Structural organization of a filamentous influenza A virus (original) (raw)
Abstract
Influenza is a lipid-enveloped, pleomorphic virus. We combine electron cryotomography and analysis of images of frozen-hydrated virions to determine the structural organization of filamentous influenza A virus. Influenza A/Udorn/72 virions are capsule-shaped or filamentous particles of highly uniform diameter. We show that the matrix layer adjacent to the membrane is an ordered helix of the M1 protein and its close interaction with the surrounding envelope determines virion morphology. The ribonucleoprotein particles (RNPs) that package the genome segments form a tapered assembly at one end of the virus interior. The neuraminidase, which is present in smaller numbers than the hemagglutinin, clusters in patches and are typically present at the end of the virion opposite to RNP attachment. Incubation of virus at low pH causes a loss of filamentous morphology, during which we observe a structural transition of the matrix layer from its helical, membrane-associated form to a multilayered coil structure inside the virus particle. The polar organization of the virus provides a model for assembly of the virion during budding at the host membrane. Images and tomograms of A/Aichi/68 X-31 virions show the generality of these conclusions to non-filamentous virions.
Keywords: electron cryomicroscopy, matrix protein, ribonucleoprotein particles hemagglutinin, neuramindase
Influenza A virus is a lipid-enveloped orthomyxovirus and a cause of human disease by strains that arise through seasonal variation and through pandemic infection resulting from viral reassortment or adaptation that introduces new influenza viruses into the human population. The virus genome contains eight separate RNA segments of negative polarity that encode eight structural proteins and four additional proteins. The genome segments are packaged in the virus in complex with the nucleoprotein (NP) as ribonucleoprotein particles (RNPs) (1). The RNPs are also associated with the viral polymerase (composed of the PA, PB1, and PB2 subunits) (2), which is necessary for viral replication. The virus acquires a membrane envelope containing two types of spike glycoprotein, the hemagglutinin (HA) and the neuraminidase (NA), by budding through the host plasma membrane. The matrix protein (M1) is the most abundant protein in the virus and forms a layer associated with the inside of the envelope. An ion channel (M2) is also present in the virus envelope (3).
During infection, the virus attaches to cell surface receptors containing sialic acid, which are recognized by the HA protein (4), and the virus enters the cell by receptor-mediated endocytosis. At the low pH of the endosome, a large structural rearrangement of the HA exposes a hydrophobic fusion peptide and mediates fusion of the virus membrane with the host endosomal membrane (4), releasing the packaged contents of the virus (RNPs) into the host cytoplasm. In addition, acidification of the virus core through the M2 ion channel is necessary for membrane fusion and release of the RNPs (5, 6). The M1 protein also has an RNP-binding function that regulates transport of RNPs to the cell nucleus where replication and transcription of viral gene products can occur and subsequently may also transport RNPs to the plasma membrane where virus assembly occurs (7). The NA glycoprotein is a receptor-destroying enzyme that removes sialic acid from potential receptors and may have roles in both virus entry and exit (8). High resolution x-ray crystal structures of the HA trimer in the neutral pH conformation (9), proteolytic fragments of the HA in the low pH conformation (10, 11), and the NA tetramer (12, 13) have provided a molecular understanding of the function of these proteins.
Further understanding of the virus life cycle requires 3D studies of ultrastructure that can identify the specific molecular interactions that govern virus self-assembly. In addition, ultrastructural changes that are essential to membrane fusion and virion disassembly during cell entry remain to be described. Electron cryomicroscopy of influenza virions that are vitrified by rapid plunge-freezing (14–17) shows contrast from virion structures themselves and not from stain distributions, which can also distort or disrupt virus structure. However, an understanding of the structural organization of influenza virus has remained difficult because of its pleomorphic nature. Electron cryotomography can be used to directly determine the structure of pleomorphic virions, although at a resolution limited by the accumulated radiation damage and restricted angles of the tilt series.
In the present study, we investigate the structural basis for filamentous morphology in influenza virus. Filamentous morphology is typical of clinical isolates of influenza virus, whereas a spherical morphology is observed in many laboratory-passaged influenza strains (18, 19). Taking advantage of the surprising structural regularity displayed by an influenza virus (A/Udorn/72) of known, persistent filamentous phenotype (20), we combined electron cryotomography of frozen-hydrated virions with analysis of higher resolution projection images to elucidate the structural organization of influenza virus. We show that the M1 protein forms a helix associated with the uniform cylindrical structure of filamentous Udorn virions. A polar organization of the virion is evident in both the packaging of the genome and in the structure of the surrounding envelope, and applies generally to influenza virus based on comparison with images and tomograms we obtained for the non-filamentous influenza A/Aichi/68 X-31. We also studied acid-treated Udorn virus by electron tomography to image virus ultrastructure at the low pH of the endosome and observed a structural reorganization of the matrix layer associated with a change in virus morphology.
Results
Cryomicroscopy of Frozen-Hydrated Influenza Virions (H3N2) at pH 7.
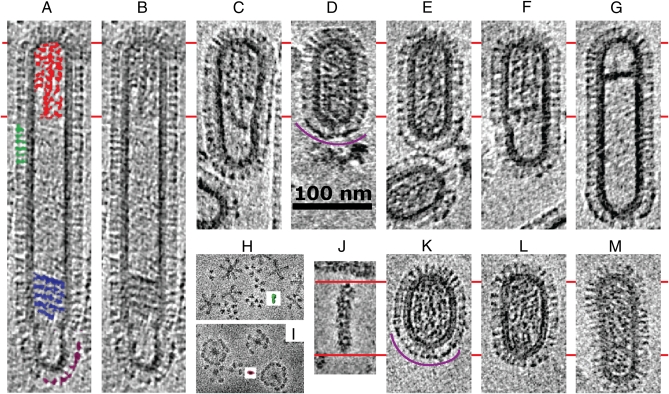
Fig. 1 shows a gallery of images of both Udorn and X-31 virions aligned by in-plane rotation to emphasize common features.
Fig. 1.
Tomogram sections of frozen-hydrated influenza A virions recorded in a single tomogram field. (A_–_G) A/Udorn/72 virions from single field shown in Fig. S1_A_ and Movie S1. Virion in A is identical to that in B, but has been manually colored to indicate features corresponding to RNPs (red), multilayered-coil (blue), NA (purple), and HA (green). (K, L, and M) A/Aichi/68 X-31 virions from single field shown in Fig. S1b and Movie S2. Negative stain images of (H) HA rosette (green HA trimer model inset), (I) NA rosette (purple NA head domain tetramer model inset), and (J) RNP purified from X-31. Red lines are used for comparison of RNP length. Purple arcs in D and K indicate typical NA clusters at one end.
Udorn virions (Fig. 1 A_–_G), recorded in a tomogram of a single field of view (tomogram in Movie S1 and Fig. S1_A_), show an extended morphology with capsule-shaped particles (cylindrical with a hemispherical cap at each end), typically about 120 nm in length, and longer filamentous particles, including some over 1 μm in length (Fig. S2). The virions usually have their long axis in the plane of the thin ice film, but some of the smaller capsule-shaped virions have their axis tilted with respect to the plane. The filamentous particles show a uniform diameter of about 55 nm (measured from the lipid bilayer), whereas the average diameter of the shorter capsule-shaped particles is somewhat wider at 59 nm. There is a tendency for end-to-end association of the virions like a string of sausages (Fig. S3).
X-31 virus (Fig. 1 K_–_M, Figs. S1_B_ and S4, and Movie S2) shows only occasional long filamentous particles, but, compared to a previous study by cryomicroscopy (16), we observed that a greater fraction are prolate ellipsoids with hemispherical ends. Images show 87 out of 145 particles are elongated by a factor greater than 1.5 along one axis. X-31 virions are wider (average diameter 70 nm, measured from the bilayer) than cylindrical Udorn virions.
The virions show an outer membrane containing surface glycoproteins and a dense matrix layer beneath the membrane. RNPs are visible inside many virions. The filamentous Udorn virions show that the RNPs are part of an assembly held at one end, and the rest of the interior is typically empty. Some particles appear to lack internal RNP segments yet retain a cylindrical morphology. Thus, morphology is maintained entirely by the matrix layer and the viral envelope without requiring RNPs.
The RNPs are part of an assembly that fits just within the inner diameter of the matrix layer and tapers as it enters the hemispherical cap. In filamentous particles, two RNPs extend further than the others (total length approximately 100 nm) and are similar in length to the longest purified RNP segments observed by negative stain microscopy (Fig. 1_J_). These may be tentatively assigned to genomic segments 1 and 2, which have the longest sequence. The RNPs only appear to contact the matrix layer at the tapered end of the assembly; they do not interact or make regular contacts with the matrix layer along their length. Some of the shorter virions are just long enough (120 nm) to package the longest, straight RNP segments.
Although the Udorn particles give a clearer view of the extent and asymmetry of the RNP assembly within the virion, the arrangement of RNPs within X-31 virions is highly similar (Fig. 1). However, the X-31 virions are shorter than Udorn so that the RNPs extend completely from one end to the other and follow the ellipsoidal profile of the matrix layer and membrane but still without making regular contacts. Thus, the X-31 RNP assembly appears slightly wider than the Udorn assembly.
The observed surface glycoproteins match their known crystallographic structures (Fig. 1). The tetrameric NA is slightly longer (14 nm measured from the membrane) than the HA trimer (13 nm) and shows less density closer to the membrane consistent with a narrow stalk, for which there is, as yet, no x-ray crystal structure. Although NAs may be found anywhere on the virus surface, the tomograms of Udorn virions show that NAs cluster at the end of the virion opposite to the end where the RNPs are attached (Fig. 1). NA clustering at one end is also evident in single Udorn images (72 out of 168 virion images have more than 3 NAs visible at only one end). Tomograms of X-31 also show NAs clustered at one hemispherical end of the virion (Fig. 1) and single images show clusters (at least 3 NAs) in 44 of 87 virions, and 25 of these virions show the clusters to be sited at one end of the virion. Because molecular overlap can complicate the interpretation of structures in projection, clustering at one end is most strongly demonstrated by tomography, and tomograms of filamentous Udorn particles now show that the end where clusters are found is opposite to the end where RNPs are attached.
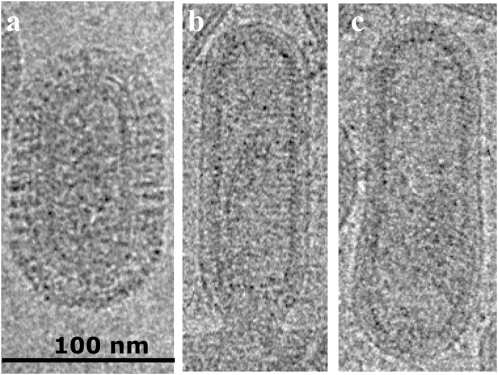
Fig. 2_A_ shows an image of an Udorn virion at defocus conditions that resolve both the bilayer and the adjacent matrix layer. Lines of density cross the bilayer and are likely to be the transmembrane regions of the HA. Fig. 2 B and C show Udorn virions where bromelain digestion has removed most of the glycoprotein layer. These reveal a clear view of the matrix layer showing thin projections toward the bilayer, which are also evident where it enters the hemispherical caps. Lines of density in the bilayer may be HA transmembrane regions that remain in contact with the matrix layer after glycoprotein removal. Because the distance between glycoprotein ectodomains is such that they do not contact each other, this implies that the spacing of the glycoproteins is attributable to interactions with the underlying matrix layer.
Fig. 2.
Low-dose images of Udorn virions. (A) Capsule-shaped virion at pH 7 and (B and C) after bromelain digestion.
Influenza Udorn Matrix Protein Forms a Helix.
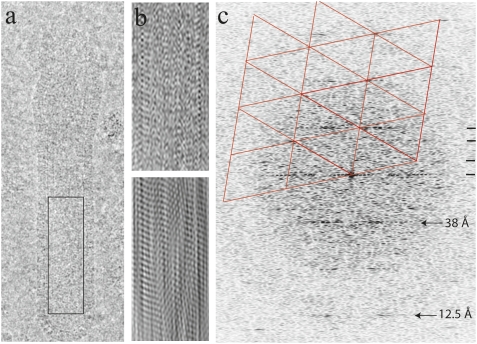
The matrix layer is a hollow cylinder that appears to have periodic features in images and tomograms. Because radiation damage limits the resolution of tomograms, we analyzed these periodic features in low dose images (Fig. 3_A_), which provide higher resolution information in projection. A diffraction pattern (Fourier transformation) (Fig. 3_C_) computed from the image shows that maxima occur along layer lines indicating a helical organization. A strong layer line at 38 Å corresponds to the pitch of a helix slightly inclined from the horizontal (although different angles are adopted in different images). Images obtained by noise filtering of the transform (Fig. 3_B_) to include data from layer lines only reveal the periodic pattern associated with the helical symmetry of the cylindrical matrix layer, consistent with a subunit of dimensions similar to M1 as derived from biophysical data and crystal structures for a proteolytic fragment of the protein (21–26). It has a similar appearance to negative stain images of coils obtained during isolation of the M1 protein (26). Thus, the helical component of the matrix layer is an organization of the M1 protein, although other viral or host proteins may associate with the matrix layer. Although a hollow cylinder of about 500-Å diameter, the helix is ordered to at least 12 Å and suggests a rigid structure.
Fig. 3.
Filamentous influenza A Udorn shows a helical organization of the matrix protein. (A) Image of a virion. (C) Fourier transform of the area within the membrane (black box) in A indicates a helical organization. A lattice (red) shows the prominent reflections arising from one side of the helix. The 38-Å reflection arises from a 7-start helix. (B) Images obtained after two-sided (Upper) and one-sided (Lower) filtering of the transform in C on three layer lines and the equator (indicated by black ticks at right).
Images recorded at specific defocus values show striations from the matrix layer that are limited by the inner radius of the membrane and continuously narrow in diameter as they enter the hemispherical cap regions (Fig. 2 B and C and Fig. S3_A_). These observations are consistent with M1 forming a spherical spiral, but it is not possible to assess the detailed molecular arrangement near the poles. Spirals have previously been identified in negative stain studies of disrupted virions (27).
Fourier transforms of occasional filamentous X-31 virions as well as ellipsoidal particles identify a similar 38-Å spacing to the matrix layer observed in Udorn, although with less order (Fig. S4), and this can be seen directly in images. The ellipsoidal shape of the matrix layer is consistent with a spiral of M1 with a radius determined by the membrane radius. Virions showing even more diversity of shape and width still show evidence of a similar, locally flat 2D lattice of the M1 protein, consistent with M1 being able to polymerize with a wide range of curvatures. A previously described mutant of the M1 protein (K102A) of A/WSN/33, obtained by reverse genetics, transforms a non-filamentous strain to a filamentous phenotype (28). In Fig. S5, we show that K102A shows a strong layer line at 38 Å, indicating a similar helical symmetry of the matrix layer to that of Udorn. Thus the helical structure is associated with a specific sequence of the M1 protein.
A Structural Transformation in the Matrix Layer.
To study changes in virus structure at the low pH encountered in the endosome, we imaged frozen-hydrated Udorn virions that were acid-treated or acid-treated and then trypsin-treated. Trypsin removes the HA1 residues 28–328 of HA molecules that have undergone the low pH-induced conformational change, which, if present, make it extremely difficult to observe individual glycoproteins. The trimeric HA2 portion of the molecule that remains after trypsin treatment (10) has previously been shown (29, 30) to be in a conformation where both its N-terminal fusion peptide and C-terminal transmembrane region are inserted into the virus membrane. The ectodomain appears as a thin stalk with a membrane distal knob, quite distinct from both the unchanged HA and the NA.
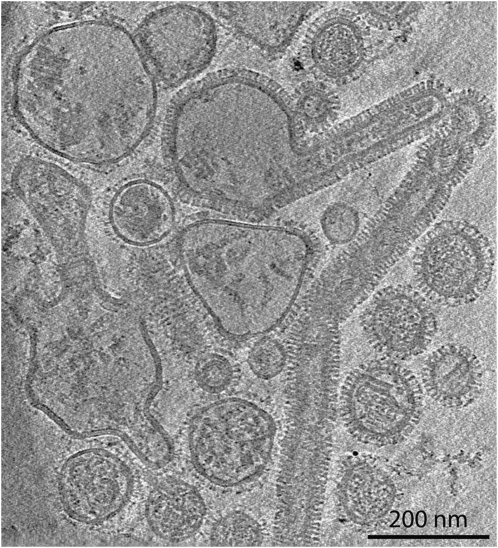
Tomogram sections (Fig. 4 and Movie S3) show a field of acid-treated, trypsin-treated Udorn virus displaying a much wider range of morphologies than is typically seen in untreated pH 7 virus. There are particles with filamentous and spherical shapes, particles of mixed morphology, and some that are likely to be the product of fusion events. The acid-induced change in shape is in agreement with previous studies, but the filamentous phenotype makes the change appear more dramatic (31). The large spherical particles likely represent particles that have lost their long filamentous shape, and the smaller spherical particles are probably former capsule-shaped particles. In Movie S4, three vesicular structures have their membranes distorted into tear-drop shapes by adjacent virions undergoing low-pH associated morphological changes.
Fig. 4.
Tomogram (also shown in Movies S3 and S4) section showing frozen-hydrated influenza A Udorn virions after acid treatment for 10 min at pH 4.9 and trypsin treatment after neutralization.
When viewing internal virus structures in the tomogram, it becomes evident that the loss of filamentous morphology is associated with disassembly of the highly organized matrix layer just beneath the membrane. The image of a spherical virion observed in Fig. 5_A_ shows a resolved lipid bilayer containing HA2 glycoproteins (low pH-induced conformational change has occurred) and no adjacent matrix layer. In contrast, the filamentous virion in Fig. 5_B_ has a matrix layer adjacent to and almost unresolvable from the lipid bilayer, and most of the HAs remain in neutral pH conformation. We observe a correlation between loss of filamentous shape, disruption of the matrix layer, and conformational change of the HA glycoprotein.
Fig. 5.
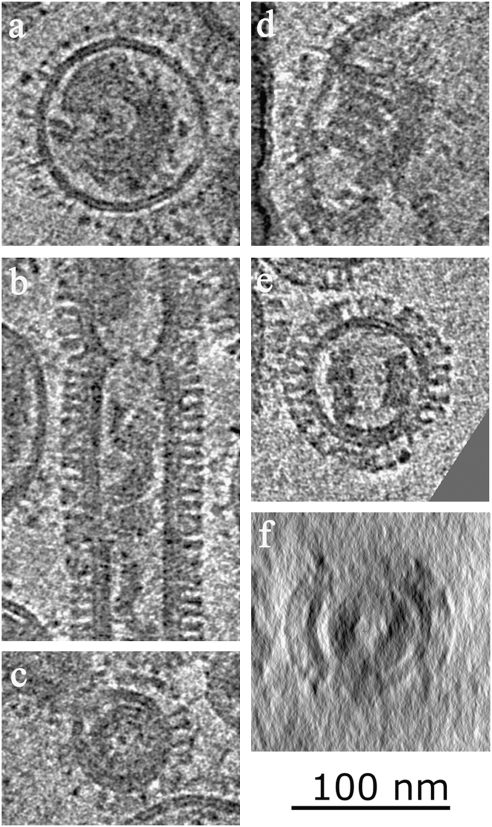
Gallery of multilayered coils from acid and trypsin-treated virions. (A) Spherical virion with multilayered coil axis perpendicular to image and resolved membrane. (B) Filamentous particle showing both intact matrix layer and matrix layer peeling off membrane to form a multilayered coil. (C) Small coil showing layered structure. (D) Multilayered coil viewed perpendicular to axis showing membrane attachment. (E) Side-on view of multilayered coil attached to membrane. (F) Same coil as E viewed down axis.
The filamentous particles show the matrix layer peeling away from the internal sides of the virions. The spherical particles are not filled with disorganized matrix layer, rather they possess large dense coiled structures, which are usually associated with the membrane in small areas where there is still an intact matrix layer adjacent to the membrane (Fig. 5 D and E). Where the matrix layer can be seen detaching from the membrane in the filamentous particles, it does so in a fashion that suggests the association of several strands of the helical matrix layer and a transition into the dense coiled structure observed in spherical particles.
Similar coil structures have been reported by negative stain microscopy on partially disrupted virions (27) and are not unique to the Udorn strain. Single images and tomograms show them to be a coiled structure of varying diameter with a typical pitch of 110 Å, thick walls and a hollow interior (Fig. 5 D and E). The multilayered structure of the coil is most easily viewed when the axis of the coil is along the vertical direction of the tomogram (Fig. 5 A and C), where the walls of the coil consist of several layers, each of similar thickness to the helical matrix layer. Fourier transform of projections perpendicular to the coil axis (Fig. S6) show spacings of 36 Å and 45 Å, similar to the spacings observed in the 3D packing of the N-terminal domain of M1 in crystal structures.
It has been suggested that these dense coiled structures (which are also occasionally apparent in pH 7 virus preparations) are composed of alignments of the RNP segments (16). In most of the particles where dense coils are seen in this tomogram, free RNP segments are clearly visible (Movie S4). The RNPs resemble those seen in filamentous pH 7 particles, and those seen in isolated and purified RNP preparations (Fig. 1_J_). They are no longer held together as a package at one end of the virion. Some do appear to be associated with the dense coils via one end, but they are separate and distinct structures. Disruption of the virus glycoprotein layer by bromelain digestion or mercaptoethanol treatment can also promote the conversion of the matrix layer to multilayered coils with identical transform properties (Fig. S7). Images of Udorn particles that are acid-treated without subsequent trypsin treatment also show a loss of filamentous shape, disruption of the matrix layer, and presence of multilayered, dense coiled structures (Fig. S8). We cannot discern the shape of individual glycoproteins, presumably due to disorder of the HA1 domains.
Discussion
The structural organization of filamentous influenza virus particles suggests a model for their assembly. We have shown that a highly ordered, helical organization of the matrix layer is associated with the cylindrical morphology of filamentous and capsule-shaped Udorn influenza virus. Previously, the M1 gene segment has been identified as a genetic determinant of filamentous morphology (20, 28, 32, 33). Although the M1 protein alone was reported to be essential for virus-like particle (VLP) formation and capable of forming intracellular tubes (34), it now appears that viral membrane proteins are required for budding (35). A helix provides a specific structural mechanism by which M1 subunits can be added to a rigid assembly that drives budding at the plasma membrane. Regular interactions between the matrix layer and membrane components suggest that recruitment of membrane glycoproteins couples M1 helix formation with budding through the membrane.
Images of filamentous influenza particles budding from cells, obtained by electron microscopy of stained, plastic sections, suggest that an RNP assembly is found on the apical end of a budding virion and distal to the virus membrane (36). It is likely that the RNP assembly may be an important trigger for budding. The M1 helix is nucleated near the point of attachment of the RNP assembly and the subsequent polymerization process drives filamentous virion budding. RNPs may influence the width of the surrounding matrix layer and also determine the minimum length of the virion before pinching-off can occur.
The sequence of the Udorn M1 protein likely confers the filamentous phenotype by stabilizing specific helical contacts in the matrix layer (28). Length variation in Udorn particles may reflect a competition between extension of the M1 helix and the completion of budding (“pinching off”) and release. The “beaded” structures that result from the end-to-end association, reminiscent of budding defects in other lipid-enveloped viruses (37), may reflect this competition. Cellular factors associated with the vacuolar sorting pathway are implicated in virus budding and release for several virus families, and some of these may form protein assemblies that regulate the physical constriction required for pinching-off (38). It is not known what host factors regulate orthomyxovirus budding (39, 40), but host factors such as actin and the virus M2 protein may contribute to the filamentous phenotype (20, 41, 42).
The ellipsoidal shape of the typical X-31 matrix layer may be thought of as two spiral structures similar to those in the hemispherical caps of the Udorn virions but without the intervening cylindrical region. The shape of the X-31 matrix layer reflects the absence of strong intrinsic helical or cylindrical polymerization of the X-31 M1 protein due to sequence differences to Udorn M1 and may be influenced by the shape of the RNP assembly or the membrane. The M1 protein’s capacity to polymerize in 2D lattices with a wide range of curvature is an important basis for influenza virus pleomorphy and reflects the use of a comparatively small building block to build a structure with a large radius.
The budding driven by polymerization of the M1 helix may lead to the observed segregation of HA and NA in the viral membrane, either by recruiting HA through specific contacts between M1 and the HA cytoplasmic domain or by exclusion of NA clusters that display lateral interactions between head-domains (Fig.1_I_, NA rosettes). NA clusters may lead to pinching-off by uncoupling matrix layer extension from glycoprotein recruitment and bud extension. The cytoplasmic domains of the HA and NA can both influence particle shape (43, 44). Membrane microdomains such as “rafts” are thought to concentrate the HA at the sites of budding (45). VLPs have also been reported with expression of the HA and NA in the absence of the matrix protein (35), but we assume these cannot possess the axial organization driven by M1 helix polymerization and glycoprotein recruitment as described here.
The organization of glycoproteins in the virus envelope may be of functional significance. The rigidity, radius of curvature, and regular spacing of the HA glycoprotein lattice may provide specific geometric constraints on receptor-binding and membrane fusion. Although clustering of NAs on the virus surface has been described before (16, 46), our observation of clustering at the end of the virion opposite the RNPs, close to the point of release from the host cell membrane and in an orientation such that the neuramindase active site is positioned to hydrolyze receptors on the host cell surface, may reflect a localized requirement for receptor destruction (46). Inhibitors of NA enzyme activity result in a virus that is stalled or aggregated at the host membrane. NA inhibitors that are anchored to the membrane could target this site of action.
In viruses incubated at the low pH of the endosome, there is a correlation between loss of filamentous shape, disassembly of the matrix layer, and conformational change of the HAs. We observe that the helical matrix layer dissociates from the virus membrane at low pH, forming an alternative, stable assembly described here as a multilayered coil. We also find intermediate structures in this coiling transition. In addition to the M2 ion channel, acidification of the virus interior could be a consequence of insertion of the fusion peptide of HA into the virus membrane. Because the multilayered coil can also form at neutral pH, particularly when the glycoproteins are disrupted, low pH is not a requirement for their formation, but rather they are a consequence of concerted dissociation of the matrix layer from the membrane. Disassembly of the matrix layer at low pH may facilitate membrane fusion by exposing bare patches of membrane bilayer or by allowing freedom of movement of the glycoproteins to form fusogenic complexes. Acidification of the virion interior through the M2 ion channel increases the rate of membrane fusion between influenza viruses and liposomes (5). Matrix disassembly and the associated morphological changes also facilitate release of individual RNP segments from the assembly attached at the apical tip of the virion.
Materials and Methods
Growth and Purification of Virus.
A/Udorn/72 (a gift from R. Compans, Emory University School of Medicine, Atlanta, GA), A/WSN/33, and M1 mutant K102A (a gift from D. Steinhauer, Emory University School of Medicine, Atlanta, GA) were grown in Madin-Darby canine kidney (MDCK) cells and purified over sucrose gradients as previously described (28). A/Aichi/68 X-31 virus was grown in embryonated hens’ eggs and purified over sucrose gradients as previously described (47).
Low pH Incubation and Trypsin Treatment of Virus.
Virus was taken to pH 4.9 with 0.1 M sodium citrate and incubated at 20 °C for 10 min and then neutralized with 1 M Tris. In the case of further trypsin treatment, virus was then incubated with trypsin (Sigma) at an enzyme:protein ratio of 1:50 for 1 h at 20 °C, before stopping the reaction with trypsin inhibitor (Sigma). Virus was then pelleted and resuspended in PBS.
Bromelain Digestion of Virus.
Virus in 0.1 M Tris buffer pH 8.0 was incubated with bromelain (Sigma) at an enzyme:viral protein ratio of 1:2 together with 50 mM β-mercaptoethanol for 3 h at 37 °C. A further aliquot of bromelain and mercaptoethanol was added (at the same enzyme:viral protein ratio) and the sample incubated at room temperature for 18 h; 40 mM iodoacetamide was added to stop the digestion and the virus pelleted (TL100 centrifuge, 55,000 rpm, 10 min) and resuspened in PBS. Control virus samples were also treated under identical conditions with either iodoacetamide or mercaptoethanol in the absence of bromelain.
Electron Cryomicroscopy.
Four microliters of virus sample was mixed with 10-nm gold particles (British-Biocell) and applied to amylamine glow-discharged 200 mesh copper Quantifoil (R2/4) grids in the environment chamber (4 °C, 90% RH) of a Vitrobot Mark III (FEI), blotted on both sides with a double layer of paper for 4 s before plunging into liquid ethane. Grids were transferred to a Gatan 626 tomography holder or Polara stage. Imaging was performed in an FEI Spirit TWIN microscope at 120 keV using a tungsten filament source and equipped with a cryobox around the sample. Images were recorded unbinned on an Eagle 2K camera (FEI) at 21 K (10.0 Å/pixel), 30 K (7.2 Å/pixel), or 52 K (4.3 Å/pixel) magnification under a range of defocus conditions. Magnification was calibrated by recording images of tobacco mosaic virus under identical conditions. Tilt series for tomography were recorded as individual low-dose images or automatically using Inspect3D (FEI) from 0 to ±66° in 3° or 4° steps, typically with a total dose less than 50 e-/Å2. An FEI G2 Polara operating at liquid nitrogen temperature and 300 keV was used to collect images and tilt series on a 224 HD detector (TVIPS) at 15 K (9.1 Å/pixel), or on film at 59 K magnification, which was digitized at 7 μm/pixel on a Z/I scanner densitometer.
Image and Tilt Series Analysis.
Tomographic tilt series were aligned using IMOD software (48). Alignment initially used cross-correlation and then used gold particles or other dense features as fiducials. Reconstructed 3D volumes were generated by back-projection or by an iterative alignment and reconstruction procedure using the Priism package (49). Images were analyzed using FFTRANS and Ximdisp programs from the MRC package (50). The filtered images (Fig. 3_B_) were obtained by masking transforms (program TRMSK) to include peaks from both sides of the indicated layer lines or from one side corresponding to points on the lattice (Fig. 3_C_) and the equator. In the tomogram in Movie S2, gold particle fiducials were computationally removed from aligned tilt series before reconstruction.
Supplementary Material
Supporting Information
Acknowledgments
This work was funded by the Medical Research Coun-cil (United Kingdom) under program code U117581334.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Coloma R, et al. The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog. 2009;5:e1000491. doi: 10.1371/journal.ppat.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Area E, et al. 3D structure of the influenza virus polymerase complex: Localization of subunit domains. Proc Natl Acad Sci USA. 2004;101:308–313. doi: 10.1073/pnas.0307127101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson DC, et al. Electron microscopic evidence for the association of M2 protein with the influenza virion. Arch Virol. 1991;118:199–207. doi: 10.1007/BF01314030. [DOI] [PubMed] [Google Scholar]
- 4.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 5.Wharton SA, Belshe RB, Skehel JJ, Hay AJ. Role of virion M2 protein in influenza virus uncoating: Specific reduction in the rate of membrane fusion between virus and liposomes by amantadine. J Gen Virol. 1994;75:945–948. doi: 10.1099/0022-1317-75-4-945. [DOI] [PubMed] [Google Scholar]
- 6.Zhirnov OP. Isolation of matrix protein M1 from influenza viruses by acid-dependent extraction with nonionic detergent. Virology. 1992;186:324–330. doi: 10.1016/0042-6822(92)90090-c. [DOI] [PubMed] [Google Scholar]
- 7.Noton SL, et al. Identification of the domains of the influenza A virus M1 matrix protein required for NP binding, oligomerization and incorporation into virions. J Gen Virol. 2007;88:2280–2290. doi: 10.1099/vir.0.82809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Eichelberger MC, Compans RW, Air GM. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature. 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 10.Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Skehel JJ, Wiley DC. N- and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil. Proc Natl Acad Sci USA. 1999;96:8967–8972. doi: 10.1073/pnas.96.16.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins PJ, et al. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature. 2008;453:1258–1261. doi: 10.1038/nature06956. [DOI] [PubMed] [Google Scholar]
- 13.Varghese JN, Laver WG, Colman PM. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 Å resolution. Nature. 1983;303:35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- 14.Booy FP, Ruigrok RW, van Bruggen EF. Electron microscopy of influenza virus. A comparison of negatively stained and ice-embedded particles. J Mol Biol. 1985;184:667–676. doi: 10.1016/0022-2836(85)90312-2. [DOI] [PubMed] [Google Scholar]
- 15.Fujiyoshi Y, Kume NP, Sakata K, Sato SB. Fine structure of influenza A virus observed by electron cryo-microscopy. EMBO J. 1994;13:318–326. doi: 10.1002/j.1460-2075.1994.tb06264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris A, et al. Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc Natl Acad Sci USA. 2006;103:19123–19127. doi: 10.1073/pnas.0607614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi M, Danev R, Nishiyama K, Sugawara K, Nagayama K. Zernike phase contrast electron microscopy of ice-embedded influenza A virus. J Struct Biol. 2008;162:271–276. doi: 10.1016/j.jsb.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Choppin PW, Murphy JS, Tamm I. Studies of two kinds of virus particles which comprise influenza A2 virus strains. III. Morphological characteristics: Independence to morphological and functional traits. J Exp Med. 1960;112:945–952. doi: 10.1084/jem.112.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilbourne ED, Murphy JS. Genetic studies of influenza viruses. I. Viral morphology and growth capacity as exchangeable genetic traits. Rapid in ovo adaptation of early passage Asian strain isolates by combination with PR8. J Exp Med. 1960;111:387–406. doi: 10.1084/jem.111.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts PC, Lamb RA, Compans RW. The M1 and M2 proteins of influenza A virus are important determinants in filamentous particle formation. Virology. 1998;240:127–137. doi: 10.1006/viro.1997.8916. [DOI] [PubMed] [Google Scholar]
- 21.Akarsu H, et al. Crystal structure of the M1 protein-binding domain of the influenza A virus nuclear export protein (NEP/NS2) EMBO J. 2003;22:4646–4655. doi: 10.1093/emboj/cdg449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arzt S, et al. Combined results from solution studies on intact influenza virus M1 protein and from a new crystal form of its N-terminal domain show that M1 is an elongated monomer. Virology. 2001;279:439–446. doi: 10.1006/viro.2000.0727. [DOI] [PubMed] [Google Scholar]
- 23.Arzt S, Petit I, Burmeister WP, Ruigrok RW, Baudin F. Structure of a knockout mutant of influenza virus M1 protein that has altered activities in membrane binding, oligomerisation and binding to NEP (NS2) Virus Res. 2004;99:115–119. doi: 10.1016/j.virusres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Harris A, Forouhar F, Qiu S, Sha B, Luo M. The crystal structure of the influenza matrix protein M1 at neutral pH: M1-M1 protein interfaces can rotate in the oligomeric structures of M1. Virology. 2001;289:34–44. doi: 10.1006/viro.2001.1119. [DOI] [PubMed] [Google Scholar]
- 25.Harris A, Sha B, Luo M. Structural similarities between influenza virus matrix protein M1 and human immunodeficiency virus matrix and capsid proteins: An evolutionary link between negative-stranded RNA viruses and retroviruses. J Gen Virol. 1999;80:863–869. doi: 10.1099/0022-1317-80-4-863. [DOI] [PubMed] [Google Scholar]
- 26.Ruigrok RW, et al. Membrane interaction of influenza virus M1 protein. Virology. 2000;267:289–298. doi: 10.1006/viro.1999.0134. [DOI] [PubMed] [Google Scholar]
- 27.Ruigrok RW, Calder LJ, Wharton SA. Electron microscopy of the influenza virus submembranal structure. Virology. 1989;173:311–316. doi: 10.1016/0042-6822(89)90248-1. [DOI] [PubMed] [Google Scholar]
- 28.Burleigh LM, Calder LJ, Skehel JJ, Steinhauer DA. Influenza A viruses with mutations in the m1 helix six domain display a wide variety of morphological phenotypes. J Virol. 2005;79:1262–1270. doi: 10.1128/JVI.79.2.1262-1270.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruigrok RW, et al. Electron microscopy of the low pH structure of influenza virus haemagglutinin. EMBO J. 1986;5:41–49. doi: 10.1002/j.1460-2075.1986.tb04175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wharton SA, et al. Electron microscopy of antibody complexes of influenza virus haemagglutinin in the fusion pH conformation. EMBO J. 1995;14:240–246. doi: 10.1002/j.1460-2075.1995.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shangguan T, et al. Morphological changes and fusogenic activity of influenza virus hemagglutinin. Biophys J. 1998;74:54–62. doi: 10.1016/S0006-3495(98)77766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourmakina SV, García-Sastre A. Reverse genetics studies on the filamentous morphology of influenza A virus. J Gen Virol. 2003;84:517–527. doi: 10.1099/vir.0.18803-0. [DOI] [PubMed] [Google Scholar]
- 33.Elleman CJ, Barclay WS. The M1 matrix protein controls the filamentous phenotype of influenza A virus. Virology. 2004;321:144–153. doi: 10.1016/j.virol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Gómez-Puertas P, Albo C, Pérez-Pastrana E, Vivo A, Portela A. Influenza virus matrix protein is the major driving force in virus budding. J Virol. 2000;74:11538–11547. doi: 10.1128/jvi.74.24.11538-11547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen BJ, Leser GP, Morita E, Lamb RA. Influenza virus hemagglutinin and neuraminidase, but not the matrix protein, are required for assembly and budding of plasmid-derived virus-like particles. J Virol. 2007;81:7111–7123. doi: 10.1128/JVI.00361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noda T, et al. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature. 2006;439:490–492. doi: 10.1038/nature04378. [DOI] [PubMed] [Google Scholar]
- 37.Yuan B, Campbell S, Bacharach E, Rein A, Goff SP. Infectivity of Moloney murine leukemia virus defective in late assembly events is restored by late assembly domains of other retroviruses. J Virol. 2000;74:7250–7260. doi: 10.1128/jvi.74.16.7250-7260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabrikant G, et al. Computational model of membrane fission catalyzed by ESCRT-III. PLOS Comput Biol. 2009;5:e1000575. doi: 10.1371/journal.pcbi.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruce EA, et al. Budding of filamentous and non-filamentous influenza A virus occurs via a VPS4 and VPS28-independent pathway. Virology. 2009;390:268–278. doi: 10.1016/j.virol.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Chen BJ, Lamb RA. Mechanisms for enveloped virus budding: Can some viruses do without an ESCRT? Virology. 2008;372:221–232. doi: 10.1016/j.virol.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts PC, Compans RW. Host cell dependence of viral morphology. Proc Natl Acad Sci USA. 1998;95:5746–5751. doi: 10.1073/pnas.95.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson-Holley M, et al. A functional link between the actin cytoskeleton and lipid rafts during budding of filamentous influenza virions. Virology. 2002;301:212–225. doi: 10.1006/viro.2002.1595. [DOI] [PubMed] [Google Scholar]
- 43.Jin H, Leser GP, Lamb RA. The influenza virus hemagglutinin cytoplasmic tail is not essential for virus assembly or infectivity. EMBO J. 1994;13:5504–5515. doi: 10.1002/j.1460-2075.1994.tb06885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin H, Leser GP, Zhang J, Lamb RA. Influenza virus hemagglutinin and neuraminidase cytoplasmic tails control particle shape. EMBO J. 1997;16:1236–1247. doi: 10.1093/emboj/16.6.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda M, Leser GP, Russell CJ, Lamb RA. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc Natl Acad Sci USA. 2003;100:14610–14617. doi: 10.1073/pnas.2235620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murti KG, Webster RG. Distribution of hemagglutinin and neuraminidase on influenza virions as revealed by immunoelectron microscopy. Virology. 1986;149:36–43. doi: 10.1016/0042-6822(86)90084-x. [DOI] [PubMed] [Google Scholar]
- 47.Skehel JJ, Schild GC. The polypeptide composition of influenza A viruses. Virology. 1971;44:396–408. doi: 10.1016/0042-6822(71)90270-4. [DOI] [PubMed] [Google Scholar]
- 48.Mastronarde DN. Dual-axis tomography: An approach with alignment methods that preserve resolution. J Struct Biol. 1997;120:343–352. doi: 10.1006/jsbi.1997.3919. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Hughes DD, Chan TA, Sedat JW, Agard DA. IVE (Image Visualization Environment): A software platform for all three-dimensional microscopy applications. J Struct Biol. 1996;116:56–60. doi: 10.1006/jsbi.1996.0010. [DOI] [PubMed] [Google Scholar]
- 50.Crowther RA, Henderson R, Smith JM. MRC image processing programs. J Struct Biol. 1996;116:9–16. doi: 10.1006/jsbi.1996.0003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information