Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 13.
Published in final edited form as: Cancer Cell. 2010 Jul 13;18(1):74–87. doi: 10.1016/j.ccr.2010.04.025
Summary
Although Bcr-Abl kinase inhibitors have proven effective in the treatment of chronic myeloid leukemia (CML), they generally fail to completely eradicate Bcr-Abl+ leukemia cells. To identify genes whose inhibition sensitizes Bcr-Abl+ leukemias to killing by Bcr-Abl inhibitors, we performed an RNAi-based synthetic lethal screen with imatinib in CML cells. This screen identified numerous components of a Wnt/Ca2+/NFAT signaling pathway. Antagonism of this pathway led to impaired NFAT activity, decreased cytokine production and enhanced sensitivity to Bcr-Abl inhibition. Furthermore, NFAT inhibition with cyclosporin A facilitated leukemia cell elimination by the Bcr-Abl inhibitor dasatinib and markedly improved survival in a mouse model of Bcr-Abl+ acute lymphoblastic leukemia (ALL). Targeting this pathway in combination with Bcr-Abl inhibition could improve treatment of Bcr-Abl+ leukemias.
Introduction
CML is a myeloproliferative disorder characterized by a t(9;22) translocation, which gives rise to a shortened chromosome 22, the Philadelphia chromosome (Ph). This translocation results in a fusion between the genes encoding the Abl tyrosine kinase and Bcr. The resultant protein, Bcr-Abl, has constitutive tyrosine kinase activity and is considered causative in the disease (Deininger et al., 2000). Ph is also found in 20-30% of ALL and is associated with poor prognosis (Faderl et al., 2002). Bcr-Abl activates a number of downstream targets including Ras, PI3 kinase, NF-κB and STAT5, resulting in cytokine-independent growth, resistance to apoptosis, and altered cellular adhesion (Sattler and Griffin, 2003).
Imatinib mesylate is a tyrosine kinase inhibitor that blocks the activity of Bcr-Abl and induces remarkable hematological and cytogenetic responses in chronic phase CML patients (Deininger et al., 2005). While imatinib is a highly effective treatment for CML, it is rarely curative. Cessation of imatinib therapy can result in relapse of the disease even in patients that show a complete response (Rousselot et al., 2007). Furthermore, a significant number of chronic phase patients on imatinib therapy eventually relapse (16% within 42 months; (Deininger et al., 2005). Relapse is often associated with mutation of Bcr-Abl. Another major concern is that advanced phase CML and Ph+ ALL are innately more refractory than chronic phase CML to imatinib therapy. Only 15% of patients in CML blast crisis and 29% of Ph+ ALL patients achieve a complete hematological response and a majority of these relapse within a few months (Deininger et al., 2005; Ottmann et al., 2002). Advanced phase CML patients and those with Ph+ ALL also fail to achieve durable emissions with the more potent second generation Bcr-Abl inhibitors nilotinib and dasatinib (Quintas-Cardama et al., 2007). These findings underscore the need to identify targets that will cooperate with Bcr-Abl inhibition to more effectively treat CML and Ph+ ALL and hopefully eradicate these diseases.
Wnt family members are secreted proteins that signal through the frizzled superfamily of G protein-coupled receptors. Activation of the canonical Wnt signaling pathway leads to nuclear accumulation of the Lef-Tcf transcriptional coactivator β-catenin (Cadigan and Liu, 2006). In addition, two noncanonical Wnt pathways have been identified that signal independently of β-catenin; the planar cell polarity pathway and the Ca2+/NFAT pathway (Veeman et al., 2003). In the Wnt/Ca2+/NFAT pathway (Figure 1A), Wnt5a-bound Frizzled (FZD), in association with a noncanonical coreceptor such as Ryk, acts through G proteins to activate phospholipase C (PLC) and phosphodiesterase (PDE) (Ahumada et al., 2002; Sheldahl et al., 2003; Slusarski et al., 1997). Activation of PLC initiates hydrolysis of membrane-bound lipid, generating two secondary messengers, diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3). DAG stimulates protein kinase C (PKC) while IP3 promotes the release of intracellular Ca2+. Increased concentrations of Ca2+ lead to activation of the Ca2+-sensitive proteins Ca2+-calmodulin-dependent protein kinase II (CaMKII; (Kuhl et al., 2000)) and the Ca2+-calmodulin-dependent protein phosphatase calcineurin and its target, the transcription factor NFAT (Saneyoshi et al., 2002). Dephosphorylation of NFAT by calcineurin promotes its translocation to the nucleus where it can activate the transcription of cytokine genes such as interleukin-4 (IL-4; (Hogan et al., 2003)). A role for the Wnt/Ca2+/NFAT pathway in leukemia cells is unclear.
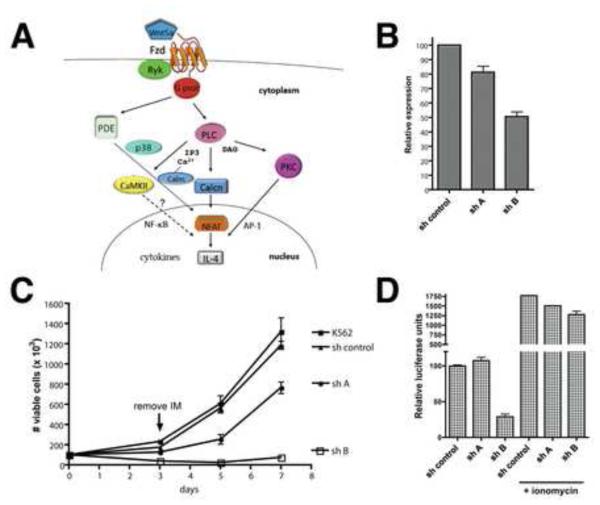
Figure 1. Knockdown of FZD-8 sensitizes CML cells to imatinib and impairs NFAT activity.
A) Diagrammatic representation of the Wnt/Ca2+/NFAT pathway. Pathway details are described in the text. B) K562 CML cells were stably transduced with shRNAs (shA or shB) targeting FZD-8 or a negative control shRNA. Real-time PCR (qPCR) was performed on resultant cell lines to examine FZD-8 expression levels normalized to 18S ribosomal RNA. C) K562 cell lines were left untreated or treated with imatinib at 0.1 μM for 72 hr, after which the cells were reseeded in the absence of drug and cultured for an additional 4 days. The number of viable cells (based on PI-exclusion) was counted by flow cytometry at the indicated time points. D) K562 cell lines were infected with an adenoviral NFAT-luciferase reporter. After 32 hr, the cells were left untreated or treated with ionomycin at 1 μg/ml for 16 hr after which the cells were harvested and luciferase activity was assayed. Error bars +/− SD. See also Figure S2.
The purpose of this study was to identify pathways that can be targeted to more effectively eliminate Ph+ leukemia cells in combination with Bcr-Abl inhibition.
Results
RNAi-based screen identifies Wnt/Ca2+/NFAT pathway genes as synthetic lethal with imatinib
In order to identify genes and pathways whose inhibition sensitizes CML cells to killing by imatinib, a large-scale RNAi-based synthetic lethal screen was performed. A lentiviral shRNA library was utilized that contains 3-5 shRNAs per target gene for ~50,000 different human transcripts. This library was transduced into K562 CML cells and the cells were selected in puromycin for two weeks to obtain a pure population of transduced cells and allow for the exclusion of shRNAs that target essential genes. The transduced cells were either left untreated or treated with imatinib at 1 μM for 72 hr, a dose that eliminated approximately 85% of the cells. The cells were cultured for an additional week, and total RNA was then isolated, reverse-transcribed, and the shRNA sequences were PCR amplified and labeled with biotin. Biotin-labeled PCR products were used to probe Affymetrix microarrays to assess representation of individual shRNAs. While few shRNAs provided resistance, a number of shRNA were deleterious to K562 cells upon exposure to imatinib (Figure S1A). Our screen identified 145 genes (Table S1) that met the following three criteria: 1) shRNAs targeting the gene were under-represented >6-fold in imatinib treated cells compared to untreated cells in ≥2/3 of comparisons 2) the observed differences had a p-value of < 0.05 and 3) at least two unique shRNAs per target gene were identified, minimizing identification of false positives due to off-target effects. We have dubbed these genes “SLIMs” for Synthetic Lethal with Imatinib Mesylate. The SLIMs fall into functional categories that include cell attachment, inflammatory signaling, cell migration, hematological system development, and hematopoietic cell proliferation and differentiation (Figure S1B and Table S2).
Among the SLIMs, the Wnt receptor FZD-8 was identified by three different shRNAs, each under-represented >116-fold in imatinib-treated cells. FZD-8 is a poorly characterized Wnt receptor whose signaling in mammalian cells remains largely unstudied. Components of the canonical Wnt/β-catenin pathway signaling were not identified; however, isoforms of both CaMKII (β) and PKC (θ), which are known to participate in the noncanonical Wnt/Ca2+/NFAT pathway, were identified. When the screen criteria were loosened to include genes that were identified by single shRNAs, canonical Wnt pathway components were still not found. Conversely, numerous potential components of the Wnt/Ca2+/NFAT signaling pathway were additionally identified (Table 1). These include the noncanonical ligand Wnt5a, FZD-2 (Ahumada et al., 2002), the noncanonical FZD co-receptor Ryk (Kim et al., 2008), p38β MAPK, PDE6C and H, PLCβ, CaMKIIγ and its substrate RARα(Si et al., 2007), calcineurin A (α and β), NFATc1 and its target genes IL-4 (Guo et al., 2008) and CCL23 (Shin et al., 2007). This list also includes several genes that, although not known to be involved in noncanonical Wnt signaling, are known or suspected to influence NFAT-dependent transcription: IL-7, RCAN1, NFATC2IP, Fra-1, EGR3 and PRMT8 (Boise et al., 1993; Gringhuis et al., 1997; Hodge et al., 1996; Liu et al., 2009; Mowen et al., 2004; Rengarajan et al., 2000). Thus, screening results suggest a role for Wnt/Ca2+/NFAT in maintaining CML survival in the face of Bcr-Abl inhibition.
Table 1. Potential Wnt/Ca2+/NFAT pathway genes identified as SLIMs.
Genes are listed in ascending order based on the fold-change of corresponding shRNAs. For genes identified by more than one shRNA, the most negative fold-change is shown. See also Tables S1 and S2 and Figure S1.
| RefSeq ID | mean FC | Hgnc symbol | description | # shRNAs |
|---|---|---|---|---|
| NM_005438 | −183.41 | FOSL1 | Fos-related antigen 1 (Fra-1) | 1 |
| NM_006257 | −154.91 | PRKCQ | Protein kinase C theta type | 2 |
| NM_031866 | −146.08 | FZD8 | Frizzled-8 precursor | 3 |
| NM_172084 | −133.08 | CAMK2B | Calcium/calmodulin-dependent protein kinase type II beta | 2 |
| NM_004430 | −131.92 | EGR3 | Early growth response protein 3 | 2 |
| NM_000589 | −106.66 | IL4 | Interleukin-4 precursor | 2 |
| NM_002751 | −54.56 | MAPK11 | Mitogen-activated protein kinase 11 (p38 beta MAPK) | 1 |
| NM_006924 | −45.85 | SFRP4 | Secreted frizzled-related protein 4 precursor | 1 |
| NM_000880 | −45.53 | IL7 | Interleukin-7 precursor | 1 |
| NM_000964 | −44.96 | RARA | Retinoic acid receptor alpha | 2 |
| NM_021132 | −34.12 | PPP3CB | Calmodulin-dependent calcineurin A subunit beta | 1 |
| NM_182734 | −31.48 | PLCB1 | Phospholipase C- beta-1 | 1 |
| NM_006888 | −31.30 | CALM1 | Calmodulin (CaM) | 2 |
| NM_006204 | −30.17 | PDE6C | cGMP phosphodiesterase 6C | 1 |
| NM_003392 | −24.94 | WNT5A | Wingless-type MMTV integration site family, member 5A | 1 |
| NM_005064 | −22.99 | CCL23 | C-C motif chemokine 23 precursor | 2 |
| NM_005822 | −22.81 | RCAN2 | Calcipressin-2 (Regulator of calcineurin 2) | 1 |
| NM_172387 | −19.10 | NFATC1 | Nuclear factor of activated T-cells, cytoplasmic 1 | 1 |
| NM_002958 | −16.78 | RYK | RYK receptor-like tyrosine kinase | 1 |
| NM_019854 | −15.79 | PRMT8 | Protein arginine N-methyltransferase 8 | 1 |
| NM_006205 | −14.95 | PDE6H | cGMP phosphodiesterase 6H | 1 |
| NM_004414 | −13.32 | RCAN1 | Calcipressin-1 (Regulator of calcineurin 1) | 1 |
| NM_001466 | −12.21 | FZD2 | Frizzled-2 precursor | 1 |
| NM_001222 | −12.18 | CAMK2G | Calcium/calmodulin-dependent protein kinase type II gamma | 1 |
| NM_032815 | −12.15 | NFATC2IP | NFATc2-interacting protein | 1 |
| NM_000944 | −11.77 | PPP3CA | Calmodulin-dependent calcineurin A subunit alpha isoform | 1 |
Inhibiting the Wnt/Ca2+/NFAT pathway sensitizes Ph+ leukemia cells to killing by Bcr-Abl inhibitors
To verify that knockdown of the FZD-8 receptor sensitizes CML cells to imatinib, two different lentiviral constructs expressing shRNAs targeting FZD-8 (shA and shB) were stably transduced into K562 cells. Analysis of FZD-8 expression using real-time PCR revealed that shB significantly reduced FZD-8 expression (~50%) relative to a control non-targeting shRNA, whereas shA failed to significantly reduce expression (Figure 1B) and thus shA serves as an additional negative control. shB did not have a substantial impact on the proliferation rate of K562 cells (Figure S2A), indicating that FZD-8 knockdown does not affect cell viability in the absence of imatinib. To examine the consequences of imatinib treatment, these cell lines were exposed to 0.1 μM imatinib for 72 hr and the number of viable cells, based on propidium iodide (PI)-exclusion, was determined using flow cytometry. The cells were then reseeded in the absence of drug and viability was monitored over 7 days. Figure 1C shows that shB-expressing cells were severely sensitized to imatinib-mediated killing relative to untransduced and control cells. This translated into a dramatic effect on long-term viability: whereas the control cells recovered from imatinib treatment and were proliferating normally by day 7, the shB cells did not significantly recover. These results suggest that even partial FZD-8 knockdown can significantly potentiate the efficacy of imatinib in killing CML cells.
FZD-8 is a largely unstudied Wnt receptor whose role in activating noncanonical Wnt/Ca2+/NFAT signaling has not been examined. Frizzled receptors are known to tranduce signals that lead to activation of β-catenin, and knockdown of β-catenin has previously been shown to sensitize CML cells to imatinib (Coluccia et al., 2007). Indeed, we also found that β-catenin knockdown decreased viability of K562 CML cells treated with imatinib, although not nearly to the same extent as knockdown of FZD-8 (Figure S2). Moreover, knockdown of FZD-8 did not affect levels of β-catenin protein in the nucleus or its transcriptional activity (Figure S2), inconsistent with a role for FZD-8 in signaling through the canonical pathway. Given that so many potential mediators of Wnt/Ca2+/NFAT signaling were identified in the screen, we wanted to determine if knockdown of FZD-8 altered the activity of NFAT. Therefore, K562 cells expressing shA, shB or control shRNA were transduced with an NFAT reporter construct that contains tandem NFAT binding sites derived from the IL-4 promoter fused to luciferase (Braz et al., 2003). Luciferase activity was severely impaired in shB cells relative to control cells (Figure 1D), and this was rescued by treatment with the calcium ionophore ionomycin suggesting that FZD-8 acts upstream of intracellular Ca2+ release, consistent with a role for FZD-8 in mediating Wnt/Ca2+/NFAT pathway signaling.
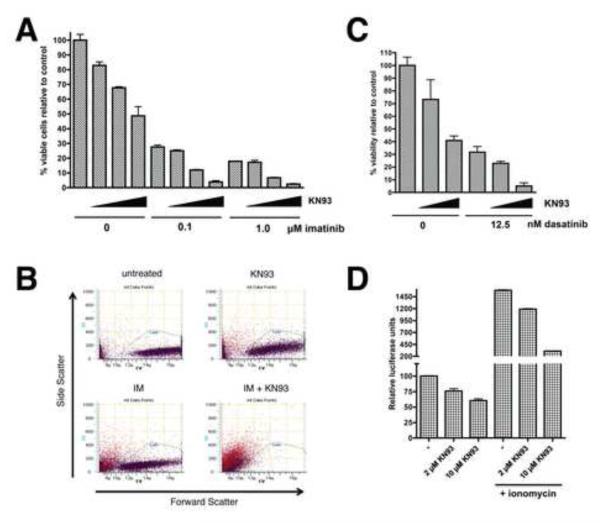
Our screen identified CaMKII β as a SLIM. Thus, we next examined whether inhibition of CaMKII activity with the CaMKII-specific inhibitor KN93 could sensitize CML cells to the effects of imatinib. After 48 hr, the number of viable cells was counted by flow cytometry (Figure 2A and B). While KN93 had some effect on cell viability by itself, at 5 and 10 μM it showed a synergistic effect with imatinib in cell elimination (combination index [CI] values are shown in Table S3). Remarkably, the combination of 0.1 or 1 μM imatinib and 10 μM KN93 resulted in near complete cell death. KN93 is known to have off-target effects including the inhibition of calcium and potassium channels (Gao et al., 2006; Rezazadeh et al., 2006). However, KN92, an analog of KN93 that retains these off-target effects but is inactive towards CaMKII, was ineffective in combination with imatinib (Figure S3), suggesting that the observed synergism with KN93 is due to CaMKII inhibition. To verify that synergism with KN93 extends to other Bcr-Abl inhibitors, we demonstrated that KN93 effectively synergized with dasatinib in eliminating K562 cells (Figure 2C). Although CaMKII is known to participate in Wnt/Ca2+ signaling, CaMKII involvement in regulation of NFAT-dependent transcription has not been established. Thus, K562 cells transduced with the NFAT-luciferase reporter were treated with KN93 for 16 hr and luciferase activity was assayed. KN93 treatment caused a dose-dependent reduction in NFAT reporter activity (Figure 2D), especially in the context of ionomycin-stimulated cells (6-7 fold inhibition), consistent with CaMKII acting downstream of intracellular calcium release.
Figure 2. Inhibition of CaMKII sensitizes CML cells to Bcr-Abl inhibition and impairs NFAT activity.
A) K562 cells were treated with imatinib and KN93 alone or in combination as indicated (increasing KN93 concentrations of 0, 2, 5, and 10 μM are indicated by the triangles) for 48 hr and viable cells (based on PI-exclusion) were counted by flow cytometry. Values were normalized to untreated (control) cells and graphed. Representative flow profiles (side scatter vs. forward scatter) from these experiments are shown in B. The “cell” gate was defined based on the scatter profile of untreated viable K562 cells (upper left panel). PI+ cells are colored in red and PI− in violet. C) K562 cells were treated with dasatinib and KN93 as in A for 48 hr and viable cells were counted. D) K562 cells were infected with an NFAT-luciferase reporter. After 32 hr, the cells were treated with the indicated concentrations of KN93 alone or together with ionomycin at 1 μg/ml for 16 hr after which cells were harvested and assayed for luciferase activity. Error bars +/− SD. See also Figure S3.
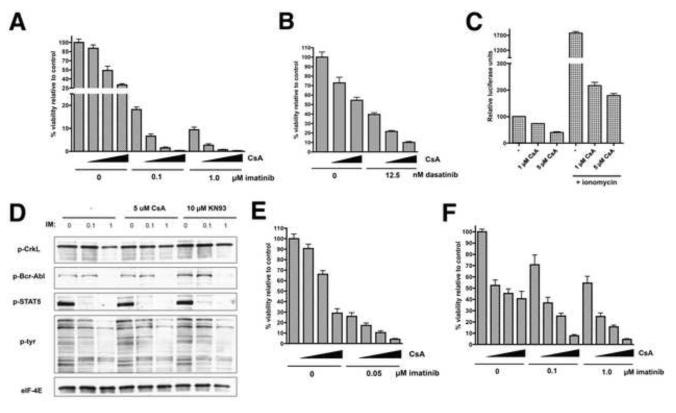
Since our results demonstrated a compelling correlation between impaired NFAT activity and sensitivity of CML cells to Bcr-Abl inhibition, we next focused on calcineurin, an essential regulator of NFAT whose activity is required for the nuclear translocation of NFAT. Calcineurin is an attractive therapeutic target because its activity can be effectively inhibited by both tacrolimus and cyclosporin A (CsA), two drugs that have been in clinical use for many years as immunosuppressants. We first utilized K562 cells to determine if calcineurin inhibition by CsA enhances CML cell killing by imatinib. K562 cells were treated with combinations of CsA and imatinib for 72 hr. The doses of CsA tested (1-5 μM) are clinically relevant as patients achieve peak serum concentrations between 1-2 μM with a standard dosing of CsA used as an immunosuppressant for organ transplantation (Halloran et al., 1999). While CsA treatment alone had only a moderate effect on cell number, a dramatic effect on viability was obvious upon combined treatment with CsA and imatinib, with 2.5 and 5 μM CsA causing near complete cell death when combined with either 0.1 or 1 μM imatinib (Figure 3A; CI values are shown in Table S3 and representative flow profiles in Figure S4A). The mechanism by which CsA enhances imatinib-mediated cell death appears to be by enhancement of apoptosis, as indicated by a dramatic increase in sub-G1 DNA content when the two drugs were combined (Figure S4B). CsA also sensitized K562 cells to the Bcr-Abl inhibitor dasatinib (Figure 3B), and thus, as with KN93, the effects of CsA are not unique to imatinib. Reporter assays verified that CsA causes a dose-dependent decrease in NFAT-driven transcription in both unstimulated and ionomycin-stimulated K562 cells (Figure 3C). Importantly, imatinib had no influence on NFAT activity in these cells (Figure S4C). These results suggest that calcineurin inhibition cooperates with Bcr-Abl inhibition to eliminate CML cells.
Figure 3. Inhibition of calcineurin-NFAT by CsA sensitizes Ph+ leukemia cells to Bcr-Abl inhibition.
A) K562 cells were treated with imatinib and CsA alone or in combination as indicated (increasing CsA concentrations of 0, 1, 2.5, and 5 μM are indicated by the triangles) for 72 hr and viable cells were counted by flow cytometry. B) K562 cells were treated with dasatinib and CsA as in A for 48 hr and viable cells were counted. C) K562 cells were infected with an NFAT-luciferase reporter. After 32 hr, the cells were treated with the indicated concentrations of CsA alone or together with ionomycin at 1 μg/ml for 16 hr after which cells were harvested and assayed for luciferase activity. D) K562 cells were treated with imatinib and CsA as indicated. After 24 hr, the cells were harvested and lysates subjected to western blot analysis for phosphorylated (p) Bcr-Abl, CrkL, STAT5 and total phosphotyrosine or EIF-4E (loading control). E) KBM7 CML cells were treated with imatinib and CsA as in A for 48 hr and viable cells were counted. F) SUP-B15 Ph+ ALL cells were treated with imatinib and CsA as in A for 72 hr and viable cells were counted. Error bars +/− SD. See also Figure S4.
In addition to calcineurin, CsA is also known to inhibit p-glycoprotein (MDR1), a drug efflux pump that has been proposed to contribute to imatinib-resistance in CML (Illmer et al., 2004). Thus, the effect of CsA, or perhaps even KN93, could potentially be due to prevention of imatinib efflux from cells, leading to improved Bcr-Abl inhibition. To examine this possibility, K562 cells were treated with imatinib and CsA or KN93, alone or in combination, for 24 hr and subjected to western blot analysis for tyrosine-phosphorylated forms of known Bcr-Abl substrates including CrkL, STAT5, and Bcr-Abl itself, in addition to global tyrosine phosphorylation. Imatinib effectively inhibited tyrosine phosphorylation of all examined Bcr-Abl substrates (Figure 3D) to varying degrees, and global tyrosine phosphorylation was also affected. However, cotreatment with CsA or KN93 did not enhance this inhibition, inconsistent with roles for CsA or KN93 in increasing intracellular imatinib concentrations. Furthermore, qPCR analysis failed to detect expression of MDR1 in K562 cells, while its expression was easily detectable in AML cell lines (not shown). Thus, the effects of CsA and KN93 on K562 cell viability are not attributable to decreased imatinib efflux through inhibition of MDR1.
The effects of the combination of CsA and imatinib on the viability of a different CML cell line, KBM7, were then assessed (Figure 3E). CsA at 2.5 and 5 μM showed synergistic elimination of KBM7 cells in combination with 0.05 μM imatinib. Upon drug removal and grown for 3 days, a synergistic effect of the drug combination on long-term viability was evident (Figure S4D). Given the effectiveness of CsA combined with imatinib in killing CML cells, we tested whether the same might also apply to Ph+ ALL cells, which are known to be more refractory to Bcr-Abl inhibition. We utilized SUP-B15 cells, a cell line established from a patient with Ph+ B-cell ALL. While neither CsA nor imatinib had a severe effect, the combination caused synergistic cell killing (Figure 3F). These results suggest that calcineurin-NFAT is a promising therapeutic target for CML and Ph+ ALL.
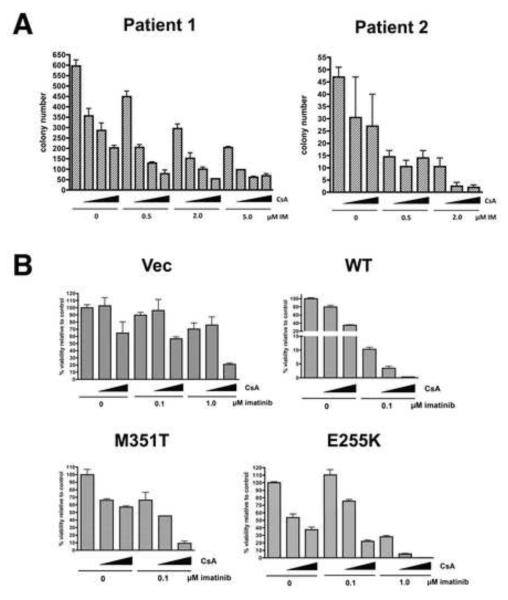
To determine whether the synergism observed with imatinib and CsA in cell lines extends to primary human tumors, CD34+ cells isolated from bone marrow of two patients with CML in chronic phase (CP) were treated with imatinib and/or CsA in colony forming assays. CsA synergized with imatinib in suppressing colony formation at all doses of imatinib tested in the “Patient 1” sample and at 2 μM IM in the “Patient 2” sample (Figure 4A; CI values, Table S3). CsA also cooperated with imatinib in inhibiting proliferation of cells from 2 additional CP CML samples as measured by an MTS assay (Figure S5). Thus, CsA is effective at sensitizing primary human Ph+ leukemia cells to the effects of Bcr-Abl inhibition.
Figure 4. CsA sensitizes primary CML cells and cells with imatinib-resistant forms of Bcr-Abl to imatinib.
A) Colony formation assays were performed on CD34+ cells purified from bone marrow of two patients with chronic phase CML in the presence of imatinib and/or cyclosporine at the indicated concentrations. B) Ba/F3 cells transduced with vector, wild-type Bcr-Abl, or imatinib-resistant mutant forms of Bcr-Abl (M351T or E255K) were treated with imatinib and CsA alone or in combination as indicated (increasing CsA concentrations of 0, 2.5, and 5 μM are indicated by the triangles) for 72 hr and viable cells were counted. Error bars +/− SD. See also Figure S5.
A fraction of CML patients who initially respond to imatinib develop resistance through mutations in Bcr-Abl that impair imatinib binding. The three most common mutations observed in patients are T315I, E255K and M351T, with the latter two Bcr-Abl mutants retaining partial sensitivity to imatinib. We have shown that CsA synergizes with imatinib at a concentration that only partially inhibit Bcr-Abl (0.1 μM imatinib). To test whether CsA can sensitize cells that express mutated Bcr-Abl to the effects of imatinib, Ba/F3 murine pro-B-cells were stably transduced with empty vector, native Bcr-Abl, or Bcr-Abl with M351T or E255K mutations. As shown in Figure 4B, Ba/F3 cells without Bcr-Abl (“Vec”) were unaffected by imatinib at 0.1 μM, and CsA at 5 μM had only a moderate effect on viability. However, upon expression of Bcr-Abl (“WT”), these cells became exquisitely sensitive to the imatinib/CsA combination, arguing that CsA synergism with imatinib is Bcr-Abl dependent. CsA also sensitized imatinib-resistant M315T mutant cells to the effects of 0.1 μM imatinib and the more resistant E255K cells to 1 μM imatinib, indicating that CsA can sensitize mutant Bcr-Abl cells to partial inhibition of Bcr-Abl by imatinib. CsA also enhanced killing of Ba/F3 vector cells by imatinib (1 μM), suggesting an off-target effect of imatinib. However, this effect was not comparable to the dramatic effect of CsA/imatinib on Bcr-Abl native or mutant-expressing cells, enforcing the hypothesis that CsA synergism with imatinib is predominantly Bcr-Abl dependent.
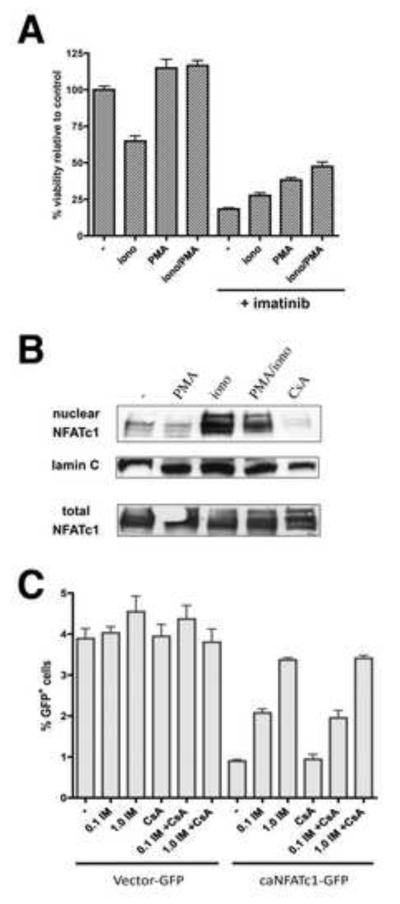
NFAT signaling promotes survival of leukemia cells upon Bcr-Abl inhibition
If CsA sensitizes Bcr-Abl+ cells to imatinib-induced cell death through inhibition of calcineurin and subsequent inhibition of NFAT, then activation of NFAT should protect cells from imatinib-induced death. NFAT-dependent transcription can be activated by treatment of cells with ionomycin, which elevates levels of intracellular Ca2+ resulting in activation of calcineurin and NFAT nuclear translocation, and/or the phorbol ester PMA, which activates protein kinase C, promoting activation of NFAT cofactor AP-1. K562 cells were treated with PMA and ionomycin alone or in combination for 6 hr followed by treatment with 1 μM imatinib for 48 hr. Treatment with either agent enhanced cell viability and the combination increased viability by 2.5-fold after exposure to imatinib compared to untreated cells (Figure 5A). We verified that NFATc1, identified in our screen as a SLIM, is expressed in CML cells and that its translocation to the nucleus is induced by ionomycin in the presence or absence of PMA (Figure 5B).
Figure 5. NFAT protects CML cells from imatinib-induced cell death.
A) K562 cells were treated with ionomycin (1 μg/ml) alone or in combination with PMA (10 ng/ml). After 16 hr, the cells were treated −/+ 1 μM imatinib for 48 hr as indicated and the number of viable cells was counted. B) K562 cells were treated with PMA and/or ionomycin, or CsA for 16 hr and harvested. Whole cells lysates and nuclear extracts were prepared and subjected to western blotting for NFATc1. The nuclear extract blot was stripped and reprobed for lamin C as a loading control. C) K562 cells were infected with retrovirus expressing GFP alone or constitutively active NFATc1-GFP. Forty-eight hours after infection, the cells were treated with imatinib at the indicated concentrations and/or CsA (5 μM) for 72 hr and the percentages of viable GFP+ (relative to all viable cells) were determined using flow cytometry. Error bars +/− SD.
To directly assess whether NFAT activation can protect Ph+ cells from imatinib, K562 cells were transduced with a retrovirus encoding a constitutively active NFATc1 co-expressed with GFP (Monticelli and Rao, 2002). Of note, in the absence of imatinib treatment, these cells are unable to maintain overexpression of constitutively active NFATc1 (data not shown), suggesting a negative impact on cell growth. However, imatinib treatment selects for cells that express NFATc1 (GFP+), resulting in a 4-fold increase in GFP+ cells relative to untreated cells (transduction efficiency: ~1%) with no effect on cells carrying empty vector (~4% GFP with or without imatinib) (Figure 5C). Selection for constitutive NFAT activity occurred in the presence and absence of CsA, consistent with complete bypass of the need for calcineurin activity. Thus, activation of NFAT can provide a major survival advantage to CML cells upon Bcr-Abl inhibition with imatinib.
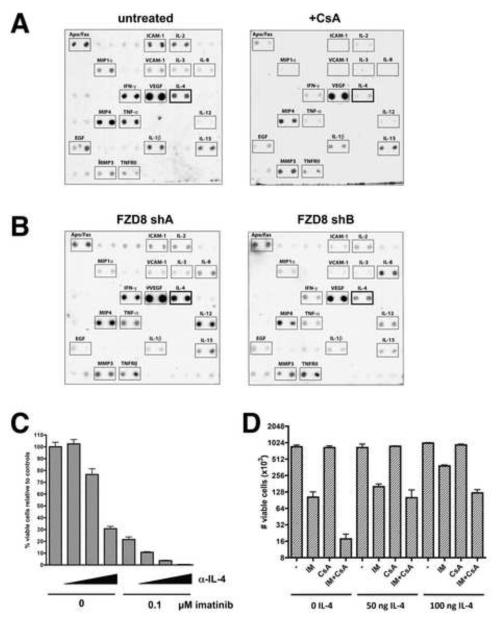
The majority of NFAT transcriptional targets are cytokines, suggesting that NFAT-driven autocrine cytokine production may protect Ph+ leukemia cells from Bcr-Abl inhibitor-mediated cell death. To probe for NFAT-dependent cytokine production by CML cells, antibody-based cytokine arrays were performed using conditioned media from untreated K562 cells or K562 cells treated with CsA for 24 hr. CsA inhibited the production of several known NFAT-regulated cytokines, most notably IL-2, IL-4, IFN-γ, and TNF-α, whereas other secreted factors not known to be NFAT-dependent, such as MMP3, TNFRII and IL-15, remained mostly unchanged (Figure 6A). To examine whether knockdown of FZD-8 affects cytokine production, consistent with Wnt/Ca2+ signaling controlling NFAT activity, K562 cells expressing shA (negative control) and shB (FZD-8 knockdown), described in Figure 2, were subjected to cytokine array analysis. Figure 6B shows that FZD-8 knockdown is not the equivalent of universal calcineurin inhibition with CsA, indicating that there is some specificity to FZD-8 signaling. However, several NFAT-dependent cytokines were commonly downregulated upon FZD-8 knockdown, including IL-4 and IFN-γ, supporting a role for FZD-8 signaling in regulating NFAT-controlled transcription of a subset of cytokines.
Figure 6. IL-4 production is NFAT and FZD-8 dependent and protects Ph+ cells from imatinib.
A) Conditioned media from K562 cells treated −/+ CsA at 5μM was used for cytokine array analysis. Cytokines that were consistently detectable are labeled. B) Conditioned media from K562 cells expressing FZD-8 shA (no knockdown) or shB (FZD-8 knockdown) was prepared and used for cytokine array analysis. C) Ba/F3 cells expressing Bcr-Abl (described in Figure 4) were treated with imatinib and neutralizing IL-4 antibody (11B11) alone or in combination (increasing 11B11 concentrations of 0, 25, 50, and 100 μg/ml are indicated by the triangles) for 72 hr and viable cells were counted. D. Ba/F3 cells expressing Bcr-Abl were treated with recombinant murine IL-4 together with imatinib and CsA at the indicated concentrations for 72 hr and viable cells were counted. Error bars +/− SD. See also Figure S6.
Given that both CsA treatment and FZD-8 knockdown impair its production and that it was identified as a SLIM, IL-4 is a good candidate for a cytokine with a role in maintaining survival of Ph+ cells upon Bcr-Abl inhibition. We asked whether the IL-4 neutralizing monoclonal antibody 11B11 could sensitize Bcr-Abl-expressing murine Ba/F3 cells to killing by imatinib. Cytokine array analysis confirmed that these cells have IL-4 production that is suppressed by CsA (Figure S6A). Treatment with 11B11 resulted in dramatic dose-dependent cell killing when combined with imatinib, similar to what was observed with CsA treatment (Figure 6C). Thus, IL-4 inhibition is sufficient to sensitize Bcr-Abl+ cells to imatinib. A monoclonal antibody that neutralizes murine CD4, which is not expressed by B-cells, had only minor effects alone or in combination with imatinib (Figure S6B). Addition of exogenous murine IL-4 protected the cells from the effects of imatinib and, in fact, largely reversed the ability of CsA to sensitize the cells to imatinib (Figure 6D). Since IL-4 is a target of NFAT, these results reinforce the conclusion that the observed synergy with CsA and Bcr-Abl inhibitors is due to calcineurin-NFAT inhibition rather than inhibition of MDR1.
NFAT inhibition with CsA enhances elimination of Bcr-Abl+ leukemia by dasatinib in vivo
Finally, we wanted to test whether inhibition of NFAT signaling by CsA could enhance the ability of a Bcr-Abl inhibitor to eliminate Ph+ leukemia cells in vivo. Transduction of bone marrow from ARF-/- mice with p185 Bcr-Abl/GFP generates an aggressive B-cell acute lymphoblastic leukemia (B-ALL), providing a tractable model that is reflective of human Ph+ ALL (Williams et al., 2007). The therapeutic response observed with dasatinib in this model mirrors that seen in patients: while dasatinib reduces leukemic burden, a significant number of dasatinib-refractory leukemia cells are maintained and the disease often relapses with resistance mutations in Bcr-Abl (Williams and Sherr, 2008). To test the effects of dasatinib combined with CsA using this model, mice were inoculated with 5 × 105 ARF-/- p185 B-ALL cells and after 3 days to allow engraftment, mice were treated with vehicle, CsA alone, dasatinib alone, or dasatinib and CsA in combination. An initial experiment, with therapy up to day 96, showed that mice treated with vehicle or with CsA alone succumbed to leukemia on day 10, whereas treatment with dasatinib alone and dasatinib/CsA resulted in 50% and 100% leukemia-free survival, respectively (Figure S7).
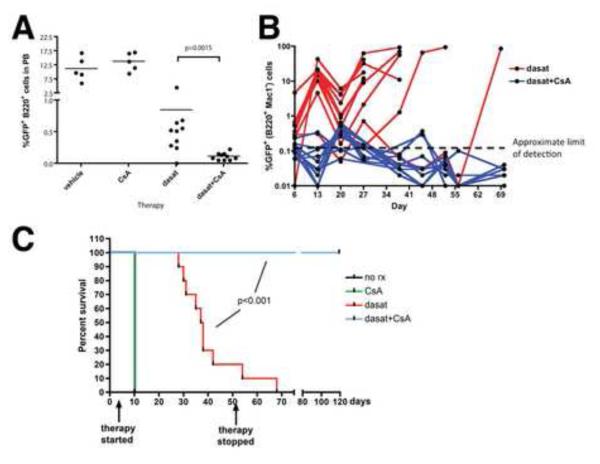
In a second in vivo experiment under the same conditions, leukemic burden in the peripheral blood was assessed by flow cytometry after 3 days of therapy. This analysis found that, while CsA by itself had no significant effect, CsA in combination with dasatinib was far more effective than dasatinib alone at rapid elimination of Bcr-Abl+ leukemic B-cells (GFP+, B220+, Mac1−) from the blood (Figure 7A). Weekly analysis of peripheral blood showed that GFP+ B-cells remained at near undetectable levels in mice receiving dasatinib/CsA, while leukemic cells eventually rose to high levels in mice receiving dasatinib alone (Figure 7B). Mice receiving vehicle or CsA alone were moribund and sacrificed on day 10 (Figure 7C). Post-mortem analysis showed extensive leukemia in the blood, bone marrow and spleen (not shown). By day 52, 8 of 10 mice receiving dasatinib alone had succumbed to leukemia and therapy was halted to determine if leukemia had been eradicated in the surviving mice. The 2 remaining dasatinib-treated mice succumbed to leukemia within 16 days, while all 10 of the dasatinib/CsA treated mice remained healthy up to day 119, when the experiment was terminated (Figure 7C). Post-mortem analysis of these mice revealed no GFP+ cells in the blood, bone marrow and spleen, and white blood cell counts were normal (not shown). Thus, under conditions where dasatinib as monotherapy was largely ineffective, adjuvant therapy with CsA resulted in complete eradication of Bcr-Abl+ leukemia.
Figure 7. CsA enhances elimination of Ph+ ALL cells by dasatinib in vivo.
C57BL/6 mice were inoculated intravenously with 5 × 105 ARF-/- p185 Bcr-Abl/GFP B-ALL cells. After 3 days, groups of mice were treated once daily by oral gavage with vehicle (80 mM citric acid, pH 3.1; n=5), CsA (25 mg/kg; n=5), dasatinib (20 mg/kg; n=10), or dasatinib and CsA combined (n=10) up to day 52 post-inoculation. A) After 3 days of therapy, peripheral blood from all mice was immunostained for B220 and Mac-1 and analyzed by flow cytometry. The percentage of GFP+ cells in the B-lineage (B220+, Mac-1−) population was determined and plotted. B) Peripheral blood was taken on the indicated days and analyzed as in A and plotted over time. The limit of detection of GFP in peripheral blood, based on analyses of blood from control C57Bl/6 mice is ~0.1%. C) Kaplan-Meier curve showing survival of mice receiving the indicated therapy. Mice were sacrificed when moribund, and all showed clear evidence of leukemia in blood, bone marrow and spleen. See also Figure S7.
Taken all together, our data indicate that signaling through the Wnt/Ca2+/NFAT pathway plays a role in maintaining survival of Ph+ leukemia cells upon Bcr-Abl inhibition and suggests that targeting this pathway in tandem with Bcr-Abl represents an effective therapeutic strategy for treatment of CML and Ph+ ALL.
Discussion
While the success of imatinib in patients with chronic phase CML has revolutionized the treatment of this disease, relapses remain common among patients with advanced CML or Ph+ ALL, and high failure rates persist with second-line dasatinib and nilotinib therapy (Milone and Enrico, 2009; Quintas-Cardama et al., 2007). Similar poor response rates are observed with tyrosine kinase inhibitor-based targeted therapy in other types of cancer, which is likely due to the increased genetic complexity of these diseases. As imatinib therapy has rarely proven to be curative, eradication of Ph+ leukemias will likely require targeting other gene products in addition to Bcr-Abl.
We applied large-scale an RNAi screening approach to identify genetic pathways that could sensitize blast crisis CML cells to Bcr-Abl inhibition by imatinib. Our screen identified numerous components of a noncanonical Wnt/Ca2+/NFAT pathway as being synthetic lethal with imatinib, suggesting this pathway plays a role in maintenance of Bcr-Abl+ leukemia cells despite Bcr-Abl inhibition. These studies highlight the strength of an unbiased synthetic lethal screen: the Wnt/Ca2+ pathway has not previously been implicated in CML or Ph+ ALL pathogenesis, and analyses of gene expression changes that accompany CML progression to advanced phases does not reveal significantly altered expression of this pathway during CML evolution (Radich et al., 2006). A pathway does not necessarily need to be differentially expressed or mutationally deregulated in a cancer to contribute to resistance to a therapy that the cancer did not evolve to endure. An unbiased screen provides a unique opportunity to uncover such pathways.
The Wnt/Ca2+/NFAT pathway is largely uncharacterized in mammalian cells, with most studies performed in Xenopus and zebrafish, and its role in human cancer remains controversial. Some studies support a role for noncanonical Wnt signaling as tumor-suppressive, while others indicate a role in promoting tumorigenesis (reviewed in Pukrop and Binder, 2008). Our data strongly support a role for the Wnt receptor FZD-8 as a mediator of Wnt/Ca2+/NFAT signaling in CML, given that FZD-8 knockdown causes impaired NFAT activity and impaired production of NFAT-regulated cytokines. FZD-8 is a poorly studied receptor that has not previously been shown to be involved in Wnt/Ca2+ signaling. Ectopic overexpression studies showed that FZD-8 is capable of activating β-catenin-dependent signaling when co-expressed with the canonical coreceptor LRP6 (Liu et al., 2005). However, studies performed in Xenopus implicate FZD-8 in β-catenin-independent Wnt signaling (Wallingford et al., 2001). Whether a given FZD receptor signals through canonical or noncanonical pathways may be dependent on what coreceptor is utilized (Cadigan and Liu, 2006).
The Wnt/Ca2+/NFAT pathway has multiple branches that lead to activation of PDE, CaMKII, calcineurin and PKC. Given that these enzymes are not known to have shared substrates, it was somewhat surprising that all components of this pathway were identified in our screen. However, multiple components of this pathway can converge on activation of NFAT. We provide evidence that inhibition of FZD-8, CaMKII and calcineurin all impair NFAT activity, which correlates with an increased sensitivity to imatinib. While calcineurin is known to control nuclear translocation of NFAT, the precise role of CaMKII in NFAT activation is less clear. CaMKII has been shown to activate the NFAT-cooperative transcription factor NK-κB in T-cells through direct phosphorylation of CARMA1 (Ishiguro et al., 2006). Exactly how CaMKII and other components of this pathway impinge upon NFAT-dependent transcription in Ph+ leukemia cells remains to be established. Nonetheless, our data strongly argue that NFAT plays a critical role in survival of Ph+ leukemia cells upon Bcr-Abl inhibition. This role is best evidenced by our findings that overexpression of constitutively active NFATc1 protects CML cells from imatinib-mediated killing and that CsA sensitizes multiple types of Ph+ cells to Bcr-Abl inhibition. In light of recent studies implicating calcineurin-NFAT signaling in survival of T-cell acute lymphoblastic leukemias (Medyouf et al., 2007), our studies support a perhaps underappreciated role of NFAT in hematopoietic and other types of cancer, since NFAT activation is implicated in tumors of epithelial cell origin as well (Medyouf and Ghysdael, 2008).
Much of the data presented in this report relies on calcineurin-NFAT inhibition by CsA. Given the importance of NFAT for T-cell development and function, CsA is commonly utilized in the clinic as an effective immunosuppressive agent after organ transplantation. However, CsA is also known to inhibit p-glycoprotein encoded by the multiple drug resistance gene MDR1. Yokota et al. tested INNO-406, an experimental Bcr-Abl inhibitor, in combination with CsA in a mouse model of Ph+ CNS leukemia where it enhanced INNO-406-mediated elimination of Bcr-Abl-expressing Ba/F3 cells from the brain (Yokota et al., 2007). Their explanation of these results was that CsA acts as a p-glycoprotein inhibitor to decrease efflux of INNO-406 at the blood-brain barrier, although CsA only moderately enhanced INNO-406 levels in the brain. Based on our data, we would argue that their observed effects are more likely due to CsA-mediated calcineurin inhibition synergizing with Bcr-Abl inhibition. In this study, we found no significant effect of CsA on the Bcr-Abl inhibitory activity of imatinib, inconsistent with a role for p-glycoprotein in efflux of imatinib from CML cells. This contention is further supported by the observations that MDR1 deletion does not enhance sensitivity of CML cells to imatinib in vivo (Zong et al., 2005) and artificial overexpression of p-glycoprotein is necessary to achieve reduced imatinib efflux in K562 cells (Illmer et al., 2004).
Our data support a scenario whereby enhanced NFAT activity in Ph+ cells, mediated by noncanonical Wnt/Ca2+ signaling, increases autocrine NFAT-dependent cytokine production that provides compensatory survival signaling upon exposure to imatinib. Cytokine signaling is increasingly found to have roles in multiple types of leukemia (Van Etten, 2007), and the noncanonical ligand Wnt5a has been shown to play a role in upregulation of multiple cytokines in hematopoietic cells (Blumenthal et al., 2006; Pereira et al., 2008). We found that IL-4 was commonly downregulated by both FZD-8 and calcineurin-NFAT inhibition, which sensitized Ph+ leukemia cells to Bcr-Abl inhibition. It is intriguing that autocrine IL-4 production was recently shown to maintain survival of colon cancer stem cells upon treatment with chemotherapeutics (Francipane et al., 2008; Todaro et al., 2007), implicating IL-4 signaling as a general mediator of cell survival in the face of therapeutic stress. Furthermore, deletion of the cytokine receptor common γ chain, through which IL-4 signals, restores the sensitivity of imatinib-refractory Bcr-Abl+ ARF-/- ALL cells to imatinib in vivo (Williams et al., 2007). In vivo, stromal cells are a likely source of pro-survival cytokines for leukemia cells. However, given that cytokine gene transcription is often NFAT-dependent, inhibiting calcineurin-NFAT should also severely impair cytokine-mediated survival signaling coming from paracrine sources.
The possibility that pro-survival cues might come from the environment of Ph+ leukemia cells necessitated the testing of a Bcr-Abl inhibitor in combination with CsA in an in vivo model of Ph+ leukemia. We chose to test our therapeutic approach in a model of Ph+ ALL, since this disease is refractory to therapy with Bcr-Abl inhibitors alone. Treatment of mice bearing Bcr-Abl+ ARF-/- ALL cells with dasatinib has been shown to be ineffective in completely eliminating leukemia cells, resulting in the rapid appearance of dasatinib-resistance (Williams and Sherr, 2008). Our in vivo results show that dasatinib combined with CsA therapy can be quite successful in eradication of Ph+ leukemia that is refractory to dasatinib therapy alone. These results could have important implications for improving clinical responses to Bcr-Abl inhibitors in Ph+ ALL and possibly advanced CML.
The results presented in this report strongly support the therapeutic strategy of combining Bcr-Abl inhibition with inhibition of NFAT by CsA or other NFAT-inhibitory approaches. Given that CsA has long been in clinical use, its pharmacodynamics and side effects are well understood. Thus, clinical trials exploring the efficacy of CsA combined with Bcr-Abl inhibitors for the treatment of Bcr-Abl inhibitor-refractory Ph+ leukemias may be warranted. The therapeutic efficacy of inhibiting specific NFAT targets, such as IL-4, also deserves exploration. Finally, our synthetic lethal RNAi-based screen, which allowed for the rapid identification of adjuvant drug targets in Bcr-Abl+ leukemia, may serve as an efficient model for the discovery of combination therapies for the treatment of various other types of cancer.
Experimental Procedures
shRNA screen and analysis
1 × 107 K562 cells were transduced with 1 × 106 ifu of the lentiviral human 50K shRNA library (GeneNet, SBI) using standard methods. Two days after infection, cells were subjected to selection in puromycin (2.5 μg/ml) for a period of 2 weeks. The cells were then divided into 6 groups of 1 × 107 cells, 3 were left untreated, and the other 3 were treated with imatinib at 1 μM for 72 hr and were cultured for an additional week. Total RNA was isolated from each group using Trizol reagent (Invitrogen) and reverse-transcribed using M-MLV reverse transcriptase (Epicentre). shRNA sequences were PCR-amplified and labeled with biotin according to the manufacturer’s instructions. 15 μg of the biotin-labeled PCR products were used for hybidization to microarrays (Affymetrix, HG-U133+ 2.0) and analysis of signal intensities was performed using GeneNet software (SBI). See Supplemental Information for details of the data normalization and determination of significant changes.
Cell culture and generation of cell lines
K562 and KBM7 cells (a gift from M. Beran, MD Anderson) were cultured in Iscove’s modified Dulbecco’s medium/10% fetal bovine serum (FBS). For generation of shRNA-expressing cell lines, lentivirus production from pLKO.1 constructs and transduction were performed as described previously (Porter and DeGregori, 2008) and cells were selected and maintained in puromycin (2.5 μg/ml). SUP-B15 cells were cultured in RPMI 1640 medium/20% FBS with 5mM β-mercaptoethanol. Ba/F3 cells were cultured in RPMI 1640 medium/10% FBS and 15% WEHI3 conditioned medium (WCM). Ba/F3 cell lines expressing Bcr-Abl and mutated derivatives (gifts of B. Deininger, OHSU) were generated by retroviral transduction using MSCV-ires-GFP constructs as previously described (Marusyk et al., 2007); GFP+ cells were sorted and maintained in media lacking WCM. Bcr-Abl+ ARF-/- ALL cells were generated as previously described (Williams et al., 2007) and were cultured in RPMI 1640 medium/10% FBS and 2x L-glutamine prior to inoculation into mice.
Quantitative real time PCR (qPCR)
qPCR was performed using the TaqMan method as described previously (Shapiro et al., 2006). Primer and probe sequences are provided in Supplemental Information.
Cell viability assays
Cells were seeded at 1 × 105/ml in triplicate wells of 24-well tissue culture plates. Where indicated, the cells (immediately after seeding) were treated with drug for a period of 48-72 hr. After or during treatment, a sample of cells from each well was stained with PI (10 μg/ml) and viable cells (PI−) were counted using a flow cytometer (Quanta SC, Beckman Coulter). For some experiments, the number of GFP+ viable (PI−) was counted in the same manner. The results from 2 independent experiments were combined and plotted +/− standard deviation (SD).
Colony forming assays
5 × 104 CD34+ cells from primary CP CML samples were plated in 0.9% MethoCult (Stem Cell Technologies Inc.) supplemented with 30% FBS, 1% BSA, 2mM L-glutamine and rhIL-3 (100ng/ml) and in the presence of the indicated drug combinations. Colony numbers were scored 14 days post plating. The frozen non-identifiable bone marrow patient specimens were obtained with informed consent from the OSU Leukemia Tissue Bank, Columbus OH, and all of the performed experiments were approved by The OSU Institutional Review Board.
Western blotting
Nuclear extracts were prepared using the protocol described at http://www.uphs.upenn.edu/ncrc/morrisey/nucextract. Preparation of whole cell lysates and western blotting were performed as previously described (Shapiro et al., 2006). Antibodies utilized were anti-NFATc1 (7A6, Santa Cruz), anti-lamin C (636, Santa Cruz), anti-β-catenin (clone14, BD Transduction Laboratories), anti-α-tubulin (DM1A; MeoMarkers), anti-phosphotyrosine (4G10; Millipore). Phospho-specific antibodies to c-Abl (#2681), CrkL (#3181) and Stat5 (#9351), and anti-eIF4E (#9742) were from Cell Signaling Technology.
Reporter assays
K562 cells were co-infected with 10 ifu/cell of adenovirus containing the 9XNFAT-luciferase reporter plus adenovirus expressing GFP. Forty-eight hr after infection, a sample of cells was analyzed by flow cytometry to determine the number of viable (PI−) GFP+ cells and lysates were subjected to standard firefly luciferase assays (Promega). Luciferase values were normalized to the number of GFP+ cells. Results shown are from 2 independent experiments; error bars +/− SD
Cytokine assays
Conditioned media was generated by incubating 2 × 106 cells in 2 ml of low serum (0.2% FBS) media for 24 hr, in the presence or absence of CsA, as indicated. Conditioned media was incubated on antibody based cytokine arrays (Panomics, Human Array 3.0) for 16 hr at 4°C. Detection was performed according to the manufacturer’s instructions. Results are representative of two independent experiments.
Mice
Female C57BL/6 mice (6-10 wk old) from the National Cancer Institute were utilized for experiments. Dasatinib was prepared by dissolving in 80 mM citric acid, pH 2.1 daily for oral gavage. Cyclosporin A (Neoral; Novartis) was added directly to vehicle or dasatinib preparation just prior to gavage. All mouse experiments were approved by the University of Colorado Denver IACUC.
Flow cytometry analysis
Flow cytometry was performed as previously described (Porter and DeGregori, 2008). Antibodies utilized for cell surface staining were anti-mouse B220-APC, anti-mouse B220-PE, and anti-mouse Mac1-PE-Cy7 (all from eBioscience). Statistical analysis of results was performed using Prism 4 software (GraphPad). Unpaired two-tailed t tests were used to determine statistical significance between 2 groups.
Accession numbers
Microarray data from the screen have been deposited at NCBI GEO under the accession number GSE21499.
Significance
The promise of targeted cancer therapies has generated much excitement with the success of Bcr-Abl inhibitors for treatment of chronic phase CML. However, targeted therapies for other cancers have typically failed to achieve durable responses. For example, patients with advanced phase CML and Bcr-Abl+ ALL do not show long term responses to Bcr-Abl inhibitors. The targeting of additional genes may be necessary to enhance the efficacy of Bcr-Abl inhibitors. The RNAi-based screen described here identified numerous components of a Wnt/Ca2+/NFAT pathway whose inhibition sensitizes leukemic cells to Bcr-Abl inhibitors. Pharmacological targeting of this pathway could improve the treatment of Bcr-Abl+ leukemias. Our screen may also serve as a model for discovery of adjuvant targeted therapies for other forms of cancer.
Highlights
- RNAi screen identifies targets that are synthetic lethal with Bcr-Abl inhibitors
- Bcr-Abl inhibition reveals a role for Wnt/NFAT signaling in leukemia cell survival
- Inhibition of NFAT signaling improves treatment of Bcr-Abl+ leukemia in mice
Supplementary Material
01
02
03
04
Acknowledgements
JD is supported by the Leukemia and Lymphoma Society (108692) and the National Cancer Institute (NCI; RO1-CA109657) and MG by the Cancer League of Colorado and the NCI (K01-CA133182). DP is supported by the NCI (CA095512) and the US Army and CML Research Program (DAMD17-03-1-0184). DP is a Scholar of The Leukemia and Lymphoma Society. RW is supported by an NIH Cancer Center Support Core Grant (CA-21765), the American Lebanese Syrian Associated Charities, and a Career Development Award from the AACR. We would like to thank Anjana Rao, Jeff Molkentin, Heide Ford, Ulli Bayer, Michael Deininger, and Charles Sherr for providing reagents, Dexiang Gao for statistical analyses, Nidal Boulos for characterization of Arf-null/p185 Bcr-Abl+ GFP+ cells, Andriy Marusyk, Christopher Porter, and Richard Gregory for comments on the manuscript, Bifeng Gao and Uma Pugazhenthi of the UC Cancer Center Gene Expression Core, and Karen Helm and Christine Childs of the UC Cancer Center Flow Cytometry Core (supported by NIH grant 2P30-CA46934).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–2010. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- Boise LH, Petryniak B, Mao X, June CH, Wang CY, Lindsten T, Bravo R, Kovary K, Leiden JM, Thompson CB. The NFAT-1 DNA binding complex in activated T cells contains Fra-1 and JunB. Mol Cell Biol. 1993;13:1911–1919. doi: 10.1128/mcb.13.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Coluccia AM, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, Benati D, Pinna LA, Gambacorti-Passerini C. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- Faderl S, Garcia-Manero G, Thomas DA, Kantarjian HM. Philadelphia chromosome-positive acute lymphoblastic leukemia- current concepts and future perspectives. Rev Clin Exp Hematol. 2002;6:142–160. doi: 10.1046/j.1468-0734.2002.00066.x. discussion 200-142. [DOI] [PubMed] [Google Scholar]
- Francipane MG, Alea MP, Lombardo Y, Todaro M, Medema JP, Stassi G. Crucial role of interleukin-4 in the survival of colon cancer stem cells. Cancer Res. 2008;68:4022–4025. doi: 10.1158/0008-5472.CAN-07-6874. [DOI] [PubMed] [Google Scholar]
- Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun. 2006;345:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, de Leij LF, Verschuren EW, Borger P, Vellenga E. Interleukin-7 upregulates the interleukin-2-gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both nuclear factor of activated T cells and activator protein-1. Blood. 1997;90:2690–2700. [PubMed] [Google Scholar]
- Guo L, Urban JF, Zhu J, Paul WE. Elevating calcium in Th2 cells activates multiple pathways to induce IL-4 transcription and mRNA stabilization. J Immunol. 2008;181:3984–3993. doi: 10.4049/jimmunol.181.6.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran PF, Helms LM, Kung L, Noujaim J. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation. 1999;68:1356–1361. doi: 10.1097/00007890-199911150-00023. [DOI] [PubMed] [Google Scholar]
- Hodge MR, Chun HJ, Rengarajan J, Alt A, Lieberson R, Glimcher LH. NF-AT-Driven interleukin-4 transcription potentiated by NIP45. Science. 1996;274:1903–1905. doi: 10.1126/science.274.5294.1903. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlagel U, von Bonin M, Pursche S, Bergemann T, Ehninger G, Schleyer E. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia. 2004;18:401–408. doi: 10.1038/sj.leu.2403257. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Green T, Rapley J, Wachtel H, Giallourakis C, Landry A, Cao Z, Lu N, Takafumi A, Goto H, et al. Ca2+/calmodulin-dependent protein kinase II is a modulator of CARMA1-mediated NF-kappaB activation. Mol Cell Biol. 2006;26:5497–5508. doi: 10.1128/MCB.02469-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GH, Her JH, Han JK. Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J Cell Biol. 2008;182:1073–1082. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- Liu G, Bafico A, Aaronson SA. The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts. Mol Cell Biol. 2005;25:3475–3482. doi: 10.1128/MCB.25.9.3475-3482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Busby JC, Molkentin JD. Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point. Nat Cell Biol. 2009;11:154–161. doi: 10.1038/ncb1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Wheeler LJ, Mathews CK, DeGregori J. p53 mediates senescence-like arrest induced by chronic replicational stress. Mol Cell Biol. 2007;27:5336–5351. doi: 10.1128/MCB.01316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H, Alcalde H, Berthier C, Guillemin MC, dos Santos NR, Janin A, Decaudin D, de The H, Ghysdael J. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007;13:736–741. doi: 10.1038/nm1588. [DOI] [PubMed] [Google Scholar]
- Medyouf H, Ghysdael J. The calcineurin/NFAT signaling pathway: a novel therapeutic target in leukemia and solid tumors. Cell Cycle. 2008;7:297–303. doi: 10.4161/cc.7.3.5357. [DOI] [PubMed] [Google Scholar]
- Milone JH, Enrico A. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Leuk Lymphoma. 2009;50(Suppl 2):9–15. doi: 10.3109/10428190903370395. [DOI] [PubMed] [Google Scholar]
- Monticelli S, Rao A. NFAT1 and NFAT2 are positive regulators of IL-4 gene transcription. Eur J Immunol. 2002;32:2971–2978. doi: 10.1002/1521-4141(2002010)32:10<2971::AID-IMMU2971>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mowen KA, Schurter BT, Fathman JW, David M, Glimcher LH. Arginine methylation of NIP45 modulates cytokine gene expression in effector T lymphocytes. Mol Cell. 2004;15:559–571. doi: 10.1016/j.molcel.2004.06.042. [DOI] [PubMed] [Google Scholar]
- Ottmann OG, Druker BJ, Sawyers CL, Goldman JM, Reiffers J, Silver RT, Tura S, Fischer T, Deininger MW, Schiffer CA, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100:1965–1971. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]
- Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- Porter CC, DeGregori J. Interfering RNA-mediated purine analog resistance for in vitro and in vivo cell selection. Blood. 2008;112:4466–4474. doi: 10.1182/blood-2008-03-146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukrop T, Binder C. The complex pathways of Wnt 5a in cancer progression. J Mol Med. 2008;86:259–266. doi: 10.1007/s00109-007-0266-2. [DOI] [PubMed] [Google Scholar]
- Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6:834–848. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, Sawyers C, Shah N, Stock W, Willman CL, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Mittelstadt PR, Mages HW, Gerth AJ, Kroczek RA, Ashwell JD, Glimcher LH. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- Rezazadeh S, Claydon TW, Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinn amyl)-N-methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther. 2006;317:292–299. doi: 10.1124/jpet.105.097618. [DOI] [PubMed] [Google Scholar]
- Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, Blanchet O, Marit G, Gluckman E, Reiffers J, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature. 2002;417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- Sattler M, Griffin JD. Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin Hematol. 2003;40:4–10. doi: 10.1053/shem.2003.50034. [DOI] [PubMed] [Google Scholar]
- Shapiro GS, Van Peursem C, Ornelles DA, Schaack J, DeGregori J. Recombinant adenoviral vectors can induce expression of p73 via the E4-orf6/7 protein. J Virol. 2006;80:5349–5360. doi: 10.1128/JVI.02016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YH, Lee GW, Son KN, Lee SM, Kang CJ, Kwon BS, Kim J. Promoter analysis of human CC chemokine CCL23 gene in U937 monocytoid cells. Biochim Biophys Acta. 2007;1769:204–208. doi: 10.1016/j.bbaexp.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Van Etten RA. Aberrant cytokine signaling in leukemia. Oncogene. 2007;26:6738–6749. doi: 10.1038/sj.onc.1210758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol. 2001;45:225–227. [PubMed] [Google Scholar]
- Williams RT, den Besten W, Sherr CJ. Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes Dev. 2007;21:2283–2287. doi: 10.1101/gad.1588607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, Sherr CJ. The INK4-ARF (CDKN2A/B) Locus in Hematopoiesis and BCR-ABL-induced Leukemias. Cold Spring Harb Symp Quant Biol. 2008 doi: 10.1101/sqb.2008.73.039. [DOI] [PubMed] [Google Scholar]
- Yokota A, Kimura S, Masuda S, Ashihara E, Kuroda J, Sato K, Kamitsuji Y, Kawata E, Deguchi Y, Urasaki Y, et al. INNO-406, a novel BCR-ABL/Lyn dual tyrosine kinase inhibitor, suppresses the growth of Ph+ leukemia cells in the central nervous system, and cyclosporine A augments its in vivo activity. Blood. 2007;109:306–314. doi: 10.1182/blood-2006-03-013250. [DOI] [PubMed] [Google Scholar]
- Zong Y, Zhou S, Sorrentino BP. Loss of P-glycoprotein expression in hematopoietic stem cells does not improve responses to imatinib in a murine model of chronic myelogenous leukemia. Leukemia. 2005;19:1590–1596. doi: 10.1038/sj.leu.2403853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
04