%) | 12 (11%) | 0.67 | 0.15–2.98 | 0.60 | 0.56 | - | - | - |
| Never | 112 (77%) | 25 (71%) | 87 (78%) | 1.16 | 0.34–3.91 | 0.81 | - | - | - | - |
| Social functioning score | ||||||||||
| ≤ 14 | 79 (54%) | 16 (46%) | 63 (57%) | 1.00 | - | - | - | - | - | - |
| >14 | 52 (36%) | 16 (46%) | 36 (32%) | 0.57 | 0.26–1.28 | 0.17 | 0.50 | - | - | - |
| Missing | 15 (10%) | 3 (9%) | 12 (11%) | 1.02 | 0.26–4.03 | 0.98 | - | - | - | - |
| Current depression, n (%) | ||||||||||
| No | 127 (87%) | 26 (74%) | 101 (91%) | 1.00 | - | - | - | 1.00 | - | - |
| Yes | 19 (13%) | 9 (26%) | 10 (9%) | 0.29 | 0.11–0.78 | 0.014 | - | 0.40* | 0.14–1.17 | 0.093 |
| Injecting drug use ever, n (%) | ||||||||||
| No | 33 (23%) | 6 (17%) | 27 (24%) | 1.00 | - | - | - | - | - | - |
| Yes | 113 (77%) | 29 (83%) | 84 (76%) | 0.64 | 0.24–1.73 | 0.38 | - | - | - | - |
| Injection drug use in previous 6 months, n (%) | ||||||||||
| No | 56 (38%) | 11 (31%) | 45 (41%) | 1.00 | - | - | - | - | - | - |
| Yes | 90 (62%) | 24 (69%) | 66 (59%) | 0.67 | 0.30–1.51 | 0.34 | - | - | - | - |
| Number of standard drinks per week | ||||||||||
| <4 drinks | 93 (68%) | 26 (79%) | 67 (64%) | 1.00 | - | - | - | - | - | - |
| ≥4 drinks | 44 (32%) | 7 (21%) | 37 (36%) | 2.05 | 0.81–5.18 | 0.13 | - | - | - | - |
| Mean estimated duration of infection at screening (wks) (SD) | 28.2 (16.6) | 23.6 (15.2) | 29.6 (16.8) | 1.02 | 1.00–1.05 | 0.063 | - | 1.03 | 1.00–1.06 | 0.035 |
| Presentation of recent HCV infection, n (%) | ||||||||||
| Acute clinical (symptomatic) | 58 (40%) | 12 (34%) | 46 (41%) | 1.00 | - | - | - | - | - | - |
| Acute clinical (ALT >400 IU/mL) | 30 (21%) | 6 (17%) | 24 (22%) | 1.04 | 0.35–3.13 | 0.94 | 0.47 | - | - | - |
| Asymptomatic seroconversion | 58 (40%) | 17 (49%) | 41 (37%) | 0.63 | 0.27–1.47 | 0.29 | - | - | - | - |
| Documented HCV seroconversion illness | ||||||||||
| No | 81 (55%) | 22 (63%) | 59 (53%) | 1.00 | - | - | - | - | - | - |
| Yes | 58 (40%) | 12 (34%) | 46 (41%) | 1.45 | 0.73–2.87 | 0.29 | - | - | - | - |
| HIV infection, n (%) | ||||||||||
| No | 97 (66%) | 23 (66%) | 74 (67%) | 1.00 | - | - | - | - | - | - |
| Yes | 49 (34%) | 12 (34%) | 37 (33%) | 0.96 | 0.43–2.14 | 0.92 | - | - | - | - |
| Peak ALT prior to enrolment (IU/L) | ||||||||||
| <400, n (%) | 60 (43%) | 16 (50%) | 44 (41%) | 1.00 | - | - | - | - | - | - |
| ≥400, n (%) | 80 (57%) | 16 (50%) | 64 (59%) | 1.45 | 0.66–3.21 | 0.35 | - | - | - | - |
| ALT at screening | ||||||||||
| <400, n (%) | 113 (77%) | 30 (86%) | 83 (75%) | 1.00 | - | - | - | - | - | - |
| ≥400 IU/L, n (%) | 33 (23%) | 5 (14%) | 28 (25%) | 2.02 | 0.72–5.72 | 0.18 | - | - | - | - |
| Log10 HCV RNA (IU/L), Mean (SD) | 4.6 (1.4) | 3.9 (1.4) | 4.8 (1.3) | 1.90 | 1.34–2.68 | <0.001 | - | 1.92 | 1.36–2.73 | <0.001 |
| HCV genotype | ||||||||||
| Genotype 1/4 | 77 (53%) | 14 (40%) | 63 (57%) | 1.00 | - | - | - | - | - | - |
| Genotype 2/3 | 62 (42%) | 18 (51%) | 44 (40%) | 0.54 | 0.24–1.21 | 0.13 | 0.17 | - | - | - |
| Unknown | 7 (5%) | 3 (9%) | 4 (4%) | 0.30 | 0.06–1.48 | 0.14 | - | - | - | - |
Overall, 113 (77%) participants had ever injected illicit drugs and 34 (23%) reported having ever received methadone or buprenorphine treatment. Among participants who reported injection drug use ever, 80% (90 of 113) injected in the six months prior to screening, with methamphetamine (52%) and opiates (41%) reported as the most often injected drugs.
The uptake of HCV treatment was 76% (111 of 146). Among those not having initiated treatment for HCV infection (n=35), the reasons for not doing so are shown in Figure 1.
Factors associated with treatment uptake in univariate analysis included tertiary education, full-time or part-time employment, no depression at enrolment, estimated duration of infection and log10 HCV RNA (Table 1). However, in multiple logistic regression analysis, factors independently associated with treatment uptake were estimated duration of infection (OR=1.03 per week, 95% CI=1.00–1.06, _P_=0.035) and log10 HCV RNA (OR=1.92 per log10 increase, 95% CI=1.36–2.73, P<0.001).
Of those not initiating treatment (n=35), nine participants were HCV RNA positive at enrolment, but were HCV RNA negative at or prior to treatment baseline (spontaneously cleared infection). To better understand non-clinical factors associated with HCV treatment, an additional analysis was conducted to evaluate factors associated with treatment among individuals who were HCV RNA positive at baseline (n=137). In multiple logistic regression analysis, factors independently associated with treatment uptake were depression (OR=0.29, 95% CI=0.10–0.86, _P_=0.026) and log10 HCV RNA (OR=1.53 per log10 increase, 95% CI=1.05–2.22, _P_=0.027).
4.0 Discussion
We have demonstrated several novel and important findings with respect to uptake of treatment for recently acquired HCV infection in the ATAHC study. First, treatment uptake was high among participants who were HCV RNA positive at enrolment (76%), despite the fact that 77% reported injecting and 80% of injectors reported recent injecting. Second, factors independently associated with HCV treatment included a longer estimated duration of infection and higher HCV RNA at enrolment. In those who were HCV RNA positive at baseline, a higher HCV RNA at enrolment and not having current depression were associated with HCV treatment uptake.
A high proportion of ATAHC participants, were interested and deemed clinically suitable to commence HCV treatment. In this study, the observed uptake of HCV treatment among participants with newly acquired HCV infection is much higher than studies in the setting of chronic HCV infection which range from 3–28% in clinic-based cohorts (Adeyemi et al., 2004; Falck-Ytter et al., 2002; Fishbein et al., 2004; Fleming et al., 2003; Groom et al., 2008; Hallinan et al., 2007; Jowett et al., 2001; Mehta et al., 2006; Morrill et al., 2005; Restrepo et al., 2005), 15–16% in community-based cohorts (Rocca et al., 2004; Stoove et al., 2005) and 1–6% among cohorts of injection drug users (Grebely et al., 2009; Hall et al., 2004; Mehta et al., 2008; NCHECR., 2008). Given that the majority of sites were tertiary care centres, there is likely a referral bias towards enrolling those more likely to be interested in receiving treatment, but the same can be said of clinic-based studies of chronic HCV. The high uptake of treatment is impressive given that the majority of participants were IDUs. It is also likely that the shorter duration of therapy for recently acquired infection and higher likelihood of response may have led to an increased proportion of patients interested in treatment. In one study evaluating attitudes toward treatment for HCV infection among IDUs at various treatment efficacy levels, willingness to consider treatment increased with efficacy of treatment scenarios, from 36% for 20% efficacy to 93% for 70% efficacy (Doab et al., 2005).
Lower HCV treatment uptake among participants with lower HCV RNA and shorter estimated duration of infection may reflect clinician-directed treatment deferral to await spontaneous viral clearance. While the natural history of acute HCV infection is unclear with respect to predictors and time-course of viral clearance (Cox et al., 2005; Glynn et al., 2005; Jauncey et al., 2004), declining HCV viral load in early infection has been associated with greater spontaneous clearance (Hofer et al., 2003). The vast majority of individuals who spontaneously clear HCV infection do so within 8–16 weeks of infection. Other demographic and injecting characteristics had no significant impact on treatment uptake.
Among those positive for HCV RNA at baseline, a higher HCV RNA and depression were the only factors independently associated with treatment uptake. Given the neuropsychiatric side-effects of PEG-IFN, it is not surprising that those with concomitant depression were two times less likely to receive treatment.
Collectively, these data provide important insights into the clinical decision making around the initiation of treatment for recently acquired HCV infection. These data suggest that at the time of presentation, decisions about whether to initiate treatment for recently acquired HCV infection were mainly driven by clinical factors, such as the estimated duration of infection and HCV RNA levels, with treatment being delayed to enable the patient time to spontaneously clear their infection. Having concurrent depression also may have influenced the clinicians’ decision to commence therapy.
The lack of an association between HCV treatment uptake and injecting drug use has important implications. First, it suggests that IDUs have a favorable outlook on HCV treatment, consistent with other research (Doab et al., 2005; Grebely et al., 2008; Seal et al., 2007; Stein et al., 2001; Strathdee et al., 2005; Walley et al., 2005). Second, it appears that clinicians involved in this study did not use recent injecting drug use as an exclusion criterion for treatment. Third, it provides a foundation to evaluate treatment for recently acquired HCV infection in IDUs, which is needed given the low numbers of IDUs in previous studies of treatment for recent infection (Gerlach et al., 2003; Jaeckel et al., 2001; Santantonio et al., 2005; Wiegand et al., 2006).
This study has several limitations. First, the results may not be generalizable to other populations of participants with recent HCV infection. Second, the study was predominantly conducted at tertiary care centres leading to a potential selection bias; participants may have had a higher level of engagement with the health care system compared with the overall population with recent HCV infection. This potentially higher level of engagement is further amplified by the fact that participants had to be tested, identified as having recently acquired HCV and recruited into a research study in a timely fashion (Walsh et al., 2008). Also, the clinicians who managed patients in this study may have more experience in working with IDUs and thus more comfortable and more likely to treat HCV in this subset of participants. An estimated 9,600 HCV infections are acquired annually in Australia, with 90% occurring in IDUs (Razali et al., 2007). A minority of cases would be detected in the initial twelve months of infection, and a small proportion of diagnosed cases would be assessed for HCV treatment (Walsh et al., 2008). Lastly, there may have been factors not measured that may have accounted for why some IDUs underwent treatment whilst others did not.
The ATAHC study has demonstrated that IDUs can be successfully recruited into a study of recent HCV infection and that a high proportion commenced HCV treatment when this was made available to them. The high rate of treatment uptake and the limited association between injecting behavioural factors and uptake indicates that both individuals with early HCV infection and their clinicians are willing to consider treatment among the predominantly IDU-acquired composition of the study population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.0 References
- Adeyemi OM, Jensen D, Attar B, Ghaoui R, Gallagher M, Wolen D, Cotler SJ. Hepatitis C treatment eligibility in an urban population with and without HIV coinfection. AIDS patient care and STDs. 2004;18:239–245. doi: 10.1089/108729104323038919. [DOI] [PubMed] [Google Scholar]
- Cox AL, Netski DM, Mosbruger T, Sherman SG, Strathdee S, Ompad D, Vlahov D, Chien D, Shyamala V, Ray SC, Thomas DL. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Wodak A, Heather N, Ward J. Development and validation of a multi-dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. Br J Addict. 1992;87:733–742. doi: 10.1111/j.1360-0443.1992.tb02719.x. [DOI] [PubMed] [Google Scholar]
- De Rosa FG, Bargiacchi O, Audagnotto S, Garazzino S, Cariti G, Veronese L, Raiteri R, Calleri G, Di Perri G. The early HCV RNA dynamics in patients with acute hepatitis C treated with pegylated interferon-alpha2b. Antivir Ther. 2006;11:165–171. [PubMed] [Google Scholar]
- Doab A, Treloar C, Dore GJ. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clin Infect Dis. 2005;40(Suppl 5):S313–320. doi: 10.1086/427446. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Hellard M, Matthews GV, Grebely J, Haber PS, Petoumenos K, Yeung B, Marks P, van Beek I, McCaughan G, White P, Ffrench R, Rawlinson W, Lloyd AR, Kaldor JM. Treatment of recent hepatitis C virus infection in a predominantly injecting drug user cohort: the ATAHC Study. Gastroenterology. 2009 doi: 10.1016/j.drugalcdep.2009.09.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin BR, Kresina TF, Raymond DB, Carden MR, Gourevitch MN, Rich JD, Cheever LW, Cargill VA. Overcoming barriers to prevention, care, and treatment of hepatitis C in illicit drug users. Clin Infect Dis. 2005;40(Suppl 5):S276–285. doi: 10.1086/427441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–292. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- Fishbein DA, Lo Y, Reinus JF, Gourevitch MN, Klein RS. Factors associated with successful referral for clinical care of drug users with chronic hepatitis C who have or are at risk for HIV infection. J Acquir Immune Defic Syndr. 2004;37:1367–1375. doi: 10.1097/01.qai.0000131932.21612.49. [DOI] [PubMed] [Google Scholar]
- Fleming CA, Craven DE, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97–100. doi: 10.1086/344907. [DOI] [PubMed] [Google Scholar]
- Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung M, Ulsenheimer A, Schraut WW, Schirren CA, Waechtler M, Backmund M, Pape GR. Acute Hepatitis C; High Rate of Both Spontaneous and Treatment-Induced Viral Clearance. Gastroenterology. 2003:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- Glynn SA, Wright DJ, Kleinman SH, Hirschkorn D, Tu Y, Heldebrant C, Smith R, Giachetti C, Gallarda J, Busch MP. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005;45:994–1002. doi: 10.1111/j.1537-2995.2005.04390.x. [DOI] [PubMed] [Google Scholar]
- Grebely J, Genoway KA, Raffa JD, Dhadwal G, Rajan T, Showler G, Kalousek K, Duncan F, Tyndall MW, Fraser C, Conway B, Fischer B. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93:141–147. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Grebely J, Raffa JD, Lai C, Krajden M, Kerr T, Fischer B, Tyndall MW. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16:352–358. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- Groom H, Dieperink E, Nelson DB, Garrard J, Johnson JR, Ewing SL, Stockley H, Durfee J, Jonk Y, Willenbring ML, Ho SB. Outcomes of a Hepatitis C screening program at a large urban VA medical center. J Clin Gastroenterol. 2008;42:97–106. doi: 10.1097/MCG.0b013e31802dc56f. [DOI] [PubMed] [Google Scholar]
- Hall CS, Charlebois ED, Hahn JA, Moss AR, Bangsberg DR. Hepatitis C virus infection in San Francisco’s HIV-infected urban poor. J Gen Intern Med. 2004;19:357–365. doi: 10.1111/j.1525-1497.2004.30613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallinan R, Byrne A, Agho K, Dore GJ. Referral for chronic hepatitis C treatment from a drug dependency treatment setting. Drug Alcohol Depend. 2007;88:49–53. doi: 10.1016/j.drugalcdep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Hofer H, Watkins-Riedel T, Janata O, Penner E, Holzmann H, Steindl-Munda P, Gangl A, Ferenci P. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology. 2003;37:60–64. doi: 10.1053/jhep.2003.50019. [DOI] [PubMed] [Google Scholar]
- Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, Pastore G, Dietrich M, Trautwein C, Manns MP. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–1457. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- Jauncey M, Micallef JM, Gilmour S, Amin J, White PA, Rawlinson W, Kaldor JM, van Beek I, Dore GJ. Clearance of hepatitis C virus after newly acquired infection in injection drug users. J Infect Dis. 2004;190:1270–1274. doi: 10.1086/423943. [DOI] [PubMed] [Google Scholar]
- Jowett SL, Agarwal K, Smith BC, Craig W, Hewett M, Bassendine DR, Gilvarry E, Burt AD, Bassendine MF. Managing chronic hepatitis C acquired through intravenous drug use. Qjm. 2001;94:153–158. doi: 10.1093/qjmed/94.3.153. [DOI] [PubMed] [Google Scholar]
- Kamal SM, Moustafa KN, Chen J, Fehr J, Abdel Moneim A, Khalifa KE, El Gohary LA, Ramy AH, Madwar MA, Rasenack J, Afdhal NH. Duration of peginterferon therapy in acute hepatitis C: a randomized trial. Hepatology. 2006;43:923–931. doi: 10.1002/hep.21197. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Genberg BL, Astemborski J, Kavasery R, Kirk GD, Vlahov D, Strathdee SA, Thomas DL. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, Moore RD, Thomas DL, Sulkowski MS. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. Aids. 2006;20:2361–2369. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCHECR. Annual Surveillance Report 2008. National Centre in HIV Epidemiology and Clinical Research; Sydney: 2008. HIV/AIDS, Viral Hepatitis and Sexually Transmissible Infections in Australia. [Google Scholar]
- Razali K, Thein HH, Bell J, Cooper-Stanbury M, Dolan K, Dore G, George J, Kaldor J, Karvelas M, Li J, Maher L, McGregor S, Hellard M, Poeder F, Quaine J, Stewart K, Tyrrell H, Weltman M, Westcott O, Wodak A, Law M. Modelling the hepatitis C virus epidemic in Australia. Drug Alcohol Depend. 2007;91:228–235. doi: 10.1016/j.drugalcdep.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Restrepo A, Johnson TC, Widjaja D, Yarmus L, Meyer K, Clain DJ, Bodenheimer HC, Jr, Min AD. The rate of treatment of chronic hepatitis C in patients co-infected with HIV in an urban medical centre. J Viral Hepat. 2005;12:86–90. doi: 10.1111/j.1365-2893.2005.00548.x. [DOI] [PubMed] [Google Scholar]
- Rocca LG, Yawn BP, Wollan P, Kim WR. Management of patients with hepatitis C in a community population: diagnosis, discussions, and decisions to treat. Ann Fam Med. 2004;2:116–124. doi: 10.1370/afm.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santantonio T, Fasano M, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, Francavilla R, Pastore G. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol. 2005;42:329–333. doi: 10.1016/j.jhep.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Seal KH, Currie SL, Shen H, Anand BS, Bini EJ, Brau N, Jeffers L, Wright TL. Hepatitis C treatment candidacy and outcomes among 4318 US veterans with chronic hepatitis C virus infection: does a history of injection drug use matter? J Clin Gastroenterol. 2007;41:199–205. doi: 10.1097/01.mcg.0000212644.82853.51. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: knowledge, perceived risk and willingness to receive treatment. Drug Alcohol Depend. 2001;61:211–215. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- Stoove MA, Gifford SM, Dore GJ. The impact of injecting drug use status on hepatitis C-related referral and treatment. Drug and alcohol dependence. 2005;77:81–86. doi: 10.1016/j.drugalcdep.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Latka M, Campbell J, O’Driscoll PT, Golub ET, Kapadia F, Pollini RA, Garfein RS, Thomas DL, Hagan H. Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis. 2005;40(Suppl 5):S304–312. doi: 10.1086/427445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, White MC, Kushel MB, Song YS, Tulsky JP. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J Subst Abuse Treat. 2005;28:181–187. doi: 10.1016/j.jsat.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Walsh N, Lim M, Hellard M. Using a surveillance system to identify and treat newly acquired hepatitis C infection. Journal of gastroenterology and hepatology. 2008;23:1891–1894. doi: 10.1111/j.1440-1746.2008.05508.x. [DOI] [PubMed] [Google Scholar]
- Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, Kauffmann W, Kallinowski B, Cornberg M, Jaeckel E, Wedemeyer H, Manns MP. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43:250–256. doi: 10.1002/hep.21043. [DOI] [PubMed] [Google Scholar]