Eosinophils In Health and Disease: The LIAR Hypothesis (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 7.
Abstract
Discussions of eosinophils are often descriptions of end-stage effector cells with destructive capabilities mediated predominantly by released cytotoxic cationic granule proteins. Moreover, eosinophils in the medical literature are invariably associated with the pathologies linked with helminth infections or allergic diseases such as asthma. This has led to an almost fatalist view of eosinophil effector functions and associated therapeutic strategies targeting these cells that would make even William of Ockham proud - eosinophil effector functions have physiological consequences that increase patient morbidity/mortality and “the only good eosinophils are dead eosinophils”. Unfortunately, the strengths of dogmas are also their greatest weaknesses. Namely, while the repetitive proclamation of dogmatic concepts by authoritative sources (i.e., reviews, meeting proceedings, textbooks, etc.) builds consensus within the medical community and lower the entropies surrounding difficult issues, they often ignore not easily explained details and place diminished importance on alternative hypotheses. The goal of this perspective is two fold: (i) We will review recent observations regarding eosinophils and their activities as well as reinterpret earlier data as part of the synthesis of a new paradigm. In this paradigm, we hypothesize that eosinophils accumulate at unique sites in response to cell turnover or in response to local stem cell activity(ies). We further suggest that this accumulation is part of one or more mechanisms regulating tissue homeostasis. Specifically, instead of immune cells exclusively mediating innate host defense, we suggest that accumulating tissue eosinophils are actually regulators of **L**ocal **I**mmunity **A**nd/or **Remodeling/R**epair in both health and disease - The LIAR Hypothesis; (ii) We want to be inflammatory (pun intended!) and challenge the currently common perspective of eosinophils as destructive end-stage effector cells. Our hope is to create more questions than we answer and provoke everyone to spend countless hours simply to prove us wrong!
Keywords: Remodeling/Repair, Immune Regulation, Tissue Homeostasis, Stem Cells, Effector Function
Origins of the accepted paradigm describing eosinophil activities and why it is incomplete
One certainly has to be cautious when suggesting that a commonly accepted perspective is incomplete, especially given the dramatic changes that have occurred in the last 20–30 years regarding the accepted role(s) of the eosinophils. Specifically, in the late 1970's/early 1980's the eosinophils were perceived as suppressors of tissue inflammation by inactivating pharmacological mediators derived from mast cells (reviewed in [1]). In contrast, in the later 1980's and early 1990's studies from several investigators led to the development of the current commonly-held paradigm that eosinophils themselves mediate tissue damage and local inflammatory events through destructive effector functions (reviewed in [2]). Thus, to initiate the discussion here lets set limits right from the beginning. Eosinophil activities are likely to have both agonist and antagonist activities on a variety of tissue resident immune cells (such as mast cells). More importantly, they are also likely to include activities that now characterize eosinophils as destructive end-stage effector cells such as the release of toxic cationic granule proteins and the elaboration of reactive oxygenated species. However, we will argue that each of these perspectives is alone too narrow in scope and that the currently common, and often cited, interpretation of eosinophil destructive mechanisms is misguided and based mostly on circumstantial evidence. Moreover, an increasing number of experimental observations are inadequately explained by this end-stage effector cell paradigm. Three specific questions are immediately apparent:
- (i)
What is the origin of the current consensus opinion? Although eosinophils are now commonly characterized as end-stage effector cells with a defined array of destructive activities, there is often little direct evidence linking these activities with induced pathologies and/or patient symptoms. In particular, eosinophil activities are linked in the literature to unique disease states with characteristic pathologies (e.g., helminth infections [3–5] and allergy/asthma [6–8]) but the vast majority of these reports are correlative rather than mechanistic in character. Surprisingly, even experimental manipulations using animal models designed to demonstrate that eosinophils are a prominent innate host defense against helminths (e.g., the available eosinophil-less strains of mice [9,10]) fail to demonstrate such a role [11]. In addition, while many clinical studies have demonstrated that eosinophils accumulate and degranulate in areas of tissue damage in asthma patients [12], a direct link between eosinophils and tissue damage has remained out of reach. Indeed, even the amelioration of hallmark pathologies/symptoms in some asthma patients following the therapeutic targeting of eosinophils with Mepolizumab® [13,14], has been interpreted as simply being due to the elimination of eosinophil destructive activities. However, although the release of eosinophil cationic secondary granule proteins has been shown to mediate cell death in vitro using tissue explants and cell cultures (see for example [15]), there is virtually no in vivo data demonstrating such activities [16,17]. Collectively, these observations suggest that there is a paucity of studies in either patients or animals models to confirm and/or define the projected outcomes of the end-stage destructive effector cell paradigm. That is, destructive eosinophil activities may contribute to asthma pathology but the data (or lack thereof) do not support a prominent role. - (ii)
What experimental data/observations are contradictory to this paradigm? Several issues highlight the need to rethink the end-stage effector cell paradigm, including the evolutionary specialization of eosinophils, acquired immunity vs innate host defense capabilities of eosinophils, and the presence of eosinophils at baseline in a variety of tissues with no obvious link to innate host defense.
The Evolution of an Innate Host Defense against Parasite Infections
On first principles, concerns arise with respect to the hypothesis that eosinophils have evolved exclusively as part of an early innate host defense against parasites. First, although eosinophils are linked with parasitic infections (see for example [18]), the association is limited to specific multicellular parasites (helminths) within this larger group of organisms (reviewed in [19,20]). As a consequence, this would imply that host organisms evolved a unique hematopoietic lineage as a defense mechanism against a select few pathogens that are generally not life-threatening. Secondly, helminths (particularly nematodes) represent an evolutionarily ancient group of organisms that evolved from free-living forms to exploit available hosts. If eosinophils evolved as an early innate cellular defense against these parasites, then one would expect to find this cell type in virtually every animal lineage, crossing even the protostoma - deuterostoma divide [21]. Indeed, a granulated ameboid cell type with characteristics similar to eosinophils is identifiable in a variety of animal species including many of the more ancient groups of invertebrates (Figure 1). However, definitive eosinophilic leukocytes are absent in nearly all of these metazoans and are present only among the five classes of vertebrates in the phylum Chordata (Figure 2). As a consequence, these observations suggest that the presence of eosinophil-like cells may, in part, be linked to innate host defense activities that date back to the origins of extant metazoans (600 million years). However, clearly this date is well before the origins of each of the classes of vertebrates and the advent of definitive eosinophilic leukocytes. Thus, although definitive eosinophilic leukocytes appear to have evolved from an early multi-functional precursor cell type(s) and therefore may retain anti-helminth, and perhaps antibacterial, activities, we suggest that this characterization of eosinophil activities in extant species is largely inadequate. That is, instead of a universal/fundamental innate host defense, the evolution of the unique eosinophilic leukocyte in vertebrates (in particular mammals) has occurred, in part, because of selective pressures associated with other functions linked to LIAR that are now mediated by eosinophils. Thus, the numerous observations in mammalian models uncoupling parasite burden, inflammatory metrics, and eosinophils (reviewed in [22]) also suggest that while eosinophils may possess anti-parasite activities, these functions are contributory and not necessarily a primary innate host defense. A third issue of concern is that among mammals the rapid and profound expansion of eosinophils from available progenitors in response to helminths appears to be uniquely dependent upon a single poietic cytokine, Interleukin-5 (see for example [23]). Specifically, as opposed to complex and often overlapping pathways that invariably arise as a consequence of pathogen-driven selective pressures, no such pressures appear to exist on eosinophil proliferation and/or IL-5 activities. That is, although in rodents IL-5 also appears to have evolved B cell agonist activities [24], these additional activities appear to be a species-specific adaptation of unknown origin as human B cells do not display similar responses to IL-5 [25]. In contrast, several cytokine pathways have been linked with rapid and profound expansion of neutrophil populations and the most significant of these neutrophilopoietic cytokines, G-CSF, also has pleiotropic consequences on a variety of cells [26]. If eosinophils were (are) a prominent innate host defense against helminths across mammal species, why haven't pathogen-driven selective pressures led to alternative and/or overlapping poietic pathways that promote the rapid and profound expansion of these cells that is dependent on a cytokine(s) in addition to and/or other than IL-5? The simplest explanation is that these pressures didn't (don't) exist and eosinophils are not a host's primary defense. Nonetheless, we recognize that the conservation of an IL-5 orthologue among mammalian species and the absence of the commonality of individual patients/strains of mice with spontaneous mutations in IL-5 suggest a degree of evolutionary selective importance that does not exclude a potential role in host defense.
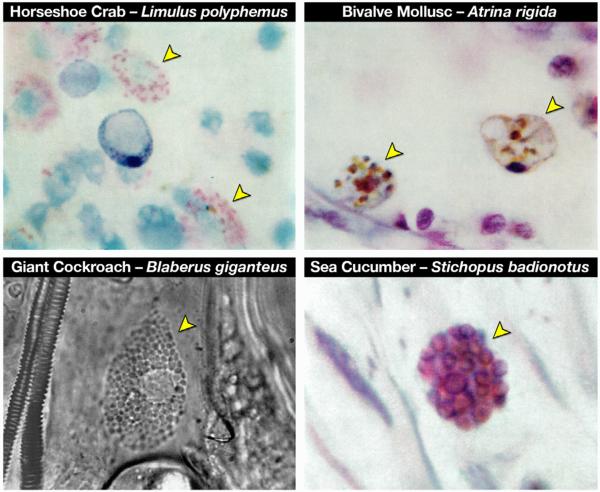
Figure 1. Photomicrographic images of “eosinophil-like” cells from invertebrates representative of three major phyla demonstrates the evolutionary conservation of this granulocyte.
Ameboid leukocytes with the distinct granulation and, in many cases, the concomitant eosin-binding characteristics (arrowheads) are found in a wide array of invertebrate species, including Arthropoda (Limulus polyphemus (horseshoe crab - Class: Crustacea, H&E) and Blaberus giganteus (giant cockroach - Class: Insecta, phase contrast microscopy)), Mollusca (Atrina rigida (clam - Class: Bivalvia, H&E), and Echinodermata (Stichopus badionotus (sea cucumber - Class: Holothuroidea, H&E). All photomicrographs were reprinted from Comparative Hematology by Warren Andrew (©1965), with permission from Elsevier)
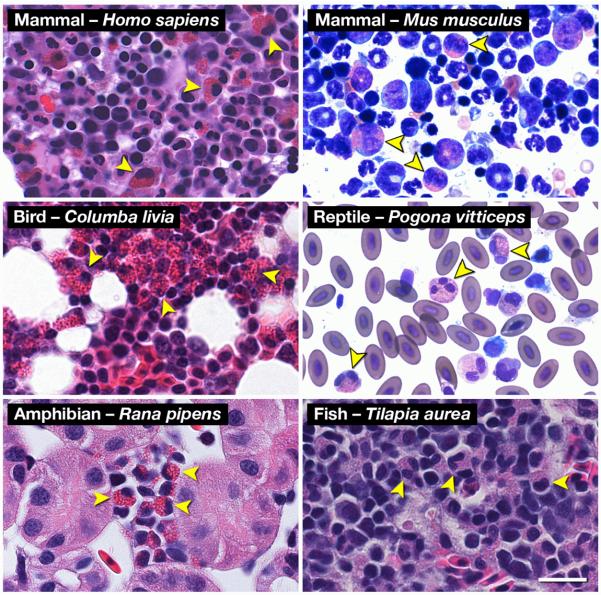
Figure 2. Hematoxylin-eosin (H&E) and Romanowsky-dye (R&D) stained preparations of hematopoietic tissues from representative animals of the five (5) classes of Vertebrata reveal the ubiquitous presence of a uniquely eosinophilic lineage in this sub-phylum.
Leukocytes displaying the unique polymorphonucleus and the eosin-binding cytoplasmic granules characteristic of eosinophils are identifiable (arrowheads) in Mammalia (Homo sapiens (human, H&E) and Mus musculus (mouse, R&D)), Aves (Columba livia (rock pigeon, H&E)), Reptilia (Pogona vitticeps (Bearded Dragon, R&D)), Amphibia (Rana pipens (leopard frog, H&E)), and Osteichthyes (Tilapia aurea (Tilapia, H&E)). Scale bar = 20μm.
Allergic Pulmonary Inflammation - Asthma
Despite nearly 100 years of intense research since clinical studies linked the pulmonary accumulation of eosinophils to the pathologies of asthma [27], a causative relationship has escaped definition. Specifically, innumerable clinical and bench-based studies either support or conflict with the hypothesis that the destructive activities mediated by eosinophils result in allergic respiratory pathologies (see for example a recent commentary written by Wenzel, S. [28]). This ambiguity alone should give one cause for concern. In addition, one might have expected that the lung pathologies alleged to be mediated by destructive end-stage effector cells would be proportional to their number infiltrating the lung. Nonetheless, even dramatic reductions in eosinophil numbers only lead to nominal improvements in patients [13,14], suggesting these results were not simply a one-for-one elimination of the destructive activities mediated by end-stage effector cells.
Resident Eosinophil Populations In Select Tissues At Homoeostatic Baseline
The presence of resident tissue eosinophils is also difficult to explain using the end-stage effector cell paradigm. In particular, why the bone marrow, gastrointestinal (GI) tract, thymus, and the endometrial lining of the uterus all maintain significant resident eosinophil populations in otherwise healthy animals/patients is difficult to explain. Most studies usually evoke arguments that suggest that these areas are either simply sites of eosinophilopoiesis (marrow and thymus) or areas that interface with the environment, representing areas in need of immunosurveillance strategies (GI tract and the endometrial lining). However, problems abound with these explanations. There are far more eosinophil lineage committed cells in the marrow relative to the periphery and the eosinophilopoietic capability of marrow is orders of magnitudes greater than that of the thymus. In addition, the energy as well as logistical difficulties associated with the proliferation, trafficking, and the maintenance of a unique cell type in outlying mucosal surfaces would appear to be extraordinarily high for only an insurance policy against a potential threat from a very limited number of non-lethal pathogens (i.e., helminths); the disconnect noted earlier between eosinophils and inflammatory metrics of parasite infection (e.g., worm burden) confirms this uncertainty. Moreover, observations linking localized tissue eosinophil accumulation with ductal differentiation of mammary glands [29] or at the lesions associated with Duchenne's Muscular Dystrophy [30] provide other enigmata given that these regions are neither sites of eosinophilopoiesis nor particularly vulnerable to parasitic infection.
- (iii)
Is there an underlying commonality between the circumstances surrounding the accumulation of eosinophils and the execution of effector functions? We propose an underlying commonality exists among disease states and, more importantly, among tissues of otherwise healthy subjects that have homeostatic baseline eosinophil populations that explain the evolutionary significance and physiological importance of eosinophils. The commonality in all of these circumstances is the co-existence of both a large pool of dying cells and a significant population of proliferating cells. Specifically, eosinophils accumulate and are restricted to defined locations in a limited number of diseases and in unique tissues of otherwise healthy subjects in response to significant cell turnover. That is, the presence of cell stress/death in a given tissue microenvironment is an initial stimulus for the recruitment of eosinophils; the co-existence of cell proliferation, with the release of eosinophil survival/differentiation factors, in the same microenvironment will, in turn, elicit a local accumulation of tissue eosinophils.
Eosinophils are unequivocal LIARs!
Our hypothesis is that eosinophils are part of host recognition pathways that identify focal bursts of cell death accompanied by cell proliferation, including possibly a mechanism for detecting local stem cell activities in outlying tissues/organs. Specifically, we are suggesting that cell turnover and/or stem cell activities together with a tissue microenvironment that supplies the necessary survival and differentiation factors will alone promote eosinophil accumulation. The fundamental role of their accumulation at these sites is to modulate **L**ocal **I**mmunity **A**nd/or **Remodeling/R**epair - The LIAR hypothesis. We suggest that the LIAR hypothesis provides a parsimonious explanation of data relative to the end-stage effector cell paradigm. That is, LIAR suggests that the unique and varied accumulation of eosinophils in a variety of tissues is not exclusively linked with innate host defense and/or simply a consequence of immune dysregulation. Instead, this accumulation occurs as part of a strategy(ies) to maintain tissue homeostasis in both healthy and pathological states.
A simplified cartoon outlines the major components of the LIAR hypothesis (Figure 3). In summary, the LIAR hypothesis postulates that a focus of cell death elicits the recruitment of eosinophils. Specifically, small molecule mediators released from stressed/dying cells represent a powerful and nearly universal signal (i.e., regardless of tissue/organ location) for the recruitment of eosinophils. For example, damage-associated molecular pattern molecules (DAMPs) such as HMGB-1 and sphingosine-1-phosphate have each been shown to be significant chemoattractants [31,32]. Nonetheless, alone this recruitment would be insufficient for the accumulation of a localized steady-state population of eosinophils. Instead, eosinophil accumulation would occur at those tissues sites also engaged in cell proliferation as a consequence of the accompanying release of growth factors/cytokines, survival factors, and perhaps even signals linked with the metabolic requirements of cell proliferation such as the local accumulation of lactate (i.e., the Warburg Effect (reviewed in [33])). Thus, in the absence of additional eosinophil agonist cytokines/growth factors and/or survival factors (reviewed in [34]), tissue-recruited eosinophils would simply turnover and/or move on. However, the concomitant presence of cell proliferation, in the context of specific immune responses and/or tissue remodeling/repair signals, will result in a much different chain of events. Under these circumstances, eosinophil numbers will accumulate to a threshold that initiates a program of eosinophil-mediated events leading to LIAR. Moreover, we further suggest that the unique character of this accumulation reflects not just cell turnover, but instead, may reflect the presence of activated stem cell populations in a given tissue [35,36]. That is, whether at homeostatic baseline or as a result of unique disease pathologies, eosinophil accumulation occurs in response to defined circumstances associated with a dynamic cell population(s) in a given tissue and not as a consequence of a specific local immune response(s).
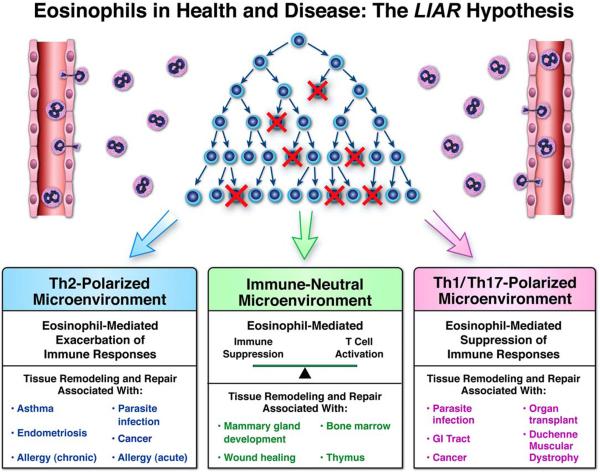
Figure 3. Schematic representation outlining the LIAR hypothesis and the outcomes-based consequences of eosinophil-mediated activities in health and disease.
Peripheral eosinophil ( ) recruitment occurs in response to the release of one or more small molecule mediators of inflammation (e.g., DAMPs) released from localized bursts of cell death (
) recruitment occurs in response to the release of one or more small molecule mediators of inflammation (e.g., DAMPs) released from localized bursts of cell death ( ). In the presence of additional eosinophil agonist growth (e.g., IL-5) and survival (e.g., GM-CSF) factors derived from concomitant cell proliferation and/or stem cell activation (
). In the presence of additional eosinophil agonist growth (e.g., IL-5) and survival (e.g., GM-CSF) factors derived from concomitant cell proliferation and/or stem cell activation ( ), these granulocytes accumulate and establish a local steady-state population. The tissue immune microenvironment subsequently dictates the downstream immune consequences mediated by eosinophil effector functions, leading either to exacerbations of local immune responses (Th2-Polarized Microenvironment), suppression of these site-specific immune responses (Th1/Th17-Polarized Microenvironment), or essentially little to no modulations of local immune responses (Immune-Neutral Microenvironment). In turn, these immune responses modulate the levels of tissue remodeling and/or tissue repair that is also characteristic of eosinophil-mediated effector functions. Thus, the immune microenvironment present upon eosinophil recruitment is a significant situational cue which drives the predominance of specific eosinophil activities. More importantly, this eosinophil-mediated **L**ocal **I**mmunity **A**nd/or **Remodeling/R**epair defines the functional roles of eosinophils in unique tissue compartments at homeostatic baseline (i.e., health) as well as within tissues associated with specific diseases.
), these granulocytes accumulate and establish a local steady-state population. The tissue immune microenvironment subsequently dictates the downstream immune consequences mediated by eosinophil effector functions, leading either to exacerbations of local immune responses (Th2-Polarized Microenvironment), suppression of these site-specific immune responses (Th1/Th17-Polarized Microenvironment), or essentially little to no modulations of local immune responses (Immune-Neutral Microenvironment). In turn, these immune responses modulate the levels of tissue remodeling and/or tissue repair that is also characteristic of eosinophil-mediated effector functions. Thus, the immune microenvironment present upon eosinophil recruitment is a significant situational cue which drives the predominance of specific eosinophil activities. More importantly, this eosinophil-mediated **L**ocal **I**mmunity **A**nd/or **Remodeling/R**epair defines the functional roles of eosinophils in unique tissue compartments at homeostatic baseline (i.e., health) as well as within tissues associated with specific diseases.
The recent demonstration that eosinophils are capable of the rapid release of a variety of pre-stored cytokines/chemokines [37] suggests that eosinophils themselves may even participate in positive feedback loops that promote their own accumulation and survival. The expanding complexity of eosinophil - T cell interactions demonstrates that eosinophils may have the ability to directly modulate local immune responses in a very direct fashion (reviewed in [38,39]). In either case, the LIAR hypothesis suggests that tissue accumulation of eosinophils is primarily a function of cell turnover and/or regional stem cell activities that initiate recruitment and the local production of eosinophil survival/differentiation factors. That is, this accumulation is not exclusively a consequence of localized immune-mediated responses; yet these responses may in some cases be a significant contributor to site-specific eosinophil accumulation (e.g., the rapid (within 2–3 hours) accumulation of eosinophils to the site of cutaneous anaphylactic reactions).
The demonstrated abilities of eosinophils to express cytokines and growth/survival factors characterized as Th1/Th17, Th2, and even acute phase inflammatory (see for example [40]) implies a degree of plasticity regarding the immunomodulatory capability of eosinophils. In addition, the LIAR hypothesis suggests that eosinophil-mediated immunomodulatory functions are, in part, dictated by the tissue immune microenvironment where they accumulate - they contribute to, but do not necessarily dominate, local immune responses. For example, in a Th2-polarized microenvironment LIAR suggests the immunomodulatory functions of eosinophils have a net exacerbating effect on immune responses through T cell activation [41–43] and/or augmentation of Th2 cytokine/chemokines and/or attenuation of Th1 responses [30,44–48]. Alternatively, in a Th1/Th17-polarized environment LIAR suggests that eosinophils suppress ongoing immune responses by either shifting the Th1/Th17 balance toward a Th2 dominated milieu (e.g., [37,44,49]) or perhaps by promoting immune suppression (e.g., [44,45]). In contrast, in an immune-neutral environment the accumulating eosinophils neither suppress nor exacerbate local immune homeostasis but instead simply become part of the complex collection of evolving site-specific immune responses induced by local inflammatory events [40]. Regardless of the immune microenvironment that accumulating eosinophils experience, the subsequent release of eosinophil-derived factors (i.e., secondary granule proteins (e.g., MBP), reactive oxygenated species, matrix modifying/degrading proteases (e.g., MMPs), and growth factors (e.g., TGF-β)) elicits/contributes to tissue remodeling/repair events (reviewed in [50]).
The fundamental role of eosinophils in the modulation of local immune responses and remodeling/repair events described by the LIAR hypothesis suggests the somewhat unorthodox view that the homeostatic baseline activities of eosinophils in otherwise healthy patients are primarily the same activities that occur during disease; only the magnitude and extent of these activities change. Thus, whether in health or disease, the recruitment and subsequent accumulation of eosinophils occurs in response to continuous site-specific signals resulting from cycles of cell turnover, local stem cell activities, or in the case of experimental model systems, the induced ectopic expression of these signals/activities. In turn, this eosinophil accumulation contributes to local immune responses and the extent of ongoing tissue remodeling/repair events.
The LIAR hypothesis – The role of resident tissue eosinophils in otherwise healthy individuals
The Bone Marrow and Other Sites of Hematopoiesis
A dominant source of resident eosinophils in otherwise healthy individuals is the bone marrow. The LIAR hypothesis suggests that the continuous signals derived from the profound and coordinated death and proliferation of stem cells occurring in the marrow facilitates/enhances eosinophil retention, survival, and/or production, leading to a steady-state population in this tissue. In turn, the demonstration of significant eosinophil degranulation occurring in the bone marrow of otherwise healthy subjects [51] suggests that the execution of eosinophil effector functions may contribute to pathways that facilitate homeostasis within the marrow, perhaps through remodeling/repair activities.
The Gastrointestinal Tract
The GI tract from the stomach to the rectum is also an area of profound (and coordinated) cell death and proliferation as well as a focal area of epithelial stem cell activities [52] that may well explain the presence of resident eosinophils and the significant eosinophil degranulation occurring in these areas [53]. Moreover, the colon exhibits myriad immune/inflammatory pathways that, in part, result from the differential gradient of bacteria across the mucosal surface (i.e., >1010 bacteria in the GI lumen to <1 bacteria in the submucosal areas). Coincidently, eosinophil accumulation is its highest in the cecum (i.e., the transitional area between the small intestine (low bacterial presence) and the colon (large bacterial presence)) suggesting a differential role for eosinophils here relative to other regions of the GI tract [54]. Multiple mechanisms have been suggested to achieve the required balance between immune and inflammatory responses to maintain homeostasis, including competing Th1 vs Th2 vs Th17 polarized immune activities as well as T regulatory cell mediated competitive suppression of immune responses [55]. The LIAR hypothesis suggests that instead of simply being an innate host defensive barrier, or sentinels of immunosurveillance, resident gut eosinophils orchestrate many of the complex and continuous immune responses and/or remodeling events in this extraordinarily large and dynamic interface with the environment. Thus, the LIAR hypothesis does not exclude the possibility that eosinophils also evolved (i.e., acquired) physiologically important roles (e.g., maintenance of epithelial barrier permeability [56]) and/or innate host defense capabilities [57], but refocuses attention to a larger and more important role in the maintenance of GI immune/inflammatory homeostasis.
A provocative example of a potential role for eosinophils in remodeling events linked with gut homeostasis unrelated to innate host defense is provided by metamorphosing larval frogs. Jordan and Speidel [58] showed that the vegetarian diet of frog tadpoles necessitates a long intestinal tract that was also missing a prominent eosinophil infiltrate. However, the initiation of metamorphosis is accompanied by a dramatic remodeling of the intestine as it shortens in length in preparation for the carnivorous diet of an adult frog. Interestingly, these remodeling events were shown to be accompanied by a massive eosinophil infiltrate and widespread eosinophil degranulation. Although their manuscript was published in 1923 (i.e., before any realistic appreciation of eosinophil effector functions) and was not intended as a study of eosinophil biology, the concepts expressed by the authors regarding eosinophils were surprisingly insightful. More importantly, these concepts were supportive of eosinophils as a responding infiltrate to cell turnover and/or regional stem cell activites that contribute to local tissue remodeling events (i.e., the LIAR hypothesis):
“In the intestinal mucosa are found many eosinophils, mostly gathered near the basement membrane of the epithelial lining. Our study of these cells suggests that they give off substances, often themselves going to pieces in the process, that aid in causing disintegration or other regressive changes of nearby tissues ….. In brief, the granuylocytes, both special and eosinophilic, appear to be mobile, unicellular glands which give off substances that have a lytic effect on tissues undergoing regressive change. The writers do not hold that the secretions of these cells are the first cause of degeneration in these regions. As Morse (1918) has pointed out, a spontaneous breaking down of tissues occurs before mobilization of the granulocytes. Whatever initiates the degeneration process, however, the granulocytes aid in furthering it.”
The Thymus
The thymus is a center for T cell education/selection. Pro-T cells (i.e., T lymphoctye progenitor/stem cells) entering the thymus undergo a massive proliferative process linked with clonal deletion of those which fail the selective criteria needed for T lymphocytes to reenter circulation as mature T cells. The LIAR hypothesis suggests that eosinophils accumulate in the thymus because of this cell turnover and may contribute to thymocyte education by modulation of T cell receptor selectivity, T cell activation, or perhaps by providing thymocytes specific cytokines/chemokines and/or survival factors. An interesting implication of the LIAR hypothesis is that eosinophils may contribute to T cell maturation and thus have a role in the immunological determination of self vs non-self; a prediction supported in a study by Throsby and colleagues [59].
The Uterus
The uterus is an organ that undergoes cyclic changes associated with fecundity. More importantly, these changes include coordinated bursts of cell death/proliferation and/or mesenchymal stem cell activities in the context of both complex immune responses associated with the self/non-self recognition (i.e., immunosurveillance) and remodeling/repair events occurring in the endometrial lining [60]. The LIAR hypothesis suggests that the cyclic burst of cell proliferation and cell death would lead to eosinophil recruitment. The ongoing immune responses in this pregnancy-associated immunologically privileged region would provide the necessary survival and growth factors for eosinophils [61]. Significantly, this hypothesis also provides an explanation as to why ectopic endometriotic lesions become foci of eosinophil accumulation and activities [62].
The LIAR hypothesis – The role of tissue eosinophils linked with disease and/or localized pathology
Helminth Parasite Infections
A recent study by Appleton and colleagues [63] investigating a primitive helminth (Trichinella spiralis) provides support for the LIAR hypothesis and explains why eosinophilic responses may be limited primarily to this subgroup of parasites. Their studies showed that eosinophils played little to no role in the initial proliferative phase of this parasite in the intestines of infected animals. Instead, later in the life cycle of Trichinella spiralis, eosinophils accumulated at host skeletal muscle sites where the organisms take-up residence. Our suggestion is that this eosinophil accumulation occurs as a consequence of activating local skeletal muscle progenitor cells (i.e., satellite cells) in response to invading parasites. In turn, these accumulating eosinophils elicit a localized suppression of immune/inflammatory responses induced by the invading parasite. Thus, eosinophil-mediated activities permitted this nematode to take-up residence in the host skeletal muscle without eliciting a potentially debilitating inflammatory response and therefore may be interpreted as being beneficial to both the host and the parasite. Furthermore, studies by Abraham and colleagues [64] demonstrated that another nematode, Strongyloides stercoralis, expresses one or more eosinophil chemoattractants, leading to the novel conclusion that helminths may have evolved unique mechanisms that actually exploit the **LIAR**-based eosinophil activities as part of their life cycle. Interestingly, these novel eosinophil mechanisms provide the host with an evolutionarily advantageous defense: If the host cannot effectively target helminths, it simply accommodates the parasite's presence with minimal immune-mediated inflammation so that it can live and, more importantly for Darwinian evolution, reproduce another day.
Significantly, a similar **LIAR**-based mechanism of immune suppression and localized remodeling/repair has been proposed to explain the presence/accumulation of eosinophils in other seemingly unconnected circumstances. In the skeletal muscle lesions in Duchenne's Muscular Dystrophy patients, accumulating eosinophils were shown to be necessary for local immune suppression and remodeling/repair events [30]. Furthermore, the **LIAR**-based suggestion that eosinophils are necessary to dampen local immune responses in the context of significant tissue remodeling/repair may also provide an explanation for the presence of eosinophils in wound healing [65].
Allergic Pulmonary Inflammation - Asthma
The eosinophil accumulation occurring in the lungs of asthmatics following allergen provocation also represents another interesting test of the LIAR hypothesis. At baseline, the pseudo-stratified columnar epithelium of the large and small airways undergo only nominal cycles of cell death and proliferation as judged by the presence of apoptotic cells and mitotic figures [66]; correspondingly, there are few resident eosinophils in healthy individuals. However, epithelial cell stress and/or death induced in patients with pulmonary disease are likely to disrupt lung homeostasis. This disequilibrium would lead to a series of immune/inflammatory events that are accompanied by increases in epithelial cell turnover and/or regional stem cell activities promoting an increase in eosinophil recruitment. Thus, we suggest that in pathogen infection or acute respiratory distress syndrome (ARDS), eosinophils are recruited to the lung. However, because these recruited leukocytes would not receive additional recruitment, survival, and/or proliferative signals necessary to accumulate they would quickly disappear [67]. Interestingly, despite the transitory character of this eosinophil infiltrate, evidence of eosinophil degranulation in the lung is nonetheless a prognostic indicator of survival in lung injury patients (manuscript in preparation). In the case of allergen challenge, we suggest that the accompanying epithelial cell stress/death (and/or the recruitment/activation of local stem cells) is accompanied by the expression of necessary eosinophil recruitment, survival, and growth factors, perhaps as a consequence of unique immune responses in the lung. As a consequence of these unique responses to allergen provocation, pulmonary eosinophil accumulation is characteristic of many asthmatics [68]. Asosingh and colleagues investigating the link between eosinophils and angiogenesis in asthmatic airway remodeling provide an astonishing affirmation of key tenets of the LIAR hypothesis [69]. These investigators show in a mouse model of allergic airways disease that allergen provocation induces the rapid recruitment of bone marrow-derived endothelial progenitor cells to the lung (i.e., prior to the arrival of eosinophils). Evidence is further provided in their report to suggest that the activities of these locally recruited progenitor cells contribute to the subsequent accumulation of eosinophils in the lung following allergen provocation; a similar observation was also presented in a cohort of asthma patients. Collectively, these studies suggest that the recruitment and activation of stems cells in the lung is an underlying response to environmental allergen exposure, providing a novel and previously unrecognized mechanism leading to the induced pulmonary eosinophilia and subsequent remodeling events associated with this airways disease.
The LIAR hypothesis goes on to suggest that if the eosinophil accumulation in the lung fails to achieve a threshold level, the tissue-immune responses would be muted and pulmonary inflammation nominal. However, if a threshold of pulmonary eosinophils is achieved, the LIAR hypothesis suggests that eosinophil activities will become a prominent contributor to the events characterized as Th2-driven pulmonary inflammation (see for example [13,14,70]). Several recent studies highlight key components of this hypothesis, including allergen-specific T cell proliferation and/or activation (e.g., [37,44,49]), modulation of the pulmonary immune microenvironment contributing to the differentiation of T cells with the skewing of induced immune responses further toward a Th2 phenotype [46–48], and allergen-specific T effector cell accumulation to the lung that exacerbates immune responses [49]. Alternatively, eosinophil-mediated expansion and recruitment of T regulatory cells may even function to quell subsequent allergen-specific responses [44]. In either case, interpretation of the data through the LIAR hypothesis suggests that once allergic respiratory inflammation achieves a threshold level of eosinophils in the various pulmonary compartments, they elicit multiple cascading immune/inflammatory responses that are not simply diagnostic of the activities mediated by other cells.
Growth of Solid Tumors and Other Cancers
The association of tumor-infiltrating eosinophils and cancer growth provides more corroborative evidence in support of the LIAR hypothesis. Solid tumors represent ectopic sites of coordinated cell death/proliferation and likely cancer stem cell activities [71] and thus the LIAR hypothesis would suggest that these areas are potential sites of eosinophil accumulation; correspondingly, reports have noted the presence of these leukocytes in a variety of tumors (see for example [31,72]). To define the extent of eosinophil infiltration of human cancers (as well as select mouse models of cancer) we systematically examined solid tumors using a state-of-the-art eosinophil specific antibody for detection of eosinophils infiltrating biopsies [73]; an example of such a survey is presented in Figure 4. These photomicrographs clearly demonstrate that eosinophils are a nearly ubiquitous leukocytic infiltrate of solid tumors. In many cases this eosinophil accumulation is quantitatively substantive but large differences exist both between tumor types and within a given tumor. These divergent observations suggest that eosinophil accumulation is responsive to multiple factors which vary between tumor types, within a given tumor, and may even vary as a function of time. Moreover, Cormier and colleagues [74] demonstrated that the accumulation of eosinophils in solid tumors occurs independent of acquired immune responses and that this accumulation is spatially restricted within solid tumors, accumulating predominately in areas of necrosis and areas of tissue remodeling. The LIAR hypothesis proposes that once recruited to a tumor, eosinophils contribute to immune responses and remodeling/repair events. That is, the eosinophil accumulation accompanying the focal cell death and proliferation associated with cancer (and/or the activation of one or more tumor stem cell populations) has the possibility of modulating host tumor immune responses by dampening cell-targeted Th1 responses, increasing tumor-mediated Th2 immune responses, and/or the activation of immune suppressing pathways leading to expansion/activation of T regulatory cells and/or T effector cell anergy toward tumor antigens [75]. Together with the potential tissue remodeling/repair activities associated with eosinophils (e.g., induction of angiogenesis [76]), the LIAR hypothesis would suggest that eosinophil-mediated reduction of tumor-targeting immune responses and promotion of remodeling events is likely to facilitate tumor growth. However, the available data do not rule out the possibility that through LIAR, or possibly through destructive end-stage effector functions, eosinophils may also negatively affect tumor growth and morphology. Interestingly, these effects are likely to be diverse and dependent on the particular tumor immune microenvironment inherited by the tumor associated eosinophils. Thus, the eosinophil-mediated activities in tumors are likely to be somewhat cancer-specific and in some cancers the focal presence of many eosinophils may provide exacerbating tumor pro-growth inflammatory signals [77] whereas in others, eosinophil activities may deter cancer growth [78]. Moreover, these effects may vary reflecting the extent of the induced eosinophil infiltrate in a given tumor. There is no reason a priori to assume these are mutually exclusive events in any given tumor and eosinophils may even switch from pro- to anti-tumorigenic activities during the development of cancer. Furthermore, we suggest that ambiguities regarding the prognostic value of tumor-associated eosinophils is a reflection of this complex dual role.
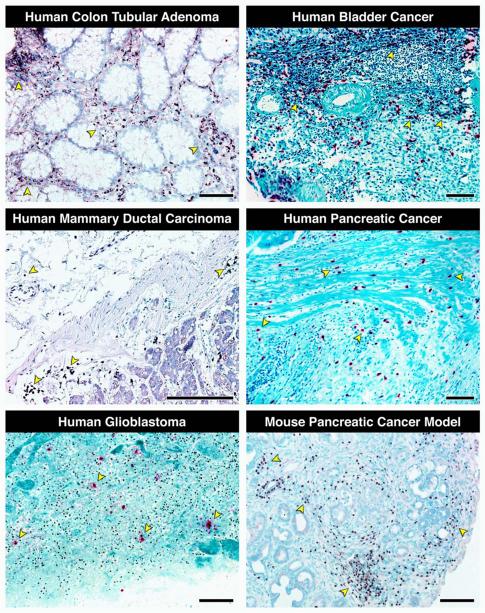
Figure 4. Histopathological assessments of biopsies from human cancers and tumors from mouse models of cancer show that eosinophil infiltration of tumors is often significant and, more importantly, a widely occurring phenomenon.
Immunohistochemistry with a unique and specific monoclonal antibody against the abundant eosinophil secondary granule protein, eosinophil peroxidase (EPX-mAb [73]) demonstrated evidence for eosinophil infiltration in multiple human cancers (darkly staining navy/black cells in each photomicrograph with representative examples noted with arrowheads), including colon tubular adenoma, bladder cancer, mammary ductal carcinoma, pancreatic cancer, and glioblastoma. In addition, staining with a monoclonal antibody specific for another abundant eosinophil secondary granule protein, major basic protein (rat anti-mouse MBP-mAb-14.7.4 [17]) demonstrated the presence of a robust eosinophil tumor infiltrate occurring in a mouse model of pancreatic cancer (Pdx1-Cre (x) KRASG12D/+ mice [82]). Scale bar = 100μm.
Organ Transplant Rejection
The implications of the LIAR hypothesis as an explanation of the induced tissue eosinophilia linked with organ transplant rejection (reviewed in [79]) is particularly significant. Specifically, the coordinated cell turnover and/or the activation of resident stem cells [80] occurring in a transplanted organ apparently provide the necessary elements to induce a local tissue eosinophilia. The LIAR hypothesis would suggest that if accumulating eosinophils reach a certain threshold level, they would be sufficient to modulate the caldron of immune host vs. graft responses that correspondingly lead to the remodeling/repair events surrounding the graft's integration into the recipient [81]. Obvious questions remain as to the relevance of eosinophil activities in organ rejection - are eosinophils part of early immunosuppressive remodeling/repair strategies or do the accumulating eosinophils actually lead to the recruitment and activation of alloreactive T effector cells that contribute to rejection? In either case, the LIAR hypothesis suggests that eosinophils are not simply diagnostic of rejection but instead are likely to be active participants.
Epilogue
The LIAR hypothesis suggests that eosinophil activities have situation-specific consequences in health and disease. LIAR also suggests that eosinophils are necessary, but not required (as evidenced by the general health and well being of eosinophil-less mice [9,10]), and thus represent a contributing mechanism(s) to organismal homeostasis. It is noteworthy that while the LIAR hypothesis suggests that most eosinophil-mediated activities are not cytocidial in character, it does not preclude that eosinophils are also end-stage effector cells with destructive capabilities linked with innate host defense. Specifically, we suggest that it is not surprising that eosinophils have maintained and/or evolved anti-parasitic [19] and anti-bacterial [57] capabilities. We speculate that eosinophils likely evolved from a primitive proto-granulocyte that may have even possessed these activities before being distinguished as a unique lineage. These additional activities may have provided an evolutionarily selective advantage to the host. Specifically, places of coordinated cell death/proliferation and/or stem cell activation are likely to be places vulnerable to opportunistic pathogens. However, in the context of the LIAR hypothesis these activities are secondary eosinophil effector functions and represent pathways that overlap with more robust host defense activities mediated by other cells. Instead, the eosinophil is a specialized multifunctional leukocyte whose true contributions to health and disease are yet to be fully realized.
ACKNOWLEDGMENTS
There is not a enough space, nor would the editors permit us the space to truly thank everyone who in small and large ways have contributed to this perspective. We simply wish to thank all of our colleagues and especially the members of the Nancy and Jamie Lee Laboratories whose enthusiasm and insights have been instrumental in driving our pathological interests in all things eosinophil. We also would to thank Marv Ruona (Mayo Medical Graphics) for the preparations of the figures as well as Linda Mardel and Shirley (“Charlie”) Kern who are an administrative staff without peers. We would like to extend particular notes of gratitude to three of our colleagues Drs. Gerald (Jerry) Gleich, Redwan Moqbel, and Michael Lotze. Jerry Gleich's passion for eosinophils has been a foundation of our laboratory group since its inception. He has been both a mentor and colleague whose friendship has enriched our lives and vastly improved our science. Redwan Moqbel's courage and single-mindedness of task have been inspirational and in many ways keeps us moving forward. Finally, this Perspective is a direct consequence of our modeling Mike Lotze's approach of trying to understand biological phenomena by asking questions and thinking about problems from EVERY viewpoint possible.
REFERENCES
- 1.Austen KF. Homeostasis of effector systems which can also be recruited for immunologic reactions. J Immunol. 1978;121:793–805. Review. [PubMed] [Google Scholar]
- 2.Smith H, Cook RM. Immunopharmacology of Eosinophils. In: Page CF, editor. The Handbook of Immunopharmacology. 1st ed. Academic Press, Harcourt Brace Jovanovich; London: 1993. p. 250. [Google Scholar]
- 3.Taliaferro WH, Sarles MP. The cellular reactions in the skin, lungs, and intestine of normal and immune rats after infection with Nippostrongylus muris. J Infect Dis. 1939;64:157–192. [Google Scholar]
- 4.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 5.Bruschi F, Korenaga M, Watanabe N. Eosinophils and Trichinella infection: toxic for the parasite and the host? Trends Parasitol. 2008;24:462–467. doi: 10.1016/j.pt.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun WJ, Sedgwick J, Busse WW. The role of eosinophils in the pathophysiology of asthma. Ann N Y Acad Sci. 1991;629:62–72. doi: 10.1111/j.1749-6632.1991.tb37961.x. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Francois-Bernard M. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. see comments. [DOI] [PubMed] [Google Scholar]
- 8.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 9.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 10.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 11.Swartz JM, Dyer KD, Cheever AW, Ramalingam T, Pesnicak L, Domachowske JB, Lee JJ, Lee NA, Foster PS, Wynn TA, Rosenberg HF. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filley WV, Kephardt GM, Holley LE, Gleich GJ. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet. 1982;ii:11–16. doi: 10.1016/s0140-6736(82)91152-7. [DOI] [PubMed] [Google Scholar]
- 13.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 14.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frigas E, Loegering DA, Gleich GJ. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Laboratory Investigation. 1980;42:35–43. [PubMed] [Google Scholar]
- 16.Denzler KL, Farmer SC, Crosby JR, Borchers MT, Cieslewicz G, Larson KA, Cormier-Regard S, Lee NA, Lee JJ. Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol. 2000;165:5509–5517. doi: 10.4049/jimmunol.165.10.5509. [DOI] [PubMed] [Google Scholar]
- 17.Denzler KL, Borchers MT, Crosby JR, Cieslewicz G, Hines EM, Justice JP, Cormier SA, Lindenberger KA, Song W, Wu W, Hazen SL, Gleich GJ, Lee JJ, Lee NA. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol. 2001;167:1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 18.Moqbel R, Macdonald AJ, Cromwell O, Kay AB. Release of leukotriene C4 (LTC4) from human eosinophils following adherence to IgE- and IgG-coated schistosomula of Schistosoma mansoni. Immunology. 1990;69:435–442. [PMC free article] [PubMed] [Google Scholar]
- 19.Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today. 2000;16:202–209. doi: 10.1016/s0169-4758(99)01620-8. [DOI] [PubMed] [Google Scholar]
- 20.Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 21.Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sorensen MV, Haddock SH, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–749. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- 22.Meeusen EN, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16:95–101. doi: 10.1016/s0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- 23.Limaye AP, Abrams JS, Silver JE, Ottesen EA, Nutman TB. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990;172:399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takatsu K, Tominaga A, Harada N, Mita S, Matsumoto M, Takahashi T, Kikuchi Y, Yamaguchi N. T cell-replacing factor (TRF)/interleukin 5 (IL-5): molecular and functional properties. Immunol Rev. 1988;102:107–135. doi: 10.1111/j.1600-065x.1988.tb00743.x. Review. [DOI] [PubMed] [Google Scholar]
- 25.Clutterbuck E, Shields JG, Gordon J, Smith SH, Boyd A, Callard RE, Campbell HD, Young IG, Sanderson CJ. Recombinant human interleukin 5 is an eosinophil differentiation factor but has no activity in standard human B cell growth factor assays. Eur J Immunol. 1987;17:1743–1750. doi: 10.1002/eji.1830171210. [DOI] [PubMed] [Google Scholar]
- 26.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Huber HL, Koessler KK. The Pathology of Bronchial Asthma. Arch Intern Med. 1922;30:689–760. [Google Scholar]
- 28.Wenzel SE. Eosinophils in asthma--closing the loop or opening the door? N Engl J Med. 2009;360:1026–1028. doi: 10.1056/NEJMe0900334. [DOI] [PubMed] [Google Scholar]
- 29.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehling-Henricks M, Sokolow S, Lee JJ, Myung KH, Villalta SA, Tidball JG. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum Mol Genet. 2008;17:2280–2292. doi: 10.1093/hmg/ddn129. Aug 2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 32.Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, Barbour SE, Milstien S, Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elsas PX, Elsas MI. Eosinophilopoiesis at the cross-roads of research on development, immunity and drug discovery. Curr Med Chem. 2007;14:1925–1939. doi: 10.2174/092986707781368487. [DOI] [PubMed] [Google Scholar]
- 35.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 36.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 37.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee NA, Gelfand EW, Lee JJ. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J Allergy Clin Immunol. 2001;107:945–957. doi: 10.1067/mai.2001.116002. [DOI] [PubMed] [Google Scholar]
- 39.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179:4840–4848. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]
- 41.Padigel UM, Hess JA, Lee JJ, Lok JB, Nolan TJ, Schad GA, Abraham D. Eosinophils act as antigen presenting cells to induce immunity to Strongyloides stercoralis in mice. The Journal of Infectious Diseases. 2007;196:1844–1851. doi: 10.1086/522968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H-B, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Ghahary A, Moqbel R. Cutting Edge: Human Eosinophils Regulate T Cell Subset Selection through Indoleamine 2,3-Dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 45.Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 46.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 47.Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rumbley CA, Sugaya H, Zekavat SA, El Refaei M, Perrin PJ, Phillips SM. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J Immunol. 1999;162:1003–1009. [PubMed] [Google Scholar]
- 49.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic Pulmonary Inflammation in Mice is Dependent on Eosinophil-induced Recruitment of Effector T Cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 51.Butterfield JH, Ackerman SJ, Scott RE, Pierre RV, Gleich GJ. Evidence for secretion of human eosinophil granule major basic protein and Charcot-Leyden crystal protein during eosinophil maturation. Exp Hematol. 1984;12:163–170. [PubMed] [Google Scholar]
- 52.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 53.Kato M, Kephart GM, Morikawa A, Gleich GJ. Eosinophil infiltration and degranulation in normal human tissues: evidence for eosinophil degranulation in normal gastrointestinal tract. Int Arch Allergy Immunol. 2001;125(Suppl 1):55–58. doi: 10.1159/000053855. [DOI] [PubMed] [Google Scholar]
- 54.Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol. 1996;9:110–114. [PubMed] [Google Scholar]
- 55.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Furuta GT, Nieuwenhuis EE, Karhausen J, Gleich G, Blumberg RS, Lee JJ, Ackerman SJ. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890–897. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- 57.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 58.Jordan HE, Speidel CC. Blood Cell Formation and Distribution in Relation to the Mechanism of Thyroid-Accelerated Metamorphosis in the Larval Frog. J Exp Med. 1923;38:529–541. doi: 10.1084/jem.38.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol. 2000;165:1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- 60.Gargett CE, Chan RW, Schwab KE. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288:22–29. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 61.Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001;142:4515–4521. doi: 10.1210/endo.142.10.8459. [DOI] [PubMed] [Google Scholar]
- 62.Blumenthal RD, Samoszuk M, Taylor AP, Brown G, Alisauskas R, Goldenberg DM. Degranulating eosinophils in human endometriosis. Am J Pathol. 2000;156:1581–1588. doi: 10.1016/S0002-9440(10)65030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. 2009;182:1577–1583. doi: 10.4049/jimmunol.182.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stein LH, Redding KM, Lee JJ, Nolan TJ, Schad GA, Lok JB, Abraham D. Eosinophils utilize multiple chemokine receptors for chemotaxis to the parasitic nematode Strongyloides stercoralis. Journal of Innate Immunity. 2009 doi: 10.1159/000233235. Published online August 5, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J, Torio A, Donoff RB, Gallagher GT, Egan R, Weller PF, Wong DT. Depletion of eosinophil infiltration by anti-IL-5 monoclonal antibody (TRFK-5) accelerates open skin wound epithelial closure. Am J Pathol. 1997;151:813–819. [PMC free article] [PubMed] [Google Scholar]
- 66.Leslie KO, Wick MR. Practical Pulmonary Pathology - A Diagnostic Approach. Churchill Livingstone; Philadelphia: 2005. [Google Scholar]
- 67.Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome - how neutrophils migrate into the lung. Crit Care. 2004;8:453–461. doi: 10.1186/cc2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vignola AM, Chanez P, Chiappara G, Siena L, Merendino A, Reina C, Gagliardo R, Profita M, Bousquet J, Bonsignore G. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999;103:563–573. doi: 10.1016/s0091-6749(99)70225-3. see comments. [DOI] [PubMed] [Google Scholar]
- 69.Asosingh K, Hanson JD, Cheng G, A.Aronica M, Erzurum SC. Allergen-Induced Eotaxin-rich Pro-angiogenic Bone Marrow Progenitors A Blood Borne Cellular Envoy for Lung Eosinophilia. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.01.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechele TL, Ansay TL, Colbert DC, Cormier SA, Justice JP, Lee NA, Lee JJ. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 71.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 72.Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol. 1981;34:1343–1348. doi: 10.1136/jcp.34.12.1343. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Protheroe CA, Protheroe C, Woodruff SA, DePetris G, Mukkada V, Ochkur SI, Janarthanan S, Lewis JC, Pasha S, Lunsford T, Harris L, Sharma VK, McGarry MP, Lee NA, Furuta GT, Lee JJ. A novel histological scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clinical Gastroenterology and Hepatology. 2009;7:749–755. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, Colbert D, Lombari TR, Constant S, McGarry MP, Lee JJ, Lee NA. Pivotal Advance: Eosinophil Infiltration of Solid Tumors Is an Early and Persistent Inflammatory Host Response. J Leukoc Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puxeddu I, Berkman N, Nissim Ben Efraim AH, Davies DE, Ribatti D, Gleich GJ, Levi-Schaffer F. The role of eosinophil major basic protein in angiogenesis. Allergy. 2009;64:368–374. doi: 10.1111/j.1398-9995.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 77.Wong DT, Bowen SM, Elovic A, Gallagher GT, Weller PF. Eosinophil ablation and tumor development. Oral Oncol. 1999;35:496–501. doi: 10.1016/s1368-8375(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 78.Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, Smyth MJ, Parish CR. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 79.Goldman M, Le Moine A, Braun M, Flamand V, Abramowicz D. A role for eosinophils in transplant rejection. Trends Immunol. 2001;22:247–251. doi: 10.1016/s1471-4906(01)01893-2. [DOI] [PubMed] [Google Scholar]
- 80.Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Alwayn IP, Weimar W, Hoogduijn MJ. Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation. 2009;87:896–906. doi: 10.1097/TP.0b013e31819b3d72. [DOI] [PubMed] [Google Scholar]
- 81.Simeonovic CJ, Townsend MJ, Karupiah G, Wilson JD, Zarb JC, Mann DA, Young IG. Analysis of the Th1/Th2 paradigm in transplantation: interferon-gamma deficiency converts Th1-type proislet allograft rejection to a Th2-type xenograft-like response. Cell Transplant. 1999;8:365–373. doi: 10.1177/096368979900800404. [DOI] [PubMed] [Google Scholar]
- 82.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]