Atypical familial hemophagocytic lymphohistiocytosis due to mutations in UNC13D and STXBP2 overlaps with primary immunodeficiency diseases (original) (raw)
Abstract
Background
Familial hemophagocytic lymphohistiocytosis is a genetic disorder of lymphocyte cytotoxicity that usually presents in the first two years of life and has a poor prognosis unless treated by hematopoietic stem cell transplantation. Atypical courses with later onset and prolonged survival have been described, but no detailed analysis of immunological parameters associated with typical versus atypical forms of familial hemophagocytic lymphohistiocytosis has been performed.
Design and Methods
We analyzed disease manifestations, NK-cell and T-cell cytotoxicity and degranulation, markers of T-cell activation and B-cell differentiation as well as Natural Killer T cells in 8 patients with atypical familial hemophagocytic lymphohistiocytosis due to mutations in UNC13D and STXBP2.
Results
All but one patient with atypical familial hemophagocytic lymphohistiocytosis carried at least one splice-site mutation in UNC13D or STXBP2. In most patients episodes of hemophagocytic lymphohistiocytosis were preceded or followed by clinical features typically associated with immunodeficiency, such as chronic active Epstein Barr virus infection, increased susceptibility to bacterial infections, granulomatous lung or liver disease, encephalitis or lymphoma. Five of 8 patients had hypogammaglobulinemia and reduced memory B cells. Most patients had a predominance of activated CD8+ T cells and low numbers of Natural Killer T cells. When compared to patients with typical familial hemophagocytic lymphohistiocytosis, NK-cell cytotoxicity and NK-cell and CTL degranulation were impaired to a similar extent. However, in patients with an atypical course NK-cell degranulation could be partially reconstituted by interleukin-2 and cytotoxic T-cell cytotoxicity in vitro was normal.
Conclusions
Clinical and immunological features of atypical familial hemophagocytic lymphohistiocytosis show an important overlap to primary immunodeficiency diseases (particularly common variable immunodeficiency and X-linked lymphoproliferative syndrome) and must, therefore, be considered in a variety of clinical presentations. We show that degranulation assays are helpful screening tests for the identification of such patients.
Keywords: familial hemophagocytic lymphohistiocytosis, mutations, UNC13D, STXBP2
Introduction
Familial hemophagocytic lymphohistiocytosis (FHL) is a genetically heterogeneous disorder of immune regulation characterized by impaired lymphocyte cytotoxicity.1–4 Four genetic defects associated with FHL have been identified. FHL-2 is caused by mutations in the PRF1 gene, which encodes Perforin 1, a pore-forming protein that is crucial for target cell lysis.5 The genes mutated in FHL-3 (UNC13D encoding MUNC13-4), FHL-4 (STX11 encoding SYNTAXIN11) and FHL-5 (STXBP2 encoding MUNC18-2) all encode proteins important for the intra-cellular trafficking and exocytosis of lytic granules containing perforin and other effector molecules of cell-mediated cytotoxicity.6–9 In the context of a relevant immunological trigger, impaired cytotoxicity leads to uncontrolled activation of cytotoxic T cells and macrophages resulting in a hyperinflammatory state characterized by T-cell and macrophage infiltration of various organs including bone marrow, liver and the central nervous system.3
FHL patients usually present within the first two years of life with hemophagocytic lymphohistiocytosis (HLH), a life-threatening disease including prolonged fever, hepatosplenomegaly, pancytopenia and neurological symptoms, as well as characteristic laboratory abnormalities such as elevated levels of serum triglycerides, ferritin and soluble interleukin 2-receptor (sCD25) and low levels of fibrinogen.10 Histomorphological demonstration of hemophagocytosis in the bone marrow or other tissues is a typical, yet initially often absent feature of the disease. Flow cytometric analysis of perforin expression and NK-cell and CTL degranulation are helpful in supporting the rapid diagnosis of FHL,7,11–13 which then needs to be confirmed by genetic analysis. Unfortunately, since there are no characteristic prodromal signs, diagnosis in patients without other affected family members is usually not established before the first HLH episode.
Due to increasing awareness of the signs and symptoms of HLH and a better understanding of the genetic basis of the disease, FHL has been increasingly diagnosed in patients presenting beyond infancy. These atypical presentations have been reported in adolescents and even in adults as late as 62 years of age.7,8,14–29 They may be associated with milder and often recurrent HLH episodes and prolonged survival in the absence of hematopoietic stem cell transplantation (HSCT), which is unusual in patients with the typical disease. “Atypical FHL” is usually associated with missense or splice-site mutations in the affected genes.15,23,30 A better characterization of these variant phenotypes of FHL can help to raise the clinical suspicion of FHL even in the absence of overt HLH. Moreover, the characterization of specific immunological parameters associated with early versus late-onset forms of FHL would facilitate an earlier diagnosis of FHL and help to guide treatment decisions, including hematopoietic stem cell transplantation. At present, it is unclear whether there is a correlation between the degree of defective NK and CTL function and the age at onset of clinical manifestations.
In this study, we report the clinical manifestations and provide a detailed immunological analysis of 8 late-onset FHL patients: 6 patients with mutations in STXBP2 and 2 patients with mutations in UNC13D. We document that late-onset FHL can present with features typically associated with primary immunodeficiencies, including characteristic immunological abnormalities that may manifest prior to the onset of HLH. In addition, we show that partial reconstitution of impaired NK-cell degranulation upon stimulation with IL-2 and retained CTL cytotoxicity are important immunological features associated with atypical rather than with typical FHL.
Design and Methods
Patients
In Germany, patients treated according to the international HLH treatment protocols (HLH-94, HLH-2004) are registered at the national study center in Hamburg, where genetic analysis for FHL is performed if indicated. Since 2008, a comprehensive immunological analysis was performed in more than 80 of these HLH patients at the Centre of Chronic Immunodeficiency in Freiburg (Germany). For the present study, we included all patients with mutations in known FHL-associated genes diagnosed since 2008 with an onset of HLH (defined according to the criteria of the Histiocyte Society) beyond two years of age and survival without HSCT until at least six years of age (hereafter termed “atypical FHL”). A group of FHL patients presenting with HLH before two years of age (“typical FHL”) and healthy blood donors were used as controls for the immunological assays. Written informed consent for genotyping, immunological studies and data collection was obtained from the patients or their legal guardians. The study was conducted according to the guidelines of the Declaration of Helsinki and has been approved by the local institutional review board.
Cells
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation (PAN-Biotec, Aidenbach, Germany). PBMCs as well as the human erythroleukemia cell line K562 and the mouse leukemia cell line L1210 (ATCC, Manassas, USA) were maintained in complete medium (IMDM, 10% fetal calf serum (FCS) and 1% penicillin/streptomycin/glutamine; all from Invitrogen, Carlsbad, CA, USA).
Antibodies and reagents
The following fluorochrome-conjugated antibodies were used for flow cytometry: anti-CD3-PerCP (clone SK7), anti-CD3-APC (clone Hit3a), anti-CD8-FITC (clone SK1), anti-CD16-PE (clone 3G8), anti-CD56-APC (clone NCAM 16.2), anti-CD107a-PE (clone H4A3), anti-HLA-DR-FITC (clone G46-6), anti-CD27-FITC (clone M-T271), anti-CD28-PE (clone CD28.2), anti-IgD-PE (clone IA6-2), anti-IgM-PE-Cy5 (clone G20-127), (all from BD Biosciences, Heidelberg, Germany) and anti-CD19-APC (clone H12153A; EuroBioSciences, Friesoythe, Germany). The antibody panel used for analysis of the TCR Vβ repertoire was obtained from Immunotech (Prague, Czech Republic). Cells were detected on a Gallios flow cytometer (Beckman Coulter, Brea, CA, USA) and data analysis was performed using Flow-Jo software (Ashland, OR, USA).
Cytotoxicity and degranulation assays
NK-cell cytolytic activity was measured in a standard 51Chromium release (51Cr) assay on K562 targets and CTL cytotoxic function was analyzed in an anti-CD3 redirected-lysis assay on L1210 target cells, as described previously.7 NK-to-target and CTL-to-target ratios were calculated by multiplying the respective effector-to-target-ratio with the percentage of NK cells or CTLs in the effector cell suspension as determined by FACS analysis. Analysis of activation-induced degranulation was performed by detection of surface expression of CD107 on NK cells and CTLs.12,13 In most patients, the degranulation assays were performed on several occasions. The mean value obtained in these assays is indicated in the figures.
Genetic analysis
Genomic DNA was isolated from peripheral blood samples using standard procedures. PCR products included the coding exons and adjacent intronic regions. Primer sequences have been published previously (STXBP2)7 or are available upon request (UNC13D). Products were directly sequenced with BigDye Terminator v1.1 and run on an ABI PRISM 3100 genetic analyzer (both from Applied Biosystems, Carlsbad, CA, USA).
Statistical analysis
The Mann-Whitney U-test was used to compare the data on CD107 expression on NK cells between early- and late-onset FHL patients.
Results
Mutations in STXBP2 and UNC13D associated with atypical familial hemophagocytic lymphohistiocytosis
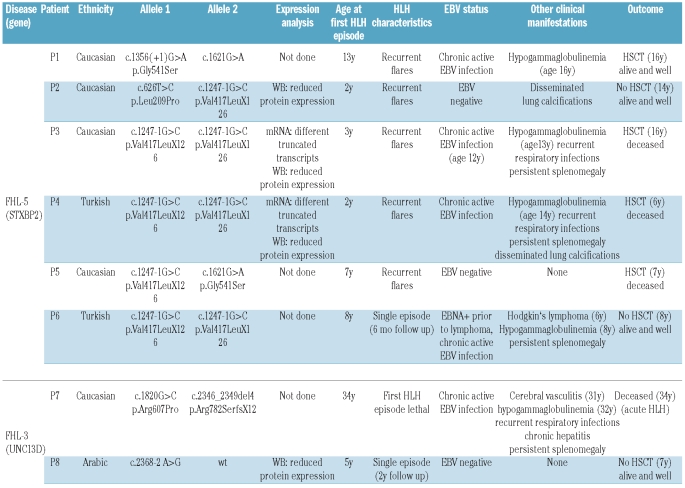
We identified 8 patients with atypical FHL carrying mutations in UNC13D (n=2) or in STXBP2 (n=6). The mutations and some functional data of patients P2, P3 and P47 and the clinical course of P1 and P4 have been reported previously.31 Five of the 6 FHL-5 patients carried the splice-site mutation c.1247 − 1G>C, either in a homozygous or a compound heterozygous state and one patient carried the mutation c.1356 + 1G>A, all of which affect exon 15 (Table 1). In P8, only one heterozygous c.2368-2 A>G mutation in the UNC13D gene could be identified. This mutation leads to a deletion of 21 nucleotides and to the use of an alternative splice-site in exon 25. Western Blot analysis revealed significantly reduced expression of MUNC13-4 (Online Supplementary Figure S1). Since mutations in the PRF, STXBP2 and STX11 genes were excluded and a degranulation defect could clearly be demonstrated (see below), this patient was included in this study although a second mutation could not be identified. Ten “typical FHL” patients, 7 with mutations in UNC13D and 3 with mutations in STXBP2 were used as controls for the cytotoxicity and degranulation assays. None of these patients carried splice-site mutations.
Table 1.
Genetic and clinical characteristics of patients with atypical FHL.
Clinical characteristics of patients with atypical FHL
The first episode of HLH occurred in the third or fourth year of life in 3 patients and in the 6th year of life or later (as late as 34 years in a patient with UNC13D deficiency) in the remaining 5 patients (Table 1). Three patients had a single episode of HLH. In the 34-year old patient, this episode was lethal, the 2 other patients had disease-free follow up for six months and two years, respectively. The remaining 5 patients had recurrent flares of the disease with long intervals of disease-free remission and mostly good responses to therapy.
Primary Epstein Barr virus (EBV) infection was a trigger for HLH in P1 and P4, while P3 initially presented with HLH-like symptoms that did not completely fulfill the HLH criteria, but developed the full clinical picture of HLH after primary EBV infection. All 3 of these patients developed chronic active EBV infection. Two other patients had apparently seroconverted to EBV without overt clinical symptoms, but eventually also developed chronic active EBV infection (P6, P7). In both of the latter patients, the development of HLH was associated with a massive increase in EBV viral load.
Of note, 3 of 8 patients initially presented with clinical features other than HLH. P3 presented with splenomegaly and fever of unknown origin three years before developing HLH, P6 presented with EBV-positive Hodgkin’s disease two years before HLH onset and P7 presented with encephalitis, splenomegaly, granulomatous hepatitis, hypogammaglobulinemia and susceptibility to bacterial infections three years before the manifestation of HLH. Persistent hepatosplenomegaly was observed in 5/8 patients and nodular pulmonary calcifications in 2 of 8 patients. At the time of writing, 3 patients are alive and well without HSCT at seven, eight and 14 years of age, respectively, and one patient has been successfully transplanted. Four of the 8 patients have died: 3 died during or after HSCT and one during the first episode of HLH.
Partial reconstitution of NK cell degranulation upon stimulation with interleukin-2 in patients with atypical familial hemophagocytic lymphohistiocytosis
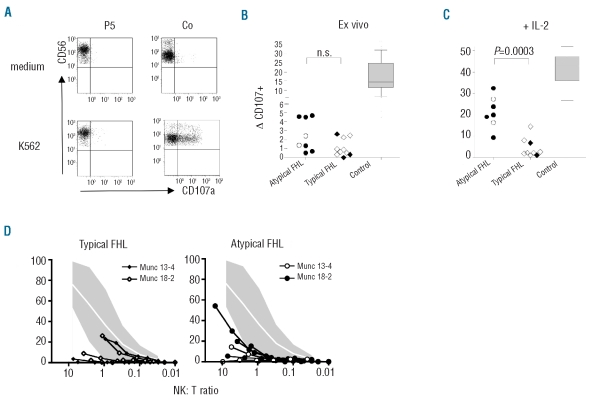
To investigate a possible correlation between the degree of defective NK-cell function and the pattern of clinical manifestations, we analyzed NK-cell cytotoxicity and NK-cell degranulation in the 8 patients with “atypical FHL” and in 9 patients with “typical FHL”. Ex vivo NK-cell degranulation was impaired to a similar extent in both patient groups, irrespective of whether they carried UNC13D or STXBP2 mutations (Figure 1A and B). Moreover, ex vivo NK-cell cytotoxicity was reduced or absent in all but one of the “typical” and all of the “atypical FHL” patients (Figure 1D). Importantly, however, addition of IL-2 could partially restore NK-cell degranulation to a level above 10% in all patients with “atypical”, but only in one of 9 patients with “typical FHL” (Figure 1C).
Figure 1.
NK cell degranulation and cytotoxicity. (A) NK-cell degranulation upon target cell recognition was examined by analyzing CD107 expression after incubation of PBMCs with K562 target cells. Plots were gated on CD3− CD56+ NK cells. (B) NK-cell degranulation ex vivo. ΔCD107 was calculated as the percentage of NK cells expressing CD107 after stimulation with K562 minus the percentage of NK cells expressing CD107 after incubation with medium. Open symbols represent MUNC-13-4 deficient patients, closed symbols MUNC-18-2 deficient patients. The box plot for controls represents mean and 25th/75th percentile, whiskers represent the 10th/90th percentile. (C) NK-cell degranulation after 48 h of prestimulation with PHA and IL-2. (D) NK-cell cytotoxicity on K562 target cells. The NK cell to target ratio was calculated based on the percentage of NK cells as determined by flow cytometry. The shaded area delineates the mean values ±2 SD observed in more than 50 healthy controls.
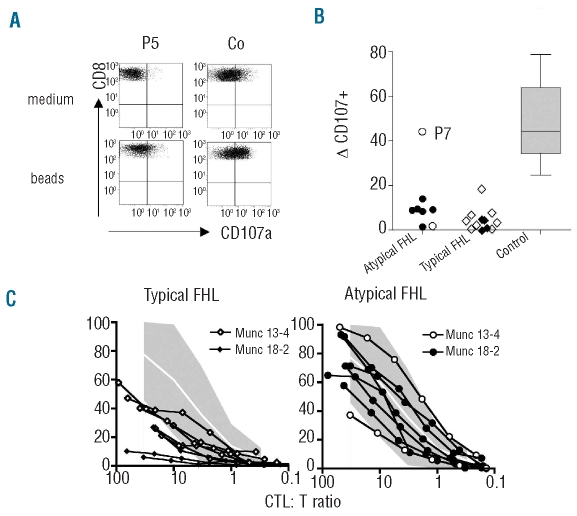
Impaired degranulation, but normal cytotoxic function of T cells in patients with atypical familial hemophagocytic lymphohistiocytosis
T-cell degranulation and T-cell cytotoxic function were analyzed using short-term (2 d and 7 d, respectively) stimulated PHA/IL-2 blasts. CTL degranulation was clearly impaired in all but one of the patients with “atypical FHL” and there was no significant difference between patients with a typical or atypical course of the disease (Figure 2A and B). In contrast, T-cell cytolytic activity was reduced or absent in “typical FHL” patients but in the normal range in all “atypical FHL” patients, irrespective of their genetic diagnosis (Figure 2C). Collectively, the data obtained in the NK-cell and T-cell cyotoxicity and degranulation studies provide a functional correlate for the atypical course of FHL in our patients.
Figure 2.
Cytotoxic T-cell degranulation and cytotoxicity. (A) CTL degranulation was examined by analyzing CD107 expression after incubation of PBMCs with PHA and IL-2 for 48 h, followed by stimulation with anti-CD3/CD28-coated microbeads. Plots were gated on CD3+ CD8+ T cells. (B) Summary of results obtained in “typical” and “atypical FHL” patients and healthy controls. Open symbols represent MUNC-13-4 deficient, closed symbols MUNC-18-2 deficient patients. (C) T-cell cytotoxicity was examined using d7–d9 PHA blasts as effectors and L1210 cells coated with anti-CD3 mAb as targets. The CTL-to-target-ratio was was calculated based on the percentage of CD3+CD8+ T cells as determined by flow cytometry of the PHA/IL-2 culture. The shaded area delineates the mean values ± 2 SD observed in more than 50 healthy controls.
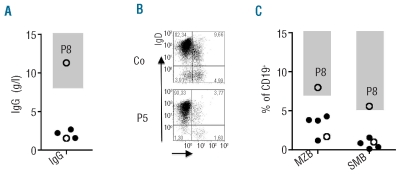
Impaired B-cell immunity in patients with late-onset familial hemophagocytic lymphohistiocytosis
Chronic hypogammaglobulinemia was documented in 4 of the 8 “atypical FHL” patients (one FHL-3 patient and 3 FHL-5 patients) (Figure 3A), in 3 cases associated with a clinically relevant susceptibility to bacterial sinopulmonary infections that improved upon immunoglobulin substitution. Interestingly, IgG levels in P4 and P6 were normal before the first episode of HLH, but persistently decreased thereafter. P7 had persistently low IgG levels and an increased susceptibility to infections before developing HLH. In patients with common variable immunodeficiency (CVID), hypogammaglobulinemia is associated with a characteristic impairment of B-cell differentiation.32 We, therefore, analyzed the B-cell phenotype in our patients. All patients were investigated outside an HLH episode. P5 and P8 (the latter being the only patient with a normal B-cell phenotype) were investigated when receiving ciclosporin and steroids, but all other patients were analyzed when they were off any immunosuppressive therapy. P1 and P3 had such a severe reduction of their circulating B cells that a reliable phenotypic analysis was impossible. Five of the 6 remaining patients had reduced percentages of IgM+ CD27+ marginal zone-like B cells and IgM− CD27+ class switched memory B cells (Figure 3B and C). Similar alterations are found in patients with CVID and suggest an impairment of marginal zone and germinal center function.
Figure 3.
Serum immunoglobulin levels and B-cell phenotype. (A) Lowest IgG serum levels documented for atypical FHL patients during the observation period. Open symbols represent MUNC 13-4 deficient, closed symbols MUNC 18-2 deficient patients. (B) Flow cytometric determination of the percentage of marginal zone like (IgD+ CD27+; MZB) and switched memory (IgD− CD27+; SMB) B lymphocytes. Plots are gated on CD19+ cells. (C) Summary of B-cell phenotyping results obtained in early- and late-onset patients. Shaded areas represent represent normal values extending to the 5th percentile.
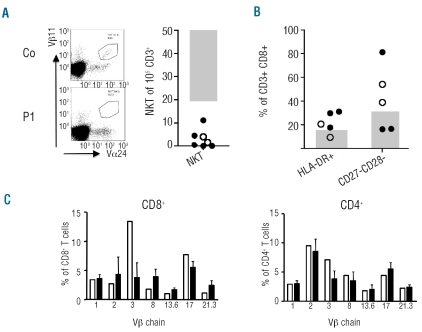
Low numbers of NKT cells and chronic CD8+ T-cell activation in patients with atypical familial hemophagocytic lymphohistiocytosis
A reduction of NKT cell numbers is a relevant feature of X-linked lymphoproliferative disease (XLP)33,34 and ITK deficiency.35 Since these diseases were relevant differential diagnoses in several of our patients, the percentage of Vα24 Vβ11 expressing NKT cells of CD3+ cells was determined and found to be below 0.02% in all 6 patients analyzed (Figure 4A). This finding suggests that low percentages of NKT cells can also be a feature of atypical FHL. Of note, SH1D1A mutations were excluded in P4 and P7 before they were investigated for FHL. In the 2 other male patients, absent NK-cell degranulation was demonstrated in the course of their immunological investigations. Since this assay is normal in patients with XLP and biallelic STXBP2 or UNC13D mutations were found, SH1D1A mutation analysis was not performed.
Figure 4.
NKT cells and CD8+ T-cell phenotype and TCR Vβ repertoire. (A) Flow cytometric enumeration of Vα24 Vβ11 CD3+ NKT cells. At least 100,000 CD3+ T cells were analyzed. The absolute number of NKT cells per 100,000 CD3+ T cells is shown. Open symbols represent MUNC 13-4 deficient, closed symbols MUNC 18-2 deficient patients. (B) Analysis of CD8+ T-cell activation markers. The percentage of CD3+CD8+ T cells expressing HLA-DR and the percentage expressing neither CD27 nor CD28 is indicated. The shaded areas represent normal values extending to the 5th percentile. (C) TCR Vβ chain expression of the 7 most frequently used Vβ chains on CD8+ and CD4+ T cells. Black columns represent control values, open columns represent data from P8.
Phenotypic analysis of CD8+ T cells outside HLH episodes revealed an increase of HLA-DR expression (5 of 6 patients) and an increased fraction of CD27− CD28− cells (3 of 5 patients) indicating chronic activation of CD8+ T cells (Figure 4B). In addition, a disturbed Vβ repertoire (defined as an at least 30% increase or decrease of at least 2 out of 7 Vβ chain families analyzed) among CD8+ T cells, but not among CD4+ T cells was documented in 4/5 patients further supporting the observation of an activated CD8+ T-cell compartment (Figure 4C). CD8+ T cells were also the predominant lymphocyte population in the pulmonary infiltrates of P4 and the liver infiltrates in P7 (data not shown).
Discussion
Several patients with late or atypical presentation of FHL have been mentioned in previous reports, but no detailed immunological evaluation of such patients has so far been presented. Late onset of HLH has been described in patients with perforin,17–20,22–25,36 SYNTAXIN11,14–16 MUNC13-427–29 and MUNC18-2 deficiency.7,8 While late-onset FHL-2 patients mostly carry missense mutations, late onset of FHL-3 is predominantly associated with splice-site mutations and in late-onset FHL-4 patients also nonsense mutations and deletions have been described. All of our patients with “atypical” FHL-5 had either homozygous or heterozygous splice site mutations consistent with a previous report.7,8 One of the 2 FHL-3 patients had a heterozygous splice site mutation, while in the other patient with severely reduced MUNC13-4 expression on Western Blot only one missense mutation has been identified so far. The functional relevance of the mutations was documented in all patients by reduced or absent NK-cell cytotoxicity and NK-cell degranulation. In contrast to patients with typical FHL-3 or FHL-5, however, NK-cell degranulation could be partially reconstituted by IL-2 prestimulation. Moreover, CTL cytotoxicity was in the low normal range in patients with “atypical FHL”, while it was impaired in patients with a typical manifestation of the disease. These functional studies indicate residual function of the affected gene products.
The discrepancy between NK-cell and CTL cytotoxicity in samples obtained from the same patients was unexpected. Since this was observed in patients with MUNC 13-4 deficiency as well as in patients with MUNC 18-2 deficiency, it cannot be ascribed to a particular molecular defect. Different molecular requirements for lytic granule release in NK cells versus CTL have so far not been described. We, therefore, consider it more likely that the differences reflect different pre-treatment of the cells. While NK-cell cytotoxicity was measured directly ex vivo, CTL had been cultured for seven days in PHA and IL-2 before target cell lysis was assessed. This interpretation is supported by the finding that NK-cell degranulation was partially restored by 48 h prestimulation with IL-2. Such restoration of NK-cell degranulation has previously been reported in FHL-4 patients.11,12 Our findings show that this effect is not specific for syntaxin-11 deficiency, but can also be observed in atypical MUNC13-4 and MUNC18-2 deficiency. Whether IL-2 stimulation improves degranulation and cytotoxicity by enhancing alternative splicing and/or exon skipping or by optimizing the residual cytotoxic potential of the affected cells remains unresolved. Whatever the mechanism, it might be relevant in vivo and thus contribute to a partially retained cytotoxicity associated with the milder and/or later presentation of our patients.
Interestingly, 5 of 8 patients (one FHL-3 patient and 4 FHL-5 patients) were diagnosed with antibody deficiency, in 3 cases associated with a clinically relevant susceptibility to bacterial sinopulmonary infections that improved upon immunoglobulin substitution. Two of the 8 patients had very low B-cell numbers and 5 of the 6 remaining patients had a reduced percentage of marginal-zone like and class-switched memory B cells. Of note, 2 patients had normal levels of immunoglobulins and specific antibodies initially, but lost them after recurrent mild episodes of HLH. Since in contrast to perforin, MUNC13-4 and MUNC18-2 are also expressed in B cells,7,9 the hypogammaglobulinemia might be explained by a role for these proteins in B-cell physiology. However, antibody deficiency and B-cell lymphopenia have not been reported in “typical FHL” patients, but have been observed in late-onset perforin deficiency,25 favoring another hypothesis. In patients with recurrent episodes of HLH, chronic activation of T cells and macrophages may have an impact on B-cell differentiation in lymphoid organs eventually resulting in a CVID-like clinical picture. The increased activation state of CD8+ T cells in our patients with non-active HLH is consistent with this explanation. Our observations show that FHL is a relevant differential diagnosis in some patients with a clinical picture of CVID, in particular when accompanied by splenomegaly, cytopenia and episodes of unexplained fever.
In male patients presenting with HLH, X-linked lymphoproliferative disease (XLP) is an important differential diagnosis to FHL. We document that the phenotypic overlap between these diseases extends to other major phenotypes of XLP, i.e. lymphoma and hypogammaglobulinemia with chronic splenomegaly.37–39 Moreover, all 5 patients infected with EBV fulfilled current diagnostic criteria for chronic active EBV infection.40 A role for EBV in the chronic activation of T cells and macrophages has been well documented. Seven out of 7 atypical FHL-patients analyzed had low numbers of NKT cells, which is also considered a characteristic feature of XLP33,37, 38,41,42 and the recently described ITK deficiency.35 This could either reflect a developmental defect of NKT cells, possibly associated with impaired intracellular trafficking of CD1d43 or a secondary phenomenon to HLH episodes. Accumulating evidence suggests a role for NKT cells in the homeostatic regulation of antiviral T-cell responses and, therefore, also possibly for the pathogenesis of HLH.44,45
In summary, our study shows that FHL is a relevant differential diagnosis not only in infants and young children, but also in older patients presenting with clinical and immunological features of a variety of primary immunodeficiencies, in particular CVID and XLP. Importantly, all patients with “atypical FHL” could be reliably identified through the study of NK-cell degranulation. Significant augmentation of NK-cell degranulation by IL-2 prestimulation and normal CTL cytotoxicity in vitro is a feature that was only observed in patients with “atypical FHL”, but not in those with “typical FHL”. These assays are, therefore, useful and complementary tools to characterize the functional impact of mutations in genes affecting lytic granule exocytosis.
Acknowledgments
we are grateful to the CCI Advanced Diagnostic team and to Sam Grieve for excellent technical assistance.
Footnotes
Funding: this study was supported by the German Federal Ministry of Education and Research (BMBF 01 EO 0803) and the European Union (FP7 CURE HLH grant agreement n. 201461).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Filipovich AH. Hemophagocytic lymphohistiocytosis and other hemophagocytic disorders. Immunol Allergy Clin North Am. 2008;28(2):293–313. viii. doi: 10.1016/j.iac.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21(5):245–53. doi: 10.1016/j.blre.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Fischer A, Latour S, de Saint Basile G. Genetic defects affecting lymphocyte cytotoxicity. Curr Opin Immunol. 2007;19 (3):348–53. doi: 10.1016/j.coi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Cetica V, Pende D, Griffiths GM, Aricò M. Molecular basis of familial hemophagocytic lymphohistiocytosis. Haematologica. 2010;95(4):538–41. doi: 10.3324/haematol.2009.019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286(5446):1957–9. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 6.zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter J-I, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Human Molecular Genetics. 2005;14(6):827–34. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 7.zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85(4):482–92. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Côte M, Ménager M, Burgess A, Mahlaoui N, Picard C, Schaffner C, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119(12):3765–73. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115(4):461–73. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 10.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 11.Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, et al. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108(7):2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 12.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110(6):1906–15. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enders A, Zieger B, Schwarz K, Yoshimi A, Speckmann C, Knoepfle E-M, et al. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood. 2006;108(1):81–7. doi: 10.1182/blood-2005-11-4413. [DOI] [PubMed] [Google Scholar]
- 14.Albayrak M, Kaya Z, Yilmaz-Keskin E, Stadt UZ, Kocak U, Gursel T. Fatal Epstein-Barr virus infection in a case of familial hemophagocytic lymphohistiocytosis with syntaxin-11 mutation. Turk J Pediatr. 2009;51(4):371–4. [PubMed] [Google Scholar]
- 15.Marsh RA, Satake N, Biroschak J, Jacobs T, Johnson J, Jordan MB, et al. STX11 mutations and clinical phenotypes of familial hemophagocytic lymphohistiocytosis in North America. Pediatr Blood Cancer. 2010;55(1):134–40. doi: 10.1002/pbc.22499. [DOI] [PubMed] [Google Scholar]
- 16.Rudd E, Goransdotter Ericson K, Zheng C, Uysal Z, Ozkan A, Gurgey A, et al. Spectrum and clinical implications of syntaxin 11 gene mutations in familial haemophagocytic lymphohistiocytosis: association with disease-free remissions and haematopoietic malignancies. J Med Genet. 2006;43(4):e14. doi: 10.1136/jmg.2005.035253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii E, Ueda I, Shirakawa R, Yamamoto K, Horiuchi H, Ohga S, et al. Genetic subtypes of familial hemophagocytic lymphohistiocytosis: correlations with clinical features and cytotoxic T lymphocyte/natural killer cell functions. Blood. 2005;105(9):3442–8. doi: 10.1182/blood-2004-08-3296. [DOI] [PubMed] [Google Scholar]
- 18.Nagafuji K, Nonami A, Kumano T, Kikushige Y, Yoshimoto G, Takenaka K, et al. Perforin gene mutations in adult-onset hemophagocytic lymphohistiocytosis. Haematologica. 2007;92(7):978–81. doi: 10.3324/haematol.11233. [DOI] [PubMed] [Google Scholar]
- 19.Solomou EE, Gibellini F, Stewart B, Malide D, Berg M, Visconte V, et al. Perforin gene mutations in patients with acquired aplastic anemia. Blood. 2007;109(12):5234–7. doi: 10.1182/blood-2006-12-063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moshous D, Feyen O, Lankisch P, Schwarz K, Schaper J, Schneider M, et al. Primary necrotizing lymphocytic central nervous system vasculitis due to perforin deficiency in a four-year-old girl. Arthritis Rheum. 2007;56(3):995–9. doi: 10.1002/art.22442. [DOI] [PubMed] [Google Scholar]
- 21.Clementi R, Locatelli F, Dupre L, Garaventa A, Emmi L, Bregni M, et al. A proportion of patients with lymphoma may harbor mutations of the perforin gene. Blood. 2005;105(11):4424–8. doi: 10.1182/blood-2004-04-1477. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg O, Yacobovich J, Dgany O, Kodman Y, Livni G, Rachmel A, et al. Prolonged course of familial hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 2006;28(12):831–3. doi: 10.1097/MPH.0b013e31802d3a96. [DOI] [PubMed] [Google Scholar]
- 23.Ueda I, Kurokawa Y, Koike K, Ito S, Sakata A, Matsumora T, et al. Late-onset cases of familial hemophagocytic lymphohistiocytosis with missense perforin gene mutations. Am J Hematol. 2007;82(6):427–32. doi: 10.1002/ajh.20878. [DOI] [PubMed] [Google Scholar]
- 24.Trizzino A, zur Stadt U, Ueda I, Risma K, Janka G, Ishii E, et al. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis due to perforin mutations. J Med Genet. 2008;45(1):15–21. doi: 10.1136/jmg.2007.052670. [DOI] [PubMed] [Google Scholar]
- 25.Katano H, Ali MA, Patera AC, Catalfamo M, Jaffe ES, Kimura H, et al. Chronic active Epstein-Barr virus infection associated with mutations in perforin that impair its maturation. Blood. 2004;103(4):1244–52. doi: 10.1182/blood-2003-06-2171. [DOI] [PubMed] [Google Scholar]
- 26.Horne A, Ramme KG, Rudd E, Zheng C, Wali Y, al-Lamki Z, et al. Characterization of PRF1, STX11 and UNC13D genotype-phenotype correlations in familial hemophagocytic lymphohistiocytosis. Br J Haematol. 2008;143(1):75–83. doi: 10.1111/j.1365-2141.2008.07315.x. [DOI] [PubMed] [Google Scholar]
- 27.Hazen MM, Woodward AL, Hofmann I, Degar BA, Grom A, Filipovich AH, et al. Mutations of the hemophagocytic lymphohistiocytosis-associated gene UNC13D in a patient with systemic juvenile idiopathic arthritis. Arthritis Rheum. 2008;58(2):567–70. doi: 10.1002/art.23199. [DOI] [PubMed] [Google Scholar]
- 28.Rudd E, Bryceson YT, Zheng C, Edner J, Wood SM, Ramme K, et al. Spectrum, and clinical and functional implications of UNC13D mutations in familial haemophagocytic lymphohistiocytosis. J Med Genet. 2008;45(3):134–41. doi: 10.1136/jmg.2007.054288. [DOI] [PubMed] [Google Scholar]
- 29.Santoro A, Cannella S, Bossi G, Gallo F, Trizzino A, Pende D, et al. Novel Munc13-4 mutations in children and young adult patients with haemophagocytic lymphohistiocytosis. J Med Genet. 2006;43(12):953–60. doi: 10.1136/jmg.2006.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda I, Ishii E, Morimoto A, Ohga S, Sako M, Imashuku S. Correlation between phenotypic heterogeneity and gene mutational characteristics in familial hemophagocytic lymphohistiocytosis (FHL) Pediatr Blood Cancer. 2006;46(4):482–8. doi: 10.1002/pbc.20511. [DOI] [PubMed] [Google Scholar]
- 31.Beutel K, Gross-Wieltsch U, Wiesel T, Stadt UZ, Janka G, Wagner HJ. Infection of T lymphocytes in Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in children of non-Asian origin. Pediatr Blood Cancer. 2009;53(2):184–90. doi: 10.1002/pbc.22037. [DOI] [PubMed] [Google Scholar]
- 32.Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99(5):1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 33.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11(3):340–5. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201(5):695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huck K, Feyen O, Niehues T, Rüschendorf F, Hübner N, Laws H, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119(5):1350–8. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clementi R, Emmi L, Maccario R, Liotta F, Moretta L, Danesino C, et al. Adult onset and atypical presentation of hemophagocytic lymphohistiocytosis in siblings carrying PRF1 mutations. Blood. 2002;100(6):2266–7. doi: 10.1182/blood-2002-04-1030. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton JK, Paquin LA, Sullivan JL, Maurer HS, Cruzi FG, Provisor AJ, et al. X-linked lymphoproliferative syndrome registry report. J Pediatr. 1980;96(4):669–73. doi: 10.1016/s0022-3476(80)80735-9. [DOI] [PubMed] [Google Scholar]
- 38.Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease) Lancet. 1975;1(7913):935–40. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- 39.Soresina A, Lougaris V, Giliani S, Cardinale F, Armenio L, Cattalini M, et al. Mutations of the X-linked lymphoproliferative disease gene SH2D1A mimicking common variable immunodeficiency. Eur J Pediatr. 2002;161 (12):656–9. doi: 10.1007/s00431-002-1083-9. [DOI] [PubMed] [Google Scholar]
- 40.Okano M. Overview and problematic standpoints of severe chronic active Epstein-Barr virus infection syndrome. Crit Rev Oncol Hematol. 2002;44(3):273–82. doi: 10.1016/s1040-8428(02)00118-x. [DOI] [PubMed] [Google Scholar]
- 41.Ma CS, Hare NJ, Nichols KE, Dupre L, Andolfi G, Roncarolo MG, et al. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115(4):1049–59. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174(6):3153–7. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 43.Cernadas M, Sugita M, van der Wel N, Cao X, Gumperz JE, Maltsev S, et al. Lysosomal localization of murine CD1d mediated by AP-3 is necessary for NK T cell development. J Immunol. 2003;171(8):4149–55. doi: 10.4049/jimmunol.171.8.4149. [DOI] [PubMed] [Google Scholar]
- 44.Roberts TJ, Lin Y, Spence PM, Van Kaer L, Brutkiewicz RR. CD1d1-dependent control of the magnitude of an acute antiviral immune response. J Immunol. 2004;172(6):3454–61. doi: 10.4049/jimmunol.172.6.3454. [DOI] [PubMed] [Google Scholar]
- 45.Ho LP, Urban BC, Jones L, Ogg GS, McMichael AJ. CD4(−)CD8alphaalpha subset of CD1d-restricted NKT cells controls T cell expansion. J Immunol. 2004;172(12):7350–8. doi: 10.4049/jimmunol.172.12.7350. [DOI] [PubMed] [Google Scholar]