Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes (original) (raw)
. Author manuscript; available in PMC: 2014 May 19.
Published in final edited form as: N Engl J Med. 2010 Jun 29;363(3):233–244. doi: 10.1056/NEJMoa1001288
Abstract
BACKGROUND
We investigated whether intensive glycemic control, combination therapy for dyslipidemia, and intensive blood-pressure control would limit the progression of diabetic retinopathy in persons with type 2 diabetes. Previous data suggest that these systemic factors may be important in the development and progression of diabetic retinopathy.
METHODS
In a randomized trial, we enrolled 10,251 participants with type 2 diabetes who were at high risk for cardiovascular disease to receive either intensive or standard treatment for glycemia (target glycated hemoglobin level, <6.0% or 7.0 to 7.9%, respectively) and also for dyslipidemia (160 mg daily of fenofibrate plus simvastatin or placebo plus simvastatin) or for systolic blood-pressure control (target, <120 or <140 mm Hg). A subgroup of 2856 participants was evaluated for the effects of these interventions at 4 years on the progression of diabetic retinopathy by 3 or more steps on the Early Treatment Diabetic Retinopathy Study Severity Scale (as assessed from seven-field stereoscopic fundus photographs, with 17 possible steps and a higher number of steps indicating greater severity) or the development of diabetic retinopathy necessitating laser photocoagulation or vitrectomy.
RESULTS
At 4 years, the rates of progression of diabetic retinopathy were 7.3% with intensive glycemia treatment, versus 10.4% with standard therapy (adjusted odds ratio, 0.67; 95% confidence interval [CI], 0.51 to 0.87; P = 0.003); 6.5% with fenofibrate for intensive dyslipidemia therapy, versus 10.2% with placebo (adjusted odds ratio, 0.60; 95% CI, 0.42 to 0.87; P = 0.006); and 10.4% with intensive blood-pressure therapy, versus 8.8% with standard therapy (adjusted odds ratio, 1.23; 95% CI, 0.84 to 1.79; P=0.29).
CONCLUSIONS
Intensive glycemic control and intensive combination treatment of dyslipidemia, but not intensive blood-pressure control, reduced the rate of progression of diabetic retinopathy. (Funded by the National Heart, Lung, and Blood Institute and others; ClinicalTrials.gov numbers, NCT00000620 for the ACCORD study and NCT00542178 for the ACCORD Eye study.)
Diabetic retinopathy, an important microvascular complication of diabetes, is a leading cause of blindness in the United States.1 Randomized, controlled clinical trials in cohorts of patients with type 1 diabetes and those with type 2 diabetes have shown the beneficial effects of intensive glycemic control2–5 and intensive treatment of elevated blood pressure6 on the progression of diabetic retinopathy. Elevated serum cholesterol and triglyceride levels have been implicated, in observational studies and small trials, as additional risk factors for the development of diabetic retinopathy and visual loss.7–14 The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study (Current Controlled Trials number, ISRCTN64783481) of participants with type 2 diabetes showed a beneficial effect of fenofibrate (at a dose of 200 mg per day) on the progression of diabetic retinopathy.15
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study was a randomized, controlled clinical trial that evaluated the effects of specific strategies for managing blood glucose levels, serum lipid levels, and blood pressure on cardiovascular events in participants with type 2 diabetes who had either established cardiovascular disease or known cardiovascular risk factors. Through the ACCORD trial, we had the opportunity to evaluate the effects of these medical strategies on the progression of diabetic retinopathy in a subgroup of trial participants (the ACCORD Eye study).
METHODS
THE ACCORD STUDY
The designs of the ACCORD study and the ACCORD Eye study are described elsewhere.16,17 Briefly, the ACCORD study was a randomized trial conducted at 77 clinical sites in the United States and Canada. Participating institutions and investigators are listed in Section 1 in the Supplementary Appendix (available with the full text of this article at NEJM.org). The trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI), and the protocol (also available at NEJM.org) was approved by a review panel at the NHLBI, as well as by the institutional review board at each center. The study drugs were donated by the manufacturers; the companies did not participate in the study design or conduct, data accrual or analysis, or manuscript preparation.
In the ACCORD trial, 10,251 participants with type 2 diabetes and a glycated hemoglobin level of 7.5% or higher were randomly assigned to undergo either intensive glycemic control (targeting a glycated hemoglobin level <6.0%) or standard therapy (targeting a glycated hemoglobin level of 7.0 to 7.9%). Of these participants, 5518 with dyslipidemia were also randomly assigned, in a 2-by-2 factorial design, to receive simvastatin (to reduce low-density lipoprotein [LDL] cholesterol levels) in combination with either fenofibrate (to reduce triglyceride levels and increase high-density lipoprotein [HDL] cholesterol levels) or matching placebo. The remaining 4733 participants were randomly assigned, in a 2-by-2 factorial design, to undergo either intensive blood-pressure control (targeting a systolic blood pressure <120 mm Hg) or standard therapy (targeting a systolic blood pressure <140 mm Hg).
The primary outcome of the ACCORD trial was the composite end point of the time until the first occurrence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. Members of the ACCORD data and safety monitoring board are listed in the Appendix, investigators participating in the ACCORD trial are listed in Section 1 in the Supplementary Appendix, and details of the study design are provided in the ACCORD protocol.
THE ACCORD EYE STUDY
The ACCORD trial participants were evaluated for eligibility for the ACCORD Eye study. Participants who, at baseline, had a history of proliferative diabetic retinopathy that had been treated with laser photocoagulation or vitrectomy were excluded. Investigators participating in the ACCORD Eye study are listed in Section 2 in the Supplementary Appendix, and details of the study design are provided in the ACCORD protocol. The writing group attests to the fidelity of the report to the protocol.
The ACCORD Eye study consisted of two comprehensive, standardized eye examinations conducted by a study ophthalmologist or optometrist, along with fundus photography of seven standard stereoscopic fields, at baseline and year 4 of follow-up. The fundus photographs were evaluated by trained graders, who were unaware of the treatment assignments, at the Fundus Photograph Reading Center (University of Wisconsin, Madison), on the basis of the photographic standards defined for the Early Treatment Diabetic Retinopathy Study (ETDRS) and graded according to an abbreviated and modified version of the ETDRS Final Retinopathy Severity Scale for Persons, which combines the severity levels from both eyes for each person.18 The scale has 17 steps, ranging from no retinopathy in either eye (step 1) to high-risk proliferative retinopathy in both eyes (step 17); details are provided in Section 3 in the Supplementary Appendix. Information collected at the annual visits in the main ACCORD trial was also used to determine whether retinal laser photocoagulation or vitrectomy had been performed to treat diabetic retinopathy during the previous year. Details of the ACCORD Eye study design are provided in the ACCORD Eye protocol. Visual acuity, measured every 2 years in all ACCORD participants, was examined for treatment effects on moderate vision loss, which was defined as worsening, in either eye, by three or more lines on the ETDRS visual acuity chart (see the protocol).
The primary outcome of the ACCORD Eye study was the composite end point of either progression of diabetic retinopathy by at least three steps on the ETDRS Severity Scale or development of proliferative diabetic retinopathy necessitating photocoagulation therapy or vitrectomy. The primary aim was to determine whether any of the three interventions evaluated in the ACCORD trial (intensive glycemic therapy, the addition of fenofibrate to a statin, and intensive blood-pressure therapy) reduced the risk of development or progression of diabetic retinopathy, as compared with the respective standard treatments.
STATISTICAL ANALYSIS
For the outcome of the rate of progression of diabetic retinopathy, we set a recruitment goal for the ACCORD Eye study to achieve a statistical power of 88% to detect a 15% relative reduction with intensive glycemic control as compared with standard glycemic control; a statistical power of 91% to detect a 20% relative reduction with lipid control with a statin and fenofibrate as compared with lipid control with a statin alone; and a statistical power of 80% to detect a 20% relative reduction with intensive blood-pressure control as compared with standard blood-pressure control. The sample size required was 3211 participants. To accommodate the potential for a mortality rate of 10%, a loss to follow-up of 10% of patients, and lack of sufficient dilation for fundus photography in 1% of patients, the recruitment goal was increased to 4065 participants. Details of the sample-size calculations have been described previously.17
Comparisons of achieved levels of glycated hemoglobin, HDL cholesterol, and triglycerides and systolic blood pressure were performed with the use of the Wilcoxon rank-sum test and the 95% rank-order confidence interval for the median. Separate models were used for the three primary hypotheses (concerning glycemic control, lipid control, and blood-pressure control). The main comparisons between the intensive-therapy groups and the standard-therapy groups, with respect to the development and progression of diabetic retinopathy over the 4 years (the results of the eye examinations at baseline and those at year 4), were made using likelihood-ratio tests from logistic-regression models with adjustment for the same study-design factors used in the ACCORD primary analysis, including previous cardiovascular events and the specific network center that supervises the clinical center. The glucose model was adjusted for the presence or absence of fenofibrate therapy and intensive blood-pressure therapy and for trial (ACCORD Lipid or ACCORD Blood Pressure). The lipid and blood-pressure models were adjusted for glycemia treatment. Cox proportional-hazards models were used to test for differences between treatment groups in visual acuity.
We performed 28 protocol-specified comparisons of subgroups defined on the basis of cutoff points that had been either previously chosen,17 used in the main ACCORD Glycemia, ACCORD Lipid, and ACCORD Blood-Pressure studies,19–21 or chosen to divide each main group into two nearly equal subgroups. Additional, post hoc comparisons were performed for the effect on glycemia between patients also enrolled in the lipid trial and patients also enrolled in the blood-pressure trial, between patients who had both high triglyceride and low HDL cholesterol levels and patients with lower triglyceride levels or higher HDL cholesterol levels (in the lipid trial), between patients with some degree of retinopathy and those without retinopathy (in the lipid trial and the blood-pressure trial), and according to categories of systolic and diastolic blood pressure and number of blood-pressure medications (in the blood-pressure trial). Tests of interaction of baseline characteristics and other variables with treatment effect were performed by adding the subgroup and the interaction term to the primary models and applying a likelihood-ratio test for the interaction. No adjustment for multiple comparisons was made.
We explored the effect of excluding from the primary outcome events not verified by photographic evidence or clinical examination. To examine the effect of missing data on our conclusions, we conducted unadjusted analyses and adjusted analyses (using the primary models) of the proportions of patients with missing data in each treatment group. Sensitivity analyses involved the use of a logistic-regression method for multiple imputation,22 as implemented in SAS software (version 9.2, SAS Institute). The imputation model included the variables in the primary models plus the variables used to define the subgroups. Imputations were done twice for each comparison: the first, separately in each treatment group, and the second, in the combined treatment groups.
RESULTS
Recruitment in the ACCORD trial began with a vanguard phase in January 2001; the main trial began in February 2003. The ACCORD Eye study began in October 2003, with 3537 participants enrolled by February 2006. Of these, 65 (1.8%) were later found to be ineligible after recruitment, leaving 3472 eligible for follow-up. Of these, 2856 (82.3%) participants had both baseline and year 4 follow-up data available for analyses (see Section 4 in the Supplementary Appendix). Because the ACCORD Eye study lagged behind the main ACCORD trial, there was insufficient time to achieve the calculated sample size.
BASELINE CHARACTERISTICS
The characteristics of the ACCORD Eye study cohort with follow-up data, the ACCORD Eye study cohort without follow-up data, and the remainder of the overall ACCORD cohort are shown in Section 5 in the Supplementary Appendix. Participants in the ACCORD Eye study with follow-up data, as compared with the remainder of the ACCORD cohort, tended to be younger, with a shorter duration of diabetes; lower LDL cholesterol level, systolic blood pressure, urinary albumin:creatinine ratio, and rate of previous cardiovascular events; slightly better visual acuity; and a higher likelihood of being white.
The baseline characteristics of the 2856 participants with follow-up data in the ACCORD Eye study are presented, according to treatment group, in Table 1. Inclusion in the ACCORD Lipid study required an HDL cholesterol level of less than 55 mg per deciliter (1.4 mmol per liter) for women and blacks and less than 50 mg per deciliter (1.3 mmol per liter) for all others; this resulted in lower HDL cholesterol levels in these participants as compared with the remaining ACCORD participants.23
Table 1.
Baseline Characteristics of the ACCORD Eye Study Participants Also Enrolled in the Glycemia, Lipid, or Blood-Pressure Trial.*
| Characteristic | ACCORD Eye (N = 2856) | ACCORD Glycemia | ACCORD Lipid | ACCORD Blood Pressure | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensive (N = 1429) | Standard (N = 1427) | P Value | Fibrate (N = 806) | Placebo (N = 787) | P Value | Intensive (N = 647) | Standard (N = 616) | P Value | ||
| Age — yr | 61.6±6.3 | 61.6±6.4 | 61.5±6.3 | 0.60 | 61.9±6.2 | 61.5±6.5 | 0.05 | 61.3±6.1 | 61.5±6.6 | 0.23 |
| Duration of diabetes — yr | 10.0±7.1 | 9.8±7.1 | 10.1±7.2 | 0.30 | 9.7±6.8 | 9.8±7.2 | 0.29 | 10.1±7.0 | 10.3±7.5 | 0.46 |
| Female sex — no. (%) | 1090 (38.2) | 538 (37.6) | 552 (38.7) | 0.57 | 247 (30.6) | 254 (32.3) | <0.001 | 310 (47.9) | 279 (45.3) | <0.001 |
| Previous cardiovascular event — no. (%) | 895 (31.3) | 452 (31.6) | 443 (31.0) | 0.74 | 263 (32.6) | 255 (32.4) | 0.35 | 180 (27.8) | 197 (32.0) | 0.03 |
| Nonwhite race — no. (%)† | 860 (30.1) | 427 (29.9) | 433 (30.3) | 0.79 | 222 (27.5) | 234 (29.7) | 0.06 | 203 (31.4) | 201 (32.6) | 0.43 |
| Glycated hemoglobin — % | 8.2±1.0 | 8.2±1.0 | 8.3±1.0 | 0.29 | 8.2±1.0 | 8.2±1.0 | 0.26 | 8.4±1.1 | 8.2±1.0 | <0.001 |
| Cholesterol — mg/dl | ||||||||||
| HDL | 41.9±11.3 | 42.0±11.4 | 41.9±11.1 | 0.93 | 38.6±7.8 | 38.5±7.9 | <0.001 | 46.3±12.8 | 46.1±13.8 | <0.001 |
| LDL | 100.7±32.7 | 100.8±33.4 | 100.7±32.1 | 0.92 | 96.5±29.7 | 97.0±30.1 | <0.001 | 107.4±37.0 | 104.1±33.5 | <0.001 |
| Triglycerides — mg/dl | 195.1±162.6 | 196.1±157.8 | 194±167.3 | 0.74 | 190.1±111.3 | 187.9±112.4 | 0.31 | 200.7±175.5 | 204.7±240.3 | 0.32 |
| Blood pressure — mm Hg | ||||||||||
| Systolic | 134.5±17.0 | 134.3±16.6 | 134.7±17.4 | 0.51 | 131.5±17.0 | 131.1±17.5 | <0.001 | 138.0±16.7 | 139.0±14.7 | <0.001 |
| Diastolic | 74.9±10.5 | 74.9±10.3 | 75.0±10.6 | 0.83 | 73.7±10.5 | 73.6±10.5 | <0.001 | 76.3±10.5 | 76.8±9.9 | <0.001 |
| Urinary albumin:creatinine ratio‡ | 71.8±253.1 | 69.5±228.9 | 74.0±275.3 | 0.64 | 62.3±180.2 | 83.2±331.5 | 0.21 | 62.5±197.6 | 79.2±269.9 | 0.29 |
| BMI§ | 32.4±5.5 | 32.4±5.5 | 32.5±5.4 | 0.41 | 32.3±5.5 | 32.6±5.4 | 0.25 | 32.7±5.7 | 32.2±5.3 | 0.15 |
| Visual acuity — no. of letters¶ | 75.9±10.2 | 75.9±10.4 | 75.9±10.0 | 0.96 | 76.2±9.7 | 76.2±10.7 | 0.39 | 75.6±10.3 | 75.5±10.2 | 0.35 |
| Smoking status — no./total no. (%) | 0.46 | 0.15 | 0.60 | |||||||
| Never smoked | 1188/2855 (41.6) | 581/1429 (40.7) | 607/1426 (42.6) | 313/805 (38.9) | 333/787 (42.3) | 279/647 (43.1) | 263/616 (42.7) | |||
| Former smoker | 1280/2855 (44.8) | 657/1429 (46.0) | 623/1426 (43.7) | 373/805 (46.3) | 352/787 (44.7) | 279/647 (43.1) | 276/616 (44.8) | |||
| Current smoker | 387/2855 (13.6) | 191/1429 (13.4) | 196/1426 (13.7) | 119/805 (14.8) | 102/787 (13.0) | 89/647 (13.8) | 77/616 (12.5) | |||
| Diabetic retinopathy status — no./total no. (%)[| | ](#TFN6) | 0.13 | 0.25 | 0.01 | ||||||
| None | 1450/2854 (50.8) | 729/1428 (51.1) | 721/1426 (50.6) | 429/806 (53.2) | 398/787 (50.6) | 328/645 (50.9) | 295/616 (47.9) | |||
| Mild | 518/2854 (18.1) | 241/1428 (16.9) | 277/1426 (19.4) | 141/806 (17.5) | 155/787 (19.7) | 105/645 (16.3) | 117/616 (19.0) | |||
| Moderate NPDR | 847/2854 (29.7) | 443/1428 (31.0) | 404/1426 (28.3) | 230/806 (28.5) | 224/787 (28.5) | 195/645 (30.2) | 198/616 (32.1) | |||
| Severe NPDR | 10/2854 (0.4) | 5/1428 (0.4) | 5/1426 (0.4) | 2/806 (0.2) | 4/787 (0.5) | 3/645 (0.5) | 1/616 (0.2) | |||
| PDR | 29/2854 (1.1) | 10/1428 (0.7) | 19/1426 (1.3) | 4/806 (0.5) | 6/787 (0.8) | 14/645 (2.2) | 5/616 (0.8) |
PROGRESSION OF DIABETIC RETINOPATHY
A total of 253 patients had end-point events at 4 years. Of these patients, 31 had laser photocoagulation only, 10 had vitrectomy only, 175 had a three-step progression on the ETDRS scale only, 1 had a three-step progression and vitrectomy, 5 had laser photocoagulation and vitrectomy, 28 had a three-step progression and laser photocoagulation, and 3 had a three-step progression, laser photocoagulation, and vitrectomy.
INTENSIVE VERSUS STANDARD GLYCEMIA THERAPY
Among the 2856 participants enrolled into the ACCORD Eye study, the baseline median glycated hemoglobin level was 8.0%. At 1 year, median levels were 6.4% among participants receiving intensive glycemia therapy, as compared with 7.5% among participants receiving standard therapy (P<0.001). A significant difference between groups was maintained throughout the follow-up period (see Section 6 in the Supplementary Appendix). After 4 years of follow-up, progression of diabetic retinopathy was seen in 7.3% of participants (104 of 1429) in the intensive glycemic control group, as compared with 10.4% of participants (149 of 1427) in the standard glycemic therapy group (adjusted odds ratio, 0.67; 95% confidence interval [CI], 0.51 to 0.87; P = 0.003) (Table 2). The rates of moderate vision loss were 16.3% (266 of 1629 patients) and 16.7% (273 of 1634) among patients receiving intensive and standard glycemia therapy, respectively (adjusted hazard ratio, 0.95; 95% CI, 0.80 to 1.13; P = 0.56) (Table 2).
Table 2.
Effects of Intensive Glycemic Control, Fenofibrate, and Intensive Blood-Pressure Control on Progression of Diabetic Retinopathy and Moderate Vision Loss.*
| Treatment | Progression of Diabetic Retinopathy | Adjusted Odds Ratio (95% CI) | P Value | Moderate Vision Loss | Adjusted Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| no./total no. (%) | no./total no. (%) | |||||
| Glycemia therapy | 0.67 (0.51–0.87) | 0.003 | 0.95 (0.80–1.13) | 0.56 | ||
| Intensive | 104/1429 (7.3) | 266/1629 (16.3) | ||||
| Standard | 149/1427 (10.4) | 273/1634 (16.7) | ||||
| Dyslipidemia therapy† | 0.60 (0.42–0.87) | 0.006 | 1.04 (0.83–1.32) | 0.73 | ||
| With fenofibrate | 52/806 (6.5) | 145/908 (16.0) | ||||
| With placebo | 80/787 (10.2) | 136/893 (15.2) | ||||
| Antihypertensive therapy | 1.23 (0.84–1.79) | 0.29 | 1.27 (0.99–1.62) | 0.06 | ||
| Intensive | 67/647 (10.4) | 145/749 (19.4) | ||||
| Standard | 54/616 (8.8) | 113/713 (15.8) |
FENOFIBRATE VERSUS PLACEBO
A total of 1593 ACCORD Eye study participants were also enrolled in the ACCORD Lipid study. The baseline median HDL cholesterol level of 38 mg per deciliter (0.98 mmol per liter) increased slightly, to a median of 40 mg per deciliter (1.03 mmol per liter), in the fenofibrate group, whereas the increased median level in the placebo group was 39 mg per deciliter (1.01 mmol per liter) in the placebo group at 1 year (P = 0.002) (see Section 6 in the Supplementary Appendix). The median baseline LDL cholesterol level of 93 mg per deciliter (2.4 mmol per liter) fell continually during the trial, as the doses of simvastatin were increased twice20; the levels were about 78 mg per deciliter (2.0 mmol per liter) in both groups at 4 years (P = 0.68). The median baseline triglyceride level of 162 mg per deciliter (1.83 mmol per liter) was decreased to 120 mg per deciliter (1.4 mmol per liter) in the fenofibrate group, as compared with 147 mg per deciliter (1.7 mmol per liter) in the placebo group at 1 year (P<0.001) (see Section 6 in the Supplementary Appendix). The rate of progression of diabetic retinopathy at 4 years was 6.5% (52 of 806 participants) in the fenofibrate group and 10.2% (80 of 787 participants) in the placebo group (adjusted odds ratio, 0.60; 95% CI, 0.42 to 0.87; P = 0.006) (Table 2). The rates of moderate vision loss were 16.0% (145 of 908 participants) and 15.2% (136 of 893 participants) in the fenofibrate and placebo groups, respectively (adjusted hazard ratio, 1.04; 95% CI, 0.83 to 1.32; P = 0.73) (Table 2).
INTENSIVE VERSUS STANDARD BLOOD-PRESSURE CONTROL
A total of 1263 ACCORD Eye study participants were also enrolled in the ACCORD Blood Pressure study. The baseline median systolic blood pressure was 137 mm Hg. At 1 year, the median systolic blood pressure was 117 mm Hg in the intensive-therapy group and 133 mm Hg in the standard-therapy group (see Section 6 in the Supplementary Appendix); these levels, and the difference between them, were stable throughout the remainder of the trial. The rates of progression of diabetic retinopathy were 10.4% (67 of 647 participants) in the group undergoing intensive blood-pressure control and 8.8% (54 of 616 participants) in the group undergoing standard blood-pressure control (adjusted odds ratio, 1.23; 95% CI, 0.84 to 1.79; P = 0.29) (Table 2). The rates of moderate vision loss were 19.4% (145 of 749 participants) and 15.8% (113 of 713 participants) in the intensive-therapy group and the standard-therapy group, respectively (adjusted hazard ratio, 1.27; 95% CI, 0.99 to 1.62; P = 0.06) (Table 2).
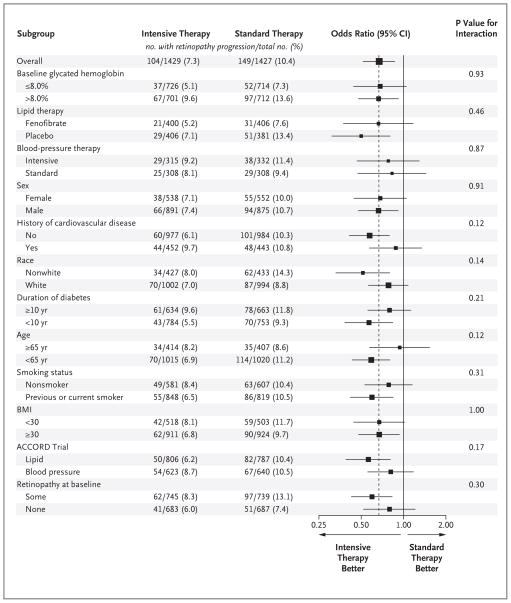
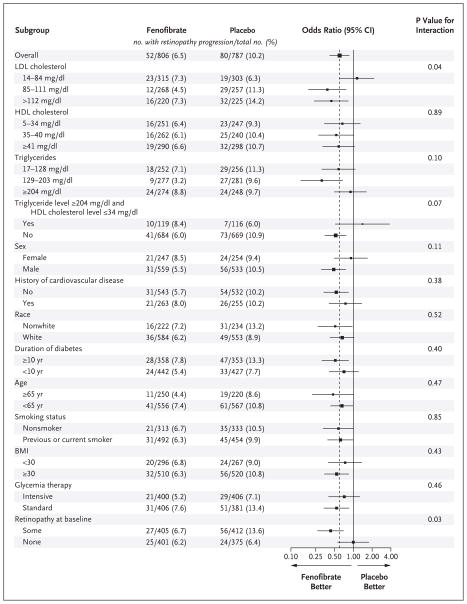
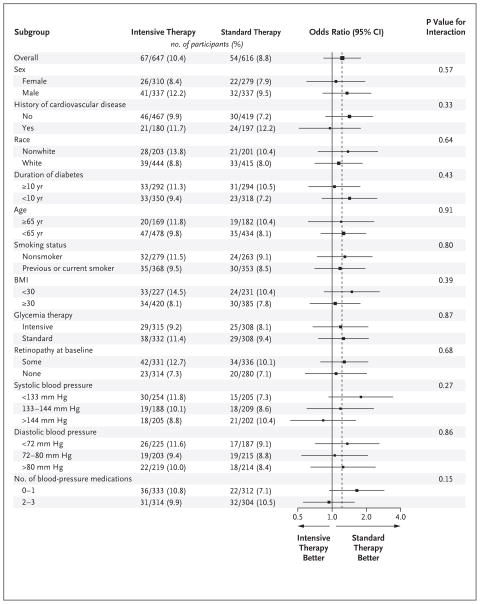
SUBGROUP ANALYSES
We found no significant interactions between treatment effect and any of the prespecified characteristics in subgroup analyses, with the exception of baseline LDL cholesterol (nominal P = 0.04) and baseline retinopathy (nominal P = 0.03) in the lipid trial (Fig. 1, 2, and 3). After any adjustment for multiple comparisons, these would not remain significant; the power of our study to detect such interactions is limited.
Figure 1. Subgroup Effects in the ACCORD Glycemia Trial.
The estimated odds ratios for progression of diabetic retinopathy are indicated as squares (with the area proportional to the sample size). The vertical dashed line is the overall treatment effect. Data were missing for some patients in some subgroups. The comparison between the subgroup enrolled in the ACCORD Lipid trial and the subgroup enrolled in the ACCORD Blood-Pressure trial was not specified within the protocol. Race was self-reported. The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. A logarithmic scale is used on the x axis.
Figure 2. Subgroup Effects in the ACCORD Lipid Trial.
The estimated odds ratios for progression of diabetic retinopathy are indicated as squares (with the area proportional to the sample size). The vertical dashed line is the overall treatment effect. Data were missing for some patients in some subgroups. Two comparisons were not specified within the protocol: the comparison between the subgroup with triglyceride levels of 204 mg per deciliter (2.3 mmol per liter) or higher and high-density lipoprotein (HDL) cholesterol levels of 34 mg per deciliter (0.9 mmol per liter) or less and the subgroup with lower triglyceride levels or higher HDL cholesterol levels, and the comparison between the subgroup with some retinopathy and the subgroup with none. Race was self-reported. The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. To convert values for cholesterol to millimoles per liter, multiply by 0.02586. To convert values for triglycerides to millimoles per liter, multiply by 0.01129. LDL denotes low-density lipoprotein. A logarithmic scale is used on the x axis.
Figure 3. Subgroup Effects in the ACCORD Blood-Pressure Trial.
The estimated odds ratios for progression of diabetic retinopathy are indicated as squares (with the area proportional to the sample size). The vertical dashed line is the overall treatment effect. Data were missing for some patients in some subgroups. The last four comparisons shown in the figure were not specified in the protocol. Race was self-reported. The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters. A logarithmic scale is used on the x axis.
SENSITIVITY ANALYSES
The exclusion of unverified events from analyses regarding the primary outcome did not qualitatively change the results (data not shown). There was no evidence for significantly different rates of missing data between the two treatment groups in the glycemia, lipid, and blood-pressure studies, either in unadjusted analyses (P = 0.55, P = 0.25, and P = 0.53, respectively) or adjusted analyses (P = 0.49, P = 0.23, and P = 0.48) (see Section 7 in the Supplementary Appendix). The findings from the imputation analyses supported those from the analyses based on patients with complete data (see Section 7 in the Supplementary Appendix).
DISCUSSION
The ACCORD trial consisted of three randomized comparisons evaluating the effect of intensive glycemia therapy versus standard glycemia therapy, simvastatin plus fenofibrate versus simvastatin plus placebo for lipid control, and intensive anti-hypertensive therapy versus standard antihypertensive therapy on cardiovascular events. Our ACCORD Eye study evaluated the effect of these same three comparisons on the progression of diabetic retinopathy.
Intensive glycemia therapy significantly reduced the risk of progression of diabetic retinopathy, defined as an increase of three or more steps on the ETDRS Severity Scale for Persons or the performance of laser photocoagulation or vitrectomy for diabetic retinopathy at 4 years (7.3% vs. 10.4% with standard therapy, P = 0.003). Two recent, smaller trials in similar patients reported nonsignificant results in the direction of a benefit with glycemic control.24–26 Similar to previous studies, our study did not show that intensive glycemic control reduces the risk of moderate vision loss. As reported elsewhere, however, there was a significant reduction in the rate of moderate vision loss in the entire ACCORD population with intensive glycemia treatment (19.1%, vs. 20.7% with standard therapy; hazard ratio, 0.91; 95% CI, 0.83 to 1.00; P = 0.047).27
As in other studies, the ACCORD trial19 showed a significantly increased risk of having a hypoglycemic event that necessitated either any assistance or medical assistance in the group receiving intensive glycemia therapy (targeting glycated hemoglobin levels <6.0%) as compared with the group receiving standard therapy (targeting glycated hemoglobin levels of 7.0 to 7.9%) (10.5% vs. 3.5%, P = 0.001). The intensive-therapy strategy was also associated with an increased rate of death from any cause after a mean of 3.5 years of follow-up, as compared with the standard strategy (5.0% vs. 4.0%). The glycemia trial was thus stopped early, potentially underestimating the reported effect of glycemia treatment on diabetic retinopathy.
We also found a beneficial effect of fenofibrate therapy on the progression of diabetic retinopathy at 4 years in participants with type 2 diabetes who were also receiving simvastatin (6.5%, vs. 10.2% with placebo; P = 0.006). The FIELD study,15 a randomized trial of monotherapy with fenofibrate (200 mg per day), showed a significant reduction in the need for laser therapy for either macular edema or proliferative retinopathy in the fenofibrate group as compared with the placebo group (3.4% vs. 4.9%, P<0.001). Our results provide further evidence that fenofibrate slows the progression of diabetic retinopathy.
We did not demonstrate a significant effect of intensive versus standard blood-pressure control on the progression of diabetic retinopathy at 4 years (10.4% vs. 8.8%, P = 0.29), nor was there a significant effect in any of the prespecified subgroups (Fig. 3). In contrast, the United Kingdom Prospective Diabetes Study (ISRCTN75451837),6 a nested trial of antihypertensive medications, showed that intensive blood pressure control (targeting a systolic blood pressure <150 mm Hg, vs. <180 mm Hg with standard control) achieved a significant reduction in the progression of diabetic retinopathy (34.0% vs. 51.3%, P = 0.004) and a significant reduction in moderate vision loss (10.2% vs. 19.4%, P = 0.004) after 7.5 years. The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study (NCT00145925)24,25 also did not show a beneficial effect of intensive blood pressure control on progression of diabetic retinopathy. However, the difference in systolic blood pressure between the treatment groups was only 5.6 mm Hg, which may account for the lack of benefit seen in the ADVANCE trial.
One limitation of our study is the collection of data on retinopathy outcomes from fundus photographs at only two time points. Another limitation is the sizable proportion of the original ACCORD Eye study population whose retinopathy status could not be assessed at 4 years. As compared with those whose retinopathy status could be assessed, these subjects were more likely at baseline to have elevated LDL levels, higher urinary albumin:creatinine ratios, and lower visual acuity scores. However, there was no evidence of significant differences regarding the amount of missing data, and the results of sensitivity analyses supported those of the primary analyses.
In summary, our study provides evidence that the beneficial effect of intensive glycemia therapy on retinopathy progression, previously shown in participants with type 1 diabetes2,3 and those with type 2 diabetes that was newly diagnosed5 or not yet accompanied by hypertension, lipid abnormalities, or established cardiovascular disease,4 applies also to patients with type 2 diabetes like those enrolled in the ACCORD trial, who were older and at greater cardiovascular risk. We also demonstrated that fenofibrate, when added to statin therapy, slows the progression of diabetic retinopathy in patients with type 2 diabetes. We did not find a significant difference in the progression of diabetic retinopathy between patients receiving standard antihypertensive therapy and those receiving intensive antihypertensive therapy according to our treatment protocols.
Supplementary Material
Supplement1
Acknowledgments
Supported by contracts (N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010) from the National Heart, Lung, and Blood Institute and the National Institutes of Health, with additional support from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Eye Institute, the National Institute on Aging, and the Centers for Disease Control and Prevention. General Clinical Research Centers provided support at many sites. The following companies donated study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca Pharmaceuticals, Bayer HealthCare, Closer Healthcare, GlaxoSmith-Kline Pharmaceuticals, King Pharmaceuticals, Merck, Novartis Pharmaceuticals, Novo Nordisk, Omron Healthcare, Sanofi-Aventis U.S., and Takeda Pharmaceuticals.
Dr. Goff reports receiving grant support or pending grant support from Merck and money for serving as a data and safety monitoring board member for a trial of a diabetes medication from Takeda; Dr. Cushman, consulting fees from Novartis, Takeda, Sanofi-Aventis, Bristol-Myers Squibb, King Pharmaceuticals, Daiichi–Sankyo, Gilead, Theravance, Pharmacopeia, and Sciele and grant support or pending grant support from Novartis, GlaxoSmithKline, and Merck; Dr. Ginsberg, advisory fees from Merck, Merck–Schering Plough, and Bristol-Myers Squibb–AstraZeneca; consulting fees from Merck, Abbott–AstraZeneca, Bristol-Myers Squibb, Roche, Isis–Genzyme, GlaxoSmithKline, Novartis, Pfizer, and Regeneron–Sanofi-Aventis; grant support or pending grant support from Merck, Isis–Genzyme, Roche, and AstraZeneca; payment for development of education presentations from Pfizer; and payment for travel and accommodation expenses from all these companies; Dr. Elam, payment for development of education presentations from Pfizer, Abbott Pharmaceuticals, and Merck–Schering Plough; and Dr. Gerstein, consulting fees from Sanofi-Aventis, GlaxoSmithKline, Eli Lilly, Novo Nordisk, AstraZeneca, Bristol-Myers Squibb, Roche, Medtronic, Merck, Bayer, Bioavail, and Jansen Ortho; grant support or pending grant support from Sanofi-Aventis, GlaxoSmith-Kline, Novo Nordisk, Merck, Pronova, and Roche; honoraria from Sanofi-Aventis, GlaxoSmithKline, Solvay, Boehringer Ingelheim, Servier, Bayer, Eli Lilly, Novo Nordisk, and Takeda; and payment for travel and accommodation expenses from all these companies; Dr. Schubart reports participating in trials sponsored by Sanofi-Aventis, Merck, and Johnson & Johnson.
APPENDIX
Members of the ACCORD data and safety monitoring board are as follows: A.M. Gotto, Jr. (chair), K. Bailey, D. Gohdes, S. Haffner, R. Hiss, K. Jamerson, K. Lee, D. Nathan, J. Sowers, L. Walters.
Members of the writing committee (Emily Y. Chew, M.D. [chair], National Eye Institute, National Institutes of Health [NIH], Bethesda, MD; Walter T. Ambrosius, Ph.D., Wake Forest University School of Medicine, Winston-Salem, NC; Matthew D. Davis, M.D., Ronald P. Danis, M.D., and Sapna Gangaputra, M.D., M.P.H., University of Wisconsin, Madison; Craig M. Greven, M.D., Wake Forest University School of Medicine, Winston-Salem, NC; Larry Hubbard, M.A.T., and Barbara A. Esser, M.S., University of Wisconsin, Madison; James F. Lovato, M.S., Letitia H. Perdue, B.A., and David C. Goff, Jr., M.D., Ph.D., Wake Forest University School of Medicine, Winston- Salem, NC; William C. Cushman, M.D., Veterans Affairs Medical Center, Memphis; Henry N. Ginsberg, M.D., Columbia University College of Physicians and Surgeons, New York; Marshall B. Elam, M.D., Ph.D., Veterans Affairs Medical Center, Memphis; Saul Genuth, M.D., Case Western Reserve University, Cleveland; Hertzel C. Gerstein, M.D., McMaster University, Hamilton, ON, Canada; Ulrich Schubart, M.D., Albert Einstein College of Medicine, Bronx, NY; and Lawrence J. Fine, M.D., National Heart, Lung, and Blood Institute, NIH, Bethesda, MD) assume responsibility for the integrity of the article.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
No other potential conflict of interest relevant to this article was reported.
References
- 1.Kempen JH, O’Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–63. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 2.The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin dependent diabetes mellitus: the Diabetes Control and Complications Trial. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 3.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–9. doi: 10.1056/NEJM199307293290502. [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 5.United Kingdom Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 6.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes. BMJ. 1998;317:703–13. Idem. [PMC free article] [PubMed] [Google Scholar]
- 7.Klein BEK, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, X: relationship of serum cholesterol to retinopathy and hard exudates. Ophthalmology. 1991;98:1261–5. doi: 10.1016/s0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- 8.Chew EY, Klein ML, Ferris FL, III, et al. Association of elevated serum lipid levels with retinal hard exudates in diabetic retinopathy. Arch Ophthalmol. 1996;114:1079–84. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 9.Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004;53:2883–92. doi: 10.2337/diabetes.53.11.2883. [DOI] [PubMed] [Google Scholar]
- 10.Davis MD, Fisher MR, Gangnon RE, et al. Risk factors for high risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy study report no. 18. Invest Ophthalmol Vis Sci. 1998;39:233–52. [PubMed] [Google Scholar]
- 11.Cullen JF, Ireland JT, Oliver MF. A controlled trial of atromid therapy in exudative diabetic retinopathy. Trans Ophthalmol Soc U K. 1964;84:281–95. [PubMed] [Google Scholar]
- 12.Harrold BP, Marmion VJ, Gough KR. A double-blind controlled trial of clofibrate in the treatment of diabetic retinopathy. Diabetes. 1969;18:285–91. doi: 10.2337/diab.18.5.285. [DOI] [PubMed] [Google Scholar]
- 13.Duncan LJ, Cullen JF, Ireland JT, Noland J, Clarke BF, Oliver MF. A three year trial of atromid therapy and exudative diabetic retinopathy. Diabetes. 1968;17:458–67. doi: 10.2337/diab.17.7.458. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137:675–82. doi: 10.1016/j.ajo.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomized controlled trial. Lancet. 2007;370:1687–97. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 16.The ACCORD Study Group. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: design and methods. Am J Cardiol. 2007;99(Suppl):21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Chew EY, Ambrosius WT, Howard LT, et al. Rationale, design, and methods of the Actions to Control Cardiovascular Risk in Diabetes Eye Study (ACCORD-EYE) Am J Cardiol. 2007;99(12A):103i–11i. doi: 10.1016/j.amjcard.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–33. [PubMed] [Google Scholar]
- 19.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Effect of intensive blood pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. doi: 10.1056/NEJMoa1001286. Idem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin DR. Multiple imputation for nonresponse in surveys. New York: John Wiley; 1987. pp. 169–70. [Google Scholar]
- 23.Ginsberg HN, Bonds DE, Lovato LC, et al. Evolution of the Lipid Trial Protocol of the Actions to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Am J Cardiol. 2007;99(Suppl):56i–67i. doi: 10.1016/j.amjcard.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 24.The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 25.Beulens JW, Patel A, Vingerling JR, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52:2027–36. doi: 10.1007/s00125-009-1457-x. [DOI] [PubMed] [Google Scholar]
- 26.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 27.Ismail-Beigi F, Craven T, Banerji M, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: a substudy of the ACCORD randomised trial. Lancet. 2010;375:26. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1