Impact of Glutamine Transporters on Pneumococcal Fitness under Infection-Related Conditions (original) (raw)
Abstract
The genomic analysis of Streptococcus pneumoniae predicted six putative glutamine uptake systems, which are expressed under in vitro conditions, as shown here by reverse transcription-PCR. Four of these operons consist of glnHPQ, while two lack glnH, which encodes a soluble glutamine-binding protein. Here, we studied the impact of two of these glutamine ATP-binding cassette transporters on S. pneumoniae D39 virulence and phagocytosis, which consist of GlnQ and a translationally fused protein of GlnH and GlnP. Mice infected intranasally with D39Δ_gln0411_/0412 showed significantly increased survival times and a significant delay in the development of pneumococcal pneumonia compared to those infected with D39, as observed in real time using bioluminescent pneumococci. In a mouse sepsis model, the mutant D39Δ_gln0411_/0412 showed only moderate but significant attenuation. In contrast, the D39Δ_gln1098_/1099 knockout strain was massively attenuated in the pneumonia and septicemia mouse infection model. To cause pneumonia or sepsis with D39Δ_gln1098_/1099, infection doses 100- to 10,000-fold higher than those used for wild-type strain D39 were required. In an experimental mouse meningitis model, D39Δ_gln1098_/1099 produced decreased levels of white blood cells in cerebrospinal fluid and showed decreased numbers of bacteria in the bloodstream compared to D39 and D39Δ_gln0411_/0412. Phagocytosis experiments revealed significantly decreased intracellular survival rates of mutants D39Δ_gln1098_/1099 and D39Δ_gln0411_/0412 compared to wild-type D39, suggesting that the deficiency of Gln uptake systems impairs resistance to oxidative stress. Taken together, our results demonstrate that both glutamine uptake systems are required for full virulence of pneumococci but exhibit different impacts on the pathogenesis of pneumococci under in vivo conditions.
Streptococcus pneumoniae, commonly known as pneumococci, are Gram-positive bacteria that colonize the human respiratory tract as harmless commensals. Pneumococci, however, may also convert to harmful pathogenic bacteria and cause serious local infections and invasive infections. In fact, pneumococci are among the most common etiologic agents of otitis media, sinusitis, and life-threatening infections such as community-acquired pneumonia, septicemia, and meningitis (7). Prior to infection, pneumococci colonize the mucosal surface of the respiratory tract by adhering to host epithelial cells. This is considered to be the initial step prior to pneumococcal translocation into the lungs and bloodstream. In these scenarios, virulence factors are essential for adherence, cell damage, and/or immune response evasion (2, 10, 26, 37). Besides virulence factors, the maintenance of pneumococcal fitness during adaptation to different host milieus is also of central importance in causing local and invasive pneumococcal diseases (IPD) in a host. Pneumococci, which grow microaerophilically, have to adapt to the different temperatures, oxygen levels, pH values, and nutrients of host microenvironments. This implies that pneumococci are able to take up and metabolize various nutrients which are available in the various host niches encountered by this versatile microorganism.
Unsurprisingly, pneumococci produce a large fraction of transporters involved in the uptake and metabolism of sugars and amino acids, and these include classical phosphotransferase systems, ATP-binding cassette (ABC) transporters, and ion gradient-driven transporters (21, 48). The regulation of sugar and/or amino acid uptake is likely crucial for the fitness of the bacteria in the nasopharynx and most likely during IPD. For example, carbon catabolite repression (CCR) is important when bacteria grow in the presence of multiple food sources. CCR is highly regulated, and it has been shown that CcpA (RegM; catabolite control protein A) controls a hierarchical sugar utilization. Remarkably, CcpA is not the sole regulator of CCR in pneumococci, but a ccpA knockout is severely attenuated for colonization and IPD (22).
More important, signature-tagged mutagenesis screens have indicated that bacterial fitness and virulence are tightly linked with the function of ABC transporter systems (15, 16, 32, 34, 39-42). The importance of ABC transporters for virulence has been shown, e.g., for the ABC-type manganese transport system (34, 40, 41, 50) and iron uptake transporters (5, 6, 24). Several of the observed effects on virulence are thought to be indirect effects caused by the altered bacterial fitness of mutants deficient in one of the ABC transporter components.
Glutamine metabolism is of central importance in bacterial physiology. Glutamine is an important resource for bacteria, and its utilization is required for the biosynthesis of a variety of nitrogen-containing compounds and for protein synthesis. As a consequence, the regulation of glutamine uptake and catabolism requires both general and specific regulators. Since glutamine uptake and regulation are important for bacterial fitness, glutamine transport is interesting for the study of metabolic pathways and links bacterial fitness with bacterial virulence (30). Strikingly, analysis of the genome of S. pneumoniae predicted at least six putative glutamine ABC transporters which are distributed over the chromosome (21). Similar to Lactococcus lactis and Bacillus subtilis, glutamine uptake in pneumococci is at least partially regulated by the nitrogen regulatory protein GlnR and the glutamine synthetase (GS) GlnA (9, 29, 31). In addition to GlnR and GlnA, pneumococci encode an orthologue of Bacillus subtilis CodY (17, 18). In B. subtilis, CodY, which is a member of the MerR family of DNA-binding regulatory proteins, is a global transcriptional regulator and many of the CodY-regulated genes are involved in nitrogen or carbon metabolism (36, 46). In pneumococci, the CodY regulon of pneumococci is essential for bacterial adherence and nasopharyngeal colonization in an experimental mouse infection model, while the glutamine-dependent regulator GlnR has no significant effect on pneumococcal virulence during colonization, pneumonia, and septicemia (17, 18). GlnA, which regulates many genes involved in amino acid metabolism, has been shown to contribute to colonization by and survival of pneumococci in the blood (18).
As the roles of the different glutamine uptake transporters have not been studied in detail so far, we investigated the impact of two out of six glutamine uptake systems on pneumococcal fitness and pathogenesis. Different mouse infection models and phagocytosis assays were used to demonstrate the role of the glutamine uptake systems SPD1098/1099, which forms an operon together with the glucose-6-phosphate dehydrogenase-encoding gene zwf, and SPD0411/0412 in S. pneumoniae D39 virulence. In these two transport systems, the membrane-spanning permease and glutamine-binding protein are translationally fused and encoded by glnHP.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. S. pneumoniae D39 (serotype 2) and isogenic mutants of D39 were cultured to mid-log phase in Todd-Hewitt broth (Oxoid, Basingstoke, England) supplemented with 0.5% yeast extract (THY; Roth, Karlsruhe, Germany) or chemically defined medium (CDM) (33, 35) or grown on Columbia blood agar plates at 37°C in 5% CO2. Escherichia coli strains were grown on Luria-Bertani (LB) agar or LB broth. Transformation of E. coli strains with plasmid DNA was carried out with CaCl2-treated competent cells according to standard procedures. Pneumococcal mutants were cultured in the presence of the appropriate antibiotics, erythromycin (5 μg/ml), spectinomycin (50 μg/ml), and/or kanamycin (150 μg/ml). To study pneumococcal growth under different conditions, freshly thawed pneumococci were cultured overnight on blood agar, followed by THY culture with a starting optical density at 600 nm (OD600) of ≤0.05. The THY culture was incubated for 6 to 8 h at 37°C and 5% CO2 without agitation.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s)a | Reference or source |
|---|---|---|
| S. pneumoniae strains | ||
| SP139 | R6x (nonencapsulated derivative of D39) | 49 |
| SP257 | Serotype 2 (D39) | NCTC 7466 |
| PN102 | D39Δ_gln0411__ 0412 (Δ_gln0411__ 0412::Sper) | This study |
| PN111 | D39Δ_cps_ (Δ_cps_::Kmr) | 44 |
| PN122 | Xen10 (A66 pAUL-A [Tn_4001_ lux ABCDE Kmr]) | Xenogen |
| PN128 | D39Δ_gln1098__ 1099 (Δ_gln1098__ 1099::Ermr) | This study |
| PN149 | D39_lux_ (bioluminescent derivative of D39) | 23 |
| PN190 | D39_lux_Δ_gln0411__ 0412 | This study |
| PN191 | D39_lux_Δ_gln1098__ 1099 | This study |
| PN199 | D39Δ_cps_Δ_gln0411__ 0412 | This study |
| PN200 | D39Δ_cps_Δ_gln1098__ 1099 | This study |
| E. coli strains | ||
| DH5α | Δ(lac)U169 endA1 gyrA46 hsdR17 φ_80_Δ(lacZ)M15 recA1 relA1 supE44 _thi_-1 | Novagen |
| M15[pREP4] | E. coli M15 with pREP4 plasmid encoding lac repressor in trans; Kmr | Qiagen |
| Plasmids | ||
| pQE30 | Protein expression vector; Apr | Qiagen |
| pQE280 | pQE30 derivative with 1,214-bp fragment of glnQ0411 for mutagenesis | This study |
| pQE356 | pQE30 derivative with glnQ0411 interrupted by Spe resistance cassette (aad9); obtained after deletion of 143 bp by inverse PCR of pQE280 and insertion of aad9 gene (1,111 bp) | This study |
| pGEM-T Easy | Cloning vector for PCR products; Apr | Promega |
| pG397 | pGEMT derivative with 3,072-bp fragment of glnQPH1098/99 for mutagenesis | This study |
| pGEM397 | pGEMT derivative with glnQPH1098/99 interrupted by Erm resistance cassette (ermB); obtained after deletion of 745 bp by MscI digestion of pG397 and insertion of ermB gene (1,051 bp) | This study |
Primers and molecular DNA techniques.
The primers used in this study are listed in Table 2 . Chromosomal DNA used as the template in PCRs was isolated and purified using the Qiagen Genomic Tip 100/G kit according to the instructions of the distributor (Qiagen, Hilden, Germany). DNA amplification was performed using AmpliTaq Gold DNA polymerase (Perkin-Elmer). PCR amplifications were carried out in 50-μl volumes, and reaction mixtures were subjected to denaturation at 94°C and 30 cycles of 94°C, primer annealing for 1 min, and elongation at 72°C. Annealing temperature depended on the primers used, and extension time depended on the length of the expected PCR product. PCR products were purified using the PCR DNA purification kit (Qiagen), and plasmids were extracted according to the QIAprep Spin Midi/Maxiprep kit protocol. DNA sequencing was carried out by using the Qiagen sequencing services. Digoxigenin (DIG)-labeled DNA probes were generated by PCR using the PCR DIG labeling mix (Roche), and Southern blot analyses were performed under stringent conditions as described recently (23).
TABLE 2.
Primers used in this study
| Primer use and name | Sequence (5′-3′)a |
|---|---|
| Insertion-deletion mutagenesis of glnQ0411 | |
| Amplification of glnQ0411 + 5′ flank | |
| glnQDABF2 | GGATCCGGAACTGCCCAAGCTTTCGG |
| glnQDABR1 | AAGCTTTTATTTGAGATAGCGTTGAAGGAAC |
| Inverse PCR of glnQ0411 + 5′ flank (pQE280) | |
| glnQ408mut1 | CCCGGGCCATCAGTTGGTGTTTCAAGTAG |
| glnQ408mut2 | CCCGGGCTGTCCTAAAACGCGAACGCAC |
| Amplification of aad9 Sp cassette gene | |
| SpcforEcoRV | GATATCATCGATTTTCGTTCGTGAATAC |
| SpcrevEcoRV | GATATCAATTAGAATGAAATATTTCCC |
| Insertion-deletion mutagenesis of glnQPH1098/99 | |
| Amplification of glnQPH1098/99 + 5′ flank | |
| 1098delfwd | GCAATTCAAGAAAGAAAAGG |
| 1098delrev | CCAATACGAGGTAGAAAAACTGC |
| Amplification of ermB Em cassette gene | |
| Eryfor-ClaI | ATCGATACGGTTCGTGTTCGTGCTG |
| Eryrev-ClaI | ATCGATCGTAGGCGCTAGGGACCTC |
| Northern blotting | |
| DNA probe for glnQ0411 | |
| NB408FW | CGTGGTGGTATCCTAGCAGTTGACA |
| NB408RV | CTAATACGACTCACTATAGGGAGAATTCATGGAGAGGGCACGAGC |
| DNA probe for glnPH1098 | |
| NB1121RV_2 | CCAACCTTTTCAAGCAACTCCAT |
| NB1121FW | GTTCGTGGTGGTATTCAGGC |
| RT-PCR | |
| glnQ0411 | |
| glnQF2 | ATGACACAAGCAATCCTTGAAATTAAACAC |
| glnQR1 | TTATTTGAGATAGCGTTGAAGGAAC |
| glnQ0531 | |
| Spr0535f | GAGCTCATGCTATCTGGTGGACAAAAACAGC |
| Spr0535r | AAGCTTTTATTTGACTTTGTCACTTTCGTGGTT |
| Spr0536f | GAGCTCATGGCTTTAGTAGAATTTAAAAACG |
| Spr0536r | AAGCTTTCACATATTTACAAATTCCAGATATT |
| glnQ0616 | |
| Spr0622f | GAGCTCATGTCTGAAACTATCTTAGAAATCAAG |
| Spr0622r | CTGCAGTTATAGATATGAGCCGAATTGGC |
| glnQ0720 | |
| Spr0728f | GAGCTCATGACAGAAACCTTGATAAAAATTG |
| Spr0728r | AAGCTTTTATAAAACCTTTCTCAAGAAATCTT |
| glnQ1099 | |
| Spr1121f | GAGCTCATGGCAAAATTAAAAATTGATGTAAATG |
| Spr1121r | AAGCTTTTAGACGTTTAAGACCTTATCTAAG |
| glnQ1329 | |
| Spr1354f | GGTACCATGTTAGAATTACGAAATATCAATAAAG |
| Spr1354r | AAGCTTCTATTTAGGTTCTACTTTCAATAATAC |
| zwf | |
| ZwfRTf | TGCGAGTGGAGACCTGGCTA |
| ZwfRTr | AATCATTGACATCATGGCTT |
| Quantitative RT-PCR | |
| glnQ0411 | |
| 0411R2 | TCCACAAGGGAGAGGTCATC |
| 0411F2 | GGAAAACCATCCCCAACTTT |
| glnP_ H0412 | |
| 0412R2 | TGACAAGCAAAACTGGTTCG |
| 0412F2 | GGAGCTGTCAAAATCGATGG |
| glnH_ P1098 | |
| 1098F2 | CGCTGAGATTAAAGGCTGGA |
| 1098R2 | ACCAACGGTTTTGCCAGTTA |
| glnQ1099 | |
| Spr1121f | GAGCTCATGGCAAAATTAAAAATTGATGTAAATG |
| NB1121RV_2 | CCAACCTTTTCAAGCAACTCCAT |
| zwf1100 | |
| zwfRTf | TGCGAGTGGAGACCTGGCTA |
| zwfRTr | AATCATTGACATCATGGCTT |
| Enolase (spd1012) | |
| RTenoF | CTCGTTACGGTGGTCTTGGT |
| RTenoR | GTAGTCAGCAGCAGCACGAG |
| GAPDH (spd1823) | |
| RTgapF | CGCATCAACGACCTTACAGA |
| RTgapR | TTCTGGATCACGTTCAGCAG |
RNA isolation, Northern blot analysis, and reverse transcription (RT)-PCR.
Wild-type and isogenic mutant S. pneumoniae D39 strains were grown in 50 ml THY medium at 37°C in 5% CO2 to mid-logarithmic phase and harvested at an OD600 of 0.3. Total bacterial RNA was isolated and purified using the RNEasy Mini kit according to the manufacturer's instructions (Qiagen). Purification included a DNase digestion step using the RNase-Free DNase Set (Qiagen). For Northern analysis, RNA preparations were treated with Terminator 5′-Phosphate-Dependent Exonuclease (Epicentre Biotechnologies) according to the instructions of the manufacturer. Northern blot analysis was performed as follows. Total RNA samples (3 to 5 μg per lane) were separated electrophoretically in denaturing agarose (1.2%) gels and vacuum transferred to Hybond-N+ nylon membrane (Amersham Biosciences). RNA was cross-linked to the membranes by incubation at 120°C for 1 h. Hybridization of the mRNA with DIG-labeled RNA or DNA probes was performed overnight at 68°C and 42°C, respectively. Hybridization signals were detected using anti-DIG-alkaline phosphatase, Fab fragments, and CDP-Star (Roche Applied Sciences). DIG-labeled probes were generated either by PCR, using the PCR-DIG Labeling Mix (Roche), for detection of spd1098/1099 mRNA or by T7 transcriptional labeling, using the SP6/T7 transcription kit (Roche), for detection of spd0411/0412 transcripts. The oligonucleotides used for generation of probes for Northern analysis are listed in Table 2. For RT, total RNA prepared form pneumococci was employed. RT was performed with SuperScript III reverse transcriptase (Invitrogen) and hexameric random primers (pd(N)6) from GE Healthcare. Briefly, 5 to 10 μg RNA (calculated using the NanoDrop ND-1000) was incubated with 10 nmol deoxynucleoside triphosphate in 20 μl RNase-free water for 5 min at 65°C. The mixture was kept on ice for 1 min, and then 4 μl First Strand Buffer (5×), 1 μl random primers, 1 μl dithiothreitol (0.1 M), 1 μl RNasin (Promega), and 1 μl SuperScript III reverse transcriptase were added to amplify the cDNA obtained. After incubation for 1 h at 50°C, the reaction was stopped by heating to 70°C for 15 min. These reaction mixtures were used as the templates in the following PCRs without further purification. Control PCRs were performed with genomic DNA and total RNA as the template, respectively (data not shown). The real-time RT-PCR was performed using StepOnePlus (Applied Biosystems), and the comparative threshold cycle (ΔΔ_CT_) standard method was used for evaluation of the results. For each gene, triplicate reaction mixtures were prepared in 96-well plates with cDNA samples generated from at least two separate RNA isolations. cDNA (2 μl) from the RT reaction (1:50 diluted) was added to SYBR green PCR Master Mix (Applied Biosystems) with the indicated primers (Table 2) at a final concentration of 100 nM, and PCRs were performed at an annealing temperature of 55°C. The expression of enolase (spd1012) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; spd1823) was used for internal normalization.
Generation of pneumococcal mutants.
Pneumococcal mutants of strain D39, D39_lux_ (23) and D39Δ_cps_ (44), were generated by insertion deletion mutagenesis. Briefly, DNA regions of spd0411/0412 and spd1098/1099, which encode annotated glutamine transporters in D39, were amplified by PCR with D39 chromosomal DNA as the template DNA. PCR products were obtained with primer pairs glnQDABF2/glnQDABR1, which contained incorporated BamHI/HindIII restriction sites, and 1098delfwd/1098delrev. The PCR product of glnQpromoDAB2/glnQDABR1 was cloned into the similarly digested pQE30 vector (Qiagen). The purified plasmid (NucleoSpin Plasmid; Macherey-Nagel) pQE280 (pQE30_gln0411_) was then used as the template for an inverse PCR with primers glnQ408mut1/glnQ408mut2 with incorporated SmaI restriction sites. This resulted in the deletion of a 143-bp fragment located at nucleotides 179 to 322 in spd0411. The deleted DNA fragment was then replaced with the aad9 gene, which was amplified by PCR using primers spcfor/spcrev, was blunt ended by a Klenow reaction, and confers spectinomycin resistance. This resulted in plasmid pQE356 (pQEΔ_gln0411spec_r). The PCR product amplified with primers 1098delfwd/1098delrev was cloned directly into vector pGEM-T easy (Invitrogen), which resulted in plasmid pGEM397. A 745-bp DNA fragment of pGEM397 was deleted by cleavage with the restriction enzyme MscI and replaced with the erythromycin resistance gene cassette (ermB) amplified by PCR. The integrity of the antibiotic resistance cassettes was verified by PCR and sequence analysis (Qiagen). Pneumococcal strains D39, D39Δ_cps_, and D39_lux_ were transformed with pQE356 and/or pGEM397 as described previously (11), using competence-stimulating peptide 1, and cultivated in the presence of the appropriate antibiotics, erythromycin (5 μg/ml), spectinomycin (50 μg/ml), and/or kanamycin (200 μg/ml). This strategy resulted in GlnQ deficiency in the glutamine transporter encoded by spd0411/0412 or GlnQPH deficiency in the glutamine transporter encoded by spd1098/1099, which was individually verified by PCR and Southern blot analysis (data not shown).
Immunoblot analysis.
Pneumococci were cultured in THY to late exponential growth phase and subsequently centrifuged. Bacterial cells were then lysed by incubation at 95°C for 10 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The total bacterial protein lysates were separated by SDS-PAGE, electrotransferred to nitrocellulose membranes, and probed with polyclonal murine or rabbit antibodies, which were previously generated against proteinase maturation protein A, PpmA (8), streptococcal lipoprotein rotamase A, SlrA (19), pneumococcal surface protein A, PspA (12), and pneumococcal surface protein C, PspC (13), diluted in phosphate-buffered saline (PBS) containing 3% bovine serum albumin. Pneumococcal enolase (4) and GAPDH (3) are essential glycolysis enzymes and were further identified as plasmin(ogen)-binding proteins located on the pneumococcal cell wall surface. Specific antibody binding was detected with secondary rabbit anti-mouse immunoglobulin-peroxidase conjugate or goat anti-rabbit IgG-peroxidase conjugate. Binding activity was detected by enhanced chemiluminescence assay (GE Healthcare).
Transmission electron microscopy (TEM).
For morphological analysis of the capsule structure, samples were fixed by the LRR fixation procedure as described earlier (14).
In vivo virulence studies in a mouse model and bioluminescent image analysis of pneumococcal infections.
All intranasal and intraperitoneal (i.p.) infection studies were performed with 6- to 8-week-old female CD-1 outbred mice (Charles River, Sulzfeld, Germany) as described recently (19, 20, 23). Bioluminescent pneumococci (23, 25) were used to infect mice, which allows monitoring of bacterial dissemination in real time using the IVIS Lumina Imaging System (Xenogen Corporation, part of Caliper Life Sciences) as described previously (23, 25).
For pneumococcal meningitis studies, 6- to 8-week-old male C57BL/6 mice were used. The animal model of pneumococcal meningitis used in this study has been previously described (27). C57BL/6 mice were examined in a blinded manner with a clinical scoring system. The score resulted from evaluation of (i) beam balancing ability (0 to 6 points), (ii) postural reflexes (0 to 3 points), (iii) vigilance, (iv) piloerection, and (v) seizures (1 point each). A score of 0 was assigned to healthy animals. Meningitis was introduced by transcutaneous injection of 15 μl of a bacterial suspension containing 107 CFU/ml S. pneumoniae D39 (n = 5), D39Δ_gln0411_ (n = 6), or D39Δ_gln1098_/1099 (n = 6) or sterile PBS (uninfected controls, n = 4) into the cisterna magna under short-term anesthesia with halothane. After 24 h, animals were scored clinically again. For hearing assessment (28), mice were anesthetized with 100 mg/kg ketamine and 5 mg/kg xylazine. Needle electrodes were placed over the left and right mastoids (negative), the vertex (positive), and the neck (ground). Impedances between the electrodes were controlled to be below 5 kΩ. Square wave click impulses (duration, 100 μs; frequency, 20 Hz) were delivered by earphones (E-A-RTONE 3A; Auditory Systems, Indianapolis, IN) that were positioned in the auditory canals. Auditory brainstem responses (ABRs) were amplified (×250,000), bandpass filtered (150 to 10,000 Hz), and averaged (n = 1,000) with a Neuroscreen Plus (Jaeger-Toennes, Hoechberg, Germany). To determine the hearing threshold, we started with a wave impulse of 105 dB and reduced the stimulus intensity in 5-dB steps. The lowest stimulus intensity that elicited ABRs was considered to be the hearing threshold. If a response could not be elicited at 105 dB, stimulus intensities of up to 130 dB were tested. Next, a catheter was placed into the cisterna magna. Cerebrospinal fluid (CSF) samples were obtained for CSF leukocyte (WBC) counting. After deep anesthesia with ketamine, animals were perfused transcardially with 15 ml ice-cold PBS containing 10 U/ml heparin. The temporal bones were dissected, fixed in 4% formalin for 4 days, decalcified in PBS containing 10% EDTA for 10 days, and embedded in paraffin. Seven-micrometer mid-modiolar sections of mouse temporal bones were deparaffinized, rehydrated, and stained with Mayer's hematoxylin and eosin (H&E). All animal experiments were approved by the Animal Care Committees of the University of Munich and by the District Governments of Upper Bavaria, Germany.
Phagocytosis experiments, antibiotic protection assays, and double immunofluorescence microscopy (DIF).
Phagocytosis assays were conducted with J774A.1 murine macrophages (DSMZ, Braunschweig, Germany) as described earlier (23, 44), and the number of intracellular and viable pneumococci was then quantified by the antibiotic protection assay as described previously (19). All experiments were performed at least three times in triplicate.
Pneumococci attached to or ingested by macrophages were also visualized by DIF microscopy. Briefly, 105 macrophages were seeded onto glass coverslips (12-mm diameter) in the wells of a 24-cell culture plate and infected the following day with pneumococci as described above. Postinfection, unbound bacteria were removed, the infected host cells were fixed on the glass coverslips with 3.7% paraformaldehyde), and DIF microscopy was carried out as described previously (19, 23). Image acquisition was performed with a confocal laser scanning microscope (Zeiss LSM510 META) and the LSM software. Each bar in the images represents 10 μm. All experiments were performed at least three times with two or more replicate wells tested for each experimental setup.
Live/dead staining.
Viability of pneumococci was investigated using the LIVE/DEAD _Bac_Light bacterial viability kit (Invitrogen). The viability of pneumococci associated with macrophages or located intracellularly was investigated 5 min, 30 min, and 60 min postinfection and immediately after starting infection (0 min). The kit contains a mixture of two fluorescence dyes which differ in the ability to penetrate intact bacterial cell walls. Green fluorescent SYTO9 stain penetrates both intact and dead bacteria and labels DNA green, while red fluorescent propidium iodide passes through only damaged bacterial membranes and reduces SYTO9 fluorescence in DNA. The excitation/emission maxima for these dyes are about 480/500 nm for SYTO9 stain and 490/635 nm for propidium iodide. The background remains virtually nonfluorescent.
Statistical evaluation.
All experiments were performed at least three times, each in duplicate, and data are expressed as the mean ± the standard deviation. Differences between the wild-type strain and isogenic mutants were statistically analyzed using the unpaired two-tailed Student t test. Survival scatter plots were compared by the Wilcoxon signed-rank test. P values for bioluminescence measurements were calculated using the unpaired one-tailed t test for differences between groups, while differences in one group between days were analyzed by the paired t test. P values for macrophage phagocytosis assays were determined by unpaired two-tailed t test. Statistical significance was confirmed by analysis of variance with Bonferroni's multiple-comparison post-hoc test. In all analyses, P values of ≤0.05 were considered statistically significant.
RESULTS
Bioinformatic analysis of gln loci.
Analysis of pneumococcal genomes deposited in the genome sequence databases (Comprehensive Microbial Resource, http://cmr.jcvi.org/tigr-scripts/CMR/shared/Genomes.cgi) of the J. Craig Venter Institute and/or GenBank of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi) predicted six putative glutamine uptake systems. Four of these putative operons consist of a glutamine-binding protein (GlnH), a glutamine permease (GlnP), and an ATP-binding protein (GlnQ) encoded by glnHPQ (Fig. 1 A). In two operons, namely, spd0411/0412 and spd1098/1099, respectively, a permease and a binding protein (GlnHP) are produced as a single protein. Downstream of and in the same orientation as spd1098/1099 lies zwf (spd1100), the gene which encodes the pentose phosphate pathway enzyme glucose-6-phosphate dehydrogenase (29). The other two operons are incomplete and lack glnH, which encodes a soluble glutamine-binding protein (Fig. 1A). Comparison of the deduced amino acid sequences (BLAST, http://blast.ncbi.nlm.nih.gov/) of the gln genes showed that the identities of glnQ, glnP, glnH, and glnHP are 36.0 to 64.0%, 25 to 36%, 22%, and 27%, respectively. Despite the low identities between GlnH proteins, domain motif searches (http://www.ebi.ac.uk/InterProScan) identified glutamine/glutamate-binding regions/sites in each of the GlnH proteins. When GlnH and GlnP are translationally fused (GlnHP), the amino-terminal part contains the GlnH domain, while the permease domain is located in the carboxy-terminal part of the protein starting at amino acid 312. Moreover, each of the GlnH proteins contains a signal peptide with a predicted cleavage site at amino acid residue 25. Sequence alignments using the KEGG database (blastn and blastp; http://www.genome.jp/) identified homologues of pneumococcal glutamine ABC transporters in the genomes of other streptococcal species and lactococci. These analyses indicated the presence of pneumococcal spd0411/0412 and spd1098/1099 homologues in all pneumococcus-related species, such as Streptococcus mitis, S. agalactiae (group B streptococci [GBS]), and S. pyogenes, while homologues of other uptake systems were present only in a subset of streptococcal species (Fig. S1). Strikingly, the spd1098/1099 homologue in GBS has been shown to be involved in fibronectin binding and virulence (47).
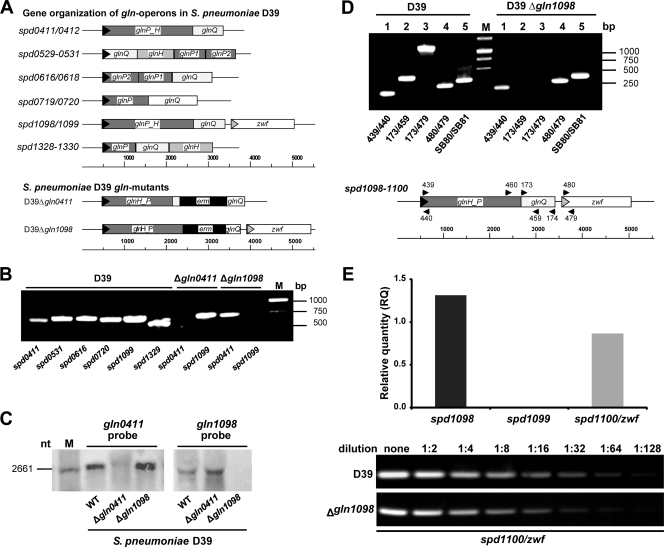
FIG. 1.
Analysis of gln gene expression in S. pneumoniae D39 and isogenic gln mutants. (A) Genetic organization of operons encoding the putative glutamine transporters in S. pneumoniae D39 and construction of gln mutants in spd0411/0412 and spd1098/1099. (B) RT-PCR of glnQ genes of S. pneumoniae wild-type D39 and gln mutants D39Δ_gln0411_ and D39Δ_gln1098_/1099. cDNAs of the different strains were used as the templates in PCRs with gene-specific primers (Table 2). (C) Northern blot analysis of glnQ0411 and glnQ1098 mRNA levels in total RNA preparations of S. pneumoniae wild-type (WT) D39 and Δ_gln0411_ and Δ_gln1098_/1099 mutants. The blots were incubated with a DIG-labeled DNA probe (gln1098/1099) and a DIG-labeled RNA probe (gln0411/0412), respectively. nt, nucleotide. (D) Comparative transcript length analysis of the putative gln1099/1100 operon of wild-type D39 and mutant D39Δ_gln1098_ by RT-PCR. (Top) PCR fragments generated from cDNAs of both strains are shown for the 5′ end of the putative operon (lanes 1), the gln Q gene (lanes 2), the region containing the glnQ gene and the 5′ end of the zwf gene (lanes 3), the 5′ end of the zwf gene (lanes 4), and the enolase housekeeping gene (lanes 5). (Bottom) Localization of primers in the gln1099/1100 operon of S. pneumoniae D39. In the D39Δ_gln1098_ mutant, the sequence between primers 173 and 459 was replaced with the ermB gene cassette (panel A). The quality of the cDNA preparations was verified by expression analysis of the eno gene (primers SB80/SB81). The purity of the RNA was assessed by using RNA as the template in PCRs, which showed no PCR products (data not shown). (E) Gene expression levels of spd1098/1099 and spd1100 (zwf) in S. pneumoniae D39Δ_gln1098_/1099 as analyzed by quantitative RT-PCR (top) and RT-PCR (bottom). The results for each transcript in the quantitative RT-PCR are presented as the relative quantity (RQ) of expression in S. pneumoniae wild-type D39. The relative change in the expression of each gene was calculated from 2−ΔΔ_CT_ by using the genes for enolase (spd1012) and GAPDH (spd1823) as internal controls. The experiment was repeated three times, and one representative result is shown. The RT-PCR for spd1100 (zwf) was generated by using serial dilutions (undiluted to 1:128) of S. pneumoniae wild-type D39 and D39Δ_gln1098_/1099 mutant cDNAs as templates, respectively.
Expression of gln genes.
In order to investigate and compare the levels of gene expression in the different gln operons, RT-PCRs were conducted using glnQ_-specific primers (Table 2). The results showed the expression of all of the annotated glnQ genes under in vitro conditions (Fig. 1B). However, quantitative PCR after RT revealed significant differences in the levels of mRNA transcripts for the gln genes of the various gln operons (data not shown). Proteome analyses of D39 showed peptide spots for ATP-binding proteins SPD0411, SPD0720, SPD1099, and SPD1329 (data not shown). Strikingly, proteome analysis of pavA knockout strain D39Δ_pavA, which is highly attenuated in virulence (20, 25, 43), demonstrated that the ATP-binding protein SPD0411 (GlnQ0411) is downregulated in the absence of a functional PavA protein (data not shown), suggesting that this protein contributes to the attenuation of the pavA mutant.
Transcriptional analysis of pneumococcal gln mutants.
To investigate the impact of the pneumococcal glutamine transport systems SPD1098/1099 and SPD0411/0412, respectively, on bacterial fitness and virulence, gln mutants were generated by allelic replacement (Fig. 1A). The mutants, designated D39Δ_gln0411_ and D39Δ_gln1098_, respectively, were confirmed by PCR and Southern blot analysis (data not shown). The absence of gln transcripts was also confirmed by RT-PCR (Fig. 1B) and Northern blot analysis using _gln1098_- or gln0411_-specific, DIG-labeled DNA/RNA probes (Fig. 1C). Northern blot analysis did not clearly demonstrate that zwf is transcribed together with the gln1098/1099 genes. However, RT-PCRs performed with different primer combinations suggested that zwf is indeed part of the gln1098/1099 transcript (Fig. 1D). Importantly, RT-PCR further demonstrated that the expression of zwf is not altered in knockout strain D39Δ_gln1098 (Fig. 1D and E).
Influence of gln mutations on growth kinetics of pneumococci.
To assess potential effects of the glutamine transport systems Gln1098/1099 and Gln0411/0412 on pneumococcal growth under in vitro conditions, wild-type pneumococci and isogenic mutants D39Δ_gln0411_ and D39Δ_gln1098_ were cultivated in complex medium THY, in CDM, or in CDM supplemented with 2% Casitone peptone (BD Biosciences, Germany) and the OD600s were isochronously measured. The results showed no differences between the growth kinetics of wild-type D39 and D39Δ_gln0411_ mutant pneumococci in all three media (Fig. 2). In contrast, the deficiency of the glutamine transport system in D39Δ_gln1098_ impaired pneumococcal growth in CDM, which could be partially restored by supplementation of CDM with Casitone (Fig. 2). These results confirmed previous findings that SPD1098/1099 is the main glutamine/glutamate ABC transporter in pneumococci (29) and demonstrated that defects in glutamine uptake can be complemented by uptake of peptides via oligopeptide transporter systems.
FIG. 2.
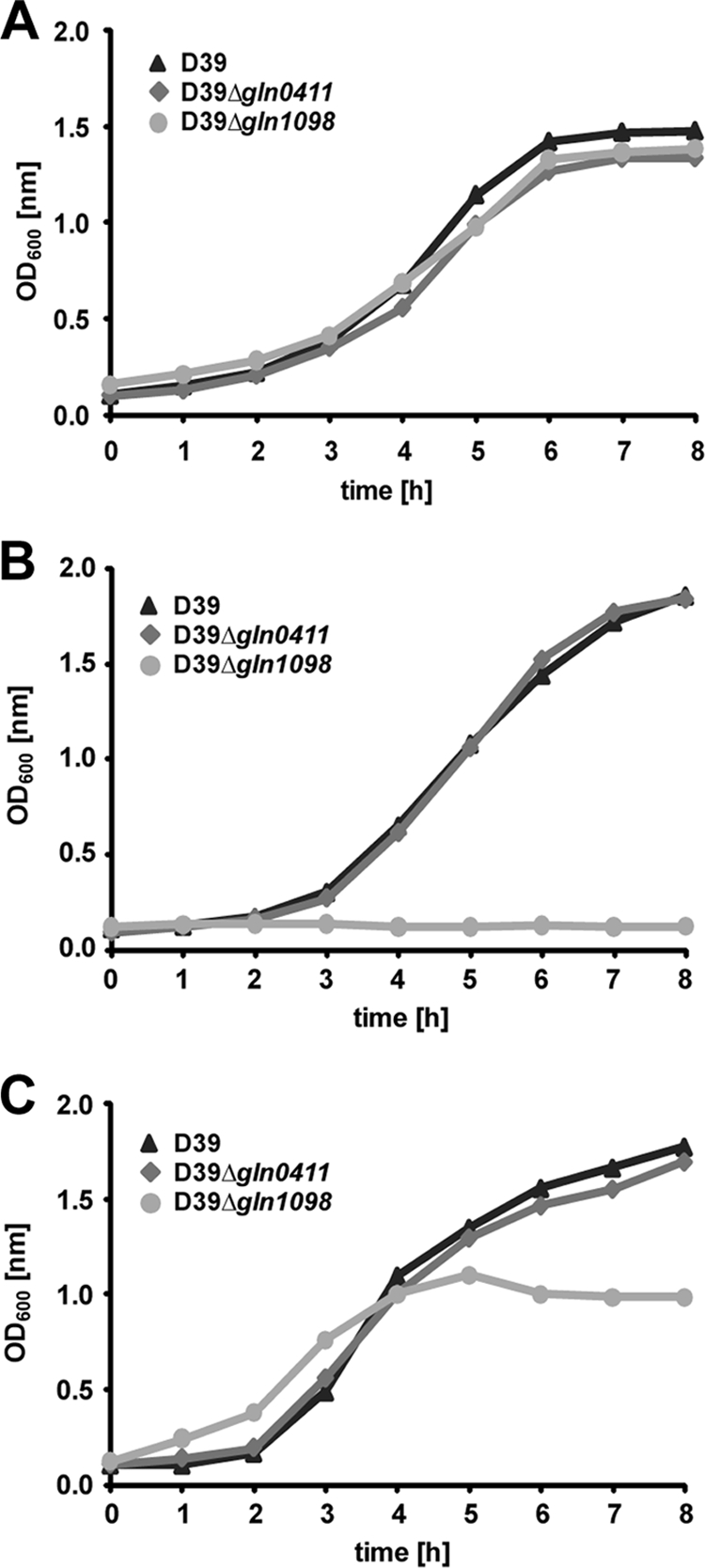
Growth behavior of pneumococcal gln mutants. S. pneumoniae strain D39 and its isogenic mutants gln0411 and gln1098 were cultured in THY (A), in CDM (B), or in CDM supplemented with 2% Casitone (C). Representative growth curves are shown.
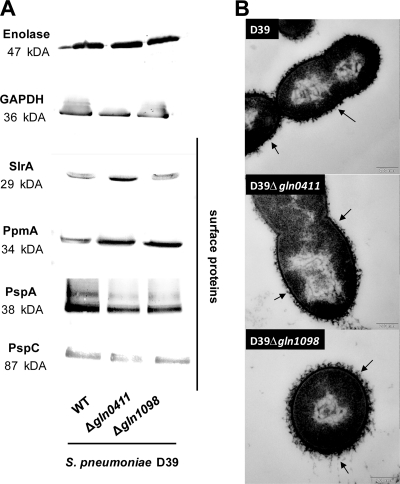
Virulence factor production is not affected in gln mutants.
To investigate exemplarily whether a deficiency in one of the glutamine uptake systems results in altered production of virulence factors exposed on the surface of pneumococci, immunoblot analyses were conducted using virulence factor-specific polyclonal antisera. The results revealed that wild-type D39 and its isogenic mutants D39Δ_gln0411_ and D39Δ_gln1098_ produced comparable amounts of lipoproteins SlrA and PpmA and choline-binding proteins PspA and PspC as well (Fig. 3 A). Moreover, a hemolysis assay (1) indicated that the pneumolysin production is not affected in the mutants (data not shown). TEM studies performed after LRR white fixation of pneumococci further indicated that the formation of the capsular polysaccharide layer is also not influenced in pneumococcal mutants deficient in one of the glutamine transporter systems (Fig. 3B).
FIG. 3.
Influence of Gln deficiencies on the expression of pneumococcal surface-exposed virulence proteins and capsule. (A) The production of surface-exposed virulence proteins (SlrA, PpmA, PspA, and PspC) and glycolytic enzymes (GAPDH and enolase) in wild-type (WT) strain D39 and its isogenic mutants D39Δ_gln0411_ and D39Δ_gln1098_ was assessed by immunoblotting using protein-specific antibodies. Eno, enolase; PpmA, putative proteinase maturation protein A; SlrA, streptococcal lipoprotein rotamase A; PspA, pneumococcal surface protein A; PspC, pneumococcal surface protein C. (B) Capsular polysaccharide of D39 and its isogenic gln mutants shown by TEM after LRR fixation as described in Materials and Methods. Black arrows show the presence of polysaccharide capsule in all three samples. Bars, 200 nm.
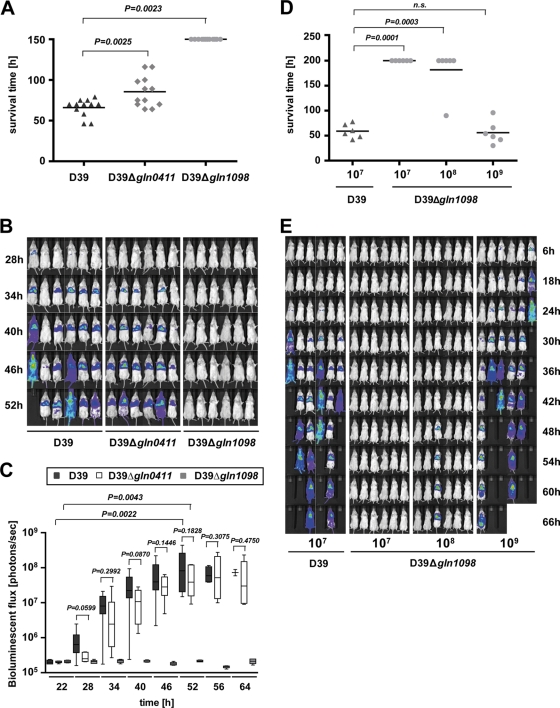
Contribution of glutamine transporter systems to pneumonia.
The impact of glutamine transporter systems GlnHPQ encoded by spd0411/0412 and spd1098/1099, respectively, on pneumonia and survival was assessed after intranasal pneumococcal infection of CD-1 outbred mice via the intranasal route. Mice (n = 12) were infected with 107 CFU of wild-type D39 or its isogenic mutants D39Δ_gln1098_ and D39Δ_gln0411_, respectively. The results revealed a mean survival time of 66 h for mice infected with wild-type D39 bacteria, while mice infected with D39Δ_gln0411_ showed significantly (P = 0.0025) longer survival times, with a mean of 85.5 h, indicating significant attenuation of this mutant. In addition, mice infected with D39Δ_gln1098_ did not develop signs of disease and consequently all of them survived (Fig. 4 A).
FIG. 4.
Impact of glutamine uptake systems on pneumococcal virulence. (A) Survival of CD-1 mice after intranasal infection with pneumococci. CD-1 mice (n = 12) were infected intranasally with a dose of 1 × 107 CFU of either wild-type strain D39 or its isogenic gln0411 or gln1098 mutant. (B) Real-time bioluminescent optical imaging of pneumococcal dissemination after intranasal infection of mice. CD-1 mice (n = 6) were intranasally infected with 1 × 107 CFU of wild-type D39 or its isogenic gln0411 and gln1098 mutants, respectively. Dissemination of pneumococci in this acute-pneumonia model was monitored for up to 144 h. (C) Determination of bioluminescent intensity at the indicated time points. Quantification of the total photon emission per second was done every 6 h by measuring the bioluminescence of CD-1 mice (n = 6) infected with wild-type D39 or its isogenic gln0411 or gln1098 mutant using the IVIS Lumina System. Data were evaluated using GraphPad Prism 4.0 software and are represented in a box whisker graph. (D) CD-1 mice (n = 6) were infected intranasally with a dose of 1 × 107 CFU of wild-type strain D39 or its isogenic gln1098 mutant using infection doses of 1 × 107, 1 × 108, or 1 × 109 CFU. n.s., not significant. (E) Monitoring of pneumococcal dissemination in the host after infection in a dose-dependent manner. Bioluminescent image analysis of CD-1 mice (n = 6) infected intranasally with a dose of 1 × 107 CFU of wild-type strain D39 or its isogenic gln1098 mutant.
Moreover, we have monitored the dissemination of pneumococci from the nasopharynx to the lungs and into the blood by employing real-time bioluminescent optical image analysis and bioluminescent pneumococci. CD-1 mice (n = 6) were infected with 107 CFU of D39_lux_, D39_lux_Δ_gln0411_, or D39_lux_Δ_gln1098_, and dissemination of the bacteria was monitored continuously in the same mice at 6-h intervals postinfection until the mice became moribund. At 34 h postinfection, mice infected intranasally with D39_lux_ or D39_lux_Δ_gln0411_ developed severe lung infections encompassing the whole of the lung. In D39-infected mice, septicemia was observed at 40 to 46 h postinfection and the first fatalities were observed at 52 h postinfection. D39Δ_gln0411_ also spread into the blood of infected mice; however, compared to wild-type-infected mice, septicemia was delayed and was first visualized at 52 h postinfection. In contrast to these infections, in vivo bioimaging showed that a dose of 1 × 107 D39_lux_Δ_gln1098_ bacteria was not sufficient to cause pneumonia and septicemia in CD-1 mice, indicating an immense attenuation of this mutant (Fig. 4B). The significant attenuation of D39Δ_gln0411_ and, hence, the delay in the development of pneumonia and septicemia, were further confirmed after calculating the luminescence intensity measured with the IVISLumina system at the indicated time points. The bioluminescent flux determined for D39_lux_Δ_gln0411_ was significantly reduced between 22 and 64 h postinfection, and the maximum bioluminescence intensity (∼109 photons/s) was reached 6 h later than in D39_lux_-infected mice (Fig. 4C). Mice infected with D39_lux_Δ_gln1098_ showed no increase in bioluminescence intensity.
To assess the limit of attenuation of D39_lux_Δ_gln1098_, two additional doses ranging from 10-fold and 100-fold greater numbers of CFU (108 and 109) were used to infect mice intranasally with this mutant. Survival and dissemination were monitored and compared to those of mice infected with 107 bioluminescent wild-type D39_lux_. All of the wild-type-infected mice died, while all of the mice infected with a dose of 107 D39_lux_Δ_gln1098_ bacteria survived. Mice infected with a 10-fold higher dose survived, with the exception of one animal, which also showed dissemination of pneumococci into the lungs (Fig. 4D and E). In contrast, mice infected with a 100-fold-increased number of CFU of D39_lux_Δ_gln1098_ developed severe disease, as visualized by the bioimaging technique, and lung infections leading to death were visible after 24 h. The mean survival time of 56 h of D39_lux_Δ_gln1098_-infected mice was similar to that of D39_lux_-infected mice (Fig. 4D).
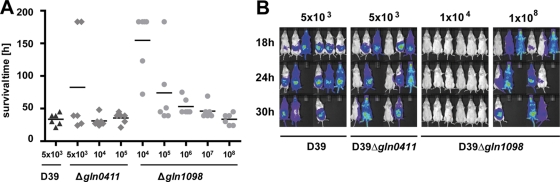
Virulence of pneumococci deficient in glutamine transporter in a systemic infection model.
To assess the impact of the two glutamine uptake systems analyzed on virulence during sepsis, the systemic infection model was employed and mice (n = 6) were infected i.p. with bioluminescent D39_lux_ and its isogenic gln mutants. All of the mice succumbed to infection when challenged with 5 × 103 wild-type pneumococci, and the mean survival time was 33.5 h. For mice infected i.p. with 5 × 103 CFU of Δ_gln0411_ pneumococci, the mean survival time increased to 80 h, while a 2-fold infection dose resulted in a mean survival time similar to that of wild-type-infected mice, indicating a slight attenuation of this mutant (Fig. 5 A). The differences in virulence between D39_lux_ and D39_lux_Δ_gln0411_ could not be visualized by the bioimaging approach (Fig. 5B). To investigate the limit of attenuation of D39luxΔ_gln1098_, mice were infected i.p. with doses of 104 to 108 CFU. The results showed that five out of six mice died when they were i.p. inoculated with a 20-fold higher number of CFU (105 pneumococci), with a mean survival time of 74 h, while mice infected with only 104 CFU survived or had increased survival times. When the challenge doses were increased to 106 CFU or even higher (107 and 108) numbers of CFU, the mice succumb to infection similar to wild-type-infected animals, and the survival times decreased, with means of 53 h (106 CFU), 45.5 h (108 CFU), and 33 h (108 CFU), respectively.
FIG. 5.
Influence of glutamine uptake systems in a septicemia mouse infection model. (A) CD-1 mice (n = 6 for each group) were infected i.p. with a dose of 5 × 103 CFU of wild-type strain D39 or with 5 × 103, 1 × 104 or 1 × 105 CFU of mutant D39Δ_gln0411_. In parallel, mice were infected i.p. with doses ranging from 1 × 104 to 1 × 108 CFU of mutant strain D39Δ_gln1098_. The vitality of mice was observed every 3 h, and mice that died were scarified. (B) Bioluminescent image analysis of CD-1 mice (n = 6) infected i.p. with wild-type strain D39 or the mutants D39Δ_gln0411_ and D39Δ_gln1098_, respectively. The data from the time point shown indicate the massive differences in pneumococcal dissemination between the groups.
Effects of gln deficiency in an experimental murine meningitis model.
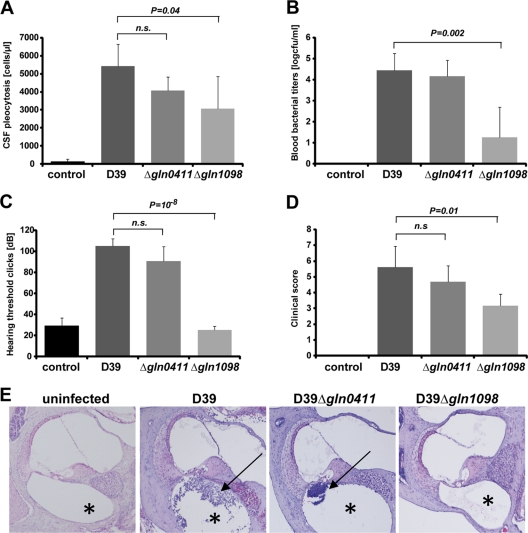
Mice were infected by intracisternal injection with 107 CFU of S. pneumoniae D39 or its isogenic gln_-deficient mutants. An equivalent volume of sterile PBS was used for an uninfected control. D39 injection results in a significant elevation of CSF WBCs (WBC count, 5,430 ± 1,213 cells/μl) compared to that of the PBS control (156 ± 124 cells/μl) at 24 h postinfection, documenting meningitis (Fig. 6 A). Mice infected with the gln0411 mutant show a nonsignificant decrease in CSF pleocytosis (4,056 ± 766 cell/μl). In contrast, intracisternal infection of mice with Δ_gln1098 pneumococci resulted in CSF WBC counts significantly lower that those of wild-type strain D39 (3,080 ± 1,771 cells/μl) (Fig. 6A).
FIG. 6.
Role of glutamine uptake systems in an experimental mouse meningitis model. (A) Cerebrospinal fluid pleocytosis. Different numbers of C57BL/6 mice were injected intracisternally with 1 × 107 CFU of wild-type strain D39 (n = 5), its gln0411 (n = 6) or gln1098 (n = 6) mutant, or the same volume of PBS (n = 4) as a control. The histogram shows mean WBC counts per μl CSF, as a marker for an inflammatory response, 24 h after injection. The experimental standard deviations are shown as bars, and two-tailed Student _t_-test analyses were performed on data sets. n.s., not significant. (B) Blood bacterial titers. Different numbers of C57BL/6 mice were injected intracisternally with 1 × 107 CFU of wild-type strain D39 (n = 5), its gln0411 (n = 6) or gln1098 (n = 6) mutant, or the same volume of PBS (n = 4) as a control. The histogram shows mean counts (log CFU/ml) of bacteria in murine blood, as a marker of bacterial migration from the subarachnoid space into blood, 24 h after injection. Experimental standard deviations are shown as bars, and two-tailed Student _t_-test analyses were performed on data sets. Experimental details are described in Materials and Methods. (C) Hearing threshold clicks. Different numbers of C57BL/6 mice were injected intracisternally with 1 × 107 CFU of wild-type strain D39 (n = 5), its gln0411(n = 6) or gln1098 (n = 6) mutant, or the same volume of PBS (n = 4) as a control. The histogram shows the mean lowest stimulus intensity that elicited ABRs 24 h after injection. Experimental standard deviations are shown as bars, and two-tailed Student _t_-test analyses were performed on data sets. Experimental details are described in Materials and Methods. (D) Clinical scores. Different numbers of C57BL/6 mice were injected intracisternally with 1 × 107 CFU of wild-type strain D39 (n = 5), its gln0411(n = 6) or gln1098 (n = 6) mutant, or the same volume of PBS (n = 4) as a control. The histogram shows the mean clinical scores 24 h after injection. Experimental standard deviations are shown as bars, and two-tailed Student _t_-test analyses were performed on data sets. Experimental details are described in Materials and Methods. (E) Presence and localization of inflammatory cells in cochleae. Shown are dissected and H&E-stained cochleae of infected mice. A representative selection of images is displayed. Wild-type strain D39 or its gln0411 or gln1098 mutant was used for infection, or the same volume of PBS was used as a control. Black arrows show the recruitment of granulocytes into the scala tympani (*). The presence of granulocytes can lead to severe granulocytic labyrinthitis.
Dissemination of pneumococci from the subarachnoid space into the blood and/or pneumococcal fitness in the bloodstream was investigated by measuring blood bacterial titers. No living bacteria were found in the blood of uninfected mice. Mice infected with D39 (4.45 ± 0.80 log CFU/ml) and the Δ_gln0411_ mutant (4.17 ± 0.75 log CFU/ml) showed similar amounts of pneumococci in the blood. In contrast, animals infected with the Δ_gln1098_ mutant showed a highly significant decrease in blood bacterial titers (1.27 ± 1.42 log CFU/ml) (Fig. 6B).
Another parameter demonstrating pneumococcal virulence is the development of hearing loss in infected mice caused by labyrinthitis. This can be functionally measured by detecting their hearing thresholds by measuring ABRs. Uninfected mice showed low hearing thresholds of 29.4 ± 7.3 dB. Mice infected with wild-type strain D39 (105 ± 7 dB) or the gln0411 mutant (90.6 ± 13.7 dB) revealed an elevation of this threshold. In contrast, _gln1098_-deficient pneumococcus-infected mice showed hearing thresholds (25 ± 3.8 dB) similar to those of uninfected mice, indicating attenuated hearing loss (Fig. 6C).
For murine fitness, physiological parameters were tested by using a clinical scoring system for meningitis. A clinical score of 0 indicates an uninfected and healthy subject, and a score of 12 indicates death. In this experiment, uninfected mice had a clinical score of 0. Mice infected with wild-type strain D39 (5.6 ± 1.3) or the gln0411 mutant (4.7 ± 1.0) had similar clinical scores (Fig. 6D). In contrast, mice infected with the gln1098 mutant showed a significantly lower clinical score of 3.2 ± 0.8 and, thus, less clinical impairment (Fig. 6D).
Histopathology of the murine inner ear shows labyrinthitis.
To demonstrate the degree of labyrinthitis, the inner ears of mice infected with D39 or isogenic Δ_gln0411_ or Δ_gln1098_ mutant pneumococci were investigated histologically. Uninfected mice showed no inflammatory cells in the cochlea. In mice infected with the Δ_gln1098_ mutant, a very mild invasion of granulocytes was observed in the cochlea. However, severe granulocytic labyrinthitis was observed in wild-type strain D39- or gln0411 mutant-infected mice (Fig. 6E).
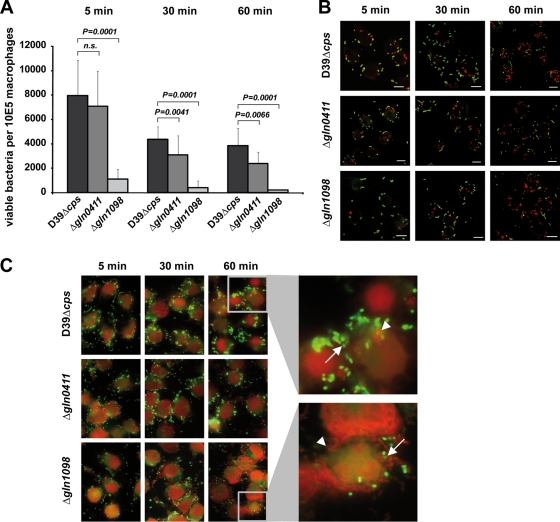
Effects of glutamine transporters on intracellular survival of pneumococci in macrophages.
To assess the impact of the two glutamine transporter systems on phagocytosis and intracellular survival, the macrophage-like cell line J774A.1 was infected with D39Δ_cps_ and isogenic gln mutants. Extracellular bacteria were killed 5, 30, or 60 min postinfection by antibiotic treatment and eliminated. Thereafter, the number of survivors recovered was determined by plating the intracellular pneumococci on blood agar plates. The results revealed an infection time-dependent decrease in the number of viable pneumococci recovered, suggesting that once phagocytosed, pneumococci have an impaired ability to survive and persist in macrophages (Fig. 7 A). The enumeration of internalized and recovered viable pneumococci further demonstrated that the number of viable intracellular D39Δ_cps_Δ_gln1098_ pneumococci was at least 7- to 17-fold lower than that of wild-type D39Δ_cps_ and 6- to 11-fold lower than that of mutant D39Δ_cps_Δ_gln0411_ (Fig. 7A). Comparison of D39Δ_cps_ and D39Δ_cps_Δ_gln0411_ showed no significant differences after 5 min of infection, while after 30 min and 60 min, the number of viable intracellular D39Δ_cps_Δ_gln0411_ bacteria was significantly lower than that of D39Δ_cps_ bacteria. To confirm these data, pneumococcal attachment to and ingestion by macrophages were visualized by DIF staining and microscopy. In general, the images showed that the number of intracellular bacteria increased over time and reached a maximum after 60 min (Fig. 7B). In addition, there seems to be no dramatic differences in pneumococcal phagocytosis between wild-type D39 and its isogenic gln mutants. However, these data seemed to be contradictory to the quantification of phagocytosed and recovered viable pneumococci determined with the antibiotic protection assay. Therefore, the viability of phagocytosed pneumococci was explored and live/dead staining of extra- and intracellular pneumococci was conducted. First, the results demonstrated that prior to the infections, the pneumococci were all alive, with only a few exceptions for strain D39Δ_cps_Δ_gln1098_. Second, the live/dead staining suggested that the ability to resist killing by phagocytes is relatively low for D39Δ_cps_Δ_gln1098_, intermediate for D39Δ_cps_Δ_gln0411_, and highest for D39Δ_cps_ (Fig. 7C).
FIG. 7.
Impact of Gln deficiencies on phagocytosis and intracellular killing. (A) Numbers of viable bacteria after J774A.1 phagocytosis assay. Murine macrophages (105) were infected with a multiplicity of infection of 50 pneumococci of strain D39 or its gln0411 or gln1098 mutant. Viable invasive bacterial numbers were determined by colony counting after 5, 15, or 30 min of infection. The histogram shows the mean values ± standard deviation of three independent experiments. n.s., not significant. (B) Immunofluorescence microscopy of host cell-attached and intracellular pneumococci. J774A.1 murine macrophages were infected with pneumococcal strain D39 or its gln0411 or gln1098 mutant for 5, 30, or 60 min. Intracellular pneumococci were stained with Alexa568 (red), whereas adherent bacteria appear yellow (Alexa488 and Alexa568). Bars represent 10 μm. (C) Live/dead staining of phagocytosed pneumococci. J774A.1 murine macrophages were infected for 5, 30, or 60 min with wild-type D39 or its isogenic mutant D39Δ_gln0411_ or D39Δ_gln1098_. Viable bacteria are only stained with SYTO9 (green fluorescence), while dead bacteria appear red fluorescent as stained with propidium iodide. Higher magnifications of selected areas 60 min postinfection are shown on the right. Viable bacteria are indicated by arrows, and dead bacteria are indicated by arrowheads.
DISCUSSION
In this study, we demonstrated the influence of two out of six glutamine ABC transporters on pneumococcal fitness and virulence by employing different in vivo and in vitro infection models. Glutamine is an important resource for bacteria and a main nitrogen donor in the cell. Nitrogen and glutamine metabolism is essential for the fitness and virulence of various bacterial species, such as Bacillus subtilis, Salmonella typhimurium, and GBS (30, 47). Lack of pneumococcal growth in CDM depleted of glutamine and glutamate indicated that pneumococci cannot synthesize these amino acids de novo (data not shown). Pneumococci encode six putative glutamine ABC transporters, each consisting of a cytoplasmic protein, GlnQ, which hydrolyzes ATP to provide energy for transport and a permease, GlnP, which contains six transmembrane domains. The gene which encodes the substrate-binding protein GlnH, which contains an amino-terminal signal peptide, is present in four of the six glutamine transporter operons and absent from operons _spd0616_-0618 and spd0719/0720. Strikingly, in two of the operons, namely, spd0411/0412 and spd1098/1099, the substrate-binding protein is translationally fused to the glutamine permease domain. The translational fusion of these two glutamine-binding proteins are also found in related species such as S. mitis, S. pyogenes, S. mutans, S. agalactiae (GBS), and L. lactis. However, L. lactis, GBS, and other streptococcal species encode a lower number of glutamine ABC transporters than do pneumococci and S. mitis. However, homologues of spd0411/0412 and spd1098/1099 are present in all of the streptococcal species analyzed. Similar to that in other bacteria, glutamine and glutamate metabolism is regulated by GlnR and GlnA, a predicted GS (29). Moreover, a global transcriptome analysis of the glnR mutant revealed that GlnR, which is regulated by GlnA, regulates glutamine operon spd1098/1099 containing the zwf gene (spd1100) downstream, while no significant changes in gene expression levels were indicated in the other glutamine operons.
A deficiency in GlnA abrogates pneumococcal adherence to host epithelial cells and attenuates pneumococci in in vivo infection models, while a deficiency in GlnR has only moderate effects (18, 29). Similar results were reported for GlnQ of GBS, which is a homologue of spd1099, and GlnA of S. suis serotype 2 (45, 47). Molecular analysis by RT-PCR and Northern blotting indicated the expression of all of the genes of pneumococcal glutamine operons. In addition, the synthesis of ATP-binding proteins SPD0411, SPD0720, SPD1099, and SPD1329 was confirmed by proteome analysis of D39 (data not shown). Remarkably, proteome analysis indicated the absence GlnQ encoded by SPD0412 in the pavA mutant D39Δ_pavA_, which is highly attenuated in virulence (20, 25, 43), suggesting that GlnQ0412 is regulated by PavA (data not shown). To demonstrate the impact of glutamine ABC transporters, SPD0411/0412 and SPD1098/1099 mutants were generated and tested for bacterial fitness and virulence. Similar to the results reported by Kloosterman et al. (29), the lack of functional ABC transporter SPD1098/1099 did not affect growth in complex medium but did affect growth in CDM, and growth was only restored by the addition of oligopeptides. In contrast, the growth of D39Δ_gln0411_ was unaffected under all of the conditions tested. These results confirm that the genes of operon spd1098/1099 encode the main glutamine ABC transporter of pneumococci, which is strengthened by the fact that these proteins show the highest homologies to glutamine uptake systems of other bacteria such as GBS. The attenuation of virulence and the reduced ability of GBS deficient in GlnQ (homologue of SPD1099) to adhere to fibronectin were attributed to the nutritional defect, namely, reduced cytoplasmic glutamine levels (47).
As metabolism is linked with virulence and virulence factor expression, the synthesis of key virulence factors was assessed by immunoblot or electron microscopic analysis. However, the results revealed no significant differences in the expression of known virulence factors, including pneumolysin and capsule, between wild-type D39 and its isogenic gln mutants.
To assess the impact of the different glutamine ABC transporters on colonization and invasive diseases, mouse infection experiments were conducted using pneumonia, sepsis, and experimental meningitis models. Moreover, dissemination and the development of pneumonia and septicemia were followed in real time using bioluminescent pneumococci. The in vivo infections demonstrated that the deficiency in the main glutamine transporter Gln1098/1099 retards dissemination from mucosal tissue to the lungs and blood, thus stopping the development of a systemic infection. Moreover, in the septicemia and meningitis infection models, D39Δ_gln1098_ mutant pneumococci were highly attenuated compared to wild-type D39 pneumococci. The deficiency in the ABC transporter Gln0411/0412 had only moderate effects on pneumococcal virulence. The pneumonia model showed a delay in the development of pneumonia and septicemia which was not significant, while the sepsis and meningitis models showed no differences in virulence. The effects on the pathogenesis of the D39Δ_gln1098_ mutant are highly similar to those reported for pneumococci deficient in PavA (20, 25, 43). PavA is thought to modulate the expression of pneumococcal factors required for colonization and virulence (43). In addition, PavA protects against phagocytosis and has been shown to be essential to induce an optimal immune response in the host (38). As the massive reduction in the virulence of D39Δ_gln1098_ in the sepsis model was not due to impaired growth of the mutant in blood (data not shown), its resistance to the immune system, in particular, to phagocytosis and killing by phagocytes, was tested. The results revealed that both mutants were efficiently phagocytosed, as shown by immunofluorescence microscopy; however, D39Δ_gln0411_ and D39Δ_gln1098_ had a significantly impaired ability to resist intracellular killing by macrophages. As expected from the in vivo infection experiments, this effect was stronger for the main glutamine transporter mutant. Further experiments suggested that the mutants were less resistant to the oxidative stress they encounter in macrophages and thus are killed highly efficiently. Hence, the phagocytosis studies suggest that the lack of multiplication and dissemination within the blood is due not to nutritional deficits within this host niche but to efficient killing by phagocytes.
In conclusion, virulence studies and phagocytosis experiments indicated that the functional inactivity of the glutamine ABC transporter Gln1098/1099 diminished bacterial fitness and virulence, while lack of Gln0411/412 had only moderate effects on virulence and survival in phagocytes. Thus, Gln1098/1099 is essential for glutamine uptake and homeostasis of cytoplasmic glutamine levels, while Gln0411/0412 is unable to complement the defect in the main glutamine transporter but probably has a role in maintaining the homeostasis of intracellular glutamine levels of pneumococci.
Supplementary Material
[Supplemental material]
Acknowledgments
This work was supported in part by the Deutsche Forschungsgemeinschaft (Ha 3125/3-1, DFG priority program SPP1316) and EU FP7 CAREPNEUMO (EU-CP223111, European Union).
We are also grateful to Gustavo Gámez and Gerhard Burchhardt (Department Genetics, Universität Greifswald) for critical discussions and reading of the manuscript. The technical assistance of Kristine Sievert-Giermann (Greifswald) is appreciated.
Footnotes
▿
Published ahead of print on 15 November 2010.
REFERENCES
- 1.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23**:**201-209. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann, S., and S. Hammerschmidt. 2006. Versatility of pneumococcal surface proteins. Microbiology 152**:**295-303. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, S., M. Rohde, and S. Hammerschmidt. 2004. Glyceraldehyde 3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 72**:**2416-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann, S., D. Wild, O. Diekmann, R. Frank, D. Bracht, G. S. Chhatwal, and S. Hammerschmidt. 2003. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49**:**411-423. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J. S., S. M. Gilliland, and D. W. Holden. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40**:**572-585. [DOI] [PubMed] [Google Scholar]
- 6.Brown, J. S., A. D. Ogunniyi, M. C. Woodrow, D. W. Holden, and J. C. Paton. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 69**:**6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright, K. 2002. Pneumococcal disease in western Europe: burden of disease, antibiotic resistance and management. Eur. J. Pediatr. 161**:**188-195. [DOI] [PubMed] [Google Scholar]
- 8.Cron, L. E., H. J. Bootsma, N. Noske, P. Burghout, S. Hammerschmidt, and P. W. Hermans. 2009. Surface-associated lipoprotein PpmA of Streptococcus pneumoniae is involved in colonization in a strain-specific manner. Microbiology 155**:**2401-2410. [DOI] [PubMed] [Google Scholar]
- 9.Fisher, S. H. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la difference! Mol. Microbiol. 32**:**223-232. [DOI] [PubMed] [Google Scholar]
- 10.Hammerschmidt, S. 2006. Adherence molecules of pathogenic pneumococci. Curr. Opin. Microbiol. 9**:**12-20. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschmidt, S., V. Agarwal, A. Kunert, S. Haelbich, C. Skerka, and P. F. Zipfel. 2007. The host immune regulator factor H interacts via two contact sites with the PspC protein of Streptococcus pneumoniae and mediates adhesion to host epithelial cells. J. Immunol. 178**:**5848-5858. [DOI] [PubMed] [Google Scholar]
- 12.Hammerschmidt, S., G. Bethe, P. H. Remane, and G. S. Chhatwal. 1999. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 67**:**1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammerschmidt, S., M. P. Tillig, S. Wolff, J. P. Vaerman, and G. S. Chhatwal. 2000. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol. Microbiol. 36**:**726-736. [DOI] [PubMed] [Google Scholar]
- 14.Hammerschmidt, S., S. Wolff, A. Hocke, S. Rosseau, E. Muller, and M. Rohde. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 73**:**4653-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45**:**1389-1406. [PMC free article] [PubMed] [Google Scholar]
- 16.Hava, D. L., J. LeMieux, and A. Camilli. 2003. From nose to lung: the regulation behind Streptococcus pneumoniae virulence factors. Mol. Microbiol. 50**:**1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriksen, W. T., H. J. Bootsma, S. Estevao, T. Hoogenboezem, A. de Jong, R. de Groot, O. P. Kuipers, and P. W. Hermans. 2008. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J. Bacteriol. 190**:**590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriksen, W. T., T. G. Kloosterman, H. J. Bootsma, S. Estevao, R. de Groot, O. P. Kuipers, and P. W. Hermans. 2008. Site-specific contributions of glutamine-dependent regulator GlnR and GlnR-regulated genes to virulence of Streptococcus pneumoniae. Infect. Immun. 76**:**1230-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermans, P. W., P. V. Adrian, C. Albert, S. Estevao, T. Hoogenboezem, I. H. Luijendijk, T. Kamphausen, and S. Hammerschmidt. 2006. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J. Biol. Chem. 281**:**968-976. [DOI] [PubMed] [Google Scholar]
- 20.Holmes, A. R., R. McNab, K. W. Millsap, M. Rohde, S. Hammerschmidt, J. L. Mawdsley, and H. F. Jenkinson. 2001. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol. Microbiol. 41**:**1395-1408. [DOI] [PubMed] [Google Scholar]
- 21.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183**:**5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer, R., N. S. Baliga, and A. Camilli. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 187**:**8340-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensch, I., G. Gamez, M. Rothe, S. Ebert, M. Fulde, D. Somplatzki, S. Bergmann, L. Petruschka, M. Rohde, R. Nau, and S. Hammerschmidt. 2010. PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways [sic] infections. Mol. Microbiol. 77**:**22-43. [DOI] [PubMed] [Google Scholar]
- 24.Jomaa, M., J. Yuste, J. C. Paton, C. Jones, G. Dougan, and J. S. Brown. 2005. Antibodies to the iron uptake ABC transporter lipoproteins PiaA and PiuA promote opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 73**:**6852-6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadioglu, A., H. Brewin, T. Hartel, J. L. Brittan, M. Klein, S. Hammerschmidt, and H. F. Jenkinson. 2010. Pneumococcal protein PavA is important for nasopharyngeal carriage and development of sepsis. Mol. Oral Microbiol. 25**:**50-60. [DOI] [PubMed] [Google Scholar]
- 26.Kadioglu, A., J. N. Weiser, J. C. Paton, and P. W. Andrew. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6**:**288-301. [DOI] [PubMed] [Google Scholar]
- 27.Klein, M., R. Paul, B. Angele, B. Popp, H. W. Pfister, and U. Koedel. 2006. Protein expression pattern in experimental pneumococcal meningitis. Microbes Infect. 8**:**974-983. [DOI] [PubMed] [Google Scholar]
- 28.Klein, M., C. Schmidt, S. Kastenbauer, R. Paul, C. J. Kirschning, H. Wagner, B. Popp, H. W. Pfister, and U. Koedel. 2007. MyD88-dependent immune response contributes to hearing loss in experimental pneumococcal meningitis. J. Infect. Dis. 195**:**1189-1193. [DOI] [PubMed] [Google Scholar]
- 29.Kloosterman, T. G., W. T. Hendriksen, J. J. Bijlsma, H. J. Bootsma, S. A. van Hijum, J. Kok, P. W. Hermans, and O. P. Kuipers. 2006. Regulation of glutamine and glutamate metabolism by GlnR and GlnA in Streptococcus pneumoniae. J. Biol. Chem. 281**:**25097-25109. [DOI] [PubMed] [Google Scholar]
- 30.Klose, K. E., and J. J. Mekalanos. 1997. Simultaneous prevention of glutamine synthesis and high-affinity transport attenuates Salmonella typhimurium virulence. Infect. Immun. 65**:**587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen, R., T. G. Kloosterman, J. Kok, and O. P. Kuipers. 2006. GlnR-mediated regulation of nitrogen metabolism in Lactococcus lactis. J. Bacteriol. 188**:**4978-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau, G. W., S. Haataja, M. Lonetto, S. E. Kensit, A. Marra, A. P. Bryant, D. McDevitt, D. A. Morrison, and D. W. Holden. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40**:**555-571. [DOI] [PubMed] [Google Scholar]
- 33.Leonard, C. G., J. M. Ranhand, and R. M. Cole. 1970. Competence factor production in chemically defined media by noncompetent cells of group H Streptococcus strain Challis. J. Bacteriol. 104**:**674-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marra, A., J. Asundi, M. Bartilson, S. Lawson, F. Fang, J. Christine, C. Wiesner, D. Brigham, W. P. Schneider, and A. E. Hromockyj. 2002. Differential fluorescence induction analysis of Streptococcus pneumoniae identifies genes involved in pathogenesis. Infect. Immun. 70**:**1422-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mickelson, M. N. 1964. Chemically defined medium for growth Streptococcus pyogenes. J. Bacteriol. 88**:**158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molle, V., Y. Nakaura, R. P. Shivers, H. Yamaguchi, R. Losick, Y. Fujita, and A. L. Sonenshein. 2003. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J. Bacteriol. 185**:**1911-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nobbs, A. H., R. J. Lamont, and H. F. Jenkinson. 2009. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 73**:**407-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noske, N., U. Kammerer, M. Rohde, and S. Hammerschmidt. 2009. Pneumococcal interaction with human dendritic cells: phagocytosis, survival, and induced adaptive immune response are manipulated by PavA. J. Immunol. 183**:**1952-1963. [DOI] [PubMed] [Google Scholar]
- 39.Obert, C., J. Sublett, D. Kaushal, E. Hinojosa, T. Barton, E. I. Tuomanen, and C. J. Orihuela. 2006. Identification of a candidate Streptococcus pneumoniae core genome and regions of diversity correlated with invasive pneumococcal disease. Infect. Immun. 74**:**4766-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orihuela, C. J., G. Gao, K. P. Francis, J. Yu, and E. I. Tuomanen. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190**:**1661-1669. [DOI] [PubMed] [Google Scholar]
- 41.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72**:**5582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polissi, A., A. Pontiggia, G. Feger, M. Altieri, H. Mottl, L. Ferrari, and D. Simon. 1998. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect. Immun. 66**:**5620-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pracht, D., C. Elm, J. Gerber, S. Bergmann, M. Rohde, M. Seiler, K. S. Kim, H. F. Jenkinson, R. Nau, and S. Hammerschmidt. 2005. PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect. Immun. 73**:**2680-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rennemeier, C., S. Hammerschmidt, S. Niemann, S. Inamura, U. Zahringer, and B. E. Kehrel. 2007. Thrombospondin-1 promotes cellular adherence of gram-positive pathogens via recognition of peptidoglycan. FASEB J. 21**:**3118-3132. [DOI] [PubMed] [Google Scholar]
- 45.Si, Y., F. Yuan, H. Chang, X. Liu, H. Li, K. Cai, Z. Xu, Q. Huang, W. Bei, and H. Chen. 2009. Contribution of glutamine synthetase to the virulence of Streptococcus suis serotype 2. Vet. Microbiol. 139**:**80-88. [DOI] [PubMed] [Google Scholar]
- 46.Sonenshein, A. L. 2005. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8**:**203-207. [DOI] [PubMed] [Google Scholar]
- 47.Tamura, G. S., A. Nittayajarn, and D. L. Schoentag. 2002. A glutamine transport gene, glnQ, is required for fibronectin adherence and virulence of group B streptococci. Infect. Immun. 70**:**2877-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293**:**498-506. [DOI] [PubMed] [Google Scholar]
- 49.Tiraby, J. G., and M. S. Fox. 1974. Marker discrimination and mutagen-induced alterations in pneumococcal transformation. Genetics 77**:**449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tseng, H.-J., A. G. McEwan, J. C. Paton, and M. P. Jennings. 2002. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 70**:**1635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]