Chemoimmunotherapy With a Modified Hyper-CVAD and Rituximab Regimen Improves Outcome in De Novo Philadelphia Chromosome–Negative Precursor B-Lineage Acute Lymphoblastic Leukemia (original) (raw)
Abstract
Purpose
The adverse prognosis of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia (ALL) prompted incorporation of monoclonal antibody therapy with rituximab into the intensive chemotherapy regimen hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone). Other modifications (irrespective of CD20 expression) included early anthracycline intensification, alterations in number of risk-adapted intrathecal chemotherapy treatments for CNS prophylaxis, additional early and late intensifications, and extension of maintenance phase chemotherapy by 6 months.
Patients and Methods
Two hundred eighty-two adolescents and adults with de novo Philadelphia chromosome (Ph)–negative precursor B-lineage ALL were treated with standard or modified hyper-CVAD regimens. The latter incorporated standard-dose rituximab if CD20 expression ≥ 20%.
Results
The complete remission (CR) rate was 95% with 3-year rates of CR duration (CRD) and survival (OS) of 60% and 50%, respectively. In the younger (age < 60 years) CD20-positive subset, rates of CRD and OS were superior with the modified hyper-CVAD and rituximab regimens compared with standard hyper-CVAD (70% v 38%; P < .001% and 75% v 47%, P = .003). In contrast, rates of CRD and OS for CD20-negative counterparts treated with modified versus standard hyper-CVAD regimens were similar (72% v 68%, P = not significant [NS] and 64% v 65%, P = NS, respectively). Older patients with CD20-positive ALL did not benefit from rituximab-based chemoimmunotherapy (rates of CRD 45% v 50%, P = NS and OS 28% v 32%, P = NS, respectively), related in part to deaths in CR.
Conclusion
The incorporation of rituximab into the hyper-CVAD regimen appears to improve outcome for younger patients with CD20-positive Ph-negative precursor B-lineage ALL.
INTRODUCTION
The prognostic relevance of immunophenotypic classification of acute lymphoblastic leukemia (ALL) relates to associations with cytogenetic and molecular aberrancies. While detection of surface antigens (eg, CD19, CD20, CD22, CD33, CD52) on lymphoblasts by flow cytometry (FC) identifies targets for monoclonal antibody (MoAb) therapy, expression of particular antigens may have prognostic implications. CD20 is a B-lineage antigen expressed on normal and malignant cells during nearly all stages of differentiation (except early B-cell precursors or plasma cells). Heterogeneity in CD20 expression among B-cell malignancies has been well-described.1 It ranges from 40% to 50% in precursor B-lineage ALL compared with 80% to 90% in mature B-cell or Burkitt-type leukemia/lymphoma.
CD20 functions as a calcium channel that influences cell cycle progression and differentiation via downstream signaling pathways, modulating levels of proapoptosis proteins, such as sarco/endoplasmic reticulum Ca2+ (SERCA3) and Bax/Bak.2 Constitutive activation of survival pathways involving nuclear factor-κB and extracellular receptor kinase (ERK1/2) results in overexpression of antiapoptotic Bcl-2 proteins and associated Bcl-2 genes.3 Expression of CD20 likely confers drug resistance via these mechanisms, resulting in persistence of leukemia subclones which eventually re-emerge.
The prognostic significance of CD20 expression in de novo precursor B-lineage ALL was initially evaluated in the pediatric setting with conflicting results. The Pediatric Oncology Group assessed CD20 expression by the traditional 20% cut point and mean fluorescence intensity.4 CD20 expression and increasing mean fluorescence intensity were independently associated with inferior event-free survival rates irrespective of known prognostic factors such as age and karyotype. In contrast, the St Jude experience suggested that CD20 expression was associated with slightly more favorable prognosis.5 It was postulated that these disparate results could be accounted for by differences in intensity of regimens and/or application of risk-adapted strategies.
The influence of CD20 expression on outcome for adults with de novo precursor B-lineage ALL was studied in the context of conventional (vincristine, doxorubicin, dexamethasone [VAD]6) or intensive (fractionated cyclophosphamide plus VAD [hyper-CVAD]7,8) chemotherapy.9 Complete remission (CR) rates were similar regardless of CD20 status (positive/negative by 20% cut point). However, CD20 expression was associated with significantly higher relapse rates (61% v 37%; P < .01) and lower 3-year CR duration (CRD) and survival (OS) rates (22% v 58%; P < .001 and 27% v 60%, P < .01, respectively) after hyper-CVAD therapy. These findings were particularly significant for the younger subsets, whereas CRD and OS rates were uniformly poor for the older group (age ≥ 60 years). Association of CD20 expression with higher cumulative incidence of relapse was subsequently confirmed in the Group for Research in Adult Acute Lymphoblastic Leukemia (GRAALL) 2003 trial, which applied a pediatric regimen to younger adults with de novo Philadelphia chromosome (Ph) –negative ALL.10
Rituximab, a chimerical MoAb directed at surface CD20, induces apoptosis, antibody-dependent cell-mediated cytotoxicity, and complement-mediated cytolysis.11 Incorporation of rituximab into first-line chemotherapy regimens has significantly improved outcome for subsets of non-Hodgkin's lymphoma such as Burkitt-type leukemia/lymphoma and mantle-cell lymphoma/leukemia.12–14 The favorable impact of chemoimmunotherapy has even extended to chronic lymphocytic leukemia, where CD20 expression of the malignant clone is lower than normal B lymphocytes.15,16
The hyper-CVAD program has proven to be an effective first-line therapy for adults with de novo ALL and lymphoblastic lymphoma (LL).7,8,17 Modifications to the regimen were implemented in order to improve on the results. Early anthracycline intensification was initially incorporated based on earlier reports suggesting that this therapeutic strategy improved relapse-free survival.18 Maintenance therapy was extended by 6 months with additional early and late intensifications to avoid relapses in close proximity to completion of therapy. Interventions targeting certain subsets included administration of induction chemotherapy in a protective environment if older to reduce early infection-related mortality; alteration in number of intrathecal chemotherapy treatments (IT) for CNS prophylaxis from four to six if classified as low CNS risk and from 16 to 8 if high CNS risk since prior isolated CNS relapse rates were 6% and 1%, respectively; and incorporation of rituximab for CD20 expression ≥ 20%. Herein, we summarize the results of modified hyper-CVAD regimens for de novo Ph-negative precursor B-lineage ALL with emphasis on outcomes by CD20 expression.
PATIENTS AND METHODS
Study Group
The diagnosis of ALL was established according to WHO criteria.19 Eligibility criteria included age ≥ 10 years without other active malignancy with expected consequent death within 12 months or known positivity for HIV 1. Adequate hepatorenal function was required unless attributable to leukemia. Protocols were approved by the institutional review board at M. D. Anderson Cancer Center. Informed consent for participation was obtained in accordance with institutional guidelines and Declaration of Helsinki. Mature B-cell ALL or Burkitt-type leukemia/lymphoma and Ph-positive ALL were treated on separate protocols with details reported elsewhere.13,20–22 Since October 2006, patients with de novo Ph-negative precursor B-lineage ALL younger than 31 years of age were allocated to first-line therapy with an augmented Berlin-Frankfurt-Münster regimen modeled after an established pediatric program.23
Therapy
Comparative details of the standard and modified hyper-CVAD 1 or 2 regimens are delineated in Table 1 and online-only Appendix.7,13,17,24 Treatment included eight or nine induction-consolidation courses of hyper-CVAD, liposomal daunorubicin with cytarabine (only if anthracycline intensification [modified hyper-CVAD 125]), and high-dose methotrexate with cytarabine. Intensive cycles were administered every 21 days or earlier (at least 14 days apart) on recovery (absolute neutrophil count [ANC] ≥ 1 × 109/L after granulocyte colony-stimulating factor discontinued for ≥ 24 hours and untransfused platelet [PLT] count 50-60 × 109/L). If marrow lymphoblasts were CD20-positive (≥ 20%), rituximab 375 mg/m2 was given on days 1 and 11 of hyper-CVAD cycles and on days 1 and 8 of liposomal daunorubicin and cytarabine or high-dose methotrexate and cytabarine cycles, for eight total doses over the first four courses. Rituximab was given with early and late hyper-CVAD intensifications during months 6 and 18 of maintenance therapy.
Table 1.
Hyper-CVAD and Modified Hyper-CVAD Chemoimmunotherapy Regimens
| Regimen | Modified Hyper-CVAD 1 and 2 (± rituximab) | Standard Hyper-CVAD (1992-1999) | |
|---|---|---|---|
| 2: Without Intensification (2001-present) | 1: With Intensification (2000-2001) | ||
| Induction | |||
| Hyper-CVAD | Y | Y | Y |
| Laminar air flow rooms if age ≥ 60 years | Y | Y | N |
| Rituximab 375 mg/m2 IV days 1, 11 if CD20 ≥ 20% | Y | Y | N |
| Consolidation | |||
| Cycle 2 (anthracycline intensification) | |||
| LDNR 150 mg/m2 IV over 12 h days 1-2 | N | Y | N |
| Cytarabine 1.5 g/m2 CI IV daily days 1-2 | |||
| Prednisone 200 mg PO days 1-5 | |||
| Cycles 2, 4, 6, 8 or cycles 3, 5, 7, 9 | |||
| MTX 200 mg/m2 IV over 2 h, then 800 mg/m2 IV over 22 h day 1 | Y | Y | Y |
| Cytarabine 3 g/m2 (1 g/m2 if age ≥ 60) IV over 2 h every 12 h × 4 doses on days 2-3 | |||
| Solu-Medrol 50 mg IV every 12 h × 6 doses on days 1-3 | |||
| Leucovorin 50 mg IV 12 h after end MTX then 15 mg IV every 6 h × 8 doses or until MTX level < 0.1 μmol/L | |||
| Acetazolamide if urine pH < 7 | |||
| Cycles 1, 3, 5, 7 or cycles 1, 4, 6, 8 | |||
| Cyclophosphamide 300 mg/m2 IV over 2 h every 12 h × 6 doses on days 1-3 | Y | Y | Y |
| Mesna 600 mg/m2 CI IV daily days 1-3 | |||
| Dexamethasone 40 mg IV or PO days 1-4, 11-14 | |||
| Doxorubicin 50 mg/m2 CI IV over 2-24 h day 4 (48 h if EF < 50%) | |||
| VCR 2 mg IV days 1,11 | |||
| Cycles 1-4 | |||
| If CD20 ≥ 20%: 8 doses rituximab 375 mg/m2 IV | Y | Y | N |
| Days 1, 11 (hyper-CVAD) | |||
| Days 1, 8 (LDNR- or MTX-cytarabine) | |||
| CNS prophylaxis | |||
| MTX 12 mg (6 mg if Ommaya) day 2 | Y | Y | |
| Cytarabine 100 mg day 7 or 8 | |||
| No. of ITs | |||
| Liposomal cytarabine in modified hyper-CVAD 2 (n = 32)24 | Y | N | |
| Risk adapted (LDH ≥ 1,400 U/L, S + G2M ≥ 14%) | |||
| High (one elevated) | 8 | 16 | |
| Indeterminate (one unknown) | 8 | 8 | |
| Low | 6 | 4 | |
| Maintenance | |||
| Oral POMP (6-mercaptopurine, VCR, MTX, prednisone) | Months 1-5, 8-17, 20-30 | Months 1-6, 8-10, 12-24 | |
| Intensification | |||
| Hyper-CVAD (plus rituximab 375 mg/m2 IV days 1, 11 if CD20 ≥ 20%) | Months 6, 18 | N | |
| Intensification† | |||
| MTX 100 mg/m2 IV day 1 weekly × 4 | Months 7, 19 | Months 7, 11 | |
| L-asparaginase 20,000 units IV day 2 weekly × 4 | |||
| Supportive care | |||
| IV/oral alkalinzation all courses; rasburicase/allopurinol for induction | |||
| G-CSF 10 μg/kg subcutaneously daily until ANC > 109/L; pegfilgastrim 6 mg subcutaneously could be substituted after 2007 | |||
| Duration of doxorubicin infusions increased for modified hyper-CVAD regimens for cardioprotection | |||
| Leucovorin rescue: 50-100 mg IV every 4-6 h if MTX levels were elevated at the end of infusion [0 h, confirmed on repeat sample] to greater than 20 μmol/L, > 1 μmol/L at 24 h, or > 0.1 μmol/L at 48 h |
Methods
FC was performed on diagnostic bone marrow aspirates (BMA) to establish lineage and CD20 expression as previously described.9 BMA specimens collected at the time of morphological CR (approximately day 21 of induction therapy) were assessed for minimal residual disease (MRD) when feasible (modified hyper-CVAD 2 cohort). BMA cells were stained with four-color antibody panel (multiparameter FC [MFC], sensitivity < 10−4) including markers for CD9, CD10, CD13, CD15, CD19, CD20, CD22, CD33, CD34, CD38, CD58, CD66c, and cytoplasmic-terminal deoxynucleotide transferase by methodology previously described (Appendix).26,27 Total DNA was extracted from BMA samples using automated methods (Autopure; Genta, Minneapolis, MN). B-cell clonality was determined using nonquantitative polymerase chain reaction (PCR) method with V consensus primers (cPCR) derived from framework 1 (FR1), framework 2 (FR2), and framework 3 (FR3) regions, in combination with mixture of fluorescently labeled J primers; sensitivity ranged from 10−2 to 10−4 depending on number of polyclonal B-cells.28
Response Criteria
CR was defined as ≤ 5% blasts in normocellular or hypercellular marrow with ANC ≥ 1 × 109/L, PLT ≥ 100 × 109/L, and resolution of extramedullary disease. CR with incomplete PLT recovery included CR criteria except for incomplete PLT recovery. Other outcomes were induction death if occurred after start of therapy without meeting definitions of CR or resistant disease, and resistant disease if survived the induction period but leukemia persisted. Relapse was defined as disease recurrence at any site after achievement of CR. Toxicity was graded according to National Cancer Institute Common Toxicity Criteria (version 3.0).
Statistical Considerations
The end points of these sequential prospective, open label, single-center, phase II trials of hyper-CVAD and its variants were response and CRD. OS was measured from initiation of therapy until death. CRD was measured from CR until relapse. Differences in CR rates or pretreatment characteristics among subgroups were analyzed by χ2 or Fisher's exact tests. Unadjusted CRD and OS analyses were performed using Kaplan-Meier plots29 with differences among them analyzed by the log-rank test.30 Goodness-of-fit was assessed by Martingale residual plots.31 The Cox proportional hazards model32 was used to assess treatment and characteristics predicting CR, CRD, and OS. Factors significant for CR, CRD, and OS outcomes by univariate analysis were analyzed further by stepwise regression using assumption of proportional hazards as suggested by Cox.32
RESULTS
Study Group
From July 1992 to August 2009, 282 adolescents and adults with de novo Ph-negative precursor B-lineage ALL were treated with standard (n = 109) or modified hyper-CVAD regimens (with [n = 47] or without [n = 126] anthracycline intensification; Table 2). Median age was 41 years (range, 13 to 83); 21% were 60 years or older and 57% were males. There were no significant differences in distribution among treatment categories by CD20 status (positive or negative), except for higher incidence of CD20 expression in the older group.
Table 2.
Characteristics of Adolescents and Adults With De Novo Philadelphia Chromosome–Negative Precursor B-Lineage ALL (n = 282)
| Characteristic | Modified Hyper-CVAD ± Rituximab | Hyper-CVAD Without Rituximab(n = 109) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2: Without Intensification (n = 126) | 1: With Intensification* (n = 47) | |||||||||||
| CD20 Negative | CD20 Positive | CD20 Negative | CD20 Positive | CD20 Negative | CD20 Positive | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. | 53 | 73 | 23 | 24 | 56 | 53 | ||||||
| Age, years | ||||||||||||
| ≤ 30 | 13 | 25 | 25 | 34 | 8 | 35 | 6 | 25 | 25 | 45 | 19 | 36 |
| 31-59 | 30 | 57 | 29 | 40 | 9 | 39 | 9 | 38 | 26 | 46 | 25 | 47 |
| ≥ 60 | 10 | 19 | 19 | 26 | 6 | 26 | 9 | 38 | 5 | 9 | 9 | 17 |
| Median | 42 | 47 | 40 | 53 | 32 | 43 | ||||||
| Range | 15-75 | 15-79 | 18-80 | 19-83 | 13-70 | 16-78 | ||||||
| Sex | ||||||||||||
| Female | 31 | 49 | 22 | 35 | 10 | 43 | 14 | 58 | 21 | 38 | 17 | 32 |
| Zubrod performance status | ||||||||||||
| 0-1 | 48 | 91 | 60 | 82 | 19 | 83 | 15 | 63 | 44 | 79 | 33 | 62 |
| 2-3 | 5 | 9 | 13 | 18 | 4 | 17 | 9 | 38 | 12 | 21 | 20 | 38 |
| Leukocyte count, ×109/L | ||||||||||||
| < 5 | 26 | 49 | 42 | 58 | 11 | 48 | 18 | 75 | 20 | 36 | 25 | 47 |
| 5-29.9 | 17 | 32 | 22 | 30 | 4 | 17 | 4 | 17 | 24 | 43 | 20 | 38 |
| ≥ 30 | 10 | 19 | 9 | 12 | 8 | 35 | 2 | 8 | 12 | 21 | 8 | 15 |
| Hemoglobin, g/dL | ||||||||||||
| < 10 | 36 | 68 | 62 | 85 | 19 | 83 | 20 | 83 | 41 | 73 | 41 | 77 |
| Platelet count, ×109/L | ||||||||||||
| < 100 | 35 | 66 | 64 | 88 | 15 | 65 | 18 | 75 | 40 | 71 | 42 | 79 |
| β-2 microglobulin, mg/dL | ||||||||||||
| ≥ 3 | 17/47 | 36 | 35/61 | 57 | 12/23 | 52 | 8/21 | 38 | 20/45 | 44 | 12/35 | 34 |
| Lactate dehydrogenase, U/L | ||||||||||||
| ≥ 1,400 | 25 | 47 | 32 | 44 | 7 | 30 | 6 | 25 | 29 | 52 | 22 | 42 |
| % peripheral blasts | ||||||||||||
| Any | 41 | 77 | 61 | 84 | 16 | 70 | 19 | 79 | 53 | 95 | 45 | 85 |
| Karyotype (except IM; n = 50;58;21;23;48;45) | ||||||||||||
| Diploid | 22 | 44 | 30 | 52 | 12 | 54 | 12 | 52 | 18 | 38 | 19 | 42 |
| Hyperdiploid | 9 | 18 | 5 | 9 | — | 5 | 22 | 4 | 8 | 6 | 13 | |
| Hypodiploid | 1 | 2 | 5 | 9 | 2 | 9 | — | 2 | 4 | 4 | 9 | |
| Translocation/other | 18 | 36 | 18 | 31 | 7 | 33 | 6 | 26 | 24 | 50 | 16 | 36 |
| CNS leukemia | ||||||||||||
| Yes | 3 | 6 | 9 | 12 | — | 1 | 4 | 1 | 2 | 3 | 6 | |
| Splenomegaly | ||||||||||||
| Yes | 9 | 17 | 15 | 21 | 2 | 9 | 3 | 12 | 11 | 20 | 18 | 34 |
| Lymphadenopathy | ||||||||||||
| Yes | 12 | 23 | 21 | 29 | 8 | 35 | 5 | 21 | 18 | 32 | 14 | 26 |
| Hepatomegaly | ||||||||||||
| Yes | 4 | 7 | 10 | 14 | 1 | 4 | 2 | 8 | 6 | 11 | 8 | 15 |
| CNS relapse risk (n = 56;60;23;24;44;45) | ||||||||||||
| Low | 17 | 30 | 24 | 40 | 6 | 26 | 5 | 21 | 12 | 27 | 18 | 40 |
| Indeterminate | 6 | 10 | 6 | 10 | 7 | 30 | 12 | 50 | 1 | 2 | 1 | 2 |
| High | 33 | 59 | 30 | 50 | 10 | 43 | 7 | 29 | 31 | 70 | 26 | 58 |
| Systemic risk classification | ||||||||||||
| Low | 13 | 25 | 26 | 36 | 2 | 9 | 6 | 25 | 18 | 32 | 16 | 30 |
| High | 40 | 75 | 46 | 64 | 21 | 91 | 18 | 75 | 38 | 68 | 37 | 70 |
Response
The overall CR rate was 95% (Table 2). Lower CR rates were observed in the older group (88% v 97%; P = .02, with failures equally divided between resistant disease and induction death). CR rates by pretreatment characteristics are provided in Table 3. There were no significant differences in CR rates by regimen or CD20 expression. Assessments of MRD were performed on BMAs collected at the time of CR in 93 of 126 patients (74%) treated with modified hyper-CVAD 2. The incidence of MRD-negativity by MFC (< 0.01%) was higher (81% v 58%; P = .02) for the CD20-positive subset (n = 57) treated with rituximab compared with the CD20-negative group (n = 36). The respective rates for MRD-negativity by cPCR for immunoglobulin heavy chain gene rearrangements in 91 evaluable patients were 85% versus 70% (P = .1). Discordance between MFC and cPCR was observed in 21 of 81 BMAs with concurrent assessments (5 of 56 [9%] MRD-negative by MFC were positive by cPCR whereas 16 of 25 [64%] MRD-positive by MFC were negative by cPCR).
Table 3.
Outcome for Adults With De Novo Philadelphia Chromosome–Negative Precursor B-Lineage Acute Lymphoblastic Leukemia by Pretreatment Characteristics (n = 282)
| Characteristic | No. | % | % CR | P (χ2) | % CRD | P | % OS | P (χ2) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 Year | 5 Year | 3 Year | 5 Year | |||||||
| Age, years | ||||||||||
| Overall | 282 | 95 | — | 60 | 50 | — | 58 | 50 | — | |
| ≤ 30 | 96 | 34 | 99 | .02 | 63 | 50 | NS | 70 | 64 | < .001 |
| 31-59 | 128 | 45 | 98 | 66 | 55 | 60 | 50 | |||
| ≥ 60 | 58 | 21 | 88 | 53 | 47 | 29 | 21 | |||
| Sex | ||||||||||
| Male | 282 | 57 | 96 | NS | 57 | 60 | .1 | 57 | 48 | NS |
| Female | 120 | 43 | 96 | 69 | 45 | 57 | 50 | |||
| Performance status | ||||||||||
| 0-1 | 219 | 78 | 96 | NS | 65 | 55 | .04 | 60 | 50 | NS |
| 2-3 | 63 | 22 | 97 | 50 | 40 | 50 | 44 | |||
| Leukocyte count, ×109/L | ||||||||||
| < 5 | 142 | 50 | 96 | NS | 65 | 54 | < .001 | 60 | 49 | < .01 |
| 5-29.9 | 91 | 32 | 98 | 65 | 54 | 64 | 54 | |||
| ≥ 30 | 49 | 17 | 94 | 47 | 44 | 42 | 35 | |||
| Hemoglobin, g/dL | ||||||||||
| < 10 | 219 | 78 | 97 | NS | 60 | 62 | NS | 55 | 45 | NS |
| ≥ 10 | 63 | 22 | 94 | 67 | 48 | 59 | 55 | |||
| Platelet count, ×109/L | ||||||||||
| < 100 | 214 | 76 | 95 | NS | 60 | 48 | .03 | 53 | 43 | < .01 |
| ≥ 100 | 68 | 24 | 98 | 68 | 61 | 69 | 67 | |||
| Serum creatinine, mg/dL | ||||||||||
| < 1.3 | 252 | 89 | 97 | < .01 | 64 | 54 | .06 | 60 | 56 | < .01 |
| ≥ 1.3 | 30 | 11 | 87 | 51 | 35 | 43 | 25 | |||
| Serum bilirubin, mg/dL | ||||||||||
| < 1.3 | 254 | 91 | 96 | NS | 64 | 50 | NS | 59 | 50 | NS |
| ≥ 1.3 | 27 | 10 | 93 | 45 | 45 | 46 | 41 | |||
| Serum albumin, g/dL | ||||||||||
| < 3 | 232 | 83 | 94 | NS | 42 | 39 | .01 | 45 | 40 | .04 |
| ≥ 3 | 49 | 17 | 97 | 65 | 53 | 60 | 50 | |||
| β–2 microglobulin, mg/dL (n = 232) | ||||||||||
| < 3 | 128 | 55 | 98 | NS | 70 | 55 | NS | 65 | 55 | .05 |
| ≥ 3 | 104 | 45 | 95 | 53 | 55 | 52 | 46 | |||
| Lactate dehydrogenase, U/L | ||||||||||
| < 1,400 | 161 | 57 | 95 | NS | 60 | 48 | NS | 57 | 50 | NS |
| ≥ 1,400 | 121 | 43 | 98 | 60 | 52 | 57 | 48 | |||
| Systemic risk classification | ||||||||||
| Low | 81 | 29 | 99 | NS | 64 | 52 | NS | 72 | 65 | < .001 |
| High | 200 | 71 | 95 | 63 | 50 | 51 | 43 | |||
| Karyotype (n = 275) | ||||||||||
| Diploid | 113 | 40 | 96 | NS | 68 | 55 | NS | 60 | 50 | .06 |
| Hyperdiploid | 29 | 10 | 97 | 75 | 75 | 65 | 65 | |||
| Hypodiploid | 14 | 5 | 100 | 48 | 39 | 55 | 42 | |||
| t(1;19) | 8 | 3 | 100 | 85 | 68 | 62 | 62 | |||
| del 9p | 13 | 5 | 92 | 67 | 45 | 69 | 61 | |||
| t(4;11), t(11;19), 11q | 18 | 6 | 94 | 14 | 14 | 20 | 10 | |||
| Other | 44 | 16 | 93 | 64 | 56 | 59 | 48 | |||
| Insufficient metaphases | 36 | 13 | 94 | 49 | 39 | 50 | 42 | |||
| CD20 expression | ||||||||||
| Positive | 150 | 53 | 94 | NS | 53 | 48 | .002* | 60 | 52 | NS |
| Without rituximab | 55 | 93 | 40 | 40 | 45 | 39 | ||||
| With rituximab | 95 | 96 | 67 | 53 | 61 | 49 | ||||
| Negative | 132 | 47 | 96 | 69 | 52 | 54 | 46 | |||
| Myeloid marker expression (n = 269) | ||||||||||
| Positive | 125 | 46 | 94 | NS | 64 | 50 | NS | 55 | 45 | NS |
| Negative | 144 | 54 | 97 | 53 | 50 | 57 | 52 | |||
| Regimen | ||||||||||
| Hyper-CVAD | 109 | 39 | 96 | NS | 53 | 46 | < .01 | 55 | 49 | NS |
| Modified hyper-CVAD 1 | 47 | 17 | 99 | 54 | 44 | 55 | 44 | |||
| Modified hyper-CVAD 2 | 126 | 45 | 94 | 78 | 56 | 60 | 50 |
Remission Duration and Survival Outcomes
With a median follow-up of 64 months (range, 4 to 200), the overall 3-year CRD and OS rates were 60% and 58%, respectively (Table 3). Favorable predictors of longer CRD included better performance status, lower WBC, higher PLT, and higher serum albumin before initiation of therapy. Worse OS rates were noted for older age, higher WBC, lower PLT, elevated serum creatinine levels, lower albumin levels, elevated β-2 microglobulin levels, and high systemic risk classification. Multivariable analysis for OS identified younger age, lower WBC, higher PLT, and therapy with rituximab as independent predictors of favorable outcome.
Treatment with modified hyper-CVAD 2 was associated with improvement in 3-year CRD rates compared with modified hyper-CVAD 1 or hyper-CVAD (78%, 54%, and 53%, respectively; P < .01). When analyzing the entire CD20-positive precursor B-lineage ALL group, incorporation of rituximab into the modified hyper-CVAD regimens was associated with significant improvement in 3-year CRD rates (67% v 40%; P = .002), but not OS rates (61% v 45%; P = not significant [NS]). Relapse rates declined from 60% to 37% (P = .003; Table 4). In the CD20-positive group treated with modified hyper-CVAD 2 (n = 57), absence of detectable MRD by MFC at the time of CR was associated with significantly better 3-year CRD rates (82% v 24%; P = .002) but not OS rates (70% v 27%; P = NS), in part related to deaths in CR (n = 10) for the MRD-negative group.
Table 4.
Outcome With Hyper-CVAD and Modified Hyper-CVAD Regimens in De Novo Philadelphia Chromosome–Negative Precursor B-Lineage Acute Lymphoblastic Leukemia by Therapy and CD20 Status
| Characteristic | Modified Hyper-CVAD ± Rituximab | Hyper-CVAD Without Rituximab (n = 109) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2: Without Intensification (n = 126) | 1: With Intensification (n = 47) | |||||||||||
| CD20 Negative | CD20 Positive | CD20 Negative | CD20 Positive | CD20 Negative | CD20 Positive | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. | 53 | 73 | 23 | 24 | 56 | 53 | ||||||
| Response, overall | ||||||||||||
| CR | 50 | 94 | 67 | 92 | 22 | 96 | 24 | 100 | 55 | 98 | 50 | 94 |
| CRp | 1 | 2 | 1 | 1 | 1 | 4 | — | — | — | |||
| NR | 1 | 2 | 2 | 3 | — | — | 1 | — | ||||
| ED | 1 | 2 | 3 | 4 | — | — | — | 3 | 6 | |||
| Follow-up, months | ||||||||||||
| Median | 41 | 90 | 132 | |||||||||
| Range | 4-81+ | 24-106+ | 6-200+ | |||||||||
| Relapse rate | 10 | 19 | 20 | 28 | 12 | 52 | 11 | 46 | 31 | 56 | 30 | 60 |
| Time to relapse, months | ||||||||||||
| Median | 30 | 27 | 10 | 25 | 30 | 11 | ||||||
| Range | 4-72 | 2-75 | 2-47 | 7-38 | 5-140 | 2-124 | ||||||
| Deaths in CR | ||||||||||||
| Overall | 4 | 8 | 13 | 18 | 4 | 17 | 3 | 13 | 5 | 9 | 2 | 4 |
| No. age ≥ 60 years | 0 | 9 | 4 | 3 | 2 | 1 | ||||||
| % 3-year CRD | ||||||||||||
| Overall by therapy | 78 | 53 | 54 | |||||||||
| % 3-year CRD | ||||||||||||
| Overall | 84 | 73 | 50 | 54 | 68 | 37 | ||||||
| Age, years | ||||||||||||
| ≤ 30 | 84 | 75 | 62* | 40* | 75 | 26 | ||||||
| 31-59 | 89 | 75 | 45* | 67* | 62 | 48 | ||||||
| ≥60 | 71 | 65 | 0* | 45* | 50* | 50* | ||||||
| % 3-year OS | ||||||||||||
| Overall by therapy | 60 | 55 | 55 | |||||||||
| % 3-year OS | ||||||||||||
| Overall | 63 | 57 | 44 | 65 | 65 | 45 | ||||||
| Age, years | ||||||||||||
| ≤ 30 | 77 | 75 | 63* | 80* | 84 | 47 | ||||||
| 31-59 | 56 | 66 | 55* | 78* | 58 | 48 | ||||||
| ≥ 60 | 60* | 15 | 0* | 45* | 20* | 34* |
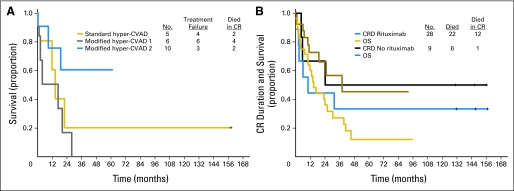
Since our prior analysis established the lack of prognostic significance of CD20 expression in the older group,9 a subset analysis excluding these patients was conducted to assess influence of rituximab therapy in the younger CD20-positive subsets (Table 4; Figs 1A, 1B, 1C). Significant improvements in 3-year CRD (70% v 38%; P < .001; Fig 1A) and OS (75% v 47%; P = .003; Fig 1B) rates favoring rituximab were observed. The outcomes were either similar or superior to those for their CD20-negative counterparts within the age subcategories (Table 4). There were no significant differences in rates of OS by regimen for younger CD20-negative precursor B-lineage ALL (Fig 1D), in contrast to better OS rates for modified hyper-CVAD and rituximab regimens within the CD20-positive group (Fig 1C). Notably there was no benefit to anthracycline intensification (modified hyper-CVAD 124), rather there appeared to be an inferior outcome, particularly in the CD20-negative elderly group (Table 4; Fig 2A).
Fig 1.
Outcomes by therapy for younger patients (age younger than 60 years) with Philadelphia chromosome–negative precursor B-lineage acute lymphoblastic leukemia. In the CD20-positive subset, (A) complete remission (CR) duration and (B) survival by inclusion or exclusion of rituximab therapy, and (C) survival by hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) regimen (standard, modified hyper-CVAD 1 with rituximab inclusive of anthracycline intensification, or modified hyper-CVAD 2 with rituximab eliminating anthracycline intensification) are depicted. (D) Survival by regimen (without rituximab) for the CD20-negative group is also depicted.
Fig 2.
Outcomes by therapy for older patients (age 60 years or older) with Philadelphia chromosome–negative precursor B-lineage acute lymphoblastic leukemia. (A) In the CD20-negative subset, survival by regimen is depicted. (B) In the CD20 positive subset, complete remission (CR) duration (CRD) and survival by rituximab therapy is depicted. OS, overall survival.
Outcome in Elderly Subset
The overall CR rate for 58 patients age 60 years or older was 88%; 3-year CRD and OS rates were 53% and 29%, respectively (Table 4; Appendix Table A1, online only). Eighteen (62%) of 29 older patients treated with modified hyper-CVAD 2 were assessed for MRD by MFC at the time of CR. Patients in the CD20-positive subset treated with rituximab had a higher rate of MRD-negativity than their CD20-negative counterparts (11 of 12 [92%] v three of six [50%]; P = .05). However, this did not translate into improved CRD or OS outcomes compared with the older CD20-positive subgroup treated with standard hyper-CVAD, in part related to deaths in CR (Appendix Table A1; Fig 2B; Appendix).
Treatment Delivery and Toxicity
Toxicity profiles of the chemoimmunotherapy regimens were similar to previous reports detailing modified hyper-CVAD 1 for LL and hyper-CVAD and rituximab for Burkitt-type leukemia/lymphoma.8,13,17,25 There was no difference in time to CR with the addition of rituximab (median 23 v 21 days). There was no increase in induction mortality for the CD20-positive subset treated with rituximab, rather, overall early mortality rates in older patients treated in the protective environment declined from 11% to 2% (P = NS).
Treatment realization for the intensive phase of chemotherapy was reviewed for 121 (96%) of 126 patients treated with modified hyper-CVAD 2. Seventy-eight of 121 patients (64%) completed eight cycles of chemotherapy within a median time (start of induction to start of cycle 8) of 5.8 months (range, 4.4 to 10 months). Nineteen patients (16%) received four or fewer cycles; 12 (63%) were CD20-positive and 10 (53%) were 60 years or older. Reasons for early discontinuation of intensive chemotherapy (after four cycles or less in 19, after five cycles in eight, after six cycles in 13, after seven cycles in three patients) included relapse (n = 9), deaths in CR (n = 7; 6 were CD20-positive), or infectious-related toxicity (n = 2). Five patients underwent allogeneic stem-cell transplantation (SCT) in first CR; four for t(4;11)(q21;q23) karyotype and one for persistent MRD. An additional 20 patients were transitioned to 6-mercaptopurine, vincristine, methotrexate, and prednisone maintenance phase chemotherapy early owing to recurrent life-threatening infections (n = 13), persistent cytopenias (n = 2), CNS toxicity (n = 2, ventriculoperitoneal shunt; cerebrovascular accident), and myocardial infarction (n = 1).
Despite similar toxicity profiles and recovery times from myelosuppression (data not shown), the number of deaths in CR was higher for the CD20-positive subset treated with modified hyper-CVAD 2, predominantly related to infections with multidrug resistant organisms in the older group (n = 6) during consolidation chemotherapy, before implementation of rotating antibacterial antibiotic prophylaxis (online-only Appendix). Other causes of death in this subgroup included complications related to secondary myelodysplastic syndrome (n = 2), cardiovascular events (n = 2), or seizure-related anoxic encephalopathy (n = 1); two others were not on active therapy at the time of their demise (Appendix Table A1).
DISCUSSION
The hyper-CVAD regimen, modeled after a pediatric treatment designed for childhood Burkitt lymphoma, has proven to be an effective program for de novo ALL and LL.7,8,17,33 Further improvements in outcome have been observed with incorporation of targeted agents such as tyrosine kinase inhibitors (eg, imatinib) for Ph-positive ALL and MoAb therapy (eg, rituximab) for Burkitt-type leukemia/lymphoma.13,20
The results of our study suggest that the addition of rituximab to hyper-CVAD-based regimens for CD20-positive precursor B-lineage ALL significantly improved 3-year rates of CRD (70% v 38%; P < .001) and OS (75% v 47%; P = .003). In striking contrast to the Burkitt-type leukemia/lymphoma experience,13 where outcome was significantly improved for the older subset, the benefit of rituximab in precursor B-lineage ALL appears to be confined to the younger group (3-year rates for CRD 45% v 50%; OS 28% v 32% if 60 years or older). These results are not inconsistent with our previous findings that outcome was uniformly poor in the elderly subset treated with hyper-CVAD in the prerituximab era, regardless of CD20 expression. It is plausible that alternative dosing schemas using rituximab in conjunction with lower intensity therapy in the postinduction phase (to avoid infectious complications of myelosuppression) may yield better survival outcomes for this older group.
Although these prospective analyses are comparative in nature (ie, nonrandomized), the absence of significant differences in outcome among the standard or modified hyper-CVAD regimens in the contemporaneously treated CD20-negative precursor B-lineage ALL subset suggests that other factors such as improvements in supportive care cannot account for these findings.
Preliminary results of other regimens incorporating rituximab for de novo CD20-positive precursor B-lineage ALL have been presented. In 26 elderly patients (> 55 years) treated with chemoimmunotherapy (eight applications of standard-dose rituximab) according to the German Multicenter Study Group for Adult ALL (GMALL) 2002 protocol, the CR rate was 63% with 1-year OS rate of 54%.34 Younger patients were treated per GMALL 07/2003 according to risk group. If high-risk (HR; WBC > 30 × 109/L or late CR), standard-dose rituximab was administered with three phases of induction (I, II) and first consolidation chemotherapy followed by allogeneic SCT. Patients with standard risk (SR) received eight treatments with standard-dose rituximab before induction (phase I, II), reinduction, and six consolidation chemotherapy cycles. Preliminary results were presented for 185 patients (133 SR; 52 HR) with CD20-positive precursor B-lineage ALL; 117 (63%) received rituximab and were compared with 70 treated with the same chemotherapy regimen without rituximab.35 In the SR subset, addition of rituximab improved 3-year CRD and OS rates (64% v 48%, P = .009 and 75% v 54%, P = not reported, respectively). In the HR group, allogeneic SCT was performed in 66% of cases with improvement in 3-year OS rates after rituximab (75% v 40%; P = not reported). Rates of death in first CR in this younger group were similar (4% v 3%).
Prospective randomized clinical trials incorporating rituximab into first-line therapy for precursor B-lineage ALL (some irrespective of CD20 expression) are planned. In our prior analysis, lower cut points of CD20 expression (eg, 10%) were discriminatory in predicting outcome.9 Upregulation of CD20 expression during induction chemotherapy in ALL cases deemed CD20-negative at baseline suggests that anti-CD20 MoAb therapy may be beneficial even with low levels of expression.36–38 Similar to the non-Hodgkin's lymphoma experience, extended dosing of rituximab during 6-mercaptopurine, vincristine, methotrexate, and prednisone maintenance therapy may further reduce relapses. Incorporation of novel agents which not only have antileukemic activity but also counter mechanisms of resistance to rituximab (eg, anti-Bcl-2 agents, mammalian target of rapamycin inhibitors, hypomethylating agents) should be explored.36 Higher-affinity novel antibodies directed against different CD20 epitopes (eg, ofatumumab) or other lymphoblast surface antigens such as epratuzumab (targeting CD22) and alemtuzumab (targeting CD52) may further improve outcome.39–43
Appendix
Study group.
From July 1992 to August 2009, 440 adolescents and adults with de novo Ph-negative acute lymphoblastic leukemia (n = 391; 89%) or lymphoblastic lymphoma (n = 49; 11%) were treated with hyper-CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone; n = 210) or modified hyper-CVAD regimens (with [n = 69] or without [n = 161] anthracycline intensification). Median age was 38 years (range, 13 to 92); 18% were 60 years or older and 62% were male. In the precursor B-lineage acute lymphoblastic leukemia subgroup (n = 313), data on CD20 expression was available in 282 patients (90%; limited immunophenotypic analyses in 31 patients treated with hyper-CVAD). This group forms the basis of the detailed subset analysis (no significant differences in distribution of characteristics or outcomes noted with inclusion/exclusion of the group with unknown CD20 status, data not shown).
Intensive therapy.
The first course in all regimens was accompanied by appropriate intravenous hydration and alkalinization (eg, dextrose water or one half normal saline with 75 to 100 milliequivalents of sodium acetate per liter to run at 50-100 mL/h) and allopurinol to reduce the incidence of tumor lysis syndrome. Oral sodium bicarbonate supplemented the intravenous formulation on days 1 to 3. Rasburicase could be substituted for allopurinol in cases with high WBC count or bulky disease at presentation. The duration of the doxorubicin infusion for the hyper-CVAD cycles was extended from 2 to 24 hours (48 hours if left ventricular ejection fraction lower than 50%) for the modified hyper-CVAD regimens owing to the addition of anthracycline-based intensifications during maintenance therapy. The high-dose methotrexate (MTX) and cytarabine courses incorporated automatic dose reduction of the cytarabine from 3 g/m2 to 1 g/m2 for patients age 60 years or older. Intravenous alkalinization was utilized to promote excretion of MTX (as for course 1 above) at a rate of 100 to 125 mL/h with prehydration period of at least 4 hours before MTX until urine pH was at least 7.0. An algorithm of additional leucovorin rescue (50 to 100 mg IV every 4 to 6 hours) was implemented if serum MTX levels were greater than 20 μmol/L at the end of infusion (0 hour, confirmed on repeat sample), more than 1 μmol/L at 24 hours, or more than 0.1 μmol/L at 48 hours. Oral acetazolamide 250 mg was administered by mouth twice daily if the urine pH was lower than 7.0 until serum MTX level lower than 0.1 μmol/L.
CNS therapy.
CNS prophlyaxis with intrathecal chemotherapy (IT) was administered according to CNS risk (Table 1). From January 2005 to December 2005, 32 patients received IT prophylaxis with liposomal cytarabine.24 Therapy for active CNS leukemia at presentation included alternating IT MTX and cytarabine twice weekly during the induction course until cerebrospinal fluid cell count normalized and cytological examination was negative for malignant cells on two sequential assessments, then weekly for 4 weeks, then according to the prophylactic schedule for all remaining cycles of intensive chemotherapy. Therapeutic radiation therapy was given if indicated (eg, 24 to 30 Gy in 10 to 12 fractions to base of the skull for cranial nerve palsies). No prophylactic cranial irradiation was administered.
Maintenance therapy.
Included treatment with 6-mercaptopurine (6-MP), vincristine (VCR), MTX, and prednisone (POMP) for 24 months with the standard hyper-CVAD regimen. Between 1992 and 1995, oral POMP was given with 6-MP 50 mg orally 3 times daily, MTX 20 mg/m2 orally or IV weekly, VCR 2 mg IV every 28 days, and prednisone 200 mg daily days 1 to 5. Thereafter, IV POMP was given with 6-MP 1 g/m2 IV over 1 hour daily for 5 days every 28 days concurrently with MTX 10 mg/m2 IV over 1 hour daily for 5 days and VCR with prednisone as just described. The 6-MP and MTX doses were reduced by 25% (to 750 mg/m2 IV and 7.5 mg/m2 IV, respectively) for moderate toxicity and by 50% (to 500 mg/m2 IV and 5 mg/m2 IV, respectively) in cases of severe toxicity. The MTX dose was usually reduced selectively for mucositis or hepatic dysfunction attributable to the agent. Oral POMP was resumed in 1999 when the intravenous formulation of 6-MP was no longer available. Two intensification courses interrupted the POMP maintenance during months 9 and 12 with VP-16 100 mg/m2 IV daily days 1 to 5 and pegylated asparaginase 2,500 U/m2 IV day 1. After July 2000, these intensifications were changed to MTX 100 mg/m2 IV day 1 and L-asparaginase 20,000 units IV day 2 weekly for 4 weeks during months 7 and 11 of maintenance therapy. For the modified hyper-CVAD regimens, the oral POMP maintenance was extended to 30 months with the addition of hyper-CVAD (with or without rituximab) to the early and late intensifications for months 6 and 18 preceeding therapy with MTX and L-asparaginase for months 7 and 19.
Guidelines for dose reductions.
Guidelines for dose reductions included (1): cytarabine 1 g/m2 for age 60 years or older, creatinine ≥ 1.5 mg/dL, or MTX level at 0 hour (repeated) ≥ 20 μmol/L;(2) VCR 1 mg for total bilirubin higher than 2 mg/dL or grade 2 persistent peripheral neuropathy or VCR eliminated for total bilirubin above 3 g/dL, grade 3 to 4 peripheral neuropathy, or grade 3 to 4 ileus; (3) doxorubicin decreased by 50% for total bilirubin 2 to 3 mg/dL, by 75% if 3 to 5 mg/dL, and eliminated if above 5 mg/dL; and (4) MTX decreased by 50% for calculated creatinine clearance 10 to 50 mL/min (eliminated if < 10 mL/min) or if pleural effusions/ascites (with thoracentesis/paracentesis as feasible) or decreased by 25% to 50% for delayed excretion, nephrotoxicity, or grade 3 or greater mucositis with prior courses.
Supportive care.
Filgastrim 10 μg/kg (rounded) subcutaneously daily commenced 24 hours after completion of the induction chemotherapy (approximately day 6) until absolute neutrophil count was greater than 1 × 109/L. The filgastrim could be delayed until 48 to 72 hours after completion of the chemotherapy for the consolidation cycles. After December 2007, pegfilgastrim 6 mg subcutaneously could be administered in lieu of the filgastrim at the discretion of the treating physician. For the modified hyper-CVAD regimens, patients age 60 years or older were placed in a laminar air flow room or protective environment for the induction course until absolute neutrophil count reached 0.5 × 109/L. Hematologic profiles were obtained at least biweekly during induction and at least weekly during consolidations. Packed RBCs were given for symptomatic and/or severe anemia. Platelet transfusions were given prophylactically for platelet count lower than 10 to 15 × 109/L or therapeutically for platelet count lower than 30 to 50 × 109/L with hemorrhage. All blood products were irradiated.
During the intensive phase, prophylactic anti-infective therapy included either a quinolone, amoxicillin-clavulanate potassium, trimethoprim-sulfamethoxazole, or other antibacterial antibiotic; fluconazole 200 mg daily with the standard hyper-CVAD regimen or itraconazole, voriconazole, or caspofungin with the modified hyper-CVAD regimens for antifungal prophylaxis as the latter agents became available; and acyclovir 400 mg twice daily or valacyclovir 500 mg daily for antiviral coverage. Since 2005, antibacterial antibiotics were rotated with each intensive chemotherapy cycle (as opposed to continuous therapy with quinolones) to reduce the incidence of infections with multidrug resistant organisms. During the maintenance phase, recommended anti-infective prophylaxis included trimethoprim-sulfamethoxazole one double strength twice daily 2 days weekly and acyclovir 400 mg twice daily (or valacyclovir 500 mg daily) to reduce the probability of Pneumocystis jirovecii infection and varicella zoster or herpes simplex infections, respectively. Neutropenic febrile episodes generally resulted in hospitalization with initiation of broad spectrum parenteral antibiotics.
Assessments.
Pretreatment evaluations included history/physical examination with documentation of extramedullary disease by imaging and/or appropriate cytological/histological evaluations; complete blood count/differential; hepatorenal function studies including lactate dehydrogenase and uric acid levels; and bone marrow aspirate/biopsy for morphology, immunohistochemical stains, immunophenotyping, and karyotyping. Analysis of cerebrospinal fluid was performed concomitantly with prophylactic IT treatment. Bone marrow aspirations were repeated on days 14 and 21 of course 1 to determine response (or later to confirm CR if needed), then every two to three courses until end of consolidation chemotherapy, then every 3 to 6 months for the first 3 years.
Methods.
Bone marrow aspirates (BMA) samples were assessed for minimal residual disease (MRD) using the panel of markers (detailed in the text). MRD was identified in comparison with the known patterns of antigen expression by normal maturing CD19 positive B-cells using an approach similar to that described by Weir et al.26 A distinct cluster of at least 20 cells showing altered antigen expression was regarded as an aberrant population. An aberrant expression of at least 2 antigens was required for definitive diagnosis of MRD. 1 × 106 bone marrow cells were stained using a standard stain-lyse-wash procedure, using Ortho Lyse Buffer (BD Biosciences, San Jose, CA). Data were acquired on FACSCalibur cytometers using CellQuest software (BD Biosciences). At least 2 × 105 cells were acquired when specimen quality permitted. Data on B-cells were selectively acquired by gating on CD19/CD34 positive cells with low side scatter.
Table A1.
Summary of Deaths in Complete Remission by Age and Therapy
| Modified Hyper-CVAD 2 (n = 126) | Modified Hyper-CVAD 1 (n = 47) | Hyper-CVAD (n = 109) | ||||||
|---|---|---|---|---|---|---|---|---|
| CD20 Positive | Age (years) | Cause | CD20 Positive | Age (years) | Cause | CD20 Positive | Age (years) | Cause |
| No | 27 | Pneumonia after SCT | No | 35 | Pneumonia | No | 40 | Unknown |
| No | 42 | HHV6 encephalitis after SCT | No | 37 | PCP pneumonia | No | 50 | Sepsis |
| No | 45 | Hepatic failure after SCT for secondary MDS | No | 68 | Peritonitis | No | 52 | Meningitis |
| No | 57 | Sudden death | No | 72 | Sepsis | No | 65 | Sepsis |
| Yes | 16 | Seizure with anoxic encephalopathy | No | 80 | Hepatic failure | No | 70 | Pneumonia |
| Yes | 49 | Sepsis (MDR Pseudomonas) | Yes | 61 | Pneumonia | Yes | 55 | Sepsis |
| Yes | 53 | Sepsis after therapy for secondary MDS | Yes | 78 | Unknown | Yes | 78 | Unknown |
| Yes | 55 | Abdominal aeortic aneurysm | Yes | 80 | Sepsis | |||
| Yes | 62 | Cardiopulmonary arrest | ||||||
| Yes | 63 | Sepsis (MDR Pseudomonas) | ||||||
| Yes | 64 | Sepsis after therapy for secondary MDS | ||||||
| Yes | 69 | Sepsis (Enterococcus) | ||||||
| Yes | 69 | Pneumonia (not on active therapy) | ||||||
| Yes | 73 | Unknown (not on active therapy) | ||||||
| Yes | 76 | Sepsis (MDR E. coli) | ||||||
| Yes | 76 | Sepsis (Staphylococcus) | ||||||
| Yes | 79 | Sepsis (MDR Pseudomonas) |
Footnotes
Supported in part by Grant No. 5K12 CA88084-2 from the National Institutes of Health and a research grant from Amgen.
Presented in part at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-8, 2008 and at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 5-8, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00671658.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Susan O'Brien, Genentech (C); Michael J. Keating, Genentech (C) Stock Ownership: None Honoraria: William Wierda, Genentech Research Funding: Deborah A. Thomas, Amgen, Genentech; Susan O'Brien, Genentech, Biogen Idec; Farhad Ravandi, Genentech; Hagop M. Kantarjian, Amgen; William Wierda, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Deborah A. Thomas
Provision of study materials or patients: Deborah A. Thomas, Susan O'Brien, Stefan Faderl, Guillermo Garcia-Manero, Alessandra Ferrajoli, William Wierda, Farhad Ravandi, Srdan Verstovsek, Jeffrey L. Jorgensen, Carlos Bueso-Ramos, Michael Andreeff, Michael J. Keating, Jorge Cortes, Hagop M. Kantarjian
Collection and assembly of data: Deborah A. Thomas, Sherry Pierce, Rebecca Garris
Data analysis and interpretation: Deborah A. Thomas, Jeffrey L. Jorgensen, Carlos Bueso-Ramos, Sherry Pierce, Rebecca Garris
Manuscript writing: Deborah A. Thomas, Jeffrey L. Jorgensen,Hagop M. Kantarjian
Final approval of manuscript: Deborah A. Thomas, Susan O'Brien, Stefan Faderl, Guillermo Garcia-Manero, Alessandra Ferrajoli, William Wierda, Farhad Ravandi, Srdan Verstovsek, Jeffrey L. Jorgensen, Carlos Bueso-Ramos, Michael Andreeff, Sherry Pierce, Rebecca Garris, Michael J. Keating, Jorge Cortes, Hagop M. Kantarjian
REFERENCES
- 1.Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51:364–369. doi: 10.1136/jcp.51.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czuczman MS, Olejniczak S, Gowda A, et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14:1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- 3.Jazirehi AR, Vega MI, Bonavida B. Development of rituximab-resistant lymphoma clones with altered cell signaling and cross-resistance to chemotherapy. Cancer Res. 2007;67:1270–1281. doi: 10.1158/0008-5472.CAN-06-2184. [DOI] [PubMed] [Google Scholar]
- 4.Borowitz MJ, Shuster J, Carroll AJ, et al. Prognostic significance of fluorescence intensity of surface marker expression in childhood B- precursor acute lymphoblastic leukemia: A Pediatric Oncology Group Study. Blood. 1997;89:3960–3966. [PubMed] [Google Scholar]
- 5.Jeha S, Behm F, Pei D, et al. Prognostic significance of CD20 expression in childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2006;108:3302–3304. doi: 10.1182/blood-2006-04-016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Walters RS, Keating MJ, et al. Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. J Clin Oncol. 1990;8:994–1004. doi: 10.1200/JCO.1990.8.6.994. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, O'Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Thomas D, O'Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DA, O'Brien S, Jorgensen JL, et al. Prognostic significance of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia. Blood. 2009;113:6330–6337. doi: 10.1182/blood-2008-04-151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maury S, Huguet F, Leguay T, et al. Adverse prognostic significance of CD20 expression in adults with Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia. Haematologica. 2009;95:324–328. doi: 10.3324/haematol.2009.010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maloney DG. Mechanism of action of rituximab. Anticancer Drugs. 2001;12(suppl 2):S1–S4. [PubMed] [Google Scholar]
- 12.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 14.Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 15.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas DA, O'Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–1630. doi: 10.1182/blood-2003-12-4428. [DOI] [PubMed] [Google Scholar]
- 18.Todeschini G, Tecchio C, Meneghini V, et al. Estimated 6-year event-free survival of 55% in 60 consecutive adult acute lymphoblastic leukemia patients treated with an intensive phase II protocol based on high induction dose of daunorubicin. Leukemia. 1998;12:144–149. doi: 10.1038/sj.leu.2400912. [DOI] [PubMed] [Google Scholar]
- 19.Brunning RD, Borowitz M, Matutes E, et al. Pathology and Genetics of Tumours of Haematopoeitic and Lymphoid Tissues. In: Jaffe ES, Harris NL, Stein H, editors. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2001. pp. 111–114. [Google Scholar]
- 20.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DA. Philadelphia chromosome positive acute lymphocytic leukemia: A new era of challenges. Hematology Am Soc Hematol Educ Program. 2007:435–443. doi: 10.1182/asheducation-2007.1.435. [DOI] [PubMed] [Google Scholar]
- 22.Ravandi F, O'Brien S, Thomas D, et al. First report of phase II study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia. Blood. doi: 10.1182/blood-2009-12-261586. [epub ahead of print on May 13, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rytting M, Thomas D, Franklin A, et al. Pediatric-based therapy for young adults with newly diagnosed lymphoblastic leukemia. Blood. 2009:114–804. abstr 2037. [Google Scholar]
- 24.Jabbour E, O'Brien S, Kantarjian H, et al. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood. 2007;109:3214–3218. doi: 10.1182/blood-2006-08-043646. [DOI] [PubMed] [Google Scholar]
- 25.Thomas D, O'Brien S, Faderl S, et al. Anthracycline dose intensification in adult acute lymhoblastic leukemia: Lack of benefit in the context of the hyper-CVAD regimen. Cancer. doi: 10.1002/cncr.25319. [epub ahead of print on June 22, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weir EG, Cowan K, LeBeau P, et al. A limited antibody panel can distinguish B-precursor acute lymphoblastic leukemia from normal B precursors with four color flow cytometry: Implications for residual disease detection. Leukemia. 1999;13:558–567. doi: 10.1038/sj.leu.2401364. [DOI] [PubMed] [Google Scholar]
- 27.Muzzafar T, Medeiros LJ, Wang SA, et al. Aberrant underexpression of CD81 in precursor B-cell acute lymphoblastic leukemia: Utility in detection of minimal residual disease by flow cytometry. Am J Clin Pathol. 2009;132:692–698. doi: 10.1309/AJCP02RPVOKTNWEC. [DOI] [PubMed] [Google Scholar]
- 28.Theriault C, Galoin S, Valmary S, et al. PCR analysis of immunoglobulin heavy chain (IgH) and TcR-gamma chain gene rearrangements in the diagnosis of lymphoproliferative disorders: Results of a study of 525 cases. Mod Pathol. 2000;13:1269–1279. doi: 10.1038/modpathol.3880232. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1965;53:457–481. [Google Scholar]
- 30.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. J R Stat Soc. 1966;50:163–170. [PubMed] [Google Scholar]
- 31.Fleming TR, Harrington DP. New York, NY: Wiley; 1991. Counting Processes and Survival Analysis. [Google Scholar]
- 32.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 33.Murphy SB, Bowman WP, Abromowitch M, et al. Results of treatment of advanced-stage Burkitt's lymphoma and B cell (SIg+) acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. J Clin Oncol. 1986;4:1732–1739. doi: 10.1200/JCO.1986.4.12.1732. [DOI] [PubMed] [Google Scholar]
- 34.Gokbuget N, Hoelzer D. Rituximab in the treatment of adult ALL. Ann Hematol. 2006;85:117–119. [Google Scholar]
- 35.Hoelzer D, Huettmann A, Kaul F, et al. Immunochemotherapy with rituximab in adult CD20 B-precusor ALL improves molecular CR rate and outcome in standard risk (SR) as well as in high risk (HR) patients with SCT. Haematologica. 2009;94(suppl 2) abstr 481. [Google Scholar]
- 36.Thomas DA, O'Brien S, Kantarjian HM. Monoclonal antibody therapy with rituximab for acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23:949–971. doi: 10.1016/j.hoc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaipa G, Basso G, Maglia O, et al. Drug-induced immunophenotypic modulation in childhood ALL: Implications for minimal residual disease detection. Leukemia. 2005;19:49–56. doi: 10.1038/sj.leu.2403559. [DOI] [PubMed] [Google Scholar]
- 38.Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: Setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982–3988. doi: 10.1182/blood-2008-06-164129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castillo J, Milani C, Mendez-Allwood D. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert Opin Investig Drugs. 2009;18:491–500. doi: 10.1517/13543780902832679. [DOI] [PubMed] [Google Scholar]
- 40.Faderl S, Thomas DA, O'Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–3415. doi: 10.1182/blood-2002-07-1952. [DOI] [PubMed] [Google Scholar]
- 41.Leonard JP, Coleman M, Ketas J, et al. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:5044–5051. doi: 10.1200/JCO.2005.13.821. [DOI] [PubMed] [Google Scholar]
- 42.Leonard JP, Schuster SJ, Emmanouilides C, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: Final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 43.Stock W, Sanford B, Lozanski G, et al. Alemtuzumab can be incoporated into front-line therapy of adult acute lymphoblastic leukemia (ALL): Final phase I results of a Cancer and Leukemia Group B Study (CALGB 10102) Blood. 2009;114:345. abstr 838. [Google Scholar]