Monocytes in atherosclerosis: subsets and functions (original) (raw)
. Author manuscript; available in PMC: 2010 Feb 1.
Published in final edited form as: Nat Rev Cardiol. 2010 Jan 12;7(2):77–86. doi: 10.1038/nrcardio.2009.228
Abstract
Chronic inflammation drives atherosclerosis, the leading cause of cardiovascular disease. Over the past two decades, data have emerged showing that immune cells are involved in the pathogenesis of atherosclerotic plaques. The accumulation and continued recruitment of leukocytes are associated with the development of ‘vulnerable’ plaques. These plaques are prone to rupture, leading to thrombosis, myocardial infarction or stroke, all of which are frequent causes of death. Plaque macrophages account for the majority of leukocytes in plaques, and are believed to differentiate from monocytes recruited from circulating blood. However, monocytes represent a heterogenous circulating population of cells. Experiments are needed to address whether monocyte recruitment to plaques and effector functions, such as the formation of foam cells, the production of nitric oxide and reactive oxygen species, and proteolysis are critical for the development and rupture of plaques, and thus for the pathophysiology of atherosclerosis, as well as elucidate the precise mechanisms involved.
Introduction
Atherosclerosis is a multifaceted, progressive, inflammatory disease that affects mainly large and mediumsized arteries. It is characterized by the formation and build-up of atherosclerotic plaques that consist of a well-defined structure of lipids, necrotic cores, calcified regions, inflamed smooth muscle cells, endothelial cells, immune cells and foam cells; consequently, atherosclerosis is associated with cardiovascular disease.1 In the 1970s, details of a comprehensive scheme of ‘response-to-injury’ began to emerge,1 in which endothelial activation is initiated by various triggers, such as altered blood rheology, modified LDL, increased homocysteine levels, and inflammation induced by bacterial antigens and membrane components. The presence of leukocytes within atherosclerotic arteries was characterized in the early 1980s.2 Initially, macrophages were reported as the predominant cell type present within atherosclerotic vessels, but T cells, B cells, neutrophils, mast cells, dendritic cells (DCs) and monocytes have all been observed in both mouse and human aortas.3-5
Transgenic mouse models have allowed researchers to directly investigate the molecular mechanisms that underlie the development of atherosclerosis.6 The roles of cell adhesion molecules, chemokines, cellular mediators and signaling molecules involved in lymphocyte and monocyte/macrophage activation, and the progression of atherosclerosis, have been described elsewhere.3-7 This Review focuses on monocyte biology in relation to atherosclerosis, and specifically discusses the recruitment of blood monocytes to plaques and their relationship with plaque macrophages. Whether and how distinct functional subsets of monocyte might have different roles in the pathogenesis of atherosclerosis is also discussed.
Monocytes and plaque growth
During homeostasis and inflammation
Monocytes circulate in the blood, bone marrow and spleen of mice in homeostasis.8,9 These cells are short-lived, and do not proliferate in the blood;10 their functions under homeostatic conditions remain unresolved. They might be involved in scavenging dead cells and toxic molecules,8 and/or have a potential role in the renewal of ‘resident’ tissue macrophages and DCs.11
During inflammation, blood monocytes migrate from blood to lymphoid and nonlymphoid tissues in response to tissue-derived signals caused, for example, by infection or tissue damage (Figure 1).11 They phagocytose other cells and toxic molecules (such as oxidized LDL [oxLDL]), produce inflammatory cytokines, and can differentiate into inflammatory DCs, macrophages or foam cells.12,13
Figure 1.
Inflammation and the role of blood monocytes. Two distinct subsets are evident in the blood circulation of mice. Gr1+/Ly6Chigh monocytes that can extravasate into tissue, differentiate into Tip-DC, M1-type or classically activated macrophages, and phagocytose pathogens, produce antibacterial products, mediate inflammation and proteolysis. Gr1−/Ly6Clow monocytes can crawl or patrol the vasculature under the steady state, but in response to inflammation, extravasate into tissue, differentiate into M2-type or alternatively activated macrophages and phagoctose pathogens and are involved in wound repair, tissue remodeling and expression of chemokines. Whether Gr1− or Gr1+ differentiated monocytes mediate foam cell formation in response to lipids requires further investigation. Abbreviations: CXCL, chemokine (C-X-C motif) ligand; iNOS, inducible nitric oxide synthase; ROS, reactive oxygen species; Tip-DC, TNF and iNOS producing dendritic cells; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
During atherosclerosis
During the pathogenesis of atherosclerosis, blood monocytes are recruited into intima and subintima (Figure 2a).14,15 Through their scavenger receptors, these cells can take up oxLDL and other lipids;16 consequently, it is thought that, when they encounter fatty deposits, they are induced to undergo activation and accumulate in the forming lesion. At an early stage of the process, monocytes differentiate into foam cells to form early plaques, termed fatty streaks, in the intima.17 Interestingly, this process occurs in atherosclerosis-prone areas of the arterial tree, such as artery branches, and is very likely to involve cellular responses to changes in fluid dynamics (discussed below).18
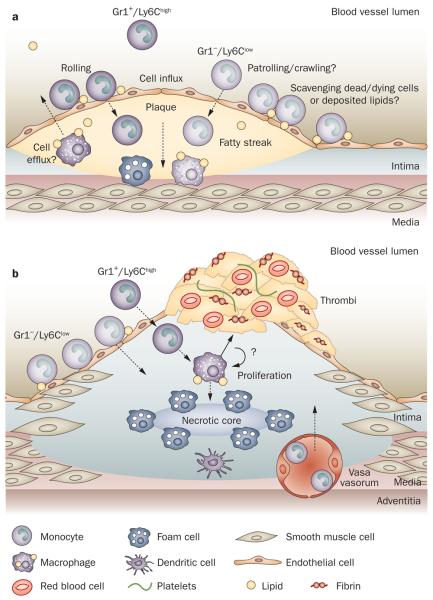
Figure 2.
Blood monocytes in atherosclerosis. a | Atherogenesis and the formation of fatty streaks. Monocytes are recruited (influx) into the intima and may differentiate into macrophages that accumulate lipids and cholesterol derivatives, form foam cells in the subintima, and make up fatty streaks. However, little is known as to which monocyte subset differentiates into a specific macrophage phenotype mediating foam cell formation. Monocytes (mainly Gr1+/Ly-6Chigh) may also give rise to dendritic cells in the plaque.47 The role of Gr1−/Ly-6Clow monocytes in this process has not been described. Gr1−/Ly-6Clow monocytes may be involved in scavenging the endothelium of lipid derivatives, dead and/or dying cells. There is evidence to suggest that interplaque cells may efflux back into the luminal blood flow. b | Maturation into atherosclerotic plaques and rupture. Continued monocyte influx is reportedly due to Gr1+/Ly-6Chigh monocytes.30 Macrophages and dendritic cells accumulate. Whether they differentiate into a specific phenotype from recruited monocytes and/or proliferate from local precursors is unknown. The role and influx of Gr1−/Ly-6Clow have not been characterized. Vasa vasorum, and neovascularization within the plaque lead to increased trafficking of leukocytes. Deposition of matrix components and recruitment of smooth muscle cells give rise to the fibroproliferative progression of the plaques. Apoptosis of macrophages and/or foam cells creates a necrotic core. Thinning and erosion of the fibrous cap in unstable plaques, through matrix degradation by proteases, ultimately results in plaque rupture and thrombosis of the artery. Egress of these monocyte/macrophages or dendritic cells from plaques has been described.28
Key points.
- ■ Atherosclerosis has long been associated with chronic inflammation
- ■ The accumulation of macrophages correlates with atherosclerotic plaque progression and plaque rupture
- ■ Monocytes accumulate at sites of inflammation and have been reported to enter atherosclerotic plaques
- ■ Macrophages and foam cell are present in plaques and might derive from monocytes or from resident macrophages
- ■ Monocytes represent a heterogeneous population of cells with differences in phenotype, function and locomotion
The initial vascular fatty deposits mature into atherosclerotic plaques following the accumulation of inflammatory cells and extracellular lipids to form a core region, which is surrounded by a cap of smooth muscle cells and a layer of collagen-rich matrix (Figure 2b). The secretion of cytokines and growth factors by plaque cells and the further deposition of extracellular matrix components contribute to the progression of plaques and, thus, the thickening of the vessel wall and the progressive stenosis of the artery. The central core of the mature plaque can become necrotic. This triggers neovascularization through the release of proangiogenic factors from inflammatory cells or by a tissue hypoxia stimulus—the exact mechanism remains to be defined.19 This neovascularization can further contribute to cellular trafficking and recruitment through the vaso vasorum.20,21
Over time, the secretion of matrix-degrading proteases and cytokines by macrophages can cause the fibrous cap that prevents contact between the blood and prothrombotic material in the plaque to thin.22 Ultimately, the cap can disintegrate and the ensuing discharge or plaque debris can release tissue factor into the blood, triggering the coagulation cascade and thrombosis.23 Thrombosis can result in acute stenosis of the artery, such as occurs in coronary syndromes, myocardial infarction and stroke, which are leading causes of death in the western world.24 Unsurprisingly, therefore, vulnerable plaques have received a great deal of attention. The generation of this specific rupture-prone plaque phenotype has been associated with increased content of inflammatory cells.25
Although monocytes are recruited into the plaque during its growth, they might also emigrate from the plaque, at least under some experimental conditions that are associated with a decrease in plaque size and, consequently, regression of atherosclerosis.26,27 Monocytes might enter lymphatic vessels and then travel to the draining lymph node, or they might migrate across the arterial endothelium towards the artery lumen to directly enter the circulating bloodstream.28
Monocytes and other leukocytes have been shown to be important in atherogenesis and atherothrombosis,5 but many basic questions still remain unanswered: Are all monocytes recruited, or do subsets with specific functions exist, and does recruitment depend on the stage of the lesions? Are distinct functions mediated by the same cells, depending on their differentiation or activation stage (plasticity), or by distinct subsets of cells (heterogeneity); in other words, are there good and bad monocytes? Do monocytes proliferate or survive once inside the plaque or do they need to be continuously replaced with blood cells? What are the mechanisms that control monocyte traffic into and out of the plaques?
Many studies have used histology to identify the cellular components of plaques,29 but adoptive transfer and fate-mapping strategies to study leukocyte recruitment and egress from plaques,30 and laser micro dissection to directly investigate the functions of plaque macro phages,31 are becoming more popular. However, the relationship between blood monocytes and plaque macrophages is an outstanding and important issue that is difficult to address, mainly owing to technical limitations.
Monocyte plasticity and heterogeneity
Monocyte migration to tissues and differentiation into various cell types, such as inflammatory DCs or macrophages, seems to be determined in part by the inflammatory milieu, which underlies the plasticity of this cell type. Cytokines are important in this phenomenon and exposure in vitro to cytokines, such as granulocyte macrophage colony-stimulating factor or macrophage colony-stimulating factor, induces monocytes to differentiate into DCs and macrophages, respectively.10 The current concept is that monocytes are phenotypically polarized by their microenvironment to undergo specific functional programs. In addition, it has become clear that monocytes are a relatively heterogeneous population, and subsets might exert different functions.10,32-34
Monocyte subsets with distinct functions can be distinguished in the circulatory system of mice by microscopy and flow cytometry, based on the differential expression of many surface markers (Figure 3a). The biological rele vance of the existence of monocyte subsets has been unclear, as monocytes as a population are highly plastic cells, as mentioned above, that can respond rapidly to their environment, at least in vitro, by differentiating into a variety of macrophages and DC-like cells.8,35
Figure 3.
Heterogeneity of mouse monocyte subsets. a | Flow cytometry assessment of mouse whole blood shows the heterogeneity of mouse monocyte subsets. A diverse pattern of Ly6C expression can be seen on Lin− (CD3, NK1.1, CD19) CD115+ CD11b+ cells in mouse whole blood (gate 3; monocytes). These populations have been color back gated as Gr1+/Ly-6Chigh (inflammatory; red) and Gr1−/Ly-6Clow subsets (patrolling; blue). Note the close CD11b and Ly6C expression profile of both NK cells (gate 1) and neutrophils (gate 4; green), which without an appropriate gating strategy can easily be interpreted as monocytes. b | Differentiation of monocyte subsets. Monocytes differentiate from HSCs through a common proliferating MDP that gives rise to CD115+ CD11b+ Gr1+/Ly-6Chigh and CD115+ CD11b+ Gr1−/Ly-6Clow subsets in the bone marrow.8,10 These subsets then exit into the blood circulation as mature CD115+ CD11b+ monocytes. Gr1+/Ly-6Chigh population can shuttle between the bone marrow and blood circulation. The developmental relationship between Gr1+/Ly-6Chigh and Gr1−/Ly-6Clow monocytes (putative CD14+ and CD16+ homolog in humans respectively) is not yet established. Abbreviations: HSC, hematopoietic stem cell; MDP, macrophage and dendritic cell precursor; NK, natural killer.
A series of in vivo experiments in mice have pushed forward the concept that, in addition to plasticity, the heterogeneity of blood monocytes is a major determinant of their effector functions.8,36 Circulating through lymphoid and nonlymphoid organs under homeostatic conditions, Gr1+/Ly6Chigh CCR2+ CX3CR1low monocytes are frequently referred to as ‘inflammatory monocytes’ (Figure 3b) as they give rise to inflammatory macrophages and DCs in a variety of infectious models and noninfectious models. A second subset of monocytes, Gr1−/Ly6Clow CCR2− CX3CR1high, ‘patrol’ the luminal side of the endothelium of small blood vessels under homeostatic and inflammatory conditions. This phenomenon has been observed in small veins and arteries in the dermis, mesentery and brain.36,37 Gr1−/Ly6Clow monocytes crawl with an average velocity of 12 μm/min. Crawling requires firm binding to the endothelium mediated by the β2-integrin LFA1 (comprising CD11a–CD18, also known as αLβ2) and by the chemokine receptor CX3CR1 (see below). Patrolling monocytes are ideally located to survey endothelial cells and surrounding tissues. However, it is not yet known whether Gr1−/Ly6Clow monocytes patrol the endothelium of larger vessels, and whether patrolling monocytes have a role in scavenging vascular endothelium perhaps of lipids, dead or dying cells and/or immune complexes. It will be important to investigate whether Gr1−/Ly6Clow patrolling monocytes might contribute to the pathogenesis of inflammatory disorders and could represent a target for their treatment.
Taken together, the results from a series of experiments (reviewed elsewhere38) support a model in which—in addition to monocyte plasticity controlled by the external milieu, including cytokines—an intrinsic dichotomy in the differentiation potential of the two main blood monocyte subsets might have a crucial role in the setting, and potentially the resolution, of inflammation.
Heterogeneity in atherosclerosis
So monocyte/macrophage heterogeneity exists, but which subset(s) are present in the plaque and responsible for atherosclerosis?
Monocytes and macrophages were the first inflammatory cells to be associated with atherosclerosis. Gerrity et al.2 identified monocytes as the main cellular component of atherosclerotic plaques in porcine specimens. Monocytes are already present in fatty streaks, the earliest visible lesion in human and experimental sclerosis.39 Depletion of monocytes from the circulation using clodronate (dichloromethylene bisphosphonate) was associated with considerably reduced plaque formation in rabbits, suggesting the importance of monocytes in atherosclerosis.40 Stoneman et al.41 showed that CD11b+ cells are critical to atherogenesis, but that, once formed, the plaque mass was not reduced by depleting CD11b+ cells. As CD11b is broadly expressed, and because clodronate also kills several macrophages subsets, these experiments do not formally prove the role(s) of monocytes in atherosclerosis. In addition, if different monocyte subsets have opposing functions, depleting all monocytes will not be sufficient to understand their roles.
Apolipoprotein E−/− mice lack the glycoprotein apolipoprotein E (apoE), which is essential for the transport and metabolism of lipids.42 The mice, which are healthy when born, provide a model for studying atherosclerosis, as they have a markedly altered plasma lipid profile compared with normal mice, and develop leukocytosis, including monocytosis, and athero sclerotic lesions when placed on a chow or high-fat diet.43-45 In these mice, Gr1+/Ly6Chi monocytosis occurs; the Gr1+/Ly6Chi monocytes were observed to adhere to activated endothelium, infiltrate lesions and become athero-sclerotic macro phages.46 Little, however, is known about how the lack of apoE affects the Gr1−/Ly6Clow population, with the numbers remaining unaffected in some studies46,47 and increasing in others.43 Of note, the cellular origin of foam cells has not been described and these cells may arise from either differentiated Gr1+/Ly6Chi or Gr1−/Ly6Clow cells.
Monocyte recruitment
Although a cholesterol-rich diet promotes murine atherogenesis at least in part through the development of monocytosis, the role of each monocyte subset in recruitment at early stages of atherogenesis and the development of the vulnerable plaque is yet to be established. Recruitment of leukocytes to sites of inflammation requires a well-orchestrated arrangement of selectins, cell adhesion molecules and chemokines (reviewed elsewhere15,48,49). However, little has been described about the mechanisms of recruitment of the Gr1−/Ly6Clow monocyte population, compared with the putative mechanisms of the Gr1+/Ly6Chigh cells (Figure 4).
Figure 4.
Monocyte recruitment. a | Gr1+/Ly6Chigh monocytes tether and roll on the endothelium through the classic adhesion cascade, involving endothelial AMs and their ligands expressed by monocytes (examples provided). After activation they can extravasate into the tissue, through slow rolling, chemokine activation (firm adhesion) and emigration. The kinetics of this movement is rapid (μm/min). b | Little is known about Gr1−/Ly6Clow monocytes. Crawling (patrolling) occurs in a random behavior and involves LFA1, CX3CR1 and the chemokine fractalkine; however, in response to inflammation, these cells can also extravasate through uncharacterized mechanisms. The kinetics of crawling in these cells is relatively slow (mm/h). Note the difference in scale for rolling versus crawling on the endothelium, describing increased distance of slow crawling (patrolling). Abbreviations: AMs, adhesion molecules; CX3CR1, CX3C chemokine receptor 1; ICAM, intercellular adhesion molecule; JAMs, junctional adhesion molecules; LFA1, Leukocyte function-associated molecule 1 (also known as β2-integrin); PECAM-1, platelet endothelial cell adhesion molecule 1; PSGL-1, P-selectin glycoprotein ligand-1; VCAM, vascular cell-adhesion molecule; VLA4, very late antigen-4 (also known as α4-integrin).
Chemokine receptors
Chemokines and their receptors have been shown to be important for leukocyte trafficking and migration related to atherosclerosis.50,51 The chemokine receptors CX3CR1, CCR2 and CCR5 are involved in Gr1+/Ly6Chigh monocyte recruitment in atherosclerosis. Both monocytosis and atherosclerosis are dramatically reduced in CX3CL1−/−CCR2−/−ApoE−/− or CX3CR1−/−CCL2−/−ApoE−/− triple knockout mice compared with controls and single knockout mice.43,52,53 However Gr1−/Ly6Clow monocyte recruitment may also be important, as CCR5 was also shown to be critical for the migration of Gr1−/Ly6Clow monocytes to aortas in hypercholesterolemic mice and the number of circulating Gr1−/Ly6Clow cells correlated with lesion size.43,47 CX3CR1 has also been shown to be important for adhesion to the endothelium, and survival, of murine Gr1−/Ly6Clow monocytes and human CD16+ monocytes.36,54,55 Chemokine receptors, in particular CCR2, are also important for the release of monocytes from the bone marrow;56 CCR2−/− mice showed a strong reduction in overall blood monocyte counts, as well as a recruitment defect.52,56
Selectins
The selectin family of adhesion molecule has been shown to mediate atherosclerosis.57 L-selectin expressed by leukocytes (such as T cells, natural killer cells and Gr1+/Ly6Chigh monocytes) is important for mediating leuko cyte rolling on the endothelium, in particular towards high endothelial venules in lymph nodes.58 Notably, Gr1−/Ly6Clow monocytes do not express L-selectin, and are not recruited to lymph nodes.32 Specific roles have been identified for P-selectin and E-selectin, which are expressed by platelets and endothelial cells in atherosclerosis. Endothelial P-selectin is rapidly upregulated in mice on an atherosclerotic diet.59 P-selectin interacts with its ligand P-selectin glycoprotein ligand-1 (PSGL-1), which is expressed by monocytes, neutrophils and lymphocytes.57 Mice deficient in P-selectin exhibit lower macrophage numbers in plaques and develop smaller fatty streaks than wild-type mice.60
E-selectin also interacts with PSGL-1 and with CD44 in mice,61 and mice lacking E-selectin show smaller plaques.62 However, a combined deficiency of E-selectin and P-selectin significantly inhibits atherosclerosis in mice, showing 80% and 40% protection in the early and late stages of disease, respectively.63 The notion that a straightforward P-selectin–PSGL-1 interaction mediates recruitment is, however, complicated by the presence of circulating soluble form of P-selectin (sP-selectin). sP-selectin dimers arise from alternatively spliced or cleaved P-selectin, and lack the cytoplasmic and trans-membrane domains; these forms have been shown to be proinflammatory, through a mechanism involving integrin activation and increased leukocyte adhesion to the endothelium.64-66 However, although raised levels of plasma sP-selectin are evident in vascular disease patients and have been shown to confer increased risk for a procoagulant phenotype in stroke models,66,67 the exact mechanism of the proinflammatory effects of sP-selectin and its role in atherosclerosis or vulnerable plaque progression remain to be established.
An et al.68 have described that Gr1+/Ly6Chigh monocytes preferentially accumulate at sites of endothelial activation and thrombosis. The authors reported that PSGL-1 expression correlates with Ly6C expression, so P-selectin (or E-selectin) might preferentially recruit this subset of inflammatory monocytes to sites of endothelial dysfunction or inflammation associated with atherothrombosis.
Integrins
Monocytic integrins and the endothelial cell family of adhesion molecules are critically involved with firm adhesion.15 VLA4 (α4-integrin) and LFA1 (β2-integrin) bind to endothelial vascular cell-adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 respectively. Both of these integrins are abundantly expressed on circulating monocytes,69 but Gr1−/Ly6Clow monocytes express higher levels of LFA1 than do Gr1+/Ly6Chigh monocytes.10 The expression of CD11c (also known as αx-integrin) is not detectable at the cell surface on mouse monocytes, in contrast to human monocytes.10 Notably, LFA1 is crucial for mediating crawling of Gr1−/Ly6Clow monocytes on the endothelium.36
Evidence exists of an involvement of these integrins and adhesion molecules in atherosclerosis. ICAM-1 and VCAM-1 are upregulated at sites of disturbed oscillatory blood flow.70 Moreover, the levels of both adhesion molecules are increased by proinflammatory cytokines, as well as by oxLDL.70 Pretreatment of atherosclerotic mice with antibodies against ICAM-1 reduced short-term macrophage infiltration into atherosclerotic lesions.71 The absence of ICAM-1 or CD18 (or both) resulted in a partial reduction in the aortic lesion size.72 Similarly, blockade of the VLA4–VCAM-1 interaction significantly lowered the recruitment of monocytes to the atherosclerotic plaque, as well as lesion size.73 Overall, these studies suggest that ICAM-1 or VCAM-1, in conjunction with their corresponding ligands, participate in the regulation of monocyte recruitment to plaques.
Biorheology and arterial recruitment
Atherosclerosis occurs within arterial vasculatures predominantly at sites of disturbed flow or localized changes in blood rheology, such as at the site of vessel bifurcation.74 Much conjecture exists about how monocytes are recruited specifically to these sites. Leukocyte–endothelial cell interactions in postcapillary venules are induced by a wide range of stimuli,15 but leukocyte interactions with the arterial endothelium are only thought to be induced by certain proatherosclerotic stimuli, such as the cytokines interleukin (IL)-1β and tumor necrosis factor (TNF), cigarette smoke, oxLDL or angiotensin II.75,76
Leukocyte recruitment to the venule microvasculature is optimized through a low shear environment;77 however, arterial walls can be subjected to almost ~100-fold greater shear stress. In arterial atherosclerosis, perhaps turbulent or oscillatory flow can induce significant decreases in shear that might facilitate monocyte recruitment; it is well documented that plaques develop at sites of low shear, although these arterial shear sizes would still be larger than venous wall shear sizes. Moreover, monocytes continue to be recruited to plaques despite high shear environments caused by luminal narrowing arising from plaque growth. Mechanisms for arterial monocyte tether ing under high shear environments must, therefore, exist. Activated endothelial cells might express ultra-large von Willebrand factor (ULVWF), which forms extremely long string-like structures to which platelets can tether.78 Formation of these strings has been reported to arise from impaired cleavage of ULVWF multimers by a disintegrin-like and metalloprotease with thrombospondin type I repeats-13 (ADAMTS13).78 Leukocytes can tether and roll on these ULVWF strings in high shear environ ments of up to 40 dyn/cm2.79 These specific stimuli and/or mechanisms, along with endothelial activation, might initiate atherogenesis; however, the mechanism(s) through which these signals are transduced to activate specific leukocyte subsets and affect their corresponding function remain to be elucidated.
As mentioned above, the Gr1−/Ly6Clow monocyte subset in the mouse can patrol, by long-range crawling, both within the venous microcirculation and arterioles in the mesenteric circulation.36 Interestingly, there seem to be no obvious differences in the main characteristics of patrolling in these vessels. By contrast, but not unexpectedly, rolling of ‘inflammatory’ monocytes (Gr1+/Ly6Chigh) under a steady state was not observed in arterioles, which, as discussed above, are thought to only tether and roll within low venous shear environments.
The patrolling paths of Gr1−/Ly6Clow monocytes in arteri oles can be described as loops, hairpins, waves and mixed patterns, which, as in venules seemed to be independent of the direction of blood flow.36 The Gr1−/Ly6Clow patrolling subset of monocytes might, therefore, be involved in the early stages of arterial atherogenesis, through mechanisms that have yet to be explored but that would most likely include CX3CR1 and β2-integrin. It remains unknown whether this subset patrols the entire vascular tree, and whether human monocytes patrol human vessels. Of note, human CX3CR1high (CD16+) monocytes were shown to bind to endothelial cells in vitro with increased avidity than CD14+ monocytes, in part through CX3CR1-dependent adhesion.54
Some evidence suggests that granulocytes, such as neutrophils, can contribute to monocyte recruitment. Pathologies associated with neutrophil defects have been proposed to be responsible for impaired monocyte chemotaxis and lower numbers of monocytes and macrophages in humans.80 However, more data are needed to confirm and validate these observations.
Scavenger receptors
Macrophages are equipped with a wide array of pattern recognition receptors, including scavenger receptors, which connect innate and adaptive immune responses during atherosclerosis. These pattern recognition receptors allow for the uptake of modified LDLs and contribute to the formation of foam cells.16 The data imply the existence of several macrophage phenotypes with distinct patterns of expression of pattern recognition receptors and metalloproteinase, which contribute to mechanisms of plaque rupture.22,81 Among the array of macrophage scavenger receptors, scavenger receptor AI (SR-AI) and CD36 (a class B scavenger receptor) are regarded as the most important in the uptake of oxLDL.82 Early studies showed that scavenger receptors are proatherogenic, on the basis that CD36−/−apoE−/− mice were protected from atherosclerosis.83 Moreover, apoE−/− mice with a combined deficiency of CD36 and SR-AI/II showed no further reduction of atherosclerosis compared with CD36−/−apoE−/− mice, providing evidence for the importance of CD36 in disease.84 In another report, however, apoE−/− mice lacking SR-A and CD36 showed an increase in aortic sinus lesions with an abundance of foam cells, which potentially indicates an alternative pathway for lipid uptake in atherogenesis.85
The relationship between scavenger receptors and monocyte or macrophage function might be further complicated by reports describing that CD36 signaling in response to oxLDL enhances macrophage spreading, inhibiting their migration and ‘trapping’ these cells in the arterial intima.86 A great deal more work is needed to elucidate the function of scavenger receptors in monocyte and macrophage subsets, in relation to their role in atherogenesis.
Neovascularization
The obvious path for continued recruitment of leuko cytes to the growing plaque is through the lumen. However, alternative mechanisms of recruitment exist, including trafficking through the vasa vasorum and neovessels that form within the plaque.87 Studies have demonstrated that neovascularisation of vasa vasorum are associated with advanced plaques in ApoE−/− mice and humans. Compounds that inhibit angiogenesis, such as TNP-470 or endostatin, can significantly reduce both plaque neovascularization and growth.88 Conversely, stimu lators of angiogenesis, such as vascular endo thelial cell growth factor or nicotine, have been shown to enhance lesion progression.89 Although these positive and negative regulators of endothelial cell function might alter plaque growth through several mechanisms, the results support the hypothesis that increased angiogenesis promotes the progression of atherosclerosis. Vasa vasorum might facilitate leukocyte entry into lesions, perfuse the vessel wall beyond the diffusion limits from the artery lumen, or cause intraplaque hemorrhage.87 How the plaque vasa vasorum contributes to monocyte trafficking remains to be fully described, and whether resident monocytes patrol the vasa vasorum also requires investigation.
Plaque survival
The continual recruitment of cells to the plaque is believed to be important for its growth. Increased cellu larity can also be obtained through proliferation of resident or differentiated macrophages, and studies using labeled monocytes or radioactive tracers have shown that the monocyte content in the aorta correlates with the surface area of the plaque.90 Monocytes might accumulate continuously during plaque formation; accumulation is proportional to atherosclerotic mass, and survival is dependent on CX3CR1 expression.55,90 However, little is known about intraplaque cell proliferation, whether recruited plaque monocytes differentiate into macrophages or foam cells in vivo, or whether resident aortic macrophages contribute to the development and, ultimately, continuation of the vulnerable plaque phenotype.
There is some evidence to indicate that an increase in apoptosis in early lesions seems to attenuate atherosclerosis; by contrast, impairment of apoptotic cell clearance in the late stage of plaque formation might contribute to secondary necrosis, leading to increased proinflammatory responses and further apoptotic signals for endo thelial cells, smooth muscle cells and leukocytes within atherosclerotic plaques.91
Human monocytes
Whereas some of the studies described above have investigated monocyte subsets in experimental studies in mice, atherogenesis in human is less well understood, mainly because of restrictions on experimentation in humans. In addition, translational studies have fallen behind because of the limited knowledge of human monocyte subsets, making it difficult to interpret results from mouse models.
However, human peripheral blood monocytes do show heterogeneity in terms of size, granularity, nuclear morphology and phenotype, similar to the existence of subsets in mice.38 Human monocytes were initially identified by their expression of large amounts of CD14 (which contributes to the lipopolysaccharide receptor). Subsequent identification of differentially expressed antigenic markers further identified CD16 (also known as FcyRIII-CD32). Two major subsets can therefore be defined: CD14hiCD16lo monocytes typically represent ~85–95% of the monocytes in healthy individuals; CD14loCD16hi monocytes comprise the remainder.92 These subsets, like those in mice, differ in many respects, including their expression of adhesion molecules and chemokine receptors.8,93,94 CD14hiCD16lo monocytes express CCR2, CD62L and CD64, and have consequently been associated with the inflammatory subset in the mouse. Conversely, CD14loCD16hi monocytes lack CCR2 and have higher levels of major histo-compatibility complex II and CD32. Both subsets express the fractalkine receptor, CX3CR1, but CD14loCD16hi monocytes express higher levels and, as such, have been associated with the resident subset in the mouse. It is, however, important to note that there is considerable hetero geneity in the CD16+ population.8
The role of each subset in human atherosclerosis remains unknown, although there are some clues. In vivo, human coronary artery lesions contain macro phage sub-populations with different gene-expression patterns,95 which points to the existence of hetero geneity. Patients with coronary artery disease have higher numbers of CD14+CD16+ monocytes than healthy cohorts.96 Furthermore, CD14+CD16+ counts correlate negatively with the concentration of HDL and positively with levels of atherogenic lipids.97 Peak levels of CD14hiCD16lo monocytes after acute myocardial infarction were found to correlate negatively with the recovery of left ventricular function.98 Finally, functional differences between subsets, for example in phagocytic activity, have been described and can be used to develop noninvasive imaging technologies, including MRI, to follow and quantify sub populations of monocytes in patients.99 These technologies might be useful for exploring the kinetics of monocyte recruitment into tissues, and might represent a prognostic tool.
Conclusions
Our understanding of the role of monocytes in atherosclerosis has progressed remarkably over past decades. We know that these cells are important for all stages of atherosclerosis, but the heterogeneous nature of blood monocytes has meant that there are many unresolved issues—most importantly, the role of each subset in various stages of disease, the mechanisms of their recruitment and migration, their expression profiles and their interaction with other immune cells within the plaque.
To understand the circuits that transform extra cellular signals into cellular responses such as monocyte recruitment, activation and differentiation in the atherosclerotic plaque, it will be necessary to investigate signal transduction, metabolic and transcriptional pathways at the cellular level, or at least the level of cellular subsets. Such a strategy will benefit from the fast-moving develop ment of molecular imaging, multiplex analysis and fate- mapping strategies.
The development of atherosclerotic models using reporter mice in which monocyte subsets can be readily detected, together with high-resolution imaging techniques (such as intravital or noninvasive imaging), is needed to investigate the role of individual monocyte subsets in recruitment, proliferation and survival, at the various stages of disease, from early atherogenesis to the generation of a vulnerable plaque through to plaque rupture and thrombosis. Indeed these studies will hopefully be translatable to human biology and perhaps generate novel therapeutic agents for the clinic.
Review criteria.
We searched for articles in PubMed and MEDLINE using the following keywords: “atherosclerosis and monocytes”, “monocyte heterogeneity”, “atherosclerosis and cell trafficking” and “monocyte recruitment”. Articles published in English-language journals were selected, but were not restricted by publication date, length or journal type.
Acknowledgments
K. J. Woollard is funded by a British Heart Foundation fellowship. Work in F. Geissmann's lab is supported by the Arthritis Research Campaign and the Medical Research Council (G0900867 Strategic Award).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Ross R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Gerrity RG, Naito HK, Richardson M, Schwartz CJ. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am. J. Pathol. 1979;95:775–792. [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 4.Galkina E, Ley K. Leukocyte influx in atherosclerosis. Curr. Drug Targets. 2007;8:1239–1248. doi: 10.2174/138945007783220650. [DOI] [PubMed] [Google Scholar]
- 5.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 6.Heeneman S, Lutgens E, Schapira KB, Daemen MJ, Biessen EA. Control of atherosclerotic plaque vulnerability: insights from transgenic mice. Front. Biosci. 2008;13:6289–6313. doi: 10.2741/3155. [DOI] [PubMed] [Google Scholar]
- 7.Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive T cell immunity in atherosclerosis. J. Lipid Res. 2009;50(Suppl.):S364–S369. doi: 10.1194/jlr.R800092-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 9.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 11.Geissmann F, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol. Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 12.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Varol C, Yona S, Jung S. Origins and tissue-context-dependent fates of blood monocytes. Immunol. Cell Biol. 2009;87:30–38. doi: 10.1038/icb.2008.90. [DOI] [PubMed] [Google Scholar]
- 14.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 15.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 16.Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res. 2009;50(Suppl.):S282–S286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu. Rev. Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler. Thromb. Vasc. Biol. 1999;19:870–876. doi: 10.1161/01.atv.19.4.870. [DOI] [PubMed] [Google Scholar]
- 20.Virmani R, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 21.Moulton KS, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc. Natl Acad. Sci. USA. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 23.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J. Am. Coll. Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 24.Primatesta P, et al. Cardiovascular surveys: manual of operations. Eur. J. Cardiovasc. Prev. Rehabil. 2007;14(Suppl. 3):S43–S61. doi: 10.1097/01.hjr.0000277988.18096.3b. [DOI] [PubMed] [Google Scholar]
- 25.Shah PK. Molecular mechanisms of plaque instability. Curr. Opin. Lipidol. 2007;18:492–499. doi: 10.1097/MOL.0b013e3282efa326. [DOI] [PubMed] [Google Scholar]
- 26.Llodra J, et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl Acad. Sci. USA. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feig JE, Quick JS, Fisher EA. The role of a murine transplantation model of atherosclerosis regression in drug discovery. Curr. Opin. Investig. Drugs. 2009;10:232–238. [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr. Opin. Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cybulsky MI, Won D, Haidari M. Leukocyte recruitment to atherosclerotic lesions. Can. J. Cardiol. 2004;20(Suppl. B):24B–28B. [PubMed] [Google Scholar]
- 30.Swirski FK, Weissleder R, Pittet MJ. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29:1424–1432. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trogan E, et al. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA. 2002;99:2234–2239. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palframan RT, et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu C, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J. Exp. Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 35.Taylor PR, Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19:2–4. doi: 10.1016/s1074-7613(03)00178-x. [DOI] [PubMed] [Google Scholar]
- 36.Auffray C, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 37.Audoy-Remus J, et al. Rod-shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J. Neurosci. 2008;28:10187–10199. doi: 10.1523/JNEUROSCI.3510-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 39.Napoli C, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ylitalo R, Oksala O, Yla-Herttuala S, Ylitalo P. Effects of clodronate (dichloromethylene bisphosphonate) on the development of experimental atherosclerosis in rabbits. J. Lab. Clin. Med. 1994;123:769–776. [PubMed] [Google Scholar]
- 41.Stoneman V, et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ. Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu. Rev. Nutr. 1995;15:495–518. doi: 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- 43.Combadiere C, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 44.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 45.Zernecke A, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 46.Swirski FK, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunon D, Piali L, Imhof BA. To stick or not to stick: the new leukocyte homing paradigm. Curr. Opin. Cell Biol. 1996;8:714–723. doi: 10.1016/s0955-0674(96)80114-1. [DOI] [PubMed] [Google Scholar]
- 49.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 50.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler. Thromb. Vasc. Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 51.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ. Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 52.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J. Clin. Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ancuta P, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landsman L, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 56.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 57.Woollard KJ, Chin-Dusting J. Therapeutic targeting of p-selectin in atherosclerosis. Inflamm. Allergy Drug Targets. 2007;6:69–74. doi: 10.2174/187152807780077345. [DOI] [PubMed] [Google Scholar]
- 58.Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 59.Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am. J. Pathol. 1994;144:952–961. [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson RC, et al. Absence of P-selectin delays fatty streak formation in mice. J. Clin. Invest. 1997;99:1037–1043. doi: 10.1172/JCI119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katayama Y, et al. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood. 2003;102:2060–2067. doi: 10.1182/blood-2003-04-1212. [DOI] [PubMed] [Google Scholar]
- 62.Collins RG, et al. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J. Exp. Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong ZM, et al. The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woollard KJ. Soluble bio-markers in vascular disease: much more than gauges of disease? Clin. Exp. Pharmacol. Physiol. 2005;32:233–240. doi: 10.1111/j.1440-1681.2005.04178.x. [DOI] [PubMed] [Google Scholar]
- 65.Woollard KJ, et al. Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ. Res. 2008;103:1128–1138. doi: 10.1161/CIRCRESAHA.108.180273. [DOI] [PubMed] [Google Scholar]
- 66.Woollard KJ, et al. Raised plasma soluble P-selectin in peripheral arterial occlusive disease enhances leukocyte adhesion. Circ. Res. 2006;98:149–156. doi: 10.1161/01.RES.0000199295.14073.69. [DOI] [PubMed] [Google Scholar]
- 67.Kisucka J, et al. Elevated levels of soluble P-selectin in mice alter blood-brain barrier function, exacerbate stroke, and promote atherosclerosis. Blood. 2009;113:6015–6022. doi: 10.1182/blood-2008-10-186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.An G, et al. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 70.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 71.Patel SS, Thiagarajan R, Willerson JT, Yeh ET. Inhibition of alpha4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation. 1998;97:75–81. doi: 10.1161/01.cir.97.1.75. [DOI] [PubMed] [Google Scholar]
- 72.Nageh MF, et al. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 1997;17:1517–1520. doi: 10.1161/01.atv.17.8.1517. [DOI] [PubMed] [Google Scholar]
- 73.Cybulsky MI, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chatzizisis YS, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 75.Eriksson EE, Werr J, Guo Y, Thoren P, Lindbom L. Direct observations in vivo on the role of endothelial selectins and alpha(4) integrin in cytokine-induced leukocyte- endothelium interactions in the mouse aorta. Circ. Res. 2000;86:526–533. doi: 10.1161/01.res.86.5.526. [DOI] [PubMed] [Google Scholar]
- 76.Alvarez A, et al. Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood. 2004;104:402–408. doi: 10.1182/blood-2003-08-2974. [DOI] [PubMed] [Google Scholar]
- 77.Caputo KE, Lee D, King MR, Hammer DA. Adhesive dynamics simulations of the shear threshold effect for leukocytes. Biophys. J. 2007;92:787–797. doi: 10.1529/biophysj.106.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chauhan AK, et al. ADAMTS13: a new link between thrombosis and inflammation. J. Exp. Med. 2008;205:2065–2074. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernardo A, et al. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J. Thromb. Haemost. 2005;3:562–570. doi: 10.1111/j.1538-7836.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 80.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 81.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2008;28:2108–2114. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 82.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Febbraio M, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuchibhotla S, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore KJ, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113:2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 88.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N. Engl. J. Med. 1984;310:175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 89.Celletti FL, et al. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat. Med. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 90.Swirski FK, et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl Acad. Sci. USA. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 92.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 93.Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 94.Weber C, et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J. Leukoc. Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 95.Salomon RN, Underwood R, Doyle MV, Wang A, Libby P. Increased apolipoprotein E and c-fms gene expression without elevated interleukin 1 or 6 mRNA levels indicates selective activation of macrophage functions in advanced human atheroma. Proc. Natl Acad. Sci. USA. 1992;89:2814–2818. doi: 10.1073/pnas.89.7.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schlitt A, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb. Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 97.Rothe G, et al. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 1996;16:1437–1447. doi: 10.1161/01.atv.16.12.1437. [DOI] [PubMed] [Google Scholar]
- 98.Tsujioka H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J. Am. Coll. Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 99.Wildgruber M, et al. Monocyte subset dynamics in human atherosclerosis can be profiled with magnetic nano-sensors. PLoS One. 2009;4:e5663. doi: 10.1371/journal.pone.0005663. [DOI] [PMC free article] [PubMed] [Google Scholar]